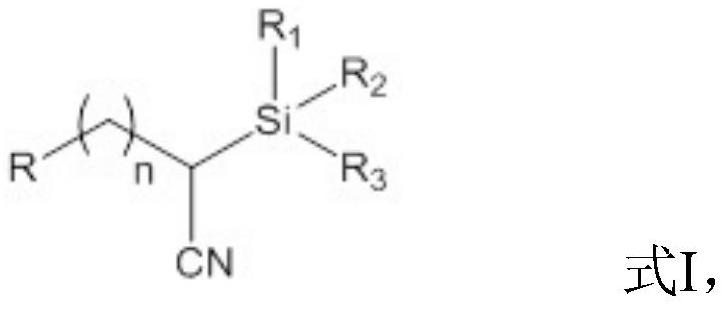
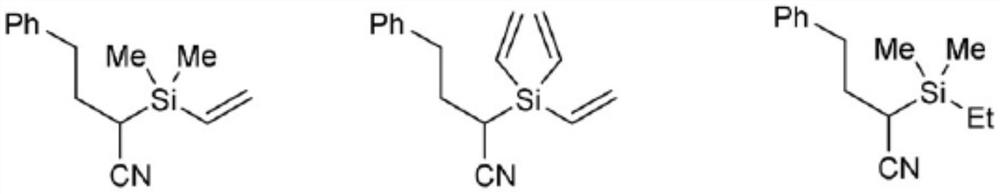
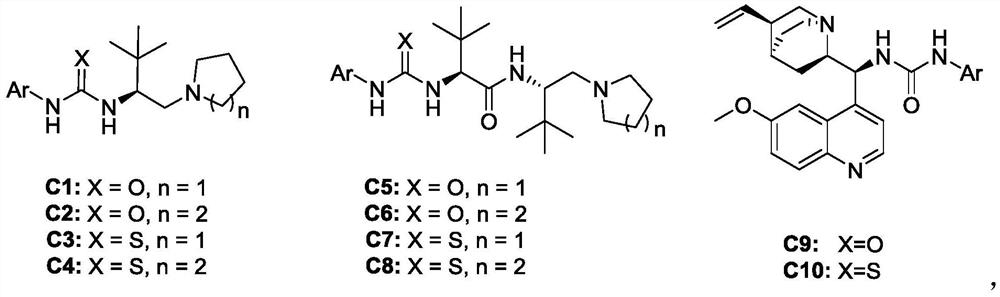
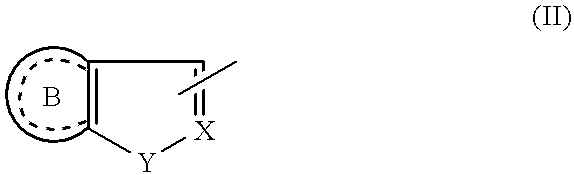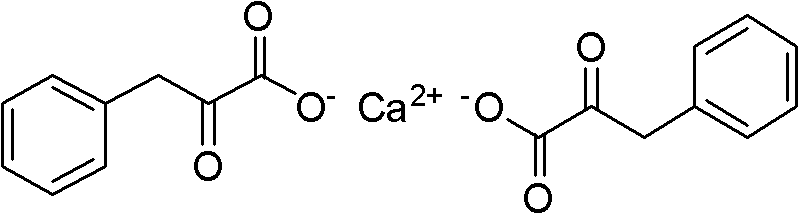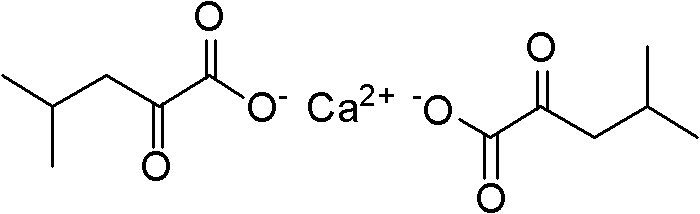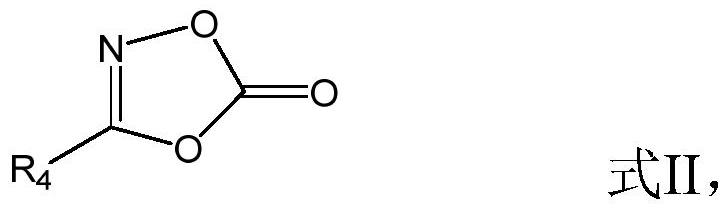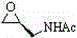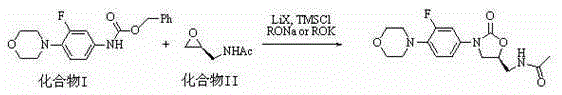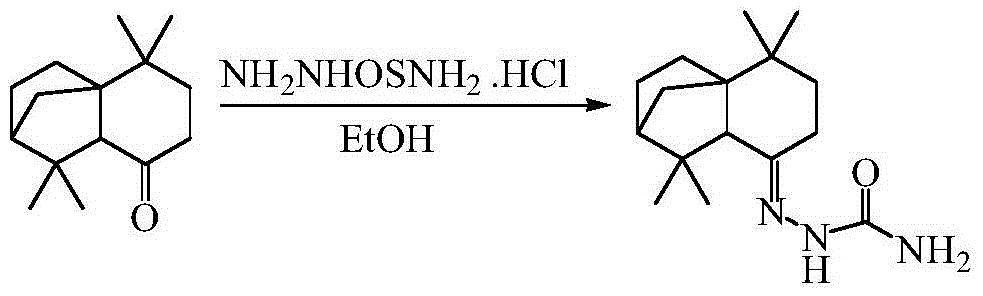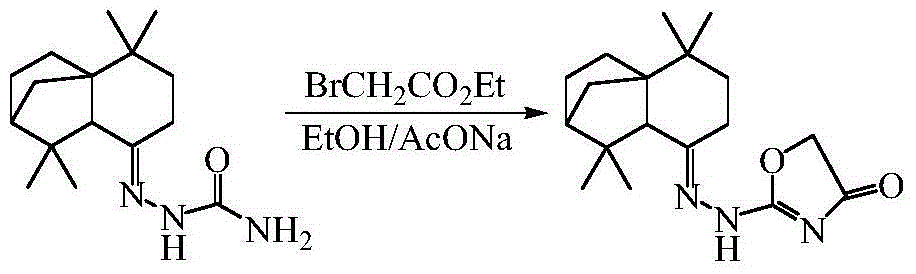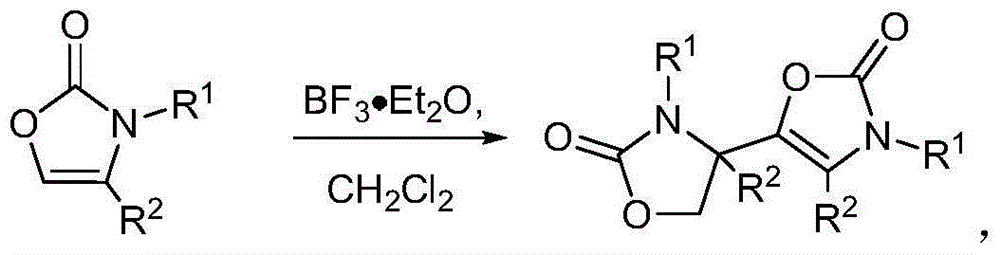Patents
Literature
117 results about "Oxazolone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
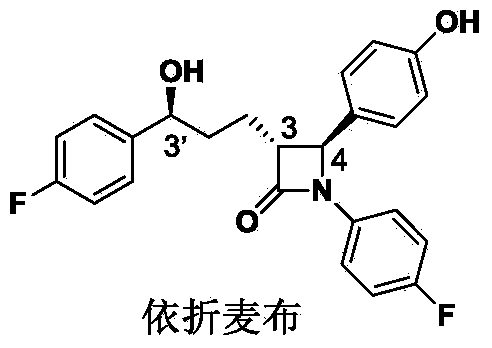
Oxazolone is a chemical compound and functional group, with the molecular formula C₃H₃NO₂. It was named in-line with the Hantzsch–Widman nomenclature and is part of a large family of oxazole based compounds.
Oxazolone derivatives and their use as anti-Helicobacter pylori agent
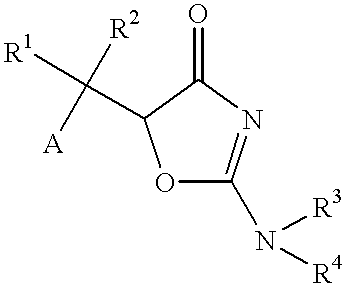
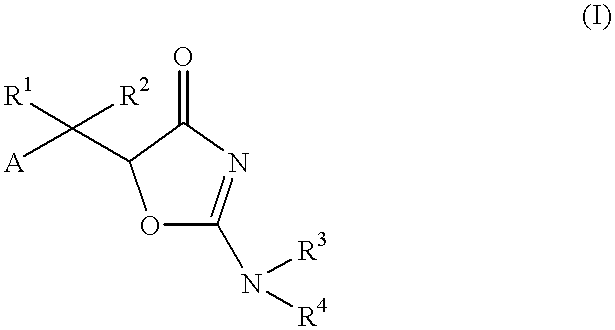
An anti-Helicobacter pylori agent comprising a compound represented by the formula:wherein A represents an aromatic ring group which may be substituted; R1 and R2, whether identical or not, each represent a hydrogen atom or a hydrocarbon group which may be substituted; R3 and R4, whether identical or not, each represent a hydrogen atom, a hydrocarbon group which may be substituted, an acyl group, a carbamoyl group which may be substituted, or a carboxyl group which may be esterified; or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
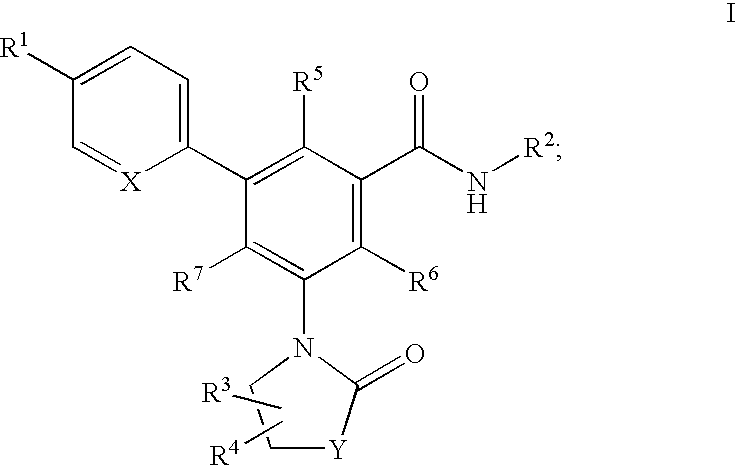
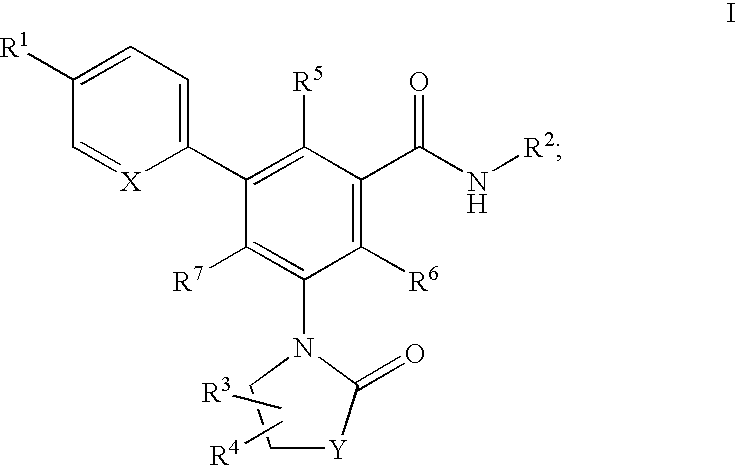
Oxazolone and pyrrolidinone-substituted arylamides as p2x3 and p2x2/3 antagonists
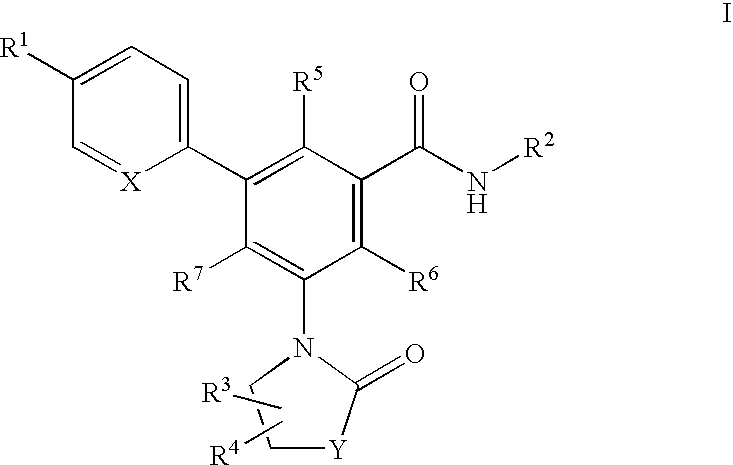
Compounds of the formula I:or a pharmaceutically acceptable salt thereof, wherein, X, Y, R1, R2, R3, R4, R5, R6 and R7 are as defined herein. Also disclosed are methods of using the compounds for treating diseases associated with P2X3 and / or a P2X2 / 3 receptor antagonists and methods of making the compounds.
