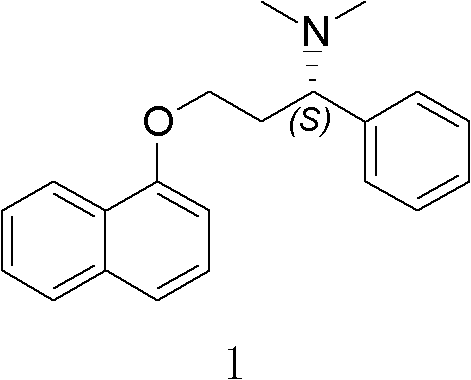
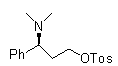
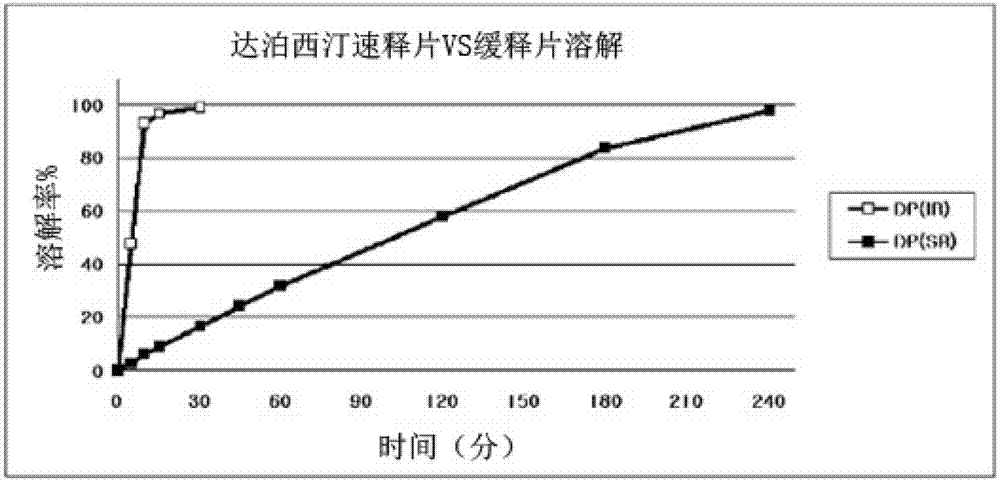
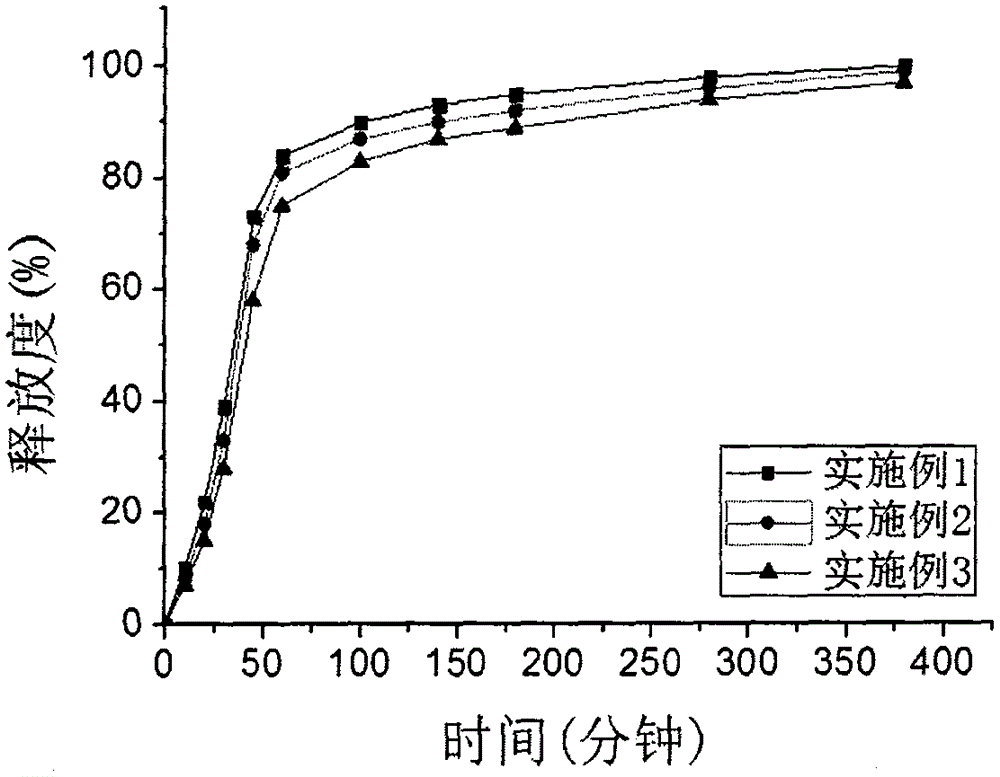
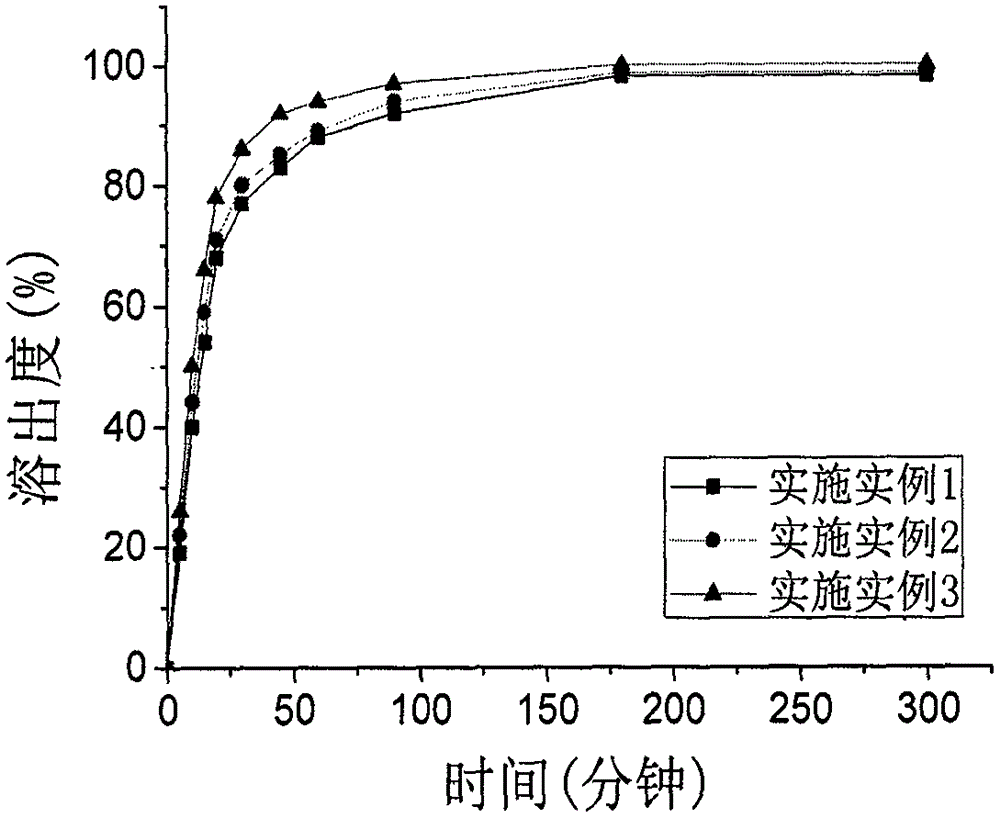
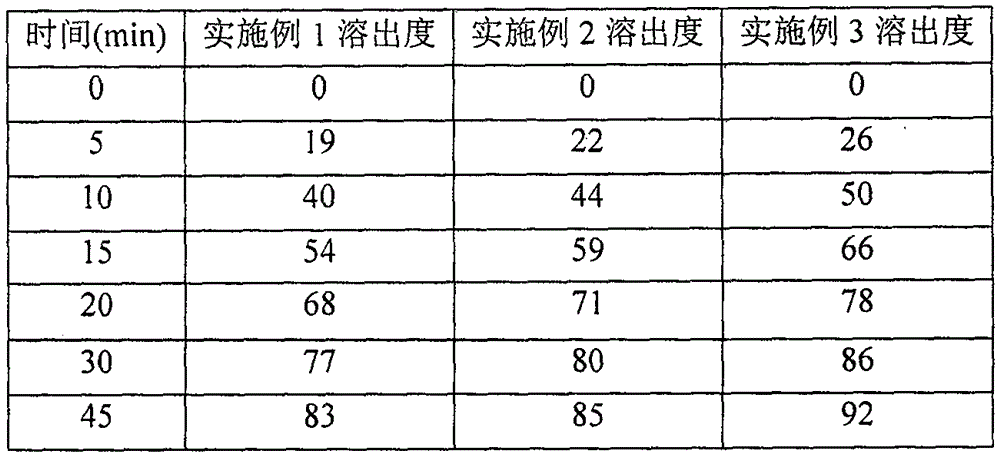
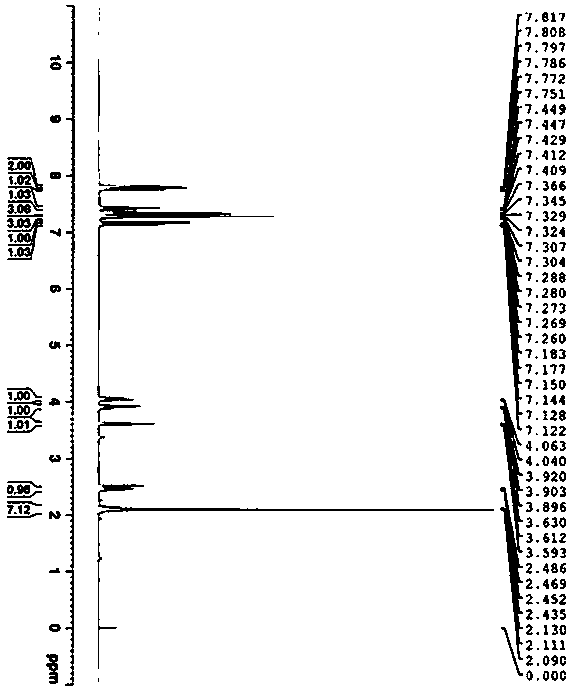
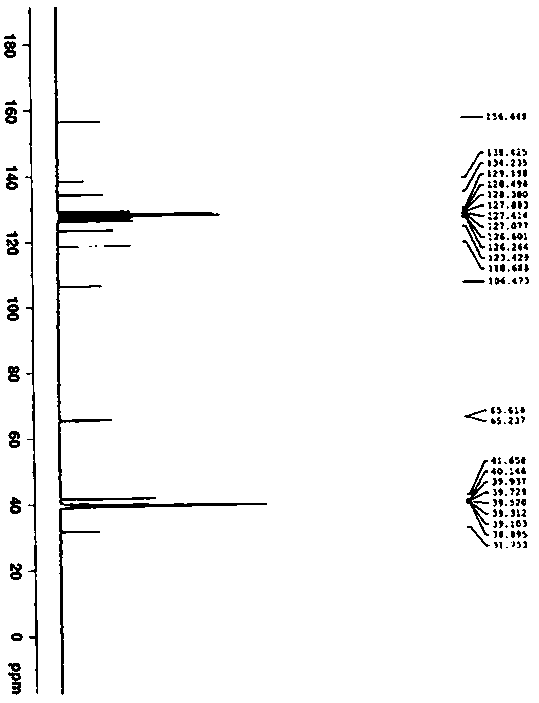
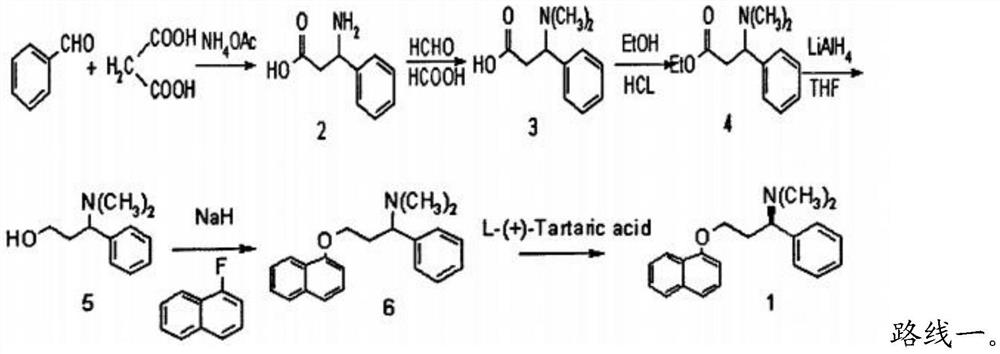
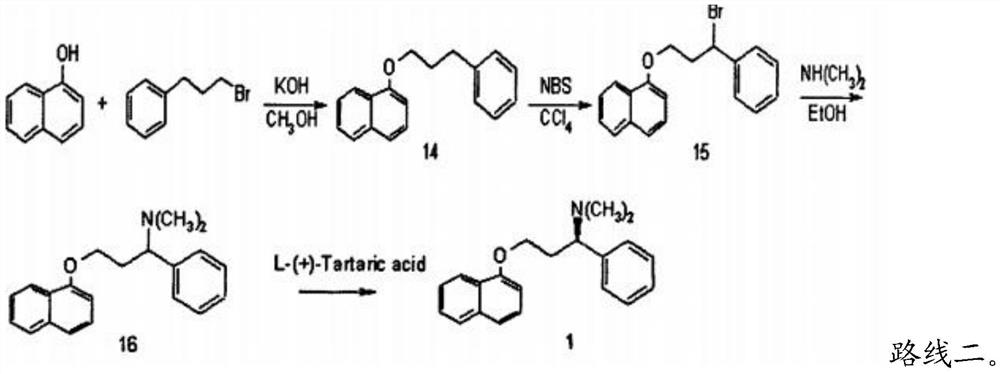
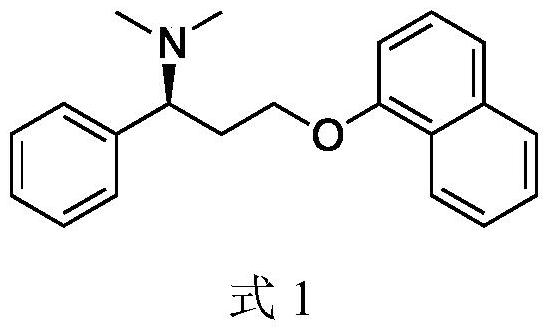
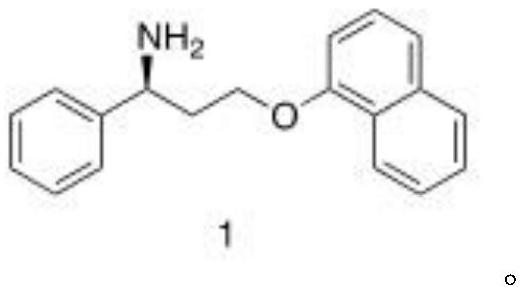
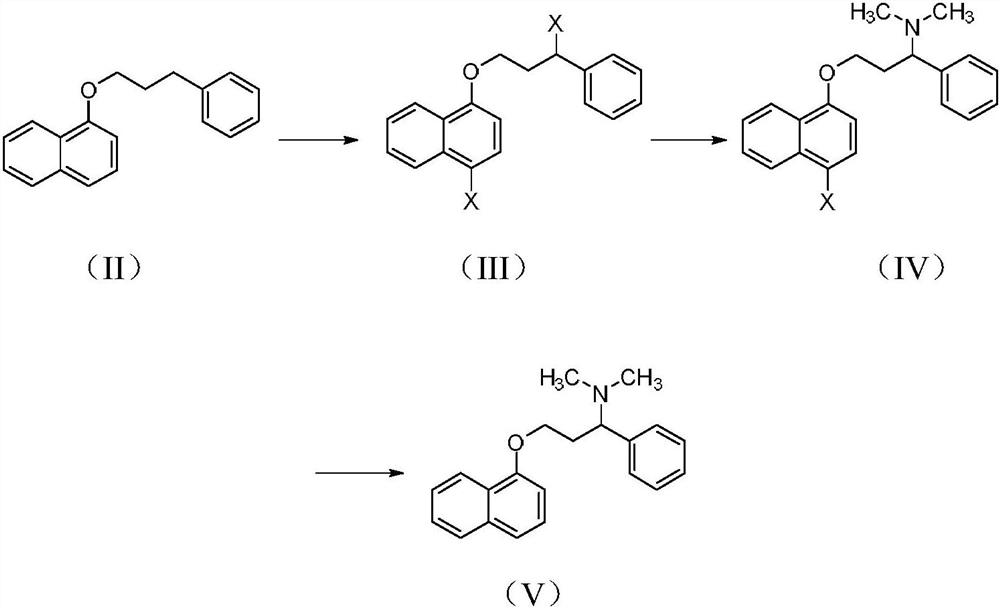
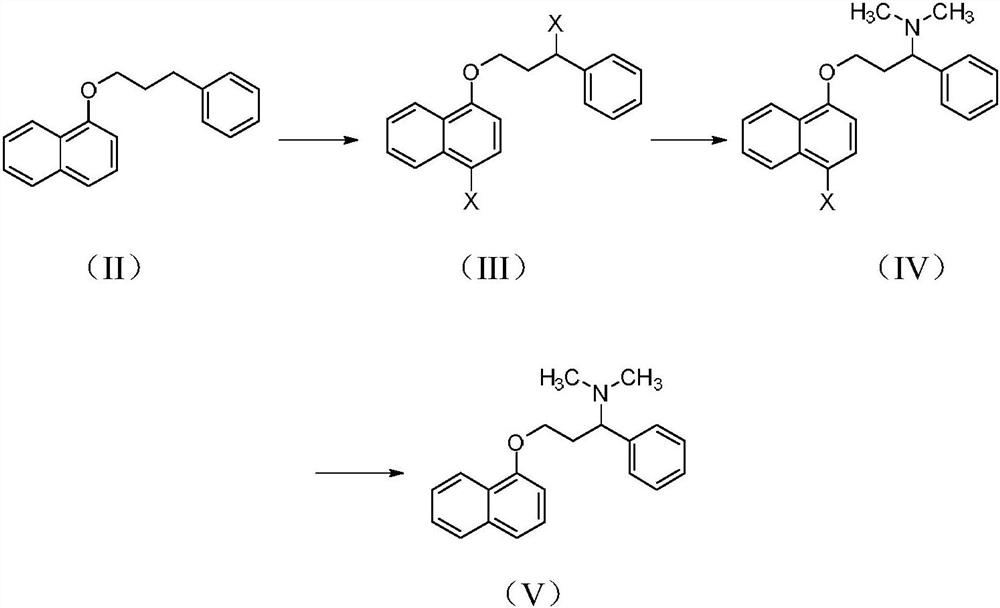
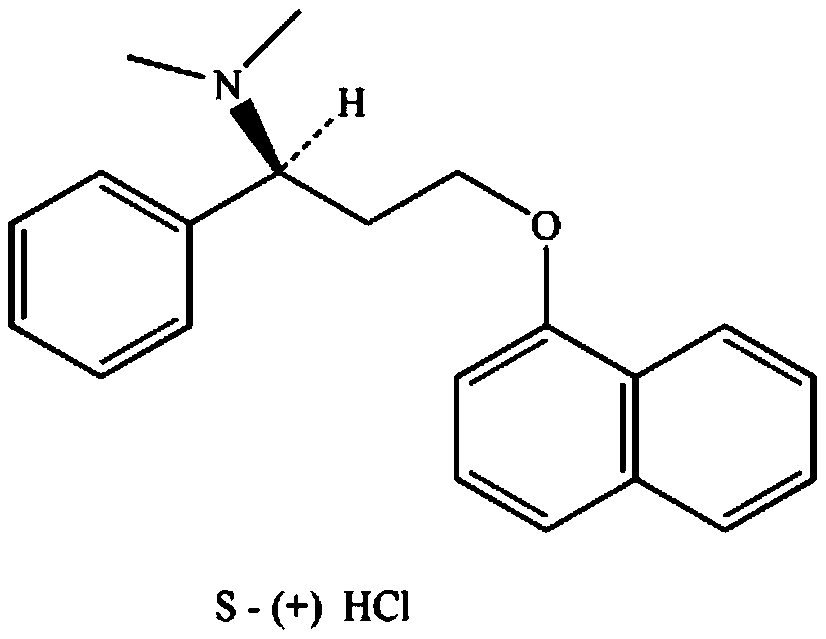
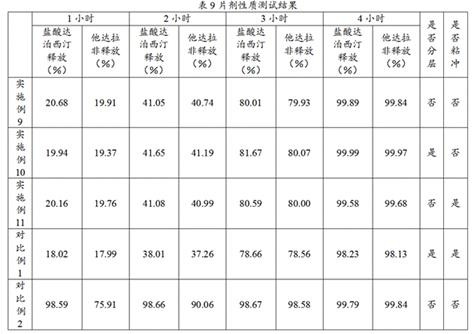
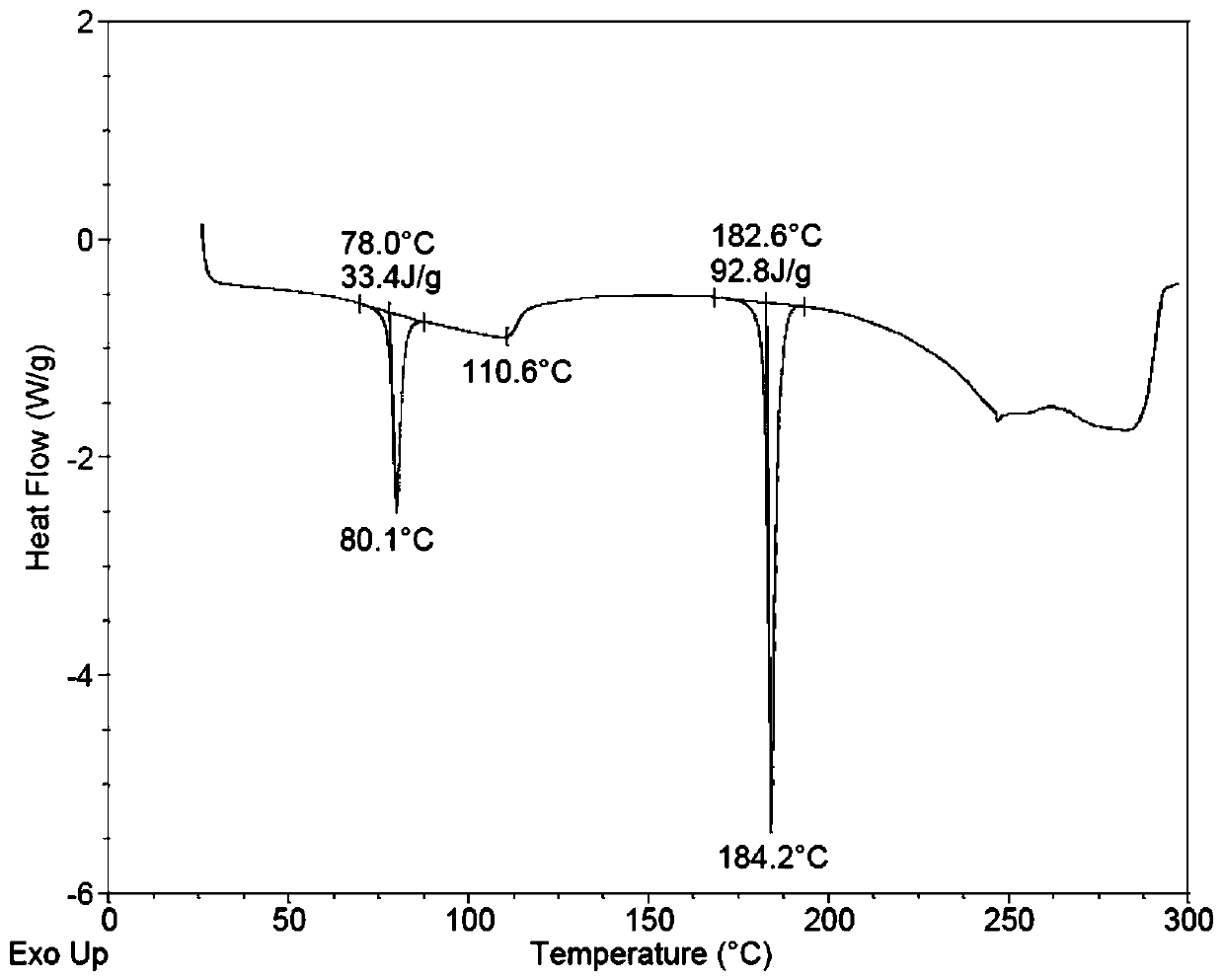
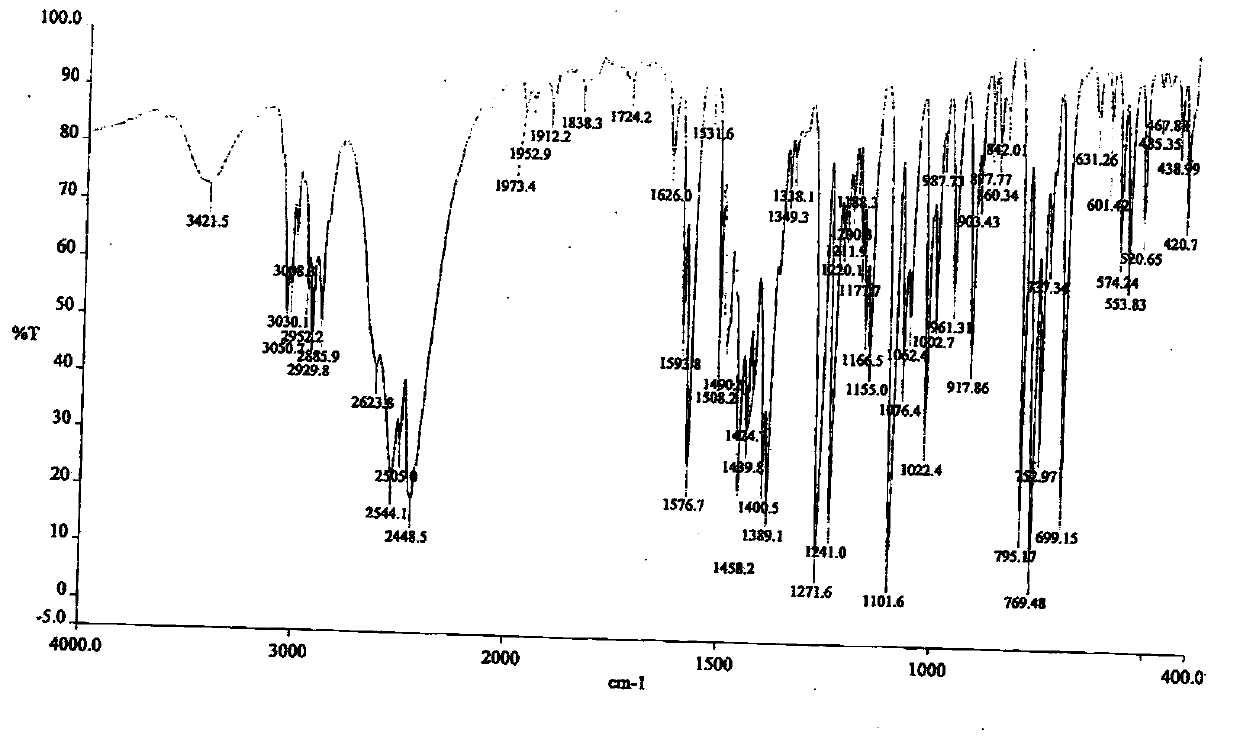
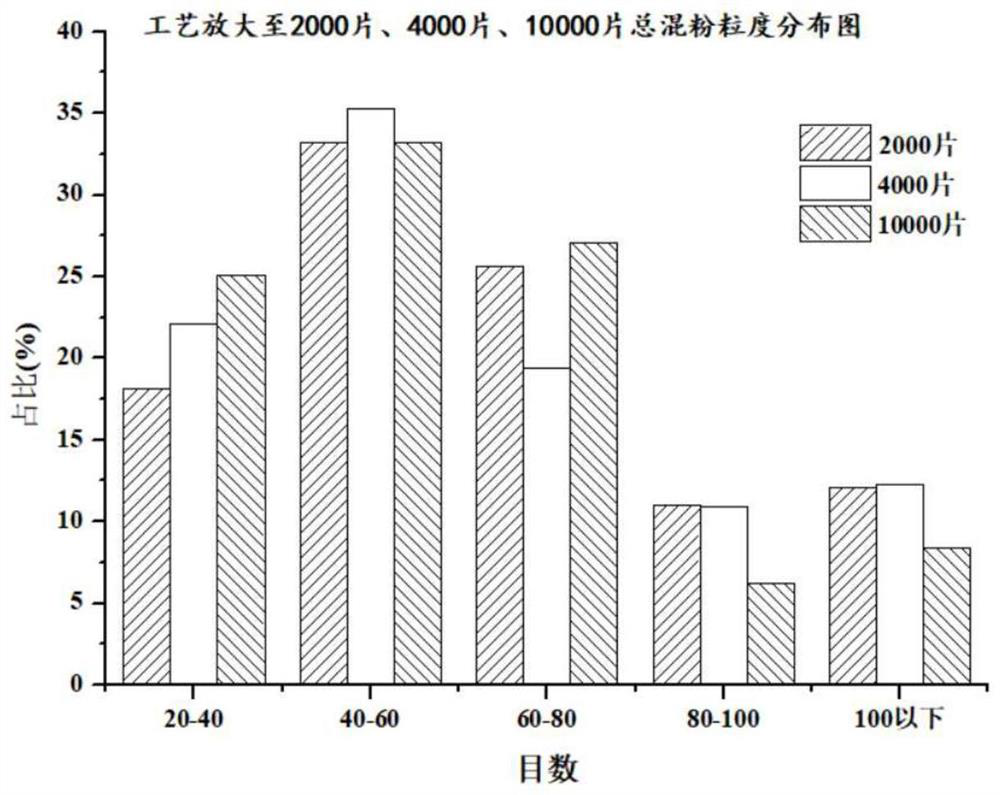
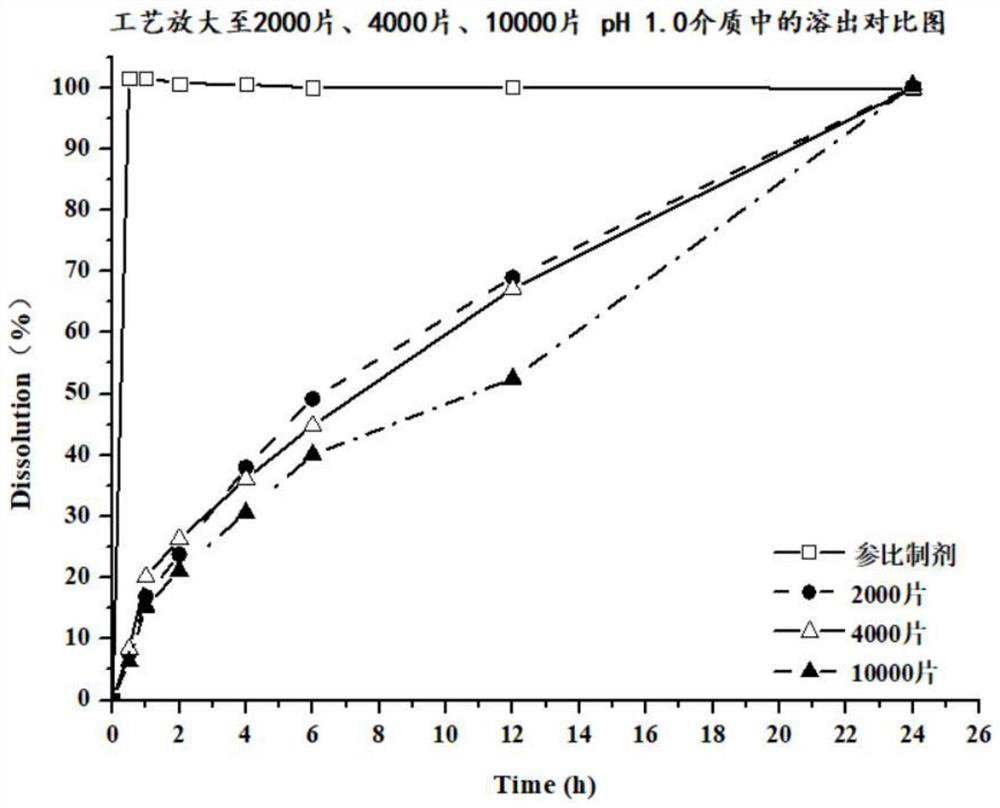
Patents
Literature
52 results about "Dapoxetine-N-oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
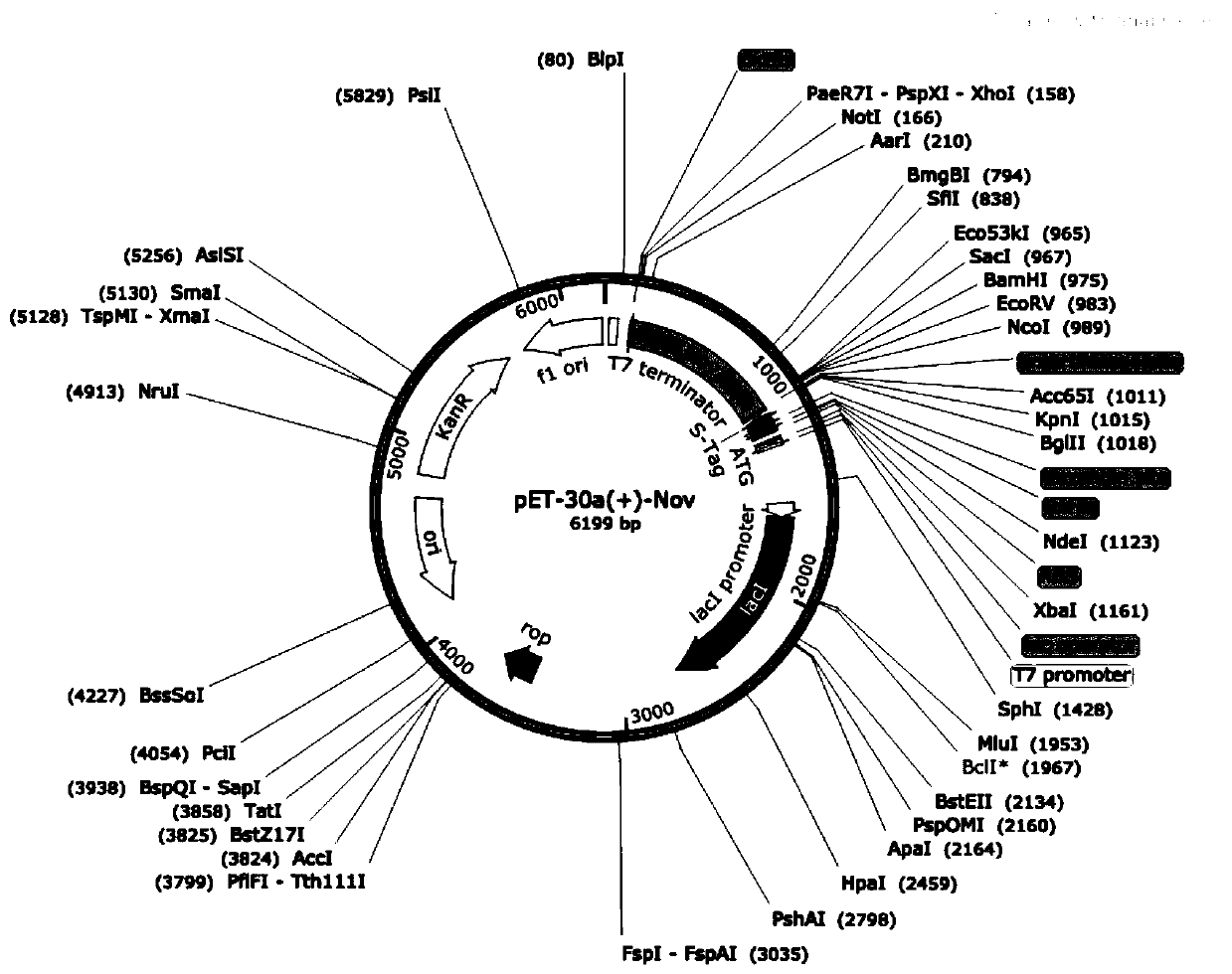
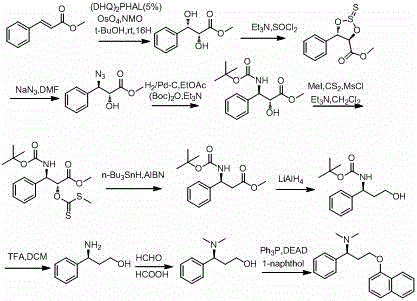
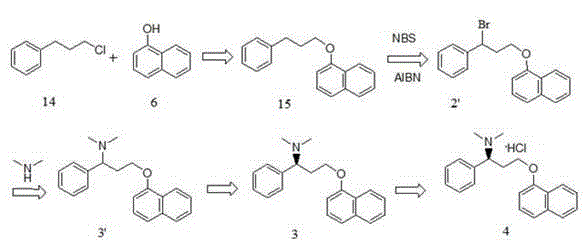
Optical pure 1,3-alkamine compound as well as preparation method and application thereof in preparing Dapoxetine and analogues thereof
ActiveCN101875666ANo wasteHigh stereoselectivityGroup 4/14 element organic compoundsCarbamic acid derivatives preparationStereochemistryDapoxetine-N-oxide
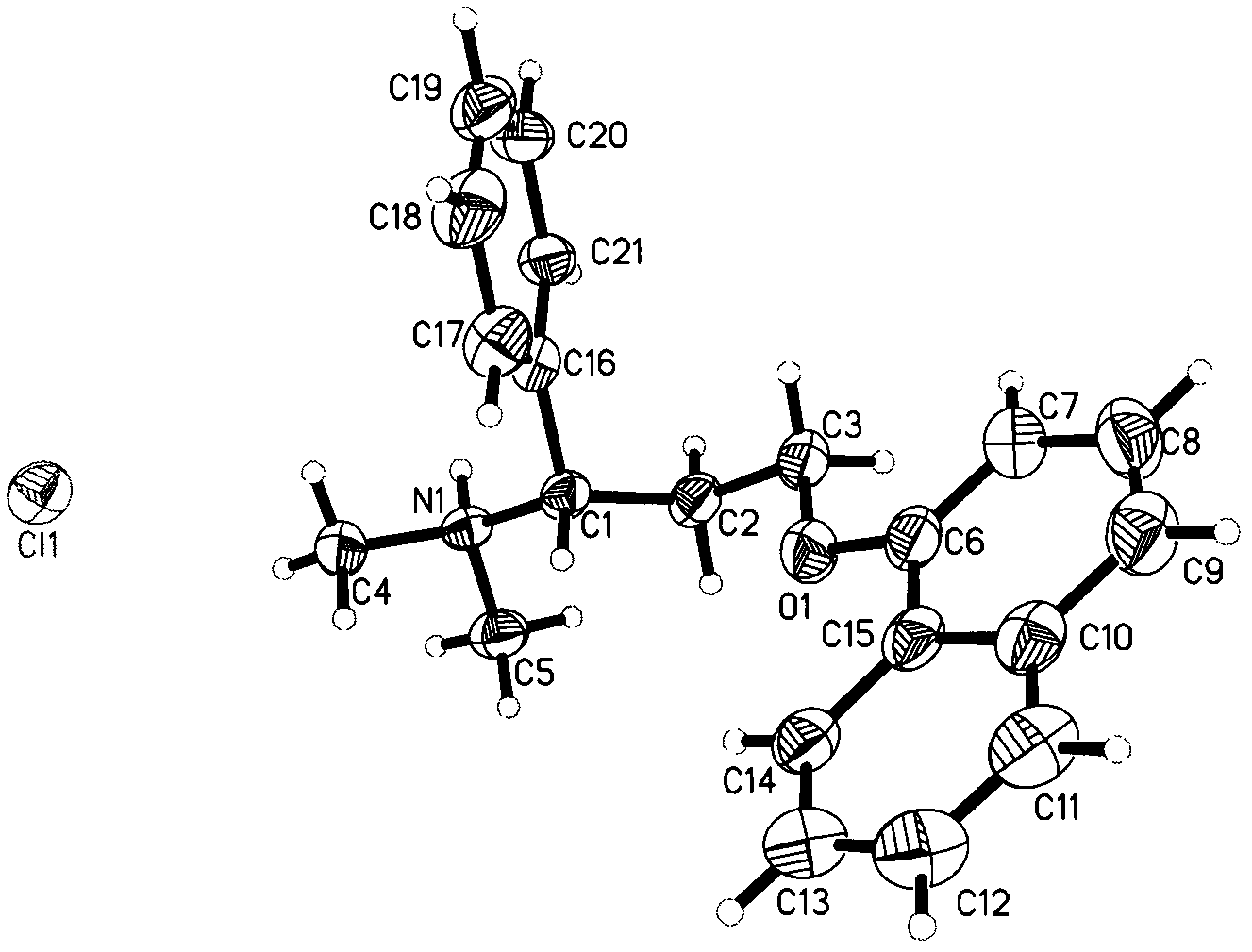
