Synthesizing method and application of 1,2,4,5-tetraamine benzene
A technology of tetraaminobenzene and synthetic method, which is applied in the direction of preparation of amino compounds, preparation of nitro compounds, chemical instruments and methods, etc., can solve the problems of waste of resources, unfavorable industrial production, high price of dichlorobenzene, etc., and achieve the benefits of industrial production, Suitable for industrial production and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
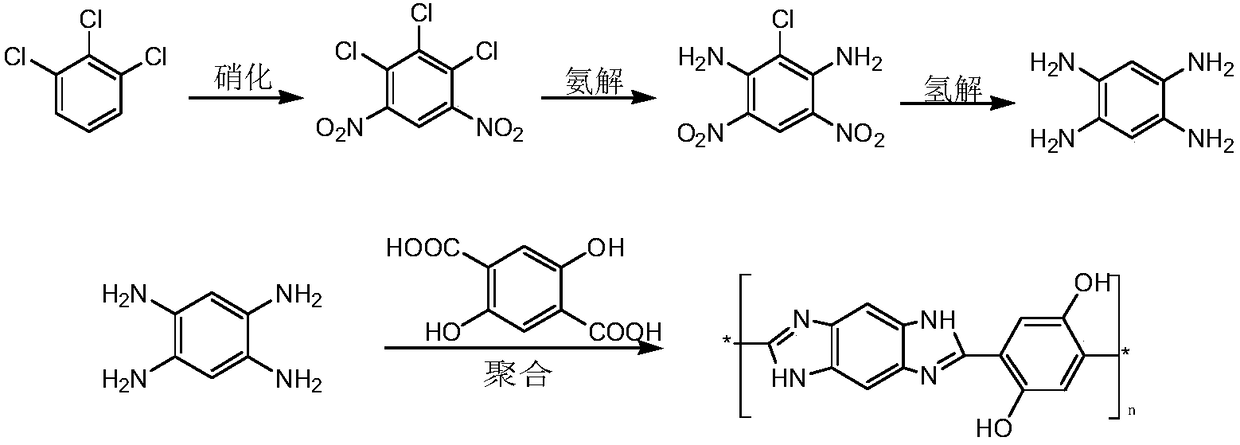
[0031] (1) Add 72g (0.4mol) of 1,2,3-trichlorobenzene and 200mL of 98% concentrated sulfuric acid into a 500mL three-necked flask, heat, stir, dissolve, add 40mL of 65% concentrated nitric acid dropwise, and the dropwise addition temperature is controlled at 55±10°C, after dropping, the reaction temperature was controlled at 70±5°C, the reaction was continued for 5 hours, and the reaction was stopped to obtain crude yellow solid 4,6,-dinitro-1,2,3-trichlorobenzene with a yield of 86.9% with a purity of 93.7%.
[0032] (2) Add 25g (0.1mol) 4,6-dinitro-1,2,3-trichlorobenzene into a 250mL autoclave, dissolve in 50mL ethylene glycol, heat to 150°C, pass ammonia gas, ammonia Pressure 1.0MPa, react for 8h, stop the reaction, cool and filter to obtain the crude product, recrystallize from absolute ethanol to obtain 4,6-dinitro-2-chloro-1,3-phenylenediamine, the yield is 84.5%, The purity is 97.6%.
[0033] (3) Add 10g (0.04mol) 4,6-dinitro-2-chloro-1,3-phenylenediamine, 50mL DMF, 0...
Embodiment 2
[0036] Adopt the method for embodiment 1, difference is only: the nitration system described in step (1) is changed into the concentrated sulfuric acid-sodium nitrate system; Ammonolysis solvent described in step (2) is methyl alcohol, and ammonolysis temperature 100 ℃, Ammonia pressure 0.2MPa, reaction time 6h; hydrogenolysis solvent described in step (3) is tetrahydrofuran, under the action of RaneyNi, hydrogenolysis temperature 75°C, hydrogen pressure 1.5MPa, reaction time 2h, the final products 1,2,4,5 -Tetraaminobenzene, the yield is 73.2%, and the purity is 92.6%.
Embodiment 3
[0038] Using the method of Example 1, the only difference is that the ammonolysis solvent described in step (2) is tetrahydrofuran, the ammonolysis temperature is 180° C., the ammonia pressure is 2.0 MPa, and the reaction time is 10 h; the hydrogenolysis described in step (3) The solvent is DMA, under the action of 3% Pt / C, the hydrogenolysis temperature is 110°C, the hydrogen pressure is 2.0MPa, and the reaction time is 10h, the final product 1,2,4,5-tetraaminobenzene has a yield of 84.2% and a purity of 86.9 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com