Owner:ROCHE PALO ALTO LLC
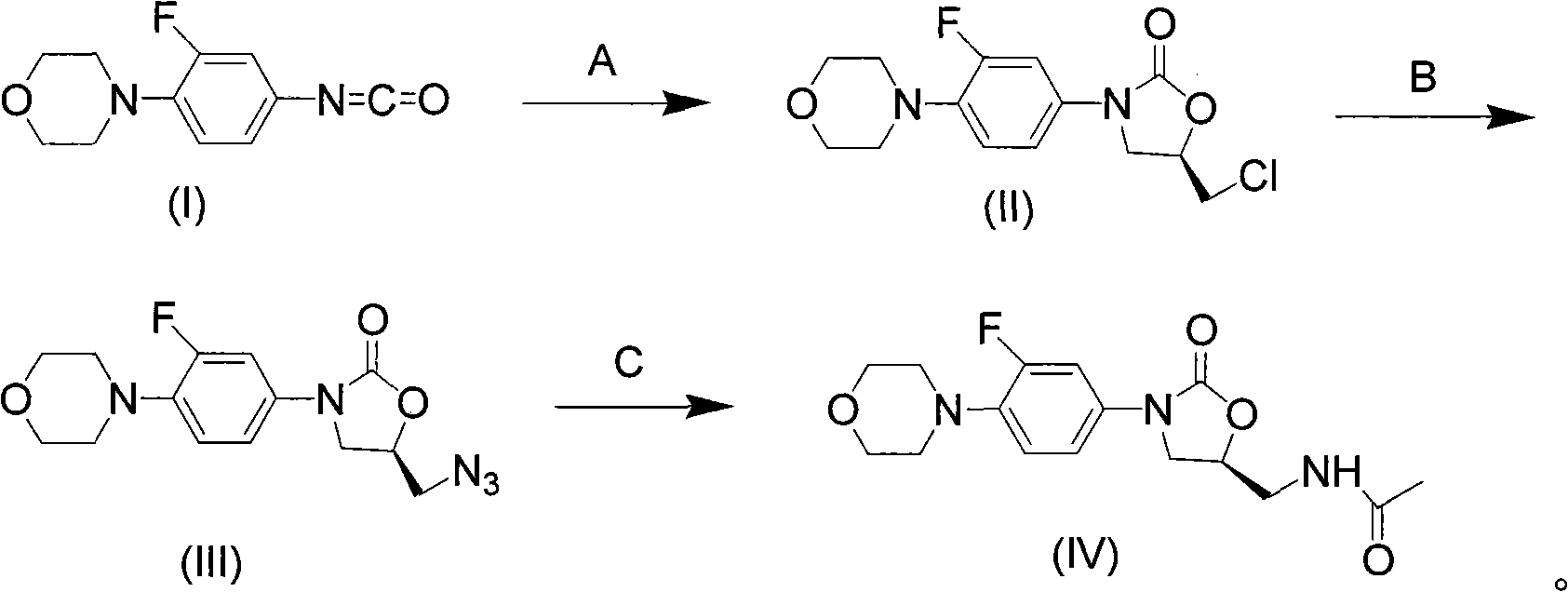
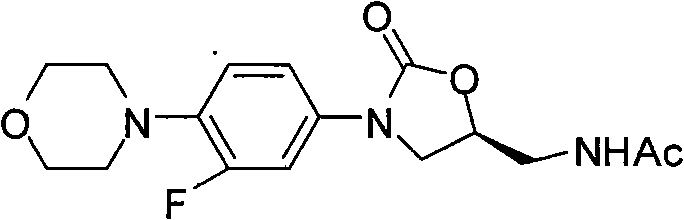
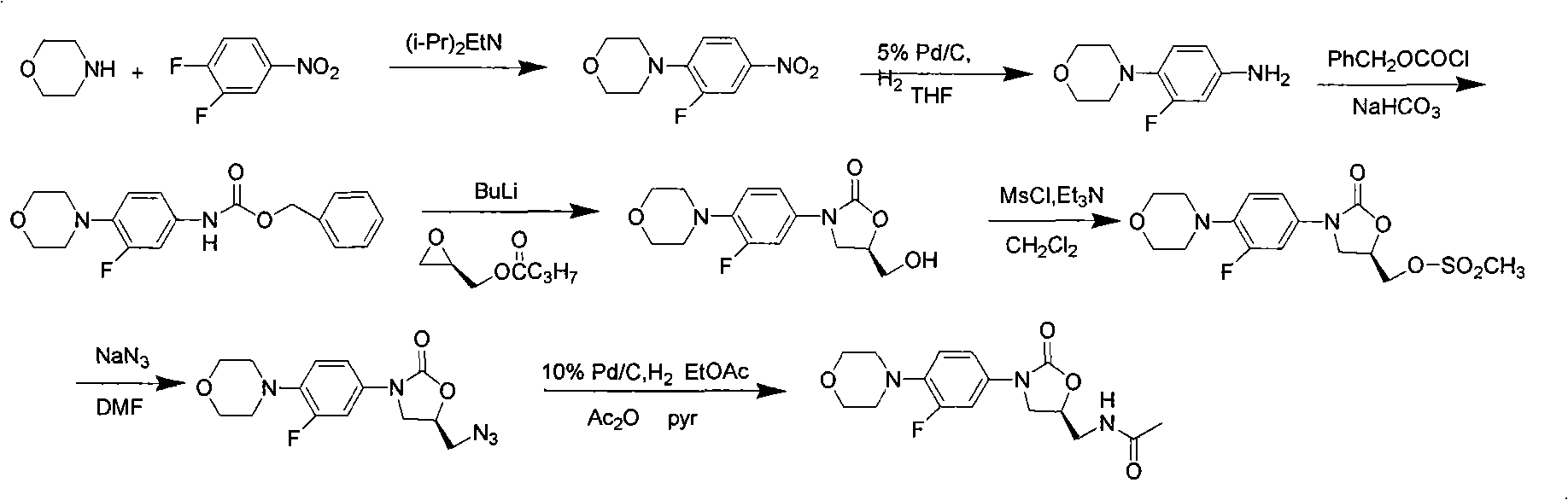
Novel preparation method of netaglinide oxazolone
InactiveCN101638392AAtom utilization is highHigh stereoselectivityAntibacterial agentsOrganic chemistryEpoxyMorpholine
The invention discloses a novel preparation method of netaglinide oxazolone shown in the formula (IV), which comprises the following steps: under the action of a catalyst A, enabling 3-fluorine-4-morpholine phenyl isocyanate shown in the formula (I) to react with (R)-epoxy chloropropane to obtain a compound (II), wherein the catalyst A is magnesium diiodide, magnesium dibromide, magnesium dichloride, magnesium perchlorate or magnesium trifluoromethanesulfonic acid; enabling the compound (II) to react with sodium azide to obtain a compound (III); reducing the compound (III) by hydrogenation, and then, acetylating the reduced compound (III) to obtain the compound (IV). In the invention, the low-cost and environment-friendly catalyst (Lewis acid magnesium) is used for catalyzing the cycloaddition reaction of the (R)-epoxy compound and the isocyanate to establish a mother nucleus structure of the netaglinide oxazolone by one step, thus the prepared netaglinide oxazolone has high stereoselectivity, does not need rigorous operation conditions, such as low temperature, no water, no oxygen and the like, has the advantages of moderate reaction conditions, simple and convenient operation, high utilization ratio of atoms, environment protection, low production cost and the like, and is suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Oxazolone analogs as amyloid aggregation inhibitors and for the treatment of alzheimer's disease and disorders related to amyloidosis
Owner:WARNER-LAMBERT CO
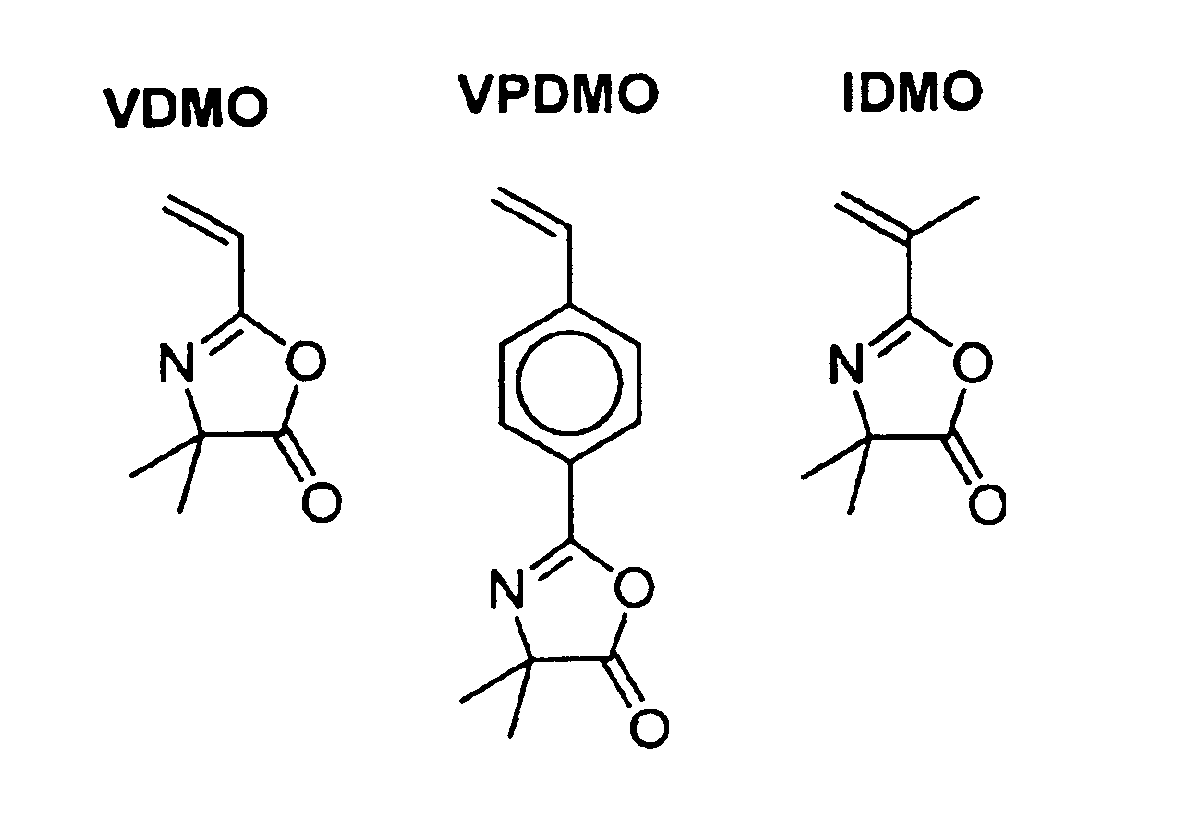
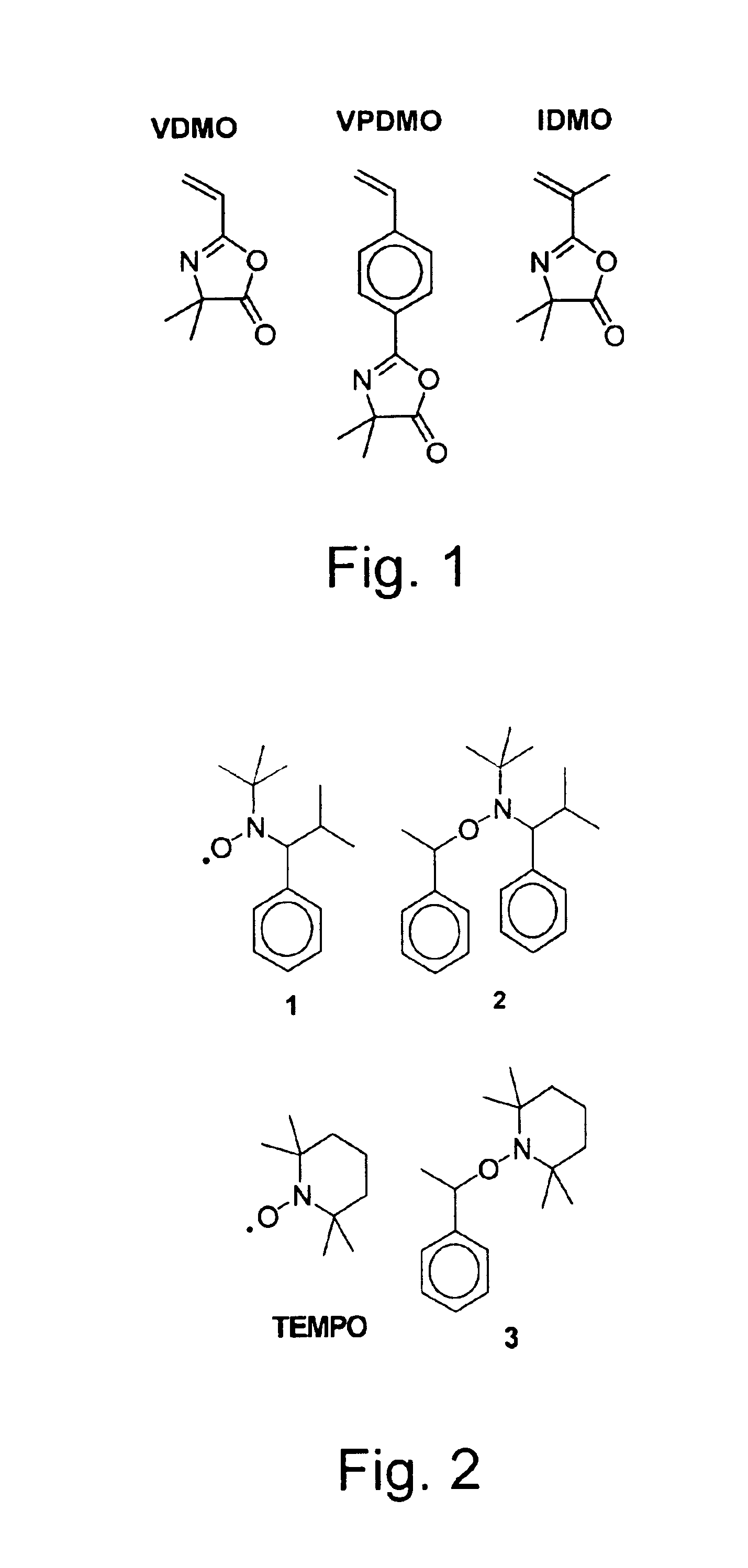
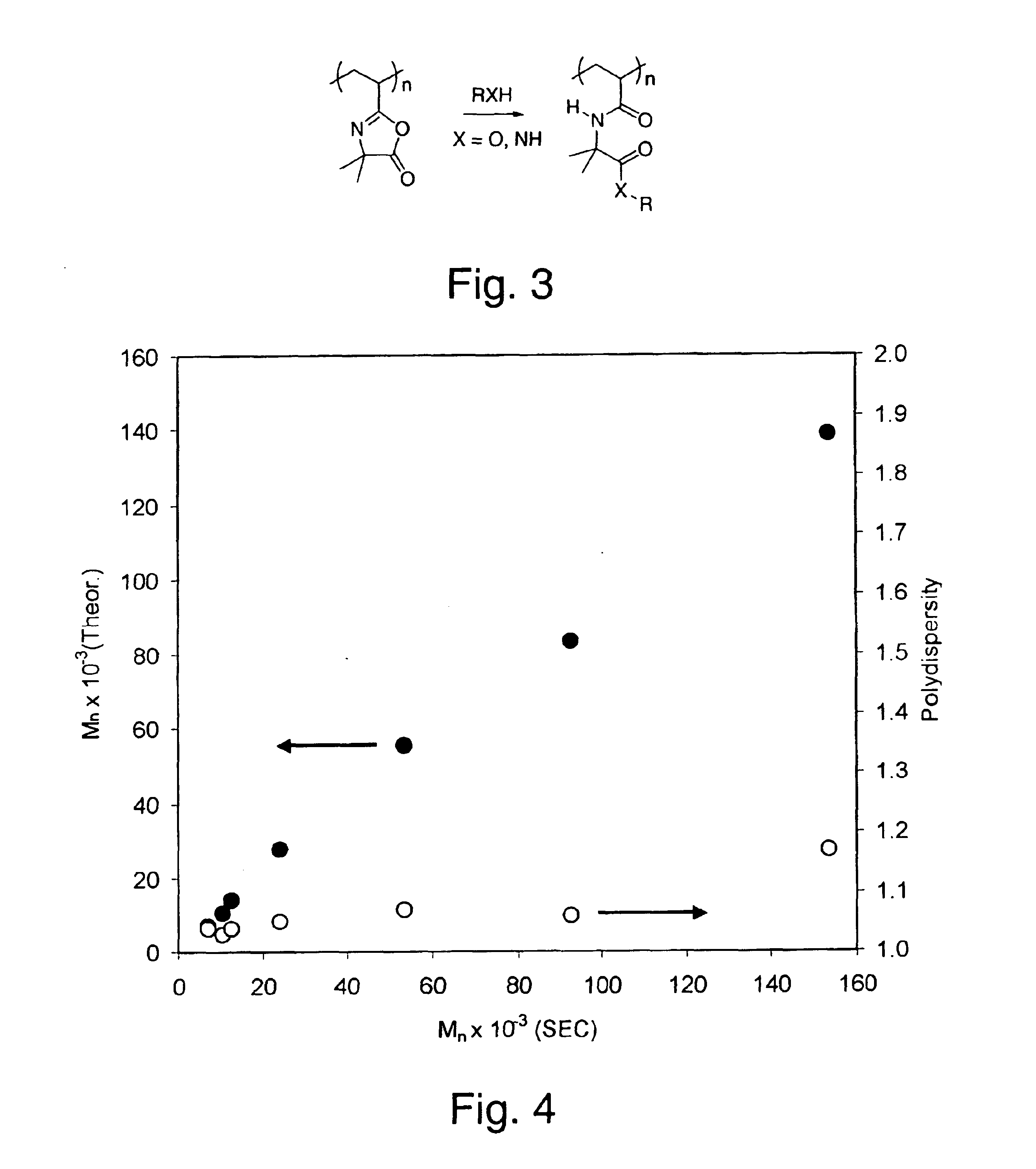
Compositions and methods of vinyl oxazolone polymerization
This invention provides novel methods for synthesis of narrow polydispersity oxazolone-containing polymers via nitroxide-mediated living free radical polymerization, as well as the products and derivatives thereof.
