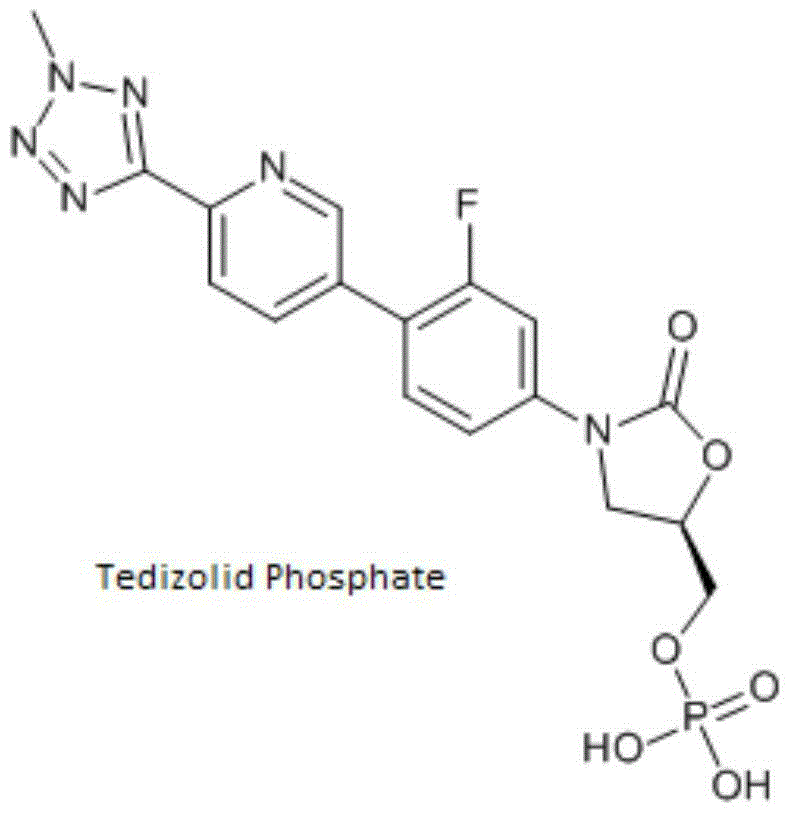
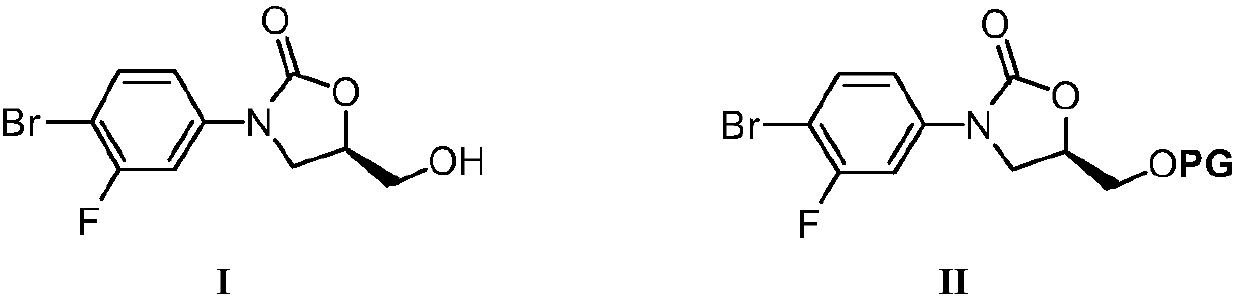Patents
Literature
66 results about "Tedizolid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
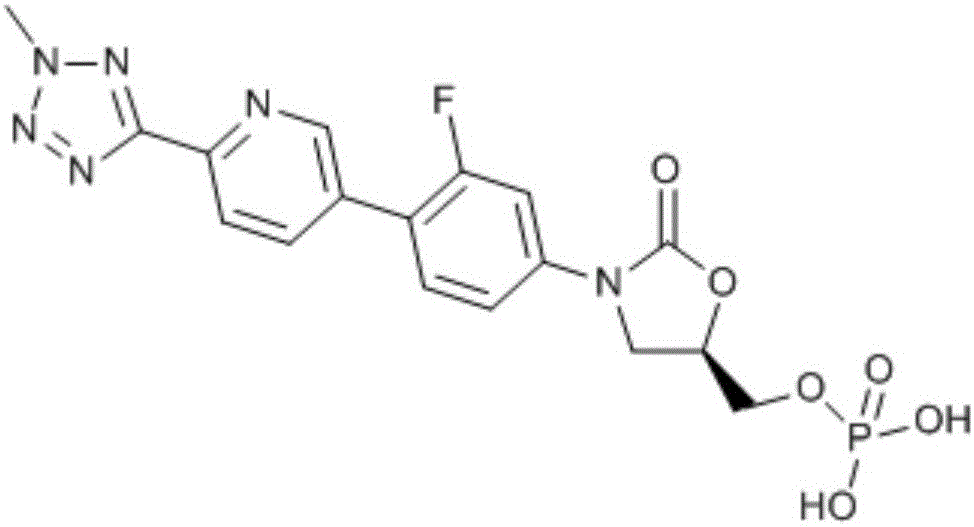
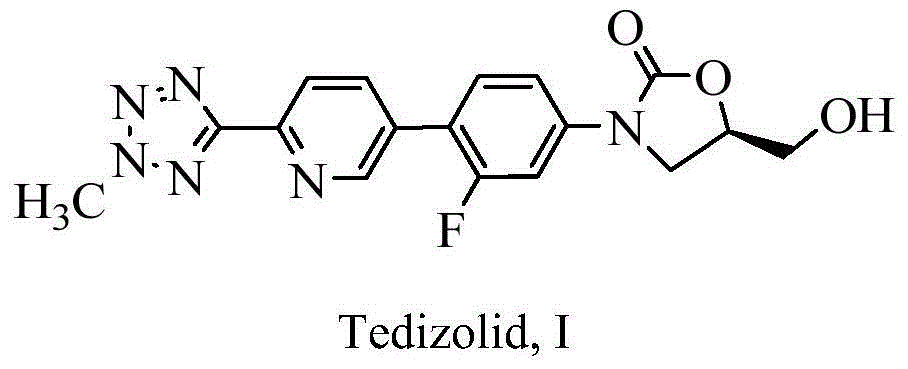
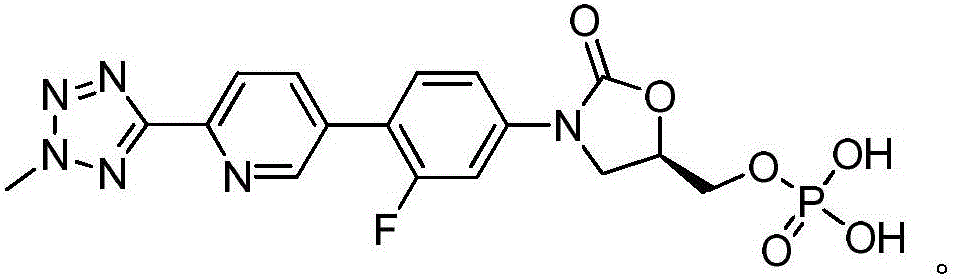
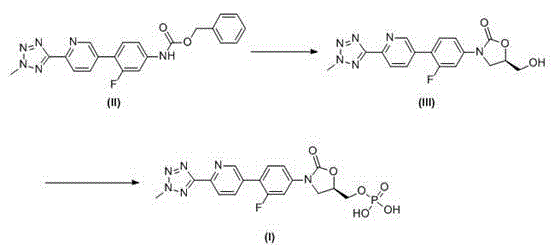
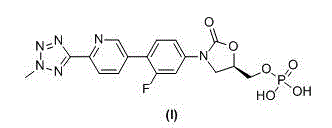
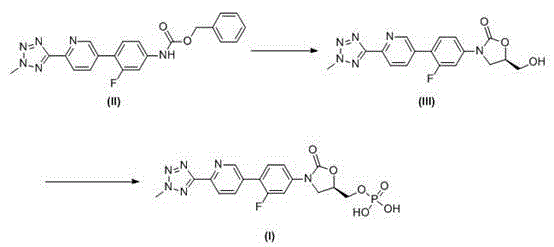
This medication is used to treat serious bacterial infections of the skin.
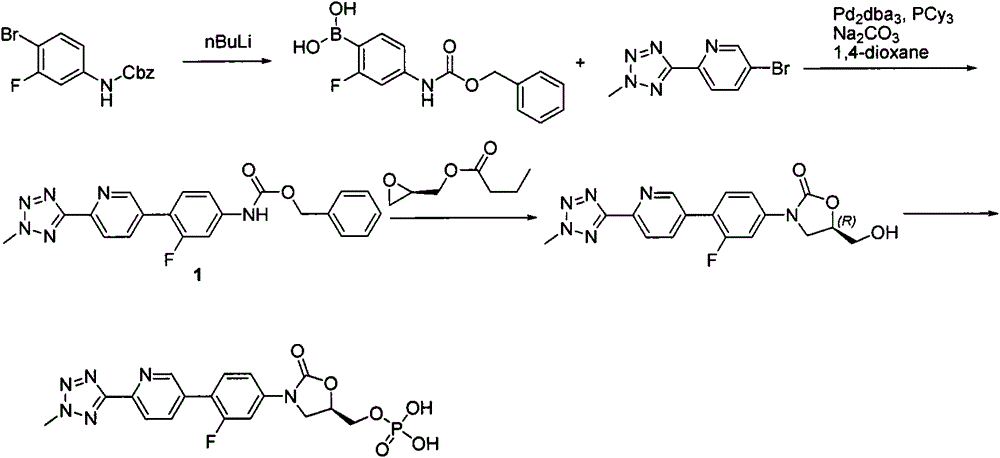
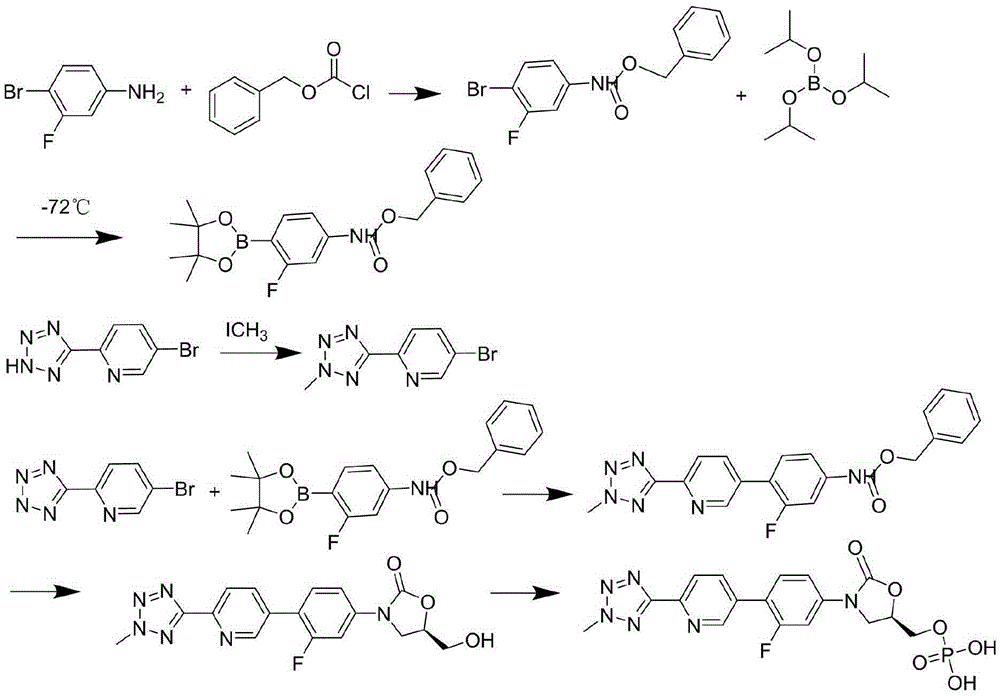
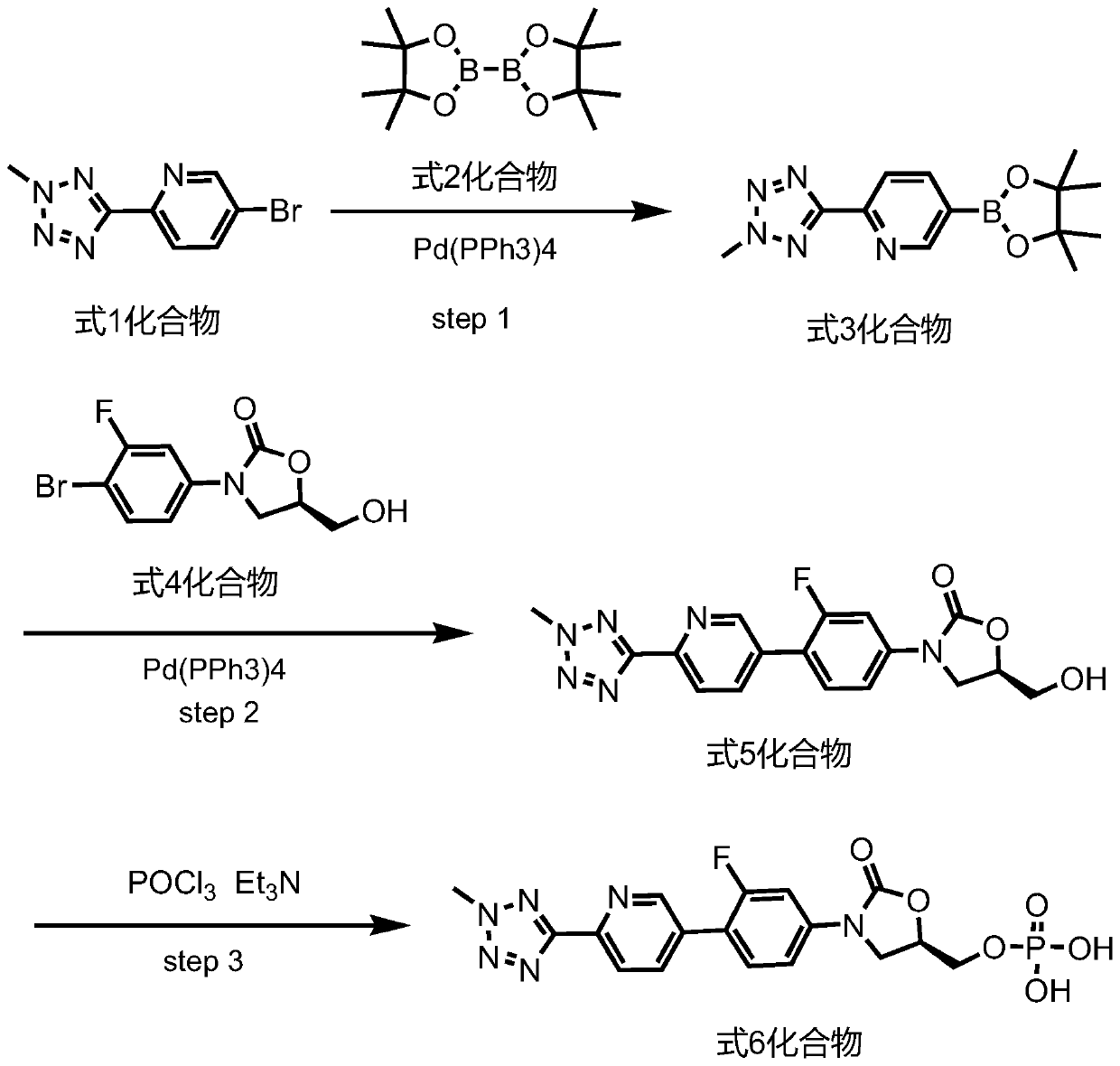
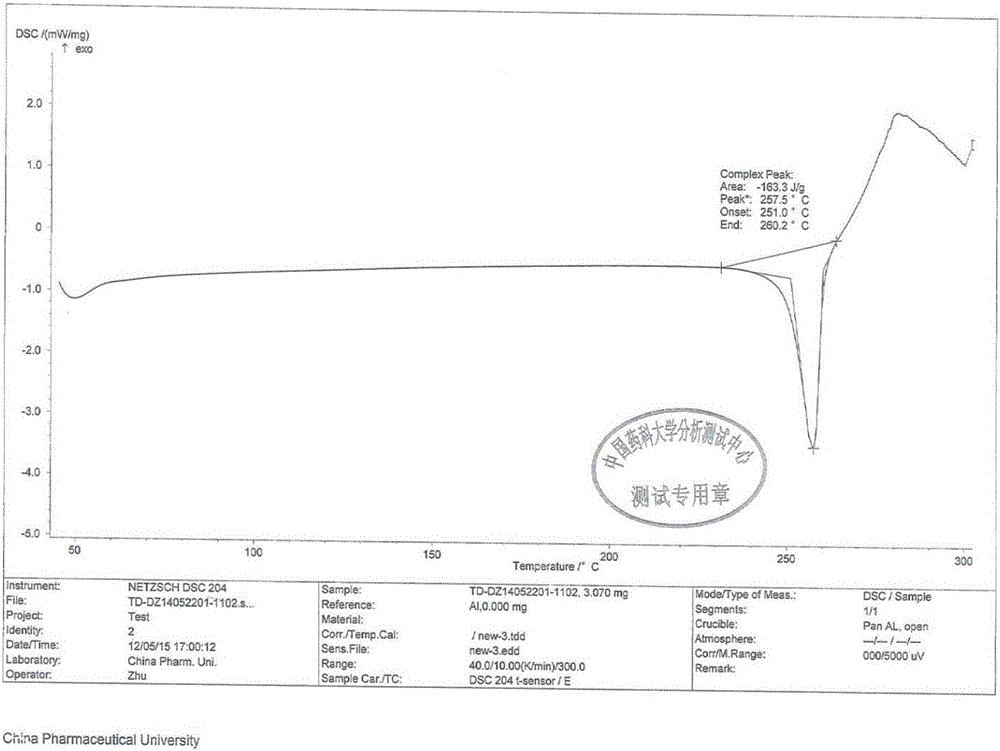
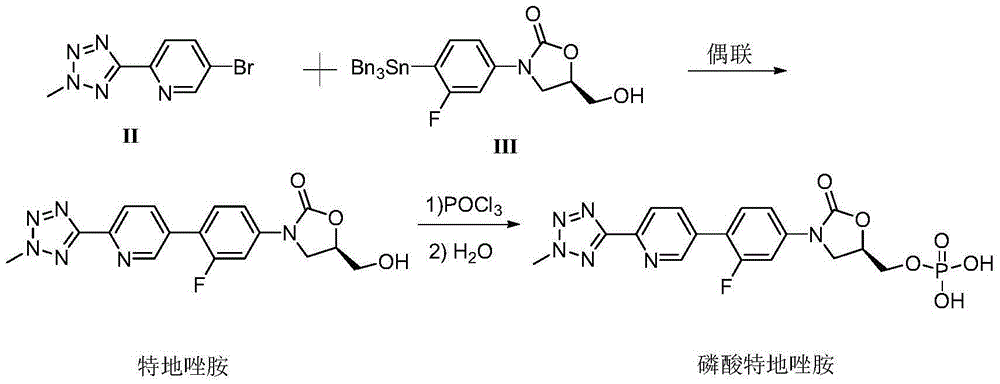
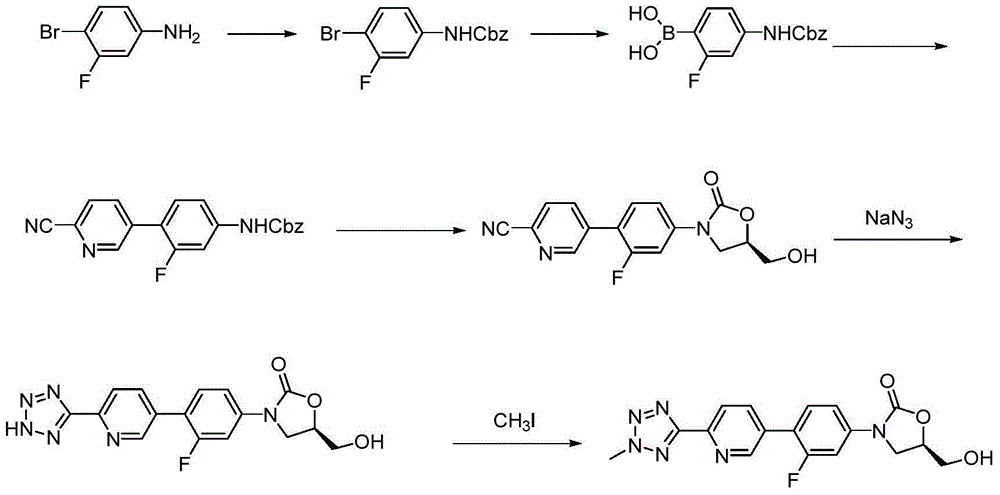
Preparation method for tedizolid
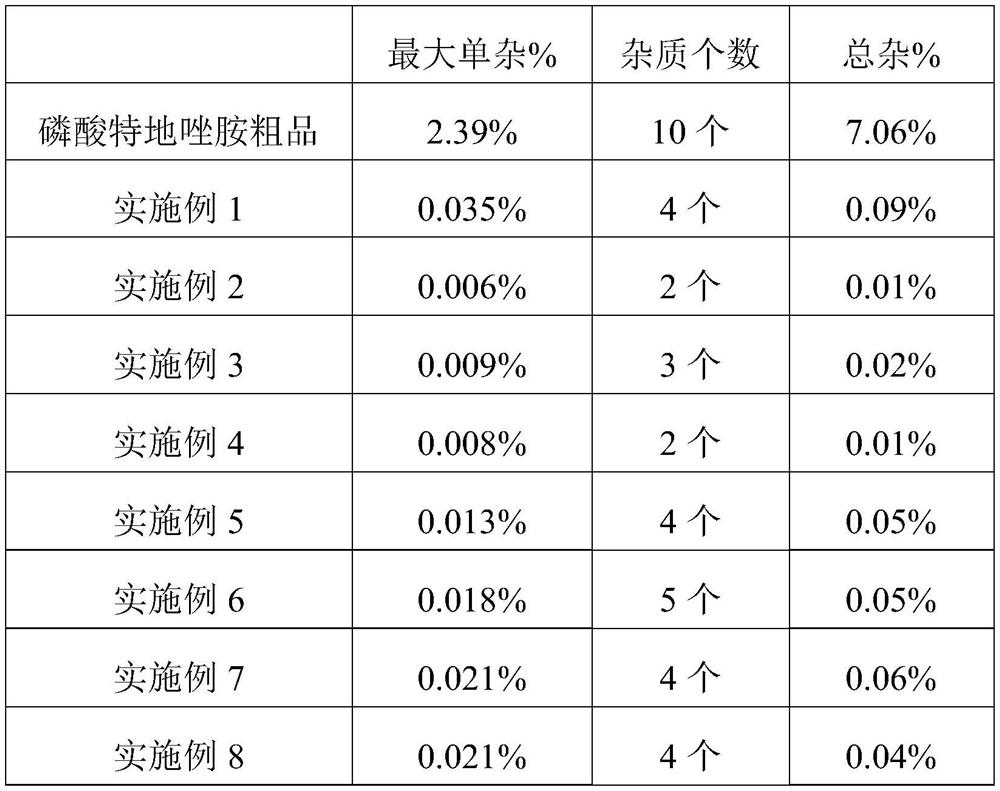
InactiveCN104892592AMild reaction conditionsMild conditionsAntibacterial agentsGroup 5/15 element organic compoundsGeneration ratePhosphate
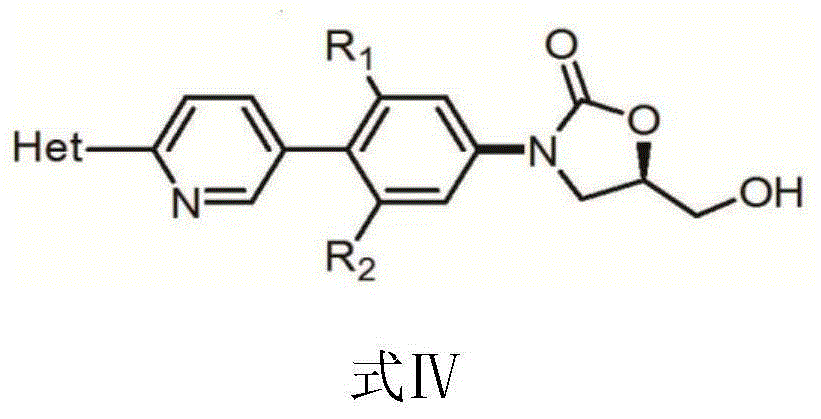
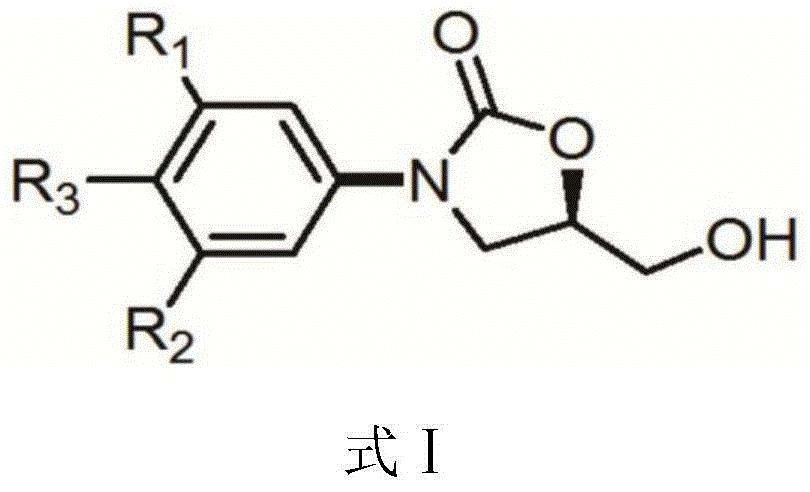
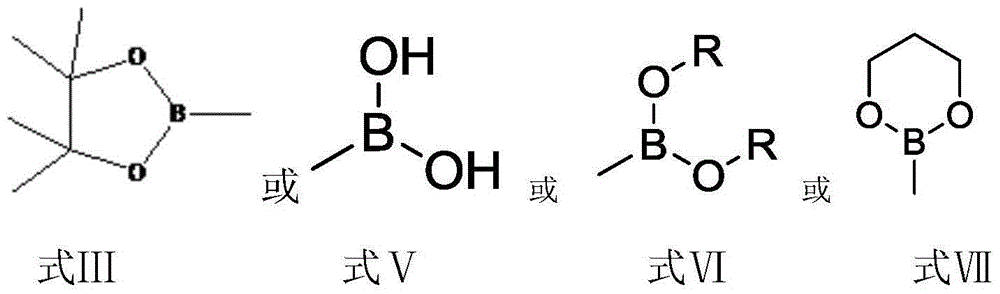
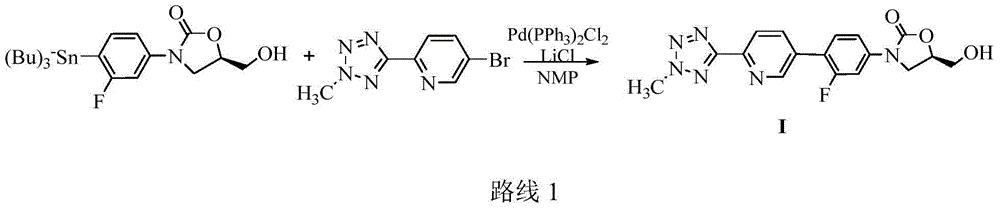
