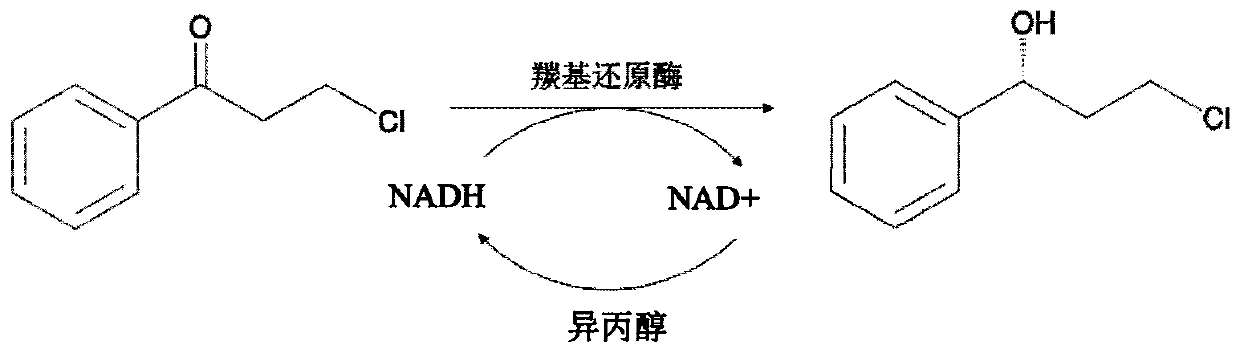
Method for preparing dapoxetine intermediate by using carbonyl reductase
A technology of dapoxetine and reductase is applied in the field of preparing dapoxetine intermediates by using carbonyl reductase, and can solve the problems of cheap preparation of unfavorable intermediates, complicated steps, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of gene encoding carbonyl reductase
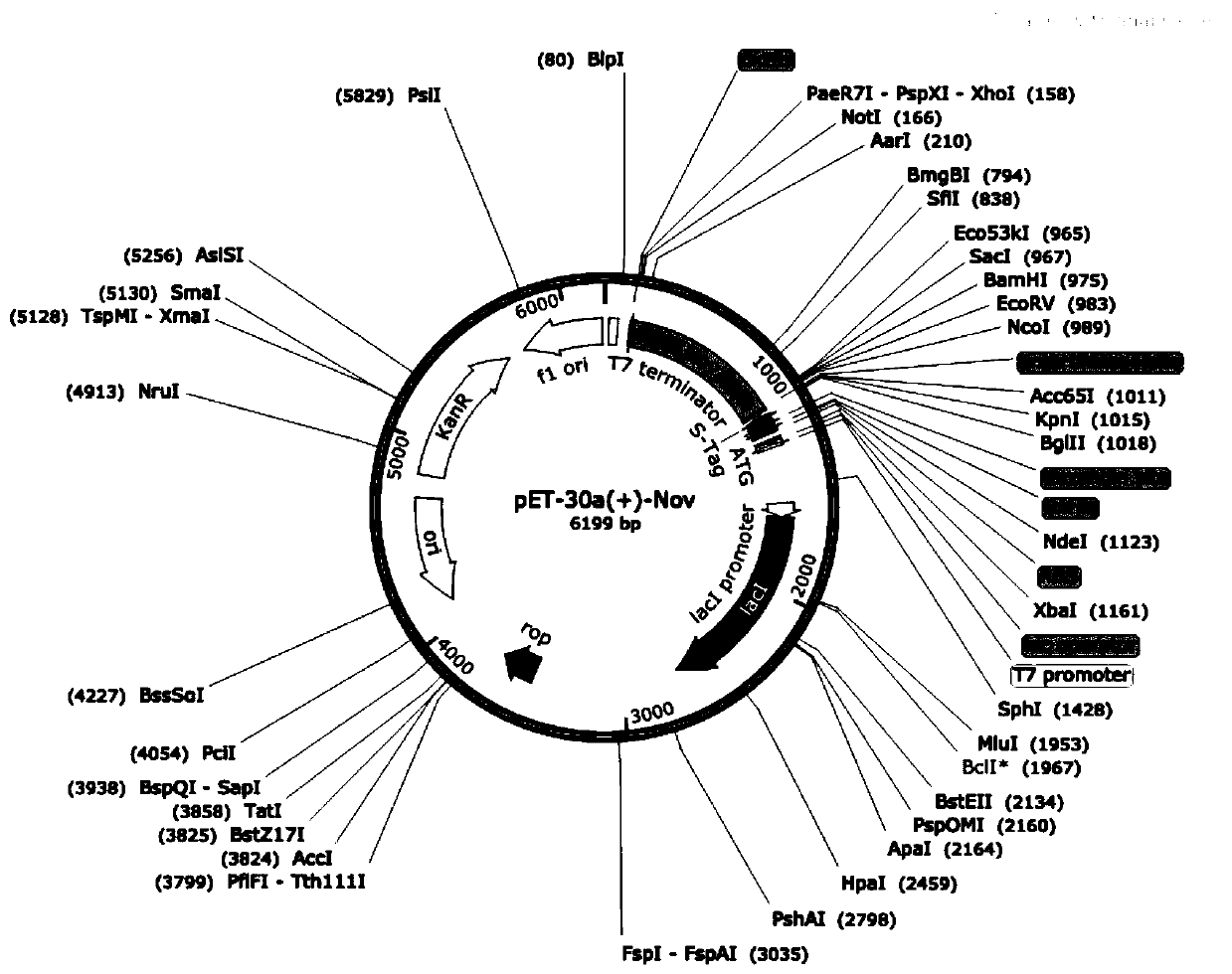
[0031] According to the original gene sequence of carbonyl reductase (NCBI Reference Sequence: WP_011906790.1), the codon optimization of the original gene sequence was carried out according to the codon analysis table of Escherichia coli and the total synthesis of the target gene was carried out. Shanghai) Co., Ltd., the obtained coding gene sequence is shown in SEQ ID NO.1, and the amino acid sequence of the translated carbonyl reductase is shown in SEQ ID NO.2. Both ends of the synthesized coding gene were cut with BamI and XhoI sites respectively and connected to pET30a(+) to obtain the recombinant expression vector pET30a(+)-Nov (see figure 2 ).
Embodiment 2
[0032] Example 2: Expression of carbonyl reductase
[0033] LB plate: peptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, 1.5% agar, solvent is deionized water, pH7.2.
[0034] LB culture medium: peptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, solvent is deionized water, pH7.2.
