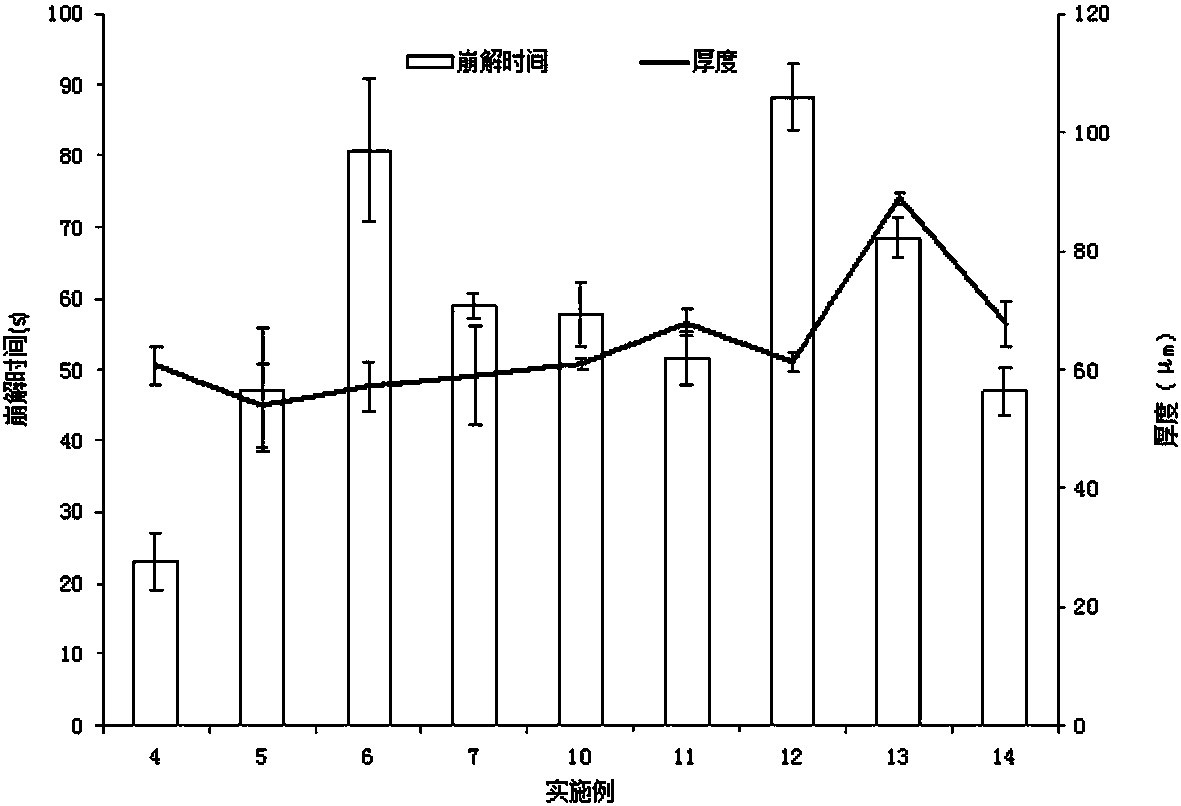
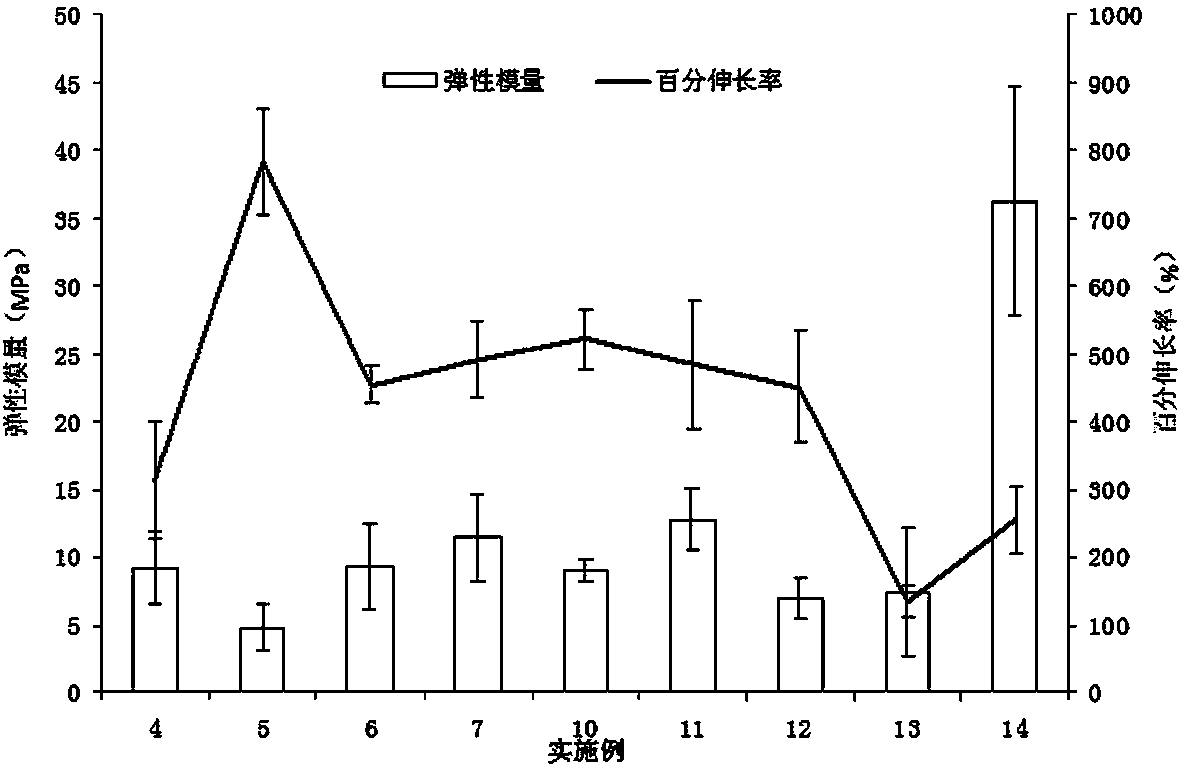
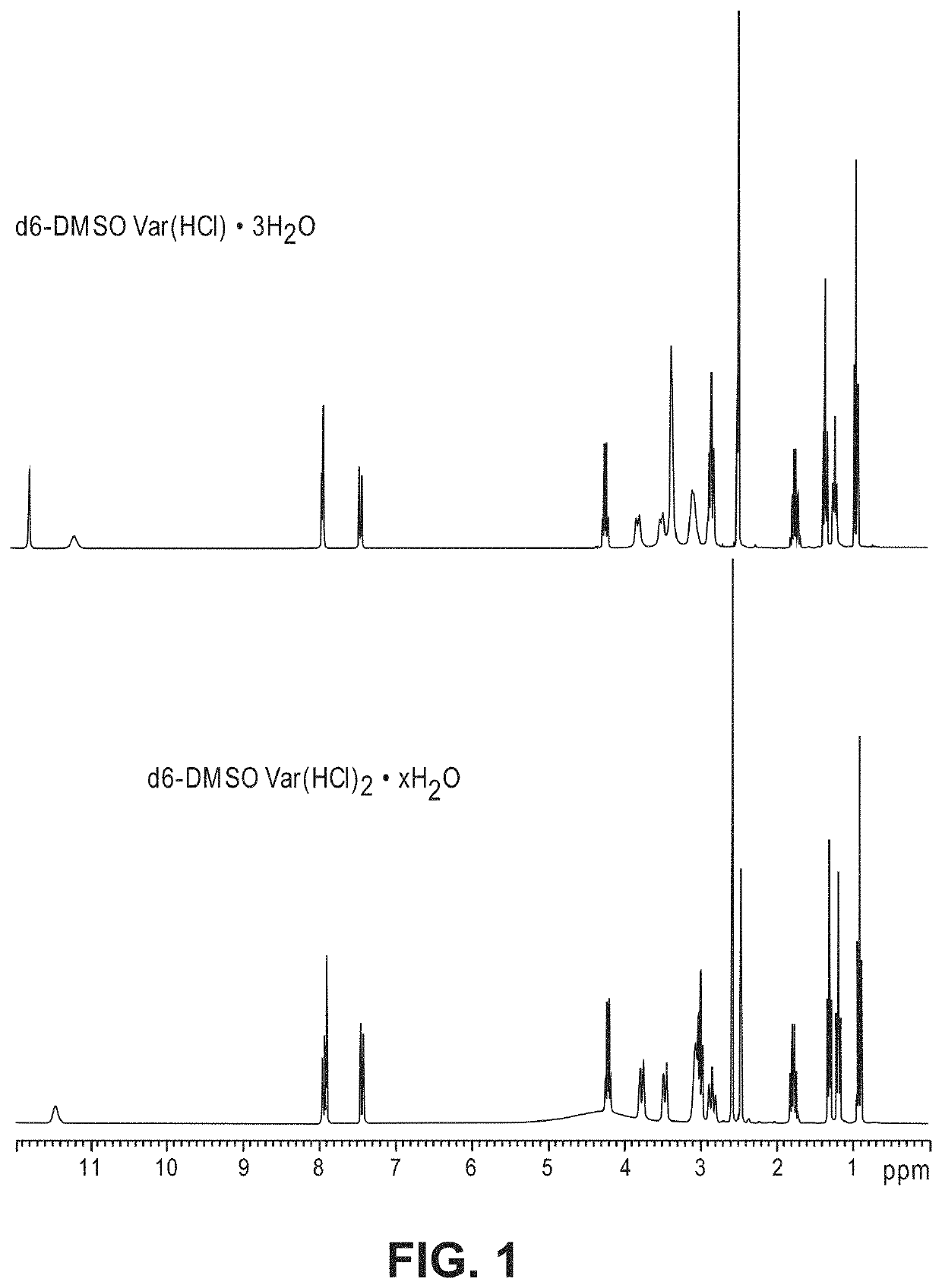
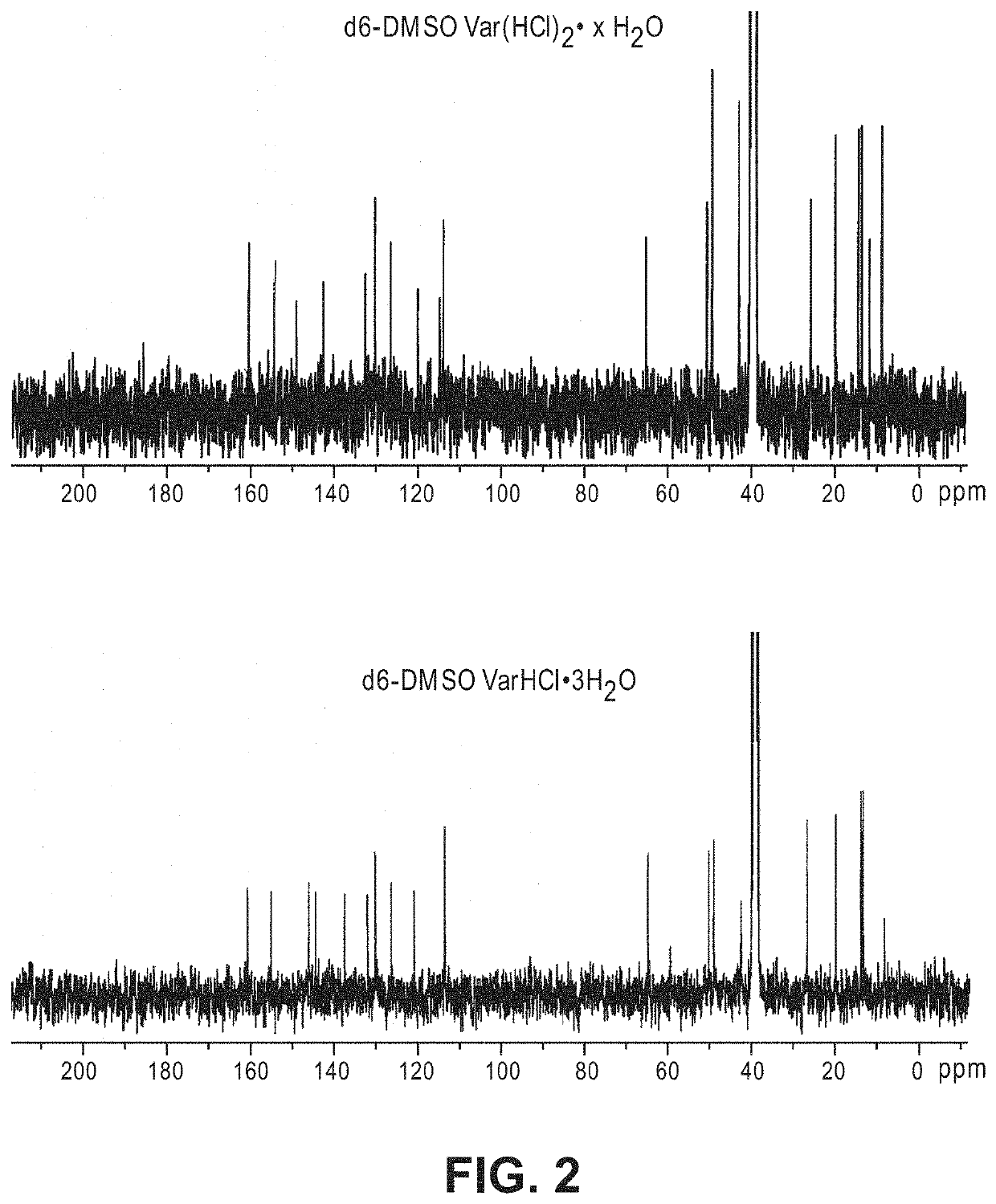
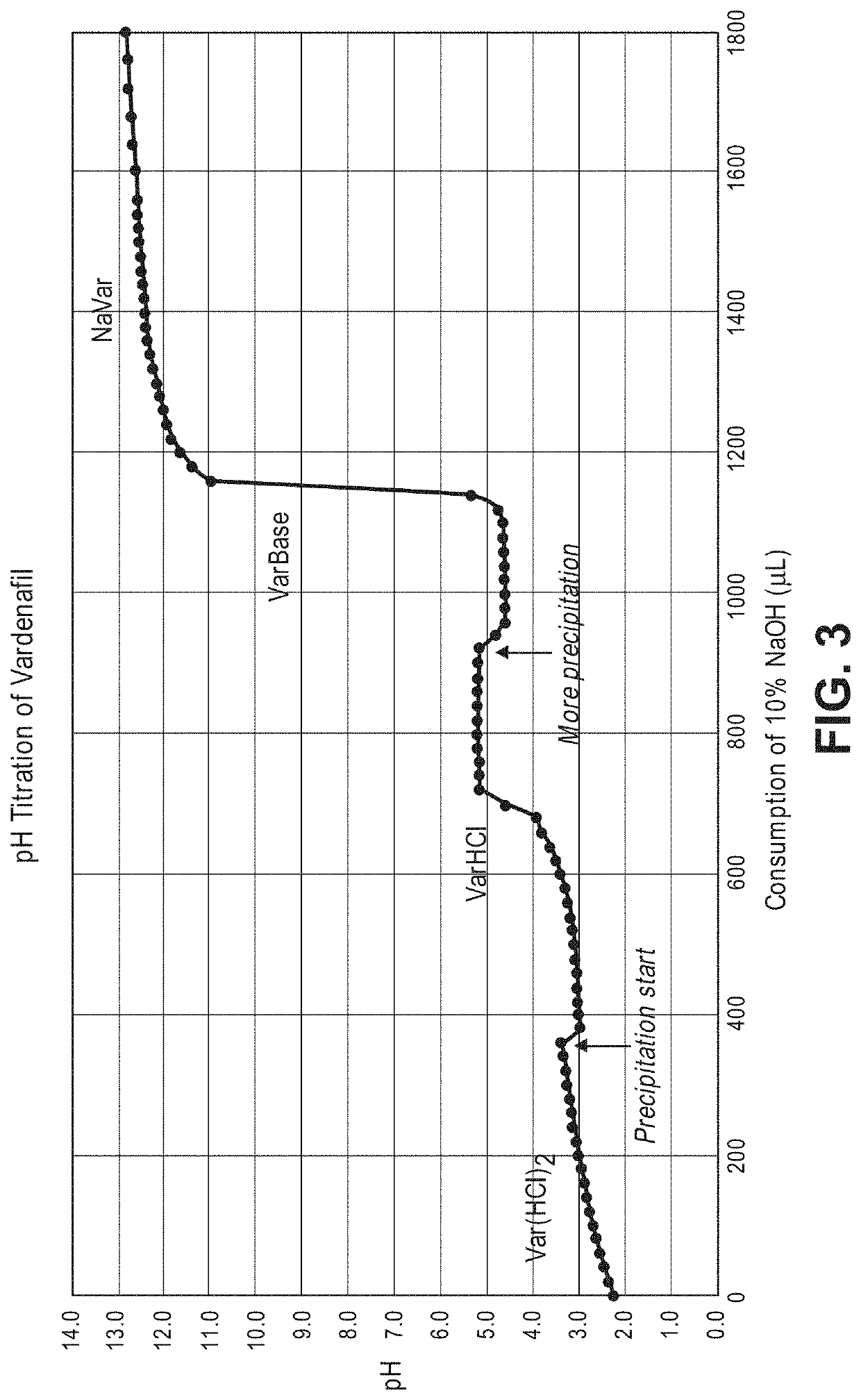
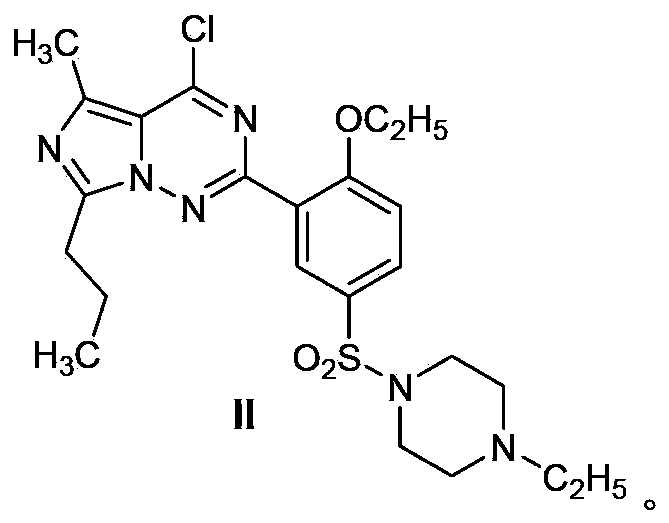
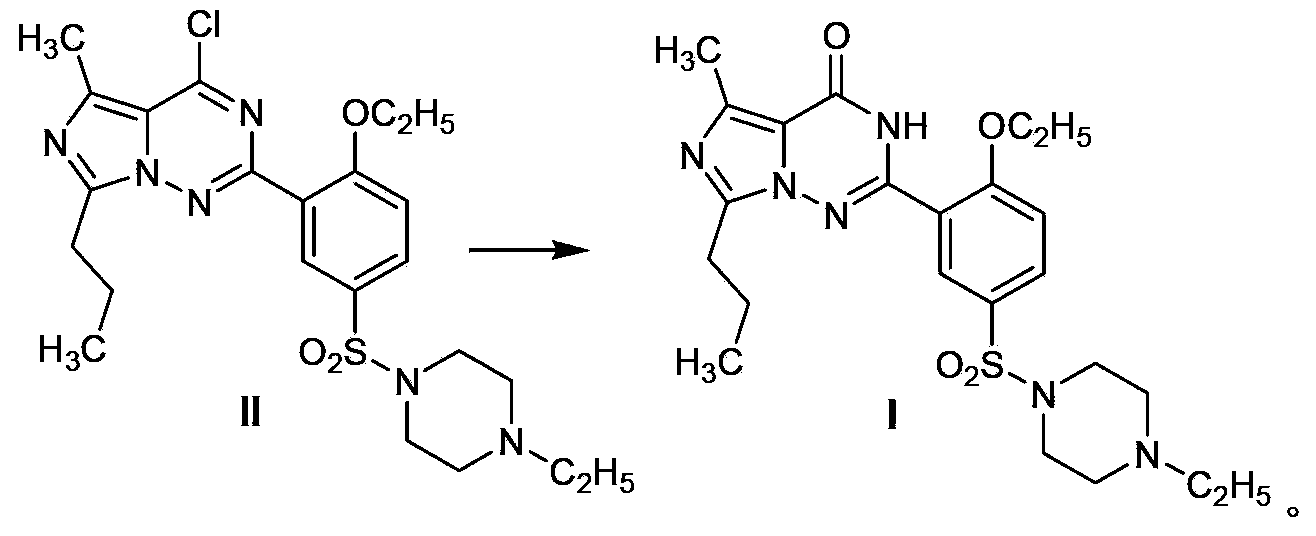
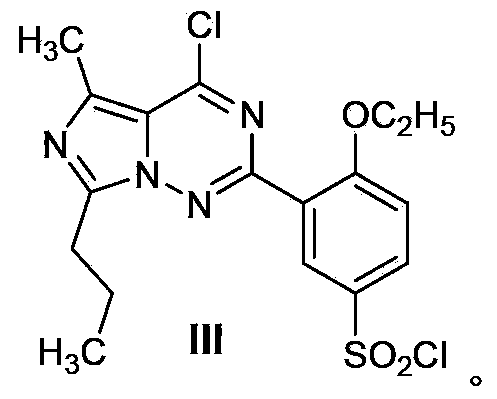
Patents
Literature
