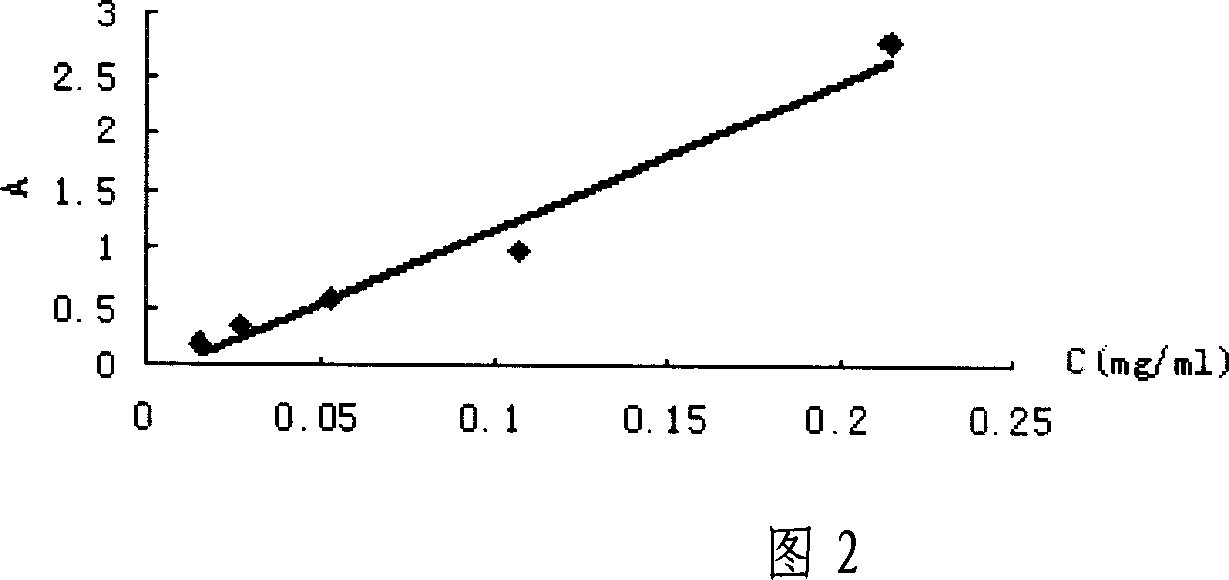
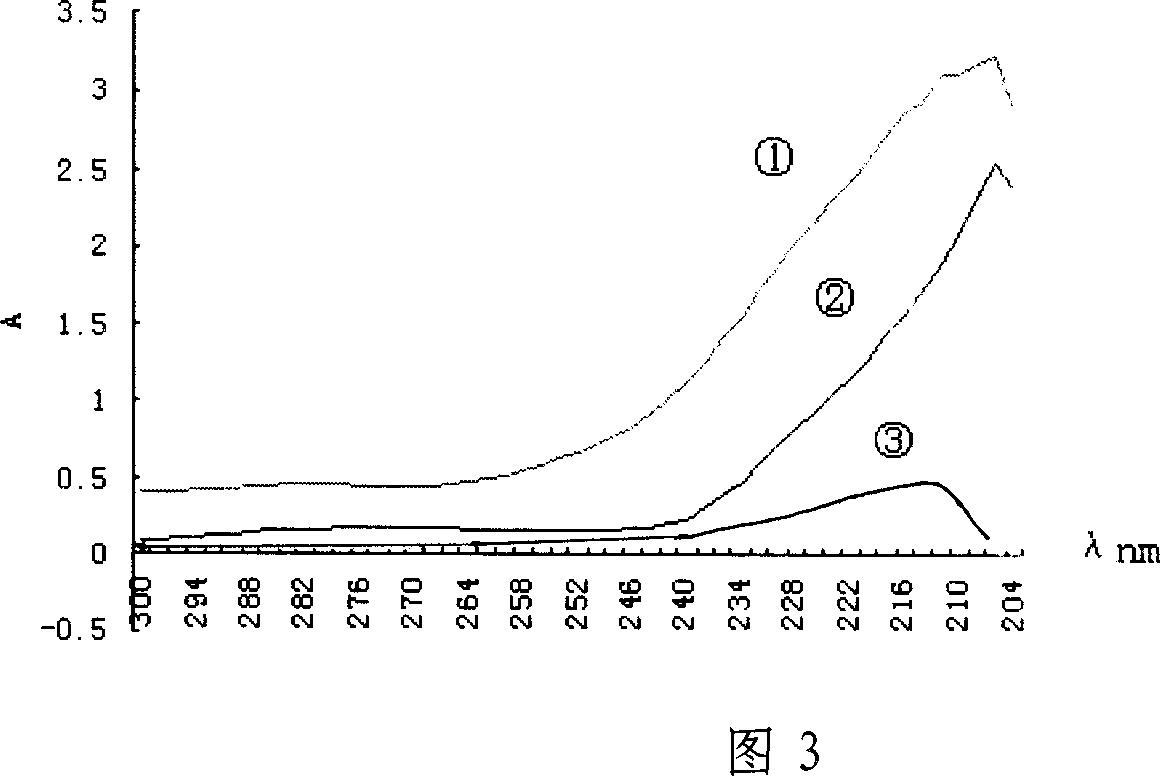
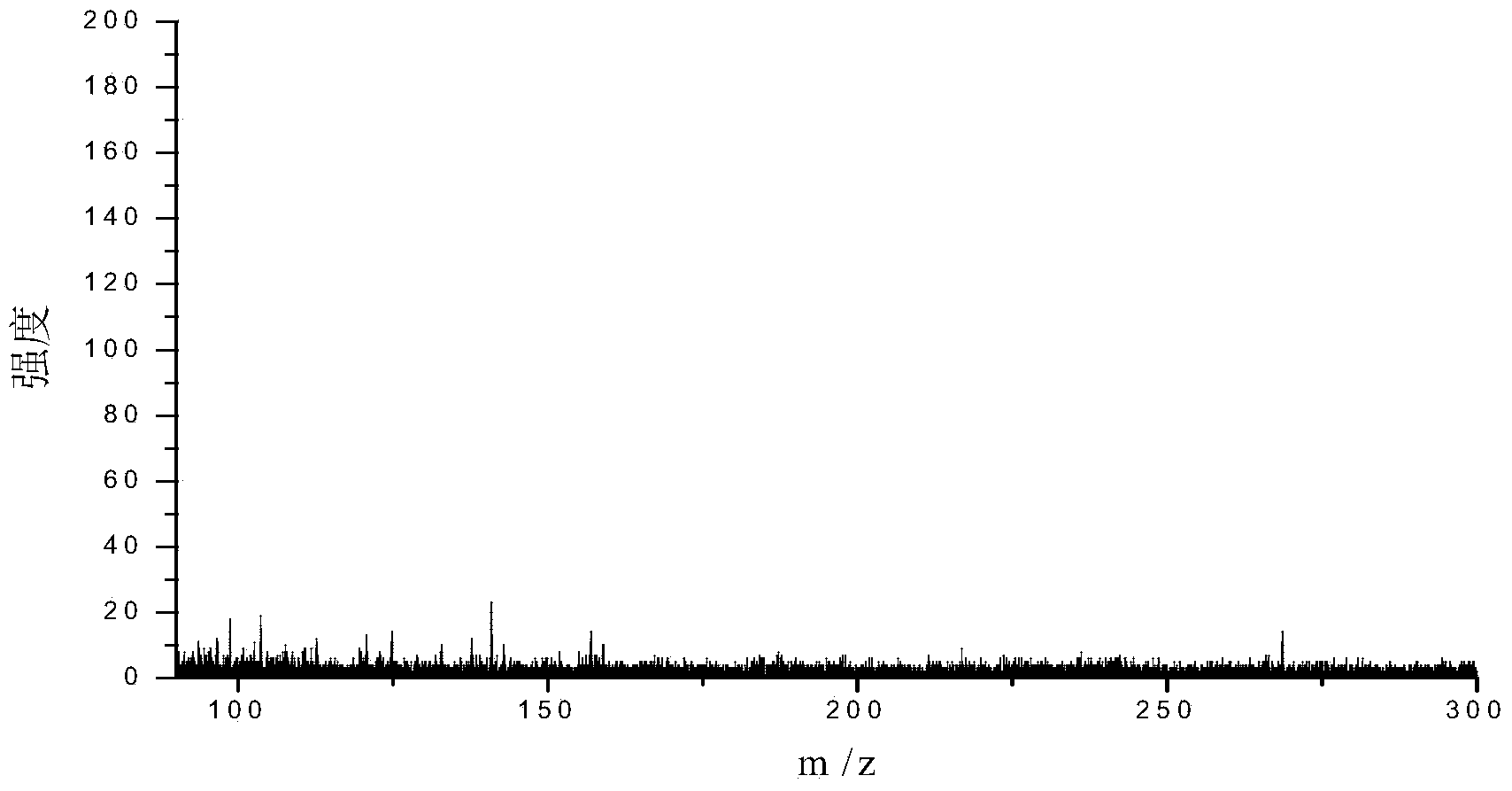
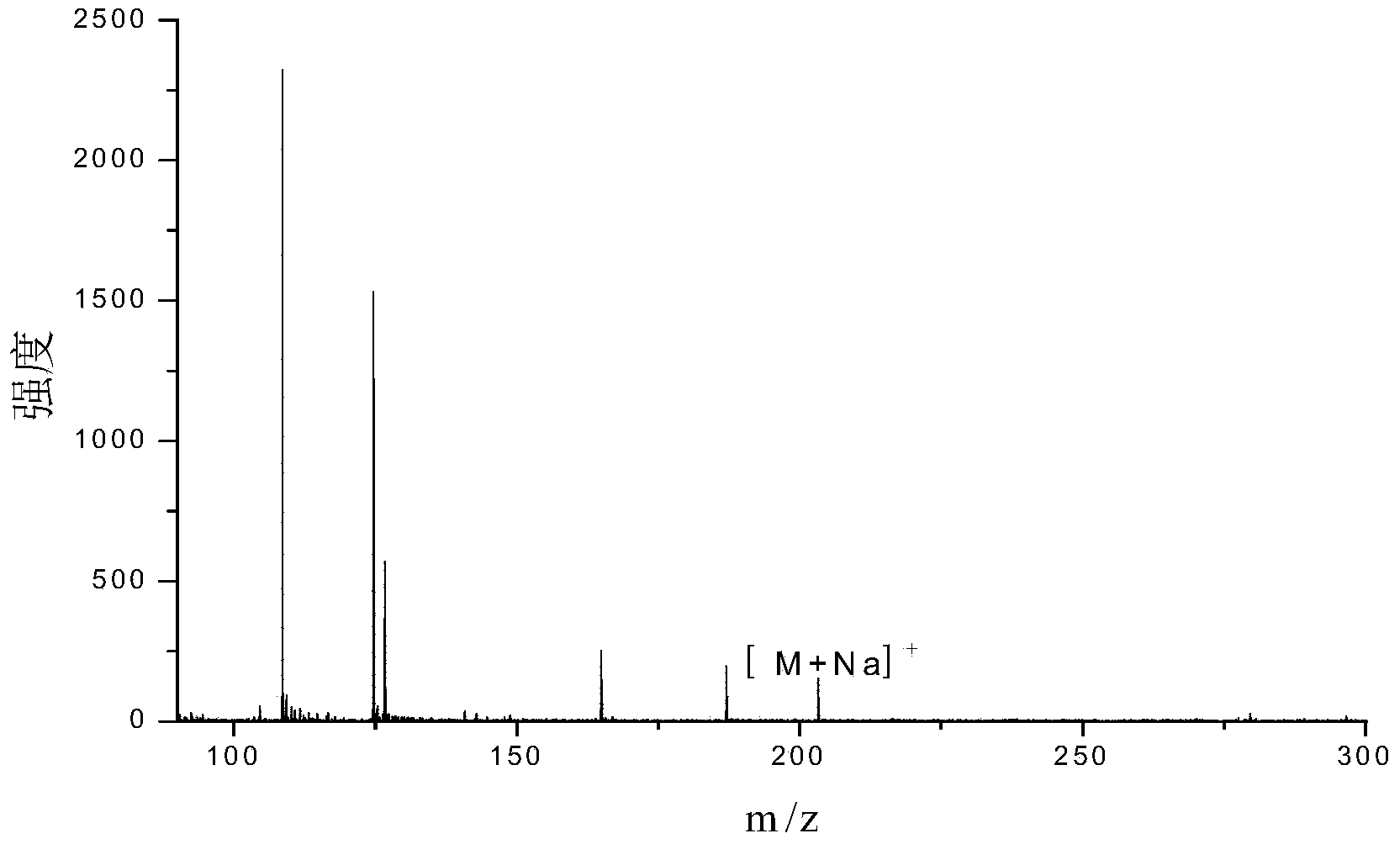
Patents
Literature
711 results about "Vinylbital" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vinylbital, also known as butylvinal, is a sedative hypnotic drug which is a barbiturate derivative. It was developed by Aktieboleget Pharmacia in the 1950s.
Silicone hydrogels comprising n-vinyl amides and hydroxyalkyl (meth)acrylates or (meth)acrylamides
The present invention relates to a process comprising the steps of reacting a reactive mixture comprising at least one silicone-containing component, at least one hydrophilic component, and at least one diluent to form an ophthalmic device having an advancing contact angle of less than about 80°; and contacting the ophthalmic device with an aqueous extraction solution at an elevated extraction temperature, wherein said at least one diluent has a boiling point at least about 10° higher than said extraction temperature.
Owner:JOHNSON & JOHNSON VISION CARE INC
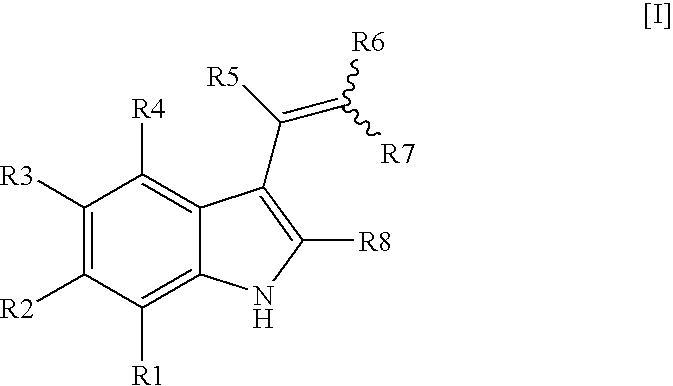
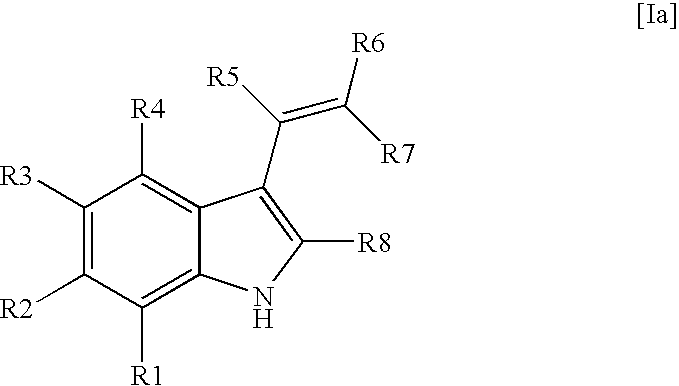
Vinyl-phenyl derivatives for inflammation and immune-related uses
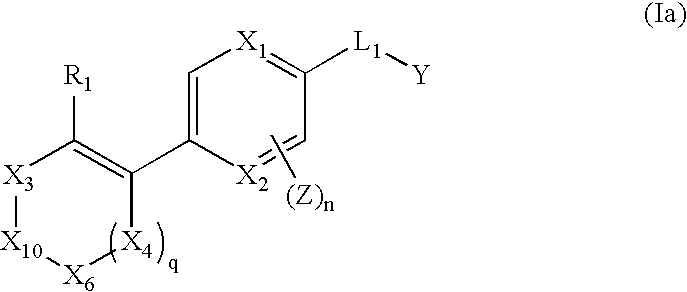
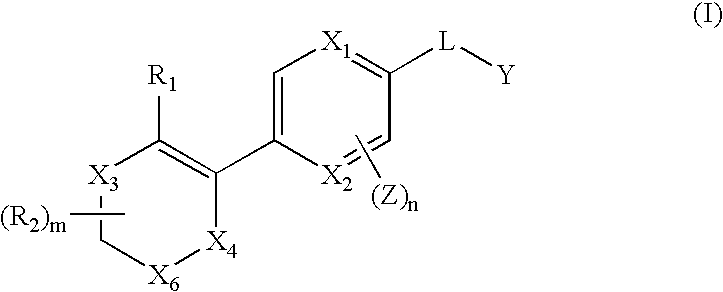
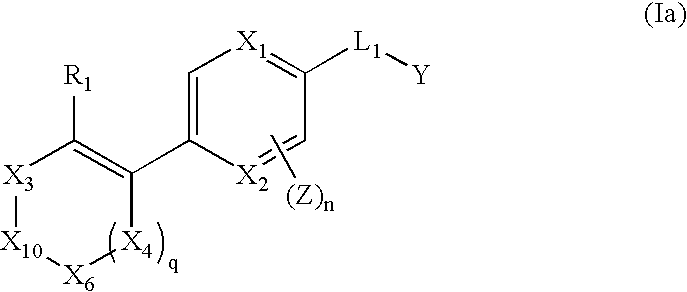
The invention relates to compounds of structural formula (Ia): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof, wherein X1, X2, X3, X4, X6, X10, R1, Y, Z, L, and n are defined herein. These compounds are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.