Owner:IRM
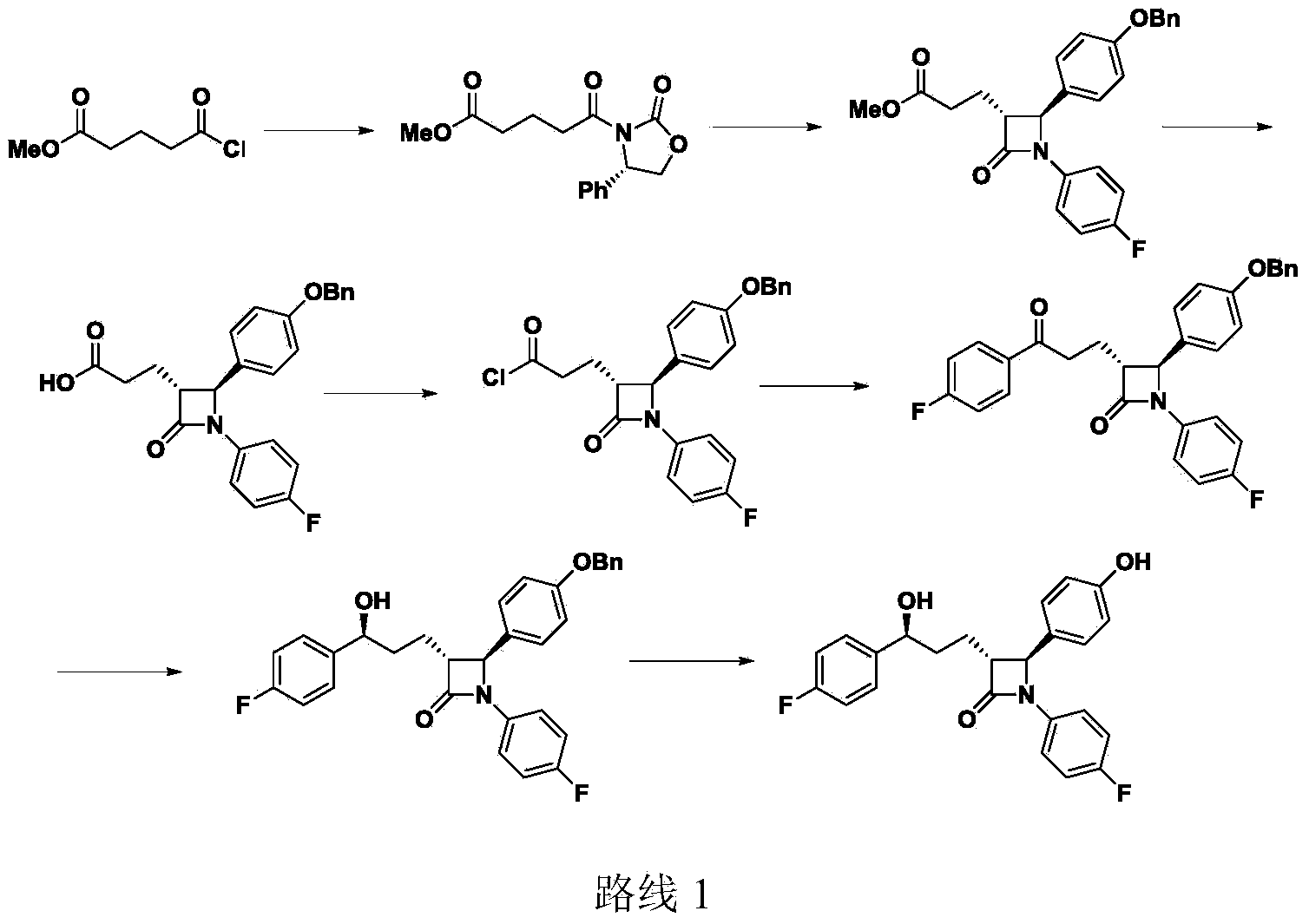
Preparation midbody for Ezetimibe and preparation method of preparation midbody
ActiveCN104230978AEasy to manufactureGroup 4/14 element organic compoundsBulk chemical productionChlorobenzeneAcyl group
The invention provides a preparation midbody for Ezetimibe which is shown in a general formula I, wherein PG is an acetyl group, a T-butyloxycarbonyl group, a benzyl group, a benzyloxycarbonyl group, a trityl group, a trimethylsilyl group or a diphenylmethyl group silicon group. The invention further provides a preparation method of the midbody I, and the application for preparing the medicine Ezetimibe. The method for preparing the Ezetimibe by adopting the compounds shown in the general formula I is different from the method in the conventional document, the newer chirality assistant (S)-4-(2-chlorphenyl)-2-oxazolone is adopted, the productive rate reaches 91%, and the optical purity reaches 100%, so that the productive rate and the optical purity are higher than those of the previously applied (S)-4-phenyl-2-oxazolone. Besides, the selected chirality assistant (S)-4-(2-chlorphenyl)-2- oxazolone can be conveniently prepared by the original commercialized ((S)-2-chlorobenzene glycine potassium).
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
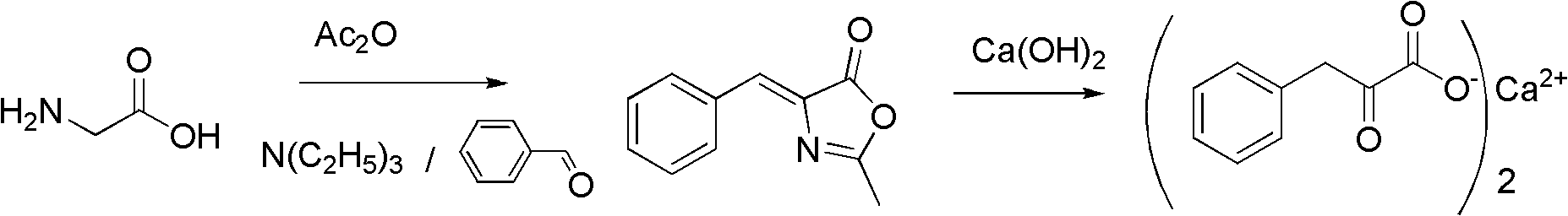
Method for preparing Alpha-keto-phenylalanine calcium
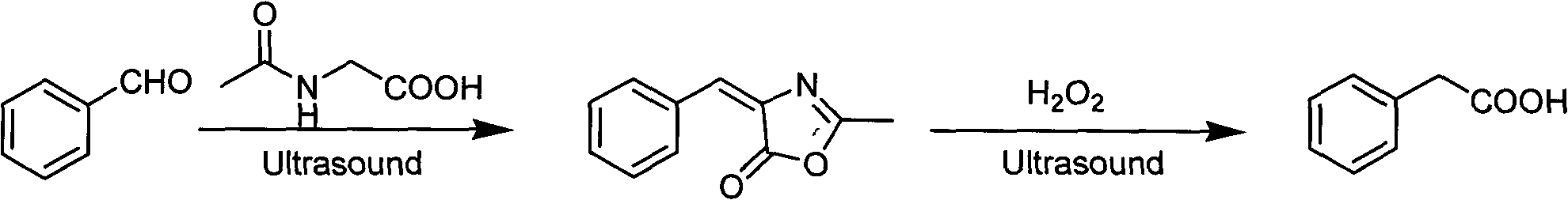
InactiveCN102050725AEasy to produceEasy to buyCarboxylic acid salt preparationChemical recyclingAcetic anhydrideBenzaldehyde
The invention discloses a method for preparing a medical material, namely Alpha-keto-phenylalanine calcium. In the conventional methods, some methods are complicate in operation and has low yield, and oxydol is used in the oxidizing process, which is not favorable for safety production; and some methods have long reaction time, harsh conditions and low hydrolysis yield. The preparation method includes the following steps: glycine, benzaldehyde, acetic anhydride and organic base catalyst are taken as raw materials, and 4-benzal-2-methyl dihydride oxazolone is obtained by adopting one-pot method for catalytic cyclization reaction; and 4-benzal-2-methyl dihydride oxazolone and calcium hydroxide conduct ring-opening and hydrolysis reaction in a pressure kettle to obtain Alpha-keto-phenylalanine calcium. The cyclization reaction adopting the one-pot method has low equipment investment and operating cost; organic amine catalyst is easy for reclamation and indiscriminate use; no waste water generates; and the hydrolysis reaction adopting pressurizing reaction has low possibility of side reaction, and the reaction conditions are mild and are easy to control, so that the reaction yield is improved.
Owner:ZHEJIANG NHU CO LTD
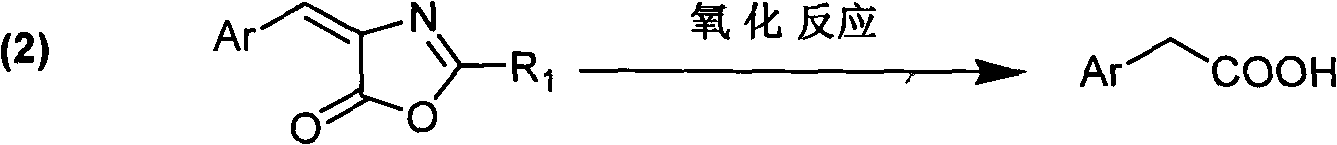
Preparation method for aryl acetic acid derivative
InactiveCN102070433AReduce pollutionMild reaction conditionsOrganic compound preparationCarboxylic compound preparationSodium acetateAcetic anhydride
The invention discloses a preparation method for an aryl acetic acid derivative. The preparation method comprises the following specific steps of: (1) adding sodium acetate, acetic anhydride and a cyclization agent into aryl methanal or alkyl original aliphatic aldehyde having more than four carbon atoms, and performing cyclization reaction to obtain an aryl oxazolone derivative, wherein the cyclization agent is N-acyl-glycine or an analogue thereof; and (2) uniformly mixing the aryl oxazolone derivative and aqueous alkali, adding an oxidizing agent and performing oxidization reaction to obtain the corresponding aryl acetic acid derivative, wherein the alkali is sodium hydroxide solution or potassium hydroxide, and the oxidizing agent is peroxy acid. Compared with the prior art, the method is mild in reaction condition, short in reaction steps, short in preparation cycle, low in cost, simple in posttreatment and low in environmental pollution, and is applicable for preparation of the aryl acetic acid derivative; by the method, the raw materials are readily available; moreover, the product prepared by the method is high in purity and stable in quality, and the method is suitable for large-scale industrial production.
Owner:GUILIN NORMAL COLLEGE
Chiral 3, 4-dihydrogen-2(1H)-quinolinone compound and preparation method
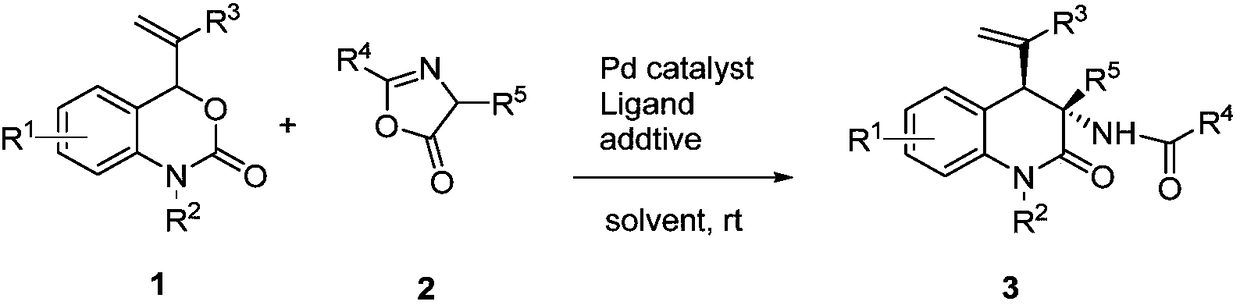
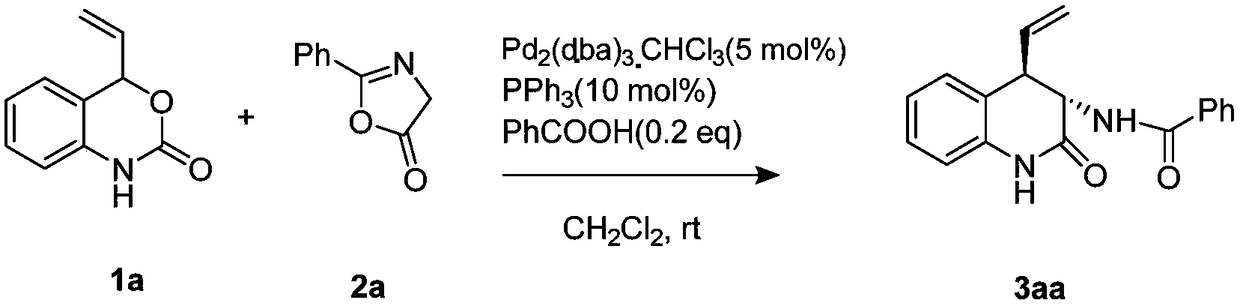
ActiveCN109354583AEasy to operateMild reaction conditionsOrganic chemistryPalladium catalystRoom temperature
The invention discloses a chiral 3, 4-dihydrogen-2(1H)-quinolinone compound and a preparation method and belongs to the technical field of compound preparation. The preparation method includes: takingvinyl benzoxazinone and oxazolone as a synthetic building block, adding a metal palladium catalyst, phosphorus-ligand and acid-alkali additive, and allowing reaction at room temperature to obtain thecompound. The preparation method is mild in reaction condition, high in reaction speed, simple in aftertreatment and wide in substrate range. A method for selective synthesis of diastereomer of 3, 4-dihydrogen-2(1H)-quinolinone having extensive physiological and pharmacological activities like resistance to tumor, high blood pressure and tuberculosis and pain stopping is brand-new and efficient.
