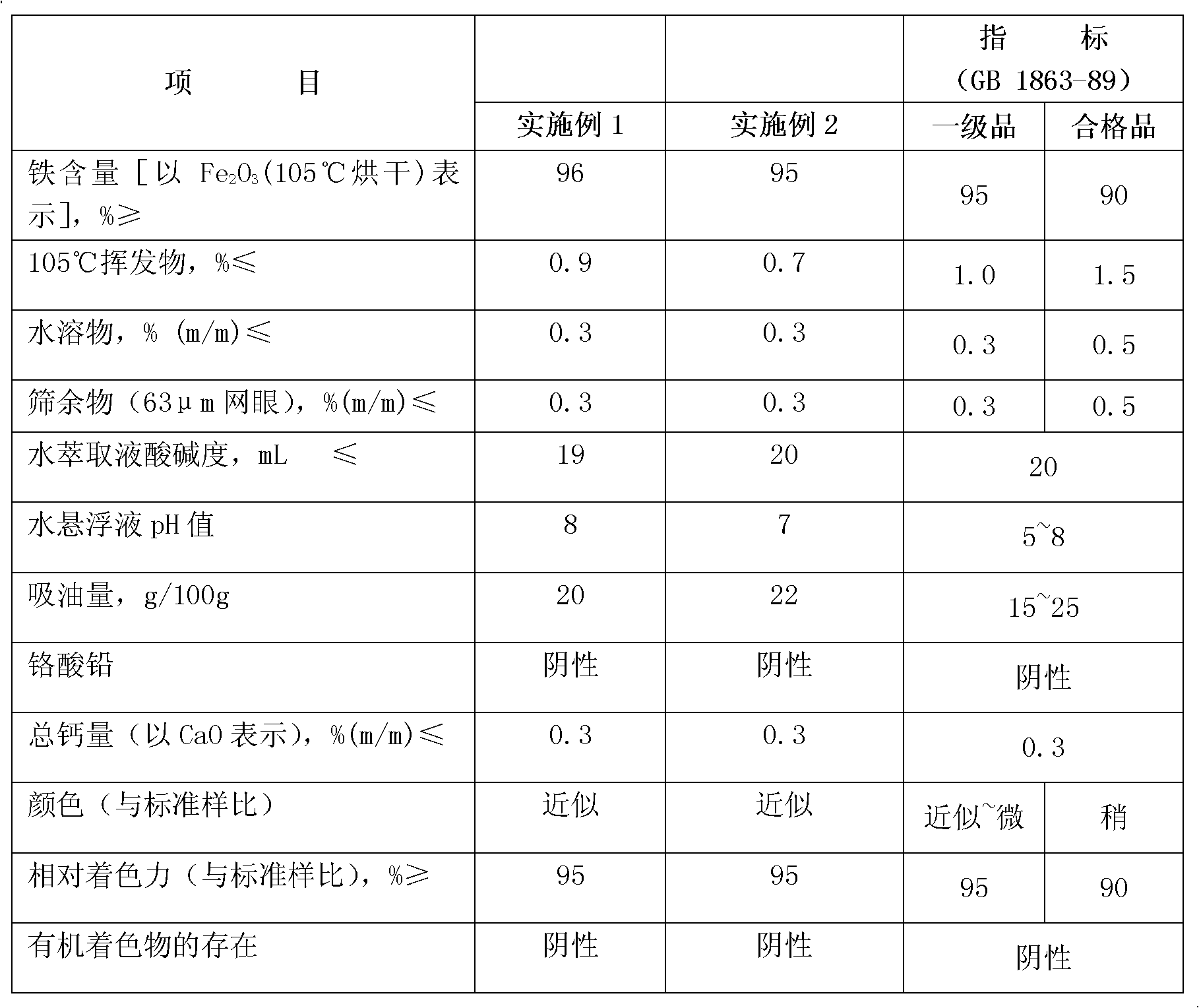
Patents
Literature
249 results about "Nitro reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
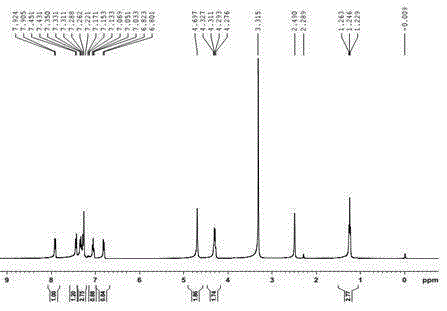
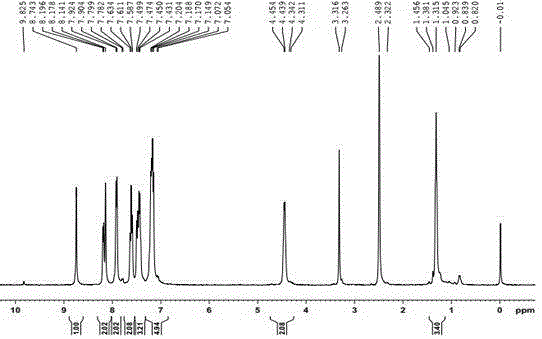
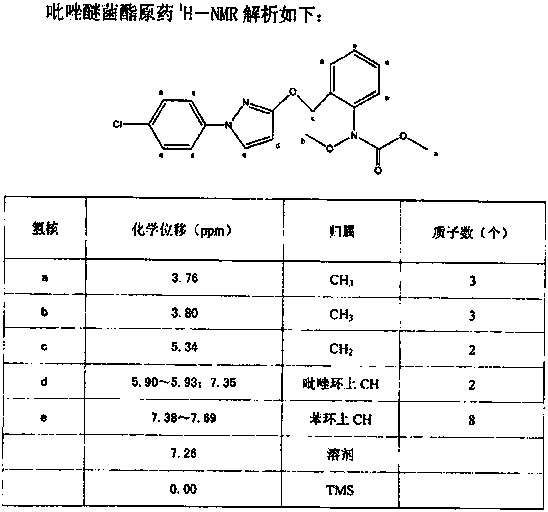
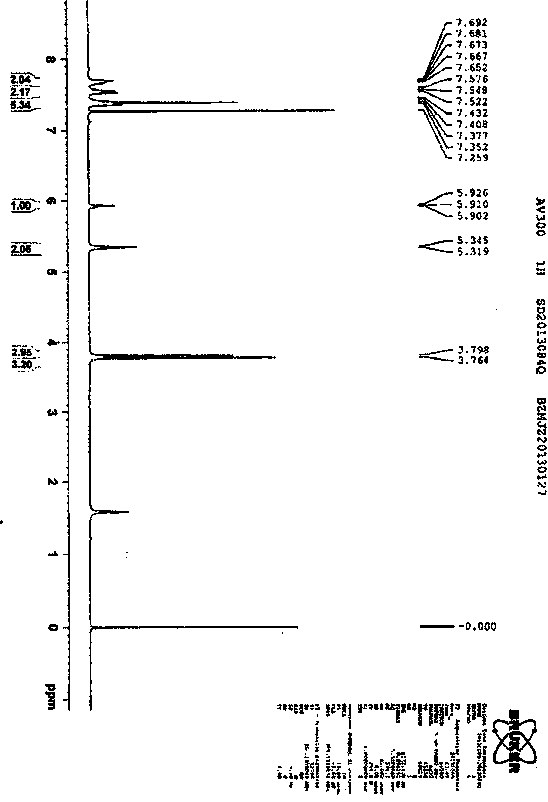
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
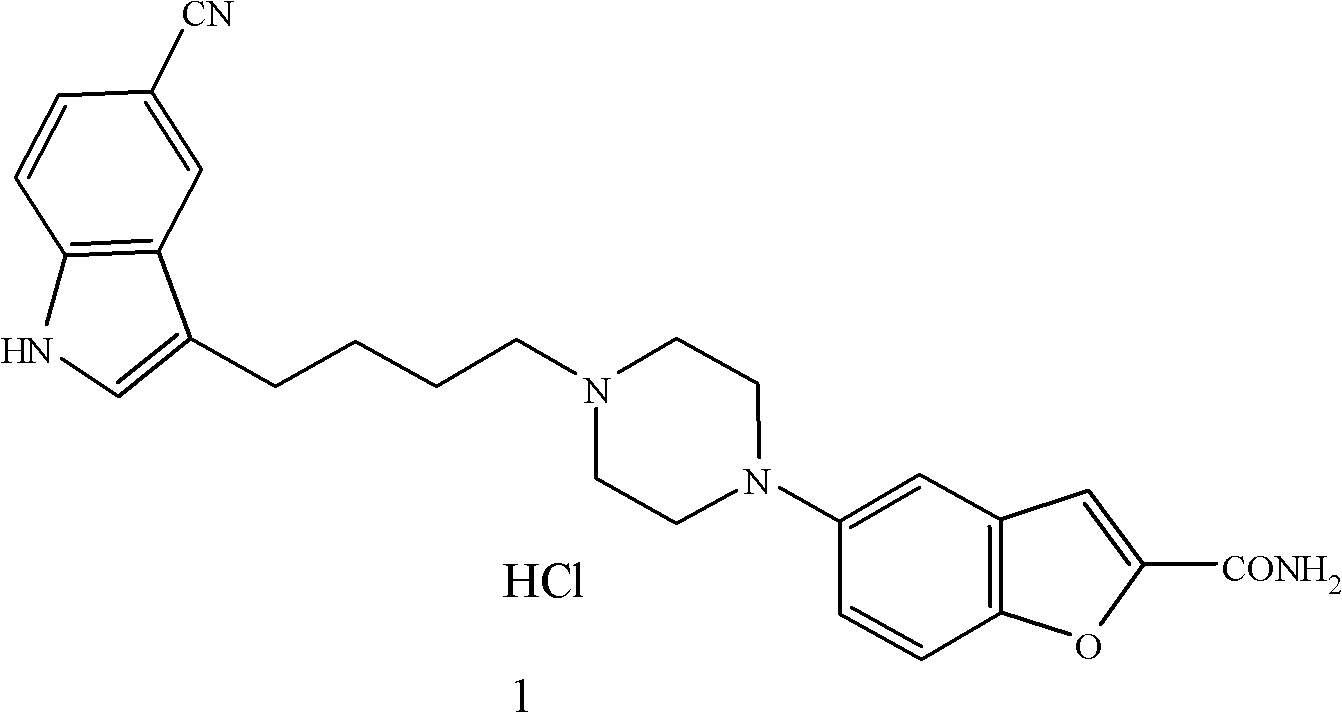
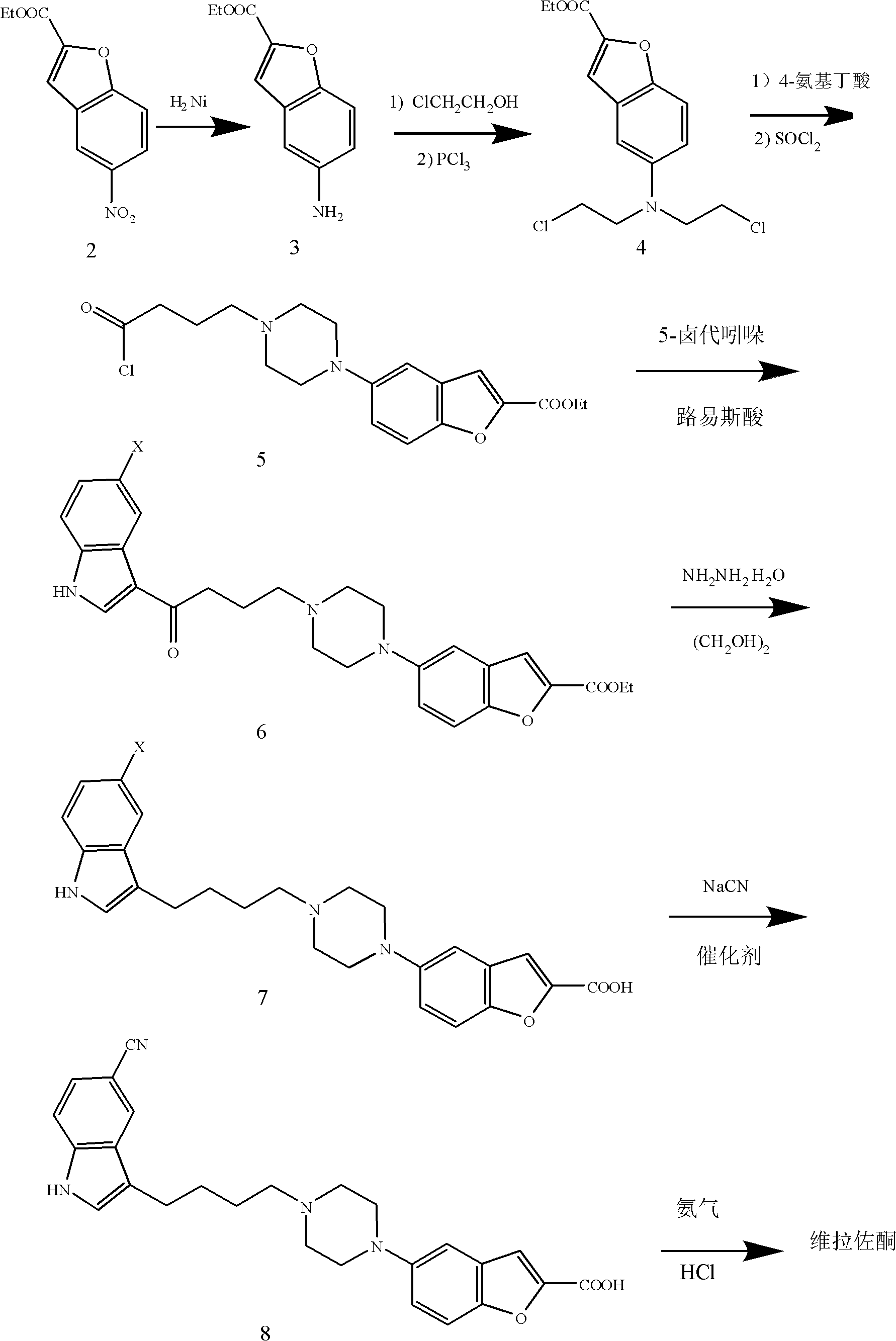
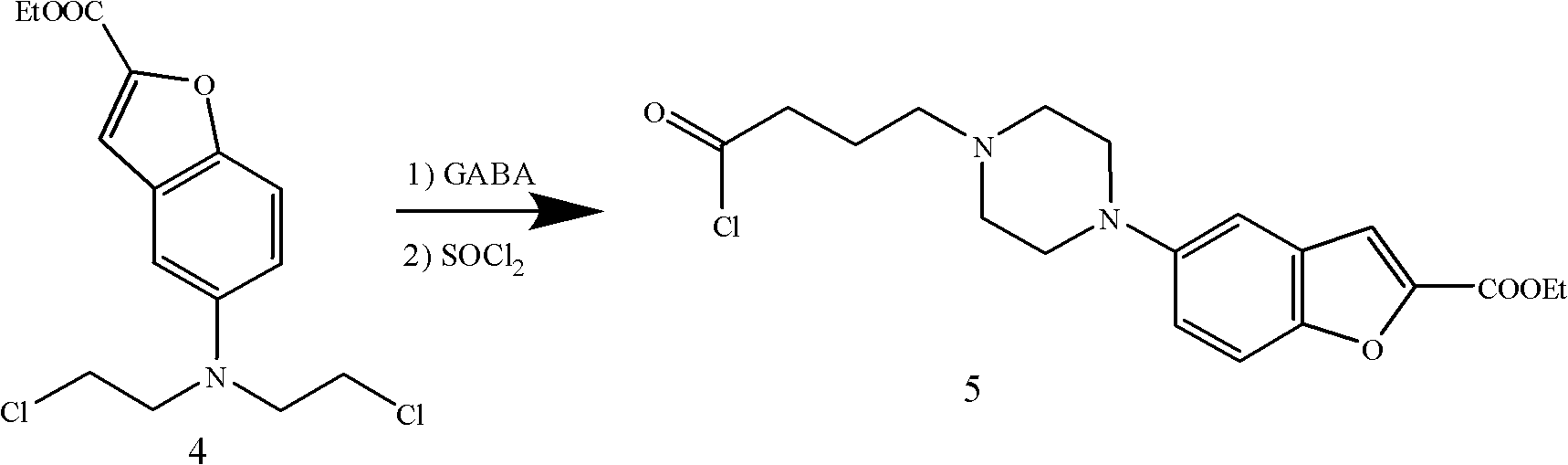
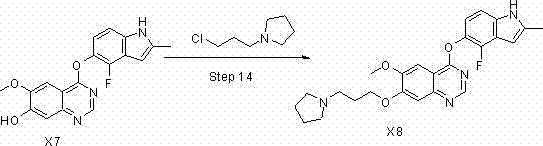
Method for preparing anti-depression medicine vilazodone
InactiveCN102180868AOvercome costsHigh reaction yieldOrganic chemistryCarboxylic acidPhosphorus trichloride
The invention provides a method for preparing an anti-depression medicine vilazodone, which comprises the following steps of: performing nitro reduction on 5-nitrobenzofuran-2-ethyl carboxylate serving as a raw material, conjugating with chlorohydrin, chlorinating by using phosphorus trichloride, cyclizing with 4-aminobutyric acid to generate a piperazine ring, performing acylchlorination, condensing with halogenated indole, performing carbonyl reduction, cyaniding, performing amidation, and the like to obtain the vilazodone. The method overcomes the defects in the prior art, is suitable for industrial production and has high application value, the used raw material is readily available, operating cost is low, and reaction yield is high.