The invention specifically relates to an optical pure 1,3-alkamine compound as well as a preparation method and application thereof in preparing corresponding optical pure 1,3-alkamine and further preparing Dapoxetine and analogues thereof, belonging to the technical field of organic chemistry. The 1,3-alkamine compound is as shown in a formula I.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Novel synthesizing method of dapoxetine
InactiveCN103304434AHigh yieldHigh stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationHydroxylaminePtru catalyst
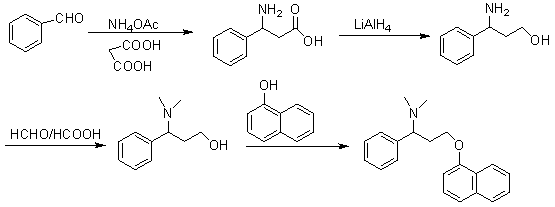
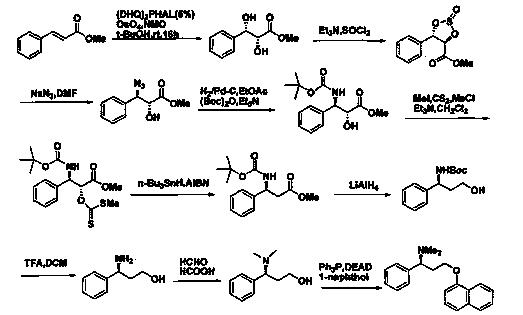
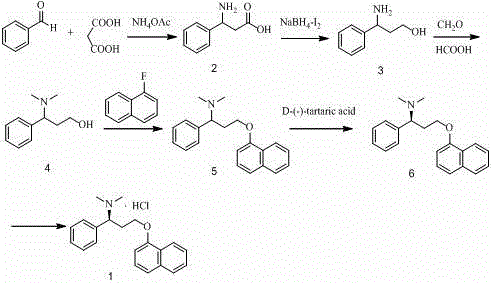
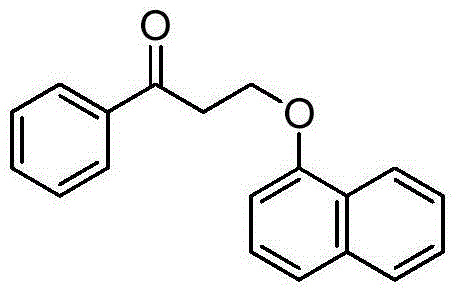
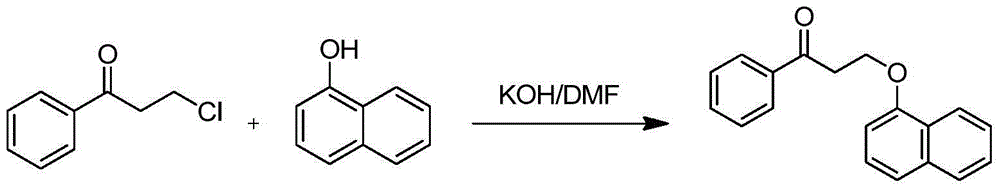
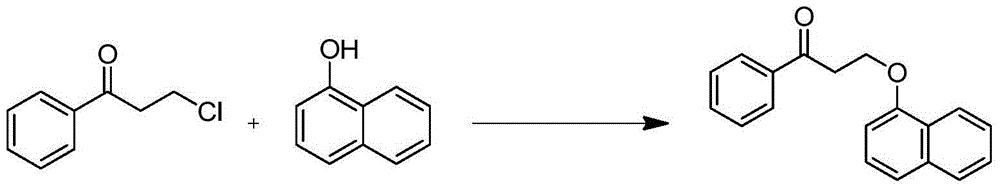
The invention discloses a novel synthesizing method of dapoxetine, wherein a series of reactions are carried out on trans-cinnamaldehyde and N-carbobenzoxy hydroxylamine which serve as initiative raw materials under the action of a self-prepared catalyst (s)-alpha-alpha-diisopropyl dimethyl tert-butyl silicon oxygroup prolinol, so that an important intermediate of the dapoxetine, namely an important chiral intermediate (S)-3-amino-3-phenyl propyl alcohol (compound 4), is obtained. The invention further discloses a preparation method of capecitabine, wherein the compound serving as a raw material is methylated and protected by hydroxyl and then reacts with 1-naphthol to form a salt, so that the capecitabine is obtained. The preparation method is easy in raw material obtainment, good in stereoselectivity and high in yield, thereby being suitable for industrial production.
Owner:湖南欧亚药业有限公司
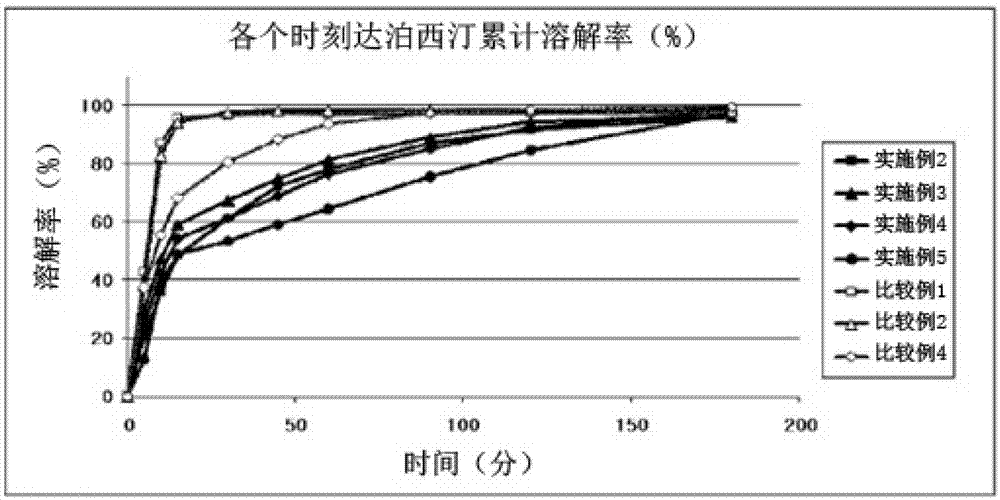
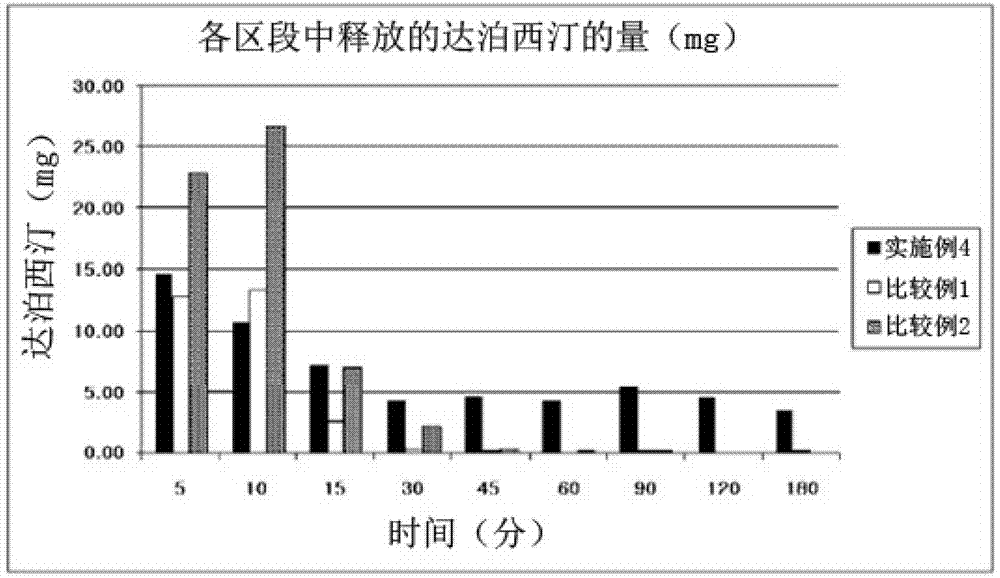