46 results about "Vardenafil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
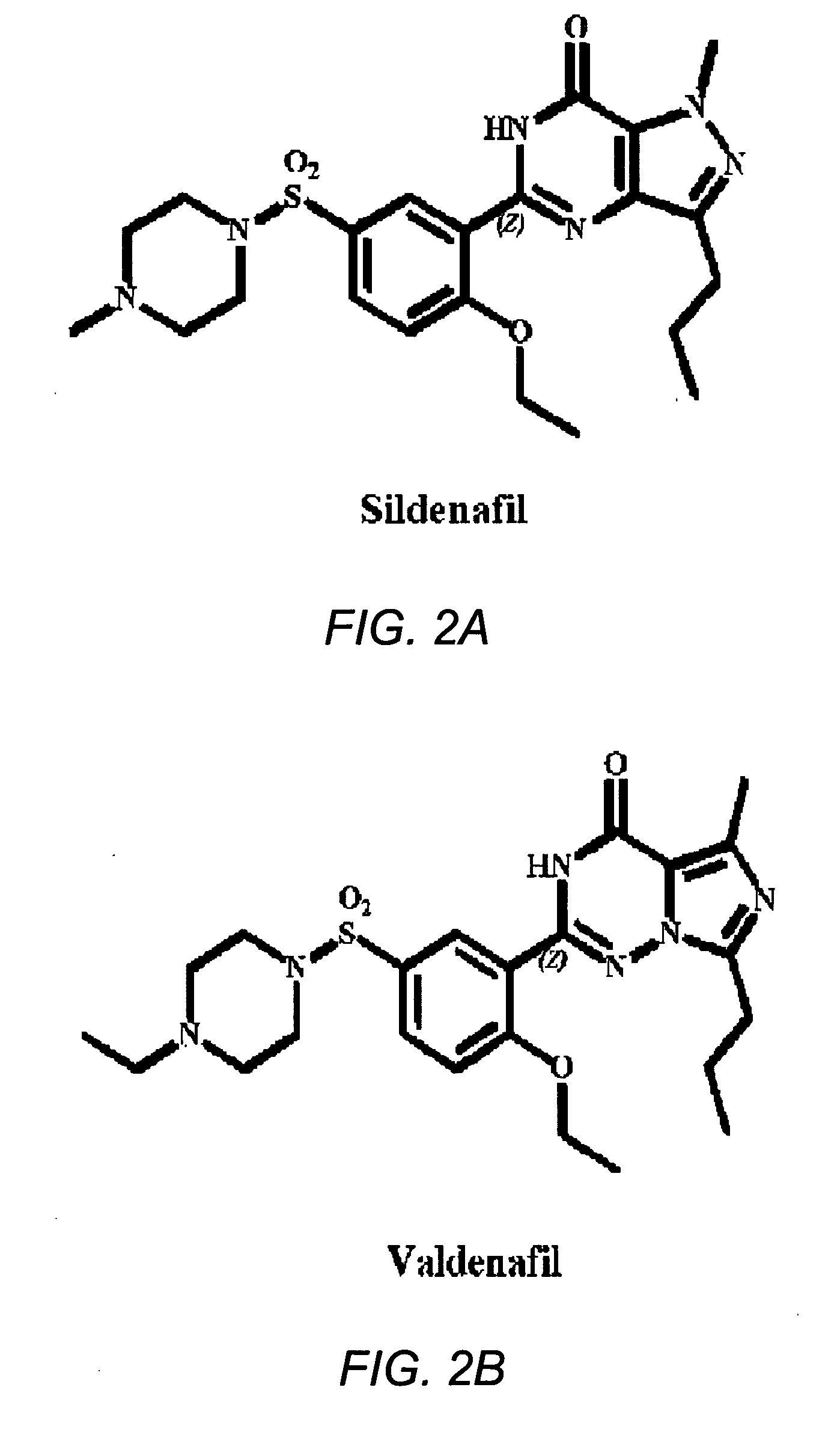
Vardenafil is used to treat male sexual function problems (impotence or erectile dysfunction-ED).
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
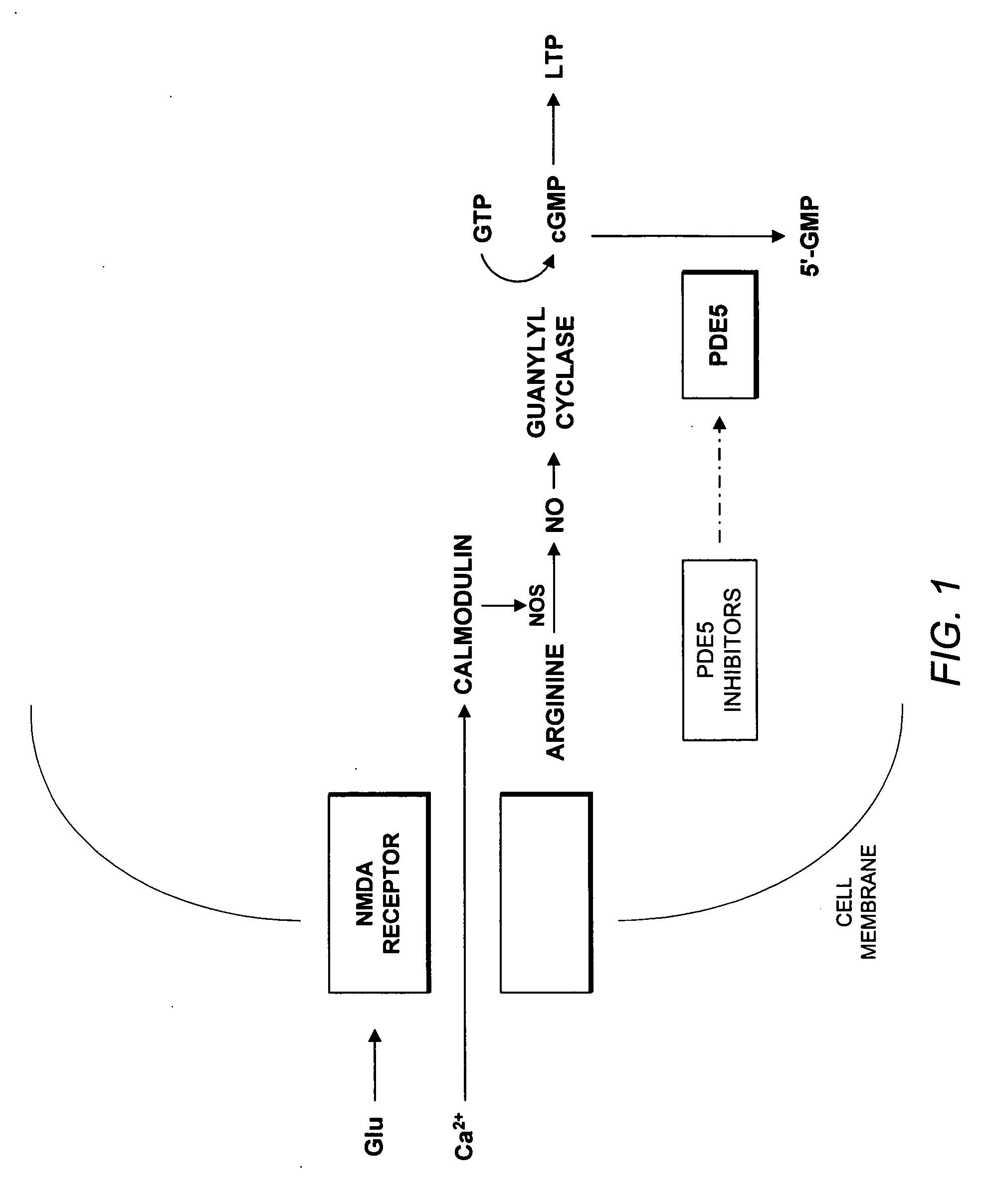
Use of phosphodiesterase 5 (PDE5) inhibitors in the treatment of schizophrenia
The use of phosphodiesterase 5 (PDE5) inhibitors for treatment of schizophrenia is described. Suitable PDE5 inhibitors for use for treatment of schizophrenia include sildenafil, vardenafil, tadalafil, E-8010, zaprinast, and E-4021. In one embodiment, for example, a method is described for treating schizophrenia in a patient which comprises treating the patient with an effective amount of a PDE5 inhibitor, or a pharmaceutically acceptable salt, solvate, or composition thereof. The PDE5 inhibitor may be administered orally. The PDE5 inhibitor may also be administered together with one or more conventional antipsychotic medications such as risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine, haloperidol, and fluphenazine.