Owner:SCI GENERAL MATERIAL & CHEM
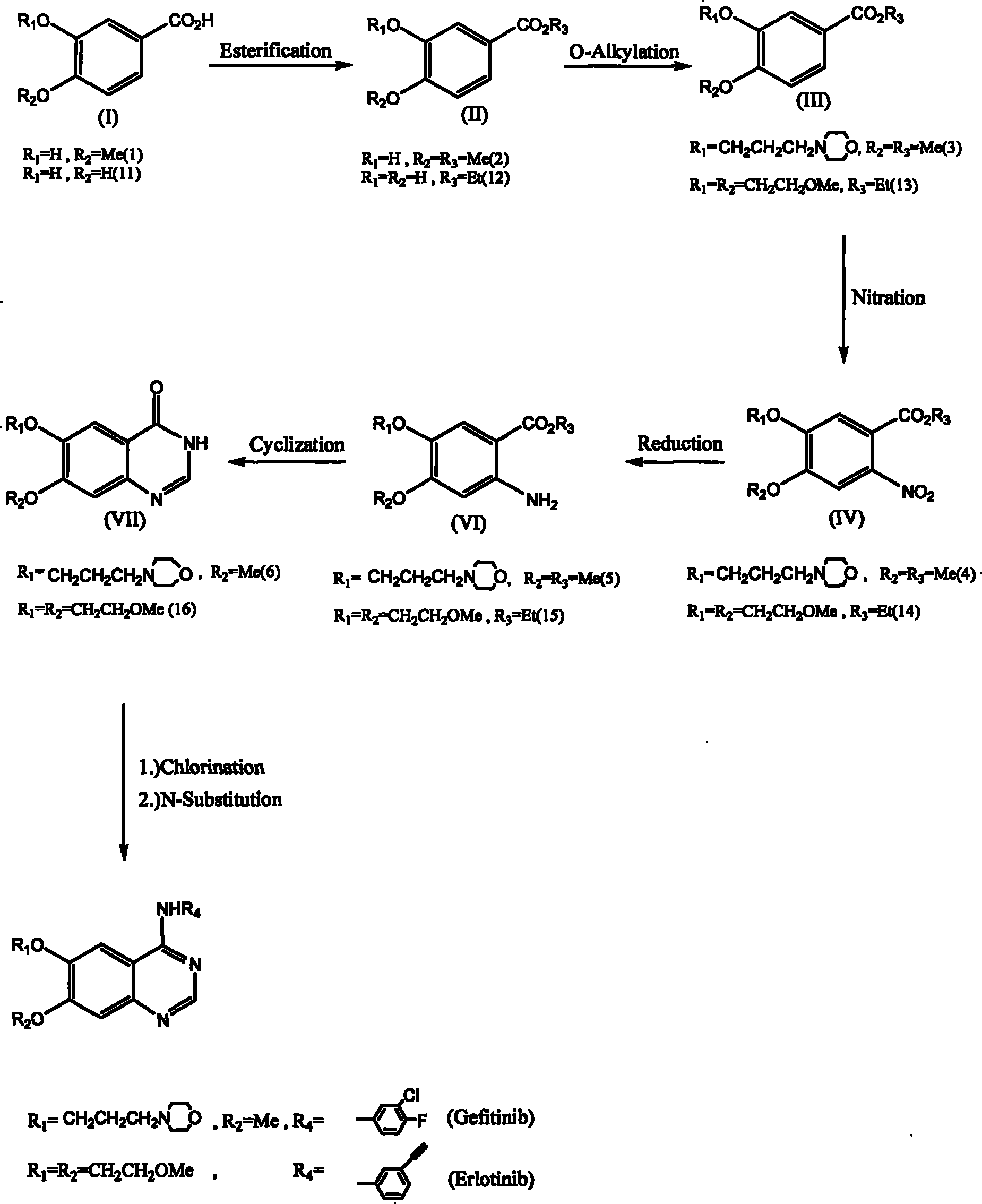
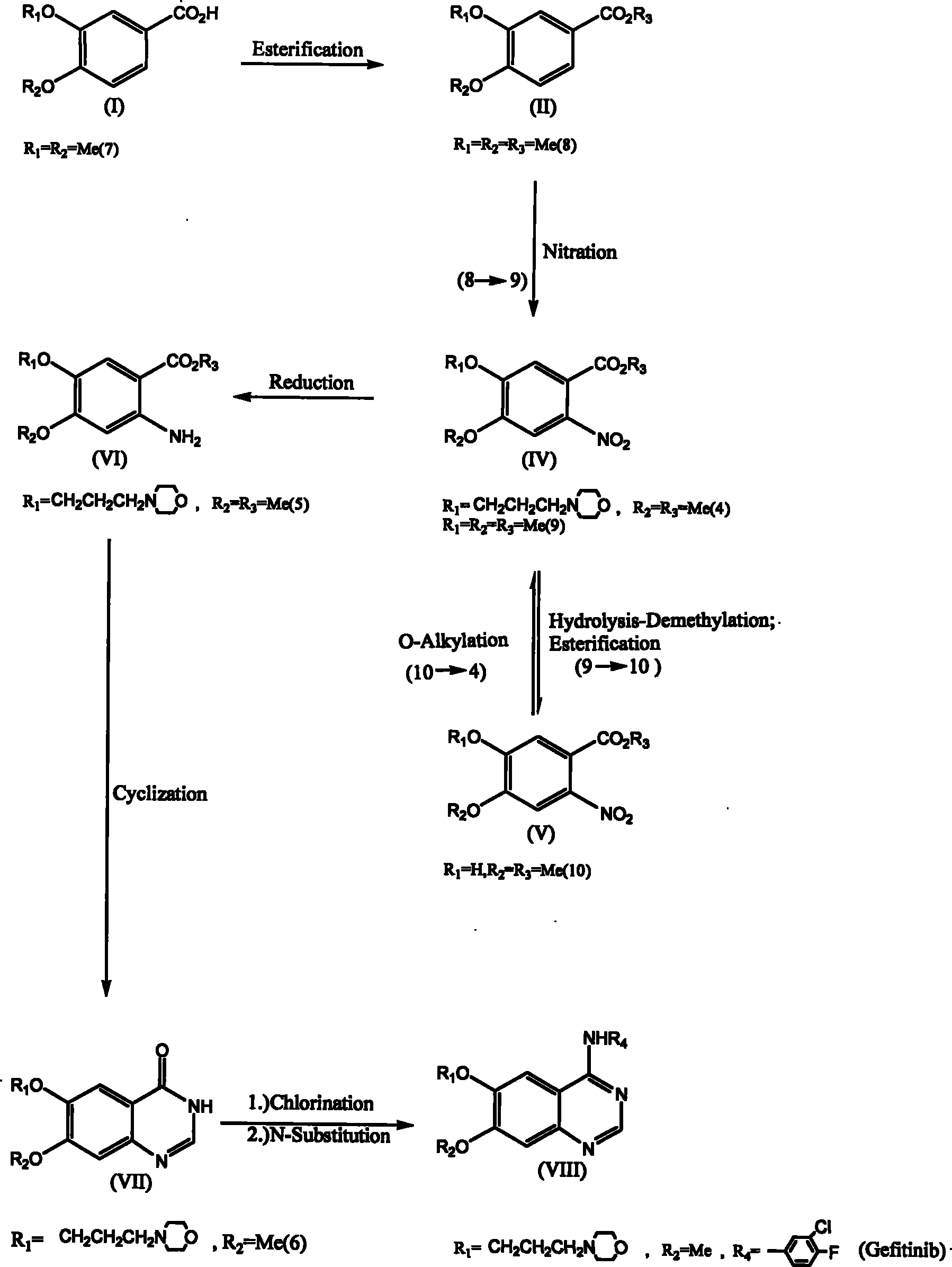
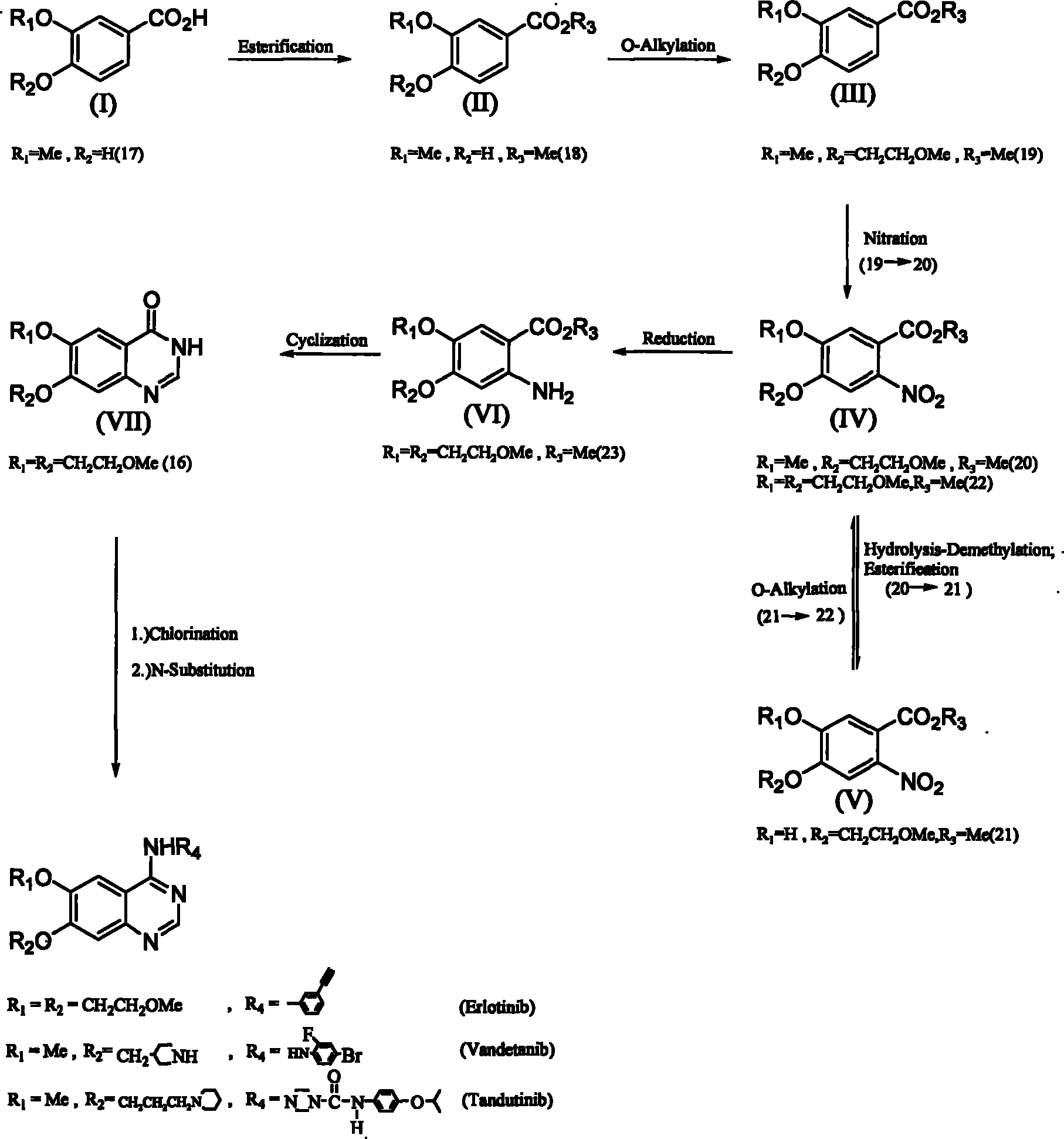
Synthesis method of 6,7-substituted-4-aniline quinazoline
InactiveCN101863844AReduce manufacturing costOrganic chemistryAntineoplastic agentsBenzoic acidSynthesis methods
The invention relates to a synthesis method of 6,7-substituted-4-aniline quinazoline. In the method, substances such as 3,4-substituted benzoic acid, and the like are used as starting materials, and the 6,7-substituted-4-aniline quinazoline is prepared through a reaction path of esterification reaction, oxygen-alkylation reaction, nitration reaction, nitro reduction reaction, ring closing reaction and two-in-one reaction or a reaction path of esterification reaction, oxygen-alkylation reaction or nitration reaction, hydrolysis-demethylation reaction after the nitration reaction, esterification reaction, oxygen-alkylation reaction, nitro reduction reaction, ring closing reaction, two-in-one reaction, and the like. The invention also provides a preparation method of a 4-aniline quinazoline derivative with lower starting material cost and higher productivity, which can effectively reduce the production cost so as to improve the competitiveness of a 4-aniline quinazoline derivative product.
