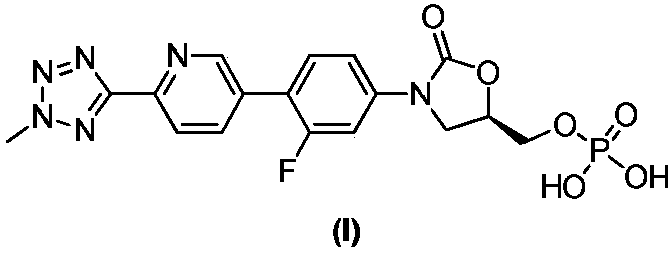
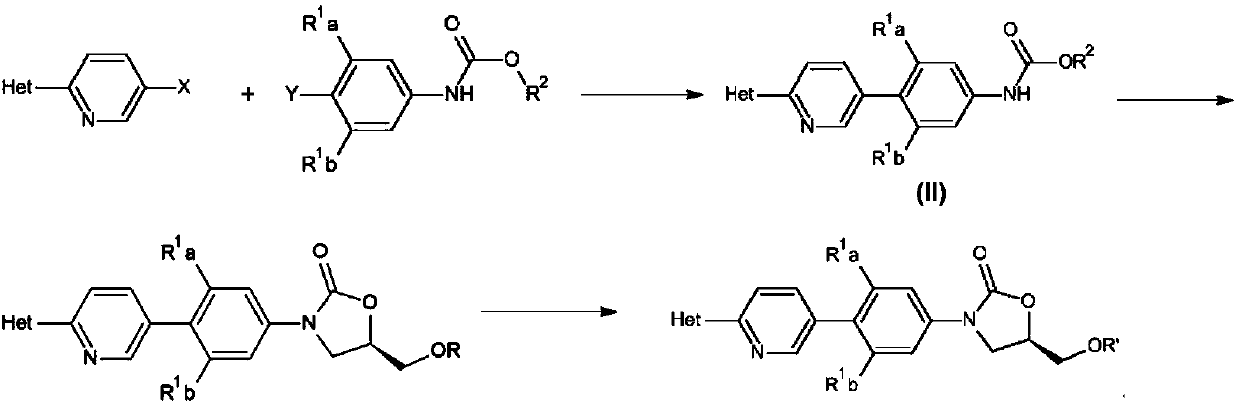
Preparation method of oxazolidinone antibiotic intermediate
An intermediate and system technology, which is applied in the field of preparation of tedizolid phosphate intermediate, can solve problems such as unfavorable industrial scale-up, and achieve the effect of easy-to-obtain raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
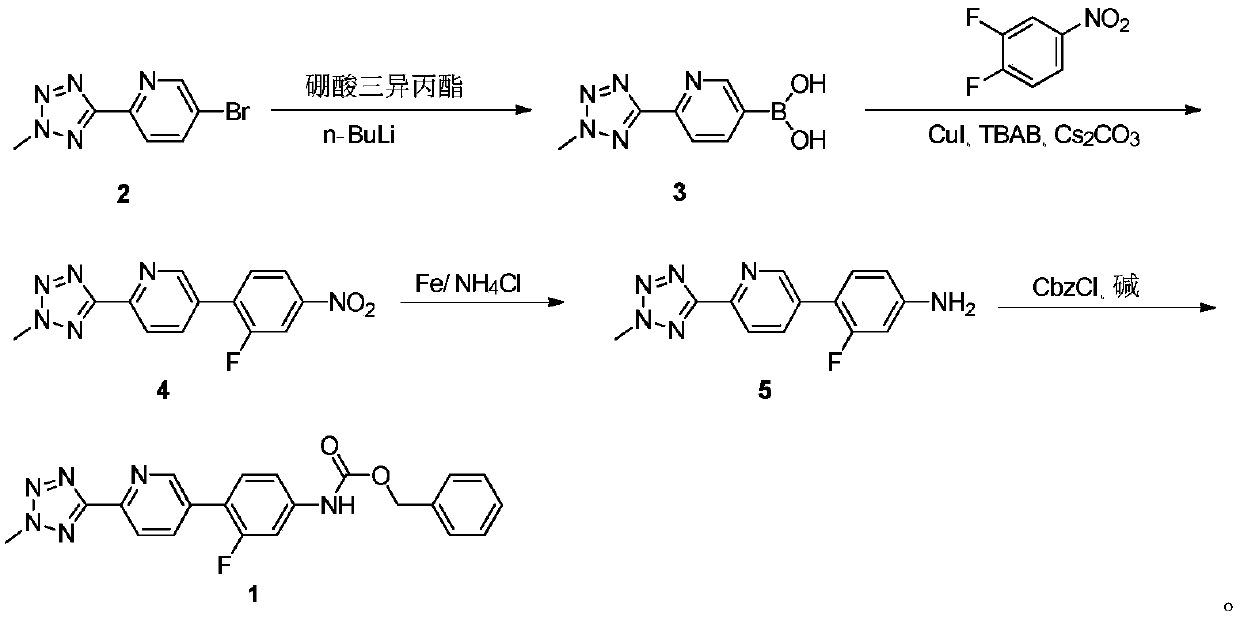
[0028] Example 1. Preparation of 2-(2-methyltetrazol-5-yl)pyridine-5-boronic acid (compound 3)
[0029]
[0030] Under a nitrogen atmosphere, add 5-bromo-2-(2-methyltetrazol-5-yl)pyridine (compound 2, 20g, 0.083mol) in a 500mL three-neck reaction flask with an additional funnel and a nitrogen inlet connector , THF (200 mL) and triisopropyl borate (17.17 g, 0.091 mol, 1.1 equiv), and the mixture was cooled to -72°C. Add n-butyllithium (15.3ml, 0.183mol, 2.2eq) in batches to the external funnel, and drop it in about 2 hours, and control the temperature not to exceed -65°C during the dropwise addition. After the completion of the reaction was determined by HPLC, the reaction solution was quenched with 100 ml of 20% (w / w) ammonium chloride aqueous solution. When the temperature of the reaction solution rose to room temperature (two-phase separation), the THF layer was separated and concentrated to dryness under reduced pressure. The crude product was slurried with 30 mL of d...
Embodiment 2
[0031] Example 2. Preparation of 5-(2-fluoro-4-nitrophenyl)-2-(2-methyltetrazol-5-yl)pyridine (compound 4)
[0032]