Owner:SHARY CIRCLE
Oral quick-dissolving film preparation and preparation method thereof
InactiveCN102824333AImprove disintegration time limitSolve the shortcomings of taking waterPharmaceutical non-active ingredientsSexual disorderVardenafilTadalafil
The invention discloses an oral quick-dissolving film preparation and a preparation method thereof. The oral quick-dissolving film comprises the following components in percentage by weight: 20 to 40 percent of medicinal active component, 40 to 75 percent of water-soluble film forming material, 10 to 25 percent of plasticizer, 0 to 25 percent of disintegrating agent, and 0.1 to 8 percent of water, wherein the medicinal active component is one of sildenafil, tadalafil, vardenafil or salts thereof. According to the oral quick-dissolving film preparation, the disintegration time limited of the film preparation can be remarkably accelerated, the problem that most oral solid preparations should be taken with water can be solved, medicine taking time cannot be delayed under a condition without water, and the taking compliance of a patient can be improved.
Owner:SUZHOU UNIV
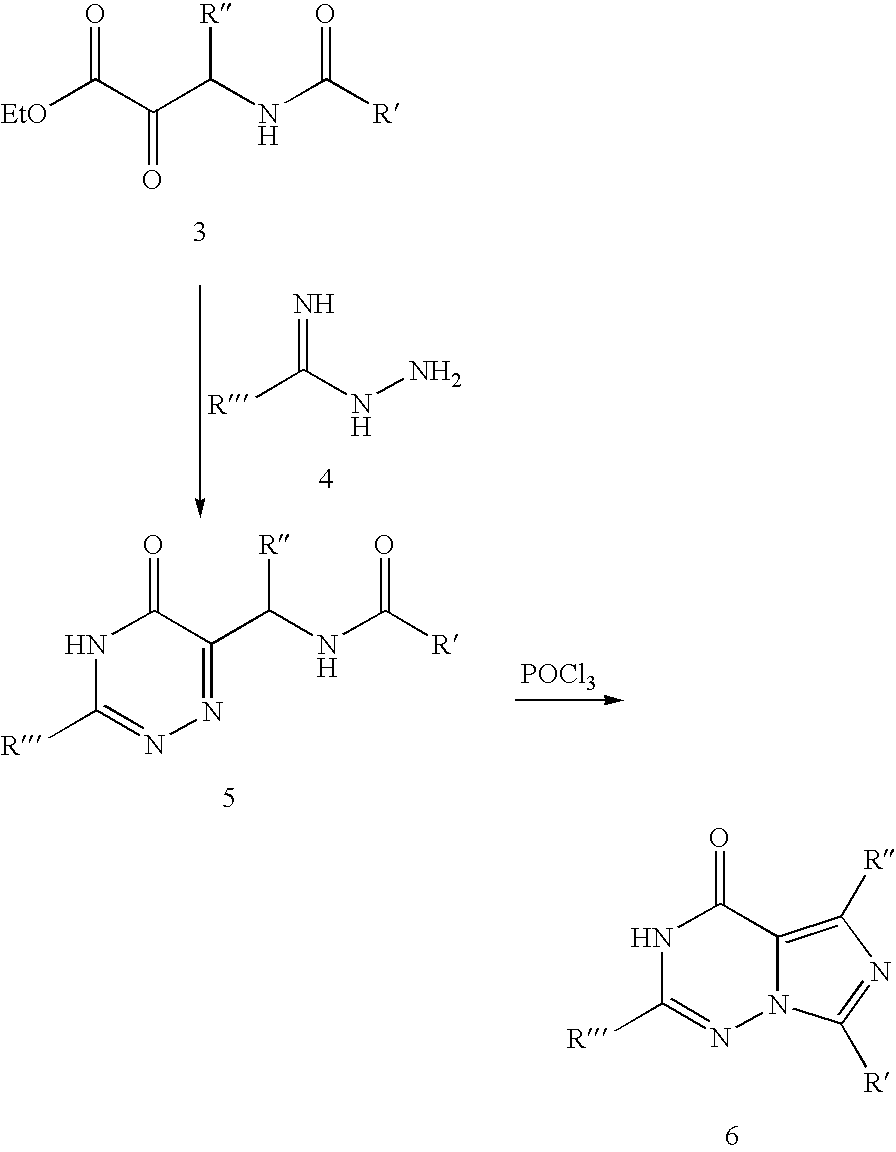
Methods for synthesizing imidazotriazinones
Methods of synthesizing imidazotriazinones, such as vardenafil, and compositions useful for the same are disclosed.