Owner:OMEGA MEDICAL TAIWAN
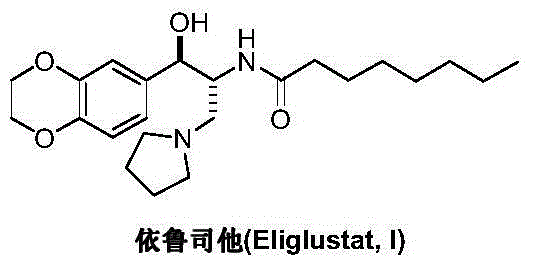
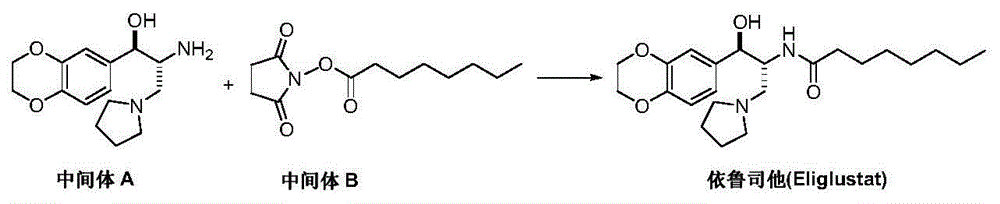
Preparation method of eliglustat
ActiveCN104557851AEase of industrial productionRaw materials are easy to getOrganic chemistryNitroethaneBenzene
The invention discloses a preparation method of eliglustat serving as a medicament for an I type Gaucher disease. The preparation method comprises the following steps: carrying out Henry reaction on 2, 3-dihydro-1, 4-benzodioxane-6-aldehyde and 2-(pyrrolidine-1-yl)-1-nitroethane in the presence of a chiral catalyst, and then carrying out nitro reduction and amidation reaction on the product to prepare the eliglustat (I). The preparation method is easy in raw material acquisition and simple and concise in process, is economical and environmentally friendly and is suitable for industrial production. Meanwhile, the invention also discloses 2-(pyrrolidine-1-yl)-1-nitroethane (III) serving as a raw material for preparing the eliglustat and a preparation method of the eliglustat.
Owner:上海御略医药科技有限公司
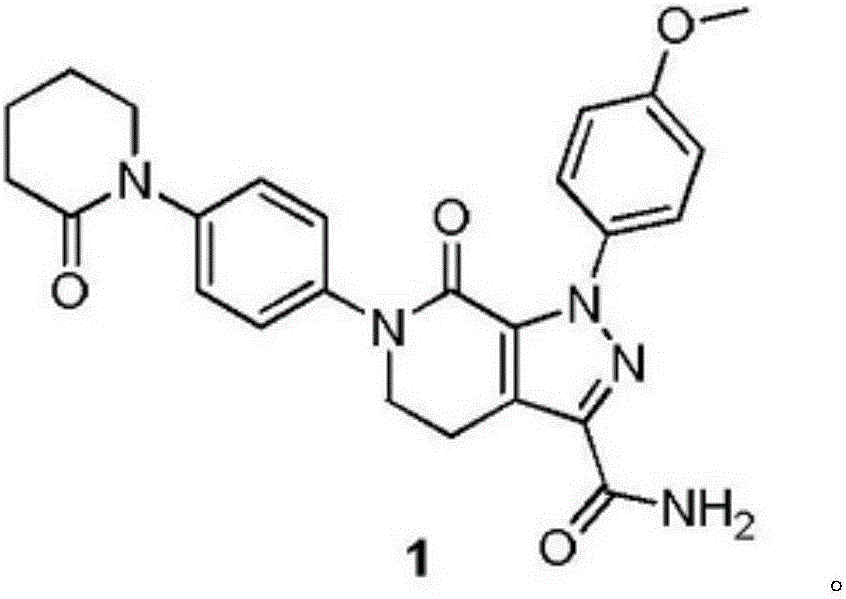
Preparation method of apixaban
ActiveCN105732622AAdaptable to conditionsFast aminolysisOrganic chemistryP-NitroanilineNitro reduction
The invention discloses a preparation method of apixaban.The method comprises the steps that paranitroaniline serves as the raw material, paranitroaniline and delta-valerolactone are subjected to amidation ring-opening, substituting and ring-closing reactions under the action of AlMe3, and a compound 8 is obtained; the compound 8 is subjected to alpha-position dichloro substituting and condensation-elimination reactions, and a compound 7 is obtained; the compound 7 and a compound 6 are subjected to [3+2] cyclization-elimination and nitro reduction, and a compound 4 is obtained; the compound 4 sequentially reacts with delta-valerolactone and ammonium chloride under the action of AlMe3, and a compound 3 is obtained; the compound 3 is subjected to substituting and ring closing, and apixaban is obtained.According to the preparation method of apixaban, paranitroaniline and delta-valerolactone which are low in price are adopted to serve as the raw materials, operation of the whole route is simple, conditions of each reaction are mild, and the synthesizing method is easy to operate, high in yield and purity and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for preparing ferric oxide red pigment by using nitryl chloride tail gas
ActiveCN102180521AReduce pollutionAchieve sustainable developmentFerric oxidesNitryl chlorideNitro reduction
The invention discloses a method for preparing ferric oxide red pigment by using nitryl chloride tail gas. The nitryl chloride tail gas is waste gas from the production process of high temperature chlorination of chloronitrobenzene. The method comprises the following steps of: adding chemical iron mud into a water absorption cell of the nitryl chloride tail gas; introducing steam to heat the water to 90 to 100 DEG C, stirring to dissolve the chemical iron mud; tracing and measuring the Fe3<+> content and the added quantity of the chemical iron mud in the solution; after the Fe3<+> content in the solution reaches 110 to 130g / L, filtering the solution, and adding ammonia water into the filtrate, adjusting the pH value to 6 to 9 and allowing trivalent iron to form iron hydroxide; precipitating; performing centrifugal separation on the precipitate; heating the solid to 105 to 115 DEG C; and decomposing the solid to obtain the ferric oxide red pigment, wherein the chemical iron mud is obtained after the nitro reduction reaction of iron powder. In the method, the harmful nitryl chloride tail gas and the solid waste iron mud obtained after nitro-reduction are used as raw materials to prepare the ferric oxide red pigment, so waste materials are changed into valuable materials, and the aims of sustainable development, energy conservation, consumption reduction and environmental pollution reduction are fulfilled.
Owner:海宁市黄湾镇资产经营有限公司
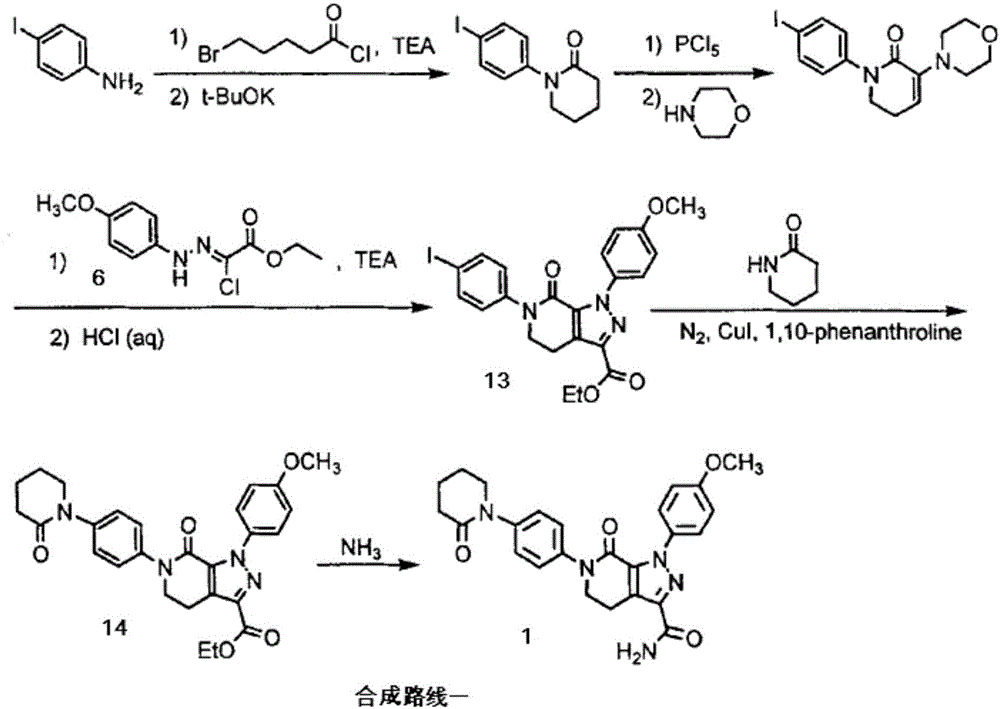
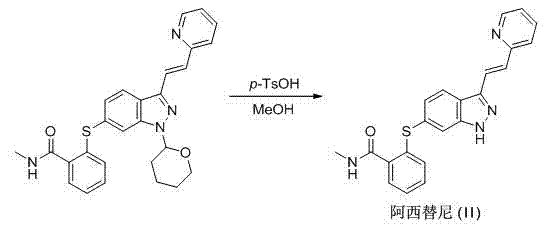
Method for preparing intermediate of axitinib and application of intermediate in preparation of axitinib
The invention relates to a method for preparing an intermediate of axitinib and application of the intermediate in preparation of axitinib. The preparation method for the intermediate of axitinib 3-iodine-6-nitro-1-(teralin-2H-pyran-2-base)-1H-indazole comprises the following steps that 6-nitroindazole and 3,4-dihydro-2H-pyran react under the action of catalyst so as to protect perssad tetralin-2H-pyran-2-base at N-H site, and the key intermediate with high yield is obtained through iodination at site 3. The application of the intermediate in preparation of axitinib is as follows: Heck coupled reaction is carried out on the intermediate and 2-vinyl pyridine, then nitro reduction and diazo-reaction for iodination are sequentially carried out, finally, the axitinib is obtained after docking of 2-sulfydryl-N-methyl benzamide and deprotection. The related main initial raw materials are easy to purchase in markets, and the method has high yield and high molecule economic efficiency, is efficient and environment-friendly, and is suitable for industrial mass production.
Owner:湖南欧亚药业有限公司
Synthesis method of cediranib
InactiveCN102603718AChange and optimize synthetic methodsReduce pollutionOrganic chemistryMetaclazepamBiochemical engineering
The invention discloses a preparation method of cediranib, which comprises the following steps of using trifluoro-nitrobenzene as a raw material and performing acetyl methyl adding, substitution, cyclization and protection to obtain a segment 1; and then using methyl vanillate as the raw material, performing benzyl bromine protection, nitro adding, reduction, unique cyclization and chlorination to obtain a segment 2; and performing nucleophilic substitution and deprotection on the two segments to obtain the final product cediranib. Compared with other methods, the preparation method of the cediranib has the advantages of mild reaction condition, high yield and scale amplification, and raw materials can be obtained easily.
Owner:CHEMPROSPECT PHARMTECH
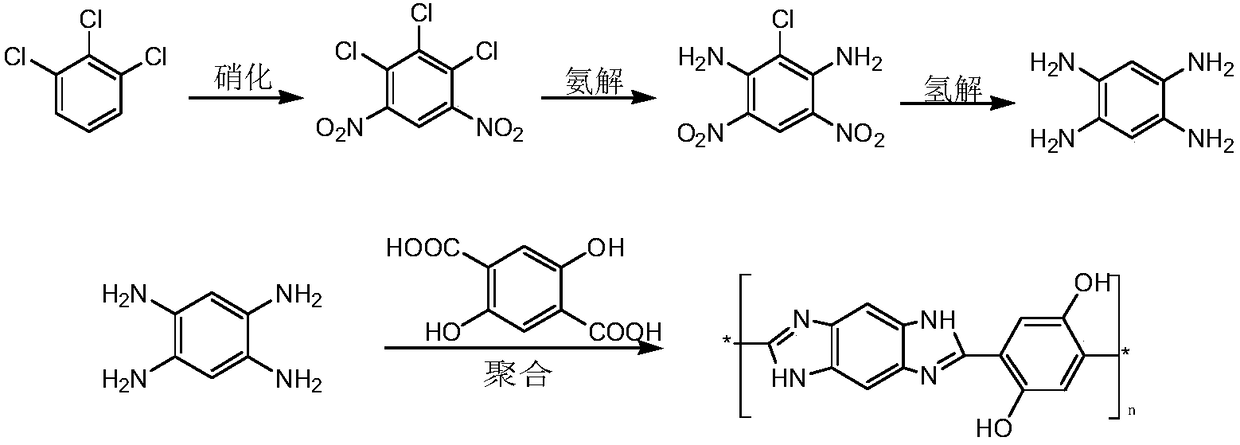
Synthesizing method and application of 1,2,4,5-tetraamine benzene
InactiveCN108191669AAvoid pollutionRealize resource utilizationOrganic compound preparationAmino compound preparationFiberChlorobenzene
The invention discloses a synthesizing method and application of 1,2,4,5-tetraamine benzene. The synthesizing method comprises the following steps: preparing a chlorobenzene byproduct which is 1,2,3-trichlorobenzene as a raw material; nitrifying to obtain 4,6-binitro-1,2,3-trichlorobenzene; further performing ammonolysis in an organic solvent A to obtain 4,6-binitro-2-chlorine-1,3-phenylenediamine; dissolving an ammonolysis product into an organic solvent B; performing catalytic hydrogenolysis to obtain 1,2,4,5-tetraamine benzene. Compared with the prior art, the method has the advantages thatthe chlorobenzene byproduct which is 1,2,3-trichlorobenzene is utilized, and moreover, nitroreduction and halogen hydrogenolysis are integrated during the hydrogenolysis process, thereby facilitatingindustrial production, and providing raw material support to development of downstream products. The application is that 1,2,4,5-tetraamine benzene is used as the raw material and polymerized with 2,5-dihydroxy terephthalic acid to obtain PDBI resin which is an engineering fiber material being high in spinnability and high in modulus.
Owner:SOUTHEAST UNIV
Preparation method and applications of hyperbranched azo polymer
InactiveCN103193967AHigh thermo-optic coefficientLow driving powerTenebresent compositionsBenzoic acidPolymer science
The invention relates to a preparation method and applications of a hyperbranched azo polymer, belonging to the organic synthesis field. The preparation method comprises the steps: firstly, performing nitroreduction and carboxyl acylation by taking p-nitrobenzoic acid as a raw material, to obtain an A2 type monomer azo-bi(benzoyl chloride) with an azobenzene structure, and then reacting the A2 type monomer azo phthaloyl dichloride with a B3 type monomer glycerol according to certain mole ratio to obtain the hyperbranched azo polymer. The materials of the obtained hyperbranched azo polymer has higher thermo-optical coefficient which is more than ten times of that of inorganic materials such as borosilicate glass, zinc silicate glass, silica glass and the like, and the material provides a possibility for developing a novel digital thermo-optical switch with low driving power.