Owner:SYNTA PHARMA CORP
Process for preparing prostaglandin derivatives and starting materials for the same
Owner:YONSUNG FINE CHEM CO LTD
Novel polyhydroxyalkanoate copolymer containing in molecule unit with vinylphenyl structure in its side chain and method of manufacturing the same
A polyhydroxyalkanoate (PHA) having a desired configuration is produced using a raw material containing omega-(4-vinylphenyl)-alkanoic acid and (omega-substituted alkanoic acid in which a group having a ring structure selected from phenyl, thienyl, and cyclohexyl structures substitutes therefor on the end thereof by producing a PHA copolymer containing the corresponding 3-hydroxy-omega-(4-vinylphenyl)-alkanoate unit and the corresponding 3-hydroxy-omega-substituted alkanoate unit by making use of a microorganism capable of producing the PHA or by oxidizing a predetermined portion of the corresponding PHA.
Owner:CANON KK
Polycationic amphiphiles as antimicrobial agents
The present disclosure provides an antimicrobial composition including a polycationic amphiphile compound, and the method of making and the method of using such a compound or composition. The compound having the formula (I) or (II) R1, R2, R3, R4, R5, R6, R10, or R11 is H or a C1-12 alkyl unsubstituted or optionally substituted with a functional group such as —OH, —OR′, —NH2, —NHR′, —NR′2, —N—C(O)R′, —N—C(O)CR′═CR′, —SH, —SR′, —O—C(O)R′, —C(O)R′, —CF3, —OCF3, halogen, benzyl, o-vinylbenzyl, m-vinylbenzyl, p-vinylbenzyl, phenyl, allyl, and substituted allyl. R7, R8 or R9 is a C1-12 alkyl unsubstituted or optionally substituted with a functional group such as —OH, —OR′, —NH2, —NHR′, —NR′2, —SH, —SR′, —O—C(O)R′, —C(O)R′, —CF3, and —OCF3. R′ is H or a C1-4 alkyl. X or Y is a halogen, m and n are integers in the range from 1 to 25.
Owner:TEMPLE UNIVERSITY +1
Band-Seals For Hard Capsules
InactiveUS20080008750A1Low production costShorten production timeN-vinyl-pyrrolidone polymer adhesivesCapsule deliveryPullulanMedicine
The present invention has as its object to provide a band-seal to manufacture a leak-tight, stable hard capsule preparation not being susceptible to deformation after having filled pharmaceuticals, foods, healthy foods, etc. in a hard capsule comprising a polyvinyl alcohol copolymer or pullulan as a base, and in more particular, relates to a band-seal intended for use in the hard capsule comprising a polyvinyl alcohol copolymer or pullulan as a base, characterized in that the band-seal is incorporated with at least one or not less than two of the below-described (a) to (c): (a) A polyvinylpyrrolidone with a molecular weight of 100,000 to 4,000,000, (b) A polyvinylpyrrolidone with a molecular weight of 10,000 to 80,000 in the proportion of not more than 90% by weight against the total weight of the band-seal; and (c) A copolymer from 1-vinyl-2-pyrrolidone and vinyl acetate.
Owner:QUALICAPS CO LTD
Electrolytic copper plating bath and plating process therewith
ActiveUS7220347B2Easy to fillSmall diameterSemiconductor/solid-state device manufacturingAnti-corrosive paintsElectrolysisPyrrolidinones
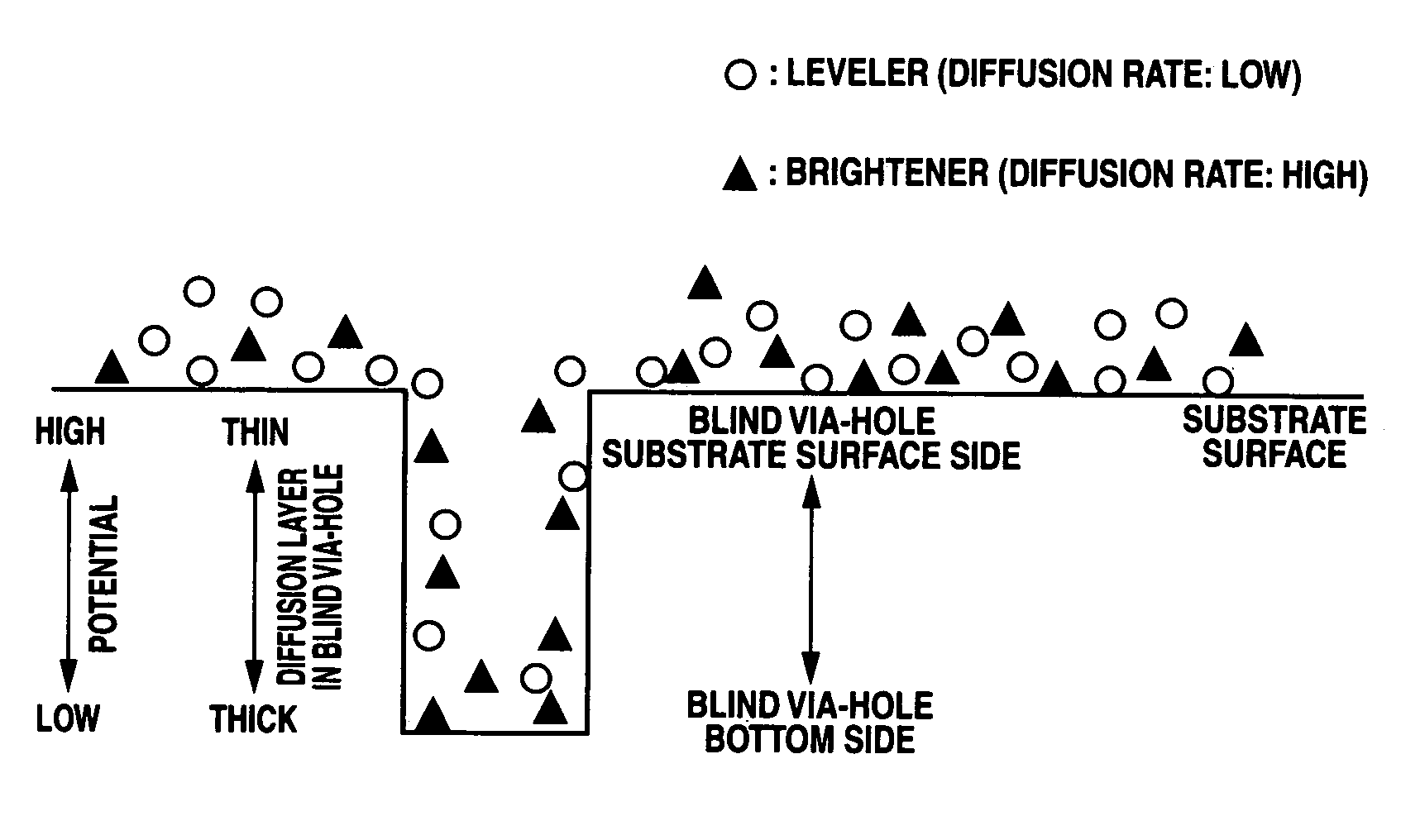
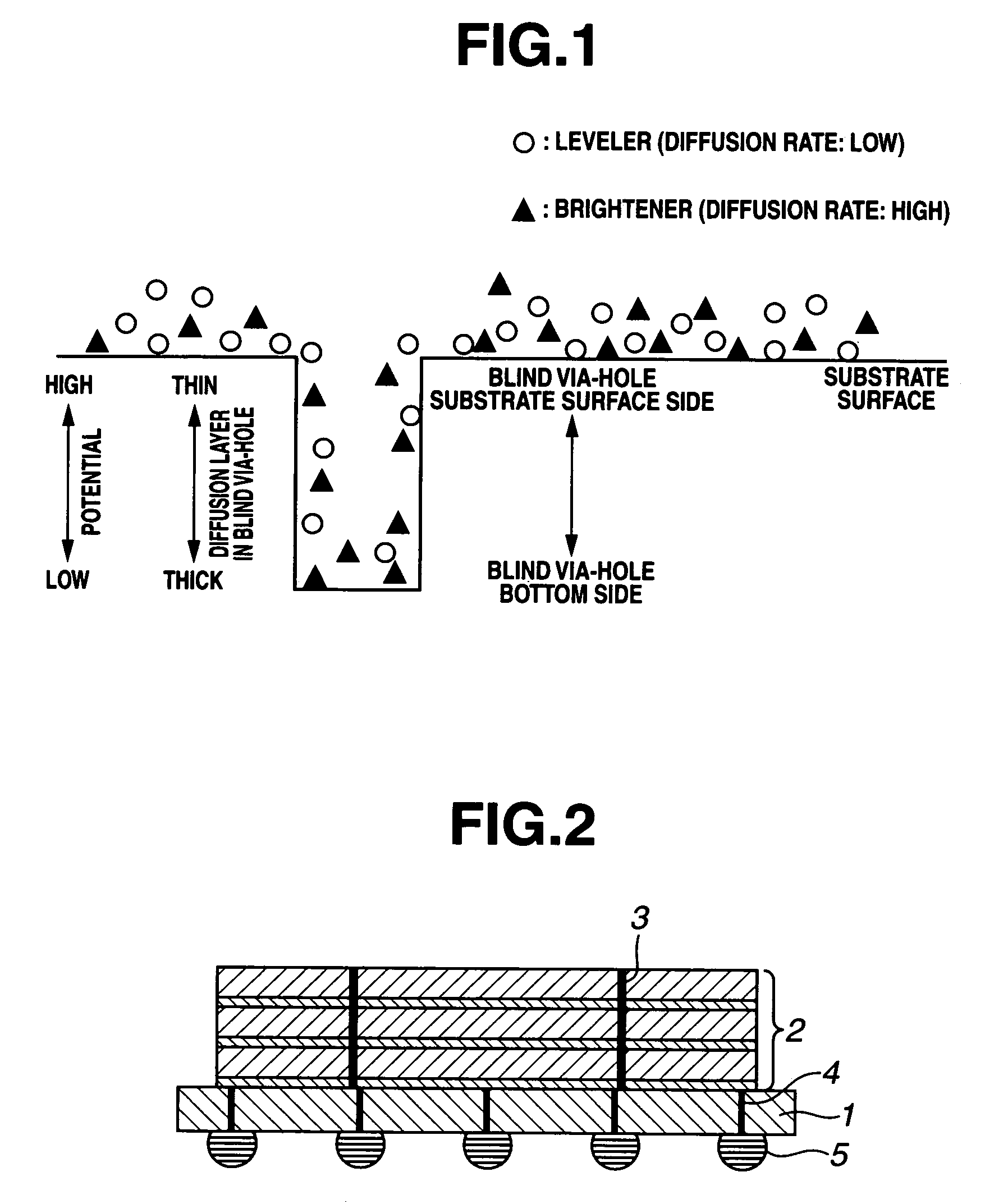
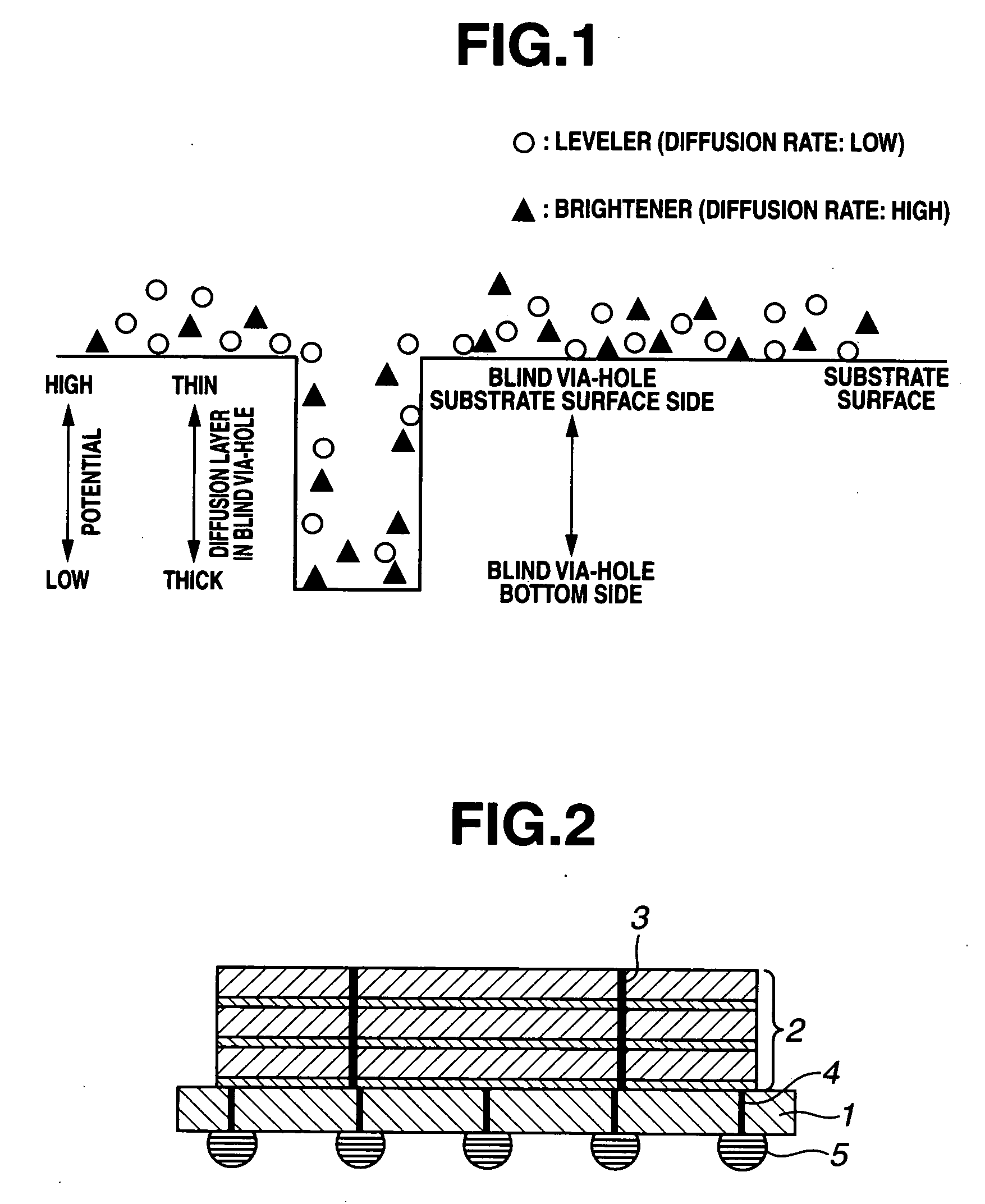
An electrolytic copper plating bath used for via-filling plating of blind via-holes formed on a substrate, containing a water-soluble copper salt, sulfuric acid, chloride ions, and a leveler as an additive, wherein the leveler is either one or both of a quaternary polyvinylimidazolium compound represented by the following formula (1) and a copolymer, represented by the following formula (2), of vinylpyrrolidone and a quaternary vinylimidazolium compound:where R1 and R2 are each an alkyl group, m is an integer of not less than 2, and p and q are each an integer of not less than 1, and a copper electroplating method for via-filling plating of blind via-holes formed on a substrate by use of the electrolytic copper plating bath.