Time-delayed sustained release pharmaceutical composition comprising dapoxetine for oral administration
InactiveCN102958513ASatisfy sexual desireLittle side effectsOrganic active ingredientsPill deliverySexual impotenceOral medication
The present invention relates to a time-delayed sustained release pharmaceutical composition for oral administration, which comprises an immediate release phase and a prolonged sustained release phase, wherein said immediate release phase and prolonged sustained release phase respectively comprise dapoxetine therein as an active ingredient.; The pharmaceutical composition of the present invention comprises dapoxetine, which is an agent for treating premature ejaculation, in both the immediate release phase and the prolonged sustained release phase thereof, to thereby immediately exhibit the effectiveness of the pharmaceutical composition of the present invention in order to enable a patient to achieve sexual satisfaction during the early stage of administration, as well as to reduce side effects by means of the time-delayed sustained release of the prolonged sustained release phase during the early stage of administration and enable a continuous in vivo absorption of dapoxetines, to thereby lengthen the duration of the effectiveness of the pharmaceutical composition of the present invention.; Further, agents for treating erectile dysfunction, such as sildenafil, tadalifil or the like can be added to the immediate release phase so as to allow for a coincidence of the durations of the effectiveness of a premature ejaculation treatment agent and erectile dysfunction treatment agents, even though a half-life difference exists between the two types of treatment agents, thus maximizing patient satisfaction.
