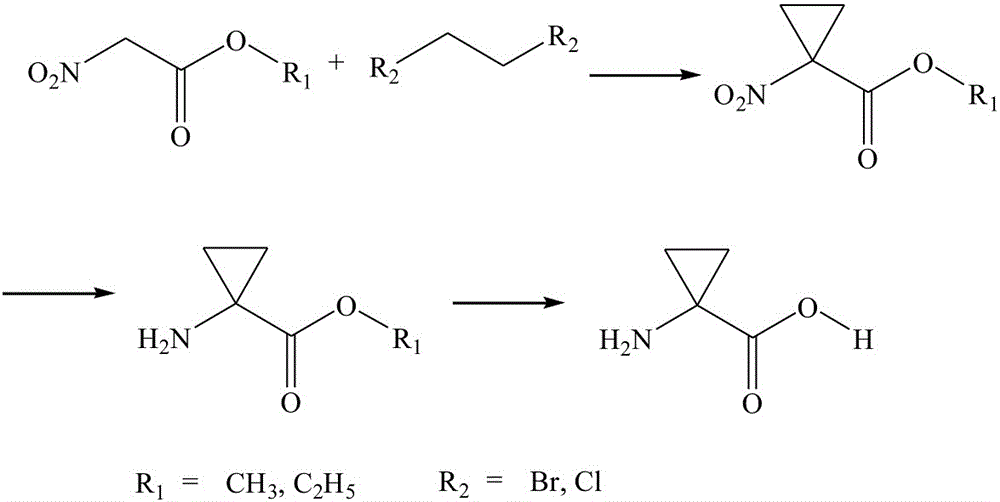
A simple synthesis process of 1-aminocyclopropane-1-carboxylic acid
A technology of aminocyclopropane and carboxylic acid, which is applied in the field of simple synthesis of natural living amino acids, can solve the problems of low total yield, high final product cost, and environmental pollution, and achieve simple equipment, green production procedures, large economy and The effect of social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthesis of embodiment 11-nitrocyclopropane-1-carboxylate ethyl ester
[0016] In a 500L stainless steel reaction kettle, add 200L of dichloromethane as a solvent, 40kg of nitroacetate and 55kg of 1,2-dibromoethane, and 2.5kg of anhydrous potassium carbonate as a catalyst in sequence, slowly raise the temperature to reflux, and continue to control the temperature After 4-5 hours of reaction, the complete reaction of the raw material nitroacetate was monitored by thin-layer chromatography, and the cyclization reaction was considered complete. Lower the temperature to less than 10°C, add water to separate layers, and wash away the catalyst. The organic phase was separated, dried, and concentrated under reduced pressure to obtain 46.2 kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate.
Embodiment 2
[0017] Synthesis of embodiment 21-nitrocyclopropane-1-methyl carboxylate
[0018] In a 500L stainless steel reaction kettle, add 200L of dichloromethane as a solvent, 40kg of nitroethyl methyl ester and 42kg of 1,2-dichloroethane as reaction materials, and 2.2kg of anhydrous sodium carbonate as a catalyst, slowly raise the temperature to reflux, and continue to control the temperature After 4-5 hours of reaction, the complete reaction of the raw material nitroacetate was monitored by thin-layer chromatography, and the cyclization reaction was considered complete. Lower the temperature to less than 10°C, add water to separate layers, and wash away the catalyst. The organic phase was separated, dried, and concentrated under reduced pressure to obtain 45.7 kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate.
Embodiment 31
[0019] Synthesis of Example 31-aminocyclopropane-1-ethyl carboxylate
[0020] In a 500L enamel reaction kettle, add 200L of reaction solvent methanol and 45kg of ethyl 1-nitrocyclopropane-1-carboxylate intermediate, stir and dissolve, cool the jacket to about 5°C, and add 70kg of tin dichloride in batches, And control the temperature at 15-20° C., stir the reaction for 3-4 hours, and monitor the raw material 1-nitrocyclopropane-1-carboxylate intermediate by thin-layer chromatography to basically disappear, and the reduction reaction is considered complete. Concentrate under reduced pressure to recover methanol, then add water and dichloromethane to extract twice, combine the organic phases, dry, and concentrate under reduced pressure to obtain 42.3 kg of crude 1-aminocyclopropane-1-carboxylate intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com