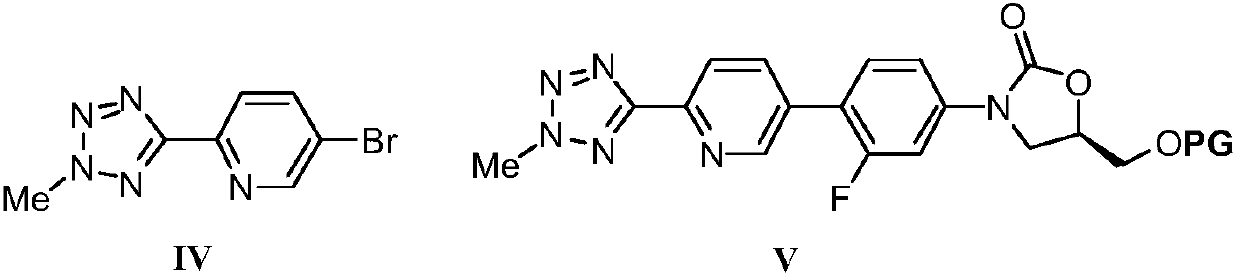
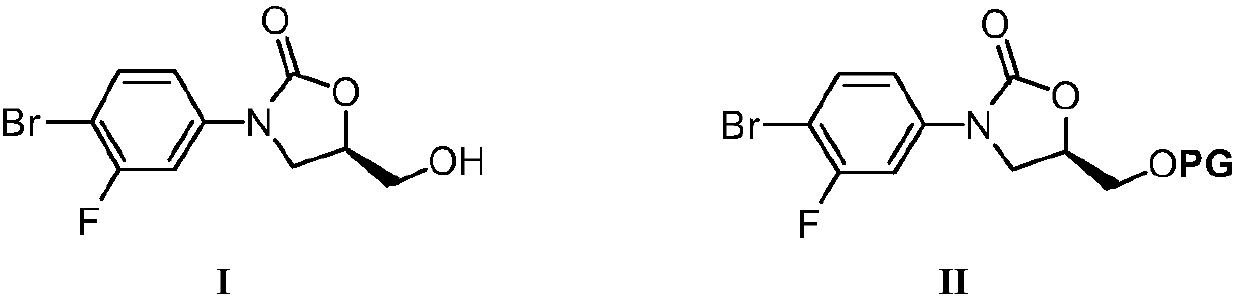Nickel catalyzed tedizolid phosphate synthesis method
A technology catalyzed by phosphate and nickel, which is applied in the fields of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of increasing operation steps and overall cost, and achieve improved synthesis efficiency, Avoid cumbersome steps, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Dissolve 5.8g (20mmol) of the compound of formula I and 2.0g (30mmol) of imidazole in 50mL of DMF, add 3.6g (24mmol) of tert-butyldimethylsilyl chloride after dissolution, and react at 60°C for 6 hours; 200mL of water was added to the solution to dilute, and a large amount of white solid was precipitated. After filtration, the filter cake was washed with water (20mL×5), dried and then recrystallized (ethyl acetate / n-hexane) to obtain white filamentous crystals, namely the compound of formula II-1 (7.9 g, 98% yield).
[0033]
[0034] 2. In the absence of water and oxygen, put 20.2g (50mmol) of the compound of formula II-1 into a 250mL eggplant-shaped reaction flask, add 60mL of dry tetrahydrofuran to dissolve, and cool to -78°C. Then slowly add 20mL of 2.5mol / L n-butyllithium n-hexane solution dropwise, after the dropwise addition, continue the reaction at -78°C for 1 hour, then slowly add 60mL of 1.0mol / L anhydrous zinc chloride solution in tetrahydrofuran, Afte...
Embodiment 2
[0043] In step 2 of Example 1, the anhydrous zinc chloride used was replaced with anhydrous zinc bromide in an equimolar amount, and the yield of the compound of formula V-1 was 86%, and other steps were the same as in Example 1.
Embodiment 3
[0045] In step 2 of Example 1, under anhydrous and oxygen-free conditions, 20.2 g (50 mmol) of the compound of formula II-1 was placed in a 250 mL eggplant-shaped reaction flask, dissolved by adding 60 mL of dry tetrahydrofuran, and cooled to -78°C. Then slowly add 20mL 2.5mol / L n-butyllithium n-hexane solution dropwise, after the dropwise addition, continue the reaction at -78°C for 1 hour, then slowly add 60mL 1.0mol / L anhydrous zinc chloride solution in tetrahydrofuran, After the dropwise addition, the stirring reaction was continued for 30 minutes to generate the intermediate shown in formula III-1. Directly, the reaction solution was slowly raised to room temperature, and then 6.1g (25mmol) compound of formula IV, 1.61g (3.0mmol) bis[(2-diphenylphosphino)phenyl]ether (DPEPhos) and 545mg (2.5mmol) were added successively. ) Nickel bromide and 5 mL of 1.0 mol / L isopropylmagnesium chloride in tetrahydrofuran. The reaction was stirred at room temperature, and the progress of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com