Owner:C UYEMURA & CO LTD
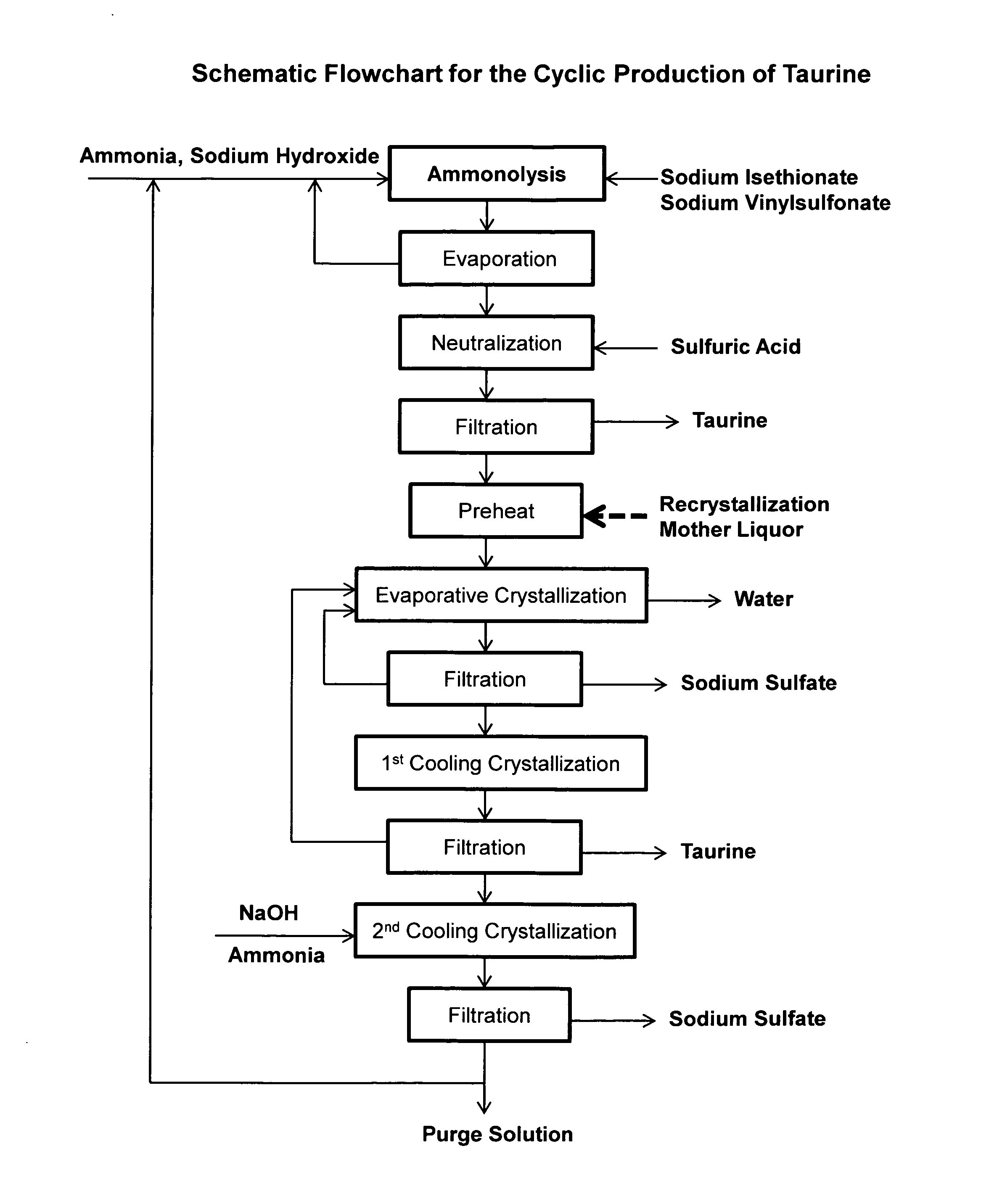
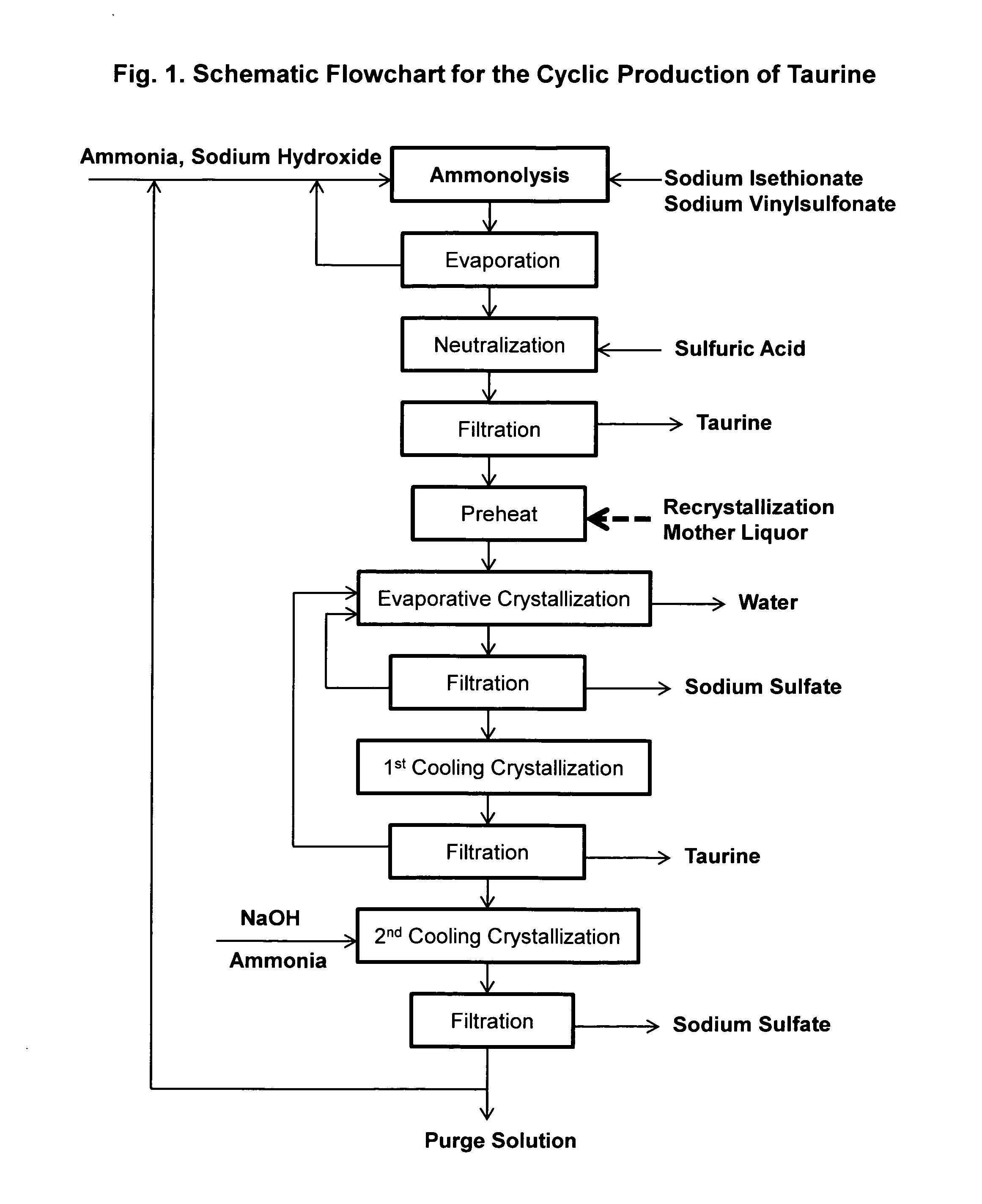
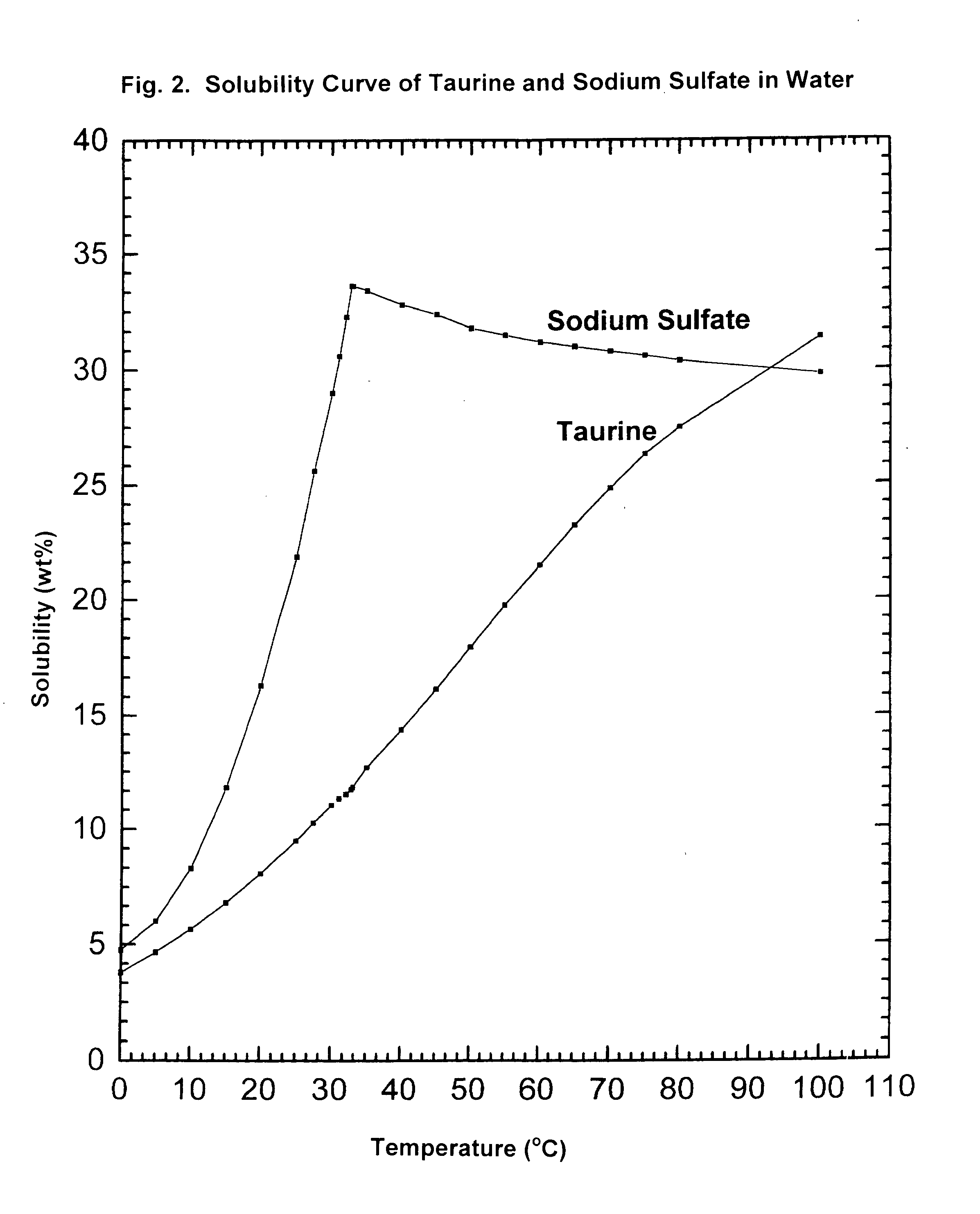
Cyclic process for the production of taurine from alkali isethionate and alkali vinyl sulfonate
ActiveUS20150299114A1Facilitate cooling crystallizationOrganic compound preparationSulfonic acids salts preparationCyclic processSulfonate
The present invention discloses a cyclic process for the production of taurine from alkali isethionate and alkali vinyl sulfonate in a high overall yield of greater than 95% by continuously converting the byproducts of the ammonolysis reaction, sodium ditaurinate and sodium tritaurinate, to sodium taurinate. Sodium sulfate and residual taurine in the crystallization mother liquor are efficiently separated by converting taurine into a highly soluble form of sodium taurinate or ammonium taurinate while selectively crystallizing sodium sulfate.
Owner:VITAWORKS IP LLC
Electrolytic copper plating bath and plating process therewith
ActiveUS20060207886A1Easy to fillHigh aspect ratioSemiconductor/solid-state device manufacturingAnti-corrosive paintsIonChemistry
An electrolytic copper plating bath used for via-filling plating of blind via-holes formed on a substrate, containing a water-soluble copper salt, sulfuric acid, chloride ions, and a leveler as an additive, wherein the leveler is either one or both of a quaternary polyvinylimidazolium compound represented by the following formula (1) and a copolymer, represented by the following formula (2), of vinylpyrrolidone and a quaternary vinylimidazolium compound: where R1 and R2 are each an alkyl group, m is an integer of not less than 2, and p and q are each an integer of not less than 1, and a copper electroplating method for via-filling plating of blind via-holes formed on a substrate by use of the electrolytic copper plating bath.
Owner:C UYEMURA & CO LTD
Curable compositions containing 1,1-di-activated vinyl compounds that cure by pericyclic reaction mechanisms
ActiveUS20180094115A1Organic chemistryNon-macromolecular adhesive additivesConjugated dienePericyclic reaction
Curable compositions containing a compound comprising a conjugated diene group and a 1,1-di-activated vinyl compound are described. The curable compositions can cure by pericyclic reaction mechanisms.