Owner:JIANGSU UNIV
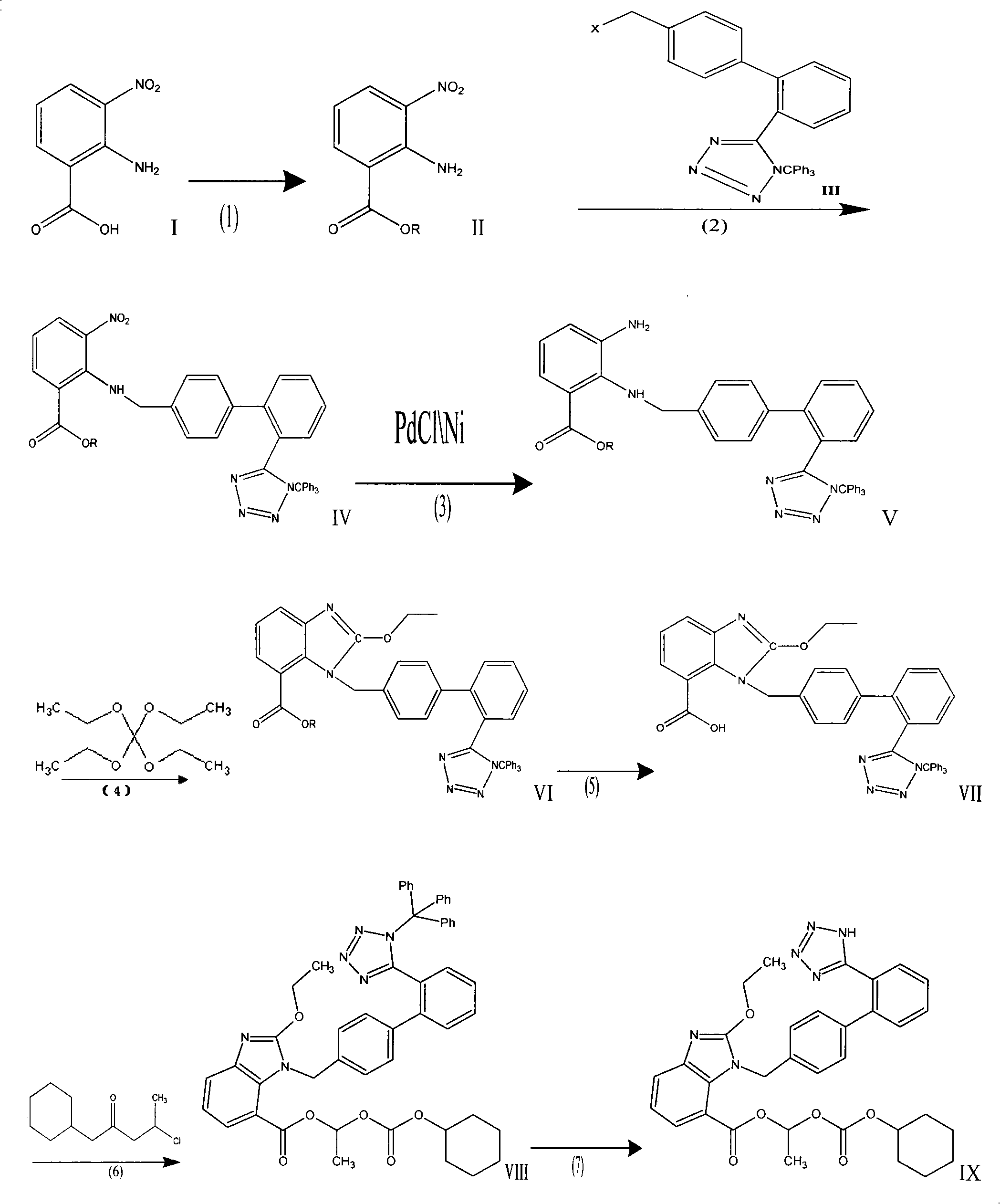
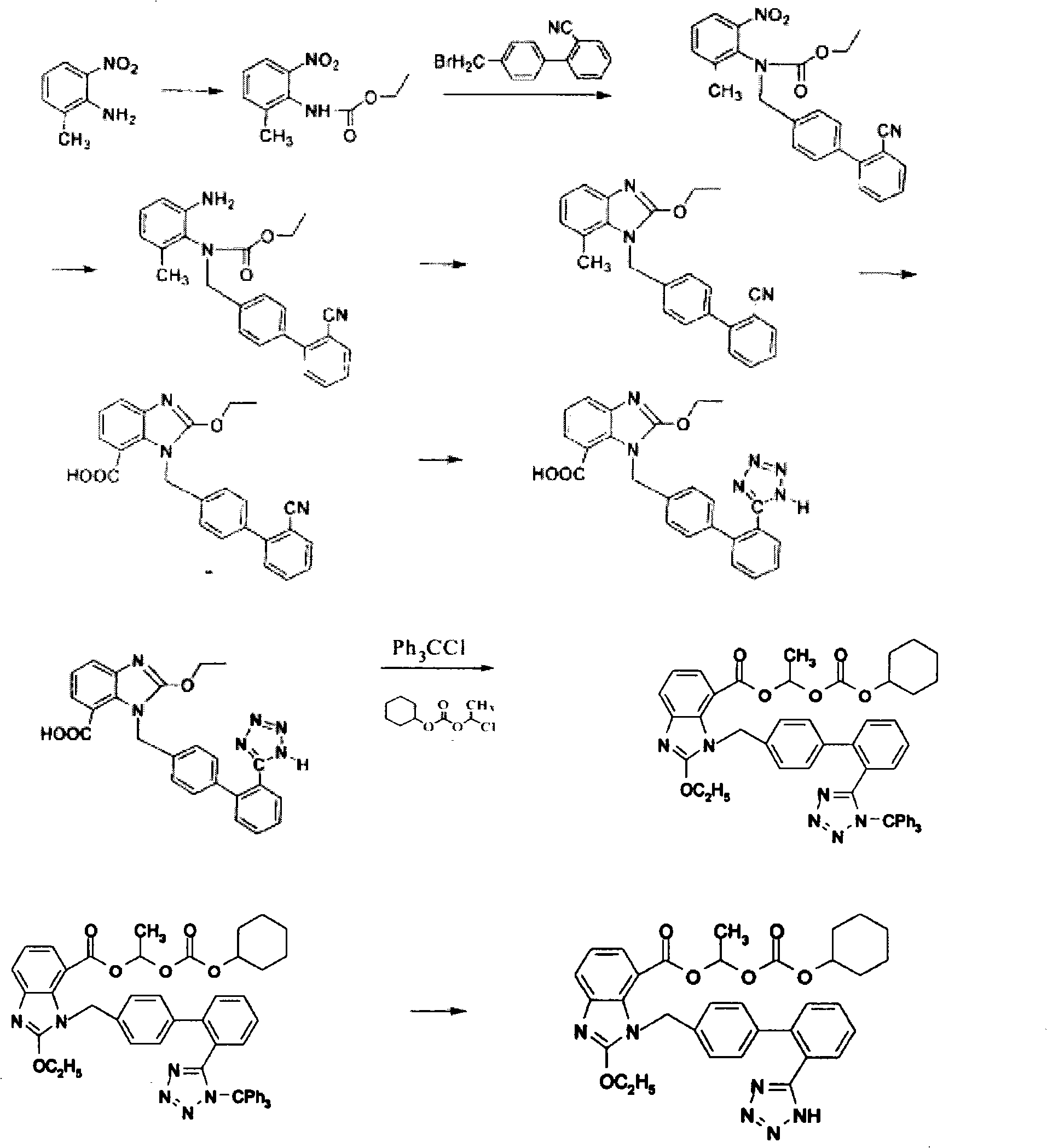
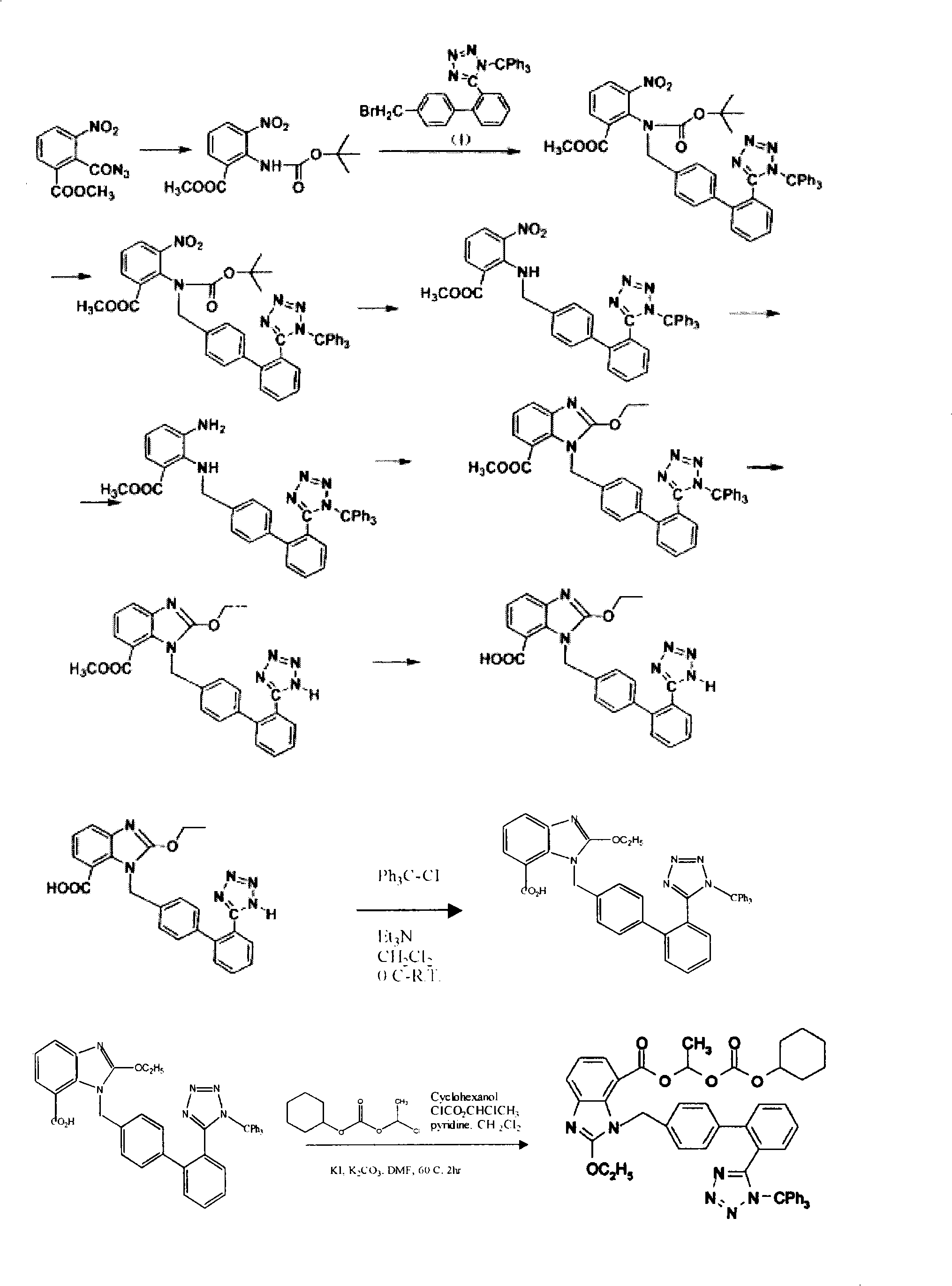
Method for preparing candesartan cilexetil
ActiveCN101781286AReduce usageHigh yieldOrganic chemistryBulk chemical productionCandesartanTetrazole
The invention provides a method for preparing candesartan cilexetil, which can solve the problems of longer reaction route, easy remaining of toxic substances in medicines and lower total yield existing in the prior art. In the method, the candesartan cilexetil is finally prepared by using 2-amino-3-nitrobenzoic acid as an initial raw material through esterification reaction, N-alkylation reaction, nitro reduction reaction, cyclization reaction, hydrolysis reaction, esterification reaction and tetrazole protecting group deprotection reaction. The synthetic method has simple steps and high yield, greatly reduces the participation and generation of toxic products in the reaction process, reduces the release of waste and is beneficial to clean production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
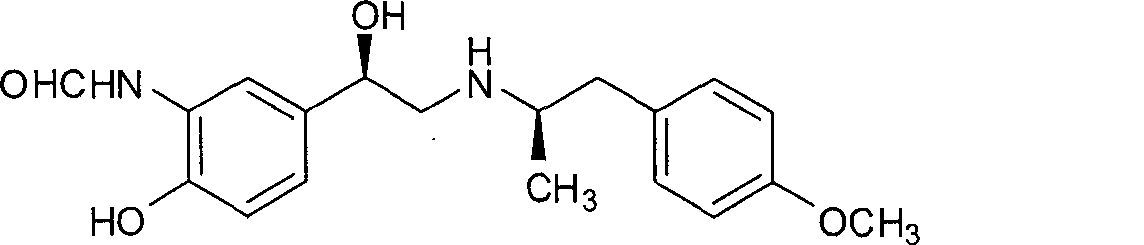
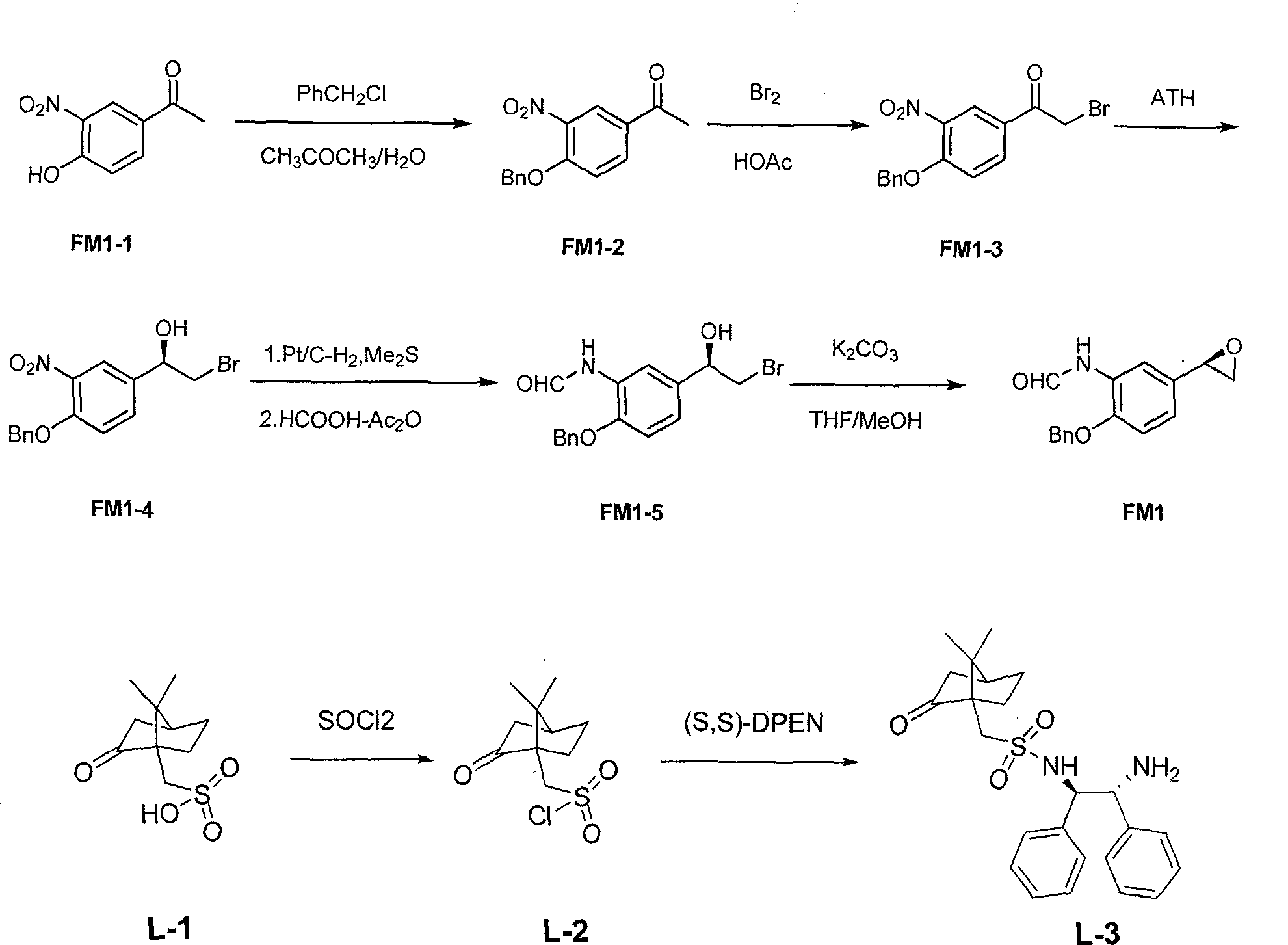
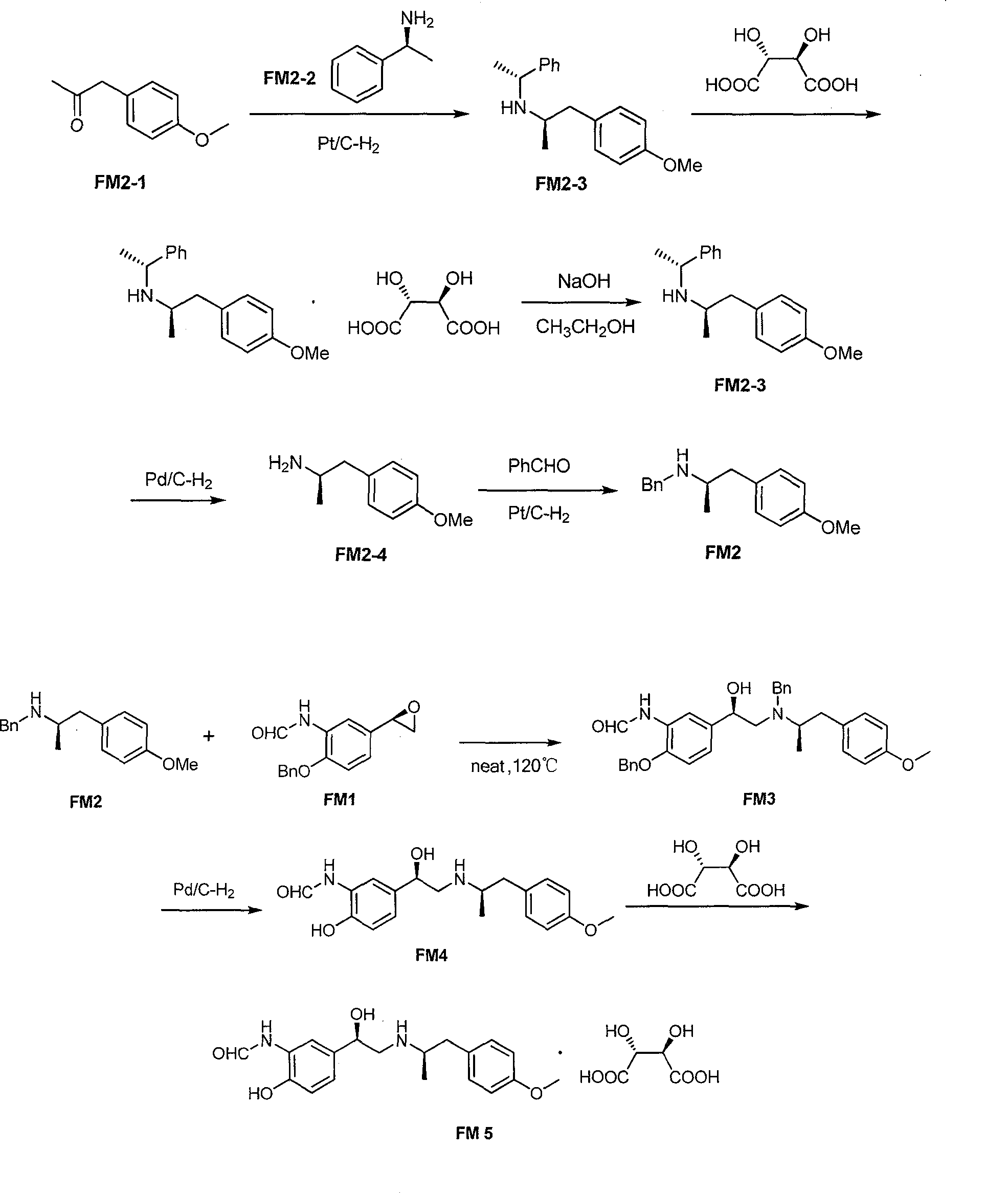
Asymmetric synthesis method of (R,R)-formoterol tartrate
InactiveCN103664677AHigh yieldHigh enantioselectivityCarboxylic acid amides optical isomer preparationCarboxylic acid salt preparationSynthesis methodsBenzaldehyde
The invention relates to an asymmetric synthesis method of (R,R)-formoterol tartrate, which comprises the following steps: by taking (S,S)-CsDPEN and transition metal complex as a catalyst, performing asymmetric hydrogen transfer reaction on alpha-bromoketone used as a raw material, thus obtaining a chiral alcohol intermediate compound; performing reaction steps of nitro-reduction, formylation, cyclization and the like, thus obtaining a key intermediate compound FM 1; by taking Pt / C as a catalyst and alpha-methylphenylethylamine as a chiral assistant, synthesizing an intermediate compound FM 2-3; performing tartaric acid salification, ionization and alpha-methylphenethyl removal, and reacting with benzaldehyde, thus preparing a chiral amine intermediate compound FM 2; reacting and coupling the two key intermediate compounds, and performing protective group removal to obtain (R,R)-formoterol FM 4; and performing tartaric acid salification on the FM 4, thus preparing the target product (R,R)-formoterol tartrate FM 5. According to the invention, the (R,R)-formoterol is synthesized through an asymmetric hydrogen transfer method by means of the chiral assistant, and high yield and favorable ee value are achieved. Compared with a chemical resolution method for synthesizing chiral formoterol, the method provided by the invention has the advantages of high overall yield, mild reaction conditions, low cost and the like, thereby being beneficial to industrial production.
Owner:SUN YAT SEN UNIV +1
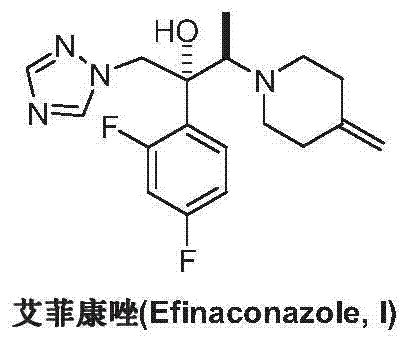
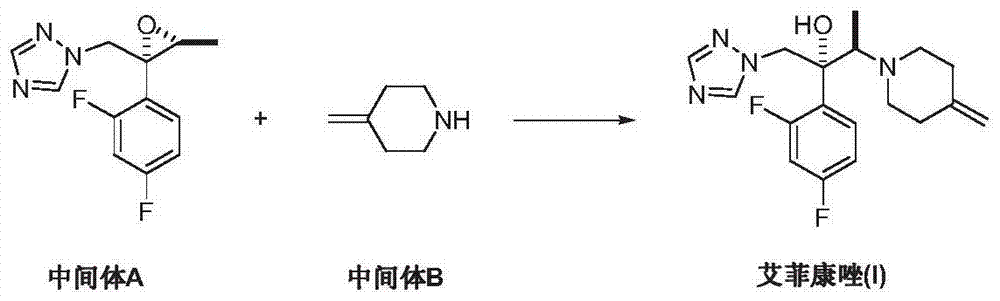
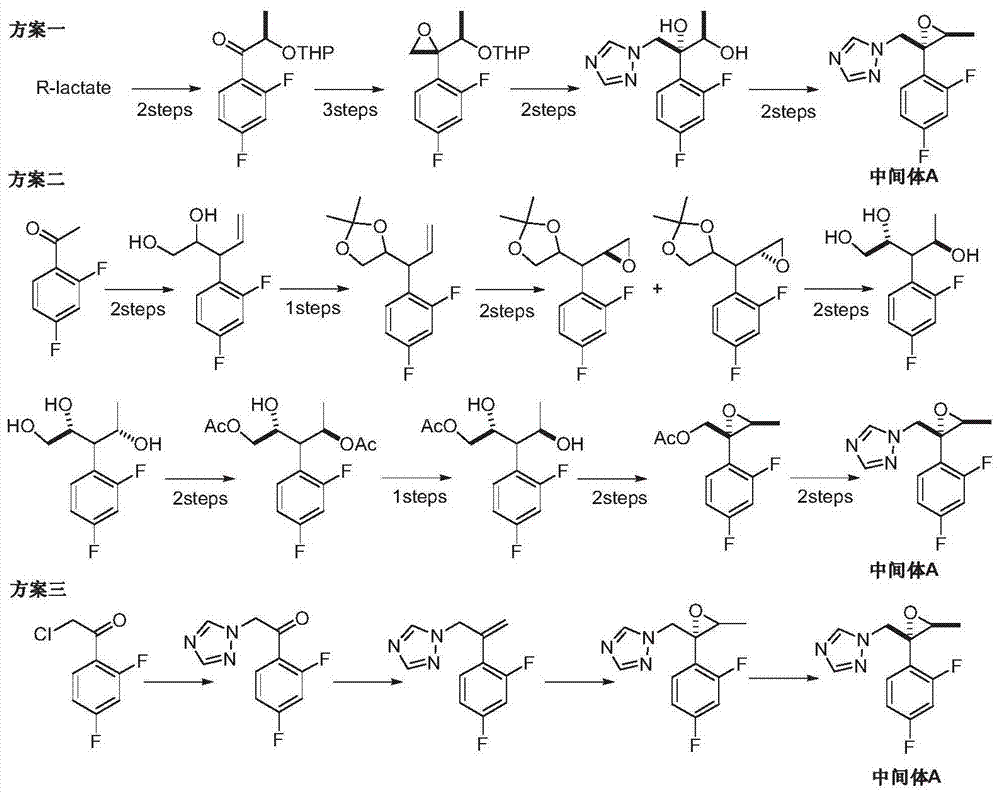
Preparation method of efinaconazole
ActiveCN104327047AEase of industrial productionRaw materials are easy to getOrganic chemistryNitroethaneBenzoic acid
The invention discloses a preparation method of efinaconazole (I). The preparation method comprises the following steps: performing asymmetric addition reaction on 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole-1-yl)aceton (II) and nitroethane in the presence of a catalyst cupreine and a promoter benzoic acid to generate (2R,3R)-3-nitro-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1-yl)-2-butanol (III); performing nitro reduction reaction on the intermediate (III) to generate (2R,3R)-3-amino-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1-yl)-2-butanol (IV); and performing cyclization reaction on the intermediate (IV) and 1,5-di-halogen-3-methylene pentane (V) to prepare efinaconazole (I). The preparation method is easily available in raw materials and concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:旌德君创科技发展有限公司
Synthetic method of mirabegron
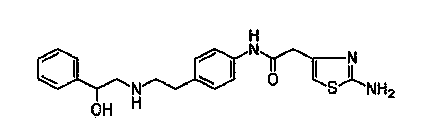
InactiveCN104016943ALow costThe synthesis process is simpleOrganic chemistry4-nitrophenethylamineAcetic acid