Owner:BEIJING UNIV OF TECH
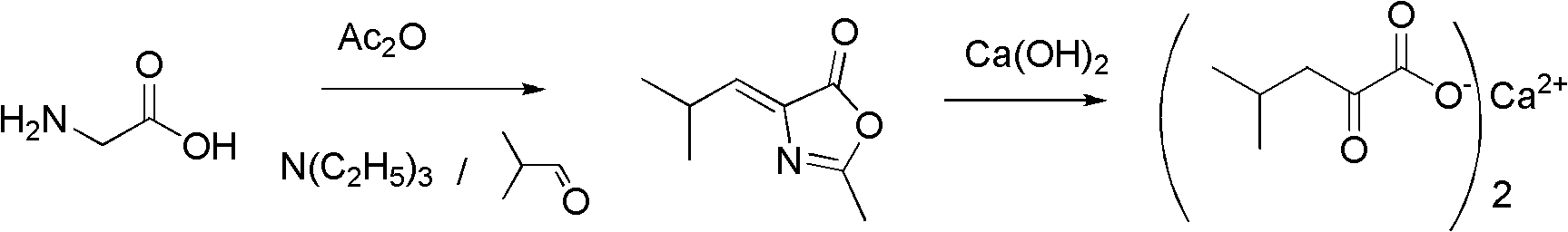
Method for synthesizing alpha-ketoleucine calcium
InactiveCN102030631AEasy to produceEasy to buyPreparation from carboxylic acid esters/lactonesChemical recyclingAcetic anhydrideOrganic base
The invention discloses a method for synthesizing alpha-ketoleucine calcium which serves as a medicinal raw material. In the conventional methods, certain methods are complex to operate, and have low yield, hydrogen peroxide is used in the oxidation process and safe production is not facilitated; and certain methods have long reaction time, severe conditions and low hydrolysis yield. The method comprises the following preparation steps of: performing catalytic cyclization reaction on glycine, iso-butyraldehyde, acetic anhydride and an organic base catalyst which serve as raw materials by a one-pot method to obtain 4-isobutylidene-2-methyl dihydro oxazolone; and performing open-loop hydrolysis reaction on the 4-isobutylidene-2-methyl dihydro oxazolone and calcium hydroxide in a pipeline reactor to obtain the alpha-ketoleucine calcium. The one-pot method is adopted in the cyclization reaction; the method has the advantages of small equipment investment, low operation cost and no wastewater and an organic amine catalyst is easy to recycle; and continuous feeding and discharging are performed by a pipeline reaction in the hydrolysis reaction, so continuous production is realized, and the efficiency is high.
Owner:ZHEJIANG NHU CO LTD
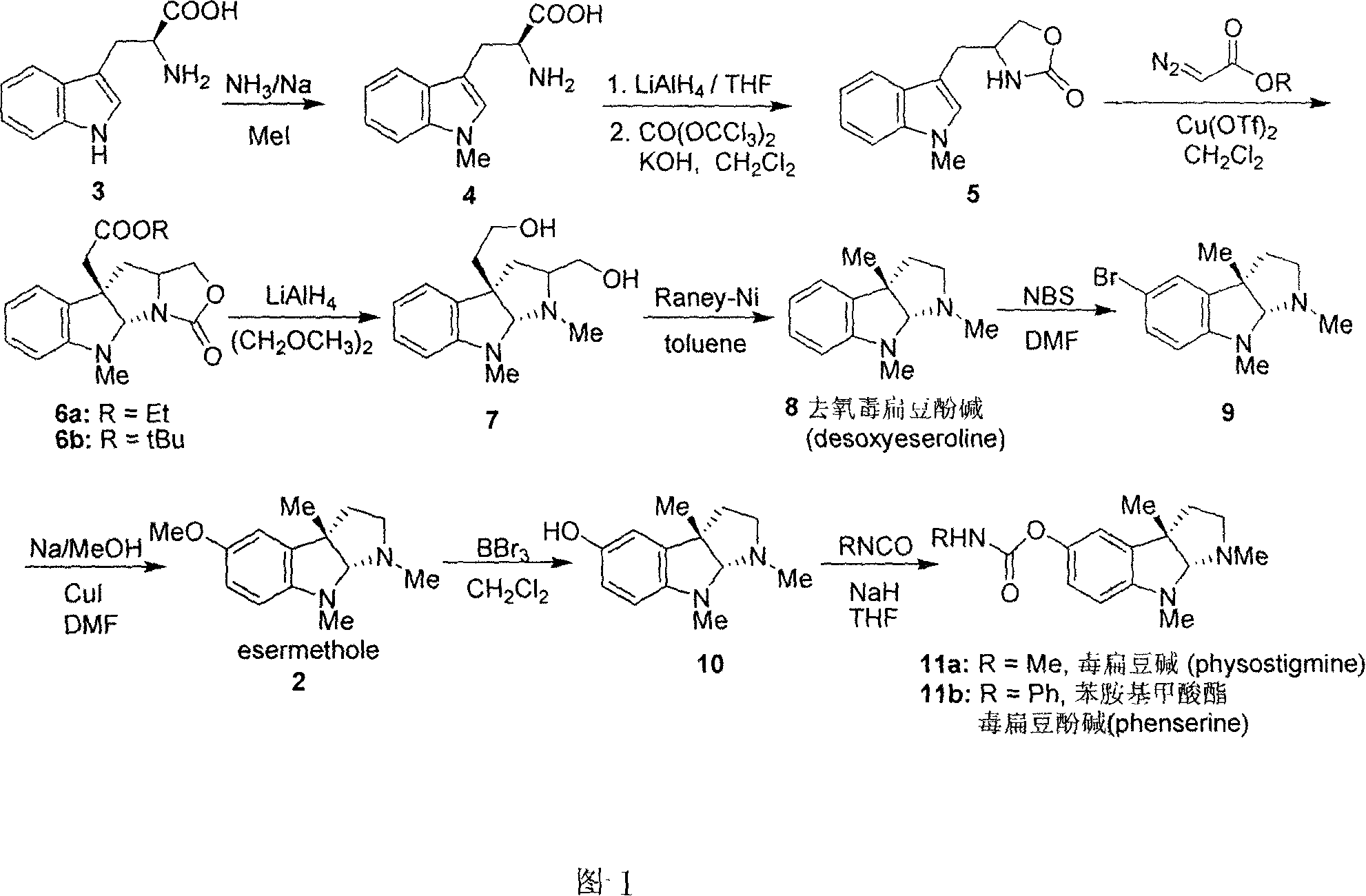
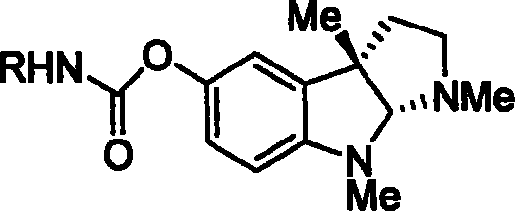
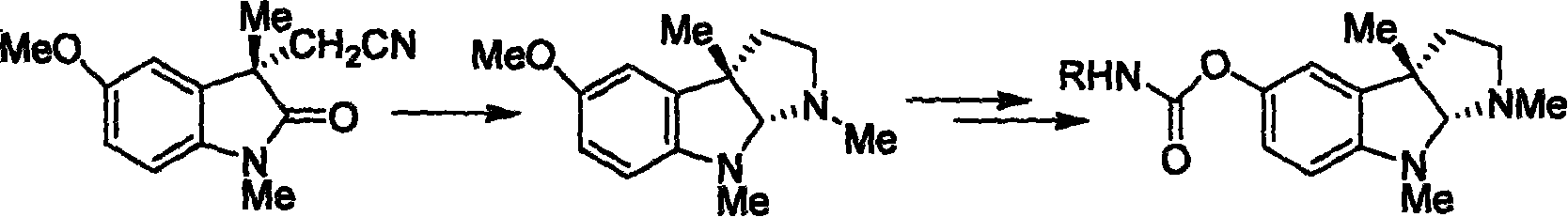
Synthesis for natural medicament physostigmine for resisting senile dementia disease and phenylaminoformic acid ester phenserine
The invention belongs to the field of drug synthesis, and relates to a new method for synthesizing physostigmine, a natural drug against senile dementia, and its derivative, anilinoformate, phenserine. The present invention uses L-tryptophan 3 as raw material, firstly prepares chiral oxazolone intermediate 5, and then through intermolecular asymmetric cyclopropionation-ring-opening-ring and three-step one-pot waterfall reaction, and diazonium Acetate reaction was prepared to obtain optically pure 3-substituted tetrahydropyrroloindole skeleton 6, which was reduced by lithium aluminum hydride to obtain diol-substituted intermediate 7, and intermediate 7 was treated with Raney nickel (Raney-Ni ) after removing the hydroxymethylene group to obtain the intermediate 8 deoxy physostigmine (desoxyeseroline), and the intermediate 8 is obtained by electrophilic bromination reaction, methyl etherification, demethylation and carbonamidation four-step reaction with high yield. Completed the synthesis of physostigmine and its derivative, anilinoformate, physostigmine, a natural drug against Alzheimer's disease. The method has the advantages of high key reaction yield, simple operation, short reaction steps, etc., and has the advantages of solving the problem of raw material source of the marketed drug physostigmine and its derivative anilinoformate phenserine. important practical significance.
Owner:SICHUAN UNIV
Non-aqueous electrolyte and lithium ion battery containing the non-aqueous electrolyte
PendingCN113823837AAvoid direct contactReduce dissolutionSecondary cellsOrganic electrolytesHigh temperature storageElectrolytic agent
The invention provides a non-aqueous electrolyte and a lithium ion battery containing the non-aqueous electrolyte. The non-aqueous electrolyte comprises a solvent, a lithium salt, an additive A and an additive B, wherein the additive A is an organic silicon nitrile compound, and the additive B is an oxazolone compound. Through the combined action of the additive A organic silicon nitrile compound and the additive B oxazolone compound, the cycle performance, the high-temperature storage performance and the low-temperature performance of the high-voltage lithium ion battery, especially the ternary high-voltage lithium ion battery, are effectively improved.
Owner:ZHUHAI COSMX BATTERY CO LTD
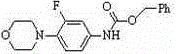
Preparation method for Linezolid
ActiveCN105348212AAtom economyMild reaction conditionsOrganic chemistryTrimethylsilyl chlorideAlcohol
The invention relates to the oxazolidinone antibiotic medicine preparation field, and concretely relates to a preparation method for Linezolid. A compound I and a compound II are employed as raw materials, halogenated lithium, alkyl alcohol sodium or alkyl alcohol potassium and trimethylchlorosilane are added to promote construction of an oxazolone ring, and furthermore a Linezolid bulk drug is prepared. The reaction chemical equation is shown in the specification. The preparation method has advantages of atom economy, easily available reagents, mild reaction conditions, environmental protection, high yield and purity and the like compared with the prior art.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Tertiary leucine derived chiral amine compound as well as preparation method and application thereof
ActiveCN103360341AStrong synergyHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsRhodanineTert-leucine
The invention discloses a tertiary leucine derived chiral amine compound as well as a preparation method and application thereof. The chiral amine compound contains a tert-butyl group, a primary amine, a secondary amine or a tertiary amine functional group and has the structural formula as shown in the specification; and chiral amine and salts thereof are prepared through simple preparation steps by taking common tert-leucine as the raw material to form the chiral amine compound. The chiral amine and the salts thereof can be used for the asymmetrical Michael additive reaction between alpha, beta-unsaturated ketone and a nucleophilic reagent such as nitrocarbol, malonic ester, substituted oxazolone and the like and the asymmetrical cascade reaction between the alpha, beta-unsaturated ketone and fifth-position unsaturated rhodanine, between fifth-position unsaturated hydantoin and the alpha, beta-unsaturated ketone; and the tertiary leucine derived chiral amine compound has very high catalytic activity and stereoselectivity as well as the highest diastereoselectivity of 30 / 1 and the highest enantioselectivity of 99%, and is wide in oligomer range.