Owner:PPG IND OHIO INC
Hydrosilation of vinyl-terminated macromonomers
Owner:EXXONMOBIL CHEM PAT INC
Application of vinyl silicone oil containing hydroxyl groups at terminal as release force regulator to preparation of release agent, release agent and release film
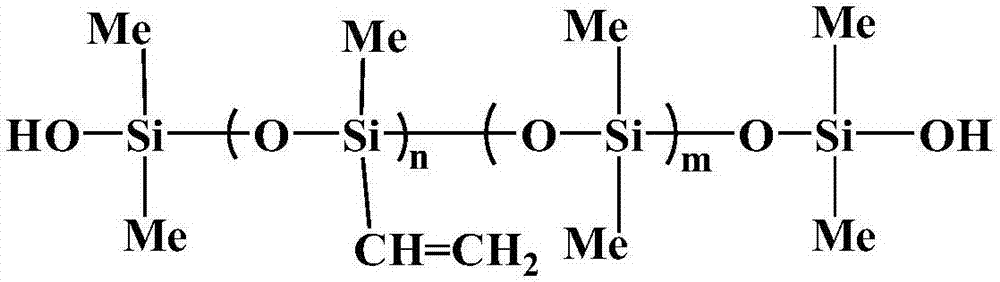
The invention belongs to the technical field of release force regulators, discloses an application of vinyl silicone oil containing hydroxyl groups at terminal as a release force regulator to preparation of a release agent, the release agent and a release film and particularly provides an application to preparation of an addition type organosilicon release agent. According to the application of the vinyl silicone oil containing the hydroxyl groups at the terminal as the release force regulator to preparation of the release agent, the structural formula of the vinyl silicone oil containing the hydroxyl groups at the terminal is shown in the description, wherein n and m represent constitutional repeating units, n ranges from 1 to 10, and m ranges from 0 to 6. The vinyl silicone oil containing the hydroxyl groups as the regulator contains double functional groups, namely, the hydroxyl groups and vinyl groups, thereby having double functions when applied to the release agent, substrate bonding firmness is enhanced by the hydroxyl groups, surface tension of the material is improved by introduction of the hydroxyl groups, the vinyl groups participate in hydrosilylation, compatibility with the release agent is enhanced, the release film with remarkably improved release force can be obtained, and the higher the content of the vinyl silicone oil is, the higher the release force of the release film is.
Owner:国科广化(南雄)新材料研究院有限公司 +2
Radiation curable ink jet composition, recorded matter, and ink jet recording method
ActiveUS20120133059A1Good adhesivenessImprove scratch resistanceSemiconductor/solid-state device detailsSolid-state devicesPolymer chemistryRadiation
The invention provides a radiation curable ink jet ink composition including: a monomer equal to or more than 20% by mass and equal to or less than 50% by mass with respect to the total mass of the ink composition, which is represented by the following formula (I); and N-vinylcaprolactam equal to or more than 5% by mass and equal to or less than 15% by mass with respect to the total mass of the ink composition:CH2═CR1—COOR2—O—CH═CH—R3 (I)wherein, R1 is a hydrogen atom or a methyl group, R2 is a divalent organic residue having 2 to 20 carbon atoms, and R3 is a hydrogen atom or monovalent organic residue having 1 to 11 carbon atoms.
Owner:SEIKO EPSON CORP
Method for hydrotreating butadiene tail gas
ActiveCN103787815AReduce loadReduce energy consumptionHydrocarbon by hydrogenationHydrocarbon purification/separationVinylacetyleneButene
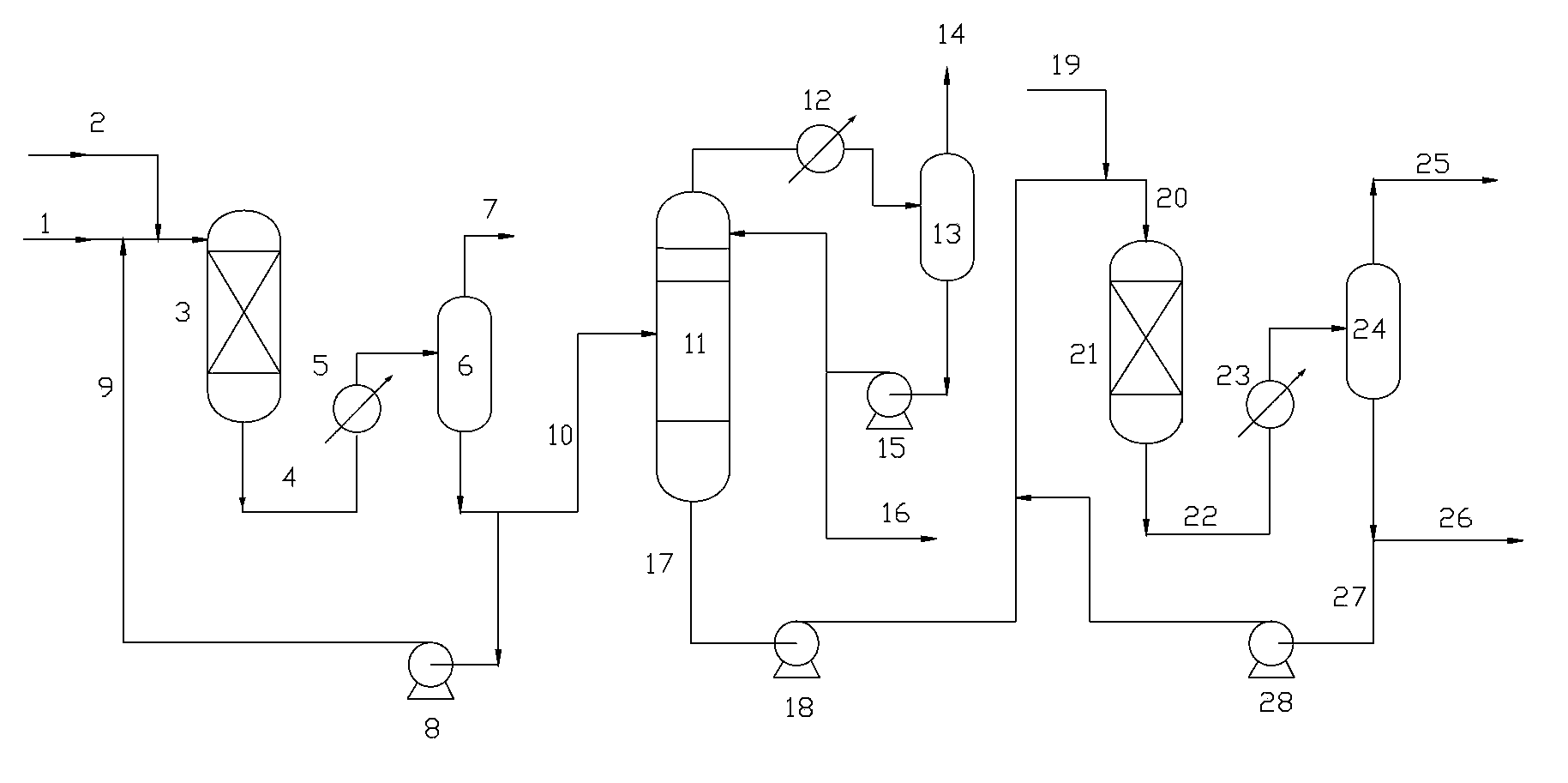
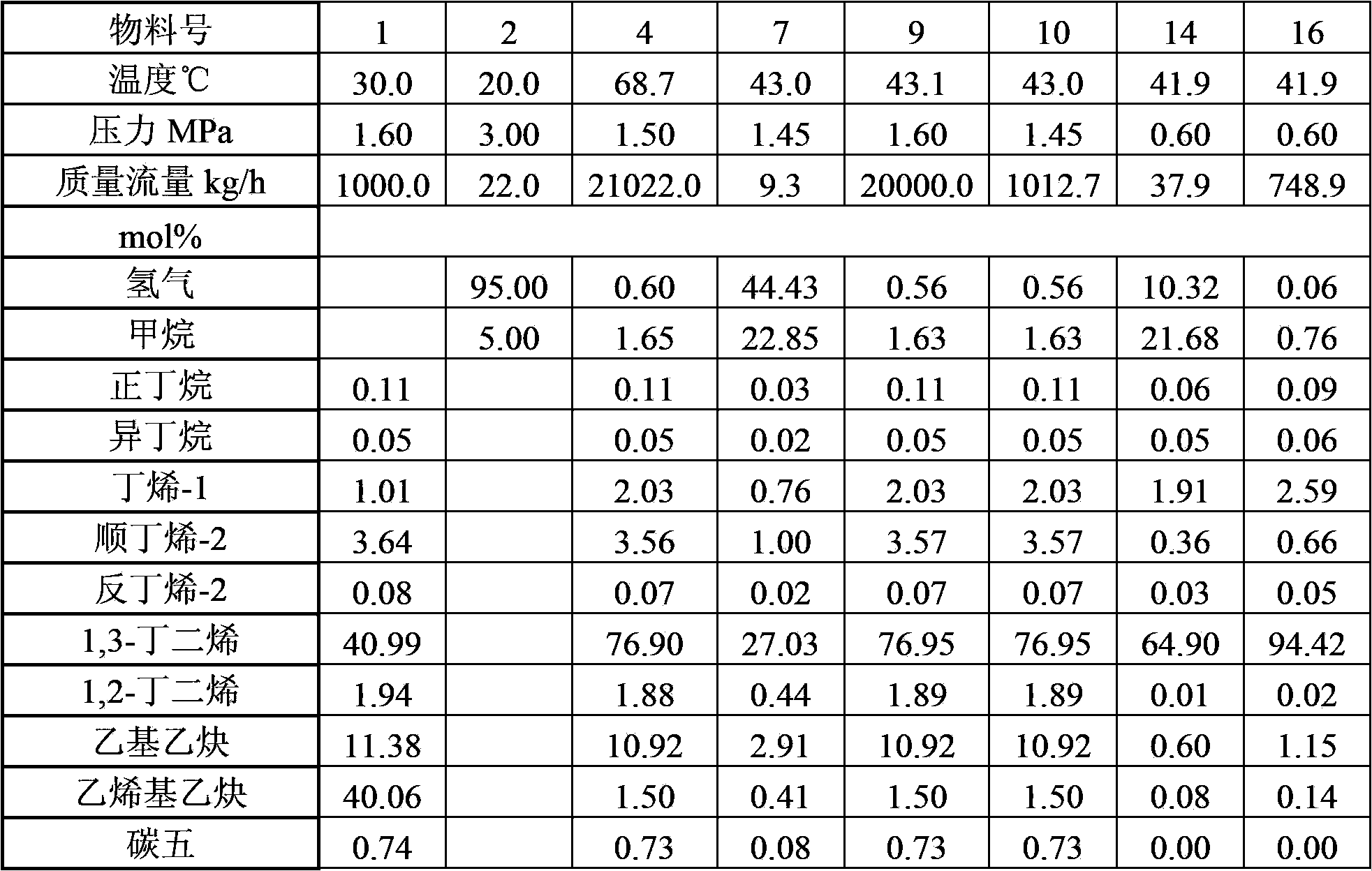
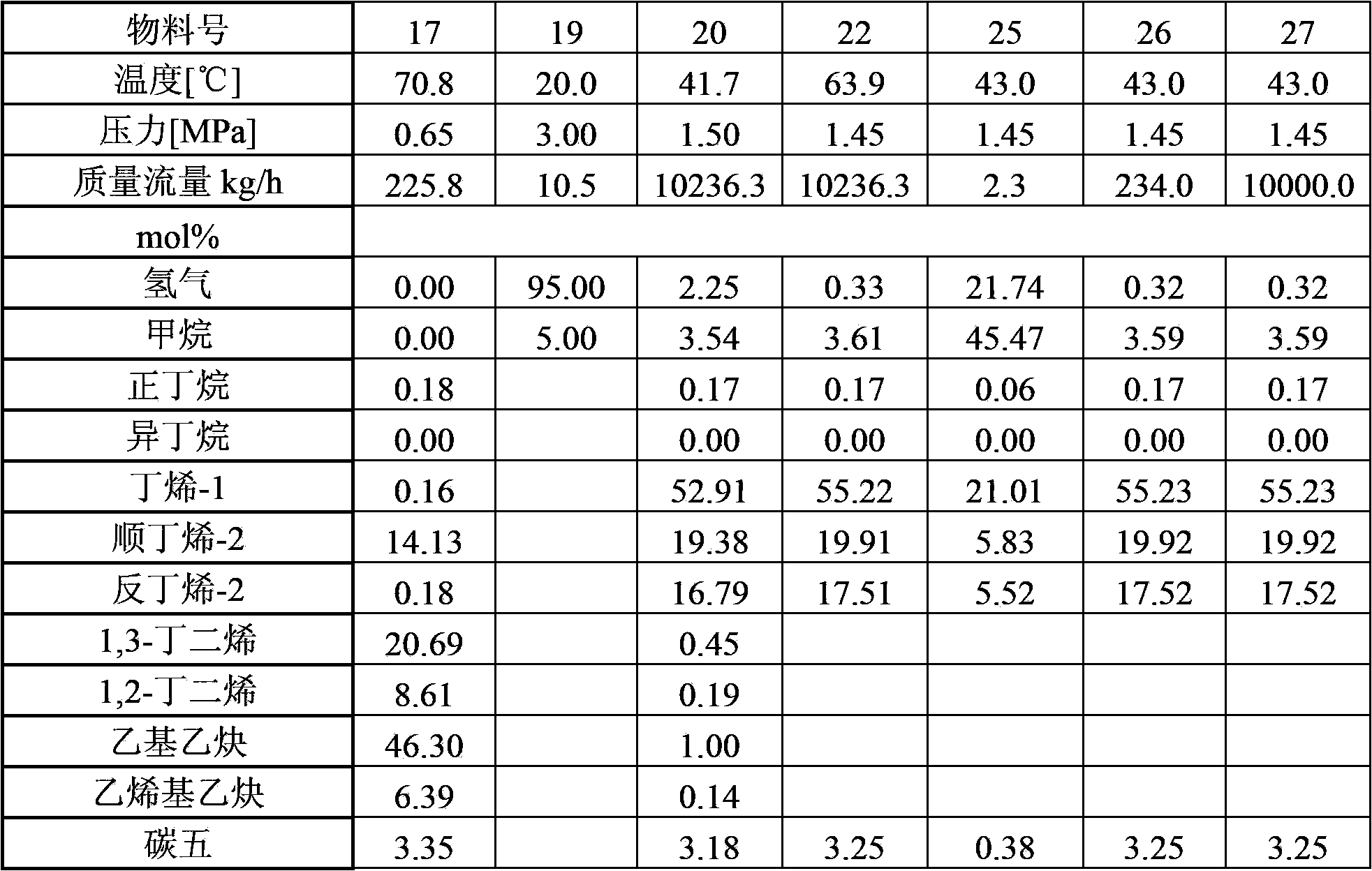
The invention provides a method for hydrotreating alkynes-rich butadiene tail gas. The method comprises the following steps: extracting butadiene tail gas, a selective hydrogenation is carried out in a one-section hydrogenation reactor, vinylacetylene is performed with hydro-conversion to 1,3-butadiene; the one-section hydrogenation reaction products are seperared, a part which is rich in 1,3-butadiene is sent to a butadiene extraction device, other part is performed with the selective hydrogenation reaction through a second-section hydrogenation reactor, alkynes and dialkene are performed with hydro-conversion to 1-butylene, two hydrogenation reaction products are sent to a recovery device of 1-butylene. The method is capable of fully recovering the valuable substances in the butadiene tail gas, and the economic benefit is obvious.