The invention discloses a synthetic method of mirabegron, which comprises the following steps: 1)amine-ester exchange reaction: taking (R)-mandelic ester and 4-nitrophenethylamine as raw materials for an ester interchange reaction to obtain a mirabegron intermediate A; 2)amide reduction: reducing amide of the mirabegron intermediate A to obtain a mirabegron intermediate B; C)nitro reduction: performing a reduction reaction of the mirabegron intermediate B and a reducing agent stannous chloride to obtain a mirabegron intermediate C; and D)condensation reaction: performing a condensation reaction of the mirabegron intermediate C and 2-aminothiazole-4-acetate under the effect of a condensing agent to obtain mirabegron. The synthetic method has the advantages of low cost of raw material and high yield of product, and is suitable for large scale industrial production.
Owner:苏州凯瑞医药科技有限公司
Main chain type azobenzene polymer and preparation method thereof
The invention discloses a main chain type azobenzene polymer and a preparation method thereof. According to the preparation method, the main chain type aromatic azo polymer is prepared by adopting nitro reduction coupling reaction for the first time. The preparation method comprises the following steps: firstly synthesizing different types of monomers through a series of reactions, namely conjugate monomers and non-conjugate monomers; secondly, synthesizing a photocatalyst required by a polymerization system, namely gold nanoparticles loaded on zirconium oxide, and characterizing the photocatalyst through a series of tests; and finally carrying out nitro reduction coupling reaction of the monomers in the presence of a methylbenzene-isopropanol mixed solvent and potassium hydroxide, thus obtaining the main chain type aromatic azo polymer. The preparation method disclosed by the invention needs few polymerization reaction components, is simple, practicable, convenient in a purification process, mild in reaction conditions and has the advantages of low toxicity, high efficiency and environmental protection. The main chain type aromatic azo polymer prepared by using the method has good solubility and functionality and high optical application value.
Owner:苏州吉尼尔机械科技有限公司
Method for preparing hydroxylamine through nitro-reduction
InactiveCN103588599ARaw materials are cheap and easy to getMild reaction conditionsFunctional group formation/introductionNitro compoundHydroxylamine
The invention discloses a method for preparing hydroxylamine through nitro-reduction and belongs to the technical field of reduction of organic nitro compounds in the organic chemistry. According to the method for preparing the hydroxylamine through the nitro-reduction, substituted nitrobenzene reacts with hydrazine, and N-phenylhydroxylamine is prepared under the catalysis of Raney Ni. According to the method, used raw materials are cheap and easy to obtain, reaction conditions are mild, the yield is higher, and the selectivity is good, so that the method is a synthetic method with the significant industrial value.
Owner:大连九港生物科技有限公司
Kutkin derivative, preparation method and application thereof
ActiveCN1923790AAnti-immune activityHas a cartilage protective effectOrganic chemistrySkeletal disorderNitrationStructural formula
The invention discloses a derivant of kutkin with chemical structural formula as formula I, wherein R1 is NO2, NH2 or amide; R2 is H, carbonyl or diaminocarbonyl. The preparing method comprises the following steps: nitrating kutkin to obtain kutkin derivant substituted by nitro group or kutkin derivant based on substituted nitro group; or kutkin derivant substituted by acetylamino.
Owner:JINAN UNIVERSITY
Aromatic azoxybenzene compound and preparation method thereof
ActiveCN107253920AMild conditionsSimple componentsOrganic compound preparationCatalystsAzoxyNitro compound
The invention discloses an aromatic azoxybenzene compound and a preparation method thereof. The preparation method comprises the following steps: firstly reducing aromatic nitro-compounds into azoxybenzene through photoinduction in an alkaline condition; firstly synthesizing different aromatic nitro-compounds; secondly, carrying out nitro reduction under the irradiation of a xenon lamp under the conditions of potassium hydroxide, methylbenzene and isopropyl alcohol, and thus synthesizing the aromatic azoxybenzene compound. The preparation method disclosed by the invention has the advantages of simple components, mild reaction conditions, low toxicity, environmental protection, high selectivity and the like; the obtained aromatic azoxybenzene compound has potential application in the aspects of dyes, liquid crystal materials, optical materials and the like.
