Polysubstituted benzochromone derivative and synthesis method thereof
A technology of benzochromone and synthesis method, which is applied in the field of multi-substituted benzochromone derivatives and their synthesis, can solve the problems of harsh reaction conditions, unstable raw materials, and reduced total yield, and achieve less side reactions and more efficient reactions. Conditions are easy to achieve, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
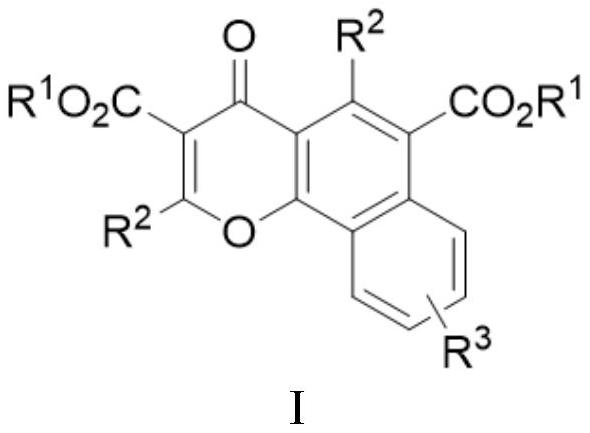
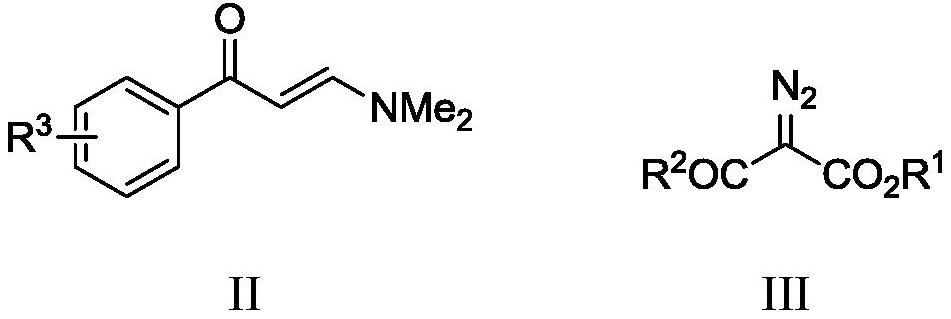
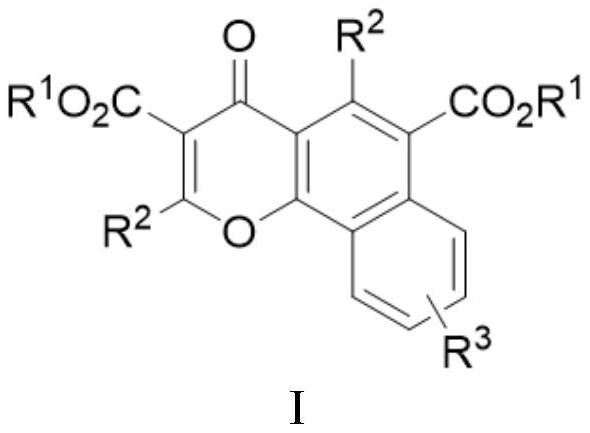
[0053] A multi-substituted benzochromone derivative has a structure shown in the following formula Ia, and the reaction scheme is as follows:
[0054]
[0055] The specific preparation steps are as follows: Add dimethylaminovinylacetophenone IIa (35mg, 0.2mmol), diazo compound IIIa (93.7mg, 0.6mmol) into a 25mL reaction tube, [Cp*RhCl 2 ] 2 (5.1mg, 0.008mmol), AgSbF 6 (11mg, 0.032mmol), AgOAc (6.7mg, 0.04mmol), Li 2 CO 3 (30mg, 0.4mmol); N 2 Under protection, dichloromethane (2 mL) was added and reacted at 100° C. for 24 hours. Then separated by silica gel column chromatography (eluent: petroleum ether (boiling point is 60-90°C) / ethyl acetate=10:1, v / v) to obtain yellow liquid Ia (58 mg, yield 78%), the target product Confirmed by NMR and high-resolution mass spectrometry.
[0056] Characterization data of polysubstituted benzochromone derivative Ia:
[0057] Multi-substituted benzochromone derivative Ia, red solid;
[0058] 1 H NMR (400MHz, CDCl 3 )δ8.40(d, J=8.3...
Embodiment 2
[0060] A kind of multi-substituted benzochromone derivatives has a structure shown in the following formula Ib, and the reaction scheme is as follows:
[0061]
[0062] The specific preparation steps are the same as in Example 1, except that the structural formula of the reaction substrate is shown as IIb (41 mg, 0.2 mmol); other steps and conditions are consistent with Example 1.
[0063] In this example, 64 mg of polysubstituted benzochromone derivative Ib was obtained, with a yield of 80%.
[0064] Characterization data of polysubstituted benzochromone derivative Ib:
[0065] Multi-substituted benzochromone derivative Ib, red solid.
[0066] 1 H NMR (400MHz, CDCl 3 )δ8.55(d, J=3.1Hz, 1H), 7.75(d, J=15.0Hz, 1H), 7.08(dd, J=15.0, 3.1Hz, 1H), 4.34(q, J=11.7Hz, 2H), 4.19(q, J=11.8Hz, 2H), 3.81(s, 3H), 2.54(s, 3H), 2.09(s, 3H), 1.28(m, 6H). 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ184.9, 171.3, 166.5, 163.3, 162.8, 151.3, 143.0, 138.2, 125.3, 123.2, 120.0, 117.7, 115.7, 111.8, 1...
Embodiment 3
[0068] A multi-substituted benzochromone derivative has a structure shown in the following formula Ic, and the reaction scheme is as follows:
[0069]
[0070] The specific preparation steps are the same as in Example 1, except that the structural formula of the reaction substrate is shown as IIc (42 mg, 0.2 mmol); other steps and conditions are consistent with Example 1.
[0071] In this example, 61 mg of polysubstituted benzochromone derivative Ic was obtained, with a yield of 75%.
[0072] Characterization data of polysubstituted benzochromone derivative Ic:
[0073] Multi-substituted benzochromone derivative Ic, red solid.
[0074] 1 H NMR (400MHz, CDCl 3 )δ8.00(d, J=15.0Hz, 1H), 7.78(d, J=3.1Hz, 1H), 7.53(dd, J=15.0, 2.9Hz, 1H), 4.34(q, J=11.7Hz, 2H), 4.19(q, J=11.8Hz, 2H), 2.54(s, 3H), 2.09(s, 3H), 1.28(m, 6H). 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ184.9, 171.3, 166.5, 163.3, 149.7, 143.2, 140.7, 137.0, 129.1, 128.3, 126.2, 125.1, 123.1, 113.9, 111.8, 61.44, 61.2, 19....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com