Owner:CHINA PETROLEUM & CHEM CORP +1
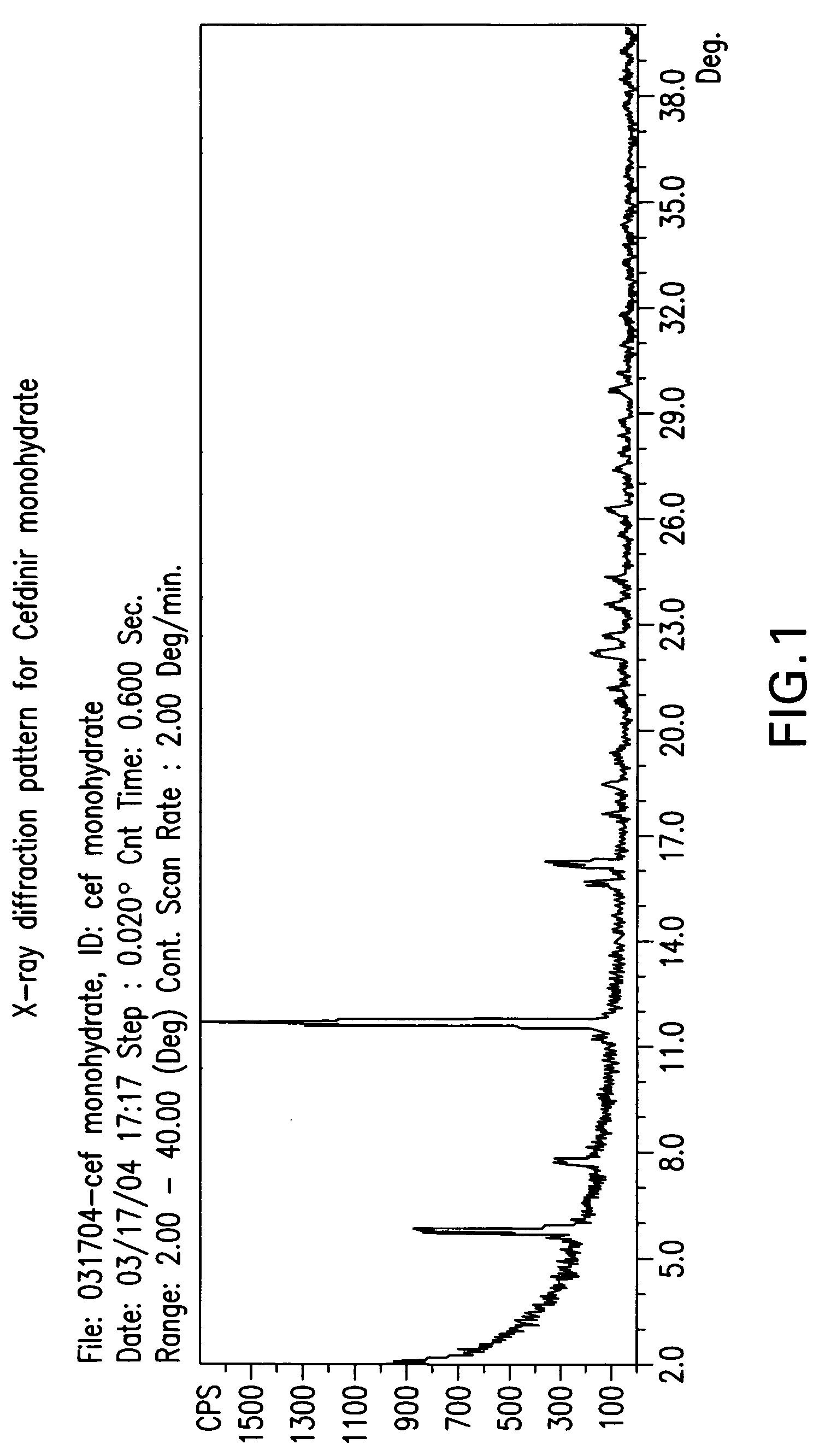
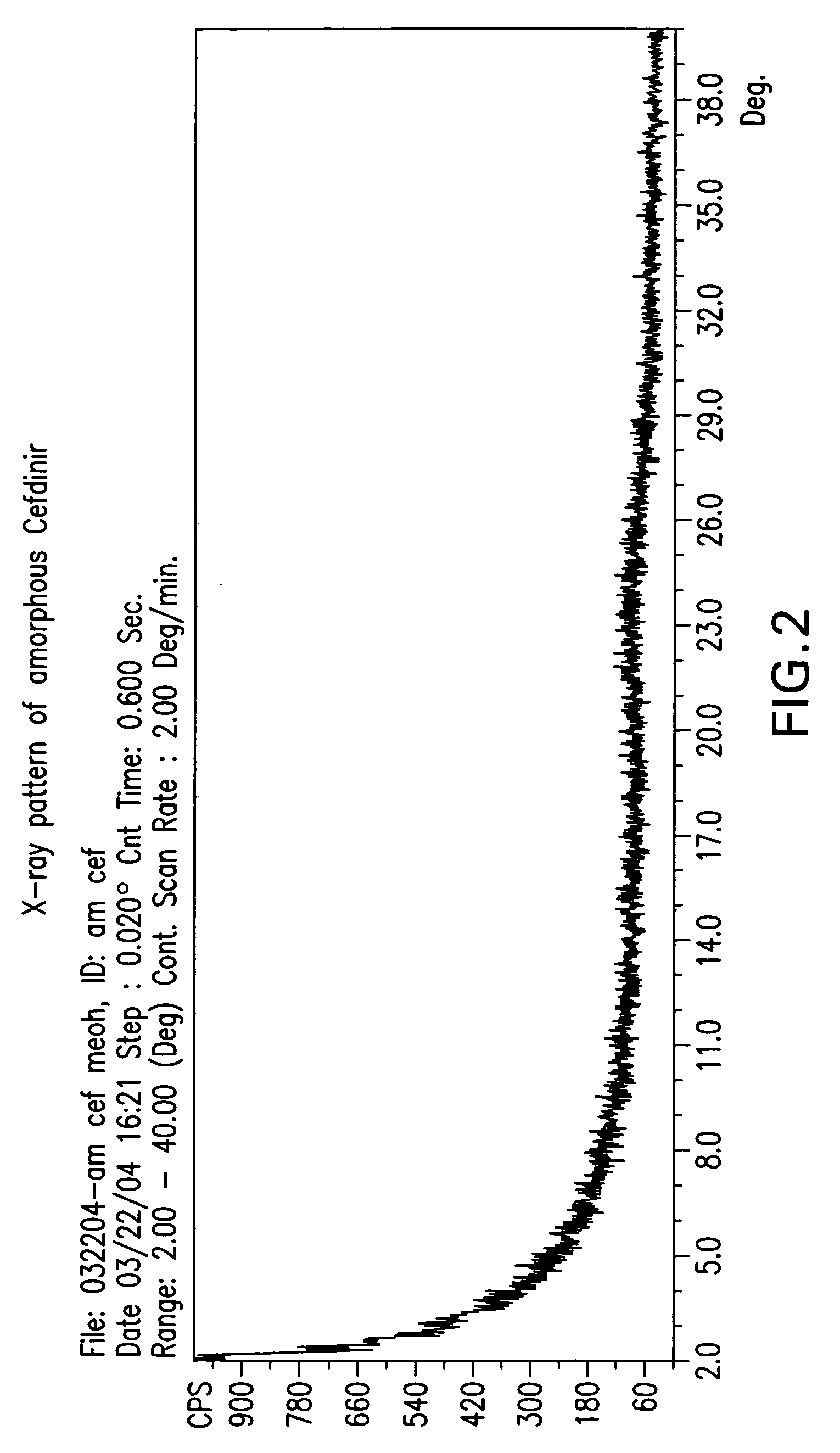
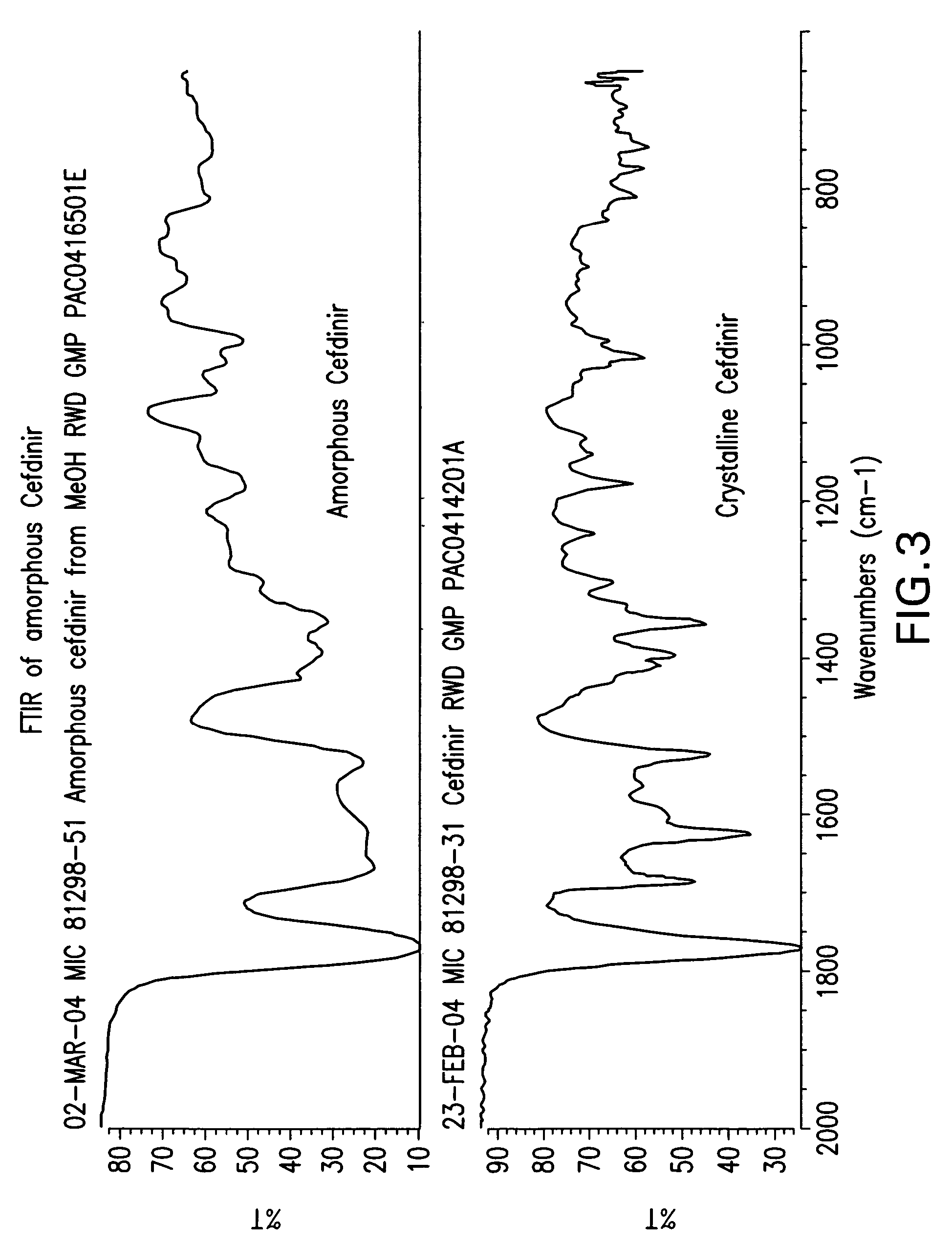
Stable amorphous cefdinir
The present invention relates to stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer), methods for its preparation, and pharmaceutical compositions comprising stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer).
Owner:ABBOTT LAB INC
Monolithic column, preparation method and application thereof
InactiveCN101537344AImprove uniformityOptimize volumeOther chemical processesPeptide preparation methodsFunctional monomerFoaming agent
The invention discloses a monolithic column, a preparation method and a application thereof. The preparation method comprises the following steps: 1) polymeric monomer, pore-foaming agent, initiator and cuprous bromide are mixed together and then added with catalyst ligand after being deoxidized; the mixture is put into a chromatographic column tube to react in a sealing way; after the reaction, the chromatographic column tube is connected with a high pressure transfer pump, and the soluble substances such as the pore-foaming agent and the like are rinsed and removed by organic solvent, so that a monolithic column with a three-dimensional continuous skeleton structure is obtained; 2) the mixture of the organic solution, catalyst, catalyst ligand and antioxidant of the functional monomer is injected into the monolithic column, and bromine group remained on the surface of the column body initiates the surface grafting polymerization reaction of vinyl monomer. The method leads polymerization reaction to be carried out at the room temperature, so as to greatly solves the problems of inhomogeneous column body structure, volume contraction and the like caused by high polymerization temperature. Compared with the traditional monolithic column, the obtained monolithic column has obviously different structure, thus being applicable to separating biological macromolecules such as steroid hormone drugs, protein or the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
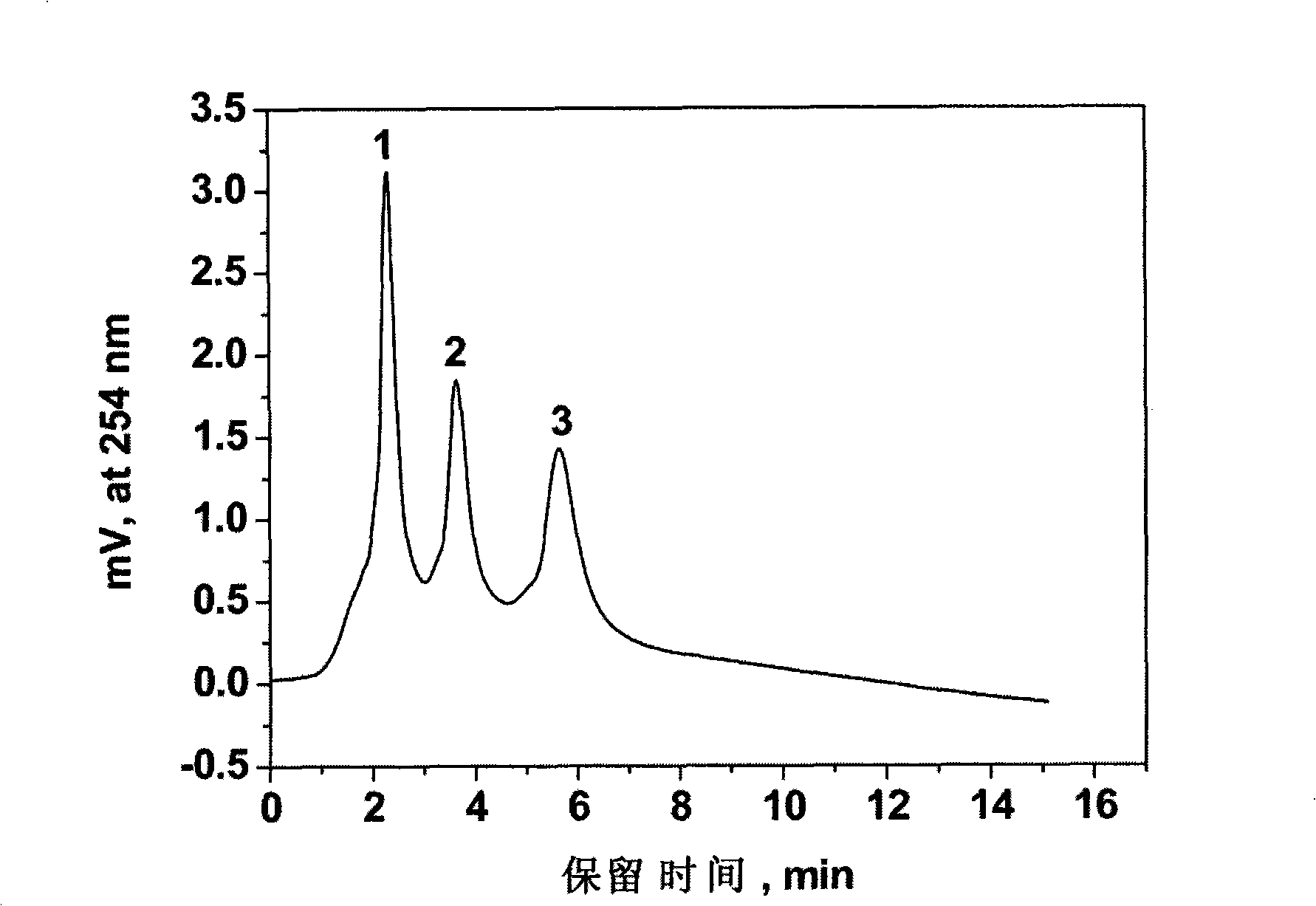
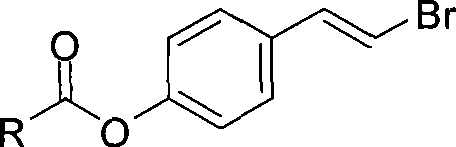
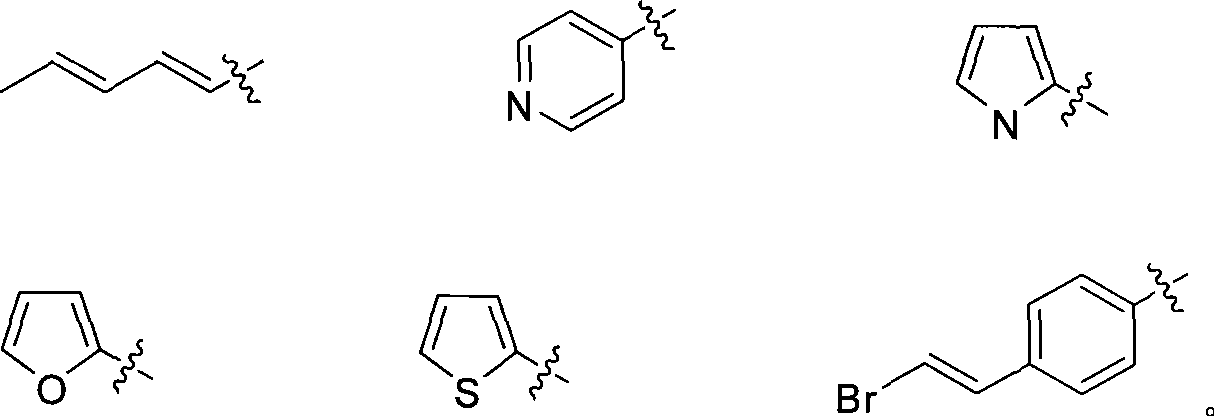
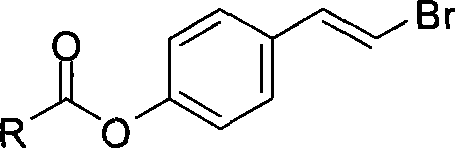
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
The invention belongs to the technical field of new drug synthesis, and in particular relates to an IDO inhibitor containing (E)-4-(beta-bromovinyl)phenoxy acyl structure, as well as a preparation method thereof. The preparation method comprises the following steps: solvent benzene, (E)-4-(beta-bromovinyl) phenol, carboxylic acid and p-dimethylaminopyridine are added to a flask respectively and magnetically stirred for 5 to 20 minutes at room temperature; then N, N'-dicyclohexylcarbodiimide is added and reacts for 2 to 24 hours at room temperature; after the reaction is completed, the solvent benzene is steamed off after decompression; residue is subjected to column chromatography separation and purification by taking ethyl acetate / petroleum ether as leacheate, and then a needed product can be obtained, wherein the molar ratio of the solvent benzene to the (E)-4-(beta-bromovinyl) phenol is 50-200:1; the molar ratio of the carboxylic acid to the (E)-4-(beta-bromovinyl) phenol is 1-1.5:1; the molar ratio of the p-dimethylaminopyridine to the (E)-4-(beta-bromovinyl) phenol is 0.1-1.5:1; and the molar ratio of the N, N'-dicyclohexylcarbodiimide to the (E)-4-(beta-bromovinyl) phenol is 1-1.2:1. The method takes the (E)-4-(beta-bromovinyl) phenol as a synthesis building block and obtains a series of the novel IDO inhibitors containing the (E)-4-(beta-bromovinyl)phenoxy acyl structure through esterification reaction, and the IDO inhibitors can be used for treating the diseases with the pathological features of IDO mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Indole derivatives for use as chemical uncoupler
InactiveUS20070010559A1Good nd harmonic generation efficiencyBiocideOrganic chemistryCompound (substance)Obesity
Novel 3-vinylsulfonyl indole derivatives are chemical uncouplers useful e.g. for treatment of obesity.