Owner:LEXICON GENETICS INC (US)
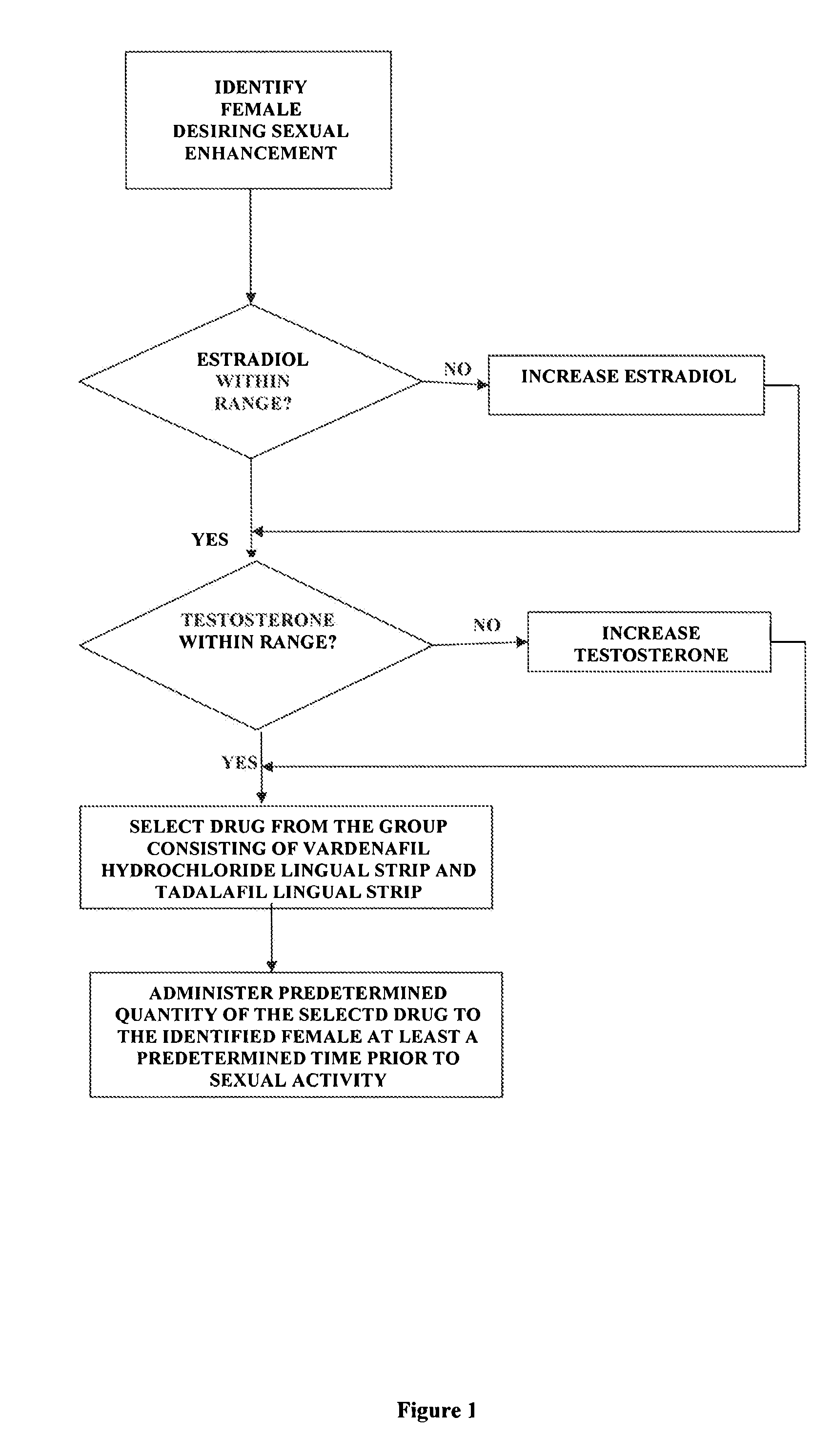
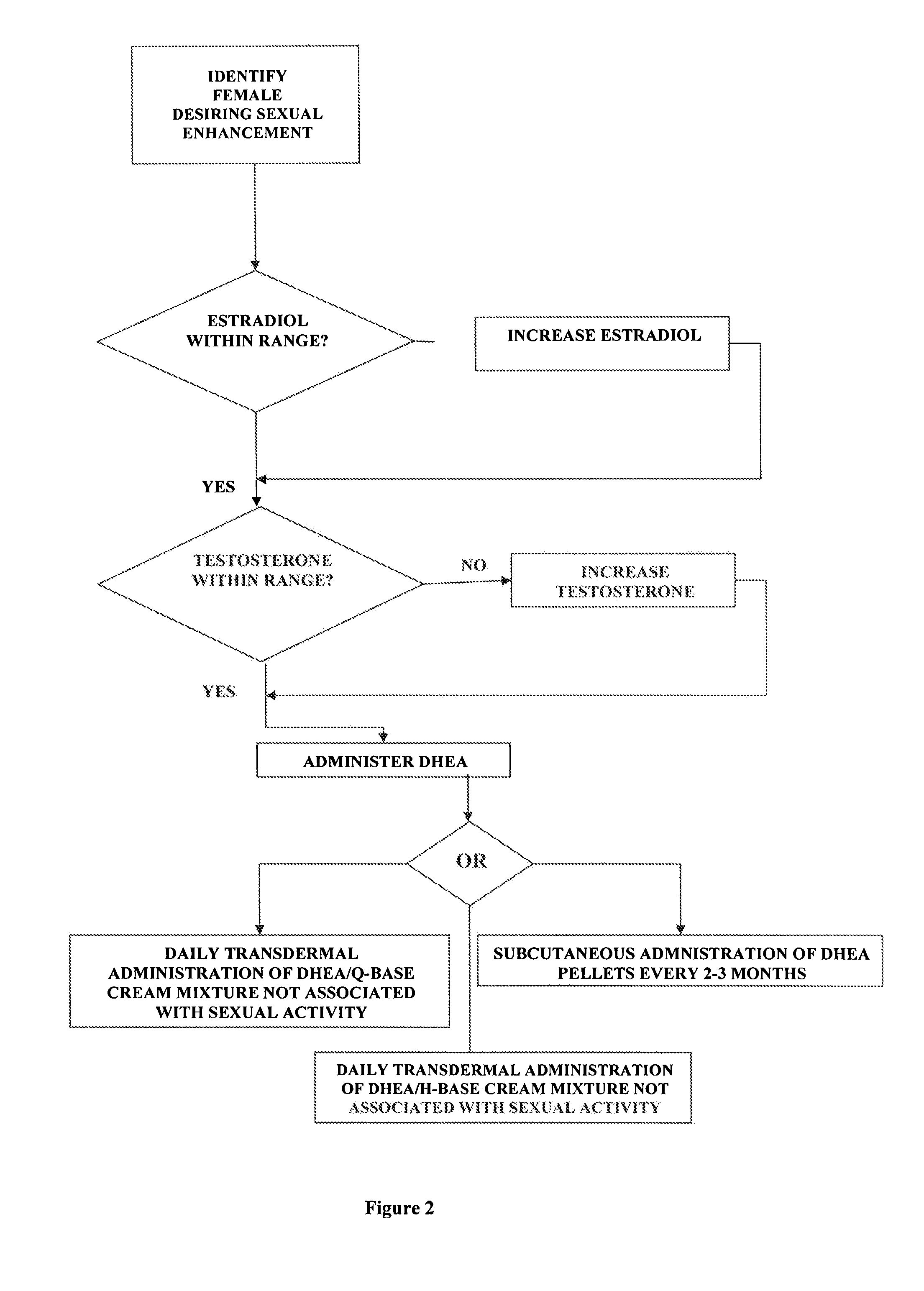
