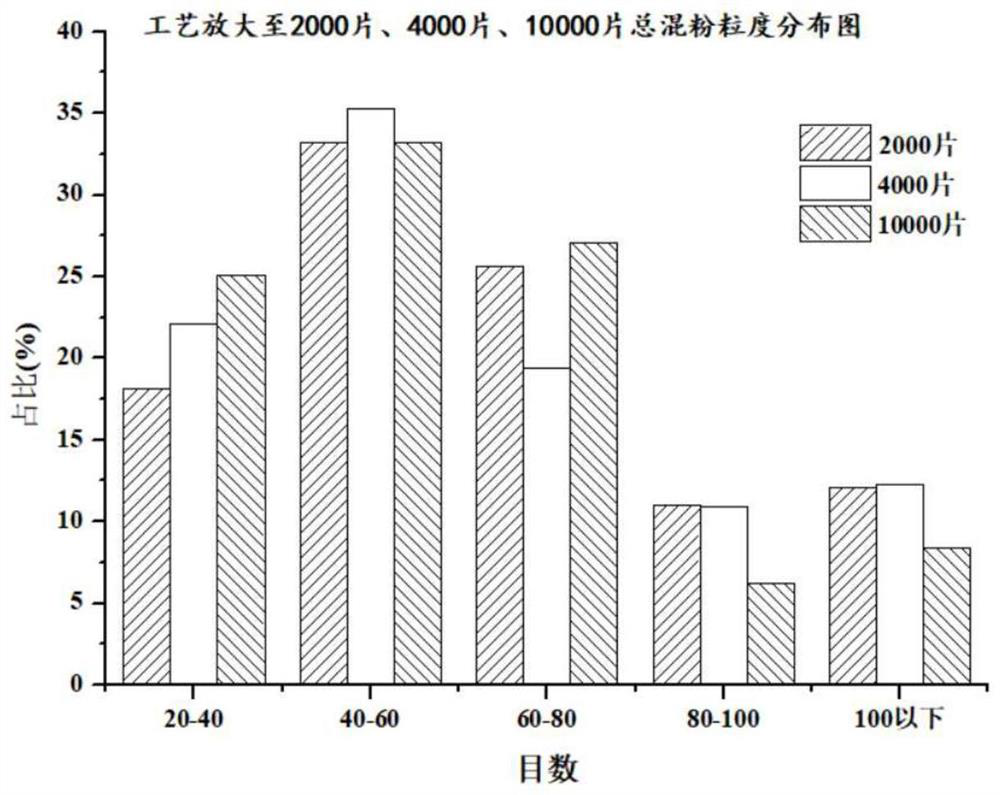
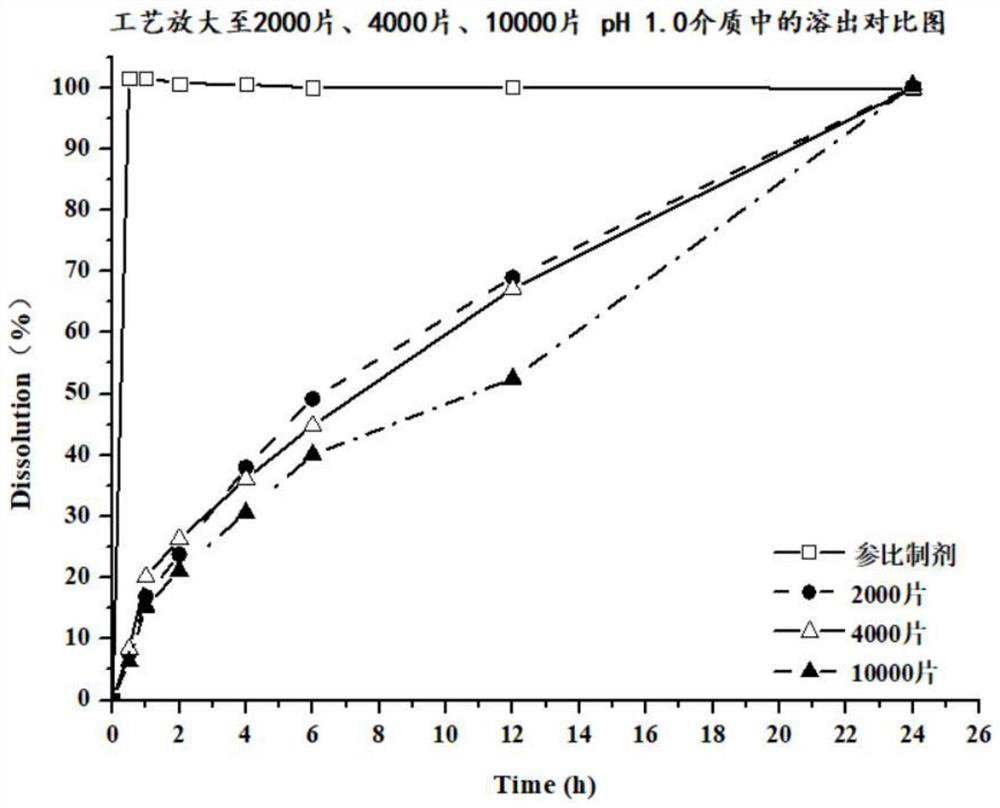
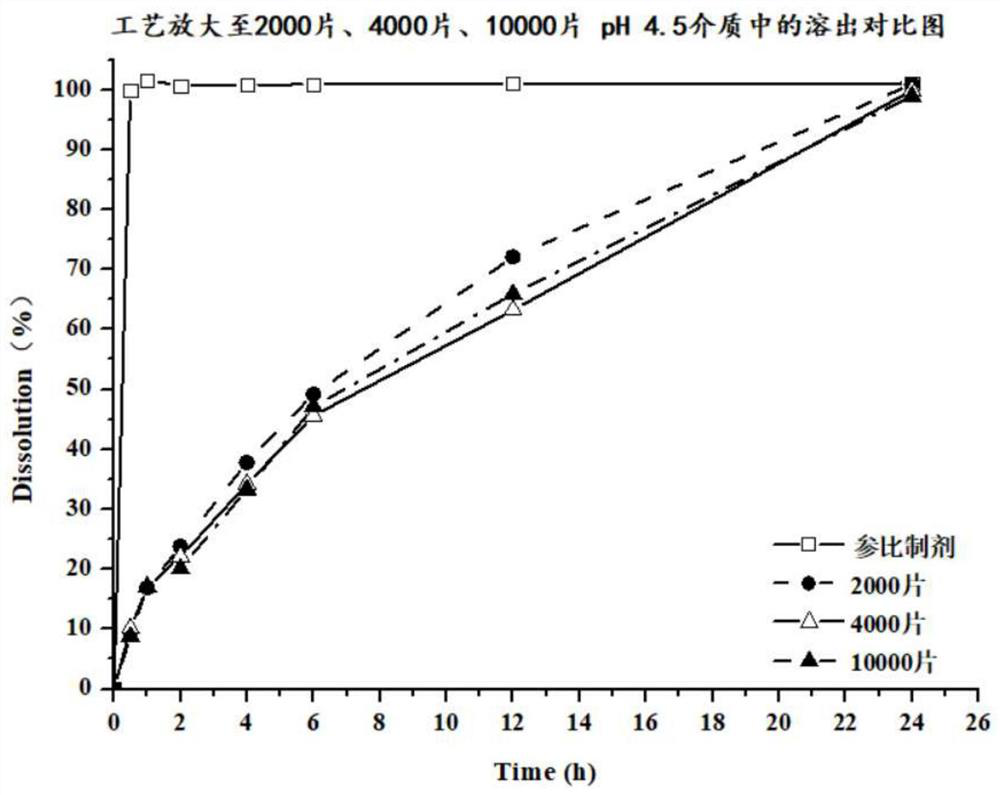
Preparation method of dapoxetine hydrochloride pellet tablet
A technology of dapoxetine hydrochloride and cetine pellets, which can be applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc., and can solve problems such as inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The prescribing information is as follows:
[0062] Specifications: 30mg, batch size is 1000 tablets.
[0063]
[0064] The preparation process is as follows:
[0065] 1. Premix: Dapoxetine hydrochloride 33.6g, croscarmellose sodium 6g, silicified microcrystalline cellulose 36g, hypromellose 50g, lactose 64.4g, sodium stearyl fumarate 1g, wet In the French granulator, the stirring speed is 300rpm, the shearing speed is 1800rpm, and the premixing is 9min;
[0066] 2. Granulation: pure water spray is added, and the spraying is required to be completed within 2 minutes, and then granulated for 1 minute;
[0067] 3. Wet granulation: Wet granulation with a 16-mesh sieve in the wet granulator swing granulator, and the materials are put into the spheronizing equipment in batches to make pellets;
[0068] 4. Drying: the air inlet temperature of the fluidized bed is 50-70°C, the material temperature is 35-45°C, and dried to a moisture content of 0.9%-2.8%;
[0069] 5. Dry...
Embodiment 2
[0074] The prescribing information is as follows:
[0075] Batches are 2000 pieces.
[0076]
[0077]
[0078] The preparation process is as follows:
[0079] 1. Premix: Dapoxetine hydrochloride 67.2g, croscarmellose sodium 24g, silicified microcrystalline cellulose 104g, hypromellose 120g, lactose 76.8g, sodium stearyl fumarate 6g, wet In the French granulator, the stirring speed is 300rpm, the shearing speed is 1800rpm, and the premixing is 9min;