Owner:EAST CHINA UNIV OF SCI & TECH
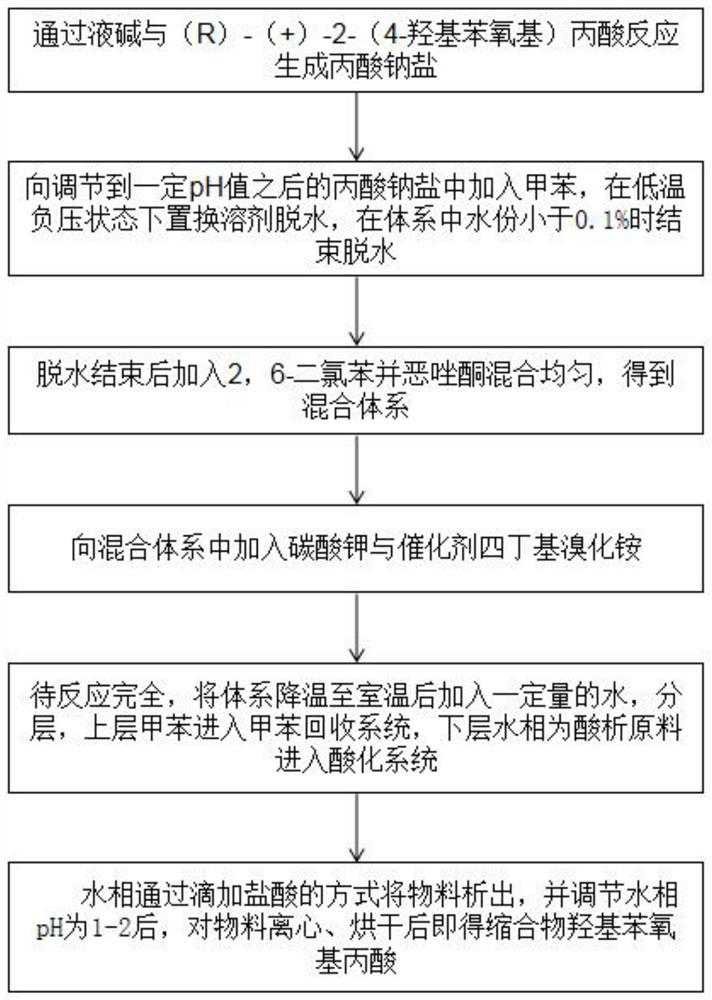
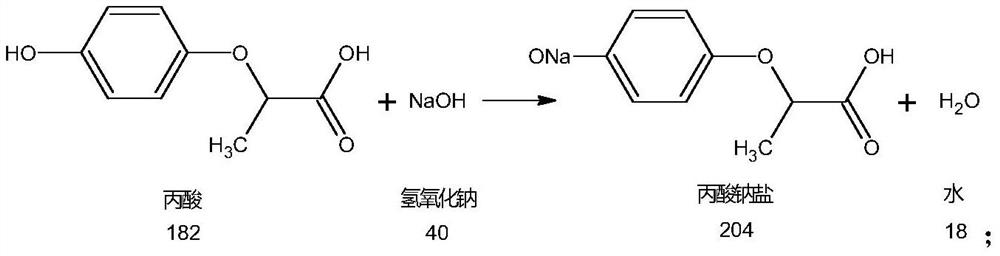
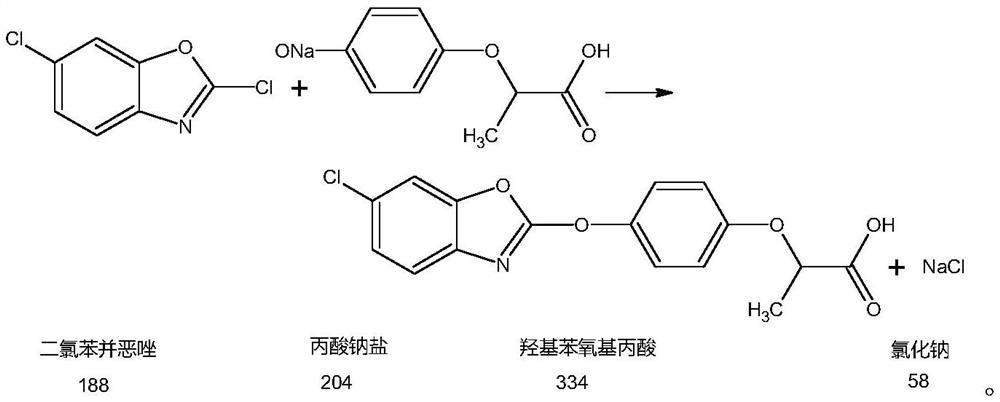
Metamifop intermediate and preparation method of metamifop
InactiveCN112409287AReduce processing costsLow costOrganic chemistryPropanoic acidPotassium carbonate
The invention provides an metamifop intermediate and a preparation method of metamifop. The preparation method comprises the following steps: reacting caustic soda liquid with (R)-(+)-2-(4-hydroxyphenoxy) propionic acid to generate sodium propionate; adding toluene into the sodium propionate after the pH value is adjusted to a certain value, carrying out solvent replacement dehydration in a low-temperature negative-pressure state, and stopping dehydration when the water content in the system is smaller than 0.1%; after dehydration is finished, adding 2, 6-dichlorobenzoxazolone, and uniformly mixing to obtain a mixed system; adding potassium carbonate and catalyst tetrabutylammonium bromide into the mixed system; after the reaction is completed, cooling the system to room temperature, adding a certain amount of water, layering, enabling upper-layer toluene to enter a toluene recovery system, and enabling a lower-layer water phase as an acidification raw material to enter an acidification system; and separating out the material from the water phase by dropwise adding hydrochloric acid, adjusting the pH value of the water phase to be 1-2, centrifuging the material, and drying to obtain condensation compound hydroxyphenoxy propionic acid.
Owner:宁夏蓝田农业开发有限公司
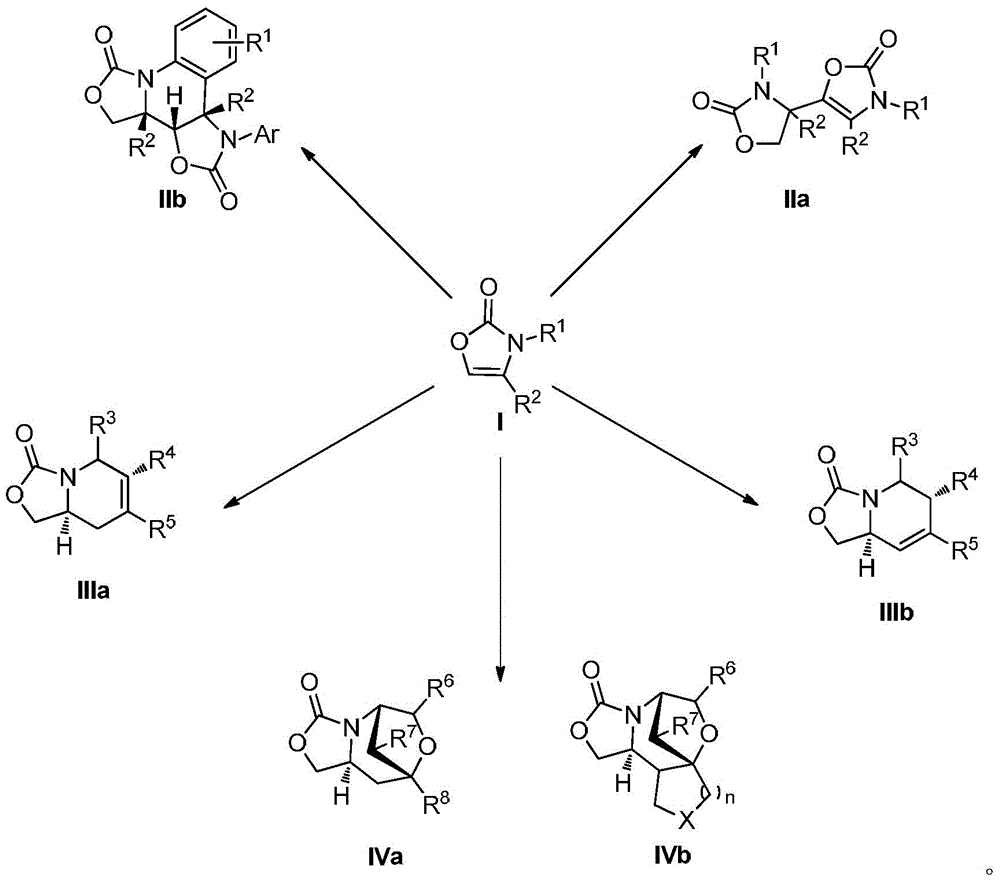
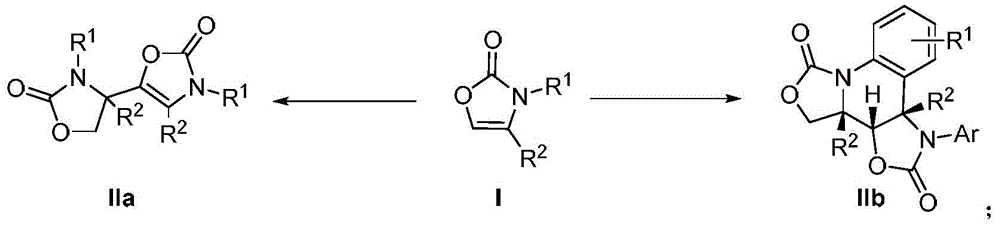
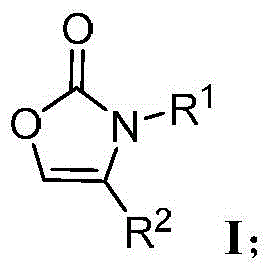
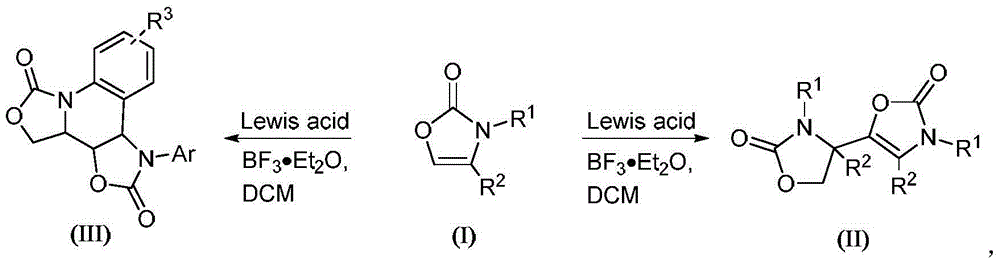
Preparation method for oxazolone heterocyclic compounds
The invention relates to a preparation method for N-substituted oxazolone polymerized compounds. According to the method, the easily available reagent, boron trifluoride ether, is used as a catalyst, and dimerization, cascaded dimerization / cyclization, terminal butylene cyclization and secondary cyclization among N-substituted oxazolone molecules are influenced under mild reaction conditions. The method is not sensitive to water in a reaction system, and identical reactions and same yield and rate may be realized under aqueous conditions. The novel preparation method with the advantages of mild reaction condition and high-efficiency conversion can be more extensively applied to synthesis of a plurality of molecules and industrial production.
Owner:CHONGQING UNIV
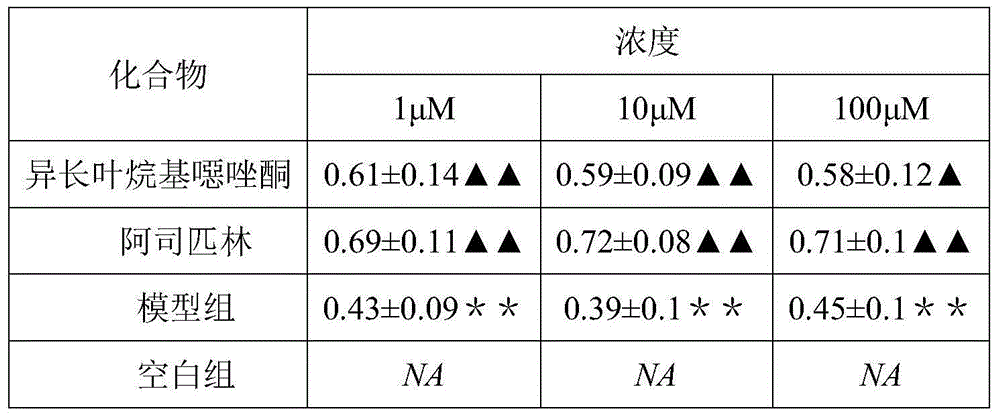
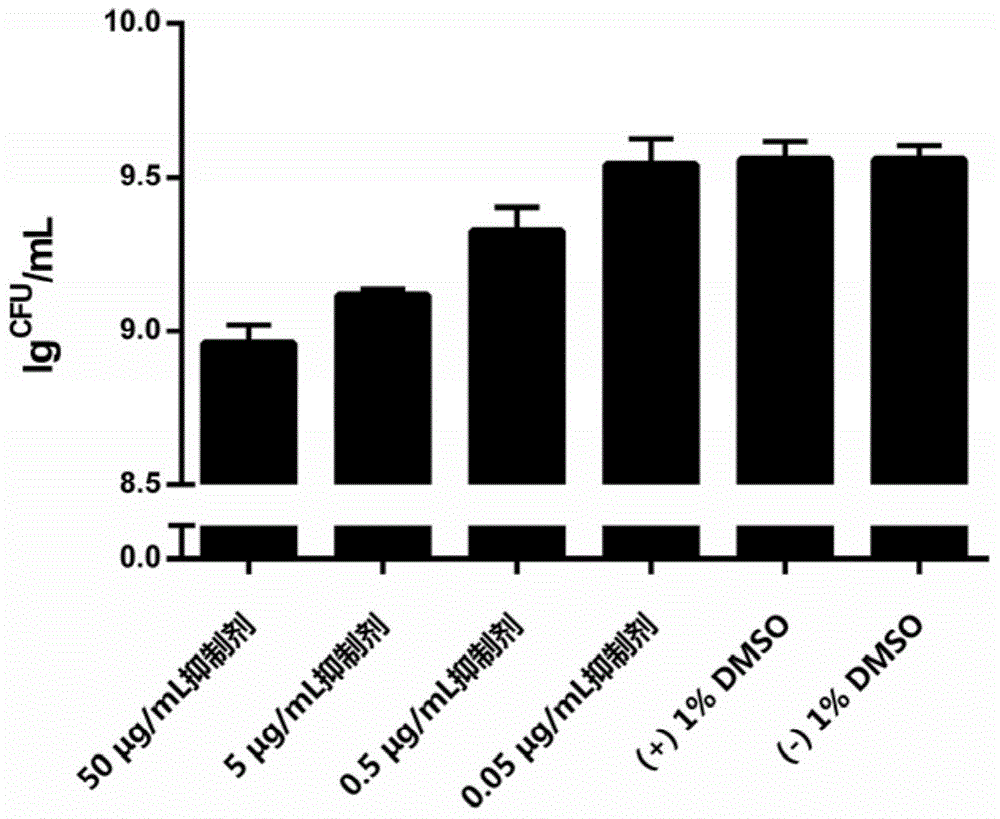
Isolongifolene alkyl oxazolone as well as synthesis method and application thereof
The invention discloses isolongifolene alkyl oxazolone as well as a synthesis method and an application thereof. The molecular formula of the isolongifolene alkyl oxazolone is C18H27N302, the molecular weight is 317.21, the physical state of the isolongifolene alkyl oxazolone is a white solid, and m.p. is 202 DEG C. The synthesis method comprises steps as follows: isolongifolanone is taken as a raw material and subjected to condensation through semicarbazide hydrochloride, and then semicarbazone is obtained; then the isolongifolene alkyl oxazolone is obtained through ring formation under the condition of reflux of absolute ethyl alcohol. The isolongifolene alkyl oxazolone shows good activity on inflammation elimination of cells and is a potential anti-inflammatory compound.
