Optical pure 1,3-alkamine compound as well as preparation method and application thereof in preparing Dapoxetine and analogues thereof
A technology of amino alcohols and compounds, applied in preparation, optically pure 1, can solve the problems of many steps, low efficiency, no ee value data and so on
Active Publication Date: 2010-11-03
ASTATECH CHENGDU BIOPHARM CORP
View PDF4 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, these splitting methods are inefficient and the defects of large waste still exist
There are also some studies on the synthesis of (S)-3-amino-3-phenylpropanol using asymmetric synthesis methods, such as L-diethyl tartrate (Tetrahedron, 2009, 65, 2605-2609) or 1,4-diol (US 6207862) as a raw material, and eventually optically active 1,3-aminoalcohols can be synthesized, but the reaction is complicated, there are many steps, the total yield is low, and there is no ee value data
Taken together, the current methods for synthesizing (S)-3-amino-3-phenylpropanol are not satisfactory, either with high cost, large waste, or insufficient optical purity, and it is necessary to further develop economical and applicable routes
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 13
Embodiment 23
Embodiment 33
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
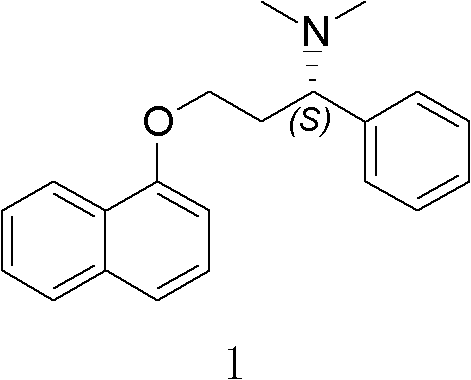
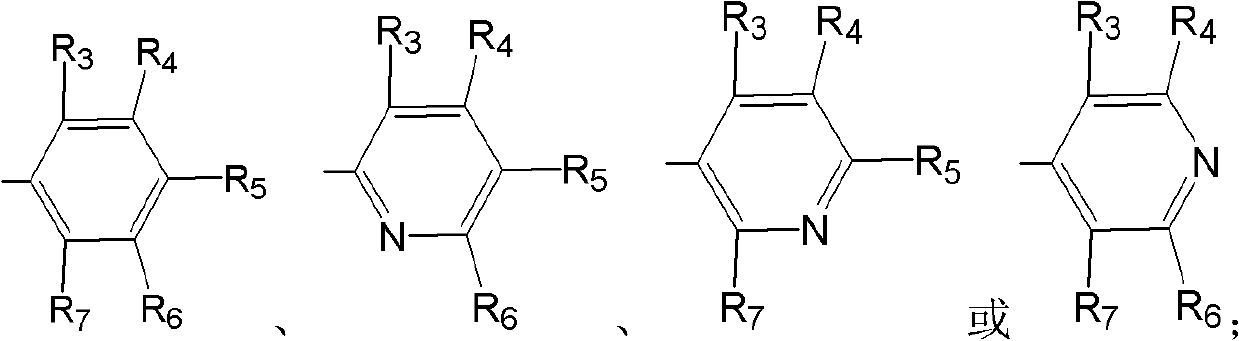
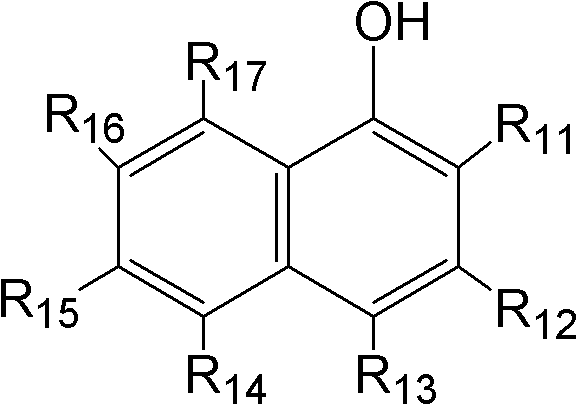
The invention specifically relates to an optical pure 1,3-alkamine compound as well as a preparation method and application thereof in preparing corresponding optical pure 1,3-alkamine and further preparing Dapoxetine and analogues thereof, belonging to the technical field of organic chemistry. The 1,3-alkamine compound is as shown in a formula I.
Description
technical field The invention belongs to the technical field of organic chemistry, and particularly relates to optically pure 1,3-aminoalcohol compounds, a preparation method and an application in preparing dapoxetine and its analogs. Background technique Dapoxetine ((S)-(+)-N, N-Dimethyl-3-(1-naphthyloxy)-1-phenylpropylamine, 1) was originally an antidepressant developed by Eli Lilly Company in 1992. It is a A selective serotonin reuptake inhibitor (SSRI). February 2009 as a drug for the treatment of male premature ejaculation (PE) (Prilig TM ) first launched in Finland and Sweden, which is the world's first oral treatment for this indication. It was listed as one of the five most promising drugs already on the market or under approval by Thomson Reuters (Thomson Reuters) quarterly report on major progress in global drug development in the first quarter of 2009. Optically pure dapoxetine mainly obtains the racemate through different synthetic routes and adopts the met...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F7/18C07C217/48C07C213/06C07C213/08C07C215/28C07C213/00C07C213/02C07C309/73C07C303/28C07C269/04C07C271/16C07D213/40
CPCY02P20/55
Inventor 谢义鹏郭鹏
Owner ASTATECH CHENGDU BIOPHARM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com