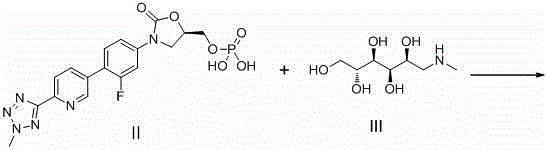
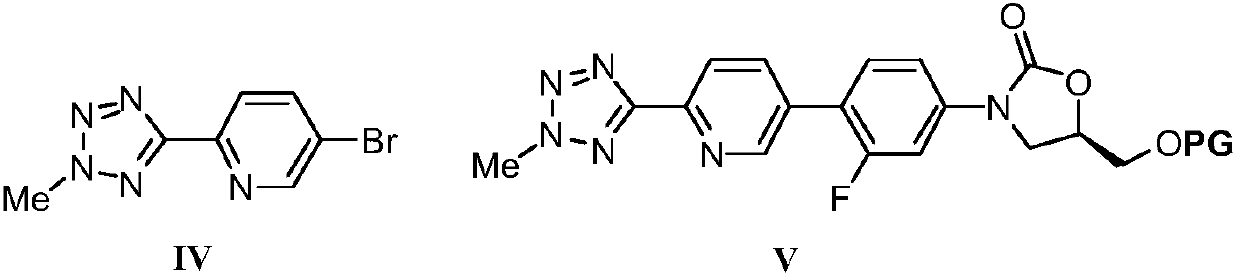
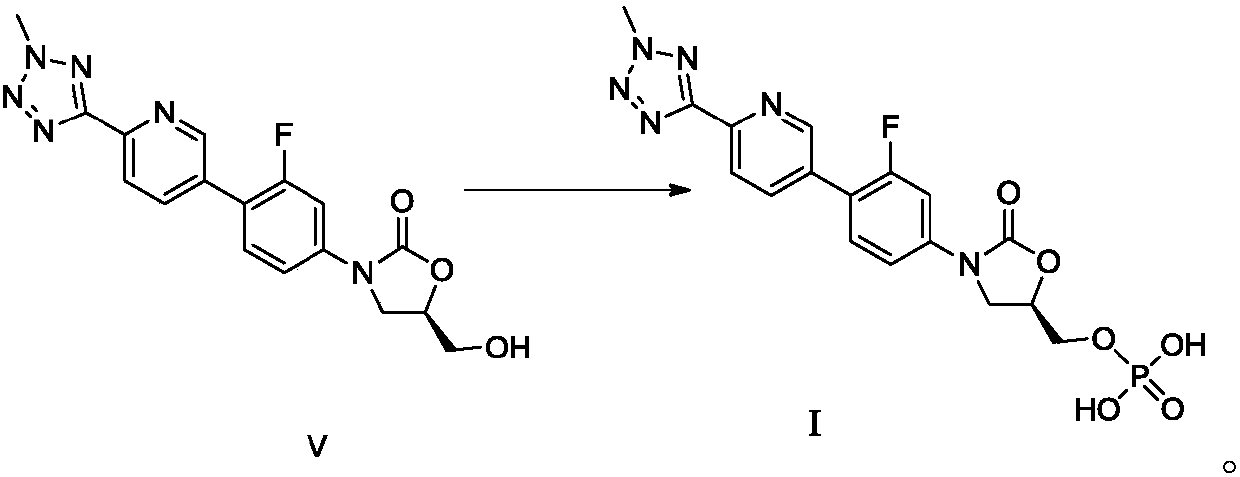
The invention discloses a preparation method for tedizolid as shown in a formula IV. According to the method, tedizolid is prepared through chemical combination of a compound as shown in a formula I and a compound as shown in a formula II via a coupling reaction; and tedizolid phosphate with medical purposes can be prepared by subjecting the obtained tedizolid to esterification by phosphate. The preparation method has the advantages of milder reaction conditions, a few produced impurities and simple post-treatment. According to the invention, the reaction mechanism of Suzuki-Miyaura coupling is employed to connect boric acid as shown in the formula I and derivatives thereof with the compound as shown in the formula II, so reaction selectivity is high, a few impurities are produced, and a synthetic ratio is more than 90%; and oxazolidinone borate is used to react with tetrazole, so conditions are milder, and the generation rate of impurities is lower.