Owner:HIGH POINT PHARMA
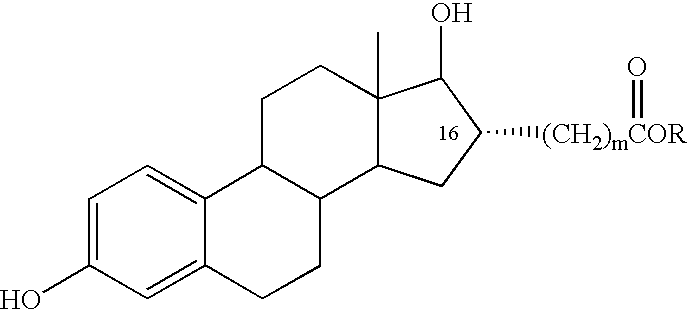
Estradiol-16alpha-carboxylic acid esters as locally active estrogens
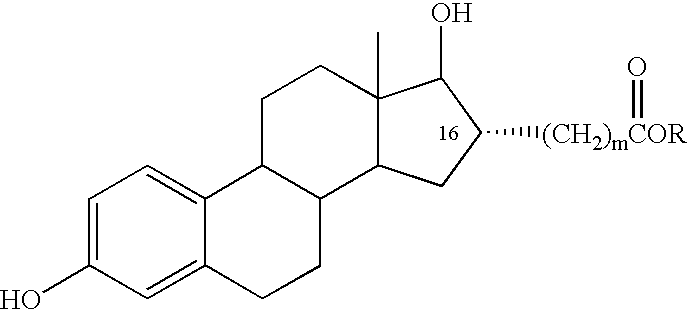
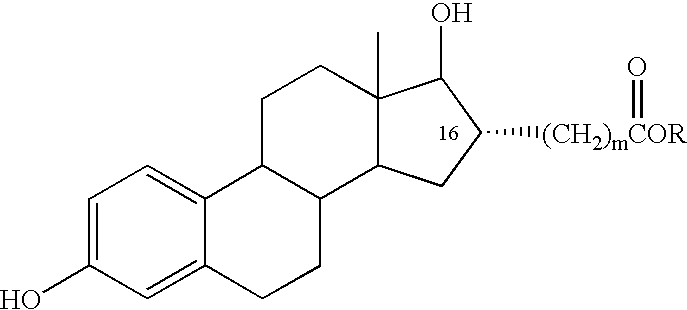
InactiveUS6476012B2Limiting deleterious effectBig advantageOrganic active ingredientsBiocideCarboxylic acidDrug biological activity
The present invention relates to analogs of estradiol, which, in their most preferred embodiment, act as locally active estrogens without significant systemic action. A series of 16alpha-carboxylic acid substituted steroids and their esters is presented which exhibit excellent biological activity for use in pharmaceutical compositions for the treatment of symptomology associated with menopause. The present invention is therefore directed to compounds according to the structure:Where R is H, a C1 to C5 alkyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3; andm is from 0-2, or a pharmaceutically acceptable salt thereof. Preferably, R is methyl, ethyl, propyl, iso-propyl, butyl, iso-butyl, pentyl, neo-pentyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0. More preferably, R is methyl, ethyl, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0.
Owner:YALE UNIV
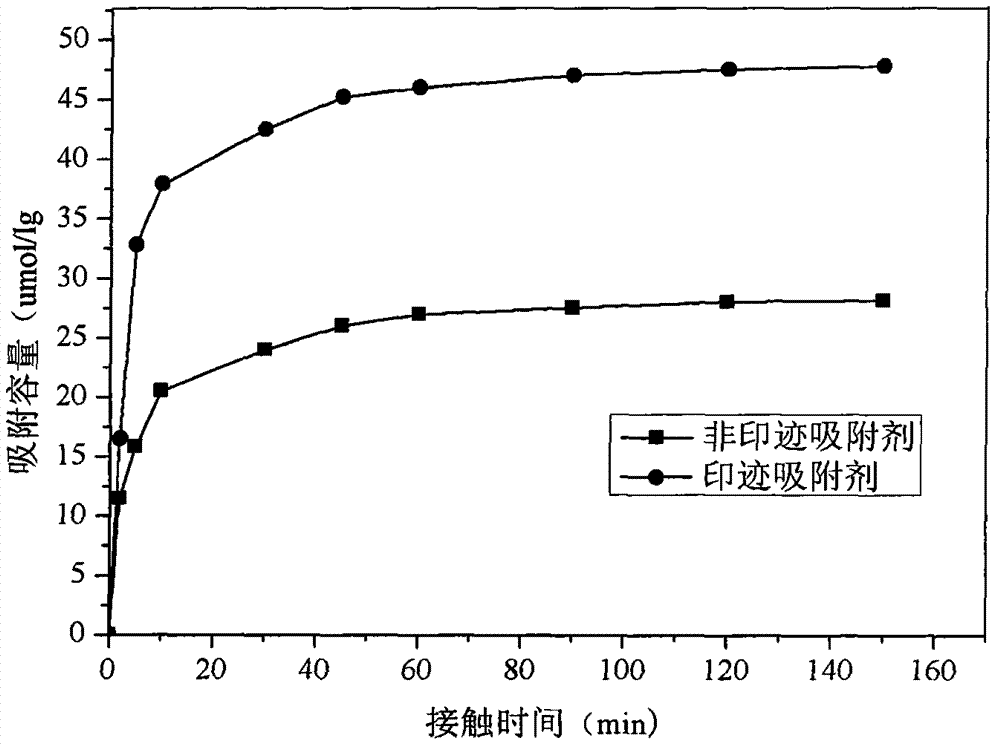
Preparation method of chloramphenicol molecular imprinted adsorbing material on surface of magnetic carbon microsphere
ActiveCN104741097AEasy to identifyHigh selectivityIon-exchange process apparatusOther chemical processesCross-linkFunctional monomer
The invention relates to a preparation method of a chloramphenicol molecular imprinted adsorbing material on the surface of a magnetic carbon microsphere. The magnetic carbon microsphere is prepared from shaddock peel by adopting a solvent thermal synthesis method, the surface of the magnetic carbon microsphere is subjected to ethylene functional modification by KH570, the treated magnetic carbon microsphere is used as a matrix material, chloramphenicol is used as a template, hydroxyethyl methylacrylate and 4-vinylpyridine are used as functional monomers, N-isopropyl acrylamide is used as a thermosensitive monomer, ethylene glycol dimethacrylate and N,N-methylene bisacrylamide are used as cross-linking agents, 2,2'-azobis[2-methylpropionamidine] dihydrochloride is used as an initiator, and the magnetic carbon microsphere surface imprinted thermosensitive adsorbing material is prepared in a methanol / water mixed system, and the adsorbing material is used for selective recognition and selective separation of chloramphenicol in a water environment. The prepared molecular imprinted adsorbing material on the surface of the magnetic carbon microsphere is low in cost, can be prepared easily, has the high recognition and selectivity properties, high separation and enrichment ability and high adsorption rate of target molecules, and is high in adsorption speed.
Owner:HENAN UNIV OF URBAN CONSTR
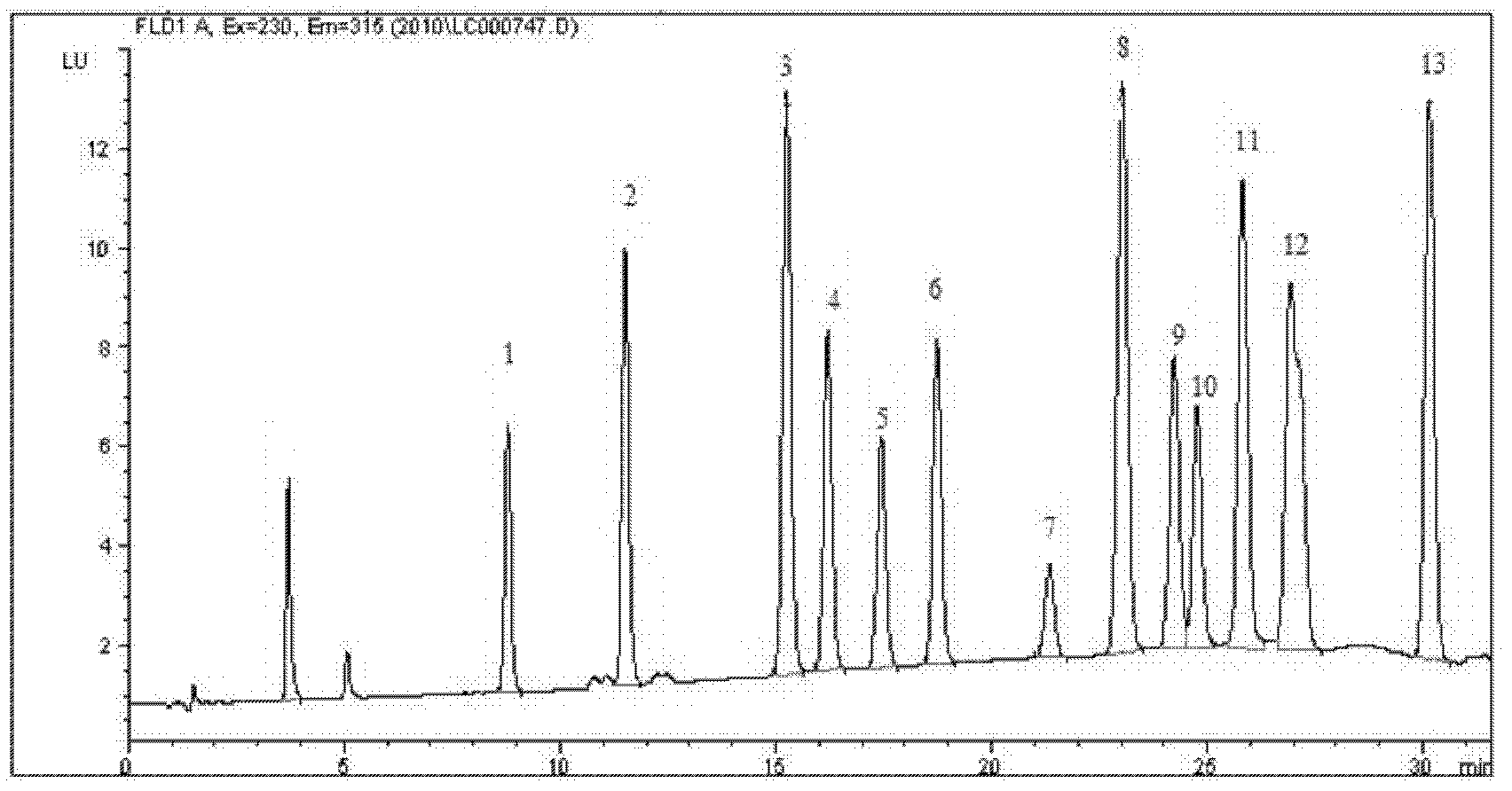
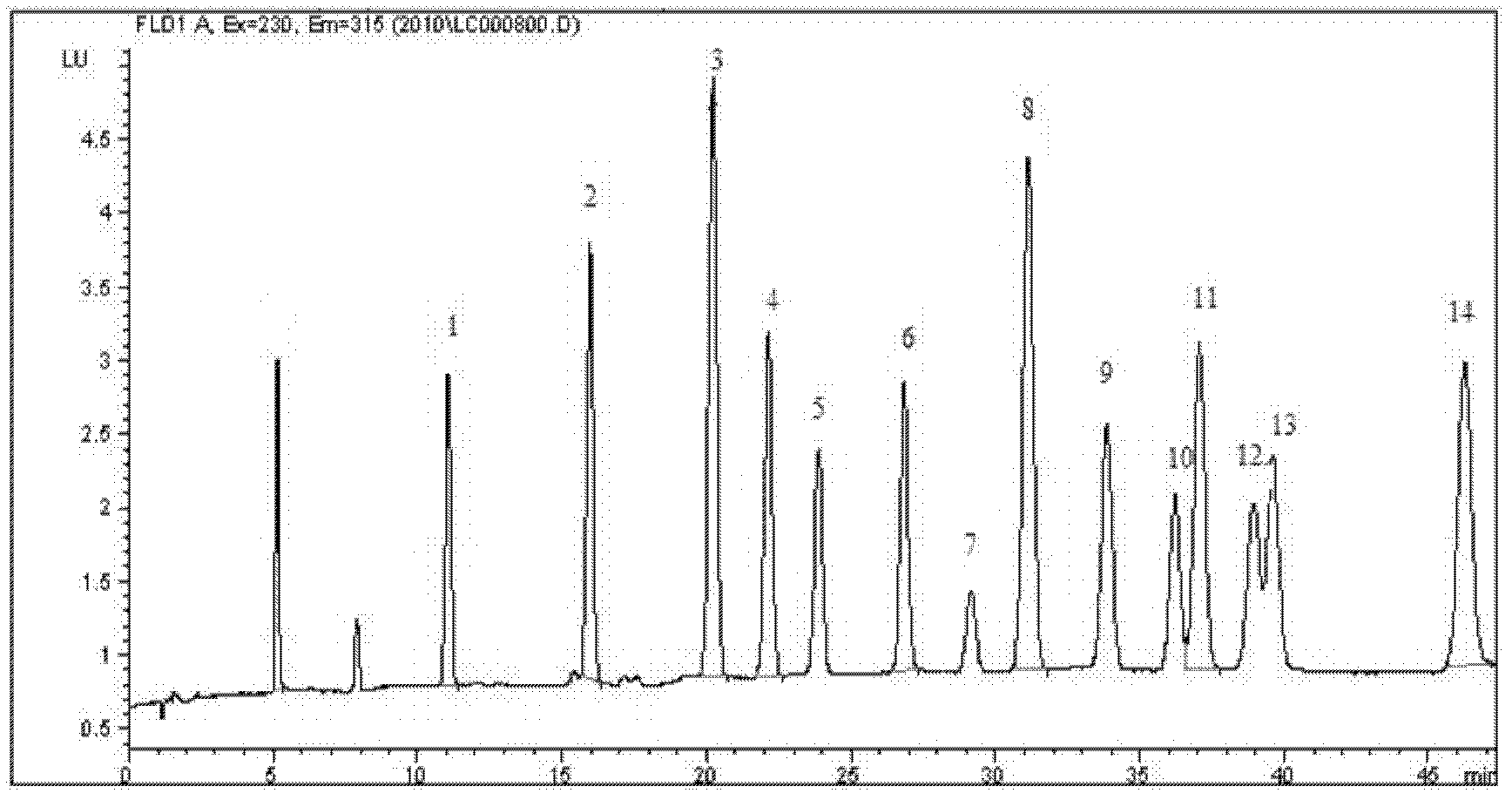
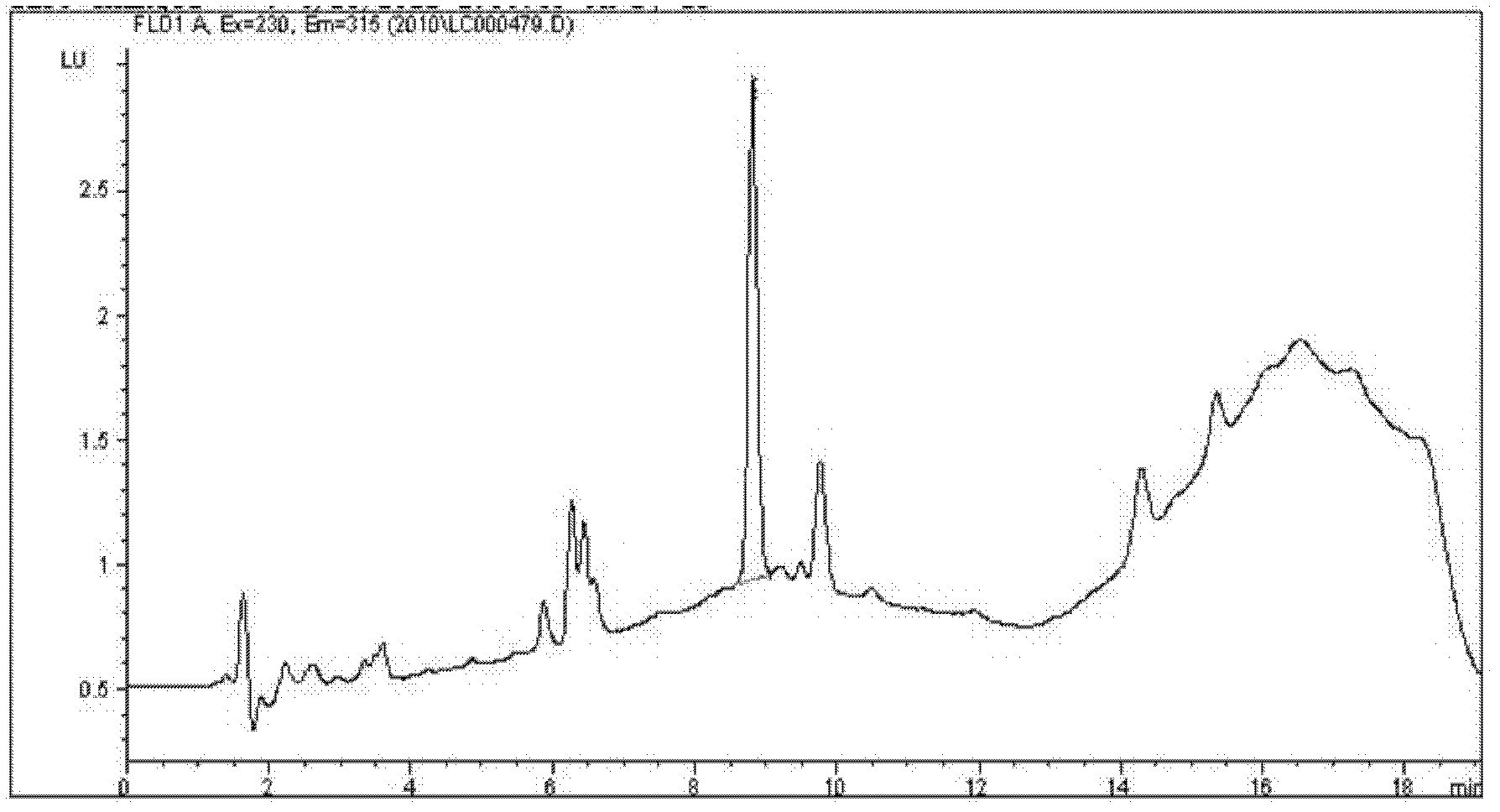
High performance liquid chromatography determination method for volatile phenolic compounds in white spirits
The invention discloses a high performance liquid chromatography determination method for volatile phenolic compounds in white spirits. By adopting a high performance liquid chromatogram-fluorescence detector (HPLC-FLD) technique to analyze volatile phenolic compounds in white spirits, the method of the invention, when used in analysis of volatile phenolic materials, can determine the following 10 volatile phenolic compounds of phenol, guaiacol, p-cresol, m-cresol, o-cresol, 4-methylguaiacol, 4-vinylphenol, 4-ethylphenol, 4-vinylguaiacol and 4-ethylguaiacol in white spirits. And the method has the advantages of no need for derivatization, high accuracy, high sensitivity and good repeatability.
Owner:GUIZHOU PROVINCIAL PRODUCT QUALITY SUPERVISION AND INSPECTION INSTITUTE +1
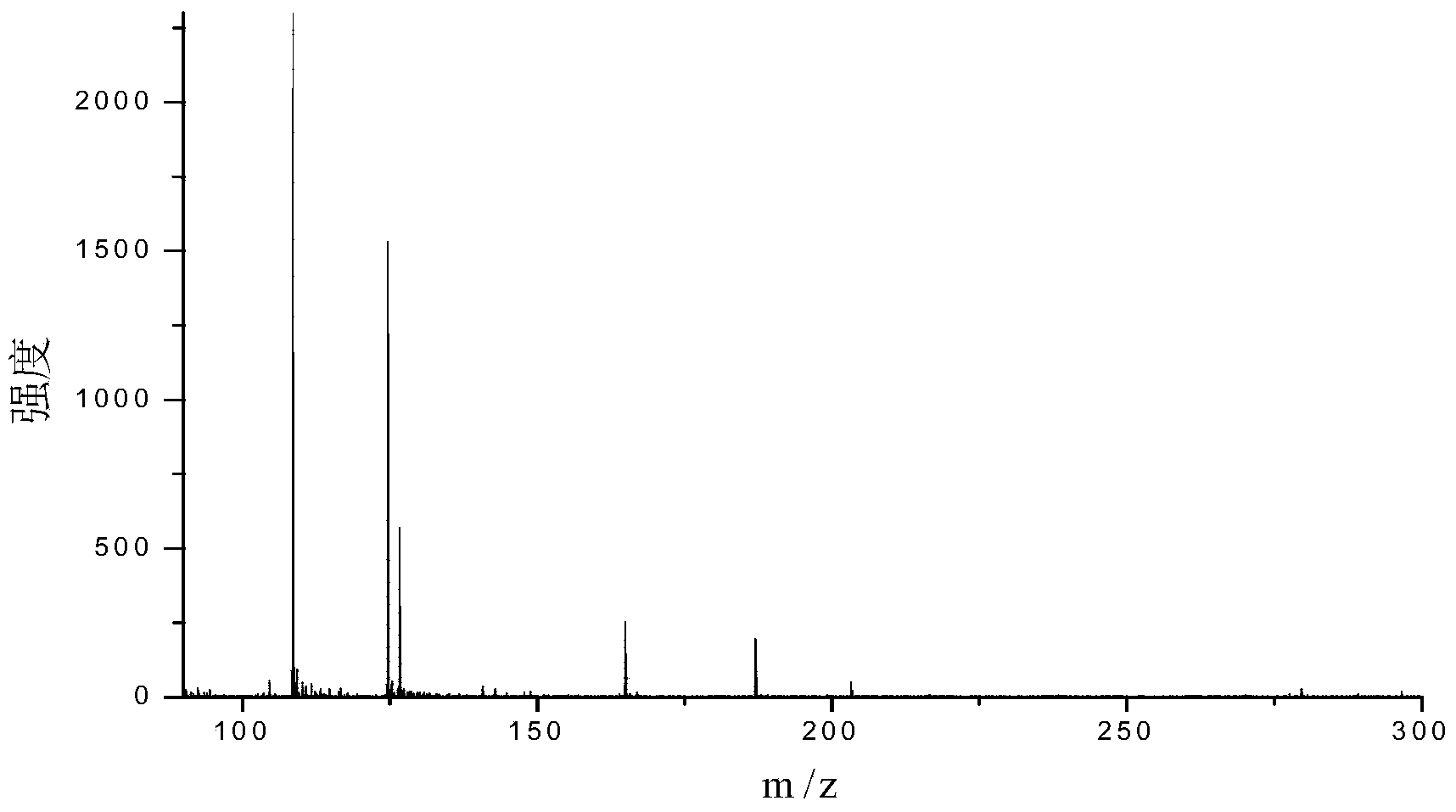
Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide