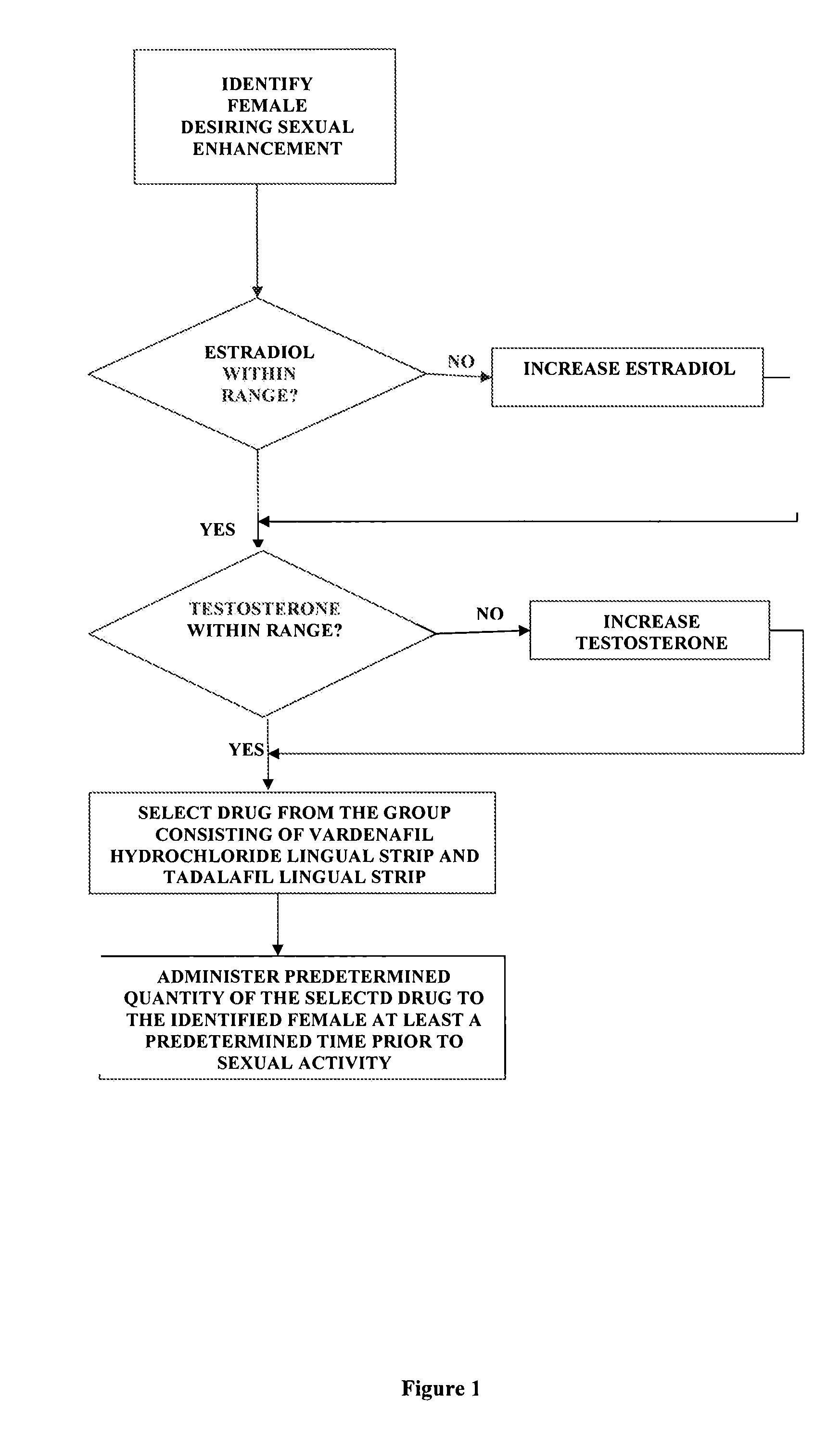
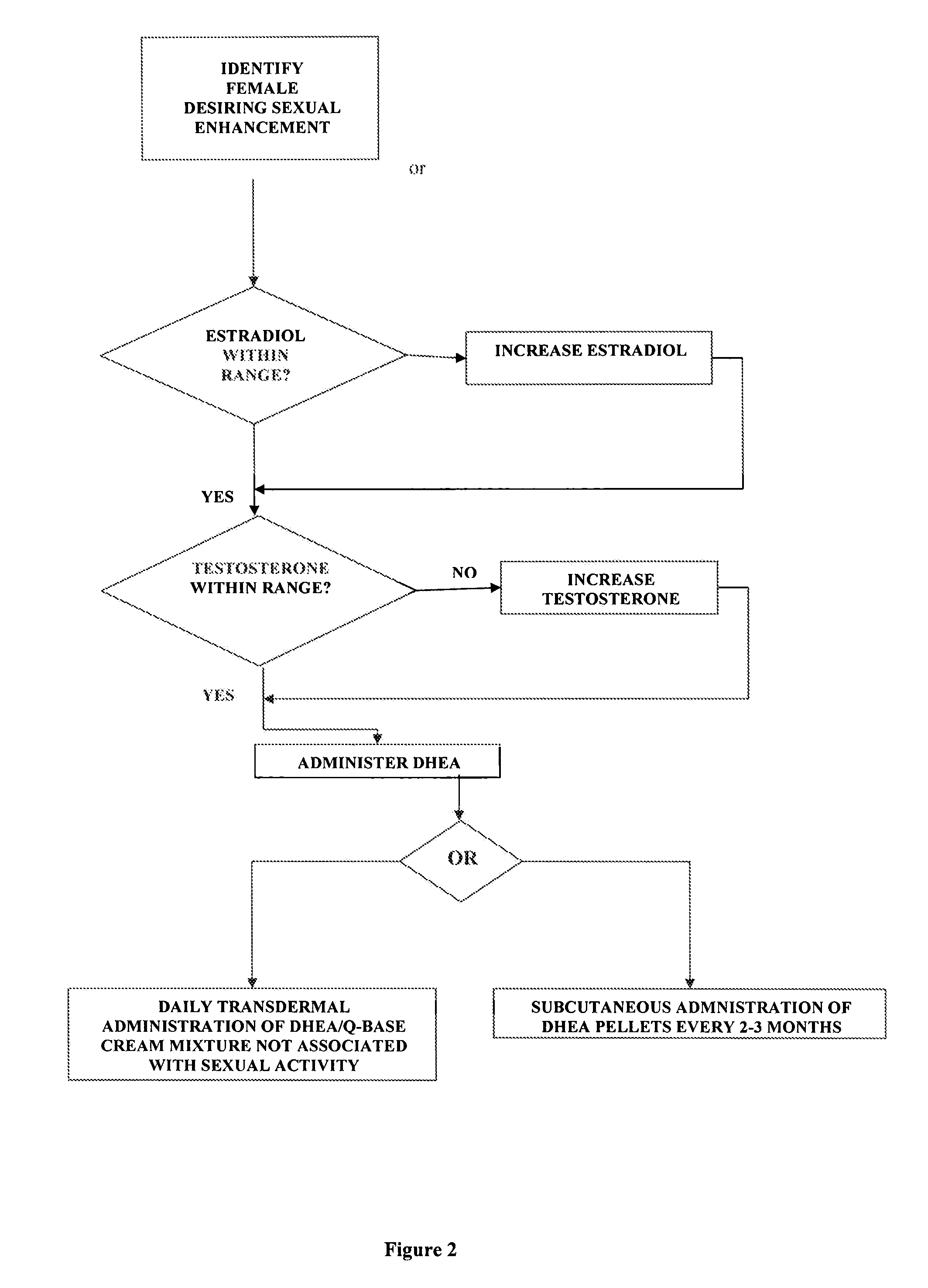