Owner:扬州胜宁信息技术有限公司
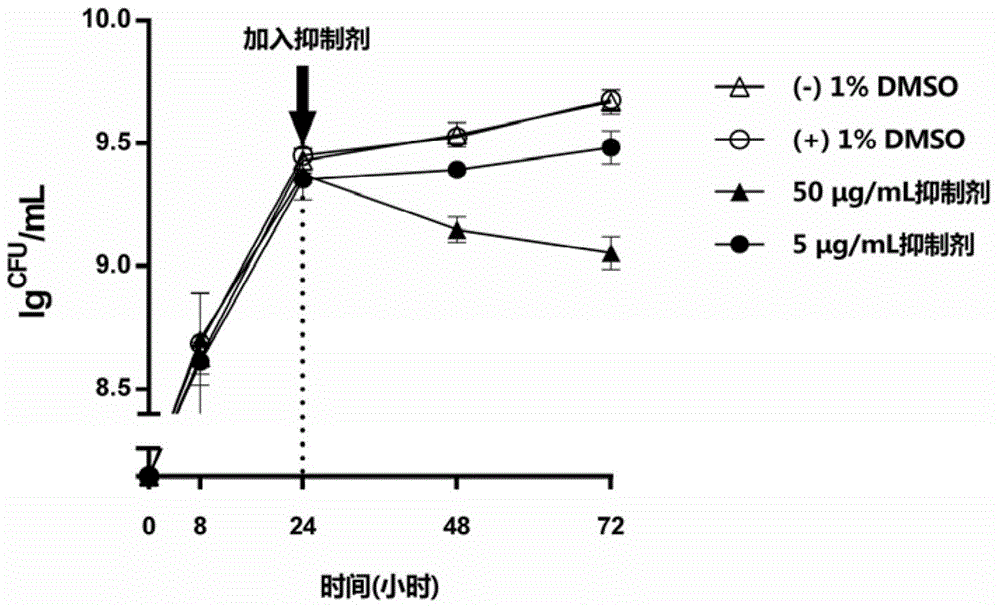
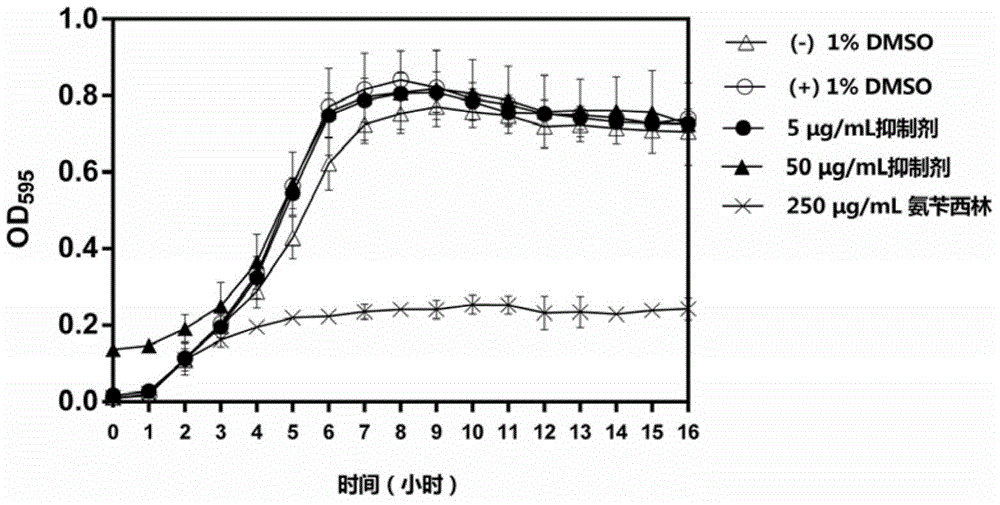
Application of oxazolone compound as streptococcus mutans biofilm inhibitor
ActiveCN104622871ASmall molecular weightSimple structureAntibacterial agentsOrganic active ingredientsBiofilmSphaerotrichia divaricata
The invention discloses an application of an oxazolone compound as a streptococcus mutans biofilm inhibitor, which belongs to the field of screening technologies of antibacterial drugs. The chemical name of the compound is 2-(4-chlorphenyl)-4-{[(6-methyl-2-pyridyl) amino] methylene}-1,3-oxazole-5((tetrahydro)-ketone, and an inhibitor formed by dissolving the oxazolone compound in DMSO can inhibit the formation and maturation of streptococcus mutans biofilms. The inhibitor has the advantages of small molecular weight, simple structure, good specificity and the like, can significantly inhibit the formation and maturation of biofilms of streptococcus mutans-a main pathogenic bacterium of caries, but has an unobvious influence on streptococcus mutans in a floating state. The inhibitor has an anti-caries potential and can be used as a candidate targeted drug of novel anti-caries strategies.
Owner:SICHUAN UNIV
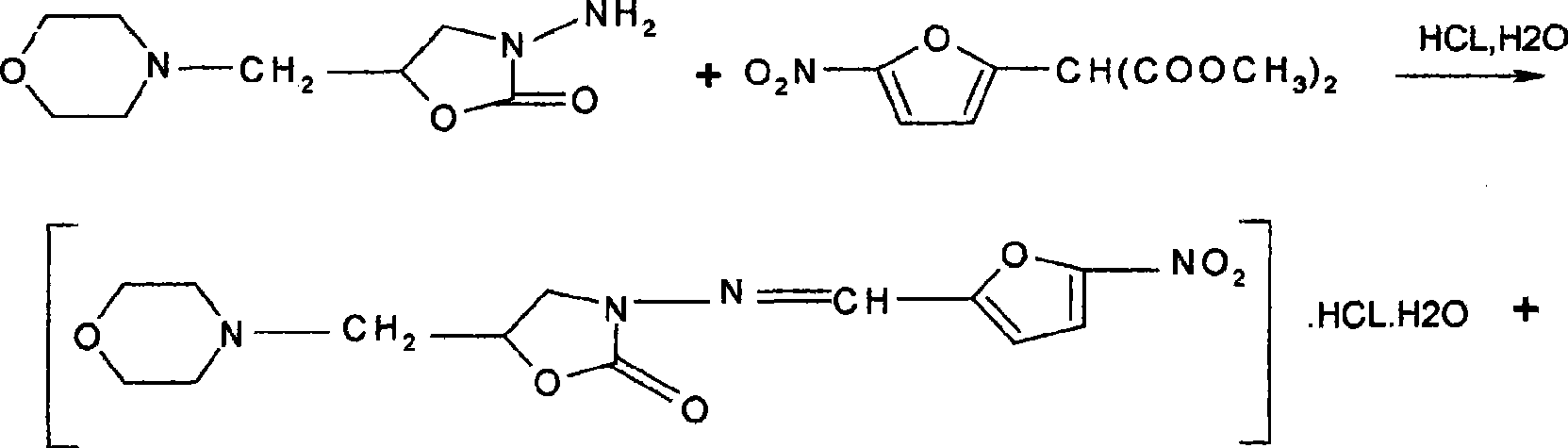
Process for preparing furaltadone hydrochloride
The invention belongs to the field of organic synthesis, and in particular relates to a process for manufacturing furaltadone hydrochloride. The technical proposal comprises the following step a condensation reaction of 5-nitro frfural diacetate and morpholinomethylene amino oxazolone is performed under the hydrochloric acid condition to generate the furaltadone hydrochloride, wherein the weight ratio of the 5-nitro frfural diacetate to the morpholinomethylene amino oxazolone is 55:95. The process has the advantages that the process has high yield, and the reaction yield is between 85 and 90 percent (calculated the morpholinomethylene amino oxazolone); and the process simplifies the operation, and reduces a two-step reaction to a one-step reaction.
Owner:SHANDONG FANGXING SCI & TECH DEV
Serine derived chiral amine compound as well as preparation method and application thereof
ActiveCN103360270AStrong synergyEfficient StereoselectivityOrganic compound preparationThiol preparationAlkanePtru catalyst
The invention discloses a serine derived chiral amine compound as well as a preparation method and application thereof. The chiral amine compound contains a primary amine, a secondary amine or a tertiary amine functional group and has the structural formula as shown in the specification; and chiral amine and salts thereof are prepared through simple preparation steps by taking common chiral serine as the raw material to form the chiral amine compound. The chiral amine and the salts thereof can be used for catalyzing the asymmetrical Michael additive reaction between alpha, beta-unsaturated ketone and nitroalkanes, malonic ester, mercaptan, substituted rhodanine, substituted hydantoin or substituted oxazolone and the cyclopropanation between the alpha, beta-unsaturated ketone and sulfur ylide; the serine derived chiral amine compound has very high catalytic activity and stereoselectivity as well as the highest diastereoselectivity of 30 / 1 and the highest enantioselectivity of 99%, and is wide in oligomer range; and the serine derived chiral amine compound disclosed by the invention is a catalyst with high efficiency as well as good selectivity and controllability.
Owner:EAST CHINA UNIV OF SCI & TECH
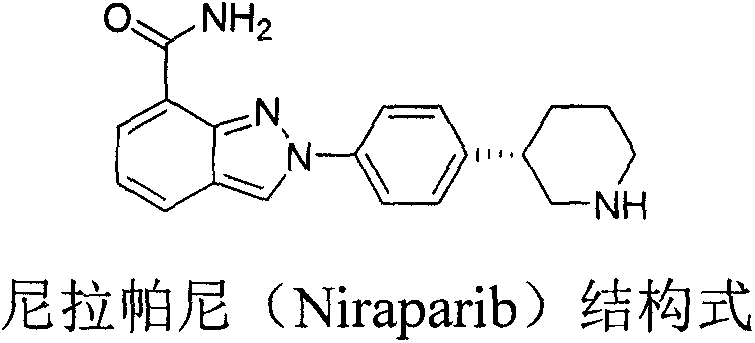
Preparation method of chiral intermediate of niraparib
ActiveCN107311911AStable in natureRaw materials are easy to getOrganic chemistryHydrolysisIntramolecular cyclization
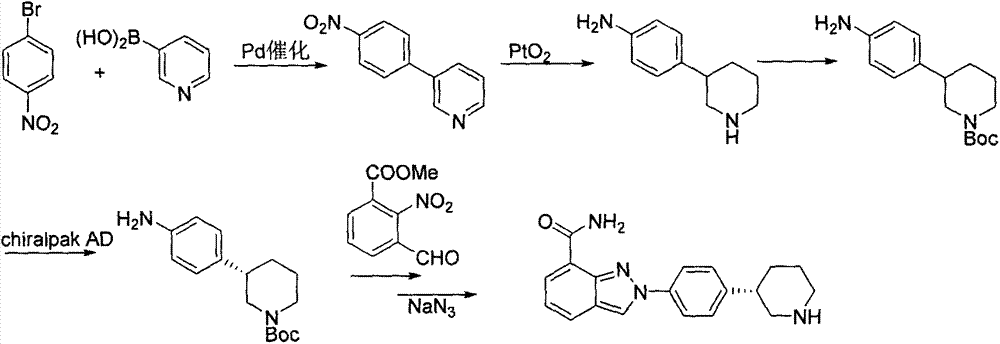
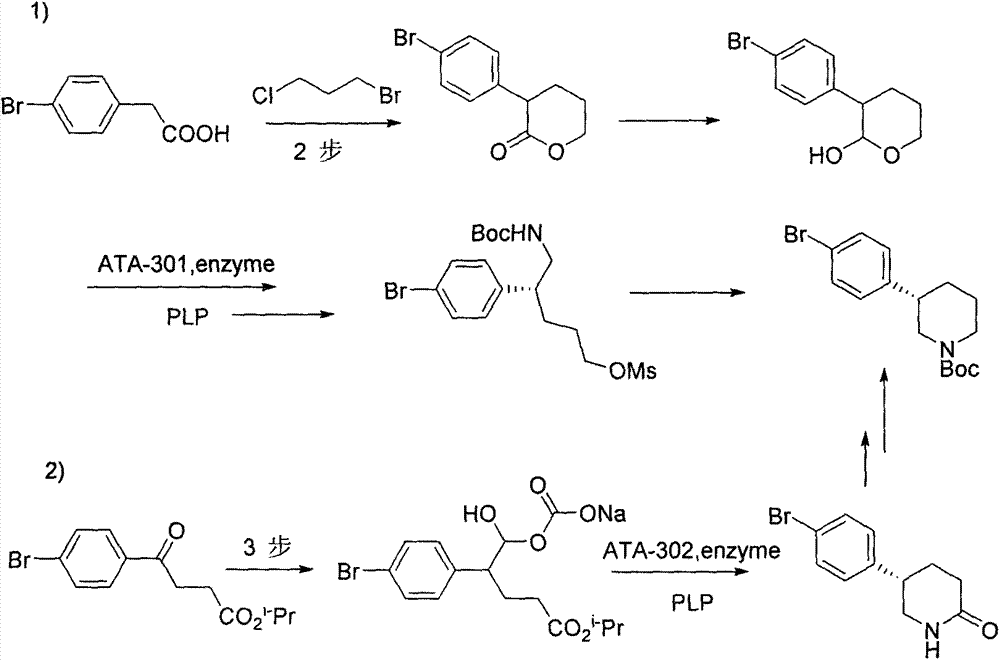
The invention discloses a novel synthetic method for preparing a chiral intermediate of niraparib. The method comprises the following steps: taking 4-bromophenylacetic acid and chiral substituted oxazolone as starting materials; and carrying out amide condensation, Michael addition, hydrolysis, reduction and intramolecular cyclization to obtain the intermediate (VII). The preparation method is low in cost, raw materials are easily obtained, the yield is high, and the synthetic method is suitable for industrialized production.
Owner:钟桂发
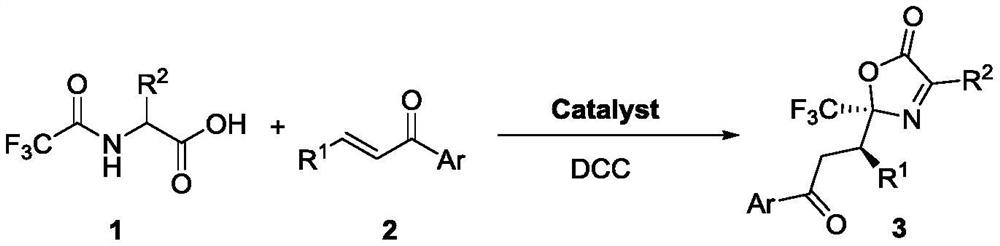
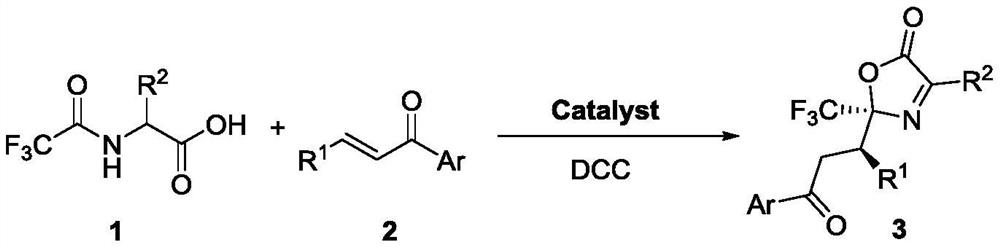
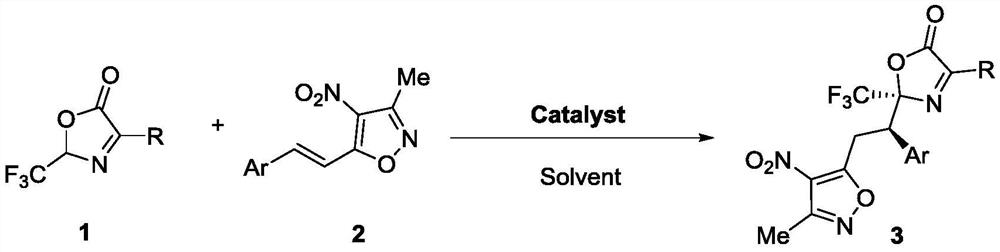
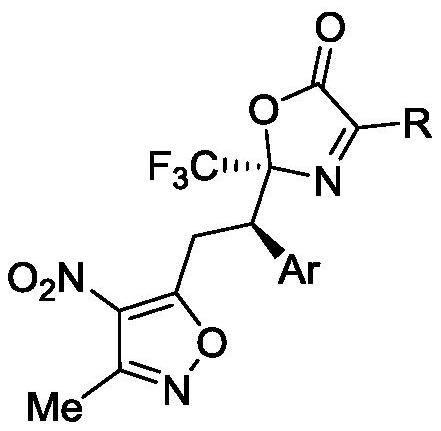
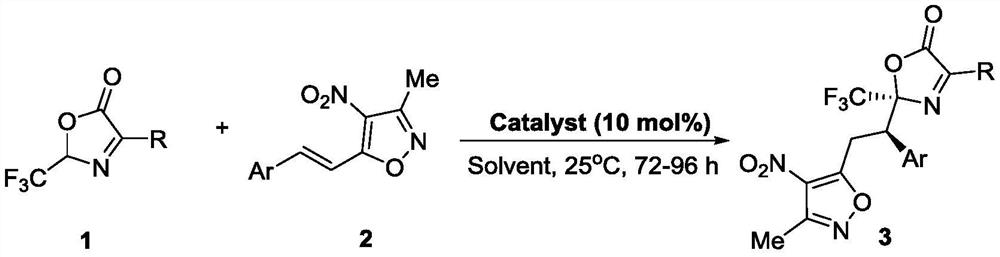
Method for synthesizing trifluoromethyl-containing oxazolone compound by one-pot method
ActiveCN112898218AHigh optical purityShorten the timeOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsMeth-Ptru catalyst
The invention discloses a method for synthesizing trifluoromethyl-containing oxazolone compounds by a one-pot method, and belongs to the field of organic chemistry. N-trifluoroacetyl amino acid (1) and alpha, beta-unsaturated ketone (2) are subjected to a one-pot high-stereoselectivity and high-enantioselectivity reaction under the action of a thiourea catalyst and DCC to obtain the trifluoromethyl oxazolone compound (3). According to the one-pot reaction, the time can be shortened, many tedious steps are omitted, the trifluoromethyl-containing oxazolone compound is obtained, and the trifluoromethyl-containing oxazolone compound has high optical purity and high stereoselectivity.