Owner:成都美域高制药有限公司
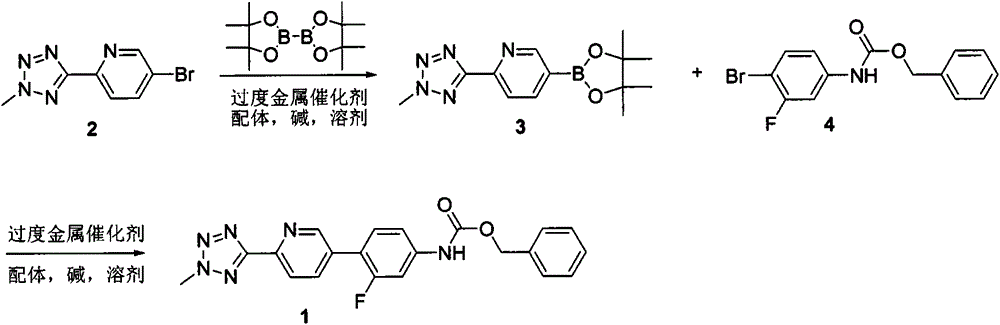
New synthesis process of oxazolinone antibiotic
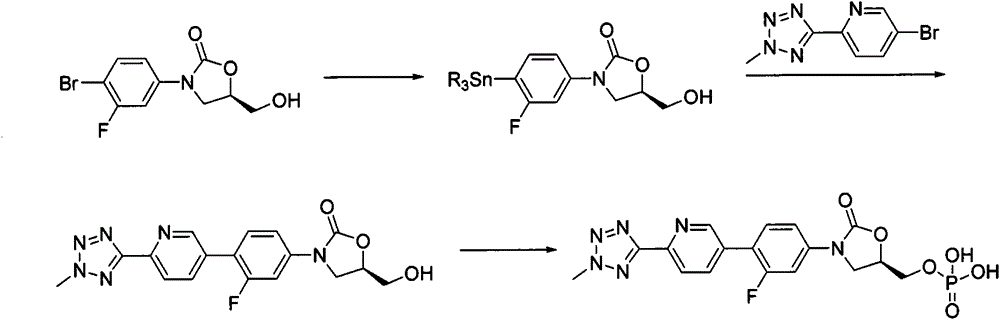
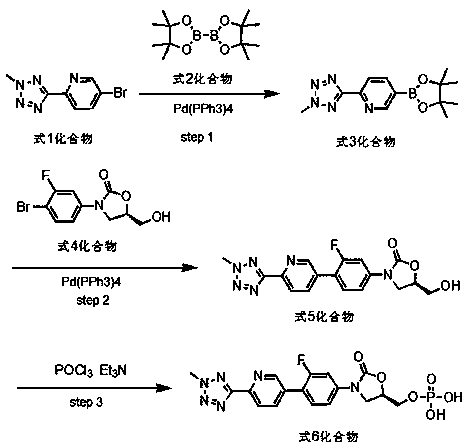
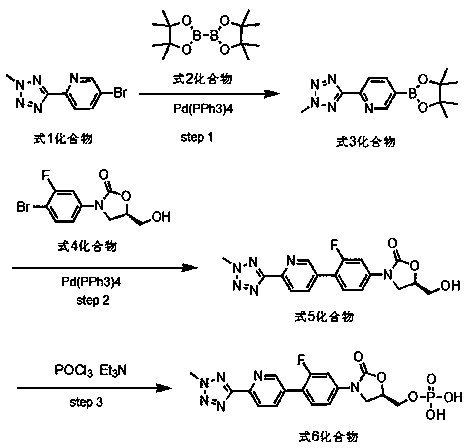
According to the method of the present invention, a methyl tetrazole pyridine bromide (2) and pinacol diboron are subjected to a reaction under catalysis of a transition metal to obtain a boronic acid pinacol ester (3), the compound (3) is separated or is not separated, and the separated compound (3) or the un-separated compound (3) and Cbz-protected bromobenzene (4) are subjected to a reaction under catalysis of a transition metal to obtain a key intermediate (1) of tedizolid. According to the present invention, the compound (3) is not subjected to separation purification, and reacts with the compound (4) in a kettle to generate the compound (1).
Owner:BEIJING CHEMPION BIOTECHNOLOGY CO LTD
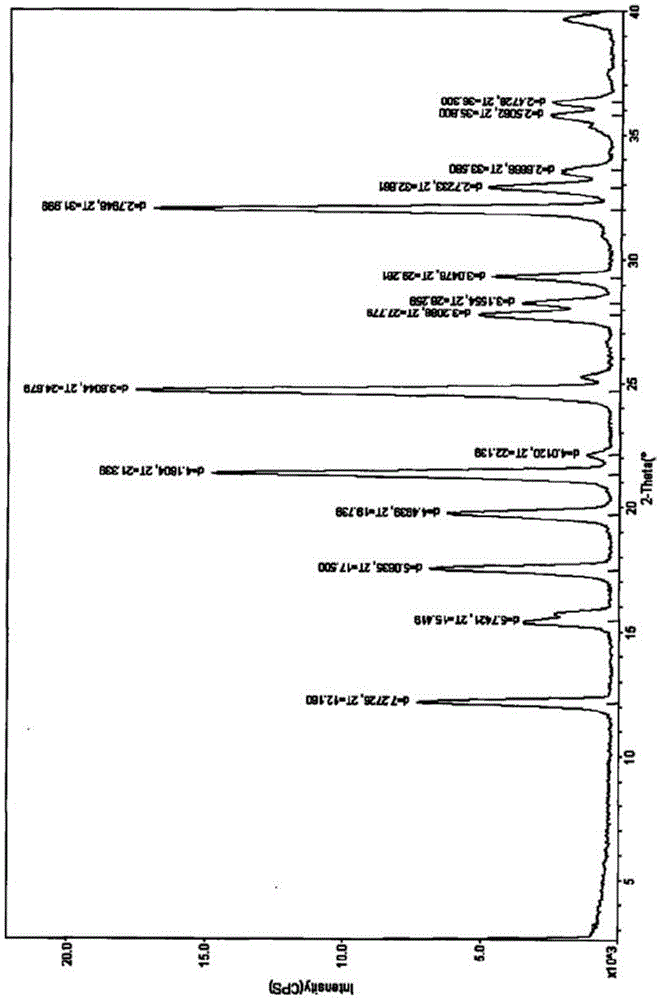
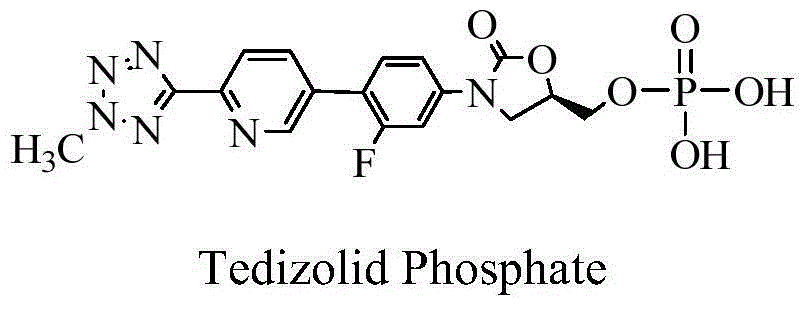
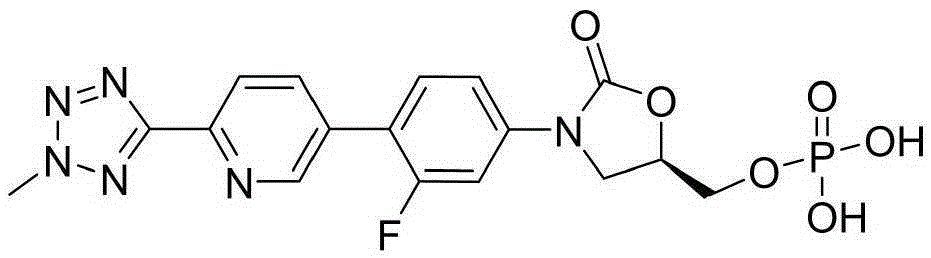
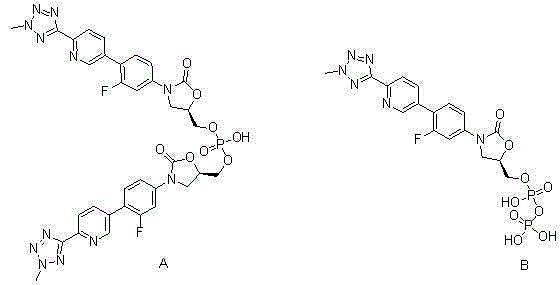
Disodium tedizolid phosphate and preparation method thereof
ActiveCN104530128AImprove stabilityReduced stabilityGroup 5/15 element organic compoundsOrganic solventFreeze-drying
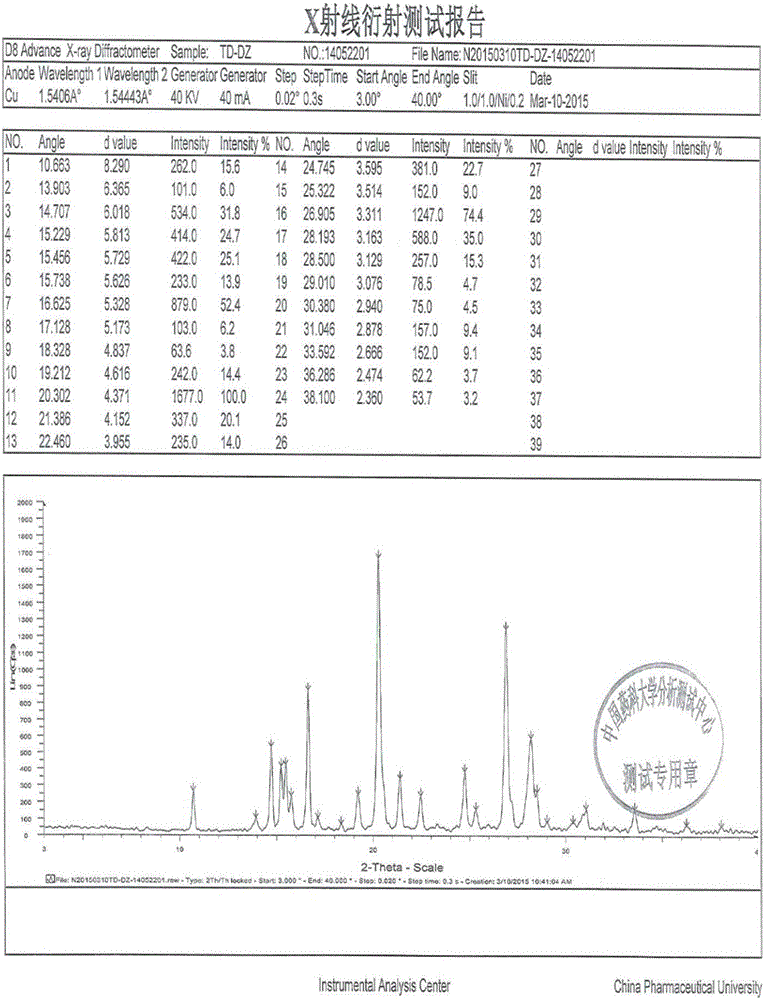
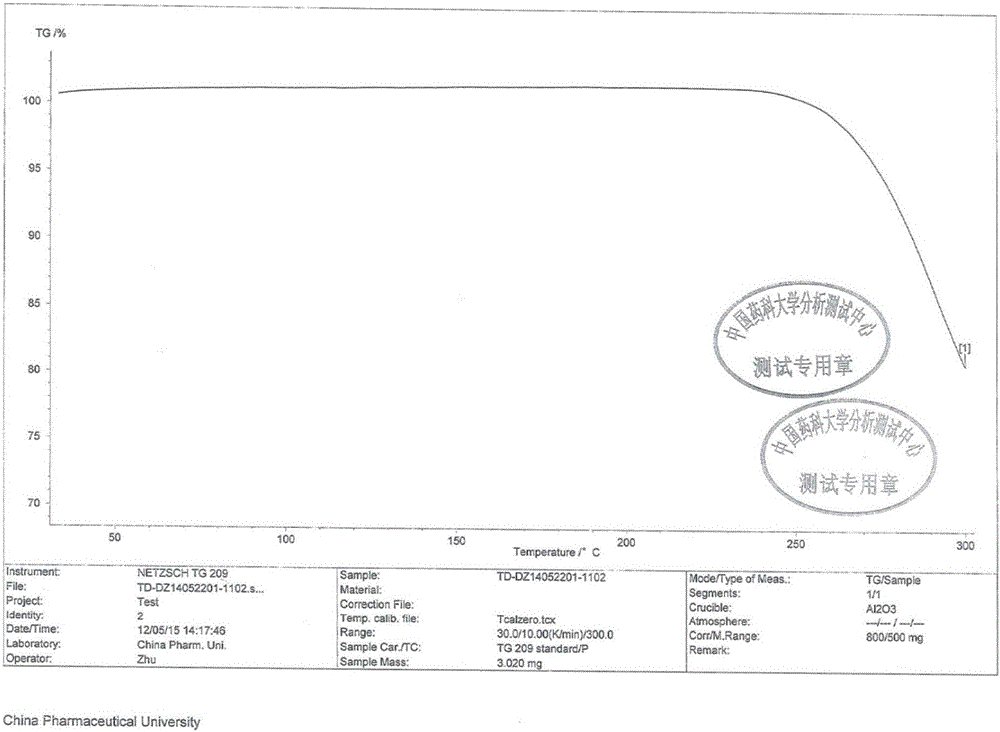
The invention relates to disodium tedizolid phosphate and a preparation method thereof and belongs to the technical field of medicines. The X-ray powder diffraction pattern of disodium tedizolid phosphate shows that the characteristic absorption peaks appear when the reflection angles 2theta are 12.2+ / -0.2 degrees, 17.5+ / -0.2 degrees, 19.7+ / -0.2 degrees, 21.3+ / -0.2 degrees, 24.7+ / -0.2 degrees, 32.0+ / -0.2 degrees. The disodium tedizolid phosphate provided by the invention has excellent stability and purity and is very suitable for producing a freeze-dried powder injection as a raw material. In addition, the preparation method of disodium tedizolid phosphate is simple, is easy to operate, has the advantage of mild reaction condition, use of a small amount and a few kinds of organic solvents, is environment-friendly, is suitable for industrial production and is an environment-friendly preparation method.