Owner:NAVIPHARM CO LTD
Separation and determination methods for dapoxetine hydrochloride intermediate SM1 and related impurities
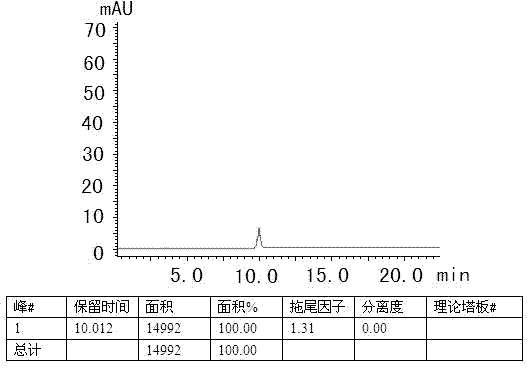
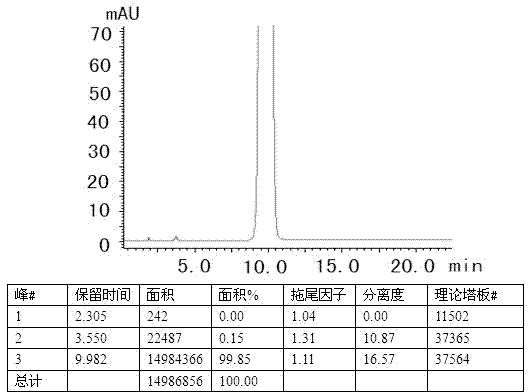
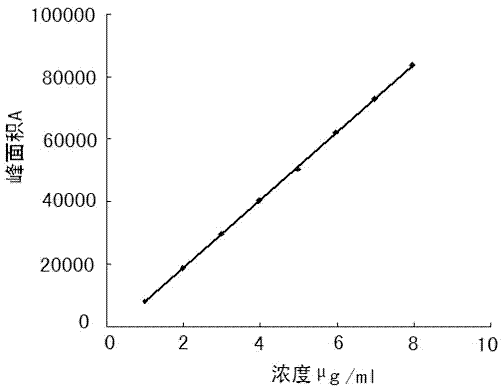
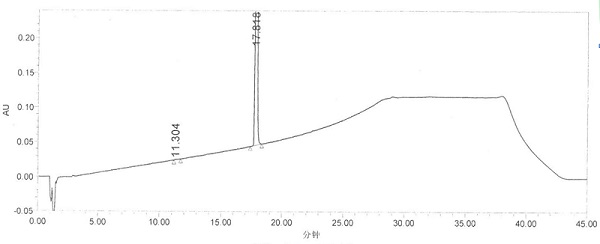
The invention specifically relates to separation and determination methods for a dapoxetine hydrochloride intermediate SM1 and related impurities, belonging to the field of analytical chemistry. The separation method employs octadecyl bonded porous silica gel and / or inorganic oxide particles as a stationary phase and an aqueous acetonitrile solution as a mobile phase for separation, and can realize effective separation of SM1 and related impurities. The determination method employs high performance liquid chromatography and comprises the following steps: preparing a test solution from a test sample; diluting the test solution by certain times so as to obtain a contrast solution; analyzing the test solution and the contrast solution by using the stationary phase and the mobile phase; recording a chromatogram; and calculating the contents of SM1 and related impurities in the test sample by using a main component self-contrast method. The separation and determination methods can realize effective separation of SM1 and related impurities and accurately measure the contents of SM1 and related impurities, and are simple to operate and high in accuracy.