Owner:SUZHOU UNIV
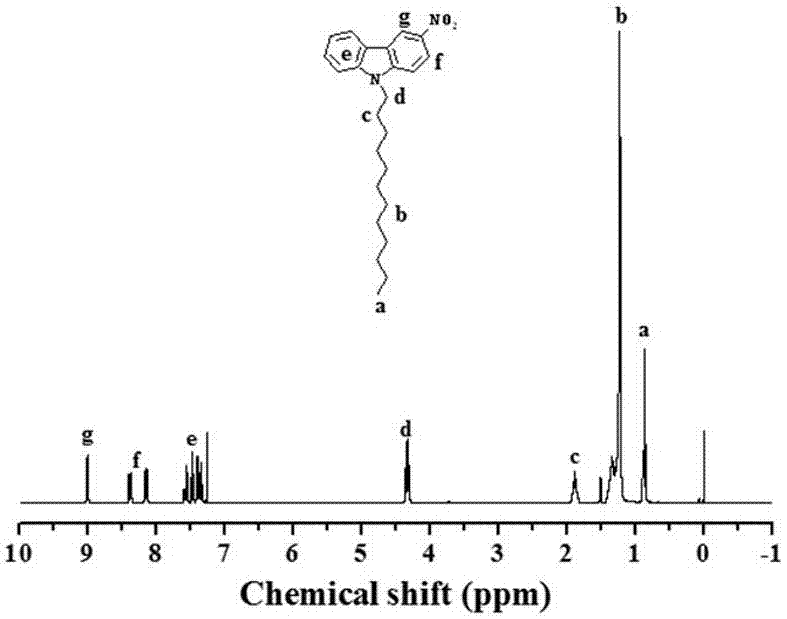
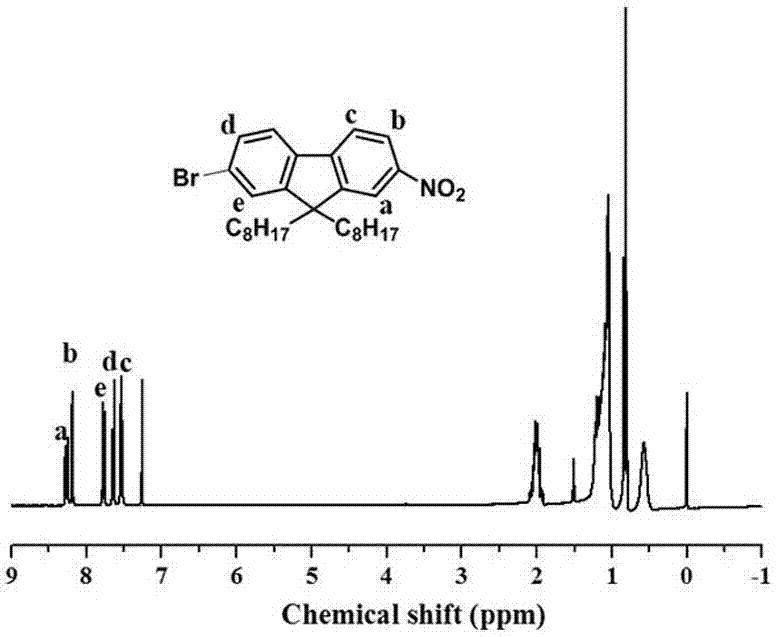
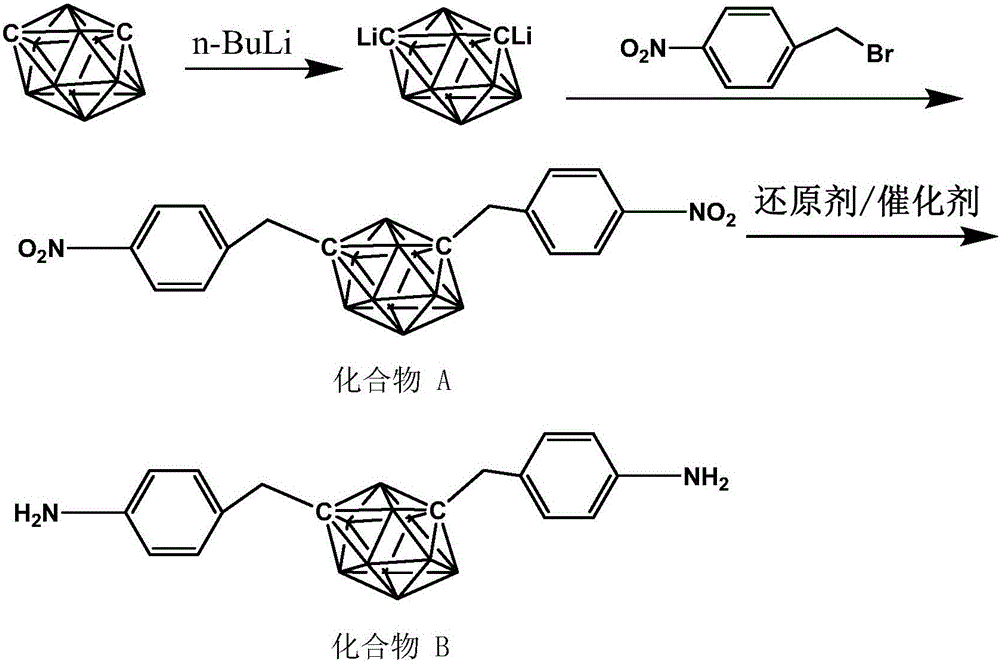
Diamine monomer containing carborane and preparation method thereof
The invention discloses a synthesis method of diamine monomer containing carborane structure. The method includes: synthesis of carborane double lithium, synthesis of carborane dinitro compound and synthesis of carborane diamine monomer. The carborane double lithium reacts with 4-nitrobenzylbromide to produce the dinitro compound containing carborane, and a nitro reduction reaction is employed for generation of the diamine monomer containing carborane. The synthesis process for diamine monomer containing carborane structure is simple, has easily controllable reaction process and high yield, can be used as an important diamine for the synthesis of high temperature resistant polyimide, and has the potential application value in high temperature and neutron protection.
Owner:CHENGDU SCI & TECH DEV CENT CHINA ACAD OF ENG PHYSICS +1
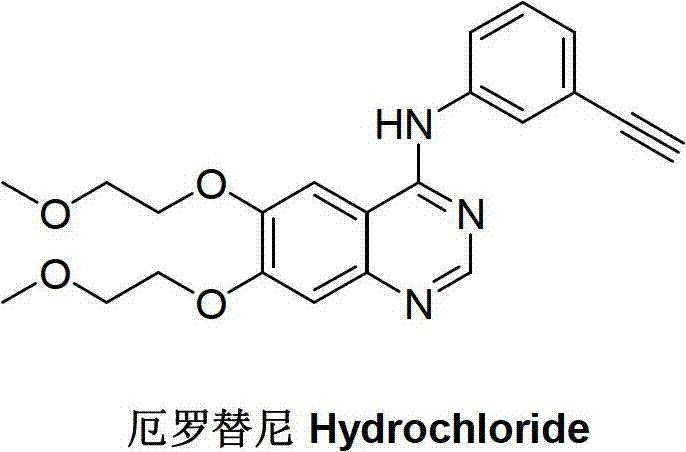
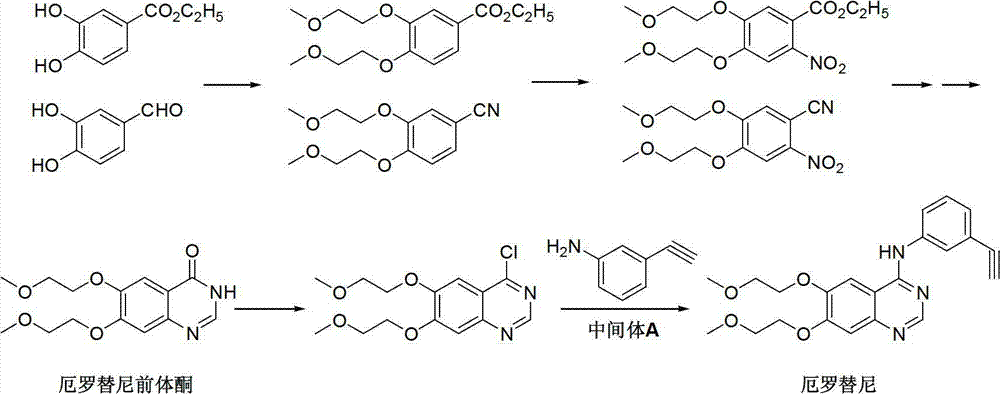
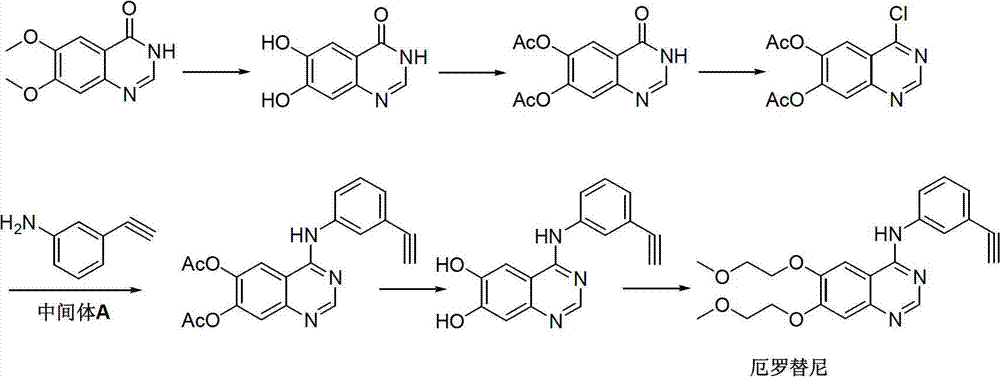
Preparation method of erlotinib intermediate, i.e., 3-aminobenzeneacetylene
InactiveCN102757350AReduce manufacturing costImprove economyOrganic compound preparationAmino compound preparationErlotinibNitrobenzene
The invention discloses a preparation method of an erlotinib intermediate, i.e., 3-aminobenzeneacetylene. The method comprises the following steps of: performing a Vilsmeier reaction on 3-nitrobenzene ethyl ketone (I) serving as an industrial raw material to obtain an intermediate, i.e., 1-chloro-1-(3-nitrobenzophenone)-2-(N,N-dimethyl formaldehyde oxime)-yl-ethylene (II) containing a halogen-substituted alkene bond; performing a dehydration reaction to obtain a formyl intermediate, i.e., 1-chloro-1-(3-nitrobenzophenone)-2-formaldehyde)-yl-ethylene (III); performing an elimination reaction to obtain an intermediate, i.e., 3-nitrophenylacetylene (IV); and performing a nitro reduction reaction on the intermediate, i.e., 3-nitrophenylacetylene (IV) to obtain the erlotinib intermediate, i.e., 3-aminobenzeneacetylene (intermediate A). The preparation method can contribute to improving the atom economy, process simplicity and environment friendliness, so that the the erlotinib intermediate, i.e., 3-aminobenzeneacetylene can be prepared easily and conveniently.
Owner:SUZHOU LIXIN PHARMA
Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol
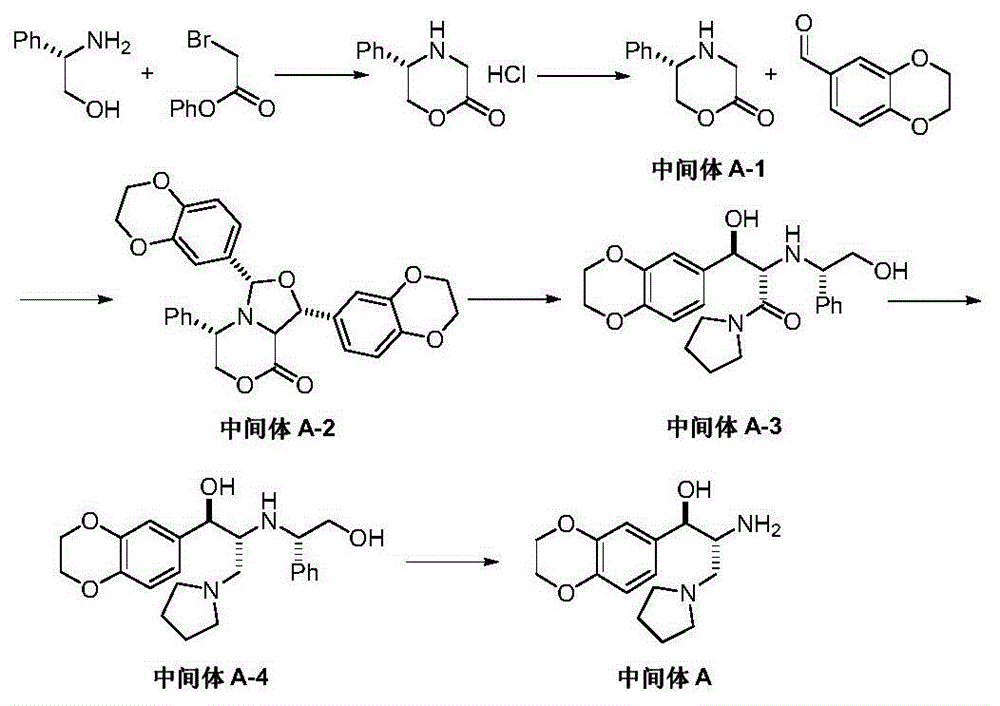
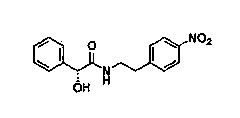
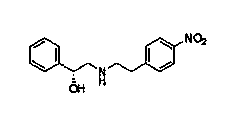
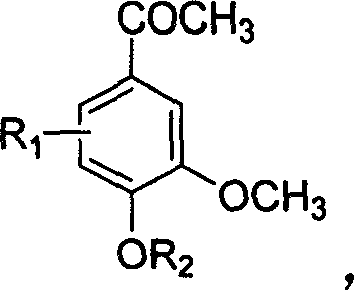
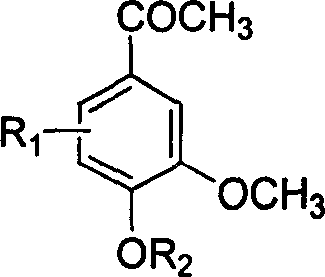
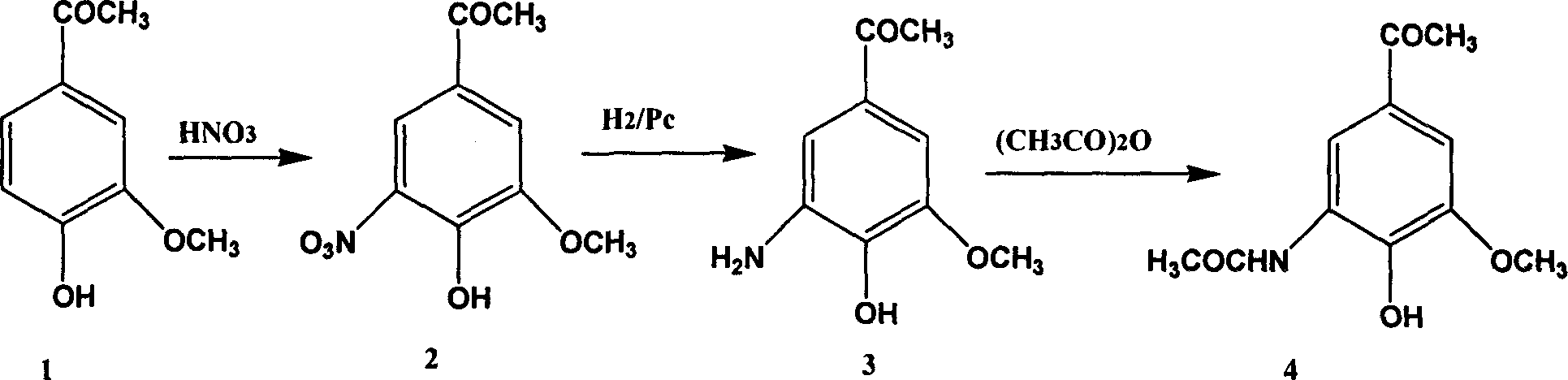
InactiveCN102050748APreparation method greenEconomical method of preparationOrganic compound preparationAmino-hyroxy compound preparationNitrogenCyclohexanol
The invention relates to a method for preparing an expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol. Nitrobenzaldehyde and trans-4-amino cyclohexanol are subjected to the combination reaction of condensation, carbon and nitrogen double-bond hydrogenation and nitro reduction to prepare the trans-4-[(2-amino benzyl) amino]-cyclohexanol in a high-yield mode. The method has the advantages of reasonable process route design, simple and convenient process, high reaction yield, low raw material cost and no harsh reaction conditions and is easy for scale production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of 5-chloro-2-methoxy cyanophenyl
InactiveCN102786439ALow priceEasy to getPreparation by cyanide reactionP-fluoronitrobenzeneNitrobenzene
The invention discloses a preparation method of 5-chloro-2-methoxy cyanophenyl, relating to the technical field of medicine intermediates. The method is characterized by using fluoronitrobenzene as an initial raw material, and conducting bromination, nitro reduction, diazotization-Sandmeyer, Williamson substitution and carbonitriding to synthesize 5-chloro-2-methoxy cyanophenyl. The 5-chloro-2-methoxy cyanophenyl obtained by the method is a white powdery solid with the purity of 99.5%, the feed stock conversion each step respectively reaches 100%, and the total yield of the whole process reaches 48%.