Owner:石药集团中诺药业(石家庄)有限公司
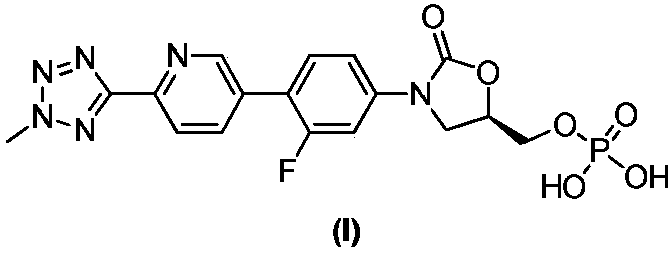
Key intermediate for preparing tedizolid phosphate, and preparation method of key intermediate
The invention discloses a key intermediate for preparing tedizolid phosphate. The key intermediate has a structure shown in formula I: R is any one of R1 or R2, and the R1 and R2 are independently selected from aryl groups or replaced aryl groups. The invention further discloses a preparation method of the key intermediate, and a method for preparing tedizolid phosphate by the key intermediate. The key intermediate disclosed by the invention is a solid compound and is easy to purify; besides, dipolymers, multimers and hydrolysis impurities cannot be generated during the preparation of tedizolid phosphat, the reaction temperature is moderate, the operation is simple, a strong-acidity reagent (phosphorus oxychloride) is prevented from being used, and the preparation method belongs to an environment-friendly production technology.
Owner:CHENGDU SINO STRONG PHARMA
Tedizolid phosphate freeze-dried powder injection
InactiveCN106344525AImprove product qualityHigh clinical safetyAntibacterial agentsOrganic active ingredientsMANNITOL/SORBITOLPhosphate
The invention discloses a tedizolid phosphate freeze-dried powder injection preparation and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. Every tedizolid phosphate freeze-dried powder injection solution contains 200mg of tedizolid phosphate, 105mg of mannitol and 2ml of water for injection. The invention aims to solve the following technical problems: the tedizolid phosphate is insoluble in water, and the preparation needs to be prepared into the freeze-dried powder injection preparation.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Process for preparing tedizolid
InactiveCN105985331AHigh yieldMild reaction conditionsOrganic chemistryState of artPalladium catalyst
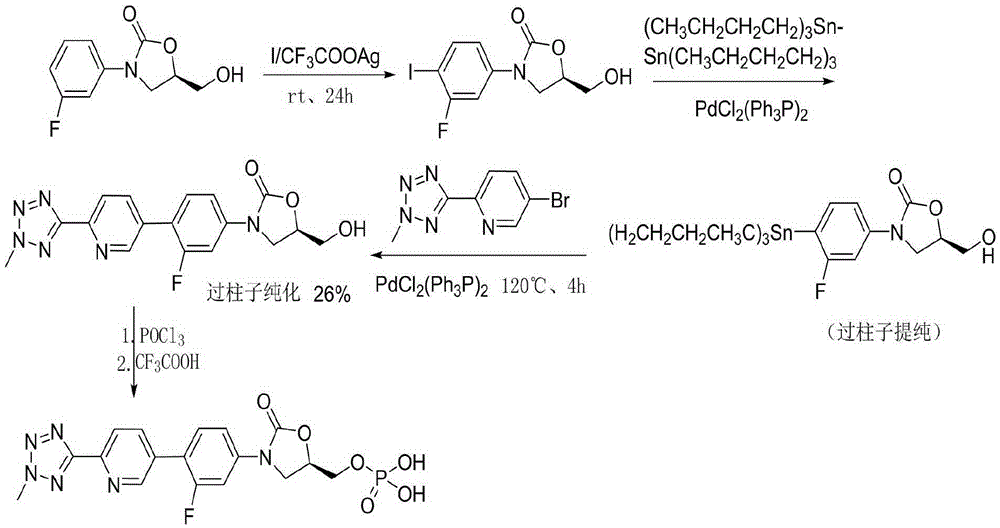
The invention provides a method for preparing tedizolid. The method comprises the step of subjecting 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxoboran-2-yl)pyridine (II) and (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolid-2-one (III) to a coupling reaction in a polar solvent in the presence of a metal palladium catalyst and alkali, so as to produce the tedizolid; and a reaction formula is as follows: formulae shown in the description. According to the method, the yield is greatly increased compared with the prior art and reaches up to 80% to 91%; in addition, the reaction conditions are mild, the operation is simple and convenient, and reagents used are moderately-priced and readily available, so that the method is adaptable to small-scale preparation of laboratories and also large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
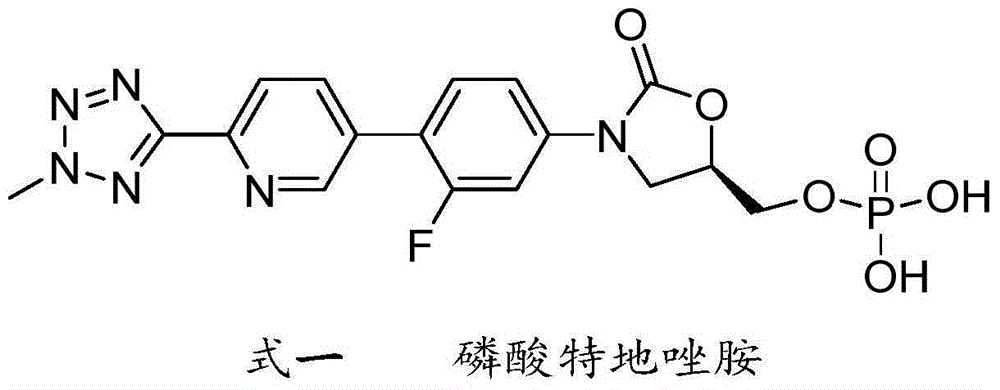
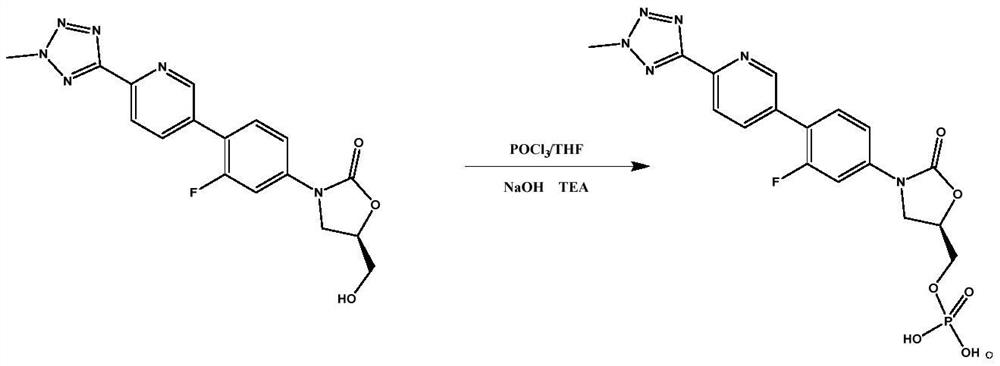
Preparation method of tedizolid phosphate
InactiveCN105418681AShort synthetic stepsEasy to operateGroup 5/15 element organic compoundsPhosphateBromine
The invention belongs to the technical field of synthesis of chemical medicines and particularly relates to a preparation method of tedizolid phosphate. (5R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one and 2-(2-methyl-2H-tetrazolyl-5-yl) pyridine-5-boronic acid pinacol ester are taken as raw materials, and (R)-3-(4-(2-(2-methyl tetrazole-5-yl) pyridine-5-yl)-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-ketone phosphate is prepared with a four-step method. The method has a mild process condition, aftertreatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
Preparation method of high-purity tedizolid phosphate
ActiveCN111518135AImprove responseHigh purityGroup 5/15 element organic compoundsPhosphoric Acid EstersPtru catalyst
The invention provides a preparation method of high-purity tedizolid phosphate. The preparation method comprises the following steps: step 1, with 2-methyl-5-(5-bromopyridin-2-yl) tetrazole as a starting material, tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) as a catalyst, carrying out reacting with bis(pinacolato)diboron to generate a compound as shown in a formula 1, namely bromine-converted pinacol borate; 2, subjecting a compound as shown in the formula 3 and (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one to Suzuki coupling under the catalytic action of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) to obtain (R)-3-[4-[2-(2-methyltetrazol-5-yl)pyrid-5-yl]-3-fluorophenyl]-5-hydroxymethyl oxazolidine; and 3, preparing tedizolid phosphate from a compound as shownin a formula 5 under the condition of phosphorylation of phosphorus oxychloride. The method for preparing tedizolid phosphate has the advantages that ultralow-temperature operation is not needed, andreaction is simple; the purity of tedizolid phosphate is high and can reach 95% or higher; byproducts are few, and yield is high and is higher than 70%; and process is stable, and operability is high.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
Novel oxazolidinone compound and preparation method thereof
InactiveCN106146558AEasy to prepareNo low physiological activityAntibacterial agentsOrganic active ingredientsPhosphoric Acid EstersDisease
The invention provides a novel oxazolidinone compound, a preparation method thereof and a medicine composition containing the compound and used for antibiotic. It is shown that the novel oxazolidinone compound has antibacterial activity on gram-positive bacteria like staphylococcus and enteric bacteria, and a new medicine choice is provided for clinical treatment of related diseases. Tedizolid phosphate reacts with N-methyl-D-glucosamine to prepare meglumine of tedizolid phosphoric acid, the technological method is simple, and the prepared compound has the advantages of being good in stability and suitable for preparing tablets and injections.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Refining method for tedizolid phosphate
ActiveCN105753904AEasy to stickLess impuritiesGroup 5/15 element organic compoundsActivated carbonPhosphate
The invention belongs to the field of medicine synthesis and relates to a refining method for tedizolid phosphate.The refining method for tedizolid phosphate includes the following steps that crude tedizolid phosphate is added into water, the pH value is adjusted with alkali liquor, tedizolid phosphate disodium salt is formed, activated carbon decoloration is conducted, acetone is slowly added into filtrate, a large number of solids are separated out, stirring crystallization is conducted, and tedizolid phosphate disodium salt is obtained after filtration; tedizolid phosphate disodium salt is dissolved in water and acidized, white solids are separated out, acetonitrile is added, stirring crystallization is conducted, and high-purity tedizolid phosphate is obtained after filtration.The purity of the product obtained through the method is 99.5% or above, the single impurity content is smaller than 0.1%, and the refining yield is not smaller than 85%; another remarkable advantage is that tedizolid phosphate A crystal form solids can be stably obtained, the particle size distribution D99 of the solids is smaller than 20 micrometers, no adhesion is caused in the pelleting process, and the solids are suitable for producing and processing preparations.