Owner:HENAN NORMAL UNIV
Oxazolone compound with bactericidal effect, and preparation method thereof
InactiveCN111559985ANovel structureImprove antibacterial propertiesAntibacterial agentsOrganic chemistryStaphyloccocus aureusAcetophenone
The invention discloses an oxazolone compound with bactericidal activity, and a preparation method thereof, and belongs to the technical field of synthesis of antibacterial drugs. The oxazolone compound is characterized in that the oxazolone compound has a structure shown in the description. 3-hydroxyacetophenone and 4-methylbenzaldehyde which are used as initial raw materials undergo a four-stepreaction to obtain the oxazolone compound with the novel structure, the synthesis method is simple, and the reaction yield is very high. An antibacterial activity test is carried out through an oxfordcup agar diffusion method, it is found that the inhibition effect of the target compound on Escherichia coli is much better than that of bixicillin, the inhibition effect on Staphylococcus aureus isclose to that of bixicillin, and the target compound has the potential of serving as a broad-spectrum antibacterial drug.
Owner:THE FIRST AFFILIATED HOSPITAL OF HENAN UNIV OF SCI & TECH
Trifluoromethyl-containing bisoxazole compound, synthesis method thereof and application of trifluoromethyl-containing bisoxazole compound in anti-cancer drugs
ActiveCN112898285AHigh stereoselectivityHigh optical purityOrganic chemistry methodsAntineoplastic agentsThioureaStomach cancer
The invention discloses a trifluoromethyl-containing bisoxazole compound, a synthesis method thereof and application of the trifluoromethyl-containing bisoxazole compound in anti-cancer drugs, and belongs to the field of organic chemistry. 4-substituted-2-trifluoromethyl oxazolone (1) and aryl alkenyl oxazole (2) are subjected to a high-stereo and high-enantioselectivity reaction under the catalysis of a thiourea catalyst to obtain the trifluoromethyl-containing bisoxazole compound (3). The compound has certain inhibition effects on liver cancer, lung cancer, stomach cancer, esophageal cancer, breast cancer and ovarian cancer. According to the method, the raw materials are easy to obtain, the conditions are mild, the reaction efficiency is high, the reaction path is short, and the obtained trifluoromethyl-containing bisoxazole compound product has high stereoselectivity and high optical purity.
Owner:HENAN NORMAL UNIV
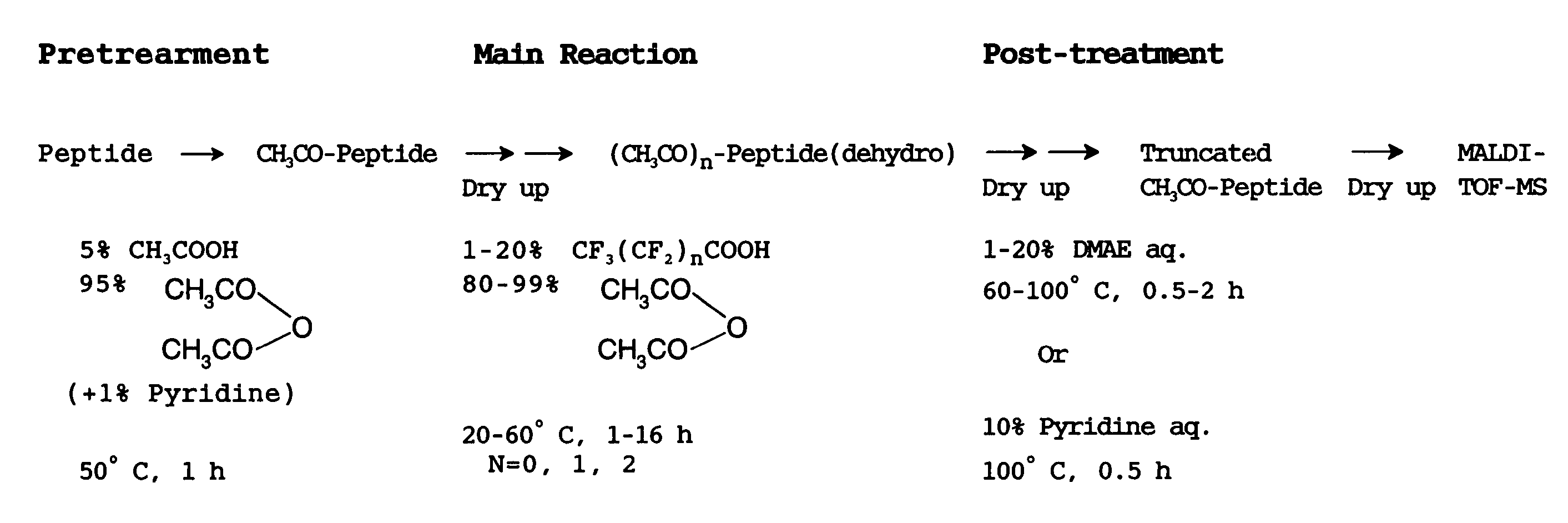
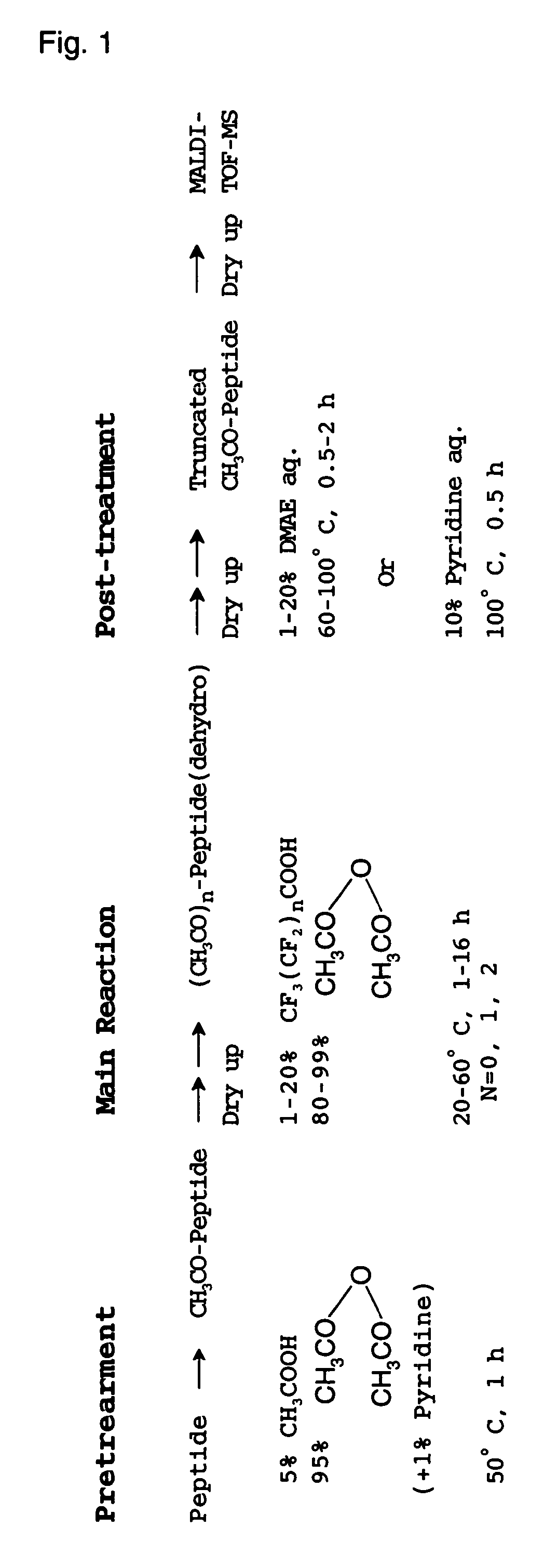
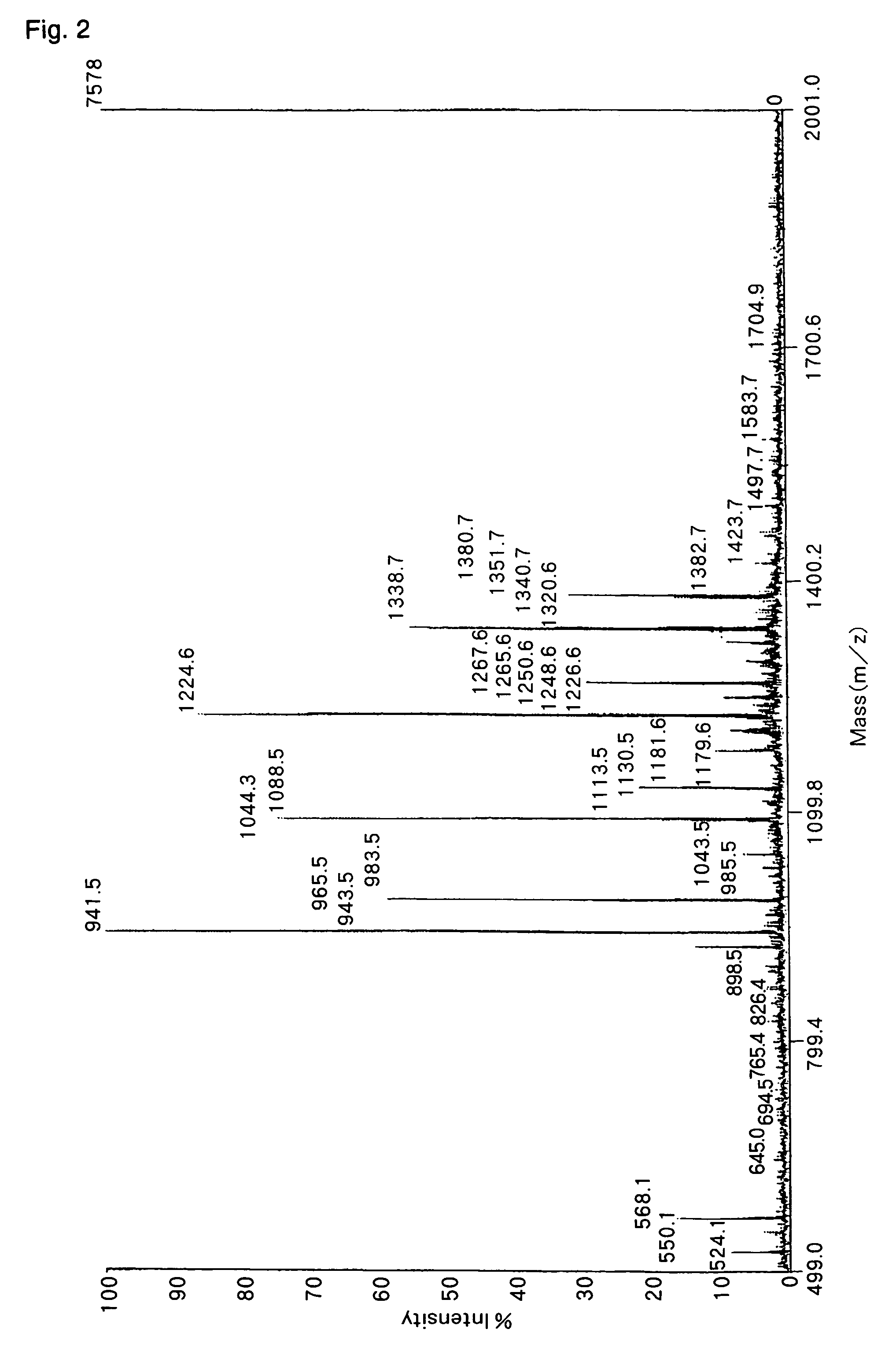
Method of analyzing peptide for determining C-terminal amino acid sequence
InactiveUS7384790B2Inhibition is effectiveHigh possibility that undesired side reactions are escalated therebyMicrobiological testing/measurementBiological testingChemical treatmentGas phase
The present invention provides a method for analyzing the C-terminal amino acid sequence of a peptide by applying reaction technique for successively releasing the C-terminal amino acids therefrom, which method can suppress, when releasing the C-terminal amino acids of the peptide in sequence, such as an undesirable side reaction as cleavage of peptide bond in the intermediate position of the peptide, and allows to carry out the chemical treatment thereof under widely applicable conditions. In the method according to the present invention, an alkanoic acid anhydride and a perfluoroalkanoic acid both of vapor phase, which are supplied from a mixture containing an alkanoic acid anhydride with a small amount of a perfluoroalkanoic acid added thereto, are allowed to act on a dry sample of the peptide to be examined in a dry atmosphere at a temperature chosen in a range of 15 to 60° C.; whereby the release of the C-terminal amino acid is resulted from successive formation of a 5-oxazolone structure being followed by cleavage of the 5-oxazolone ring; and then the C-terminal amino acids sequence is identified by analysis based on the decrease in molecular weight in a series of the reaction products obtained.
Owner:NEC CORP
Preparation method of 7-amide indole compound
ActiveCN111892528AReaction raw materials are cheap and easy to obtainInexpensive and easy to operateOrganic chemistryPivalic acidPtru catalyst
The invention discloses a preparation method of a 7-amide indole compound. The method comprises the following steps of: in an HFIP solvent, synthesizing the 7-amide indole compound at room temperatureby using [Ru (p-cymene) Cl2] 2 and AgSbF6 as catalysts, using pivalic acid as an additive and using an indole compound and an oxazolone compound as substrates. The reaction raw materials are cheap and easy to obtain; the preparation method is simple; ruthenium is used as the catalyst, so that the reaction cost is low, the yield is high, the operation is simple; and the method is suitable for synthesizing different types of 7-amide indole compounds. The method disclosed by the invention can be used for synthesizing a series of 7-amide indole compounds, and the synthesized product can be used as an intermediate compound for further constructing a complex active compound; and meanwhile, the compound has great drug activity potential.