The present invention relates to pharmaceutical compositions containing axitinib, which is known as N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide or 6-[2-(methylcarbamoyl)phenylsulfanyl]-3-E-[2-(pyridin-2-yl)ethenyl]indazole, or crystalline forms thereof, that protect axitinib from degradation, including photodegradation, as well as the therapeutic use of such compositions. The present invention also relates to novel photodegradants of axitinib.
Owner:PFIZER INC
Medical coating powder containing nano material
InactiveCN1616105AOvercome the defects that the quality is difficult to guarantee, etc.Simple and fast operationPharmaceutical non-active ingredientsDrageesUltimate tensile strengthMaterials science
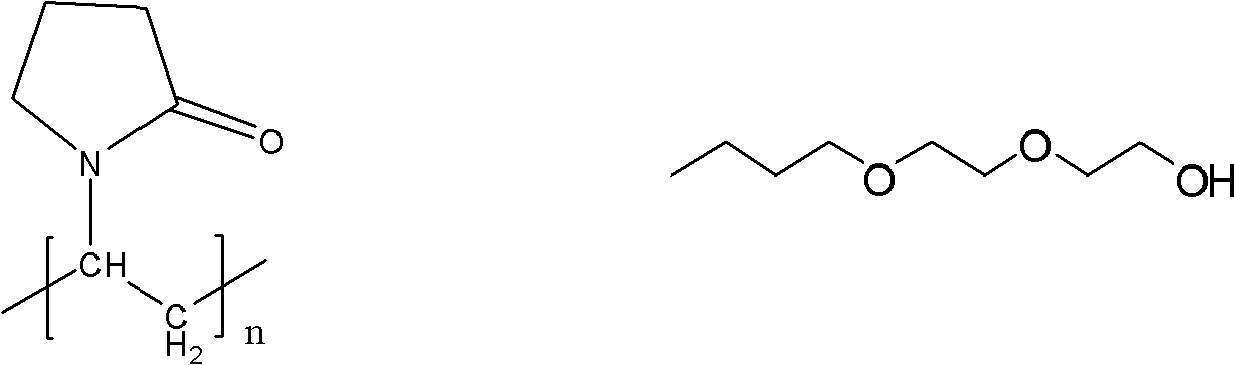
The medicinal coating powder containing nanometer material consists of hydromellose 55-65 wt%, copolymer of vinyl pyrrolidone and vinyl acetate 8 wt%, glycerin 15 wt%, Span 8 wt%, coloring agent 2-4 wt%, and nanometer titania 2-10 wt%. The medicinal coating powder containing nanometer material has simple production process, wide application, high performance / cost ratio, no physiological toxicity and many other advantages, and may meet the requirement of coating various solid Chinese medicine preparation.
Owner:GUANGDONG GUOFANG MEDICAL TECH
Vinylphenylpolysiloxane, preparation method and application
InactiveCN103819678AResidue reductionImprove stabilitySemiconductor devicesPtru catalystPhenyl group
The invention discloses a preparation method of vinylphenylpolysiloxane. The method comprises the following steps: 1) stirring a solvent, diphenyl silanediol, an alkyloxy compound and a catalyst and heating the mixture in a reaction vessel to react for 1-40h, and adding an end plate agent for blocking; 2) heating the above mixed liquor and stirring; 3) standing and filtering; and 4) heating a filtrate, removing low-boiling-point substances so as to obtain vinylphenylpolysiloxane. The invention also discloses vinylphenylpolysiloxane prepared by the above method and an application of vinylphenylpolysiloxane in LED package resin. The preparation method of vinylphenylpolysiloxane has the following advantages: firstly, resin storage stability is raised; secondly, it is convenient for industrialization; and finally, generation of industrial wastewater is avoided, and the preparation method is more environmentally friendly. Refractive index of the vinylphenylpolysiloxane disclosed by the invention is 1.50-1.57, and the vinylphenylpolysiloxane is especially applicable to be used as a strengthening agent of macromolecules in an LED packaging material.
Owner:GUANGDONG HENGDA NEW MATERIALS TECH
Preparation method of microzyme magnetic blotting composite microsphere adsorbent
InactiveCN103920471AImprove adsorption capacityImprove mechanical propertiesOther chemical processesAlkali metal oxides/hydroxidesFunctional monomerAdsorption equilibrium
The invention relates to a preparation method of a microzyme magnetic blotting composite microsphere adsorbent, and belongs to the technical field of environmentally-friendly functional material preparation. The method comprises the following steps: preparing ferriferrous oxide nanoparticles through a coprecipitation process, carrying out hydrophobic modification on the surface of the nanoparticles, preparing a stable Pickering emulsion with an oleic acid modified microzyme aqueous solution as a water phase, cyhalothrin as a template molecule, methacrylic acid and 4-vinylpyridine as functional monomers, ethylene glycol dimethacrylate as a cross-linking agent, dimethyl 2,2'-azobis(2-methylpropionate) as an initiator and hydrophobic Fe3O4 as an oil phase, and carrying out thermal-initiated polymerization to prepare the microzyme magnetic blotting composite microsphere adsorbent. Blotting microspheres obtained in the invention have a good magnetic stability, and the adsorption balance, the dynamics and the selection identification performance of the adsorbent are researched through static state adsorption experiments. Results show that the blotting adsorbent prepared in the invention has a good adsorption capacity, fast adsorption kinetics, and has a selection identification performance on LC.