Owner:NANJING GRITPHARMA CO LTD
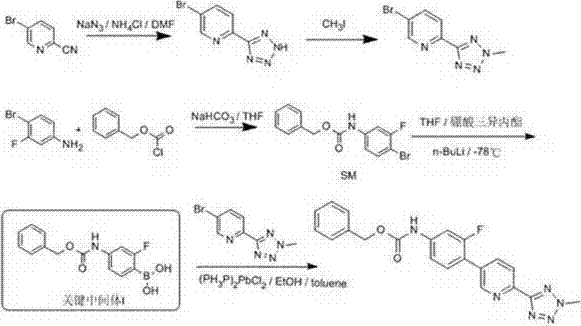
Preparation method of oxazolidinone antibiotic intermediate
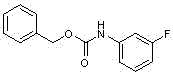
ActiveCN110938058ARaw materials are easy to getSimple processOrganic chemistryBiotechnologyCarbamate
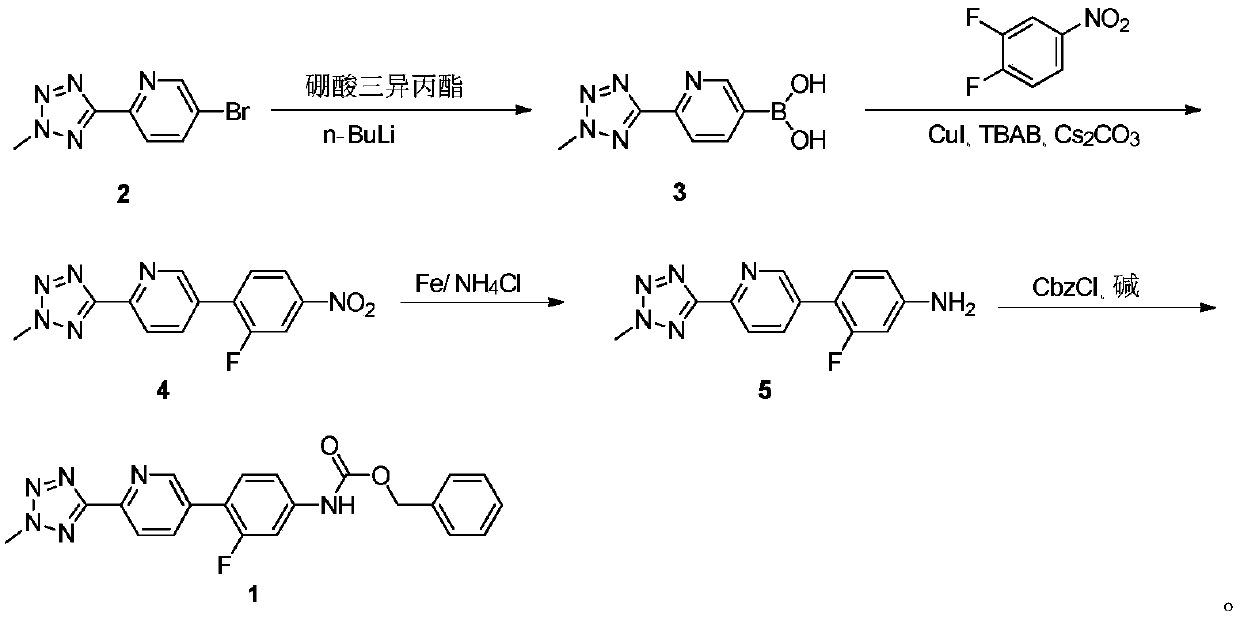
The invention provides a preparation method of a tedizolid phosphate intermediate compound 3-fluoro-4-(6-(2-methyltetrazole-5-yl)pyridine-3-yl)phenyl benzyl carbamate. The method comprises the following steps: carrying out a reaction on 5-bromo-2-(2-methyltetrazole-5-yl)pyridine as a raw material and triisopropyl borate under the action of n-butyllithium to prepare a boric acid intermediate, coupling the boric acid intermediate and 1,2-difluoro-4-nitrobenzene, and finally performing nitro reduction and amidation to obtain the required target product. Compared with the method in the prior art,the preparation method disclosed by the invention has the advantages of easily available raw materials, simple process, economy, environmental protection and suitability for industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
Preparation method for high-purity tedizolid phosphate
ActiveCN105131037AImprove production efficiencyReduce generationGroup 5/15 element organic compoundsPhosphoric Acid EstersPtru catalyst
The invention discloses a preparation method for high-purity tedizolid phosphate. The method is characterized by comprising the following steps: subjecting starting materials tedizolid and phosphorus oxychloride to a reaction so as to produce a tedizolid phosphorus oxychloride intermediate; then reacting the tedizolid phosphorus oxychloride intermediate with benzyl alcohol so as to produce a tedizolid dibenzyl phosphate intermediate; and carrying out debenzylation under the action of a catalyst so as to obtain tedizolid phosphate. Meanwhile, all the parameters of procedures are systematically adjusted. The introduced dibenzyl phosphate intermediate has good stability; benzyl protection removal reaction conditions are mild; compared with conventional preparation methods for tedizolid, by-reactions and generation of impurities are greatly reduced in the invention; the purity of the prepared tedizolid phosphate can reach more than 99.9%; and the preparation method omits the step of product purification, has more simplified steps and improves preparation efficiency of the tedizolid phosphate.
Owner:JINAN ASIA PHARMA TECH
Method for compounding tedizolid phosphate
InactiveCN105111237ASimple and fast operationSimple post-processingGroup 5/15 element organic compoundsAfter treatmentPhosphate
The invention provides a method for compounding tedizolid phosphate, which uses a compound 5 as a staring material, and a novel antibacterial agent tedizolid phosphate is prepared through a reaction. The method for compounding the tedizolid phosphate is a novel industrialized compound selectivity mode, is simple and convenient in operation simultaneously, simple in after treatment, low in cost and environmentally friendly, can obtain high quality objective product, and is beneficial for industrial manufacture.
Owner:成都维恒医药科技有限公司
Method for refining high-purity tedizolid phosphate
ActiveCN106146560AII content decreasedMeet the requirements of raw materialsGroup 5/15 element organic compoundsPhosphatePotassium hydroxide
The invention discloses a method for refining high-purity tedizolid phosphate. The method comprises the steps of 1, heating and dissolving a tedizolid phosphate crude product with dimethylsulfoxide, adding a polar proton solvent after clarification, and conducting stirring, cooling crystallization and suction filtration; 2, adding a filter cake obtained in step 1 to water, adjusting the pH value of the solution to be alkaline with sodium hydroxide or potassium hydroxide aqueous solution, then adding the solution to a water-soluble non-proton organic solvent for crystallization and suction filtration, adding an obtained filter cake to a water and tetrahydrofuran mixed solvent, adjusting pH to be 1-2 with hydrochloric acid aqueous solution, and conducting stirring crystallization and suction filtration; 3, dissolving a filter cake finally obtained from step 2 with dimethylsulfoxide, then adding the obtained solution to a water and ethyl alcohol mixed solvent, and conducting cooling crystallization, suction filtration and drying to obtain the high-purity tedizolid phosphate. The obtained product is high in purity and stable in quality, the condition for refining is mild, operation is easy, and the method is suitable for industrial application.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for preparing tedizolid phosphate
ActiveCN106317114ALow costGood reproducibilityGroup 5/15 element organic compoundsPhosphatePhosphoric acid
The invention discloses a method for preparing tedizolid phosphate, wherein the method specifically includes the steps: with N-[3-fluoro-4-[6-(2-methyl-2H-tetrazole-5-yl)-3-pyridyl]phenyl]benzyqcarbamate as a starting raw material, in the presence of R1-O-M, 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone, carrying out a reaction with R-glycidyl butyrate, and then esterfying with POCl3 phosphoric acid to obtain a tedizolid phosphate crude product; and adjusting the pH value to 7-9 with an alkali solution, converting the tedizolid phosphate crude product into a salt, and adjusting the pH value to 1-2 with hydrochloric acid to obtain the refined tedizolid phosphate. The method has the advantages of mild reaction conditions, relatively low cost, simple and convenient operation, less side reaction, high yield, good reaction reproducibility, economy and environmental protection, and is suitable for industrialized production.