Owner:WENZHOU MEDICAL UNIV
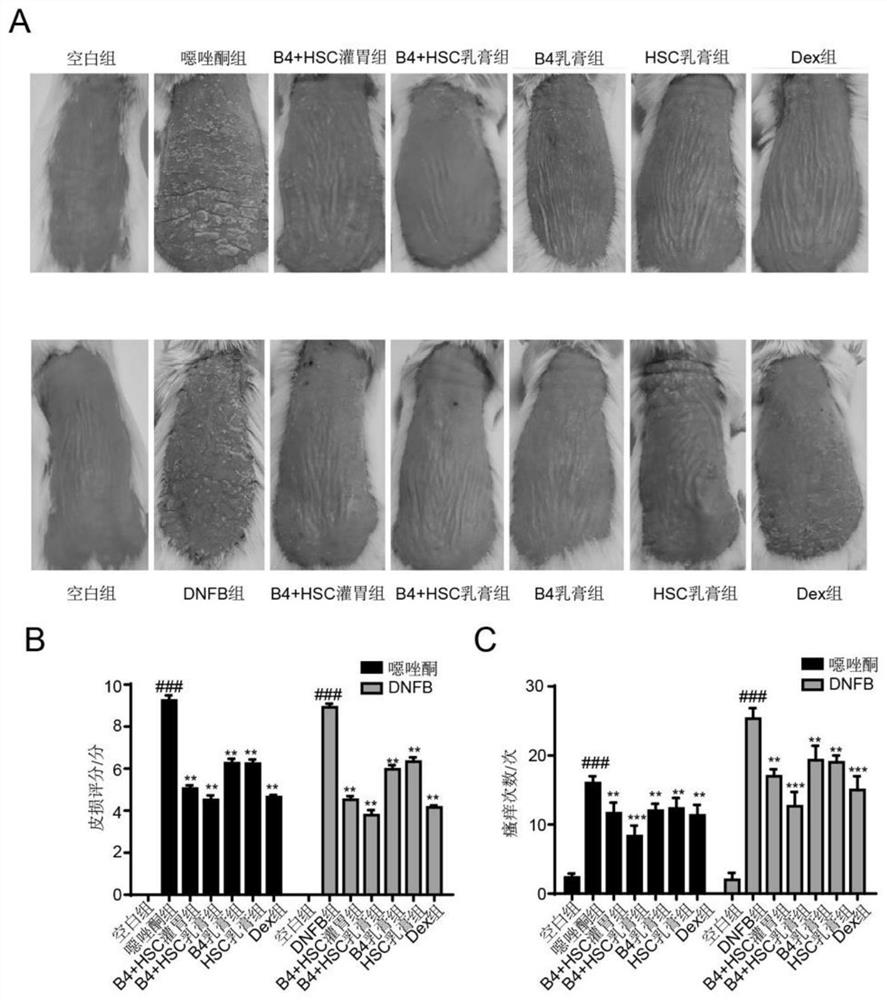
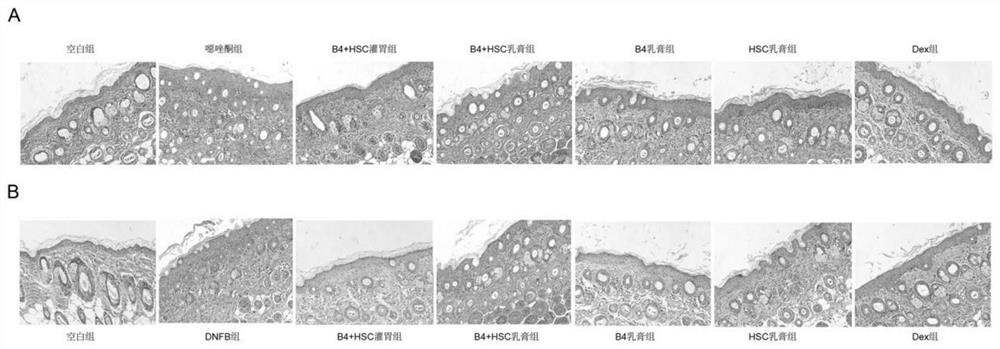
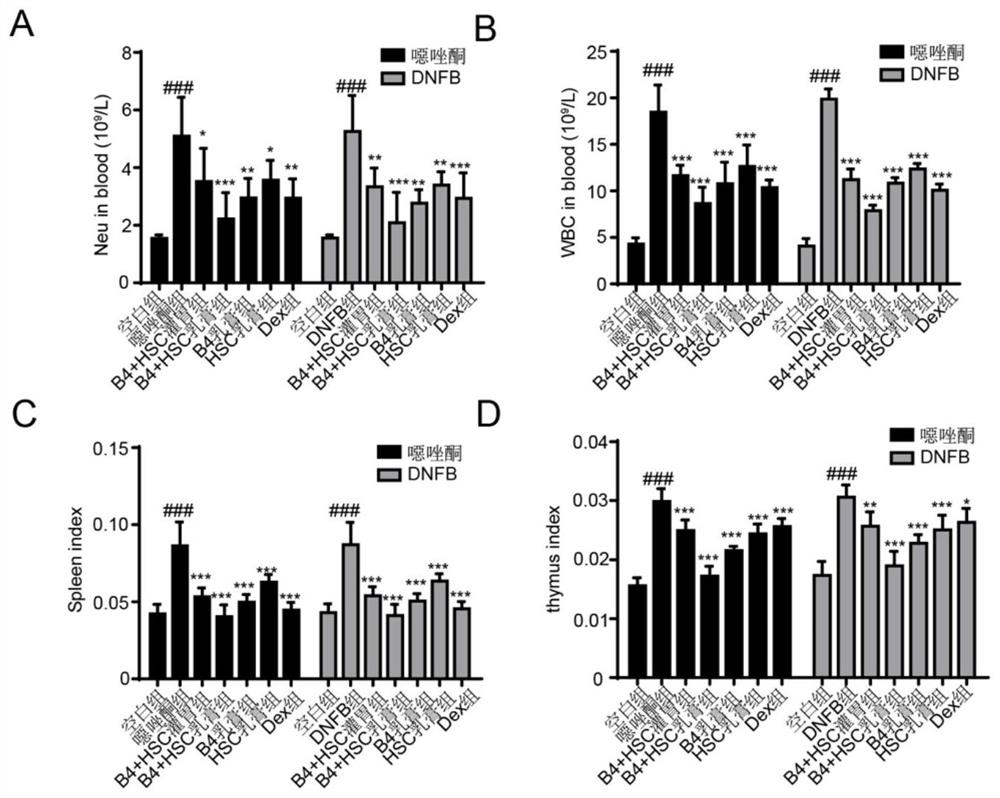
Pulsatilla saponin composition and application thereof in preparation of medicine for treating skin diseases
PendingCN114796248AGood treatment effectCombined drug effect is excellentOrganic active ingredientsAntipyreticDiseasePharmacology
The invention discloses a pulsatilla saponin composition. The pulsatilla saponin composition comprises pulsatilla saponin B4 and pulsatilla saponin B5 in a weight ratio of (3-9): 1. The invention further discloses a medicine containing the pulsatilla saponin composition and application of the medicine. According to the application disclosed by the invention, oxazolone and 2, 4-dinitrofluorobenzene (DNFB) are respectively utilized to prepare two specific dermatitis mouse models (Atopics, AD), imiquimod is utilized to prepare a mouse psoriasis model, the protective effects and possible mechanisms of pulsatilla saponin B4 and pulsatilla saponin B5 on skin diseases are discussed, and a theoretical basis is provided for treating the skin diseases.
Owner:广西馨海药业科技有限公司
Anti-radiation medicine and manufacturing method and application
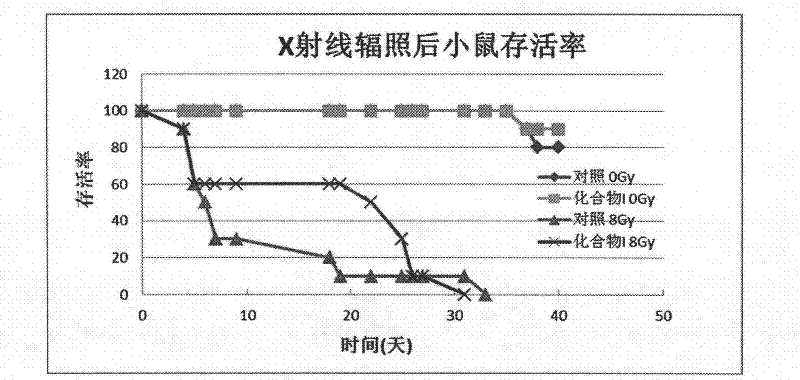
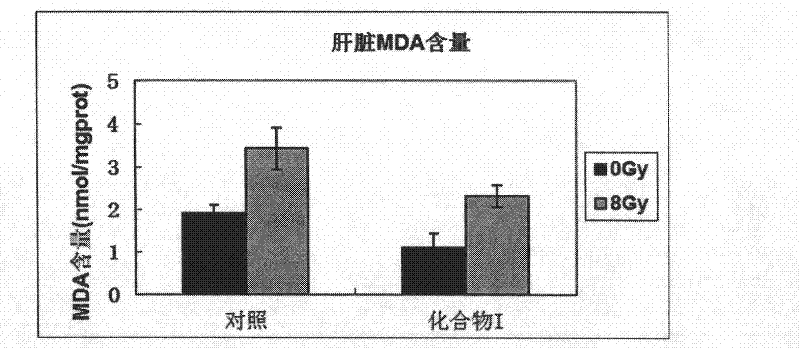
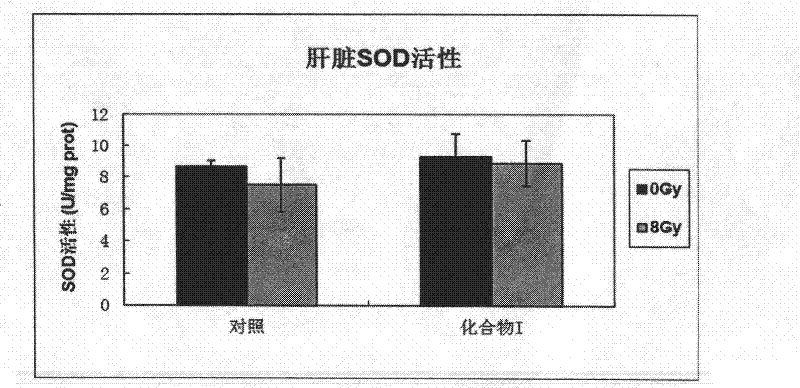
ActiveCN102499918AImprove relative survivalReduce instabilityOrganic active ingredientsOrganic chemistryNon-ionizing radiationCarbon ion
The invention belongs to the medicine technical field, and in particular relates to an anti-radiation medicine in protection of human lung embryo fibroblast and radiation injury in experimental mice. The anti-radiation medicine has a structure of 4-methoxy benzylidene-2-substituted-5(4H)-oxazolone and derivatives thereof and can be expressed in structural formula (A). The invention has the advantages that compound (A) has a significant protection effect for DNA and genome injury to human lung embryo fibroblast (MRC-5) and experimental mice caused by ionizing radiation, such as X-rays, carbon ions, etc., and non-ionizing radiation, such as ultraviolet rays and microwaves; relative survival percentage of cells irradiated by said rays can be increased significantly; DNA injury can be reduced; instability of genome is reduced; MDA (malondialdehyde) yield of mice viscera can be reduced; and SOD (Superoxide Dismutase) activity of mice viscera can be enhanced. The invention has low toxicity,wide application range and good application prospect.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
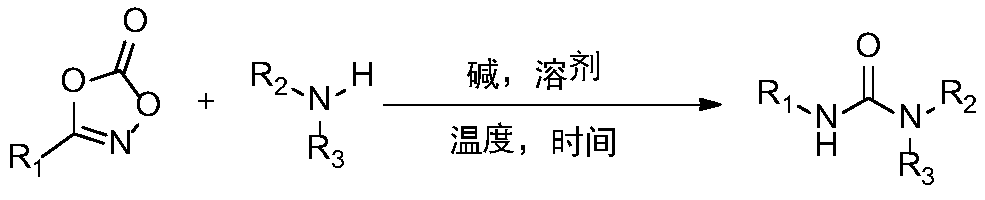
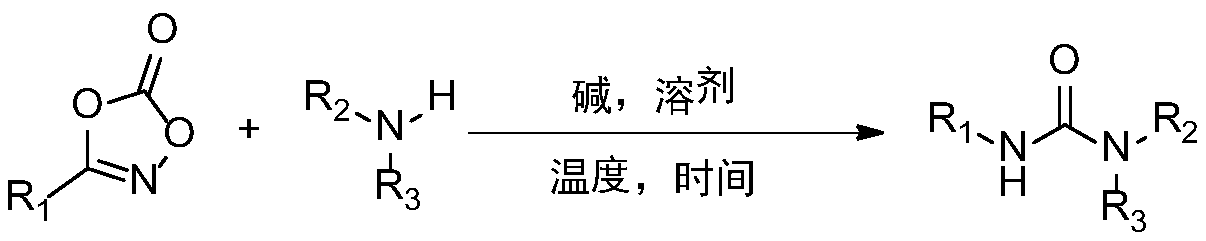
Synthetic method and application of urea compound
InactiveCN109776244ALow costSimple processUrea derivatives preparationOrganic compound preparationSodium acetateEnvironmental resistance
The invention relates to a synthetic method of a urea compound, comprising the following steps: adding substituted oxazolone and sodium acetate into a methanol solution, and adding substituted amine under the stirring condition, reacting and carrying out column chromatography to obtain the urea compound. The defect that dangerous compounds need to be used during existing synthetic process is overcome, and a one-pot method is adopted to replace an existing reaction with low yield. The method of the invention has mild reaction condition, the operation is simple, raw materials are easily available, and the substrate can be converted into various other useful molecules. The compound has strong practicality, and can be applied to synthesis of the pesticide daimuron, dieresis long and the anti-cancer drug Sorafenib. The invention relates to a green and environmentally-friendly unsymmetrical urea compound synthesis method with simple process and low cost.
Owner:ZHEJIANG UNIV
Preparation method of N-substituted oxazolone polymer derivatives
The invention relates to a preparation method of N-substituted oxazolone polymer derivatives. An available reagent boron trifluoride diethyl ether is taken as a catalyst and influences dimerization and cascading dimerization / cyclization among N-substituted oxazolone molecules under the mild reaction condition. The method is insensitive to water in a reaction system, the same reaction can be performed under the condition of water, and the same productive rate and speed are kept. The novel reaction condition and the efficient transformation mode can be more applied to synthesis of various molecules and industrial production.
Owner:CHONGQING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com