[0033] Under nitrogen atmosphere, compound 3 (12.50g, 0.061mol), 1,2-difluoro-4-nitrobenzene (7.48g, 0.047mol, 0.77 equivalent), CuI (4.48g, 0.024mol, 0.39 eq), tetrabutylammonium bromide TBAB (3.03 g, 0.009 mol, 0.15 eq), cesium carbonate (30.62 g, 0.094 mol, 1.54 eq) and DMSO (120 mL). The mixture was heated and stirred at 145-150° C. until the substrate concentration did not change as monitored by HPLC. The reaction solution was cooled to room temperature, filtered, and the filtrate was poured into 300 mL of ether for extraction. The organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a crude product. The crude product was further slurried with ethanol (30ml), filtered, and the filter cake was vacuum-dried to obtain 12.85g of compound 4, with a yield...
Embodiment 3
[0034] Example 3. Preparation of 3-fluoro-4-(6-(2-methyltetrazol-5-yl)pyridin-3-yl)aniline (compound 5)
[0035]
[0036] Add compound 4 (12.49g, 0.042mol), ethanol (120ml) and water (40ml) in reaction flask, then continue to add ammonium chloride solid (13.48g, 0.252mol, 6 equivalents) and iron powder ( 11.73 g, 0.210 mol, 5 equivalents). The reaction mixture was heated to 80° C. until the reaction was completed as monitored by HPLC. After cooling to room temperature, the reaction solution was filtered, and the filtrate was concentrated to dryness under reduced pressure. Water (50ml) was added to the obtained residue, extracted with ethyl acetate (100ml×2), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and then concentrated to dryness under reduced pressure. The resulting product was used directly in the next step without isolation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com