Trifluoromethyl-containing bisoxazole compound, synthesis method thereof and application of trifluoromethyl-containing bisoxazole compound in anti-cancer drugs
A technology of trifluoromethylbisoxazole and anticancer drugs, which is applied in the field of asymmetric synthesis, and can solve the problems of limited and reduced carbanion nucleophilicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
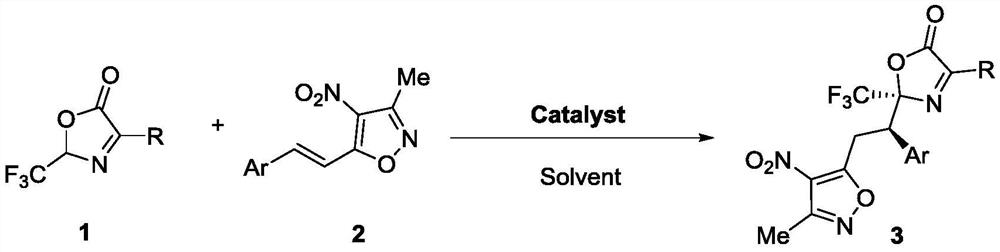
[0029]
[0030] The synthetic steps of racemic compound 3 are as follows:
[0031] Add compound 1 (1.5eq, 0.15mmol), catalyst (0.1eq, 0.01mmol), 1mL solvent and compound 2 (1.0eq, 0.1mmol) into the reaction flask, react at 25°C for 72-96h, pass through the thin-layer plate (TLC) to monitor the reaction process, after the reaction is completed, spin the solvent to dry, and separate by column chromatography (the eluent is PE:EA=400:1-200:1, then mixed with DCM according to 4:1) to obtain Racemic compound 3. The catalyst is selected from organic or inorganic bases such as triethylamine, tetramethylguanidine, DBU, and sodium hydroxide. The solvent is selected from solvents such as halogenated alkanes, nitriles, ethers, benzenes, etc. The amount of the solvent only affects the reaction rate and has no other effects. In addition, this reaction can not only be carried out at normal temperature, but also can be carried out at low temperature and high temperature.
Embodiment 2
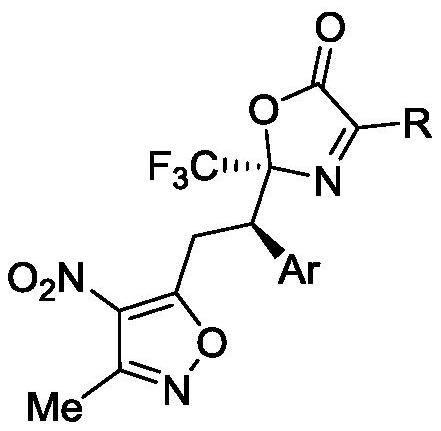
[0033]
[0034]
[0035]
[0036] a Unless otherwise stated, 1a (0.075mmol), Cat. (0.005mmol), 2a (0.05mmol), solvent (0.5mL). b separation yield. c The dr value was obtained by hydrogen spectrum analysis of the crude product. d The ee value was obtained by chiral column HPLC chiral analysis.
[0037]In the screening process of reaction conditions, the influence of different chiral catalysts on the reaction was first investigated (entries1-13), and it was found that C10 was the best chiral catalyst. In addition, in addition to the catalysts listed in the above table, there are many catalysts used Products with different ee values can also be obtained, such as amino acid derivatives, quinines, quinidines and other alkaloid catalysts. Subsequently, the influence of different solvents on the reaction was investigated (entries 14-25), and pentafluorobenzene was finally determined to be the best solvent, and then the C10 enantiomer configuration C11 was used as the ...
Embodiment 3
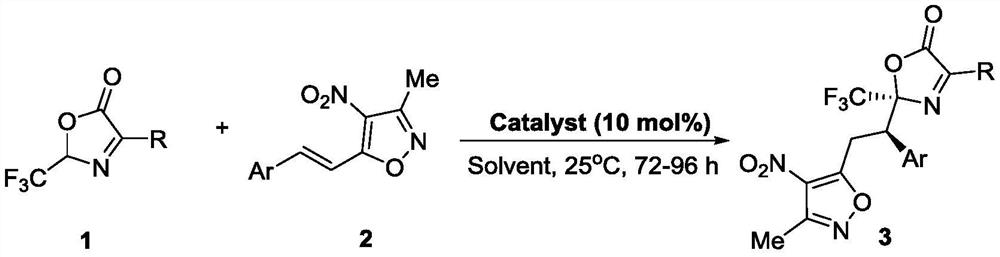
[0041]
[0042] Compound 3b synthesis steps are as follows:
[0043] Add compound 1a (0.15mmol, 1.5eq), catalyst C10 (0.01mmol, 0.1eq), 1mL pentafluorobenzene and compound 2b (0.1mmol, 1.0eq) into the reaction flask, react at 25°C for 96h, and pass through a thin-layer plate (TLC) to monitor the reaction process, after the reaction is completed, spin the solvent to dry, and separate by column chromatography (the eluent is PE:EA=400:1-200:1, then mixed with DCM according to 4:1) to obtain White solid 3b, 89% yield, 94% ee. 1 H NMR (600MHz, CDCl 3 )δ7.53(d, J=8.0Hz, 2H), 7.32(d, J=8.0Hz, 2H), 4.50(dd, J=11.7, 4.8Hz, 1H), 4.03(dd, J=15.5, 11.7 Hz,1H),3.87(dd,J=15.5,4.8Hz,1H),2.44(s,3H),1.03(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com