PDE5 inhibitor and dapoxetine coated chip and preparation method thereof
A technology of dapoxetine and PDE5, which is applied in the field of medicine and achieves the effects of good compliance, improved efficacy and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
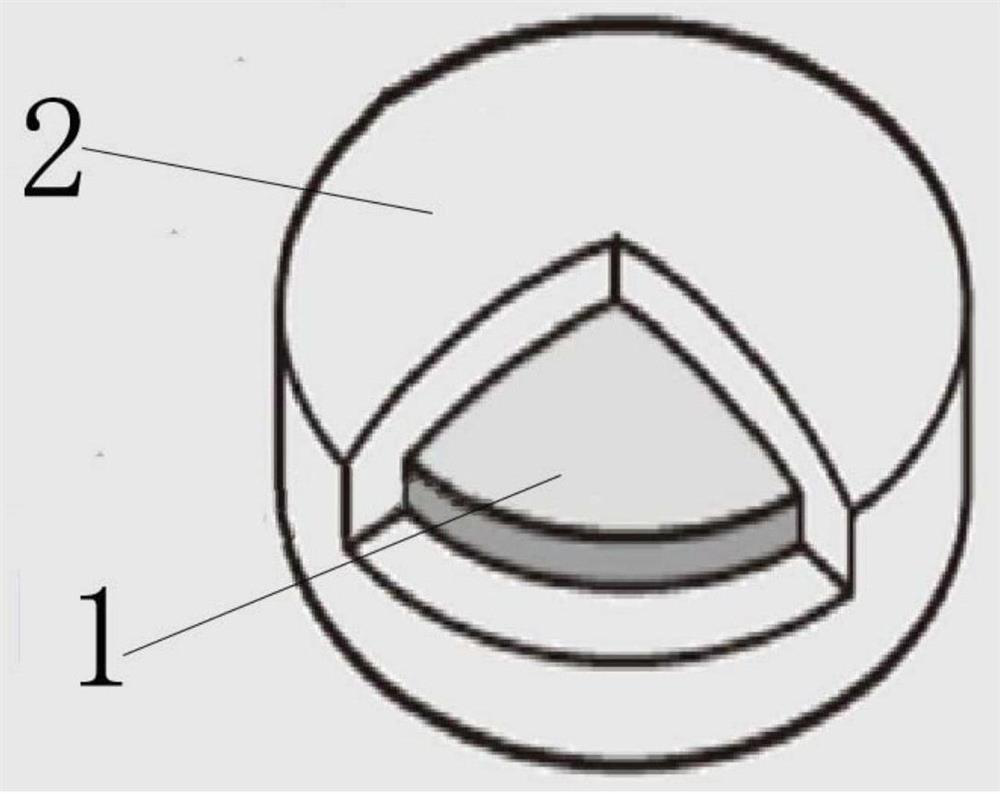
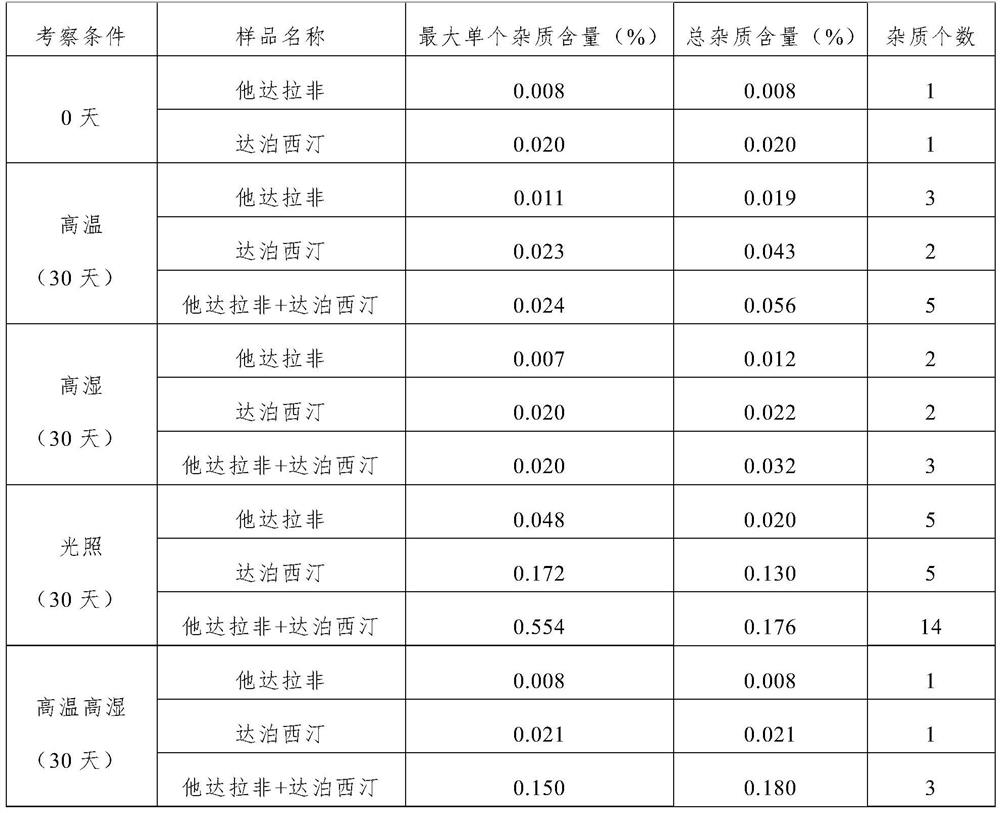
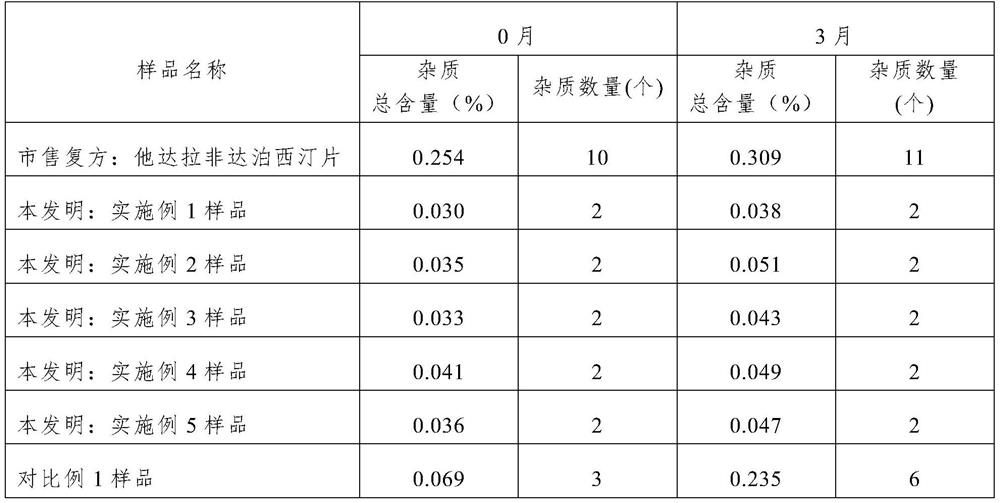
Image
Examples
Embodiment 1
[0080] Embodiment 1: Tadalafil dapoxetine pack chip
[0081] ① Preparation of tablet core
[0082] Weigh 560g of dapoxetine hydrochloride, 13g of silicon dioxide, 407g of lactose, and 10g of croscarmellose sodium, place in a hopper mixer, 10 rpm, mix for 15 minutes, add 10g of magnesium stearate, continue Mix for 5 minutes to obtain the tablet core blend material; put the tablet core blend material in the tablet press, and punch the tablet with a 5mm circular flat oblique, so that the tablet weight is about 60mg, and the hardness is 20-40N, that is, Dapoxime Ting tablets and chips. The plain tablet is placed in a coating pan, coated with gastric-soluble hydroxypropyl methylcellulose coating powder, and the weight is increased by about 3%, and the dapoxetine tablet core is obtained.
[0083] ② Preparation of shell material
[0084] Weigh 7g of hydroxypropyl cellulose, 2.8g of sodium lauryl sulfate, place in 175ml of purified water, stir to dissolve, and use it as a binder; w...
Embodiment 2
[0087] Embodiment 2: Tadalafil dapoxetine pack chip
[0088] ① Preparation of tablet core
