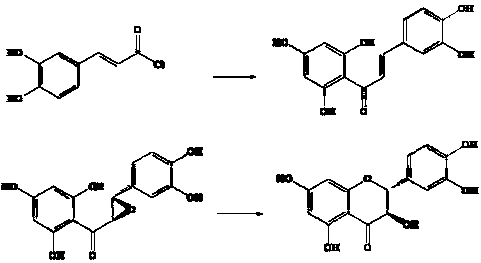
A kind of synthetic technology of natural active product dihydroquercetin
A dihydroquercetin and product technology, applied in the field of synthesis of natural active ingredient-dihydroquercetin, can solve the problems of many reaction steps, many by-products, affecting the extraction and utilization of active ingredients, etc., and achieve simple equipment , large economic and social benefits, and the effect of green production procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
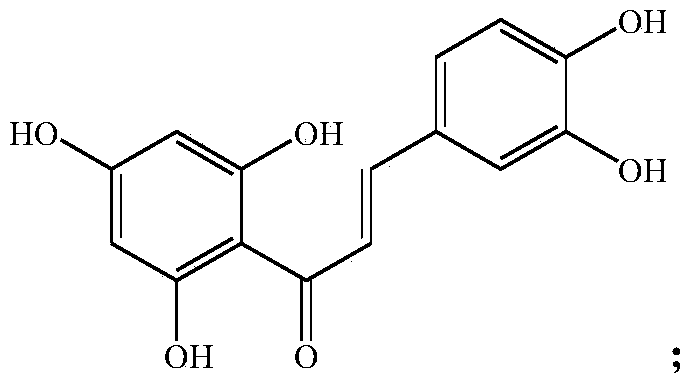
[0019] Synthesis of embodiment 11-caffeoyl-2,4,6-trihydroxyphenol
[0020] In a 500L enamel reaction kettle, add 200L of dichloromethane and 40.0kg of caffeic acid chloride as the reaction raw material, cool the jacket to about 5°C, add 13.5kg of anhydrous aluminum trichloride in batches, and control the temperature at 5-10°C. The reaction was stirred for 2h to form a complex. Continue to control the temperature within 10°C, add 26.0 kg of the raw material phloroglucinol in batches, and slowly raise to room temperature, then stir for 4 hours, and monitor the complete reaction of the raw material caffeic acid chloride by thin-layer chromatography, and the acylation reaction is considered complete. Cool down to within 10°C, slowly add 5% sodium hydroxide solution to about pH9, and hydrolyze for 0.5h. Then adjust the pH value to 6-7 with 5% dilute hydrochloric acid, and let it stand for stratification. After the organic phase was separated, it was extracted once with 100 L of d...
Embodiment 21-(3
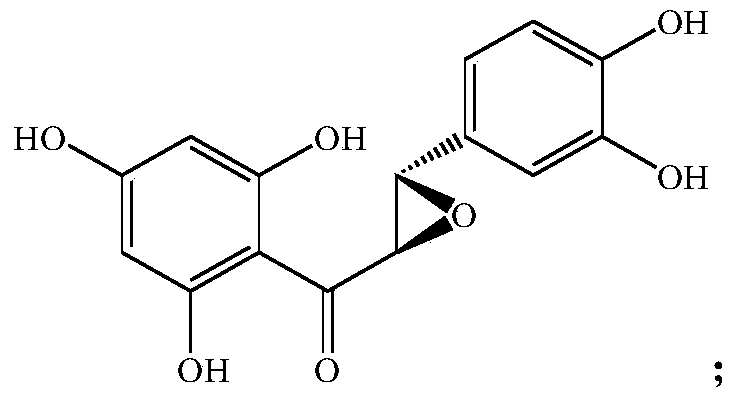
[0021] Example 21-(3,4-dihydroxy)-phenyl, 2-(2,4,6-trihydroxy)phenyl-oxirane synthesis
[0022] In a 500L enamel reaction kettle, add 55.0kg of 1-caffeoyl-2,4,6-trihydroxyphenol and stir to dissolve with 300L of methanol, cool down the jacket to about 5°C, add 40.0kg of m-chloroperoxybenzoic acid in batches, The temperature was controlled at 15° C., and the reaction was stirred for 4 hours. The raw material 1-caffeoyl-2,4,6-trihydroxyphenol basically disappeared as monitored by thin-layer chromatography, and the oxidation reaction was considered complete. Cool down to less than 10°C, slowly add 12.5 L of saturated sodium bisulfite solution to remove peroxy-m-chloroperoxybenzoic acid, concentrate under reduced pressure, and recover methanol. Add 150L of water and 200L of dichloromethane to extract twice, combine the organic phases, dry, and concentrate under reduced pressure to obtain 1-(3,4-dihydroxy)-phenyl, 2-(2,4,6-trihydroxy) The crude product of phenyl-oxirane intermedia...
Embodiment 3 2
[0023] The synthesis of embodiment 3 dihydroquercetin
[0024]In a 500L enamel reaction kettle, add 200L dichloromethane and dry 1-(3,4-dihydroxy)-phenyl, 2-(2,4,6-trihydroxy)phenyl-oxirane 50.0kg of the intermediate was stirred and dissolved, and the temperature of the jacket was lowered to about 5°C, and the acid catalyst trifluoroacetic acid 10.0L was slowly strengthened, and the temperature was controlled at 0-5°C, and the reaction was stirred for 5h. Thin-layer chromatography monitors the complete reaction of the raw material intermediate, which is considered as the completion of the ether-forming reaction. Adjust the pH value to 6-7 with saturated sodium bicarbonate solution, and let stand to separate layers. After the organic phase was separated, it was extracted once with 100 L of dichloromethane, the organic phases were combined, dried, concentrated under reduced pressure, and dried to obtain 45.8 kg of crude dihydroquercetin. The crude product was dissolved in 230L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com