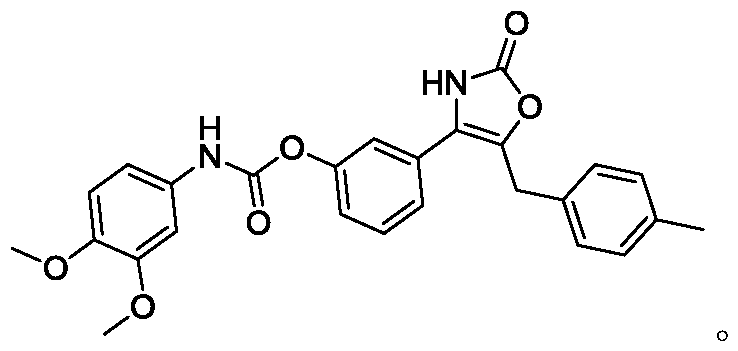
Oxazolone compound with bactericidal effect, and preparation method thereof
A compound, the technology of oxazolone, which is applied in the field of oxazolone compounds and their preparation, can solve the problems of difficult clinical treatment and difficult to effectively control the infection of new drug-resistant bacteria, and achieve good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
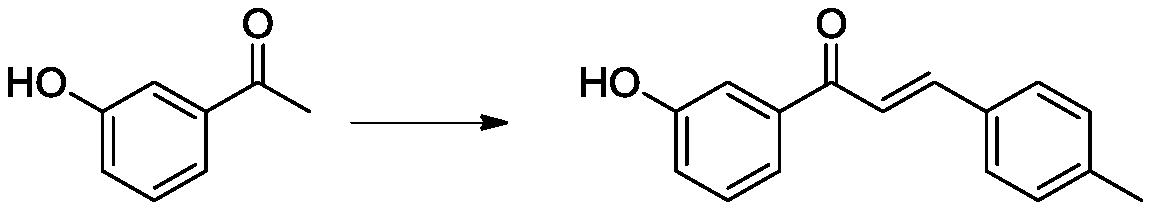
[0024] In a reaction flask with a water separator, add 13.5g of 3-hydroxyacetophenone into 150mL of toluene, stir and dissolve, add 12g of 4-methylbenzaldehyde and 11g of sodium methoxide, and slowly raise the temperature to reflux. The water generated during the reaction was discharged through the water separator. After 3 hours of reaction, TLC monitored the complete reaction of the raw materials. Under vacuum conditions, 50 mL of toluene was evaporated under reduced pressure, and then the reaction liquid was poured into 200 mL of water, and the pH of the reaction liquid was adjusted to medium properties, and then extracted 3 times with dichloromethane 100mL, combined the organic phases, and separated and purified by silica gel column chromatography to obtain 1-(3-hydroxyphenyl)-3-(tolyl)-2-enyl-1-ketone 18.1 g; LC-MS (ESI): m / z 239 [M+H] + .
Embodiment 2
[0026]In a reaction flask with a water separator, add 13.5g of 3-hydroxyacetophenone into 150mL of toluene, stir and dissolve, add 12g of 4-methylbenzaldehyde and 5.5g of sodium methoxide, slowly raise the temperature to reflux, during the reflux reaction process Drain the moisture generated in the reaction process through the water separator, keep the reflux reaction for 11h, distill 50mL of toluene under reduced pressure under vacuum conditions, then pour the reaction solution into 200mL of water, adjust the pH of the reaction solution to be neutral by dilute hydrochloric acid, and then use Dichloromethane 100mL was extracted 3 times, and the organic phases were combined, and then separated and purified by silica gel column chromatography to obtain 9.4g of 1-(3-hydroxyphenyl)-3-(tolyl)-2-enyl-1 ketone; LC- MS(ESI):m / z 239[M+H] + .
Embodiment 3
[0028] In the reaction flask, add 13.5g of 3-hydroxyacetophenone into 200mL of methanol, stir and dissolve, add 12g of 4-methylphenylacetaldehyde and 11.5g of potassium hydroxide, slowly raise the temperature to reflux, and concentrate the reaction solution after reflux reaction for 15h , after adding water 250mL, extracted several times with ethyl acetate 50mL, combined the organic phases, washed with saturated brine, concentrated and separated by silica gel column chromatography to obtain compound 1-(3-hydroxyphenyl)-3-(tolyl )-2-enyl-1-ketone 14.5g; LC-MS (ESI): m / z 239[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com