Owner:CHONGQING HUAPONT PHARMA
High performance liquid chromatography detection method for related substances in dapoxetine
ActiveCN103926335AIncrease credibilityThe detection method is accurateComponent separationO-Phosphoric AcidFluid phase
The invention discloses a dapoxetine detection method using a diode array detector and using a phosphate buffer solution (pH value is adjusted to 3.2 with phosphoric acid)-acetonitrile-methanol solution as a mobile phase. The dapoxetine detection method is as follows: taking proper amounts of dapoxetine and dapoxetine-containing relevant preparations, adding the mobile phase to prepare a solution containing 0.2mg of the dapoxetine every 1ml of the solution as a solution to be tested; diluting the solution to be tested with the mobile phase into a solution containing 0.2mug of the dapoxetine every 1ml of the solution as a contrast solution; and respectively injecting samples; wherein the sum of peak areas of impurities in a chromatogram map of the solution to be tested may not be greater than main peak area of the contrast solution. According to the detection method for the related substances in the dapoxetine and the dapoxetine-containing relevant preparations, the conditions of the impurities and degradation products of the dapoxetine can be quickly and accurately detected, and the detection method is simple in operation and high in sensitivity, and can be used to better control the quality of the product.
Owner:SHANDONG INST OF PHARMA IND
Crystal and amorphous substance of dapoxetine hydrochloride and preparation method thereof
ActiveCN103130661AImprove stabilityImprove solubilityOrganic active ingredientsOrganic compound preparationCrystallographySolubility
The invention discloses a crystal of dapoxetine hydrochloride, amorphous substance of the dapoxetine hydrochloride and a preparation method of the crystal and the amorphous substance of the dapoxetine hydrochloride. The stability of the crystal is better than that of crystal forms in the prior art, and the solubility of the amorphous substance is obvious higher than that of existing dapoxetine hydrochloride.
Owner:XIAMEN FUMAN PHARMA +1
Crystal form B of dapoxetine hydrochloride, and preparation method thereof
InactiveCN103145569AEasy to prepareGood preparation adaptabilityOrganic active ingredientsOrganic compound preparationPharmaceutical drugDapoxetine-N-oxide
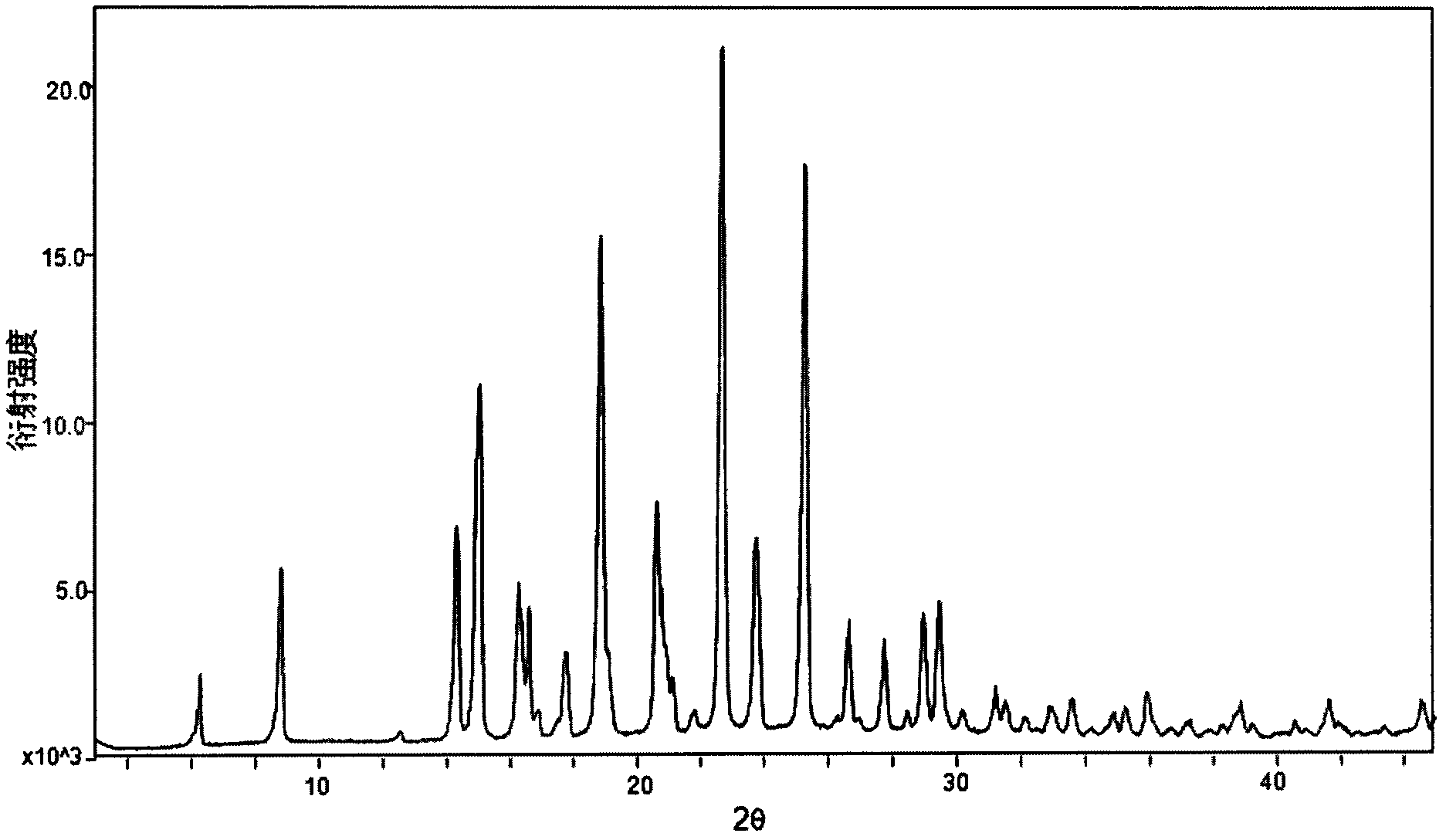
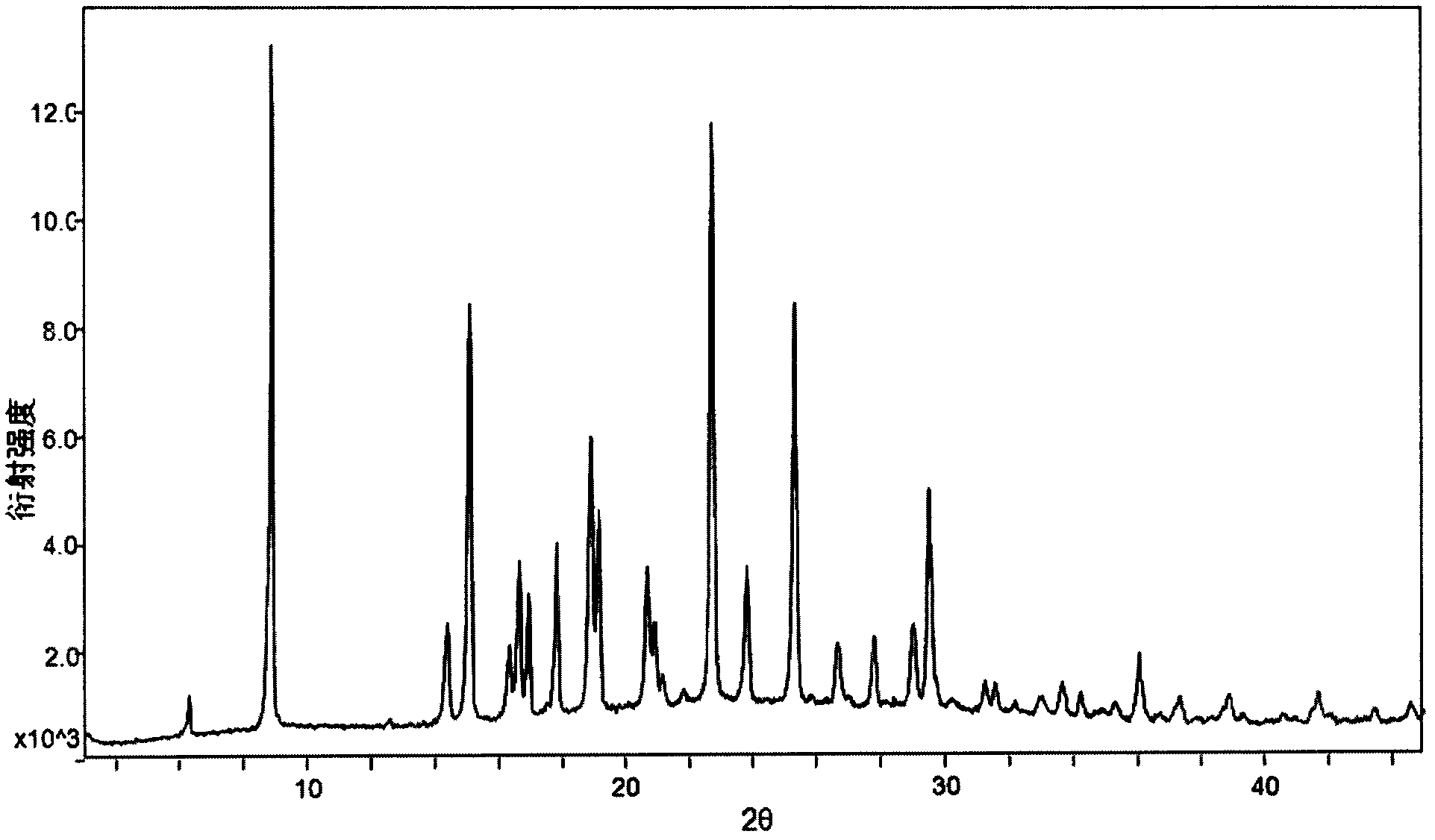
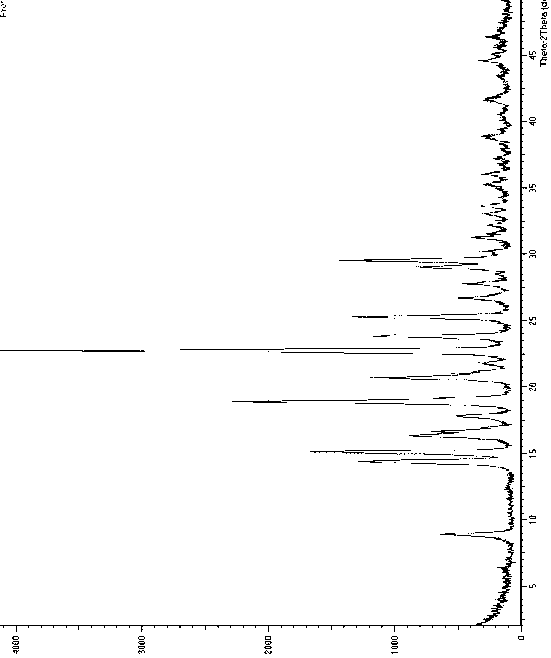
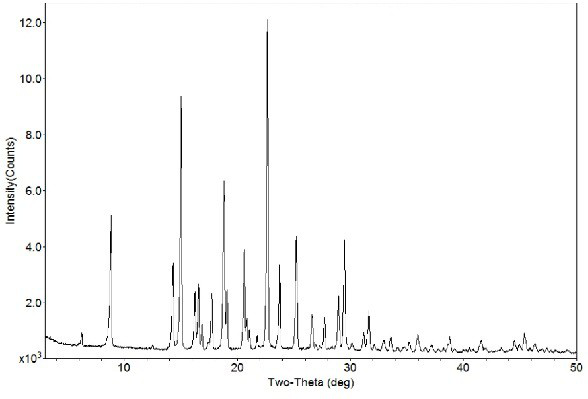
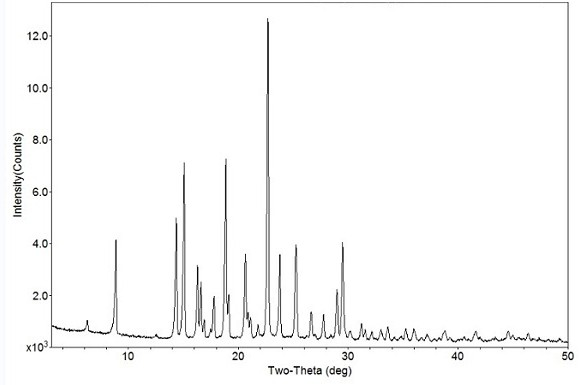
The invention relates to a crystal form B of dapoxetine hydrochloride which is a drug for treating male premature ejaculation, and a preparation method thereof. The crystal form B of dapoxetine hydrochloride is characterized in that the X-ray powder diffraction pattern thereof has corresponding characteristic diffraction peaks at positions of 2[theta] angle values being 9.2 degrees, 14.7 degrees, 16.2 degrees, 16.9 degrees, 18.0 degrees, 21.8 degrees, 23.2 degrees, 24.9 degrees, and 27.1 degrees.