Owner:CHANGZHOU UNIV
Novel method for preparing Eltrombopag intermediate
ActiveCN106146330AEasy to recycleWide variety of sourcesOrganic compound preparationAmino-carboxyl compound preparationHydrogenation processCombinatorial chemistry
The invention provides a method for preparing a compound shown as the formula I (please see the formula in the description). The method specifically comprises the following steps that 1, a compound shown as the formula (II) (please see the formula in the description) reacts with a compound shown as formula (V) (please see the formula in the description) under the alkaline condition to generate a compound shown as the formula (III) (please see the formula in the description); 2, the compound shown as the formula (III) (please see the formula in the description) reacts with a compound shown as the formula (VI) (please see the formula in the description) under the alkaline condition in the presence of palladium carbon to generate a compound shown as the formula (IV) (please see the formula in the description); 3, the compound shown as the formula (IV) (please see the formula in the description) reacts in the presence of palladium carbon and a hydrogen source under the alkaline condition to generate the compound shown as the formula (I) (please see the formula in the description). According to the method, design is ingenious, protecting group removal, dechlorination and nitro reduction are together completed in the final hydrogenation process, and the purity of the obtained compound shown as the formula (I) (please see the formula in the description) is high; the most important thing is that compared with other Suzuki coupling agents, cost of palladium carbon is lower, a source of palladium carbon is wide and easy to obtain, palladium carbon can be directly recycled and reused after being simply filtered and separated, and therefore the material cost is greatly reduced; meanwhile, emission of three wastes is reduced, and the method is quite suitable for industrialized production.
Owner:JIANGSU VCARE PHARMATECH
Preparation method of oxazolidinone antibiotic intermediate
ActiveCN110938058ARaw materials are easy to getSimple processOrganic chemistryBiotechnologyCarbamate
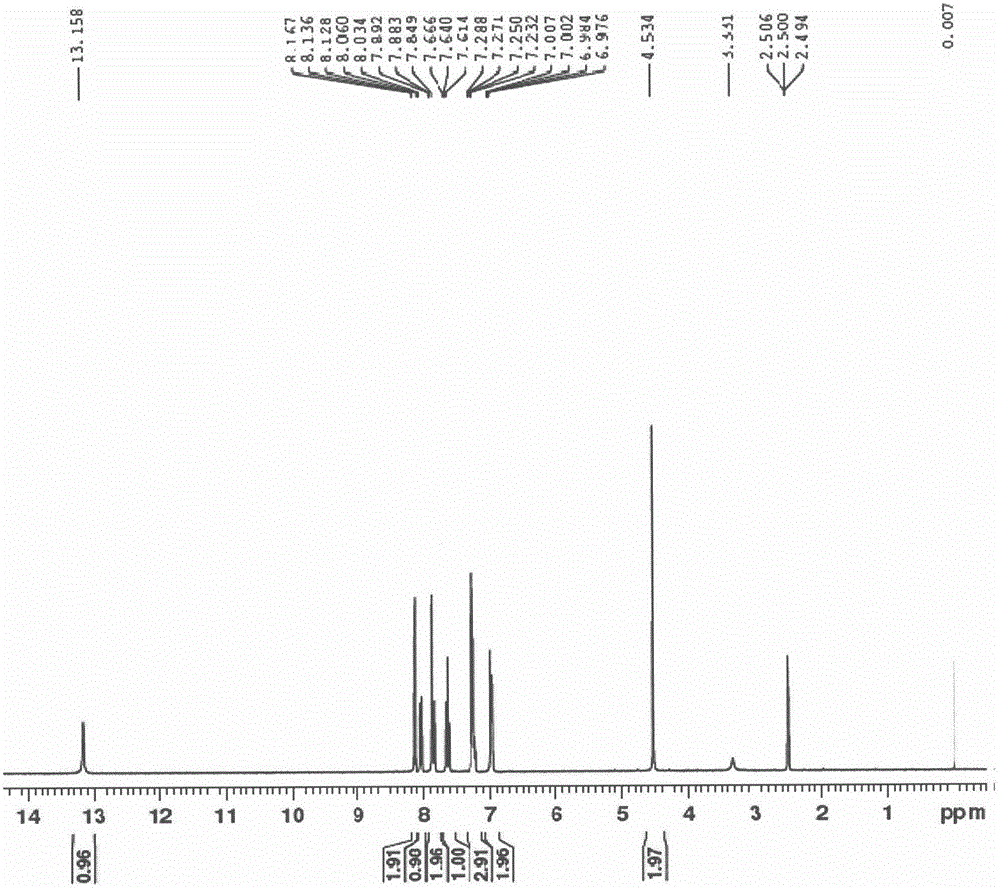
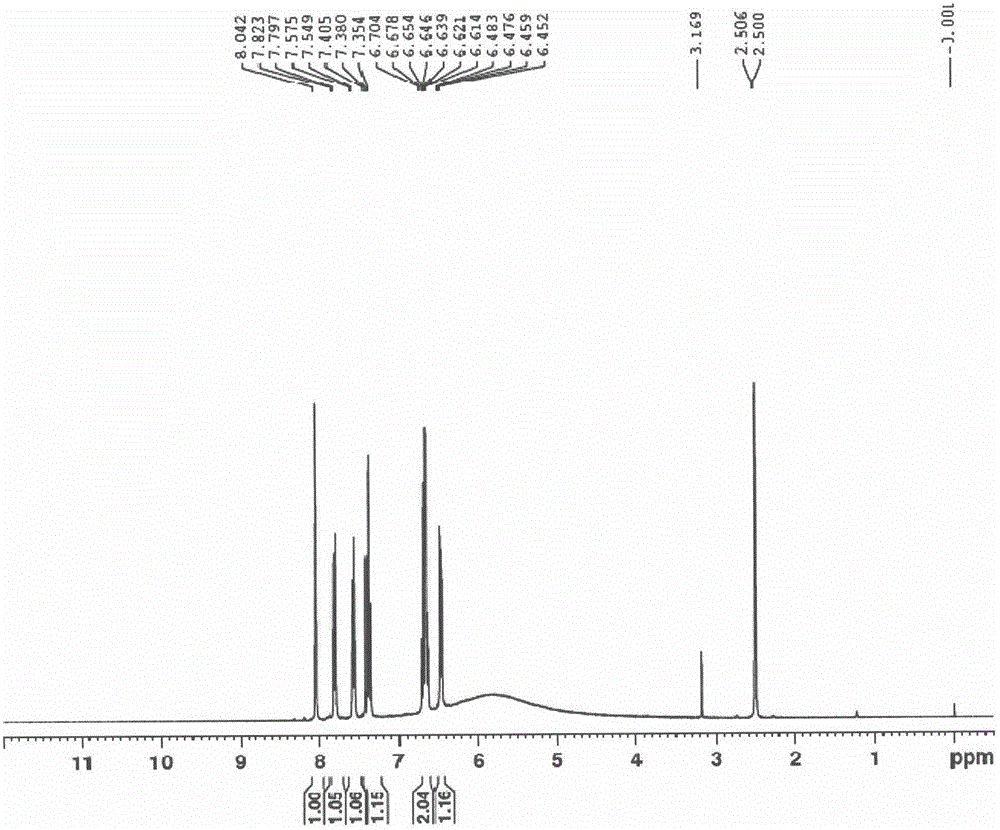
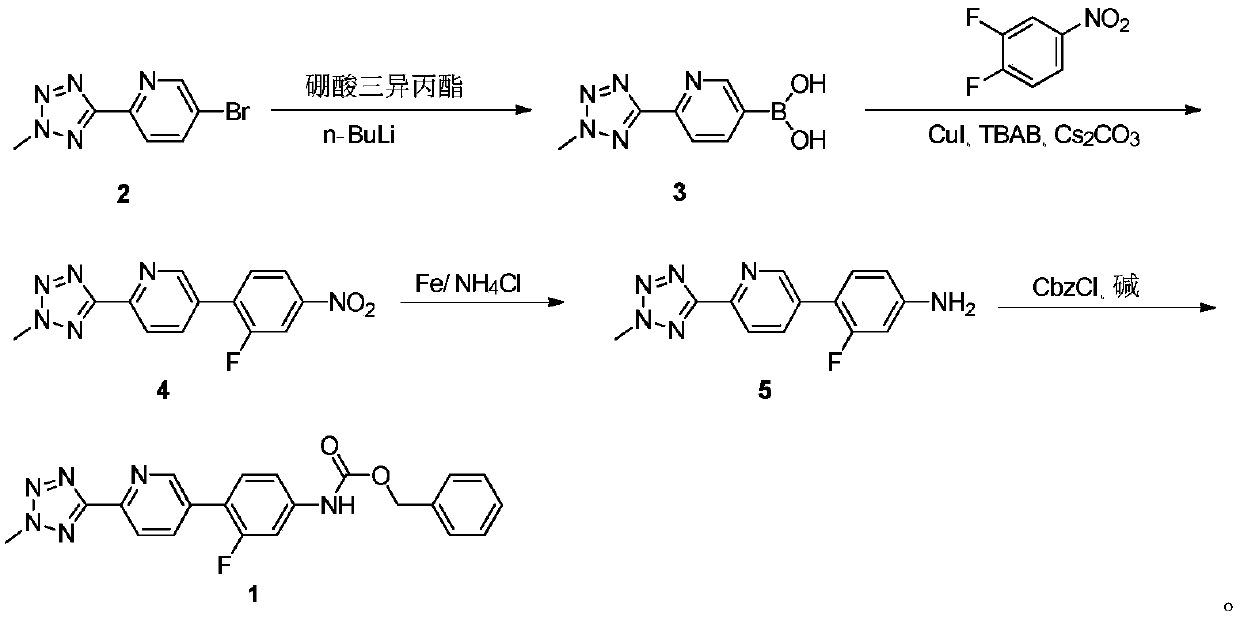
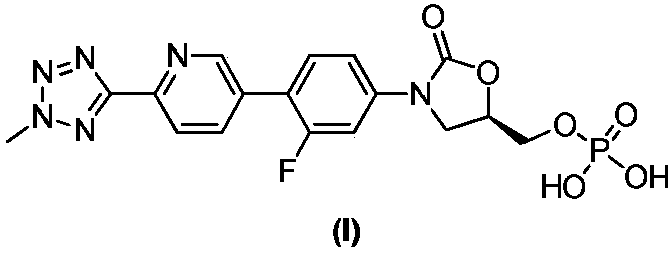
The invention provides a preparation method of a tedizolid phosphate intermediate compound 3-fluoro-4-(6-(2-methyltetrazole-5-yl)pyridine-3-yl)phenyl benzyl carbamate. The method comprises the following steps: carrying out a reaction on 5-bromo-2-(2-methyltetrazole-5-yl)pyridine as a raw material and triisopropyl borate under the action of n-butyllithium to prepare a boric acid intermediate, coupling the boric acid intermediate and 1,2-difluoro-4-nitrobenzene, and finally performing nitro reduction and amidation to obtain the required target product. Compared with the method in the prior art,the preparation method disclosed by the invention has the advantages of easily available raw materials, simple process, economy, environmental protection and suitability for industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
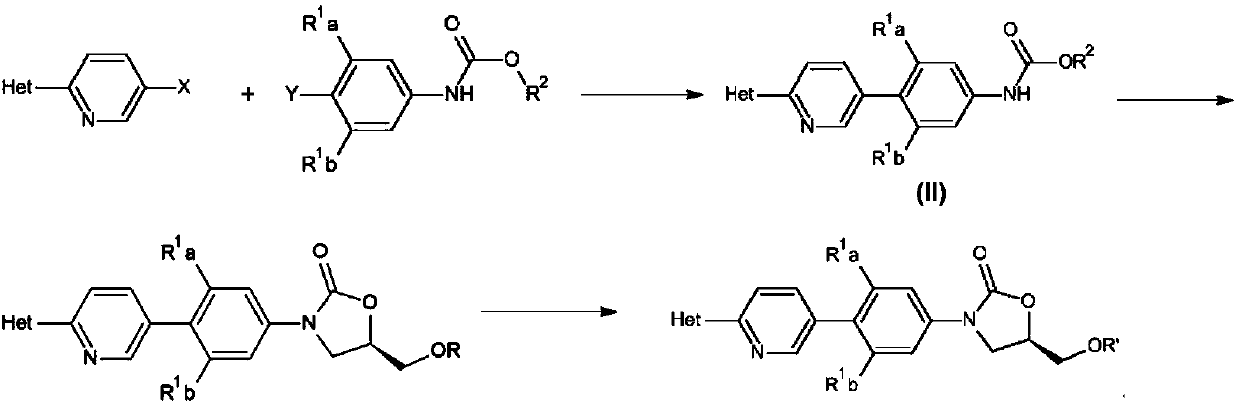
Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine
InactiveUS20070225494A1MinimizationImprove productivityNervous disorderOrganic chemistryOrganic solventThiazepine
Disclosed is a process for the preparation of 11-(4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl)-dibenzo[b,f][1,4]thiazepine. In the process, low-priced 2,2′-dithiosalicylic acid as starting material is subjected to bond formation reaction with 1-chloro-2-nitrobenzene in a basic aqueous solution, a nitro group reduction reaction is conducted, cyclization and chlorination reactions are simultaneously carried out in the presence of a equivalent amount of halogenating agent, a reaction with piperazine is continuously conducted without separation, and a reaction with 2-haloethoxyethanol is conducted, thereby it is possible to economically producing Quetiapine, that is, 11-(4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl)-dibenzo[b,f][1,4]thiazepine, in an environmentally friendly manner. Particularly, the process is advantageous in that economic efficiency is assured because of use of the low-priced starting material, use of an organic solvent is minimized because a reaction is conducted in an aqueous solution, and it is possible to achieve the environmentally friendly and economical process having high commercial usefulness because the number of reaction steps of the process is reduced and because generation of acidic waste is minimized.