Owner:JIANGSU UNIV
Method for producing 1-amino-1-alkoxycarbonyl-2-vinylcyclopropane
ActiveUS20130096339A1Easily produceCarbamic acid derivatives preparationOrganic compound preparationCarbonyl groupLeaving group
It is an object of the present invention to provide a novel method for producing (1R,2S) / (1S,2R)-1-amino-1-alkoxycarbonyl-2-vinylcyclopropane which is useful as a synthetic intermediate of therapeutic agents for hepatitis C and a synthetic intermediate thereof. According to the present invention, when a trans-2-butene derivative having a leaving group at each of the 1- and 4-positions is reacted with a malonic ester in the presence of a base, a specific amount of an alkali metal alkoxide or an alkali metal hydride is used as the base, and further a specific amount of a malonic ester is used to produce a cyclopropane diester, and further, chiral or achiral 1-amino-1-alkoxy-carbonyl-2-vinylcyclopropane and a salt thereof are synthesized using the cyclopropane diester.
Owner:API CORP (JP)
Synthesizing porcess for artificial antigen of cyanobromide chrysanthemum ester and assaying process thereof
InactiveCN1948964AMeet immunization requirementsSuitable for commercializationOrganic chemistryBiological testingBenzaldehydePhenyl group
The invention discloses Dehamethrin artificial antigen synthetic method. It includes the synthesis for (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formyl chloride, 3-(4-nitro phenoxy) benzaldehyde, [3-(4- nitro phenoxy) phenyl] cyan methanol, (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formic acid[3-(4-nitro phenoxy)phenyl] cyan methyl ester, artificial half antigen (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formic acid[3-(4-aminophenoxy) phenyl] cyan methyl ester, artificial antigen synthetic steps and its measuring.
Owner:BEIJING UNIV OF AGRI
Matrix with selectivity for micromolecule MALDI-TOF MS (Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry) detection and application thereof
InactiveCN103776892AAlleviate dispersionAlleviate the disadvantages of easy reunionMaterial analysis by electric/magnetic meansTime-of-flight mass spectrometryBromine
The invention discloses a matrix with selectivity for micromolecule MALDI-TOF MS (Matrix-Assisted Laser Desorption / Ionization Time of Flight Mass Spectrometry) detection and an application thereof. The matrix is graphene oxide grafted 4-vinylbenzeneboronic acid, and takes 4-vinylbenzeneboronic acid as a monomer; the 4-vinylbenzeneboronic acid is grafted to graphene oxide which is subjected to bromine functionalization by triggering atom transfer radical polymerization reaction on the surface, thereby preparing the matrix. The graphene oxide grafted 4-vinylbenzeneboronic acid disclosed by the invention can act as the MALDI-TOF MS matrix for selectively recognizing such micromolecule compounds containing cis-form adjacent dihydroxy structure, as cis-form adjacent dihydroxy compounds in saccharides, phenols and nucleoside; the minimum detection limit can reach 1pmol / mL around, and the selectivity and flexibility are both very high.
Owner:SHAANXI NORMAL UNIV
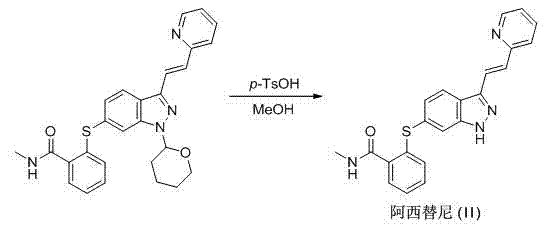
Method for preparing intermediate of axitinib and application of intermediate in preparation of axitinib
The invention relates to a method for preparing an intermediate of axitinib and application of the intermediate in preparation of axitinib. The preparation method for the intermediate of axitinib 3-iodine-6-nitro-1-(teralin-2H-pyran-2-base)-1H-indazole comprises the following steps that 6-nitroindazole and 3,4-dihydro-2H-pyran react under the action of catalyst so as to protect perssad tetralin-2H-pyran-2-base at N-H site, and the key intermediate with high yield is obtained through iodination at site 3. The application of the intermediate in preparation of axitinib is as follows: Heck coupled reaction is carried out on the intermediate and 2-vinyl pyridine, then nitro reduction and diazo-reaction for iodination are sequentially carried out, finally, the axitinib is obtained after docking of 2-sulfydryl-N-methyl benzamide and deprotection. The related main initial raw materials are easy to purchase in markets, and the method has high yield and high molecule economic efficiency, is efficient and environment-friendly, and is suitable for industrial mass production.
Owner:湖南欧亚药业有限公司
High-efficiency composite hydrate inhibitor as well as preparation method and application thereof
ActiveCN102161720ANo pollutionLow toxicity and good biocompatibilityPipeline systemsPolymer scienceBenzoyl peroxide
The invention discloses a high-efficiency composite hydrate inhibitor as well as a preparation method and an application thereof. The preparation method of the high-efficiency composite hydrate inhibitor comprises the following steps: taking N-vinyl pyrrolidone as a monomer and azodiisobutyronitrile or benzoyl peroxide as an initiator; and carrying out a polymerization reaction of a free-radical solution in a solvent to obtain the composite hydrate inhibitor, wherein the solvent is selected from at least one of the following components: carbinol, ethylene glycol, diethylene glycol, isopropanol, diethylene glycol, ethylene glycol monobutyl ether and Di(ethylene glycol) butyl ether, the volume ratio of the monomer to the solvent is (1:2)-(1:5), and the use amount of the initiator is 0.3-1.5wt% of the monomer. Compared with the method for industrially synthesizing the initiator, the method disclosed by the invention saves energy and is convenient and economic. The inhibitor synthesized by the invention can be applied to hydrate prevention and treatment in the processes of producing and transporting petroleum fluid.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


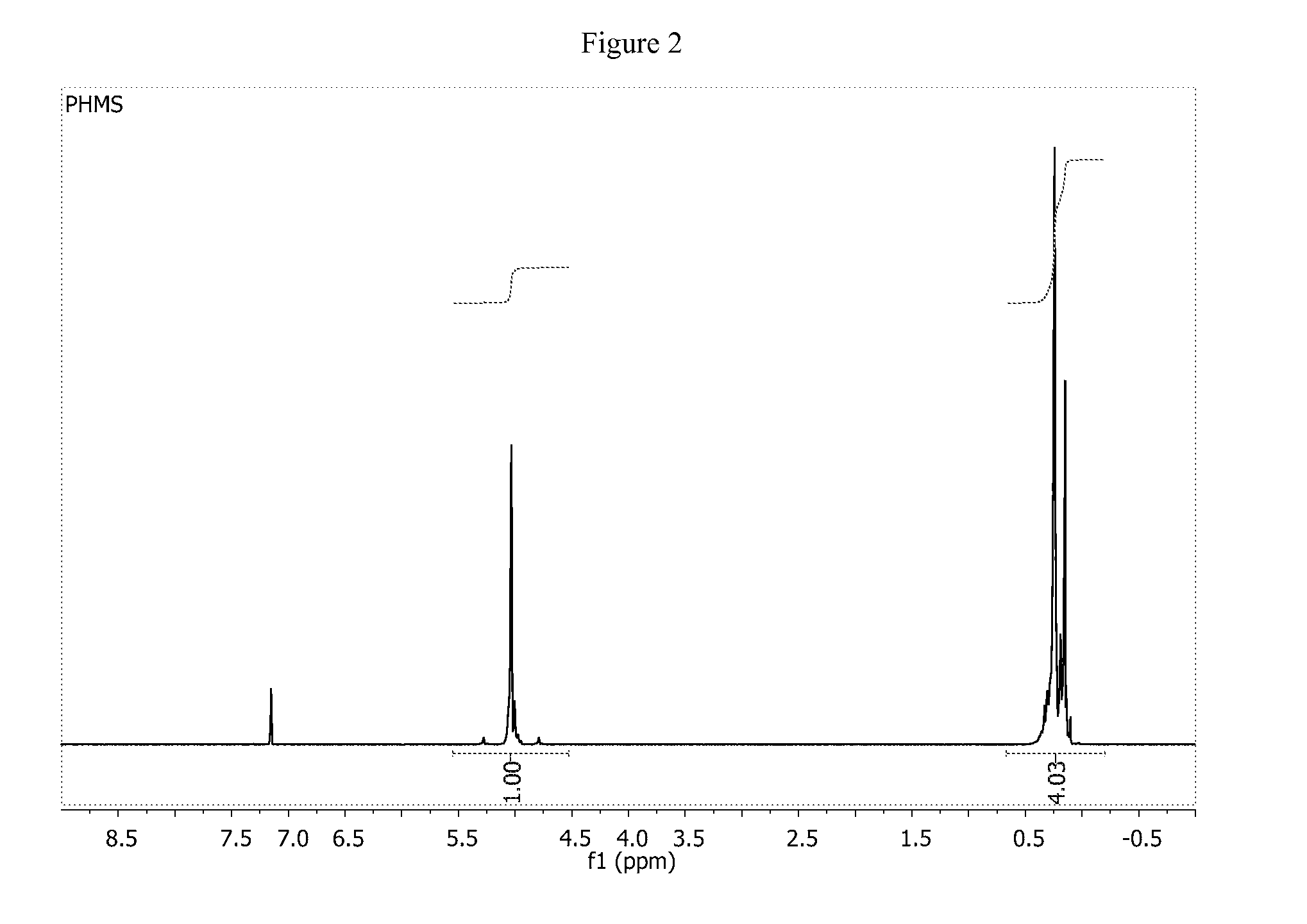

![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00001.png)
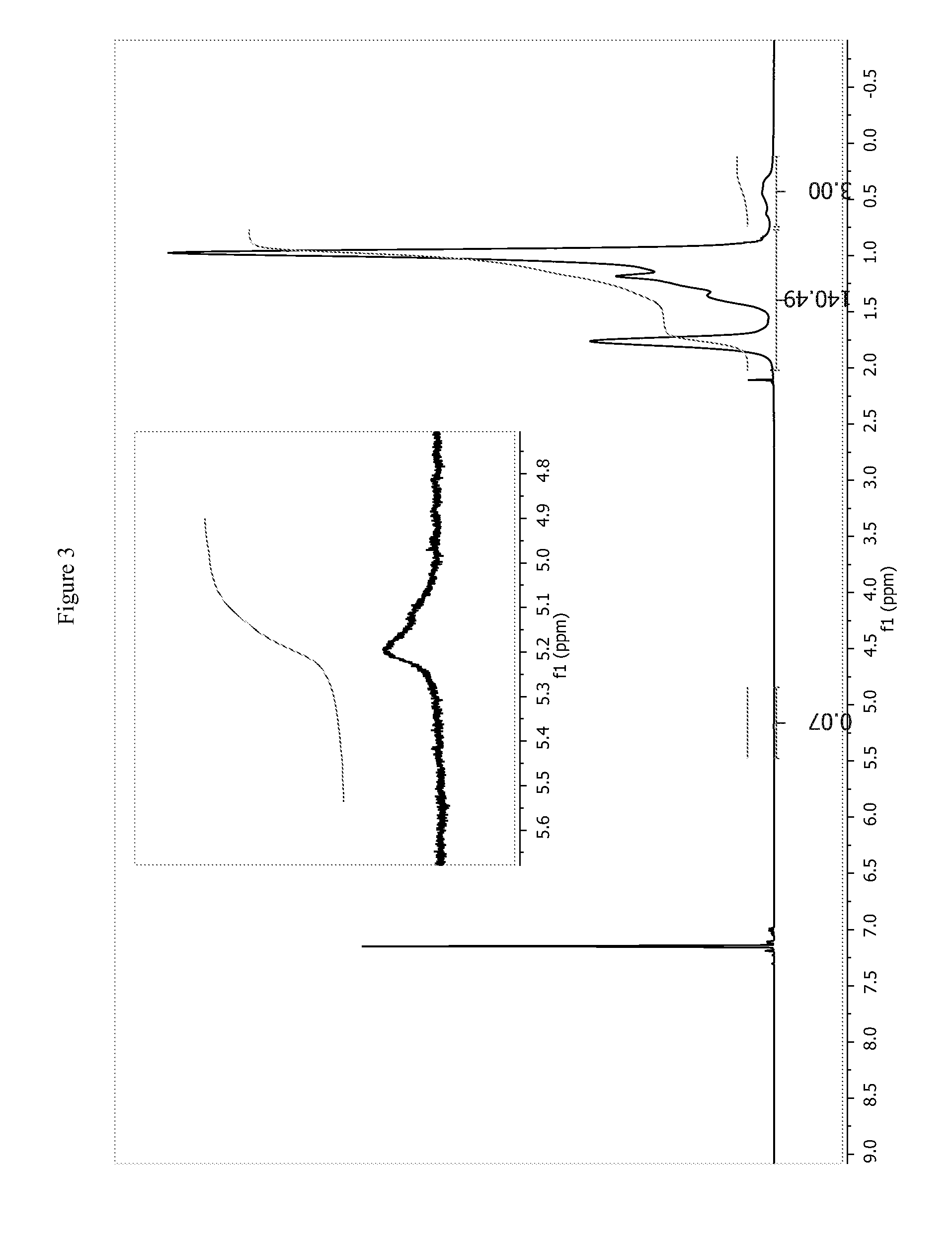
![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00002.png)
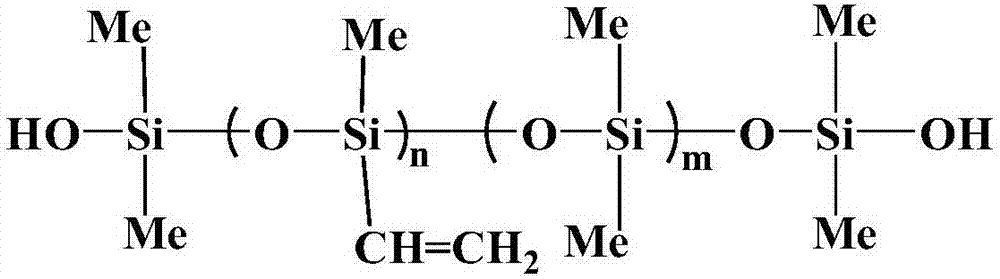
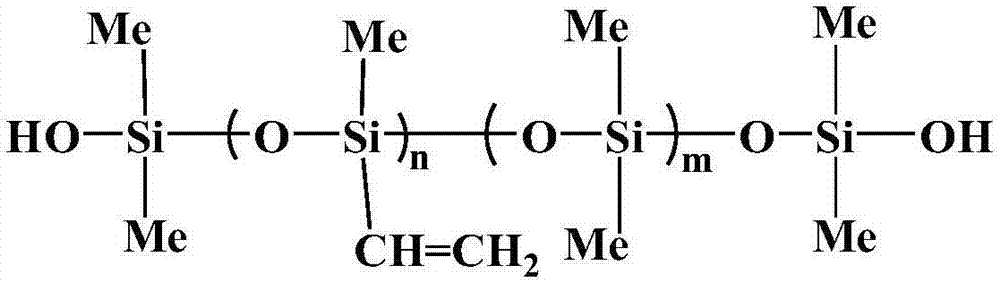
![Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide Pharmaceutical compositions of n-methyl-2-[3-((e)-2-pyridin-2-yl-vinyl)-1h-indazol-6-ylsulfanyl]-benzamide](https://images-eureka.patsnap.com/patent_img/0f92072d-341c-4606-84fb-0ef5edd7f488/US20140248347A1-20140904-D00003.png)