Owner:CHONGQING PHARMA RES INST
Compound sildennafil dapoxetine slow-release capsule and preparation method thereof
InactiveCN106511312AGood slow releasePromotes quick releaseOrganic active ingredientsGranular deliveryTime delaysImmediate release
The invention discloses a compound sildennafil dapoxetine slow-release capsule and a preparation method thereof. Each capsule of the preparation contains immediate-release particles and slow-release particles. The immediate-release particles contain an active substance sildennafil or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient or auxiliary materials; the slow-release particles contain an active substance dapoxetine or the pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient or auxiliary materials. The preparation is prepared by the following steps: preparation of excipient immediate-release particles; preparation of dapoxetine slow-release particles; preparation of the compound sildennafil dapoxetine slow-release capsule. In the compound preparation, sildennafil is used as an immediate-release component, drug effects are rapidly performed, sexual desire of patients is satisfied in a short time, dapoxetine is used as a slow-release component for time-delay release, the effects are lasting for patients, and the capsule is suitable for treating erectile dysfunction and premature ejaculation of male.
Owner:扬子江药业集团江苏紫龙药业有限公司
Method for preparing dapoxetine intermediate by using carbonyl reductase
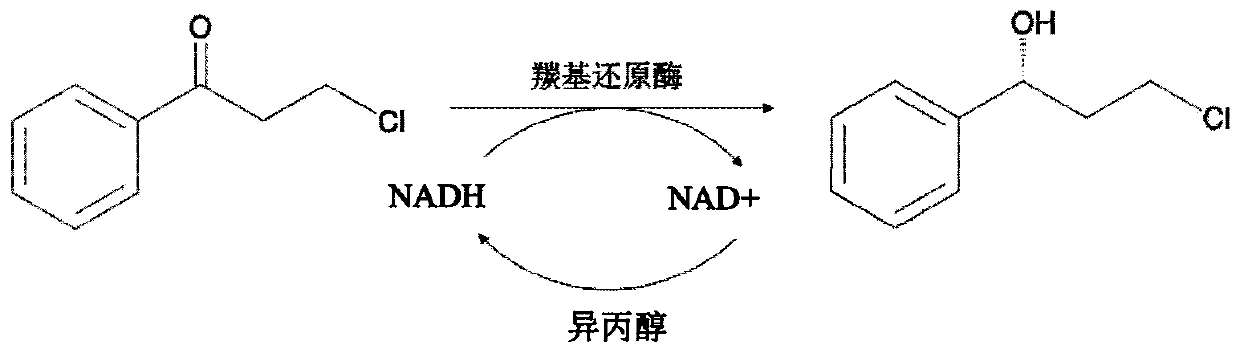
InactiveCN111378694AReduce usageMild reaction conditionsOxidoreductasesFermentationChlorobenzenePtru catalyst
The invention discloses a method for preparing dapoxetine intermediate by using carbonyl reductase. In the method, a crushed mixed solution of wet cells obtained by induction culture of engineered bacteria containing the carbonyl reductase gene after ultrasonication is used as a catalyst, and 3-chlorophenylacetone is used as a substrate, and NADH and isopropanol are added, and a phosphate buffer with a pH of 6.5. -7.5 is used as a reaction medium to constitute a reaction system. The reaction system is complete with magnetic stirring at 30-40 DEG C. The reaction solution is separated and purified to obtain (R)-3-chlorophenylpropanol. The invention adopts a new type of carbonyl reductase to catalyze a reduction reaction, has the advantages of small amount of biocatalyst, mild reaction conditions (30-40 DEG C), short reaction time (6-12 hours), 95.2% of yield coefficient, and good optical purity (greater than 99), and the like.
Owner:ZHEJIANG UNIV OF TECH
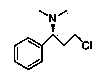
(S)-3-chloro-N, N-dimethyl-1-phenyl-1-propylamine and method for preparing dapoxetine by using same as intermediate
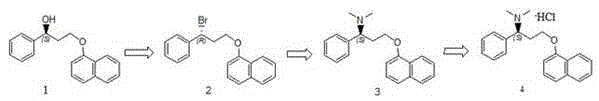
The invention discloses a method for preparing a dapoxetine intermediate (S)-3-chloro-N, N-dimethyl-1-phenyl-1-propylamine, and preparation of dapoxetine hydrochloride by using the intermediate through condensation and salification. The preparation of dapoxetine hydrochloride is shortened in reaction steps, reduced in cost, simple in operation and is applicable to industrial production.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD
Dapoxetine hydrochlorate crystal form A and preparation method thereof
InactiveCN103130658AEasy to prepareImprove stabilityOrganic active ingredientsOrganic compound preparationMedicinePharmaceutical drug
The invention relates to a crystal form A of dapoxetine hydrochlorate which is a drug for treating male premature ejaculation, and a preparation method thereof; the crystal form A is characterized in that the crystal form A has characteristic diffraction peaks corresponding to positions with 2theta values of about 8.9 degrees, 14.4 degrees, 15.1 degrees, 16.3 degrees, 16.6 degrees, 18.9 degrees, 20.7 degrees, 22.7 degrees, 23.8 degrees, 25.3 degrees, 29.0 degrees, and 29.5 degrees on an X-ray powder diffraction pattern, and the new crystal form has the characteristics of high purity, good stability, and the like.
Owner:CHONGQING PHARMA RES INST
Synthesis, separation-purification, and salt forming method of dapoxetine
InactiveCN106748825AReduce usageLower initial costOrganic compound preparationOrganic chemistry methodsBenzaldehydeBiochemical engineering
The invention provides a novel synthesis, gradient separation-purification, and salt forming method of dapoxetine. Easily available and cheap benzaldehyde is taken as the primary raw material of the synthesis route. The whole reaction conditions are mild. The synthesis route is short. No highly toxic or explosive raw material is used. The problem of chiral separation is well solved in the route. During the separation process, the product is purified. Finally, chlorinated hydromethyl tert-butyl ether which does not have any side or toxic effect is used to carry out salt forming. A large amount of labor, material, and time is saved. The production cost is reduced. The synthesis does not need any special equipment. The operation is simple and convenient. The method has a good industrial application prospect.
Owner:LIAOCHENG UNIV
High-purity dapoxetine preparation method suitable for industrialization
ActiveCN104628584AMeets requirementsHigh selectivityOrganic compound preparationAmino-hyroxy compound preparationPropanolBiochemical engineering
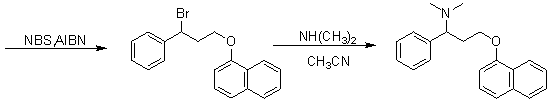
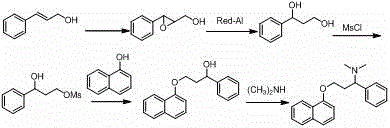
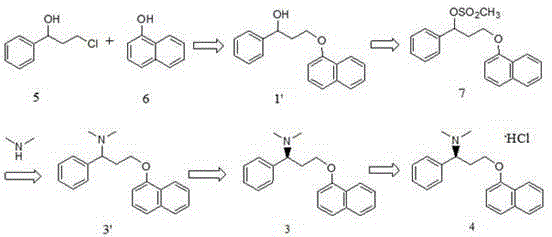
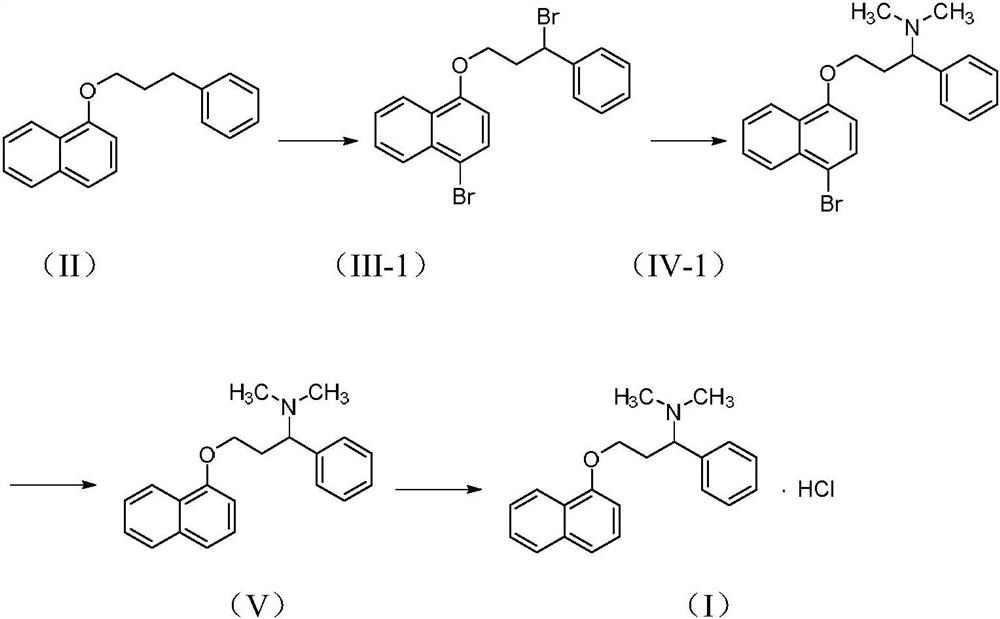
The invention relates to a high-purity dapoxetine preparation method suitable for industrialization. In the preparation, S-3-(1-naphthoxy)-1-phenyl-1-propanol is taken as the raw material and is subjected to bromination, coupling, and salt-forming reactions so as to obtain dapoxetine; or racemic 3-(1-naphthoxy)-1-phenyl-1-propanol is taken as the raw material and is subjected to bromination, coupling, resolution, and salt-forming reactions so as to obtain dapoxetine. The provided preparation method overcomes the shortages of long reaction route, tedious technology, and high cost in the prior art, and has the advantages of mild reaction conditions, no requirement of high pressure and toxic reagent, short route, high conversion rate, and low cost. The prepared drug raw materials have better quality, and the preparation method is very practical in industry.
Owner:REGENEX PHARMA LTD
Preparation method of tadalafil and dapoxetine hydrochloride mixed tablet
PendingCN113181185AAchieving the goal of synergistic administrationExperiment operation is simpleOrganic active ingredientsSexual disorderSexual impotenceTadalafil
The invention discloses a preparation method of a tadalafil and dapoxetine hydrochloride mixed tablet, and belongs to the field of pharmaceutical preparations. The tadalafil and dapoxetine hydrochloride mixed tablet disclosed by the invention is prepared from, by weight, 10.00 to 30.00 percent of tadalafil solid dispersion, 30.00 to 55.00 percent of dapoxetine hydrochloride, 8.00 to 57.00 percent of a filler, 2.00 to 6.00 percent of a disintegrating agent and 1.00 to 3.00 percent of a lubricating agent. The prepared tadalafil and dapoxetine hydrochloride mixed tablet can be used for synergistically treating erectile dysfunction and premature ejaculation, and integrates the delay effect of dapoxetine and the erection assisting effect of tadalafil. The mixed tablet formed by mixing the tadalafil and the dapoxetine has a better treatment effect.