Owner:NANJING YOKO PHARMA +2
Nickel catalyzed tedizolid phosphate synthesis method
ActiveCN107827927ALow priceGuaranteed yieldGroup 5/15 element organic compoundsBulk chemical productionSynthesis methodsPhosphoric acid
The invention discloses a nickel catalyzed tedizolid phosphate synthesis method. The method includes that methyl tetrazolium bromopyridine serving as an electrophilic reagent and N-oxazolidinone phenyl zinc halide serving as a nucleophilic reagent are subjected to nickel catalyzed Negishi coupling reaction and in-situ deprotection to obtain pharmaceutically active molecular tedizolid through a two-step one-pot process, and tedizolid is subjected to phosphoric acid esterification to obtain tedizolid phosphate. The method has advantages that 1) by in-situ synthesis and utilization of the organiczinc reagent, a complex step of metal reagent separation is avoided; 2) a cheap nickel catalyzing system is applied to establishment of a tedizolid phosphate core framework for the first time, and efficiency of nickel catalyzed Negishi coupling reaction is equivalent to that of palladium catalyzed Suzuki coupling reaction in current industrial synthesis of tedizolid phosphate. The remarkable advantages are beneficial to improvement of efficiency in tedizolid phosphate synthesis and production cost reduction, and the nickel catalyzed tedizolid phosphate synthesis method has a promising industrial application prospect.
Owner:陕西思尔生物科技有限公司 +1
Preparation method of high-purity tedizolid phosphate
InactiveCN111116652AImprove responseHigh purityGroup 5/15 element organic compoundsPhosphoric Acid EstersPtru catalyst
The invention discloses a preparation method of high-purity tedizolid phosphate. The preparation method comprises the following steps: 1, with 2-methyl-5-(5-bromopyridin-2-yl)tetrazole as a starting material and tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) as a catalyst, and reacting the starting material and the catalyst with bis(pinacolato)diboron to generate a compound as shown in a formula 1, namely bromine-converted pinacol borate; 2, subjecting a compound shown in a formula 3 and (5R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one to Suzuki coupling under the catalyticaction of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) to obtain (R)-3-[4-[2-(2-methyltetrazol-5-yl)pyridin-5-yl]-3-fluorophenyl]-5-one; and 3, preparing tedizolid phosphate from a compound as shown in a formula 5 under the condition of phosphorylation of phosphorus oxychloride.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
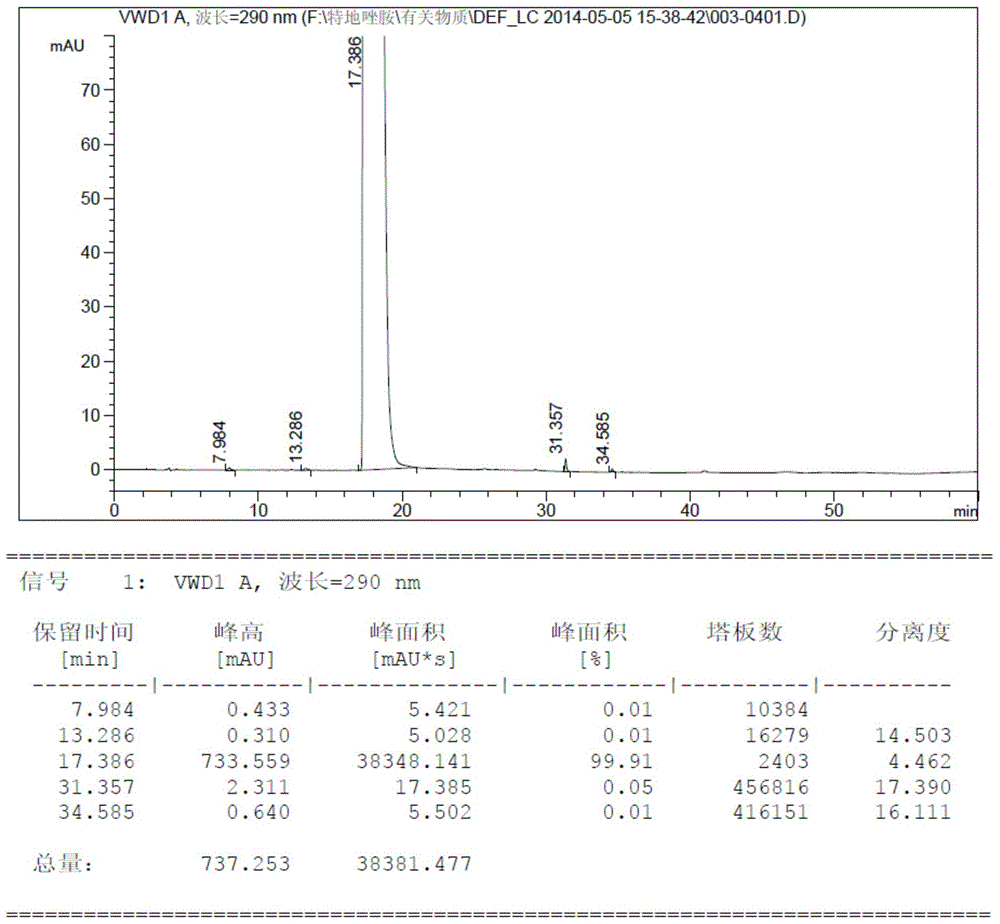
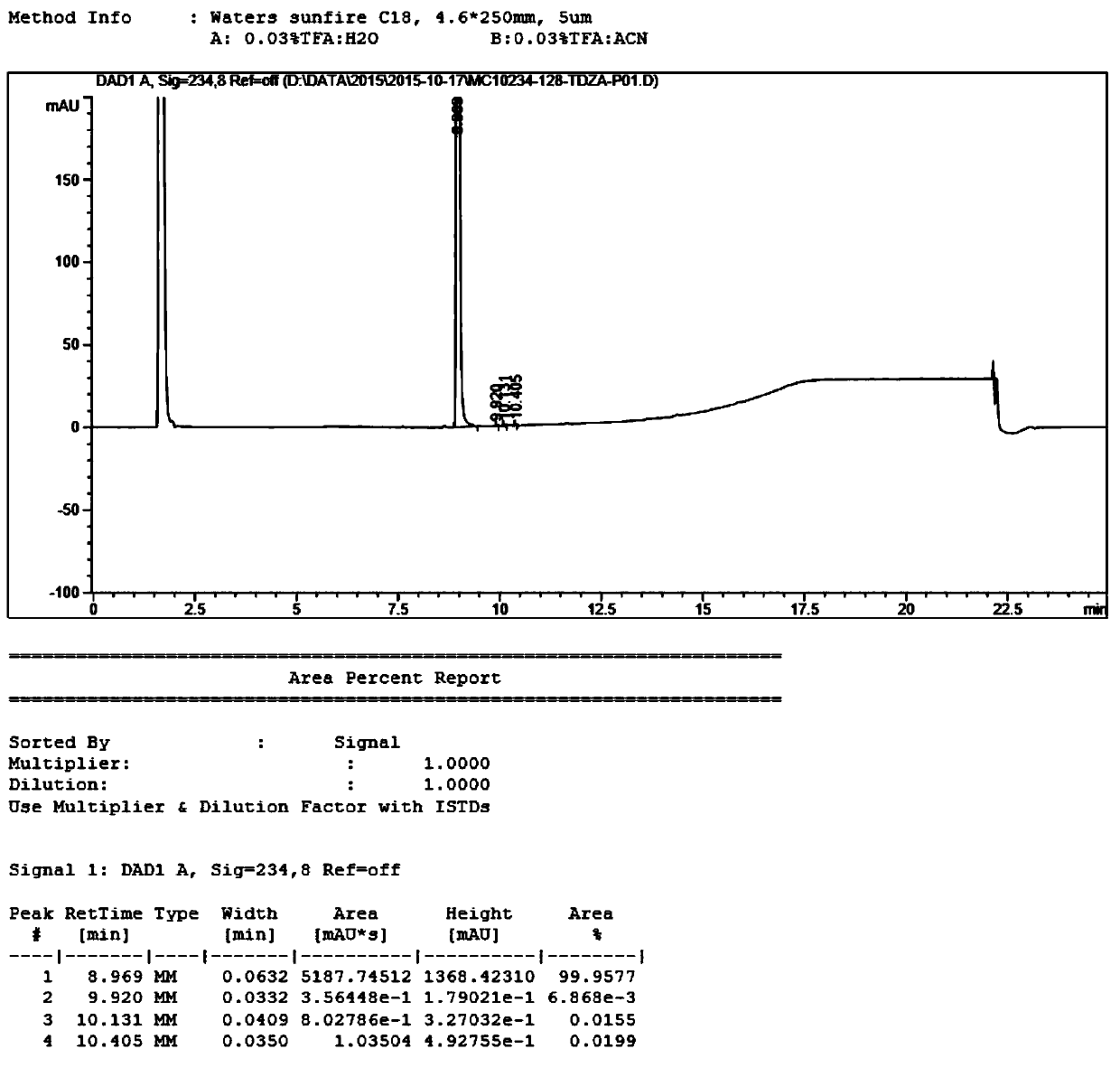
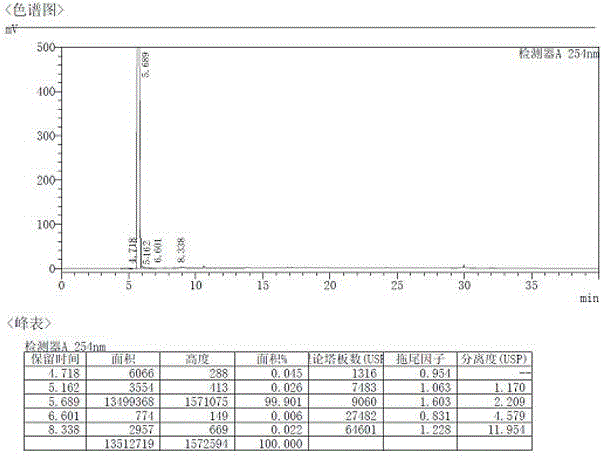
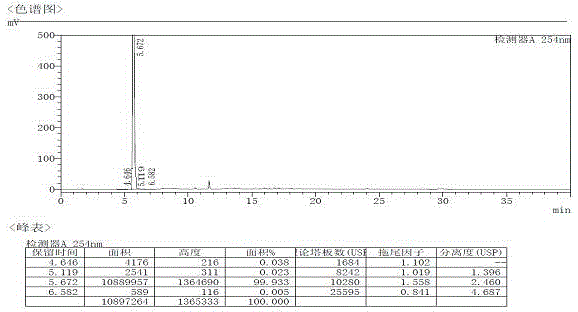
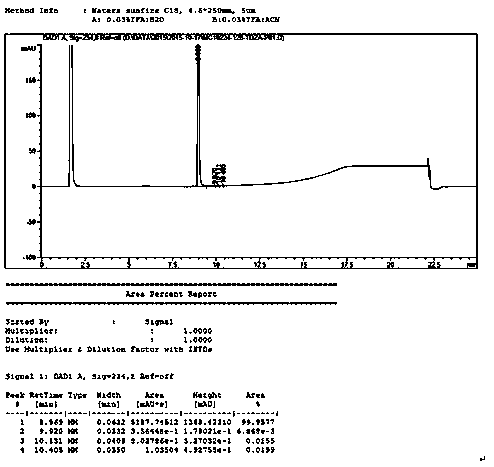
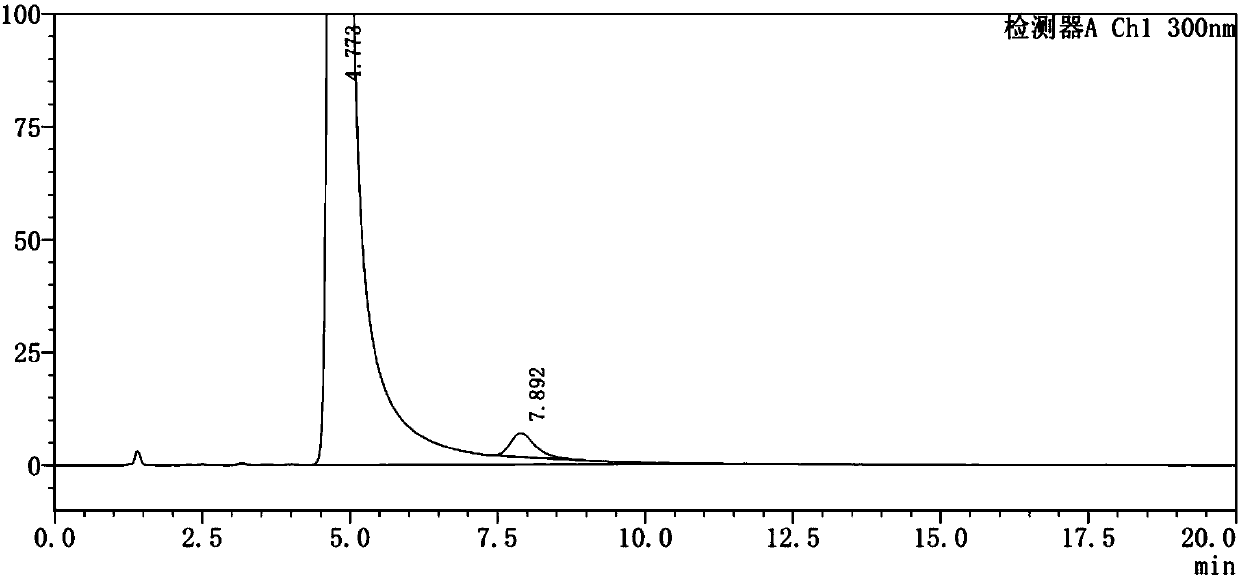
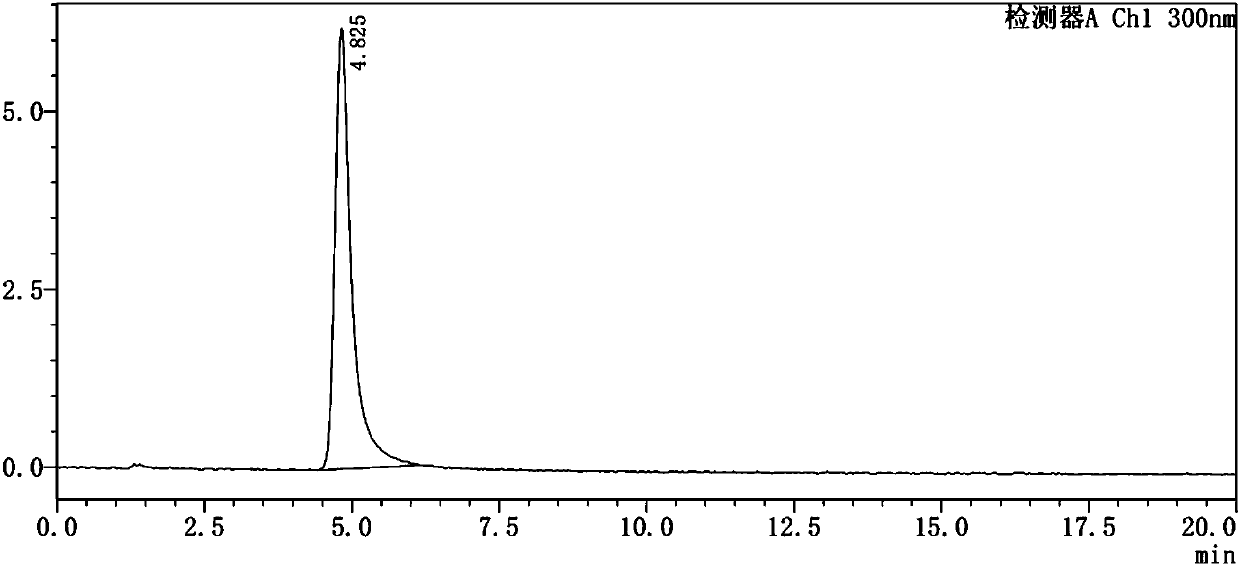
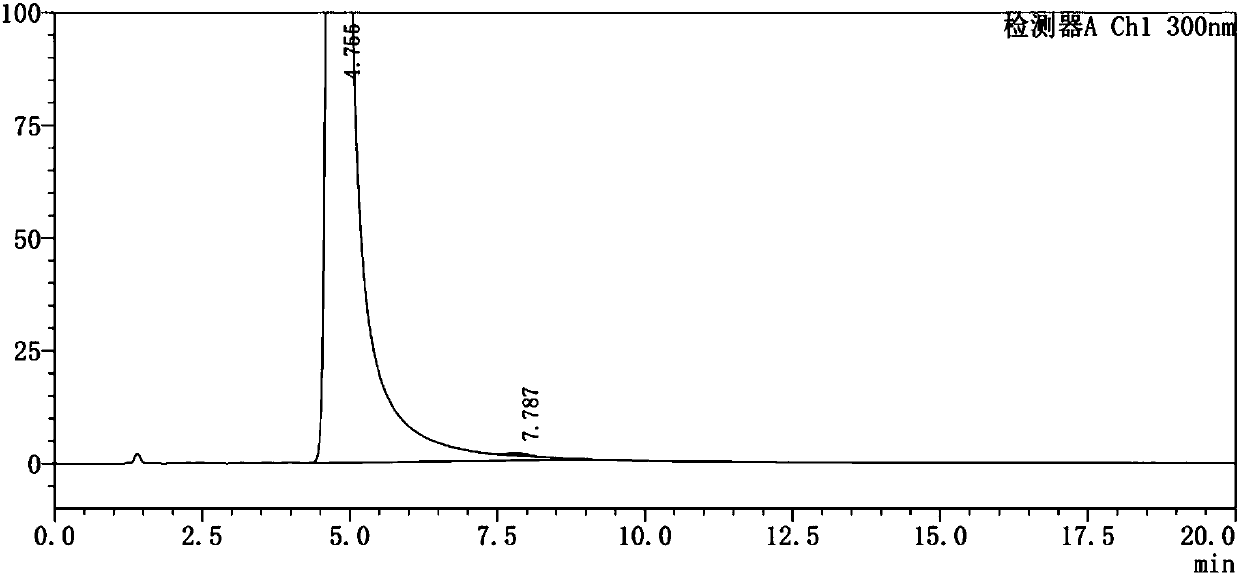
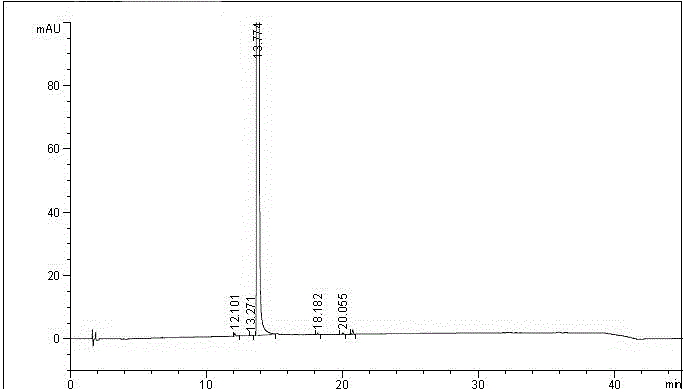
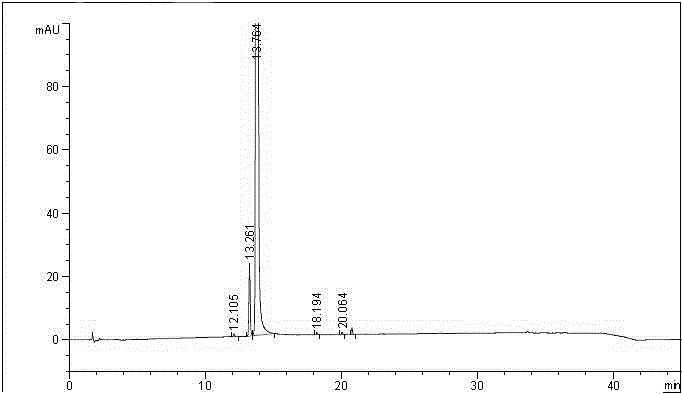
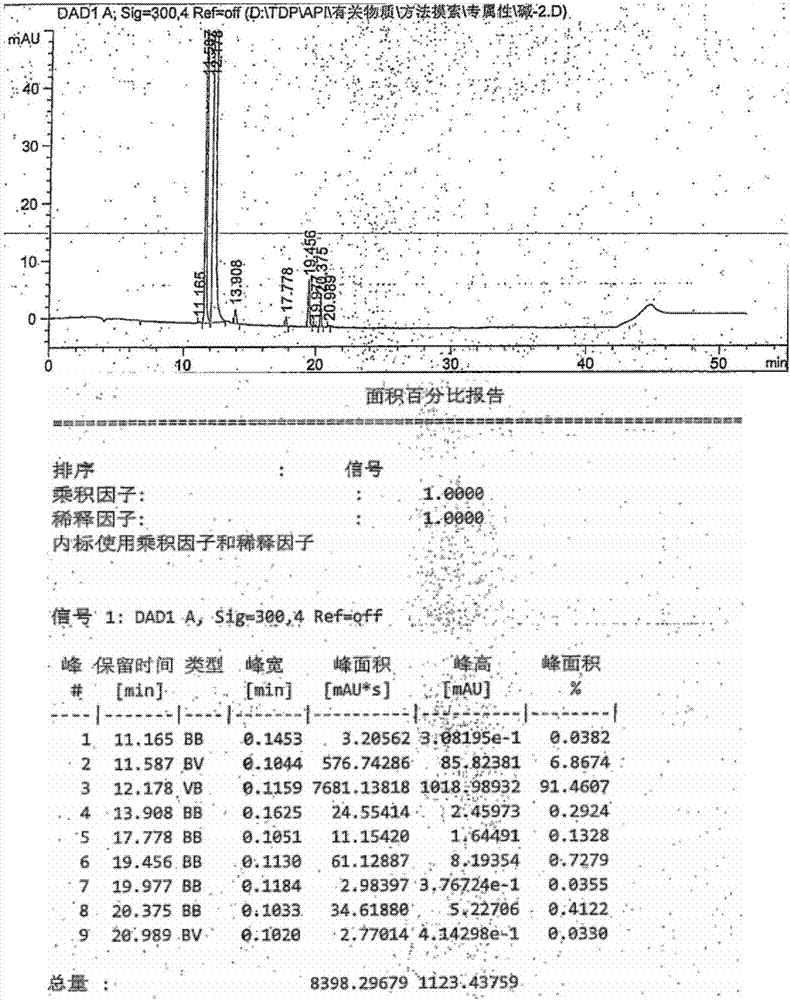
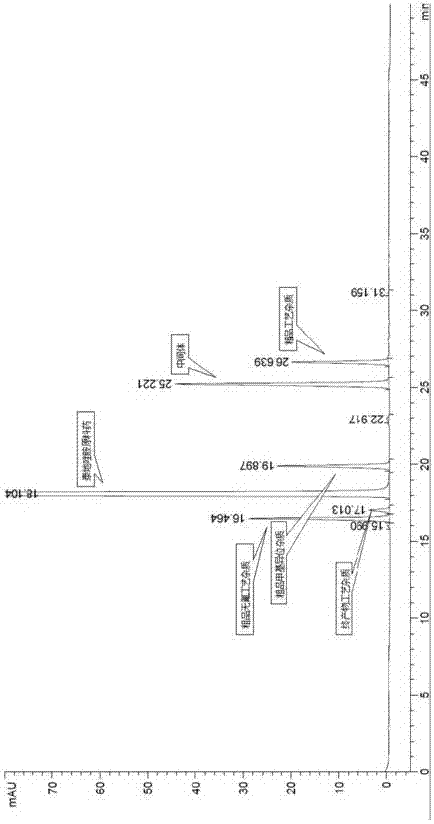
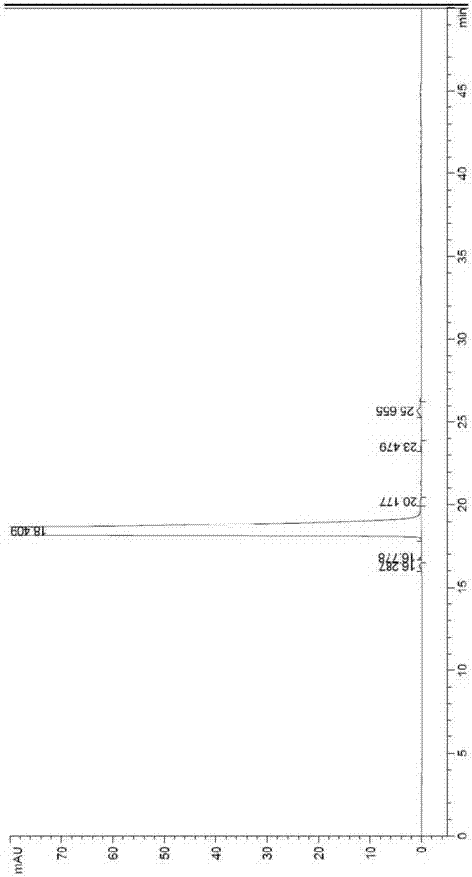
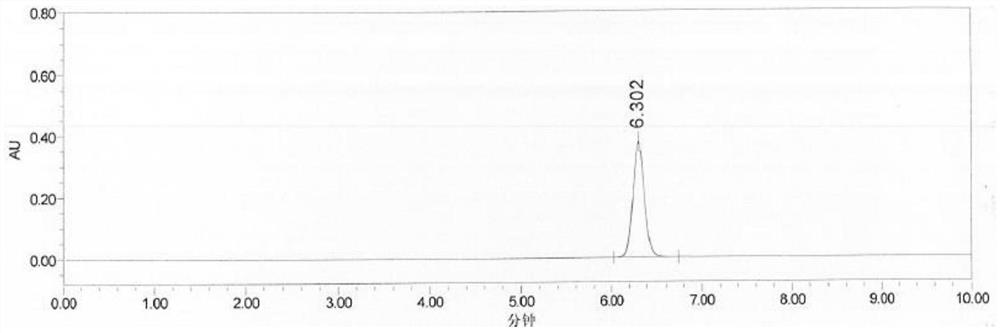
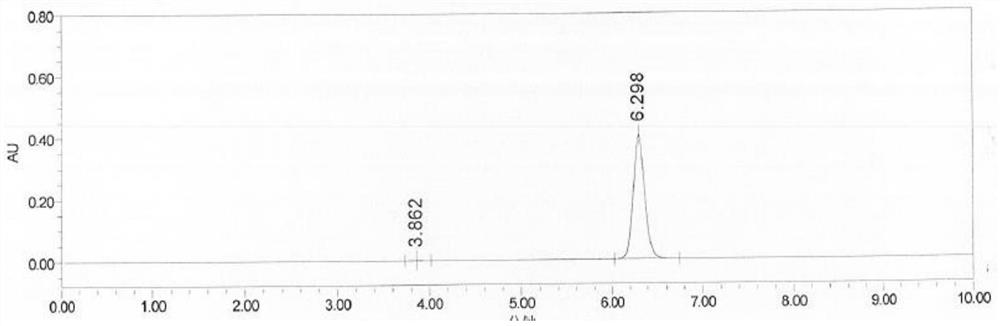
Method for testing content of tedizolid phosphate enantiomer through high performance liquid chromatography
The invention discloses a method for testing the content of tedizolid phosphate enantiomer through high performance liquid chromatography. The method comprises the following steps: (1) preparing a test solution, namely precisely weighing tedizolid phosphate, diluting with a mobile phase, and uniformly shaking so as to obtain the test solution; (2) preparing a control solution, namely precisely weighing the test solution, diluting with the mobile phase, and uniformly shaking so as to obtain the control solution; (3) testing the content, namely respectively injecting the control solution and thetest solution into a liquid chromatography meter, recording spectrums, and calculating the content of the tedizolid phosphate enantiomer by using a self-contrasted method, wherein the mobile phase ismethanol-acetonitrile-acetic acid-ammonium acetate. By selecting spectrum conditions, tedizolid phosphate and enantiomer impurities of the tedizolid phosphate can be simply, rapidly and accurately separated and detected, and controllable quality of tedizolid phosphate and a preparation of the tedizolid phosphate is achieved. Development of synthesis processes is instructed, and quality standard formulation of tedizolid phosphate raw material medicines can be facilitated.
Owner:成都美域高制药有限公司
One-pot synthesized tedizolid
The invention relates to a preparation method of tedizolid indicated in the formula I. The preparation method includes: taking 5-bromine-2-(2-methyl-2H-tetrazole-5-radical) pyridine indicated in the formula II as a raw material to be reacted with boric acid indicated in the formula III to obtain 2-(2-methyl-2H-tetrazole-5-radical)-5-(4,4,5,5-tetramethyl-1,3-dioxane-2-radical) pyridine indicated in the formula IV under the action of a palladium catalyst; allowing compound without separation to be directly in Suzuki coupling reaction with (5R)-3-(4-bromine-3-fluorophenyl)-5-hydroxymethyl oxazolane-2-ketone indicated in the formula V under the action of the palladium catalyst to obtain the tedizolid indicated in the formula I. The preparation method of the one-pot synthesized tedizolid is easy in obtaining of the raw materials, simple in production process, short in production cycle, less in discharge of three wastes (waste gas, waste water and industrial residue), and a one-pot method is high in yield and has great practical value.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Stable tedizolid phosphate medicine composition