[0089] Weigh 448g of dapoxetine hydrochloride, 13g of silicon dioxide, 30g of croscarmellose sodium, and 1g of red iron oxide, place them in a material bag for premixing, and pass through a 40-mesh sieve to obtain a premixed material; Place in a hopper mixer, add 498g of lactose, 10 rpm, mix for 15 minutes, add 10g of magnesium stearate, and continue mixing for 5 minutes to obtain the tablet core blend material; put the tablet core blend material into the tablet , with a 5mm round flat oblique punching tablet, so that the tablet weight is about 50mg, the hardness is 20-40N, and the dapoxetine tablet core is obtained.
[0090] ② Preparation of shell materials
[0091] Weigh 7g of hydroxypropyl cellulose, 2.8g of sodium lauryl sulfate, place in 175ml of purified water, stir to dissolve, and use it as a binder; weigh 40g of tadalafil, 715.2g of lactose, and croscarmellose Sodium 36g, hy...
Embodiment 3
[0094] Embodiment 3: Tadalafil dapoxetine pack chip
[0095] ① Preparation of tablet core
[0096] Weigh 224g of dapoxetine hydrochloride, 10g of silicon dioxide, 30g of croscarmellose sodium, and 1g of red iron oxide, place them in a material bag for premixing, and pass through a 40-mesh sieve to obtain a premixed material; Place in a hopper mixer, add 730g of lactose, 10 rpm, mix for 15 minutes, add 5g of magnesium stearate, and continue mixing for 5 minutes to obtain the tablet core blend material; put the tablet core blend material into the tablet , use a 5mm round flat oblique punching tablet, so that the tablet weight is about 50mg, and the hardness is 20-40N, that is, the dapoxetine tablet core is obtained.
[0097] ② Preparation of shell material
[0098]Weigh 7g of hydroxypropyl cellulose, 2.8g of sodium lauryl sulfate, place in 175ml of purified water, stir to dissolve, and use it as a binder; weigh 40g of tadalafil, 715.2g of lactose, and croscarmellose Sodium 36...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com