Owner:SK BIOTEK
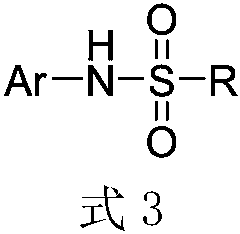
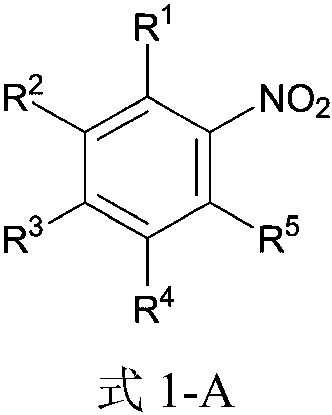
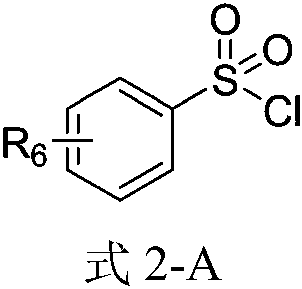
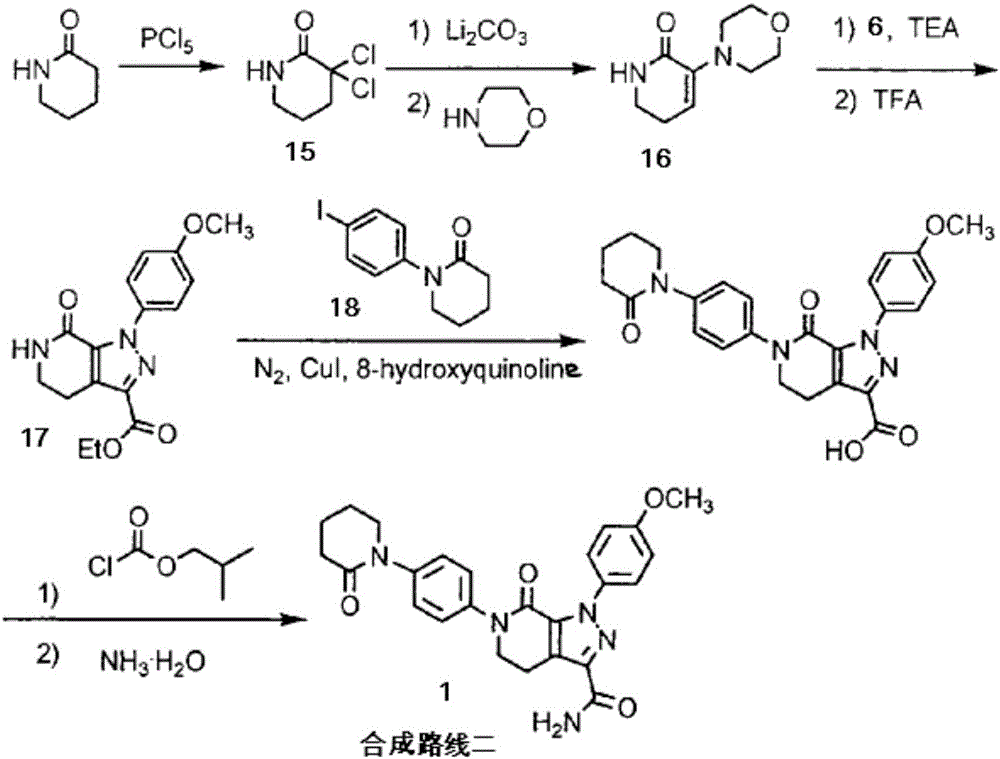
Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide
ActiveCN104387299ALow costImprove stabilitySulfonic acid amide preparationP-nitrobenzenesulfonyl chlorideNitro reduction
The invention discloses a method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide. The method comprises the following steps: S1: enabling L-phenylalanine and diazomethane to react to obtain a diazo methyl ketone intermediate product, and enabling the diazo methyl ketone intermediate product and haloid acid to react to obtain a compound A; S2, conducting carbonyl deoxidation on the compound A to obtain a compound B; S3, under the existence of iso-butylamine, conducting cyclization reaction and ring-opening reaction on the compound B in sequence to obtain a compound C; S4, enabling the compound C and nitrobenzenesulfonyl chloride to react to obtain a compound D; S5, conducting nitro reduction on the compound D to obtain the 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide. The method is simple in course, low in cost, mild in condition, and higher in intermediate product stability, and is beneficial for industrial application.
Owner:ASYMCHEM LAB TIANJIN +4
Simple synthesis process of 1-aminocyclopropane-1-carboxylic acid
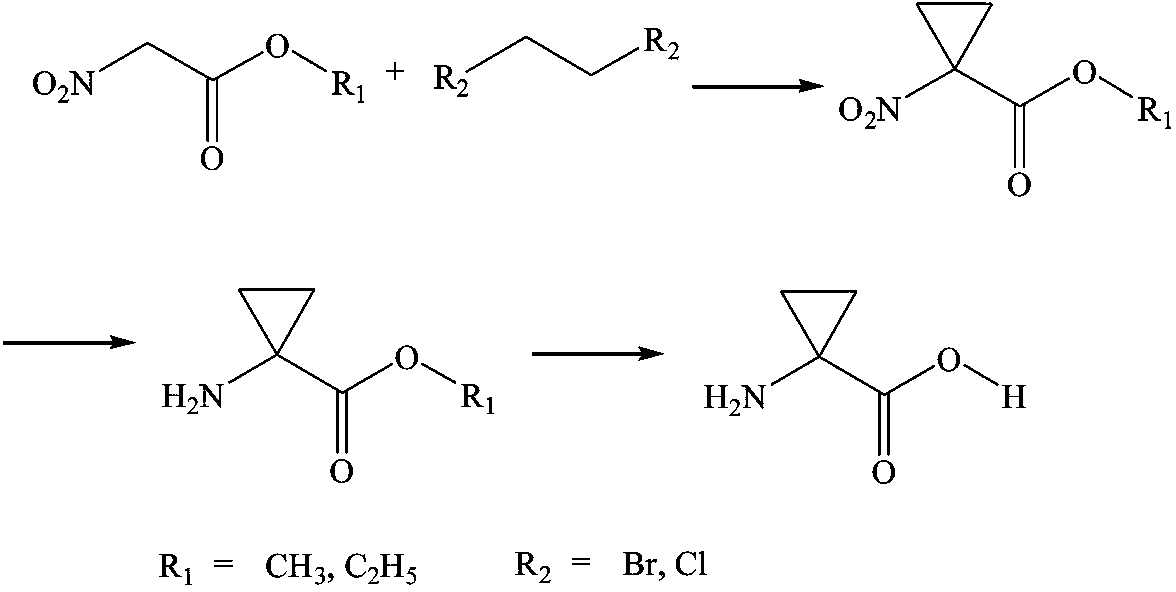
ActiveCN103864635AOvercome the disadvantages of the preparation processLow costOrganic compound preparationAmino-carboxyl compound preparation1-Aminocyclopropane-1-carboxylic acidSocial benefits
The invention relates to a synthesis process of 1-aminocyclopropane-1-carboxylic acid. According to the process, the 1-aminocyclopropane-1-carboxylic acid is synthesized from nitro acetate and 1,2-dihalo ethane through the reaction of alkylated cyclization, nitro reduction and carboxyl hydrolysis, and a high-content and high-purity product is obtained through carrying out separation, purification and crystallization after the reaction is ended. In the aspects of reagents and raw and auxiliary materials used during the reaction, both environment-friendliness and efficiency are taken into account. The process disclosed by the invention has the advantages of high atom economical efficiency, simple equipment and environment-friendly production procedure and has very large economic and social benefits.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Method for synthesizing S-3-(piperidine-2-yl)-azacyclo-azetidine-3-alcohol
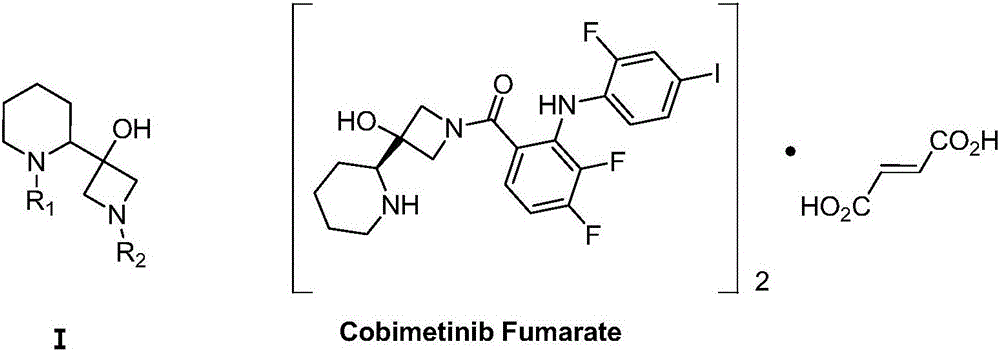
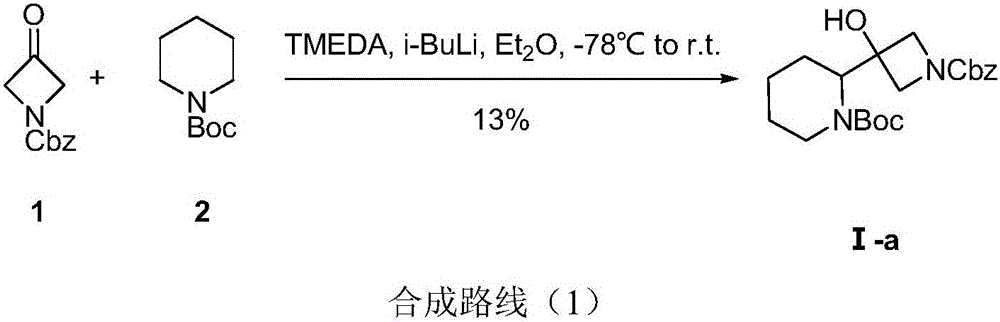
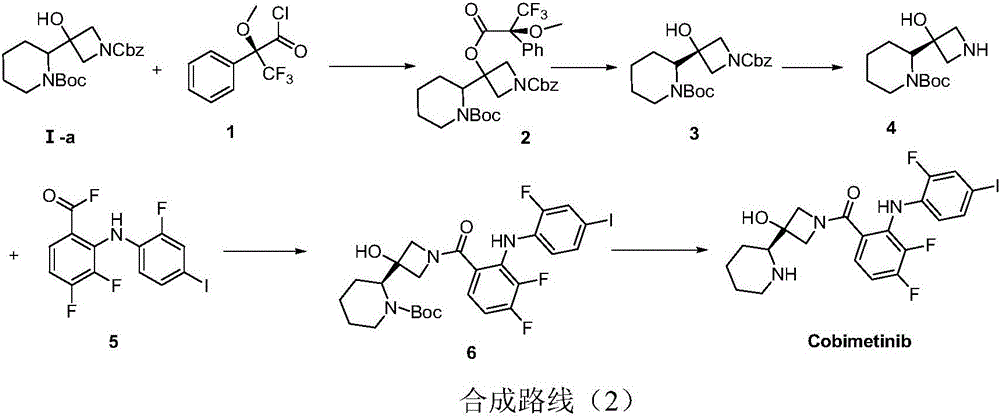
ActiveCN106220607AShort process routeAvoid the disadvantages of low yield of chiral resolutionAntineoplastic agentsAsymmetric synthesesGrignard reagentNitromethane
The invention discloses a method for synthesizing S-3-(piperidine-2-yl)-azacyclo-azetidine-3-alcohol. The method comprises the following steps: condensing a compound S-N-Boc-piperidine-2-formic acid of a formula A and CDI so as to obtain a compound of a formula B; condensing the compound of the formula B and nitromethane under an alkali condition so as to obtain a compound C; enabling the compound C and a halogen methyl Grignard reagent to react so as to obtain a compound D; performing nitro reduction and cyclization on the compound D so as to obtain a compound I; condensing a compound of a formula I and a compound of a formula II in the alkali environment so as to obtain a compound of a formula III; performing deprotection on the compound of the formula III under an acid condition, thereby obtaining the S-3-(piperidine-2-yl)-azacyclo-azetidine-3-alcohol. The method disclosed by the invention is cheap and easily obtainable in raw material, simple and convenient in process operation, small in waste pollution, green and economic and easy in industrialization; by adopting the method disclosed by the invention, use of a conventional chiral resolution method is avoided, and a feasible technical scheme is provided for large-scale production of Cobimetinib.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Method for ultrasound-assisted synthesis of N-arylsulfonamide
ActiveCN108822002AAbundant and easy to obtainLow priceSulfonic acid amide preparationSulfonyl chlorideNitro compound
The invention discloses a method for ultrasound-assisted synthesis of N-arylsulfonamide. N-arylsulfonamide compound is synthesized by series reaction of nitro reduction / sulfonyl chloride reduction / sulfonamidation with taking aromatic nitro compounds, sulfonyl chloride and iron powder as raw materials under ultrasonic stirring conditions. Water is used as a reaction medium and a hydrogen source inthe reaction. The method has the advantages of cheap and easily obtained raw materials, simple and mild reaction conditions, environmental friendliness, saving energy, high reaction selectivity and high yield, excellent compatibility of substrate functional groups and high application value.
Owner:CENT SOUTH UNIV
Novel Schiff base compound taking triphenylamine as center and preparation of novel Schiff base compound
InactiveCN105294540AEasy post-processingEasy to makeOrganic chemistryLuminescent compositionsPolymer scienceElectron delocalization
The invention provides a novel Schiff base compound taking triphenylamine as a center and having a structural formula I, and a preparation method of the novel Schiff base compound. The preparation method comprises steps of: reacting triphenylamine with DMF and POCl3 to prepare 4,4-diformyltriphenylamine; preparing N-ethylcarbazole from carbazole and bromoethane; nitrifying N-ethylcarbazole to obtain 3-nitro-N-ethylcarbazole; further reducing nitro to obtain 3-amino-N-ethylcarbazole; and finally reacting 4,4-diformyltriphenylamine with 3-nitro-N-ethylcarbazole to obtain the Schiff base compound. The prepared Schiff base compound has carbon-nitrogen double bonds (C=N) and a relatively large pi electron delocalization skeleton, which provides the compound with a relatively large fluorescence absorption coefficient. Due to the presence of lone pair electrons of C=N, the novel Schiff base compound has a potential application to detection of metal ions.
Owner:QILU UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

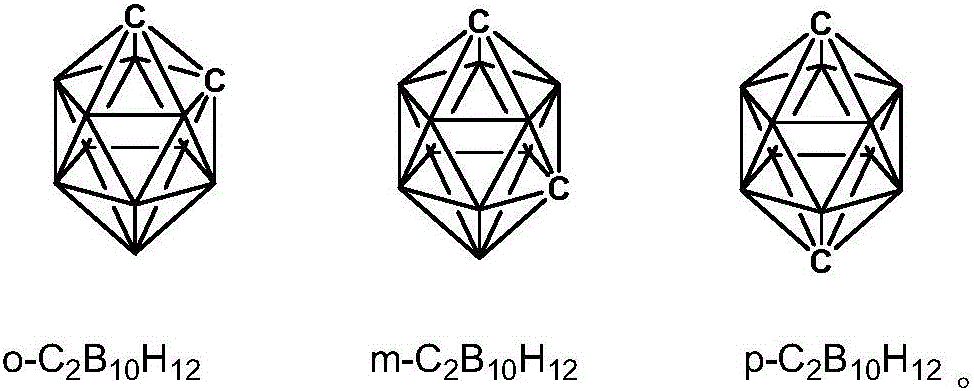
![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka.patsnap.com/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100011.PNG)
![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka.patsnap.com/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100021.PNG)
![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka.patsnap.com/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100031.PNG)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka.patsnap.com/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00001.png)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka.patsnap.com/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00002.png)
![Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine Process for the Preparation of 11-(4-[2-(2-Hydroxyethoxy)Ethyl]-I-Piperazinyl)Dibenzo[b,f][I,4]Thiazepine](https://images-eureka.patsnap.com/patent_img/6d36962c-963c-4422-b2f6-648f84065f1f/US20070225494A1-20070927-C00003.png)
![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka.patsnap.com/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000011.PNG)
![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka.patsnap.com/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000021.PNG)
![Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide Method for preparing 4-amino-N-[(2R,3S)-3-amino-2-hydroxy-4-benzene butyl]-N-isobutyl benzsulfamide](https://images-eureka.patsnap.com/patent_img/a8d980e4-fe1e-488c-8f1b-65059c0e6bf2/HDA0000592763380000031.PNG)