InactiveCN105640903AAvoid degradationKeep dryAntibacterial agentsOrganic active ingredientsPhosphateCurative effect
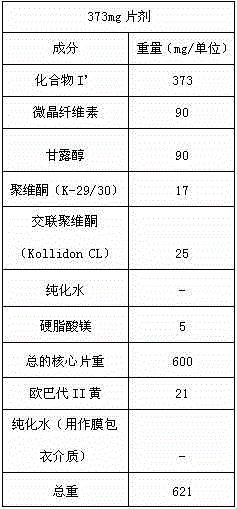
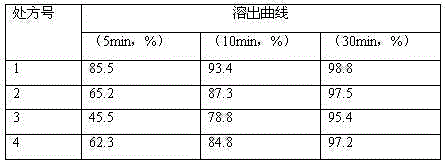
The invention discloses a stable tedizolid phosphate medicine composition, which is characterized in that a recipe for preparing 1000 tablets comprises the following ingredients including 200 to 400g of tedizolid phosphate, 70 to 140g of microcrystalline cellulose, 0.5 to 1g of superfine silica gel powder, 50 to 100g of croscarmellose sodium and a proper amount of a 10-percent amylum pregelatinisatum solution. The invention also relates to a preparation method of a tedizolid phosphate tablet. The tedizolid phosphate prepared by the recipe and the preparation method provided by the invention has the advantages of high dissolution rate, high bioavailability and good curative effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Purification method of Tedizolid phosphate
ActiveCN106279282AEasy to operateSuitable for industrial productionGroup 5/15 element organic compoundsPurification methodsPhosphate
The invention discloses a purification method of Tedizolid phosphate. The method comprises the following steps: mixing an aqueous solution of Tedizolid phosphate crude product and a weak base solution, filtering, and adjusting pH to 1-2 to obtain purified Tedizolid phosphate. The method provided by the invention is simple to operate and is low-cost. The prepared Tedizolid phosphate has high purity. The method is suitable for industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Analytical method of tedizolid phosphate and related substances thereof
ActiveCN107121503AAvoid frequent replacementImprove work efficiencyComponent separationPhosphateAdditive ingredient
The invention provides an analysis and detection method capable of simultaneously determining tedizolid phosphate and related substances thereof. Through a high performance liquid chromatograph, a sample is dissolved by using an ammonium bicarbonate water solution, an acetonitrile-tetrahydrofuran mixed solvent is taken as an organic phase, an ammonium salt water solution or a mixed liquor of an ammonium salt water solution and alkali selected from liquid ammonia, ammonium hydroxide or triethylamine is taken as a buffer solution, and gradient elution is carried out on an octadecyl silane bonded silica gel chromatographic column. The method provided by the invention is good in specificity, high in sensitivity and good in degree of separation, repeatability, degree of precision and linear relation respectively, the substances can be effectively detected when being equivalent to more than 0.03% of main ingredients, the substances can be accurately quantified when being equivalent to less than 0.05% of the main ingredients, the method is applicable to impurity control, and the requirement of related substance checking can be met.
Owner:NANJING YOKO PHARMA +2
Analysis method for tedizolid phosphate related substances
ActiveCN106855548AImprove detection abilityTimely analysisComponent separationPhosphateActive ingredient
The invention relates to an analysis method for tedizolid phosphate related substances. Timely, effective and accurate analysis and determination on product content and related substances need to be carried out in a bulk drug production process. The analysis method established by the invention is simple and practicable, sensitive, accurate and highly precise, has high detection efficiency on tedizolid phosphate process impurities, can be applied in mass production to control product quality more effectively.
Owner:TIANJIN CHASE SUN PHARM CO LTD
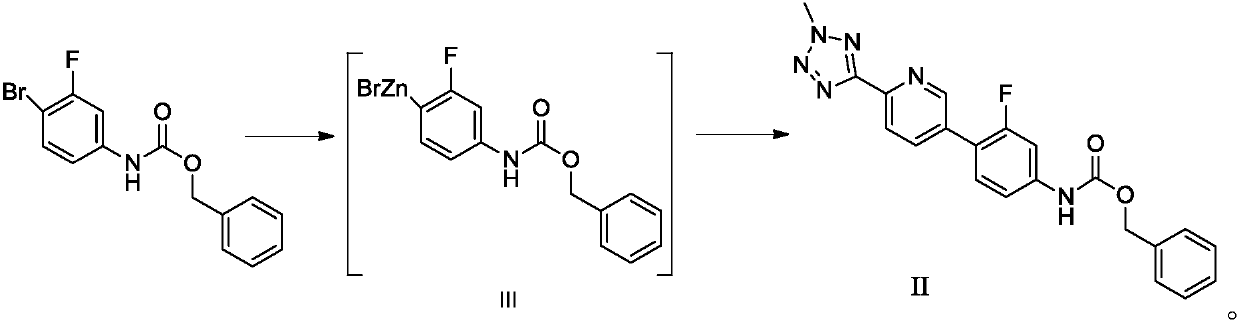
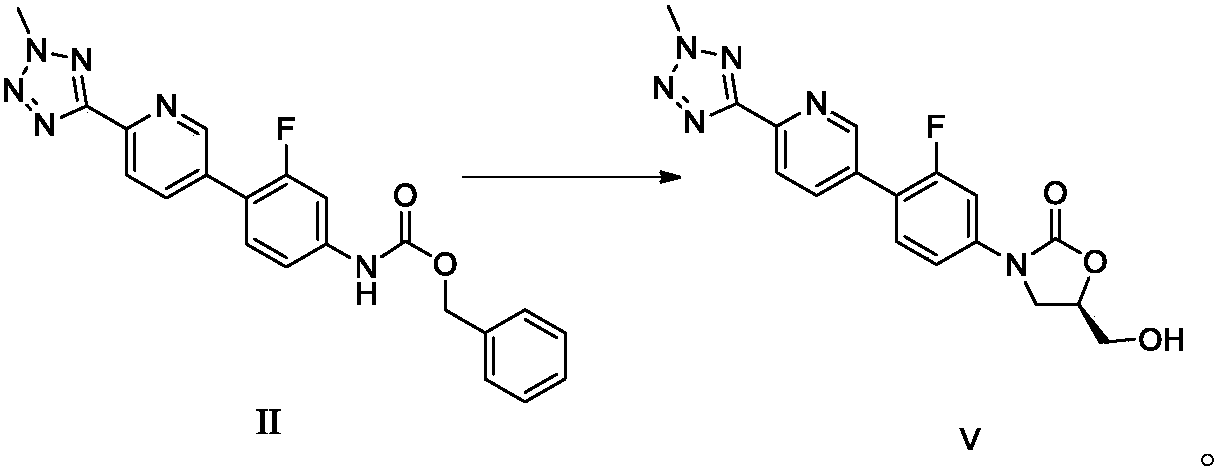
Preparation method of tedizolid phosphate and intermediate thereof
ActiveCN110804038AHigh yieldMild reaction conditionsGroup 5/15 element organic compoundsOrganic chemistry methodsPtru catalystTetrazole
The invention discloses a preparation method of tedizolid phosphate and an intermediate thereof. The preparation method of a tedizolid phosphate intermediate II comprises the following steps: 1, in anorganic solvent, under a condition of presence of a catalyst and zinc powder, performing a reaction on 3-fluorine-4-bromophenyl amino benzyl formate so as to obtain a solution of a tedizolid phosphate intermediate III; and 2, in an organic solvent, under a condition of presence of a palladium catalyst and a base, performing a coupling reaction on the solution of the tedizolid phosphate intermediate III obtained in the step 1 with 2-methyl-5-(5-bromopyridine-2-yl) tetrazole, so as to obtain the tedizolid phosphate intermediate II. The preparation method disclosed by the invention is free of toxic reagent, mild in reaction condition, safe to operate, environmentally friendly, high in yield, high in prepared product purity and low in production cost. Tedizolid phosphate prepared from the tedizolid phosphate intermediate II disclosed by the invention is high in yield, high in purity, capable of meeting raw material medicine standards and applicable to industrial production.