Owner:苏州康恒研新药物技术有限公司
Dapoxetine intermediate and preparation method thereof
ActiveCN105732339ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarbonyl compound preparation by hydrolysisAfter treatmentBromine
The invention discloses a dapoxetine intermediate and a preparation method thereof and provides a preparation method for the compound represented in the formula (2). The preparation method includes a step of performing a nucleophilic substitution reaction to the compound represented in the formula (3) with 1-naphthol in a solvent in the presence of alkali to prepare the compound represented in the formula (2). The R1 and the R2 are independently C1-C4 alkyl groups, or that the R1, the R2, oxygen atoms connected thereto respectively and carbon atoms connected to the oxygen atoms form a five-membered saturated ring structure or six-membered saturated ring structure, wherein X is Cl, Br or I. The raw materials are low in cost and easy to obtain. The preparation method is mild in reaction conditions, is high in conversion rate, is high in yield, is simple in after treatment, is low in production cost, is high in chemical purity of product, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation and detection method of dapoxetine hydrochloride isomer impurities
ActiveCN111018723AHigh stereoselectivityThe synthesis process is simpleCarbamic acid derivatives preparationOrganic compound preparationEnantiomerCombinatorial chemistry
Owner:盖天力医药控股集团制药股份有限公司
Synthesis method of dapoxetine and dapoxetine hydrochloride
InactiveCN113461553AHigh yieldReduce pollutionOrganic compound preparationSulfonic acid esters preparationStyrene oxideSulfonyl chloride
The invention provides a synthesis method of dapoxetine and dapoxetine hydrochloride, and belongs to the technical field of organic synthesis of medicines. The synthesis method of dapoxetine provided by the invention comprises the following steps: 1-naphthol and paraformaldehyde generate 1-naphthol bromomethyl ether under the action of hydrogen bromide, the 1-naphthol bromomethyl ether reacts with magnesium to obtain a 1-naphthol bromomethyl ether Grignard reagent, the 1-naphthol bromomethyl ether Grignard reagent reacts with R-styrene oxide to obtain R-3-(1-naphthyloxy)-1-phenyl propanol, and the R-3-(1-naphthyloxy)-1-phenyl propanol reacts with a bromination reagent to obtain S-1-bromo-1-phenyl-3-(1-naphthyloxy)propane; or the S-1-bromo-1-phenyl-3-(1-naphthyloxy)propane reacts with sulfonyl chloride to obtain S-1-sulfonyloxy-1-phenyl-3-(1-naphthyloxy) propane, or and the S-1-sulfonyloxy-1-phenyl-3-(1-naphthyloxy)propane reacts with sulfonyl chloride to obtain dapoxetine hydrochloride. The synthesis method provided by the invention has the advantages of short synthesis route, high product yield, good product quality, easily available raw materials, low cost and small environmental pollution, and is suitable for industrial production.
Owner:上海科利生物医药有限公司
Dapoxetine hydrochloride orally disintegrating tablet, preparation method thereof an application of orally disintegrating tablet
InactiveCN110833530AFast absorptionEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletTraditional medicine
The invention discloses a dapoxetine hydrochloride orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet comprises dapoxetine hydrochloride, fillers, disintegrating agents, dried binding agents, penetration promoting flavoring agents and lubricating agents. The orally disintegrating tablet is prepared by a direct tablet pressing method. The orally disintegrating tablet has the advantages that the orally disintegrating tablet is simple in preparation process, low in cost and convenient to take and has high effect on male premature ejaculation. The orally disintegrating tablet contains 0.1%-2% of penetration promoting flavoring agents, the taste of the orally disintegrating tablet is improved, stability of the orally disintegrating tablet is improved, absorption of the orally disintegrating tablet is further improved, the maximum plasma concentration (Cmax) is achieved within 0.5-1 hour after the orally disintegrating tablet is taken, effect taking time becomes earlier, and bioavailability is remarkably improved.
Owner:盖天力医药控股集团制药股份有限公司
A kind of biosynthesis method of dapoxetine intermediate and intermediate thereof
ActiveCN110078632BNovel synthetic routeLow costOrganic compound preparationCarbonyl compound preparationCombinatorial chemistryDapoxetine-N-oxide
The invention discloses a biosynthesis method of a dapoxetine intermediate and an intermediate thereof. The method uses compound (2) and compound (3) as starting materials, and prepares compound (4) through a phase transfer catalytic substitution reaction , the compound (4) finally obtains the dapoxetine intermediate compound (1) through a biological enzyme conversion reaction, and its reaction formula is as follows: The biosynthesis method of the dapoxetine intermediate of the present invention has a novel synthetic route, Simple and easy to implement, low cost, high synthetic yield, high yield, good product purity, good product quality, cheap and easy-to-get raw materials, and suitable for industrial production. The preparation of poxetine provides a new intermediate raw material.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Preparation method of dapoxetine hydrochloride racemate
InactiveCN111646910AHigh yieldEasy post-processingOrganic compound preparationAmino-hyroxy compound preparationDapoxetine-N-oxideMedicinal chemistry
Owner:CHINA PHARM UNIV
Preparation method of dapoxetine impurity reference substance
PendingCN113861046AOrganic compound preparationCarbonyl compound preparationSulfonyl chlorideChlorobenzene
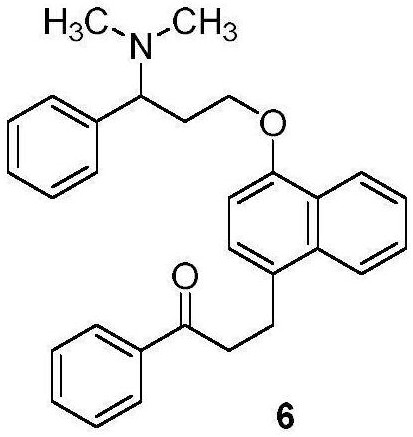
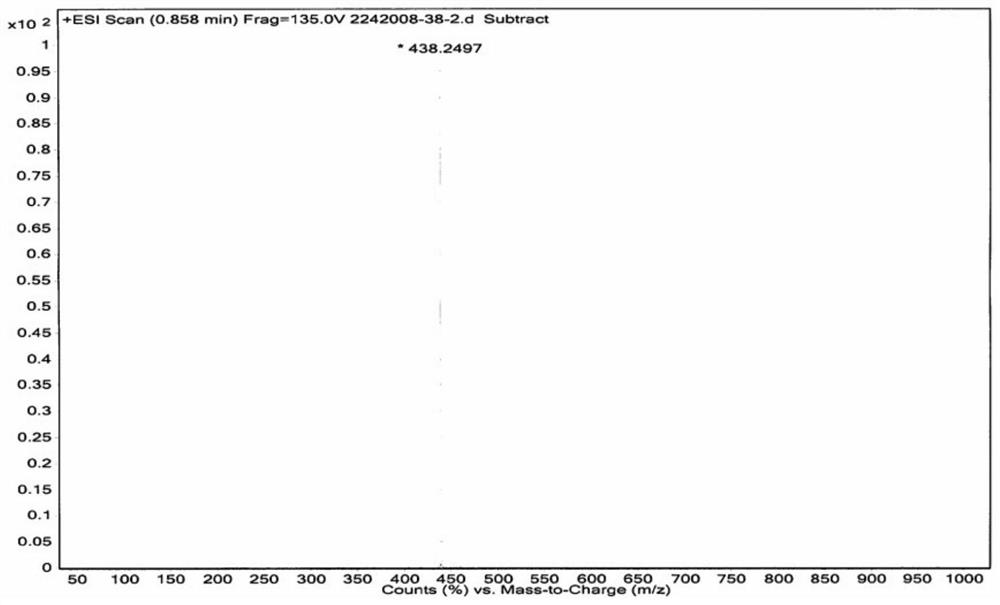
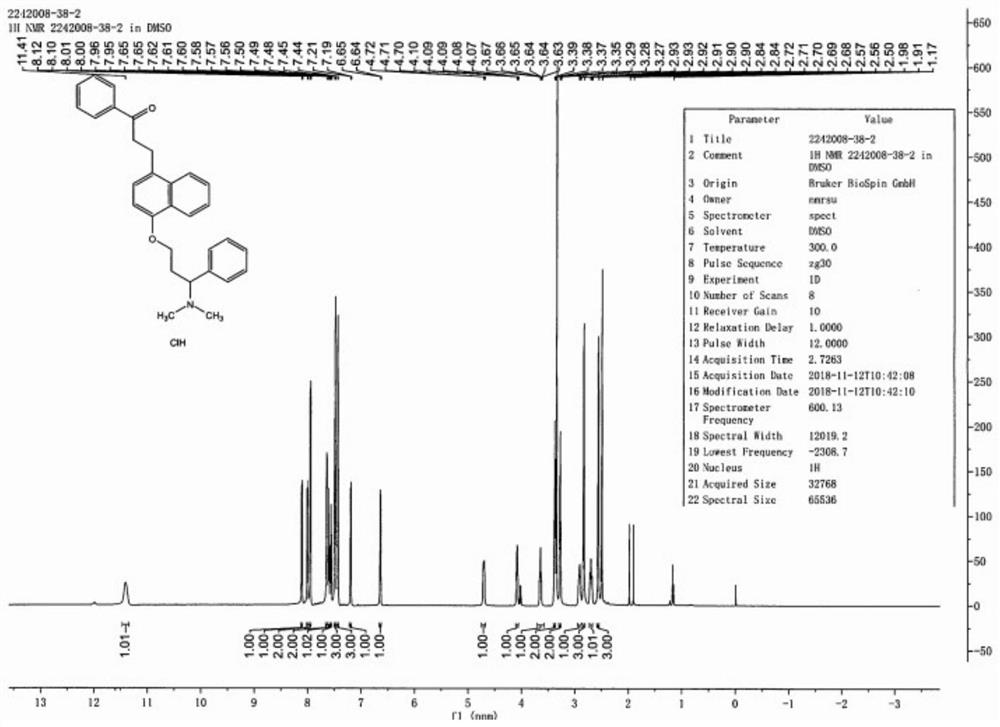
The invention discloses a preparation method of a dapoxetine impurity reference substance, which comprises the following steps: (1) reacting 3-(naphthalene-1-yloxy)-1-phenylpropan-1-ol with a p-methoxybenzyl reagent to obtain a compound 7; (2) reacting the compound 7 with 3-chloro-1-phenylpropyl-1-ketone to obtain a compound 8; (3) removing a p-methoxybenzyl protecting group in the compound 8 by using an oxidizing agent to obtain a compound 4; (4) reacting the compound 4 with alkyl sulfonyl chloride, and then reacting the compound with dimethylamine to obtain the dapoxetine impurity reference substance as shown in a formula 6. The dapoxetine impurity reference substance shown in the formula 6 is prepared for the first time.
Owner:南京卓康医药科技有限公司
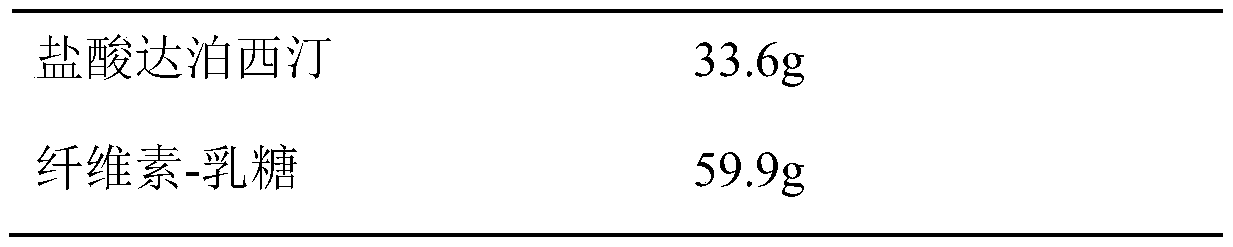
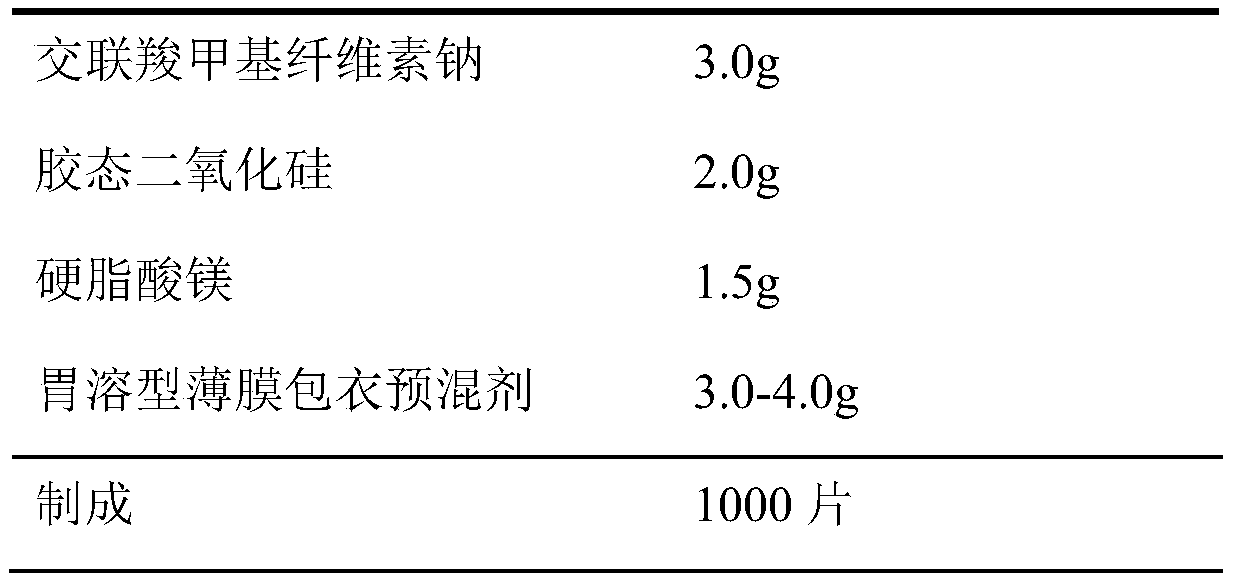
Dapoxetine hydrochloride tablet and preparation method thereof
InactiveCN110974799ASimple production processReduce stepsOrganic active ingredientsPharmaceutical non-active ingredientsMedicineCurative effect
The invention belongs to the field of pharmaceutical preparations. The dapoxetine hydrochloride tablet is composed of 33.6 mg of dapoxetine hydrochloride, less than 18 mg of microcrystalline cellulose, 40-45% of a filler, 2-8% of a disintegrating agent, 1-5% of a flow aid, 0.5-3% of a lubricant, 1-5% of coating powder and a special packaging material. The key composition auxiliary materials and the packaging material can greatly improve the stability of the medicine as well as the rate and degree of in-vivo absorption into blood, so that the curative effect is influenced.
