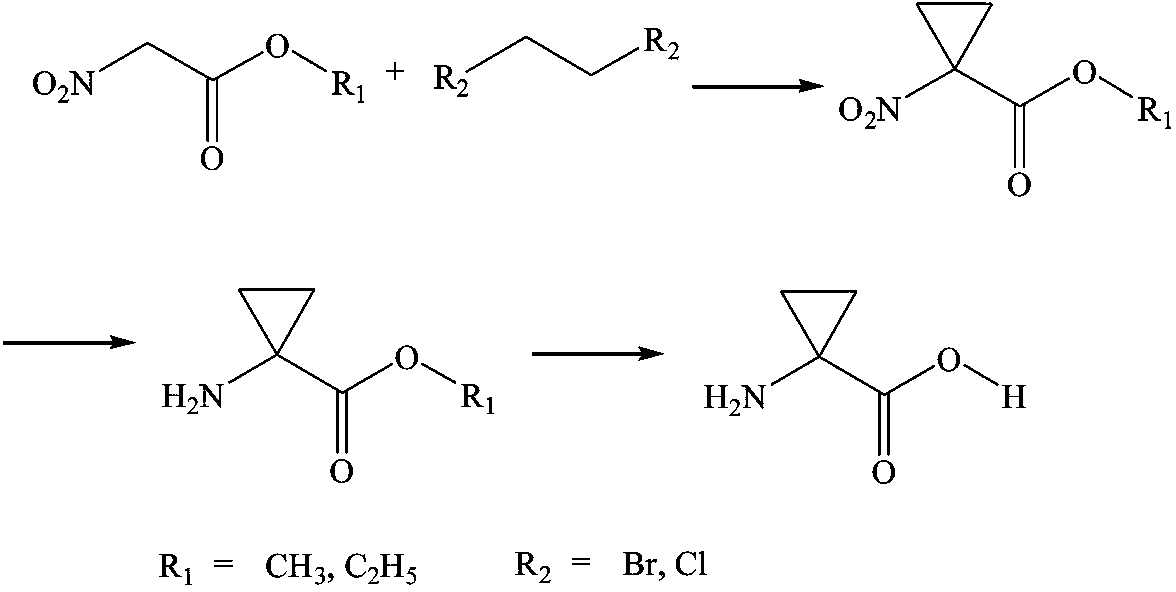
Simple synthesis process of 1-aminocyclopropane-1-carboxylic acid
A technology of alkylation cyclization and nitroacetate, applied in the field of simple synthesis of natural living amino acids, can solve the problems of high final product cost, low total yield, pollution of the environment, etc., and achieve green production procedures and simple equipment , large economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Synthesis of ethyl 1-nitrocyclopropane-1-carboxylate
[0016] In a 500 L stainless steel reactor, sequentially add 200 L of solvent methylene chloride, 40 kg of nitroacetate and 55 kg of 1,2-dibromoethane, 2.5 kg of catalyst anhydrous potassium carbonate, and slowly heat up to reflux , continue to control the temperature and react for 4-5 h, and monitor the complete reaction of the raw material nitroacetate by thin-layer chromatography, which is considered as the completion of the cyclization reaction. Lower the temperature to less than 10°C, add water to separate layers, and wash away the catalyst. The organic phase was separated, dried, and concentrated under reduced pressure to obtain 46.2 kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate.
Embodiment 2
[0017] Example 2 Synthesis of 1-nitrocyclopropane-1-carboxylic acid methyl ester
[0018] In a 500 L stainless steel reaction kettle, add 200 L of solvent dichloromethane, 40 kg of nitroethyl methyl ester and 42 kg of 1,2-dichloroethane, and 2.2 kg of catalyst anhydrous sodium carbonate in sequence, and slowly heat up to reflux , continue to control the temperature and react for 4-5 h, and monitor the complete reaction of the raw material nitroacetate by thin-layer chromatography, which is considered as the completion of the cyclization reaction. Lower the temperature to less than 10°C, add water to separate layers, and wash away the catalyst. The organic phase was separated, dried, and concentrated under reduced pressure to obtain 45.7 kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate.
Embodiment 3
[0019] Example 3 Synthesis of ethyl 1-aminocyclopropane-1-carboxylate
[0020] In a 500 L enamel reaction kettle, add 200 L of reaction solvent methanol and 45 kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate, stir to dissolve, cool the jacket to about 5 °C, and add dichloride in batches Tin is 70 kg, and the temperature is controlled at 15-20 °C, and the reaction is stirred for 3-4 h. The raw material 1-nitrocyclopropane-1-carboxylate intermediate basically disappears as monitored by thin-layer chromatography, and the reduction reaction is considered complete. Concentrate under reduced pressure to recover methanol, then add water and dichloromethane to extract twice, combine the organic phases, dry, and concentrate under reduced pressure to obtain 42.3 kg of crude 1-aminocyclopropane-1-carboxylate intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com