Owner:上海新礼泰药业有限公司
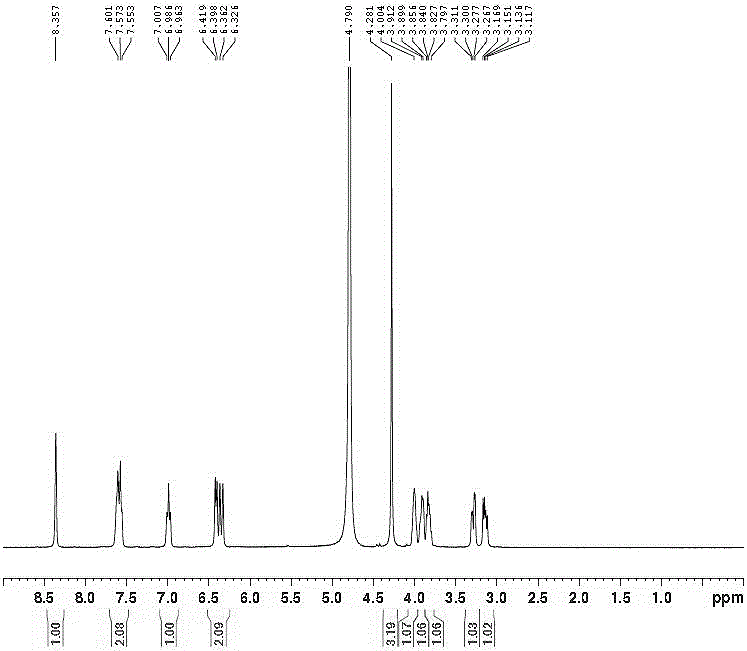
Tedizolid crystal and preparation method thereof
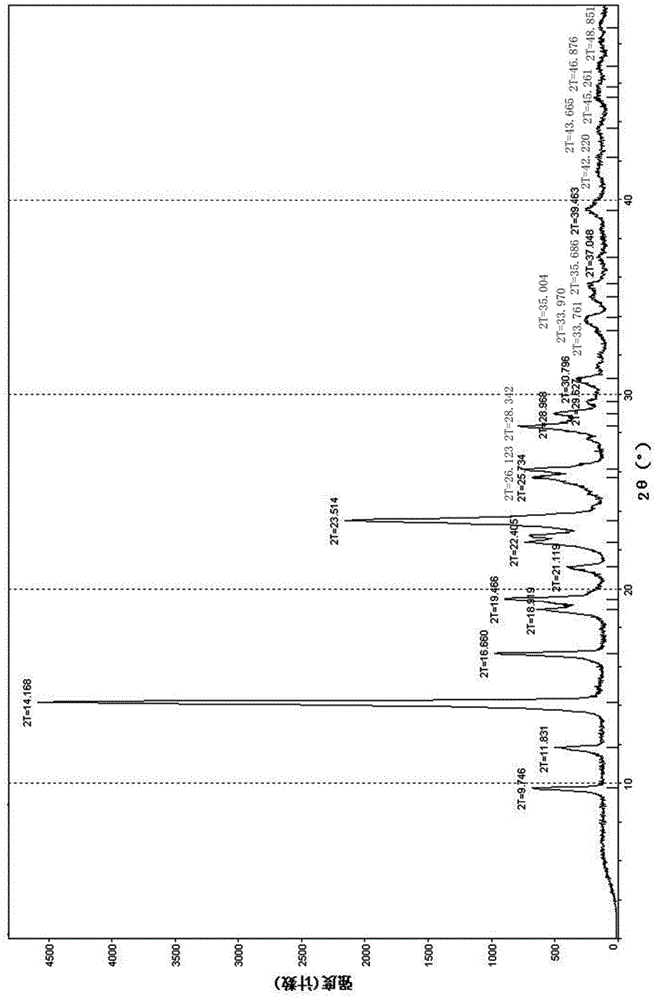
The invention discloses a tedizolid crystal and a preparation method thereof. The preparation method comprises that pinacol 2-(2-methyl-2H-tetrazyl-5-yl)pyridyl-5-boronate shown in the formula I and (R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one shown in the formula II undergo a SUZUKI coupling reaction to produce tedizolid. Under X-ray powder diffraction, the tedizolid crystal has a main characteristic peak with intensity of 100% at a diffraction angle 2 theta of 14.1+ / -0.2 degrees. The preparation method has the advantages of simple processes, high product purity, stable quality and large scale production easiness and has a significant value of tedizolid phosphate raw material industrial production and corresponding preparation quality guarantee.
Owner:SHANGHAI DESANO CHEM PHARMA +1
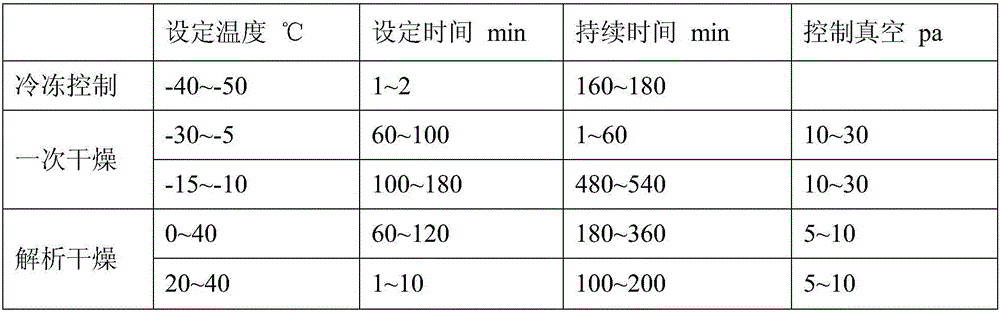
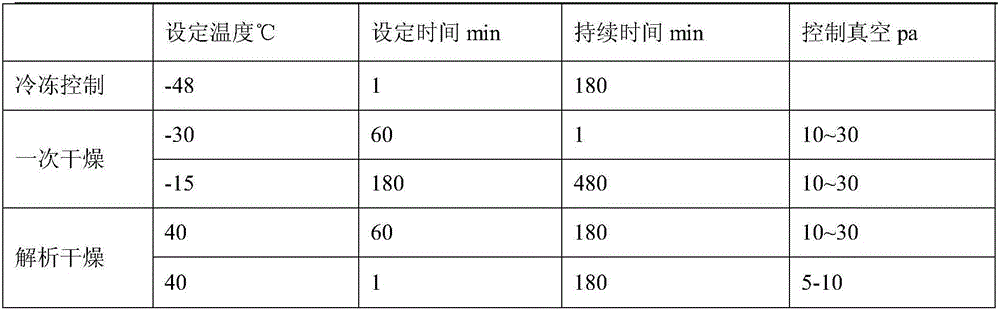
Preparation method of tedizolid phosphate freeze-dried preparation for injection
ActiveCN112826801AUniform crystal sizeEasy to operateAntibacterial agentsPowder deliveryPhosphateDrugs preparations
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a preparation method of a tedizolid phosphate freeze-dried preparation for injection. The preparation method of the tedizolid phosphate freeze-dried preparation for injection comprises the following steps: a, adding tedizolid phosphate and a freeze-drying protective additive into water for injection to obtain tedizolid phosphate liquid medicine; b, sequentially cooling the liquid medicine to -8 to -10 DEG C and -40 to -50 DEG C, and keeping for a specific time; heating the liquid medicine to -15 DEG C to -10 DEG C, and cooling the liquid medicine to -40 DEG C to -50 DEG C, and keeping the temperature for a certain period to obtain a freeze-dried product; c, under the vacuum condition, heating the freeze-dried product to -30 to -10 DEG C and 40 to 50 DEG C, and keeping the temperature for a certain period, so as to obtain the tedizolid phosphate freeze-dried preparation for injection. The preparation method is simple to operate, the time cost for preparing the tedizolid phosphate freeze-dried preparation for injection is remarkably reduced, and the high-quality tedizolid phosphate freeze-dried preparation for injection, which is low in impurity and moisture content, can be obtained.
Owner:SHIJIAZHUANG NO 4 PHARMA
Purification method of tedizolid phosphate
InactiveCN112961186AHigh purityEase of industrial productionGroup 5/15 element organic compoundsAlcoholPhosphate
The invention relates to the technical field of medicinal chemistry, in particular to a purification method of tedizolid phosphate. The method comprises the following steps: firstly, crude tedizolid phosphate is creatively mixed with absolute ethyl alcohol, impurities with lower polarity than tedizolid phosphate in the crude tedizolid phosphate are dissolved in the absolute ethyl alcohol, then the impurities are removed by filtering, and tedizolid phosphate is left in a filtered solid; and other impurities can be removed by combining the subsequent steps of converting tedizolid phosphate solid into tedizolid phosphate disodium salt, acidifying, crystallizing and the like. The tedizolid phosphate obtained through purification is high in purity, the sodium hydroxide solution is clear, and a high-boiling-point solvent which is difficult to remove does not need to be used in the purification process of the purification method. The method is simple, convenient and beneficial to industrial production.
Owner:HAINAN GENERAL & KANGLI PHARMA
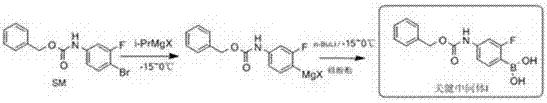
Novel synthesis method for key intermediate of tedizolid
ActiveCN107488188AHigh yieldEase of industrial productionGroup 3/13 element organic compoundsLithiumGrignard reagent
The invention relates to a novel synthesis method for a key intermediate of tedizolid. The method is characterized in that a starting material SM is dissolved in an anhydrous solvent; then a Grignard reagent isopropyl magnesium halide is added to prepare a Grignard reagent of SM; then n-butyllithium is added to prepare a SM magnesium-lithium complex; and then the SM magnesium-lithium complex is reacted with borate to prepare the target intermediate. The method provided by the invention overcomes the problem of extremely low reaction temperature in the prior art, greatly improves reaction yield, and is easy to realize industrialized production.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Preparation method of tedizolid phosphate
InactiveCN107722056ALow costReduce the temperatureGroup 5/15 element organic compoundsChemical industrySolubility
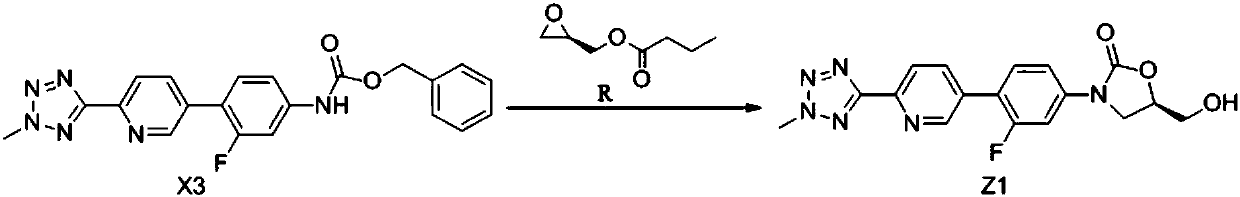
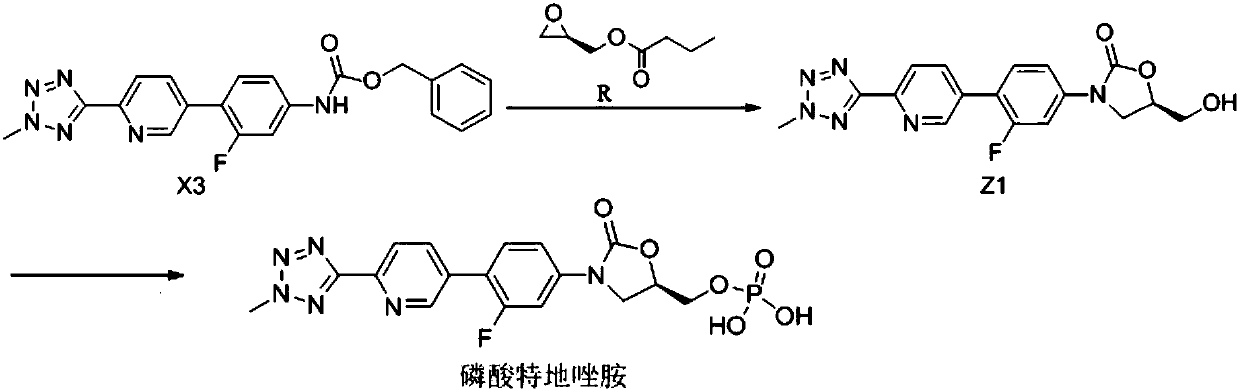
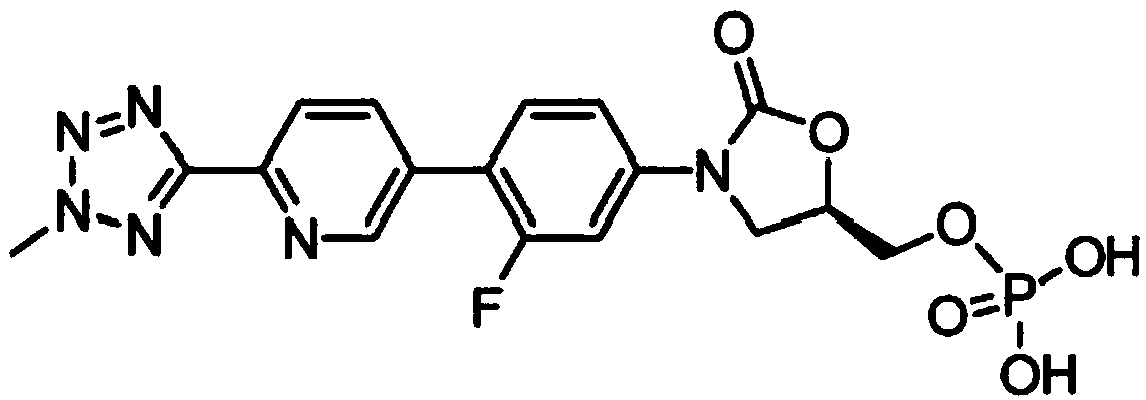
The invention belongs to the field of pharmaceutical and chemical industry, and specifically relates to a preparation method of tedizolid phosphate. The compound shown as a formula Z1 is a tedizolid phosphate precursor, and is obtained in a cyclization reaction through catalyst catalysis by using X3 as a raw material. The preparation method of the compound shown as the formula Z1 comprises the following steps: carrying out the cyclization reaction on a compound shown as a formula X2 and a compound shown as a formula R under the existence of a catalyst and a solvent, so as to obtain the compound shown as a formula Z1; carrying out a phosphorylation reaction on the compound shown as the formula Z1 so as to obtain tedizolid phosphate. According to the preparation method disclosed by the invention, the compound with high boiling point such as DMPU is prevented from being used as the catalyst, and the dissolubility of the reaction system is improved through the addition of a mixed solvent,so that the reaction time is greatly shortened, and the compound is applicable for industrial production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Refining process of tedizolid and preparation method of tedizolid phosphate
ActiveCN113214239APromote precipitationAvoid influenceGroup 5/15 element organic compoundsBiochemical engineeringPhosphate
The invention belongs to the field of medicine synthesis, and discloses a refining process of tedizolid. According to the invention, a mixed solution of dichloromethane, toluene and ethanol is prepared, tedizolid generated through Suzuki reaction is purified through steps of liquid separation, filtration and the like, and the obtained tedizolid pure product is used for tedizolid phosphate synthesis. While the purity of tedizolid is ensured, the consumption of tedizolid in the refining process is reduced, the yield of tedizolid is improved, the utilization rate of raw materials is improved, the production cost is saved, the tedizolid with higher purity participates in synthesis of tedizolid phosphate, the side reaction in the tedizolid phosphate synthesis process is reduced, the purification difficulty of tedizolid phosphate is reduced, and the purity and the yield of tedizolid phosphate are improved. The method is suitable for refining tedizolid compounds, and is especially suitable for refining an intermediate tedizolid in a tedizolid phosphate synthesis process.
Owner:HAINAN GENERAL & KANGLI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com