Owner:SHANDONG HUBBLE KISEN BIOLOGICAL TECH CO LTD
PDE5 inhibitor and dapoxetine coated chip and preparation method thereof
PendingCN114344270AAchieve separate slicesImprove complianceOrganic active ingredientsPill deliveryCoated tabletsPhosphodiesterase 5 inhibitor
The invention relates to the technical field of medicines, in particular to a compound preparation for treating male erectile dysfunction and premature ejaculation and a preparation method thereof. The PDE5 inhibitor and dapoxetine coated tablet comprises a tablet core and a shell layer, the PDE5 inhibitor is arranged in the tablet core, and the dapoxetine is arranged in the shell layer; or dapoxetine is disposed within the tablet core and a PDE5 inhibitor is disposed within the shell layer; the weight ratio of the dapoxetine to the PDE5 inhibitor is (4-70): (2.5-100); according to the dosage form of the core-coated tablet, the PDE5 inhibitor and dapoxetine can be respectively placed in the shell layer and the tablet core, the mode that the tablet core is independently tableted and the shell layer material is prepared into the shell layer is realized, and the problems of compatibility reaction, influence on the drug effect and generation of more impurities caused by direct powder pressing of a common double-layer tablet are avoided.
Owner:福建瑞泰来医药科技有限公司 +1
Pharmaceutical combination tablet containing dapoxetine hydrochloride and preparation method thereof
PendingCN114404378AThe outer dissolution profile is stableSmall batch-to-batch varianceOrganic active ingredientsPharmaceutical non-active ingredientsLactoseStearic acid
The invention relates to a pharmaceutical composition tablet containing dapoxetine hydrochloride and a preparation method thereof. The tablet comprises 20-35 parts of dapoxetine hydrochloride (main drug), 30-40 parts of lactose (filler), 1-2 parts of croscarmellose sodium (filler), 18-20 parts of microcrystalline cellulose (lubricant), 1-2 parts of silicon dioxide (lubricant) and 1-2 parts of magnesium stearate (lubricant). The preparation method comprises the following steps: S1, mixing dapoxetine hydrochloride which is half of the formula amount with silicon dioxide, sieving, adding into a mixer, adding the rest dapoxetine, and stirring and mixing; s2, adding a filling agent and microcrystalline cellulose into a mixing machine for mixing; s3, adding magnesium stearate into the mixer, mixing, and tabletting; s4, preparing a coating solution by using coating powder and purified water, and pre-coating the tablets in S3 by using the coating solution; and S5, drying after the pre-coating is finished.
Owner:海南卓力制药有限公司
Compound tablet of dapoxetine hydrochloride and tadalafil and preparation method thereof
ActiveCN114788818ANo interactionTo achieve the effect of synchronous releaseOrganic active ingredientsPill deliveryTadalafilDrugs preparations
The invention provides a dapoxetine hydrochloride and tadalafil composite tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The compound tablet comprises dapoxetine hydrochloride pellets, tadalafil pellets, a tabletting aid and a lubricant, and by virtue of the arrangement of a coating structure, tadalafil with a relatively long half-life period and dapoxetine hydrochloride with a relatively short half-life period can act synchronously, so that a relatively good synergistic effect is achieved, and the treatment effect is improved.
Owner:SHANDONG HUBBLE KISEN BIOLOGICAL TECH CO LTD
Synthetic method of dapoxetine and intermediate thereof
ActiveCN110845369AHigh yieldGood chiralityCarbamic acid derivatives preparationOrganic compound preparationPolymer sciencePtru catalyst
The invention discloses a synthetic method of dapoxetine and its intermediate, i.e., (S)-3-(tert-butyloxycarbonyl)amino-3-phenylpropanol as shown in a formula 5 which is described in the specification. The synthetic method of (S)-3-(tert-butyloxycarbonyl)amino-3-phenylpropanol is as shown in a synthesis route which is described in the specification, wherein a compound 3 and acetaldehyde are subjected to a Mannich reaction in an organic solvent under the action of a supramolecular catalyst constructed by a chiral catalyst and a polymer so as to obtain a compound 4, and the polymer is at least one selected from of the group consisting of PEG 200, PEG 400, PEG 600, MeOPEG 750, PEG 800, PEG 1000, PPG 800 and PPG 1000. The dapoxetine is synthesized from the (S)-3-(tert-butyloxycarbonyl)amino-3-phenylpropanol prepared by using the above method according to steps as shown in the synthesis route. The synthetic method of dapoxetine and the intermediate thereof has the characteristics of usage of cheap and easily available raw materials, high yield and low cost, and is more beneficial to industrial production.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of dapoxetine hydrochloride sustained release tablet
PendingCN113143879AProcess stabilityAct as a slow releaseOrganic active ingredientsNervous disorderExtended release tabletsCombinatorial chemistry
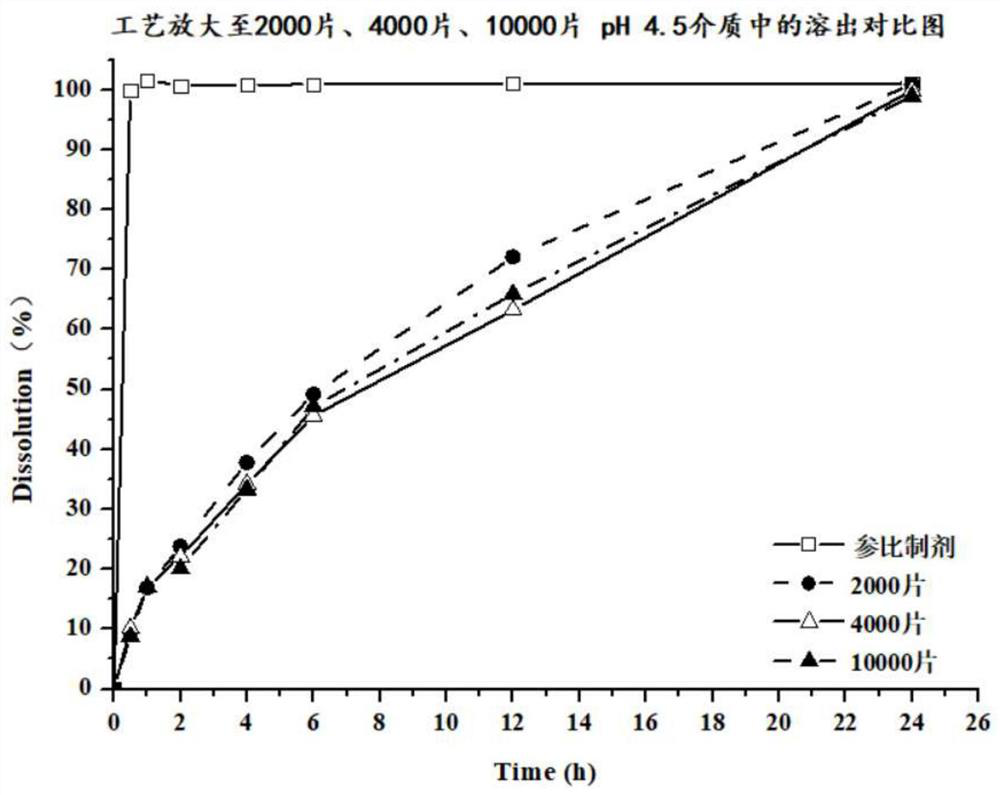
The invention discloses a preparation method of a dapoxetine hydrochloride sustained release tablet, and belongs to the field of pharmaceutical preparations. The dapoxetine hydrochloride sustained release tablet is prepared from the following raw materials, by weight, 30-34% of dapoxetine hydrochloride, 2-5% of a disintegrating agent, 5-15% of a skeleton type sustained release material, 1-3% of a flow aid and 43.4-58.4% of a filling agent. According to the method, the insoluble skeleton type material is used as a framework carrier to prepare dapoxetine sustained-release granules, the sustained-release granules are pressed into round tablets under proper pressure, finally, coating is performed, and the weight gain of the coating is 2%-5%. The dapoxetine hydrochloride sustained release tablet is stable in process, can play a good sustained-release role, is stable in release within 24 hours, has no burst release and difficulty in dissolution, is amplified to 2-4 thousands of tablets, has good results, and meets the requirements.
Owner:苏州康恒研新药物技术有限公司
Dapoxetine hydrochloride monohydrate and its preparation method and use
ActiveCN108264465BImprove solubilityExcellent dissolution propertiesOrganic active ingredientsOrganic compound preparationSexual functioningDapoxetine-N-oxide
The invention discloses a dapoxetine hydrochloride monohydrate and a preparation method thereof, a composition containing the dapoxetine hydrochloride monohydrate, and uses of the composition in preparation of drugs for treatment of sexual dysfunction, wherein the dapoxetine hydrochloride monohydrate is represented by a formula I. According to the present invention, the dapoxetine hydrochloride monohydrate overcomes the disadvantages of easy moisture absorption, poor stability, high requirements on storage conditions and difficult long-term preservation of the existing crystal form or the amorphous form of dapoxetine hydrochloride, and is suitable for industrial scale-up and preparation production. The formula I is defined in the specification.
Owner:SUZHOU KELUN PHARMA RES CO LTD
Preparation method of dapoxetine hydrochloride pellet tablet
PendingCN113181127AProcess stabilityAct as a slow releaseOrganic active ingredientsPharmaceutical non-active ingredientsLactoseDapoxetine-N-oxide
The invention discloses a preparation method of a dapoxetine hydrochloride pellet, and belongs to the field of pharmaceutical preparations. The dapoxetine hydrochloride pellet tablet is prepared from, by weight, 16-17% of dapoxetine hydrochloride, 2-8% of a disintegrating agent, 15-35% of a hydrophilic gel skeleton type sustained-release material, 1-3% of a lubricant, 10-25% of silicified microcrystalline cellulose, and the balance of lactose. The dapoxetine hydrochloride pellet tablet prepared by the preparation method has the advantages that the process is stable, the slow release effect can be well achieved, the release within 24 hours is stable, the phenomena of burst release and difficult dissolution are avoided, the tablet is amplified to 10,000 tablets, the result is good, and the requirement is met.
Owner:苏州康恒研新药物技术有限公司
Synthesis method of dapoxetine
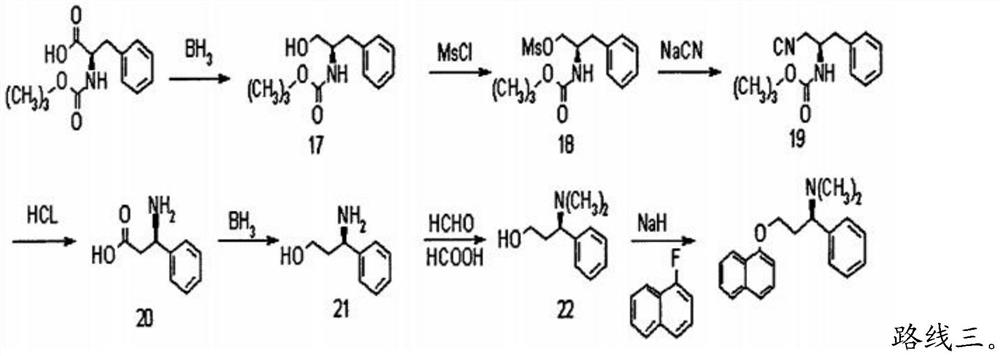
PendingCN113880721AAvoid splittingSolve the serious problem of lossOrganic compound preparationOrganic chemistry methodsPropanoic acidDapoxetine-N-oxide
The invention provides a synthesis method of dapoxetine. The method comprises: S1, dispersing (s)-3-amino-3-phenylpropionic acid or an ester compound thereof in a solvent, and carrying out a reflux reaction under the effect of a reducing agent to obtain (s)-amino-3-phenylpropanol; S2, dissolving (s)-amino-3-phenylpropanol in a formic acid aqueous solution, adding paraformaldehyde, conducting heating, and carrying out a reaction so as to obtain (s)-3-dimethylamino-3-phenylpropanol; and S3, dissolving (s)-3-dimethylamino-3-phenylpropanol in a solvent, dropwise adding the (s)-3-dimethylamino-3-phenylpropanol into an alkali solution for reaction at a relatively high temperature under the protection of nitrogen, and then adding 1-fluoronaphthalene for carrying out Williamson etherification reaction to obtain (s)-N,N-dimethyl-3-(1-naphthyloxy)amphetamine, namely dapoxetine. The synthesis method of dapoxetine has the advantages of cheap and easily available raw materials, no use of toxic and dangerous reagents, no reaction aggregation and material spraying phenomenon, simple process, and suitableness for industrial production.
Owner:HUNAN JIUDIAN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com