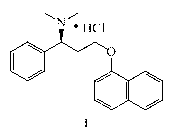
Dapoxetine hydrochlorate crystal form A and preparation method thereof
A technology of dapoxetine hydrochloride and crystal form, applied in the field of organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Dapoxetine Hydrochloride Form A
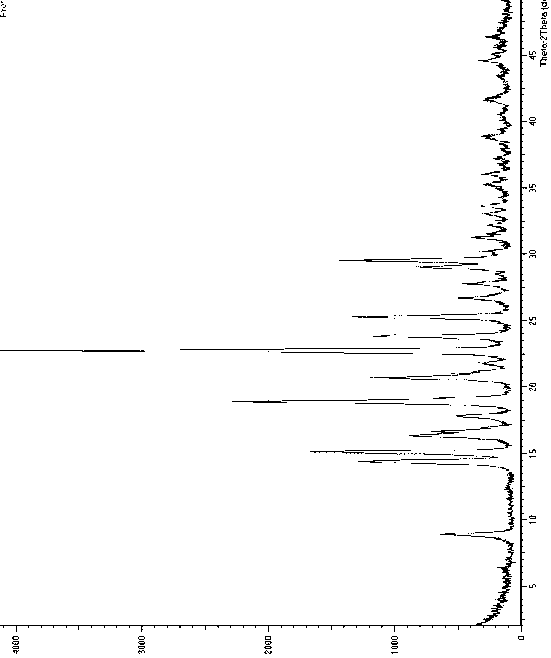
[0050] Dissolve 2g of dapoxetine hydrochloride solid in 20ml of isopropanol, heat to reflux to dissolve and clarify, let stand and cool to room temperature, add to 150ml of cyclohexane under stirring, after addition, stir at room temperature for about 50min, filter, and The filter cake was dried under vacuum at 40-50°C to obtain dapoxetine hydrochloride Form A, whose X-ray powder diffraction pattern is shown in figure 1 , the main test data values are listed in the following table (list the test data with relative strength greater than or equal to 4%).
[0051] 2θ angle (°) measured value d(?) Measured value Relative Strength(%) 8.88540 9.94422 14 14.3676 6.15980 30 15.0761 5.87188 39 16.3200 5.42702 20 16.6400 5.32337 14 16.9600 5.22364 4 17.7987 4.97934 10 18.8786 4.69688 57 19.2600 4.60472 8 20.6811 4.29140 25 21.2000 4.18752 5 21.76...
Embodiment 2
[0053] Preparation of Dapoxetine Hydrochloride Form A
[0054] Dissolve 2g of dapoxetine hydrochloride solid in 16ml of absolute ethanol, heat to reflux to dissolve and clarify, filter, add the filtrate to 100ml of ether with stirring, after the addition is complete, stir at room temperature for about 30min, then cool to 5°C and stir for about 30min , filtered, and the filter cake was dried under vacuum at 40~50°C to obtain dapoxetine hydrochloride crystal form A, and the X-ray powder diffraction pattern was within the error range and figure 1 unanimous.
Embodiment 3
[0056] Dissolve 5g of dapoxetine hydrochloride sample in 50ml of methanol, heat to reflux to dissolve and clarify, add 200ml of n-pentane / methyl acetate (volume ratio 3:1) mixture to the reaction solution, after the addition is complete, cool to 0 ~5°C for static crystallization for about 60 minutes, filter, and vacuum-dry the filter cake at 50~60°C to obtain dapoxetine hydrochloride crystal form A, whose X-ray powder diffraction pattern is within the error range and figure 1 unanimous.
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com