Preparation method of dapoxetine hydrochloride racemate
A technology of cetine racemate and hydrochloric acid, applied in the field of preparation of dapoxetine hydrochloride racemate, can solve the problems of high cost, complicated operation, long steps and the like, and achieve the effects of convenient post-processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
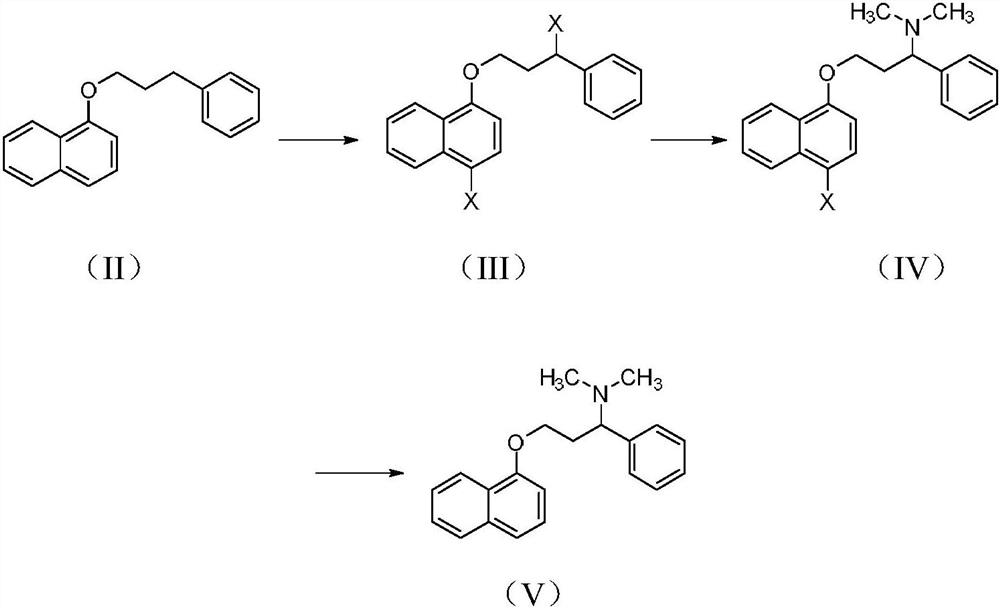
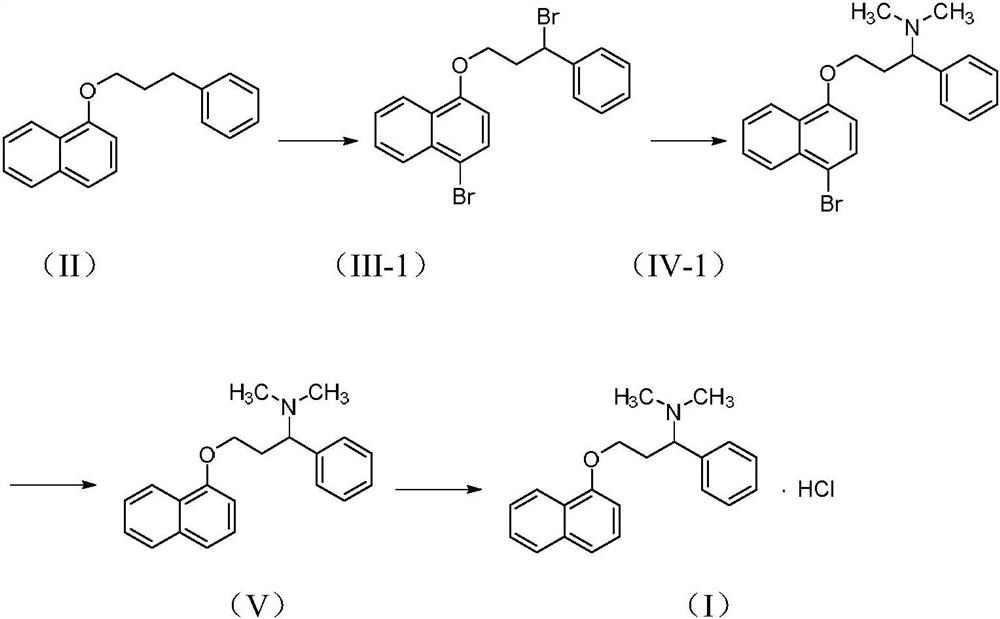
[0033] Synthesis of N,N-dimethylamino-3-(naphthyl-1-oxyl)-1-phenylpropan-1-amine hydrochloride (I)
[0034] Synthesis of 1-bromo-4-(3-bromo-3-phenylpropoxy)naphthalene(III-1)
[0035] Compound 1-(3-phenylpropoxy)naphthalene (II) (26.24g, 0.10mol) was dissolved in carbon tetrachloride (250mL), and benzoyl peroxide (0.25g) and NBS (35.6g , 0.20mol), under the light of tungsten light, slowly heated to reflux within 1 hour, and kept reflux reaction for 4 hours, after the TLC detection reaction was complete, the mixture was cooled, the insolubles were removed by suction filtration, and the filtrate was concentrated under reduced pressure to obtain 41.5 g of reddish-brown oil, yield 98.7%, was directly put into the next reaction without purification.
[0036] Synthesis of 3-((4-bromonaphthalen-1-yl)oxy)-N,N-dimethyl-1-phenyl-propan-1-amine (IV-1)
[0037] Dissolve compound III-1 (41.5g, 98.78mmol) in 280ml of acetone, add 33% dimethylamine solution (33.7g, 246.6mmol), stir for 1 h...
Embodiment 2
[0043] Synthesis of 1-chloro-4-(3-chloro-3-phenylpropoxy)naphthalene(III-2)
[0044] Compound 1-(3-phenylpropoxy)naphthalene (II) (26.24g, 0.10mol) was dissolved in carbon tetrachloride (250mL), and benzoyl peroxide (0.25g) and NCS (29.4g , 0.22mol), under the light of tungsten light, slowly heated to reflux within 1 hour, and kept the reflux reaction for about 4 hours. After the TLC detection reaction was complete, the mixture was cooled, the insolubles were removed by suction filtration, and the filtrate was concentrated under reduced pressure. 30.8 g of reddish-brown oil was obtained with a yield of 93.0%, which was directly put into the next reaction without purification.
[0045] Synthesis of 3-((4-bromonaphthalen-1-yl)oxy)-N,N-dimethyl-1-phenyl-propan-1-amine (IV-2)
[0046] Dissolve compound III-2 (17.0g, 51.3mmol) in 120ml of acetone, add 33% dimethylamine solution (21.0g, 153.7mmol), stir for 1 hour under ice bath, and then reflux for about 5 hours under slight press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com