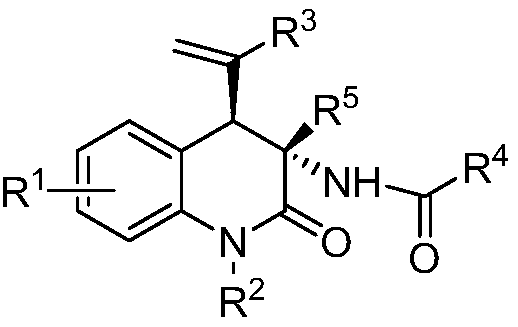
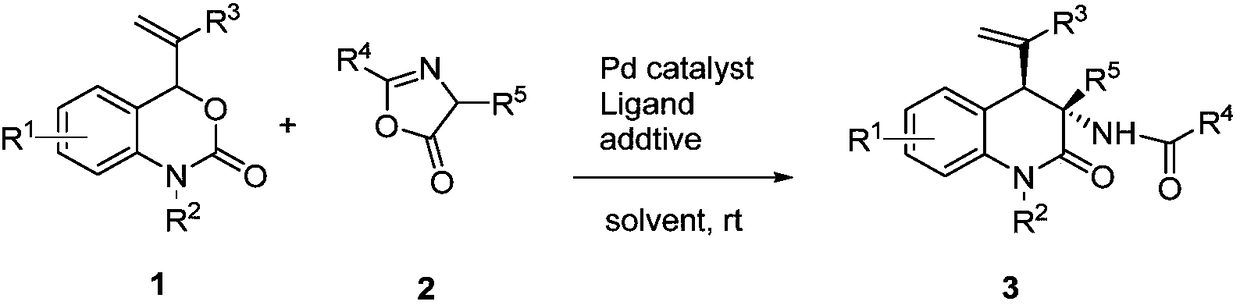
Chiral 3, 4-dihydrogen-2(1H)-quinolinone compound and preparation method
A technology for quinolinones and compounds, which is applied in the field of chiral 3,4-dihydro-2-quinolinones and their preparation, and achieves the effects of a wide range of substrates, convenient and easy-to-obtain raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
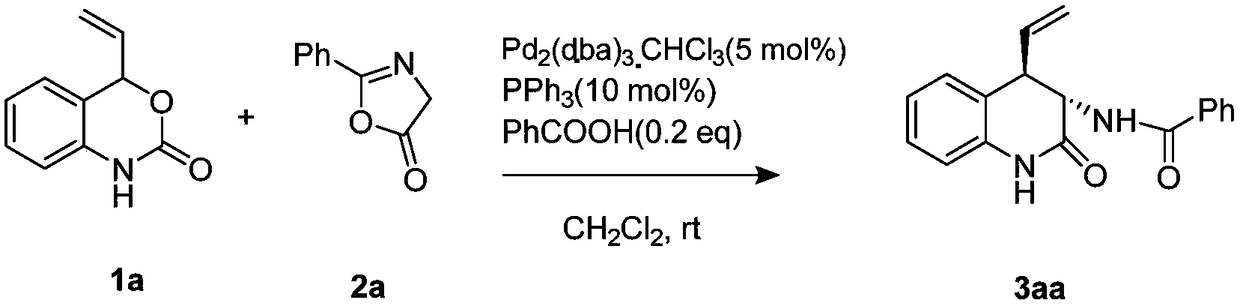
[0034] Weigh 1a (17.5mg, 0.1mmol), 2a (19.2mg, 0.12mmol), palladium catalyst (5.2mg, 0.005mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1.0mL of dichloromethane, added benzoic acid (2.4mg, 0.02mmol) and stirred for 4 hours (detected the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluted The solvent is selected as petroleum ether / ethyl acetate mixed solution with a volume ratio of 1:1) to obtain the target product 3aa (23.9 mg), with a yield of 82%.
[0035] Characterization and analysis of the target object: yellow solid, 1 H NMR (400MHz, CDCl 3 ):δ8.72(s,1H),7.84–7.82(m,2H),7.55–7.50(m,1H),7.46–7.42(m,2H),7.23-7.20(m,2H),7.09-7.05 (m,1H),6.95(d,J=8.2,1H),6.87-6.85(m 1H),6.04-5.95(m,1H),5.38(dd,J=10.0Hz,1H),5.26(dd, J=17.2Hz, 1H), 5.00(dd, J=13.2 1H), 3.69(dd, J=13.2 1H) ppm; HRMS(ESI) m / z: C 18 h 16 N 2 o 2 [M+H] + The theoretical calculation value is 293.1285, and the measured ...
Embodiment 2
[0037]
[0038] Weigh 1b (19.3mg, 0.1mmol), 2a (19.2mg, 0.12mmol), palladium catalyst (5.2mg, 0.005mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1.0mL of dichloromethane, added benzoic acid (2.4mg, 0.02mmol) and stirred for 4 hours (detected the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluted The solvent is selected as petroleum ether / ethyl acetate mixed solution with a volume ratio of 1:1) to obtain the target product 3ba (21.7 mg), with a yield of 70%.
[0039] Characterization and analysis of the target object: yellow solid, 1 H NMR (400MHz, CDCl 3):δ8.86(s,1H),7.85-7.83(m,2H),7.56-7.51(m,1H),7.49-7.44(m,2H),7.20-7.16(m,1H),6.86(d ,J=8.0,1H),6.77(td,J=8.4,1H),6.63(dd,J=9.2,1H),6.01-5.92(m,1H),5.40(dd,J=10.0Hz,1H) ,5.28(d,J=17.2Hz,1H),4.99(m,1H),3.66(dd,J=13.2 1H)ppm; HRMS(ESI)m / z:C 18 h 15 FN 2 o 2 [M+H] + The theoretically calculated value is 311.1190, and the measured value is 311...
Embodiment 3
[0041]
[0042] Weigh 1c (20.9mg, 0.1mmol), 2a (19.2mg, 0.12mmol), palladium catalyst (5.2mg, 0.005mmol) and ligand PPh 3 (2.6mg, 0.01mmol) was dissolved in 1.0mL of dichloromethane, added benzoic acid (2.4mg, 0.02mmol) and stirred for 4 hours (detected the reaction with TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluted The agent is selected as petroleum ether / ethyl acetate mixed solution with a volume ratio of 1:1) to obtain the target product 3ca (17.9 mg), with a yield of 55%.
[0043] Characterization and analysis of the target object: yellow solid, 1 H NMR (400MHz, CDCl 3 ):δ8.62(s,1H),7.83(d,J=7.6,2H),7.56-7.52(m,1H),7.50-7.44(m,2H),7.17(d,J=8.0 1H), 7.07(d, J=7.21H), 6.90(s, 1H), 6.81(d J=8.0 1H), 6.02-5.93(m, 1H), 5.43(d, J=10.0Hz, 1H), 5.00(dd ,J=17.2Hz,1H),5.05-4.98(m,1H),3.70-3.65(m,1H)ppm; HRMS(ESI)m / z: C 18 h 15 ClN 2 o 2 [M+H] + The theoretically calculated value is 327.0895, and the measured value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com