[0080] 2. Granulation: pure water spray is added, and the spraying is required to be completed within 2 minutes, and then granulated for 1 minute;
[0081] 3. Wet granulation: Wet granulation with a 16-mesh sieve in the wet granulator swing granulator, and the materials are put into the spheronizing equipment in batches to make pellets;
[0082] 4. Drying: the air inlet temperature of the fluidized bed is 50-70°C, the material temperature is 35-45°C, and dried to a moisture content of 0.9%-2.8%;
[0083] 5. Dry granulati...
Embodiment 3
[0088] The prescribing information is as follows:
[0089] Batches are 4000 pieces.
[0090]
[0091] The preparation process is as follows:
[0092] 1. Premix: Dapoxetine hydrochloride 134.4g, croscarmellose sodium 24g, silicified microcrystalline cellulose 120g, hypromellose 280g, lactose 185.6g, sodium stearyl fumarate 4g, wet In the French granulator, the stirring speed is 300rpm, the shearing speed is 1800rpm, and the premixing is 9min;
[0093] 2. Granulation: pure water spray is added, and the spraying is required to be completed within 2 minutes, and then granulated for 1 minute;
[0094] 3. Wet granulation: Wet granulation with a 16-mesh sieve in the wet granulator swing granulator, and the materials are put into the spheronizing equipment in batches to make pellets;
[0095] 4. Drying: the air inlet temperature of the fluidized bed is 50-70°C, the material temperature is 35-45°C, and dried to a moisture content of 0.9%-2.8%;
[0096] 5. Dry granulation: dry gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com