[0035] The recombinant expression vector pET30a(+)-Nov constructed in Example 1 was transformed into Escherichia coli BL21(DE3) by transformation method to obtain recombinant Escherichia coli BL21(DE3)-Nov. Spread onto LB plates containing 50 μg / mL kanamycin and culture overnight at 37°C. On the next day, pick a single colony, inoculate the single colony into 5 mL of LB culture solution supplemented with 50 μg / mL kanamycin, and cultivate overnight at 37° C. with shaking. The next day, take 1 mL of the bacterial liquid and add it to 100 mL of LB culture solution containing 50 μg / mL kanamycin, and culture it with shaking at 37 °C until the OD600 reaches 3.0, then add IPTG to a final co...
Embodiment 3
[0036] Example 3: Kinetic parameters of carbonyl reductase
[0037] Under standard conditions (20° C., 101 KPa), the enzyme activity was measured by changing the concentration of the substrate in the reaction system of Example 4, and the corresponding kinetic constants were calculated according to the double reciprocal plotting method. The substrates used in the calculation of kinetic constants and their concentrations are as follows: (3)-chloro-(1)-propiophenone (0-50.0 mM). The apparent kinetic parameters of the corresponding substrates are shown in Table 1. Each unit of enzyme can catalyze 4.945mM substrate, and it only takes 0.11 seconds to catalyze 1 molecule of substrate.
[0038] Table 1 Apparent kinetic parameters of carbonyl reductase Nov
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com