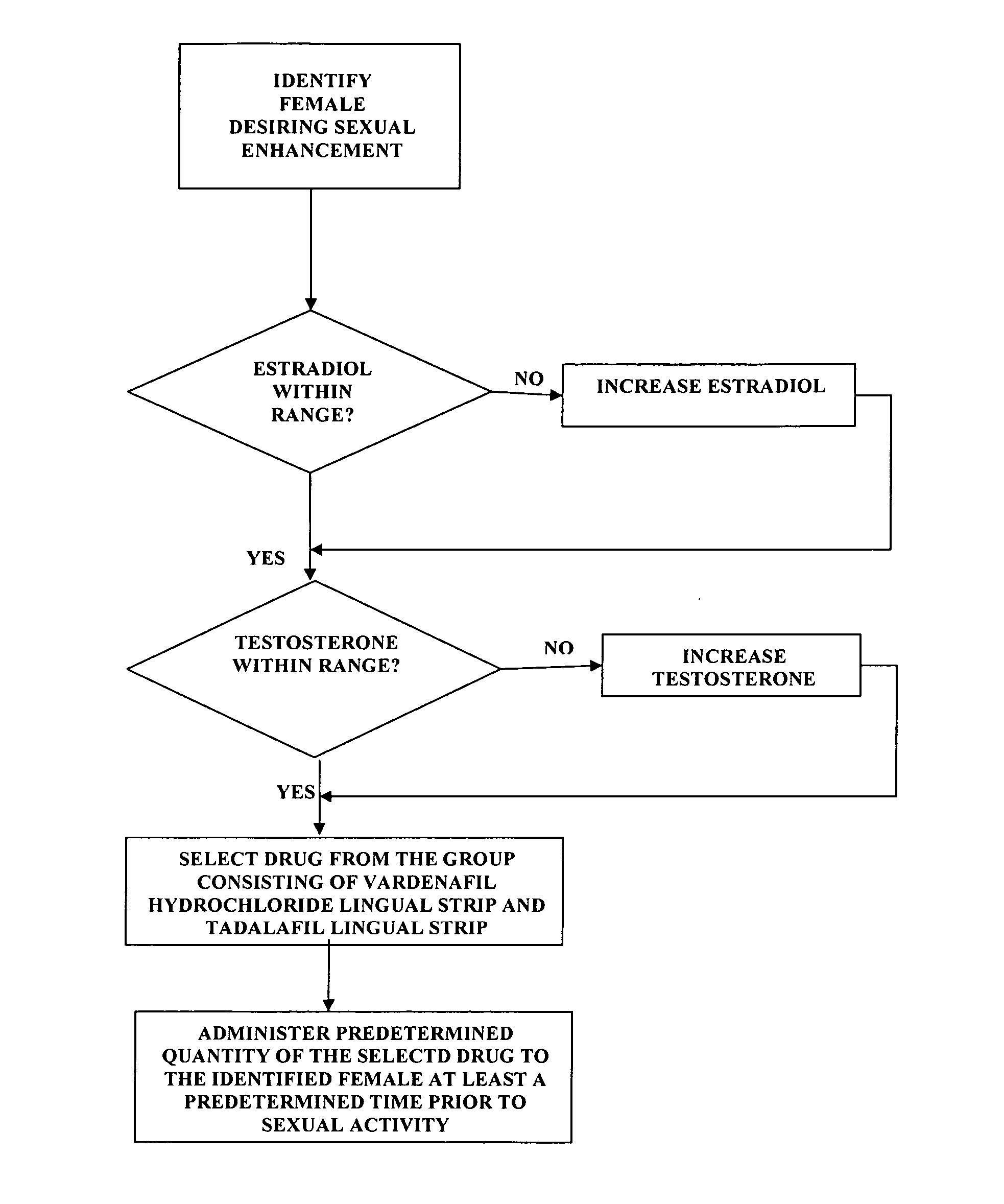
Methods of female sexual enhancement
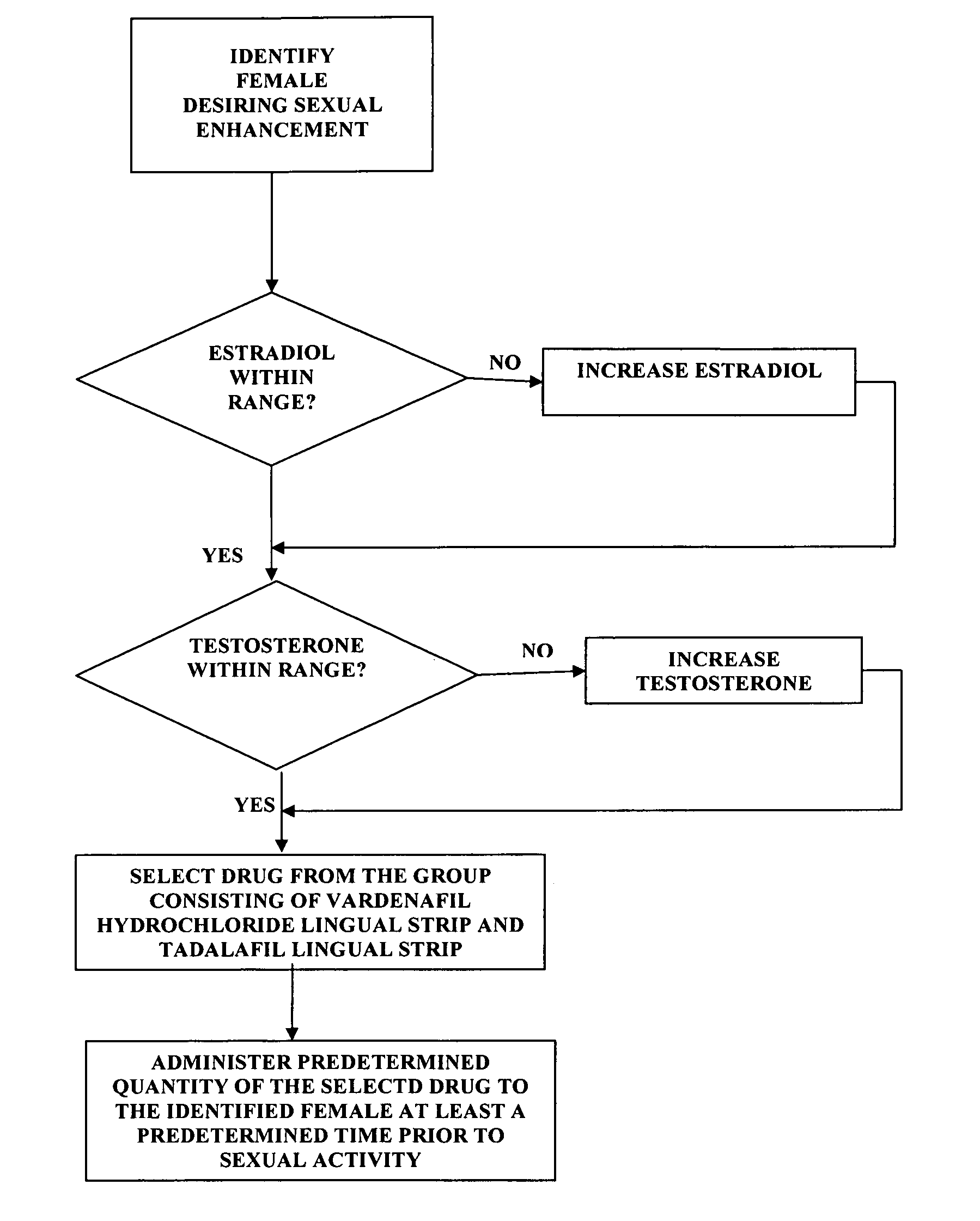
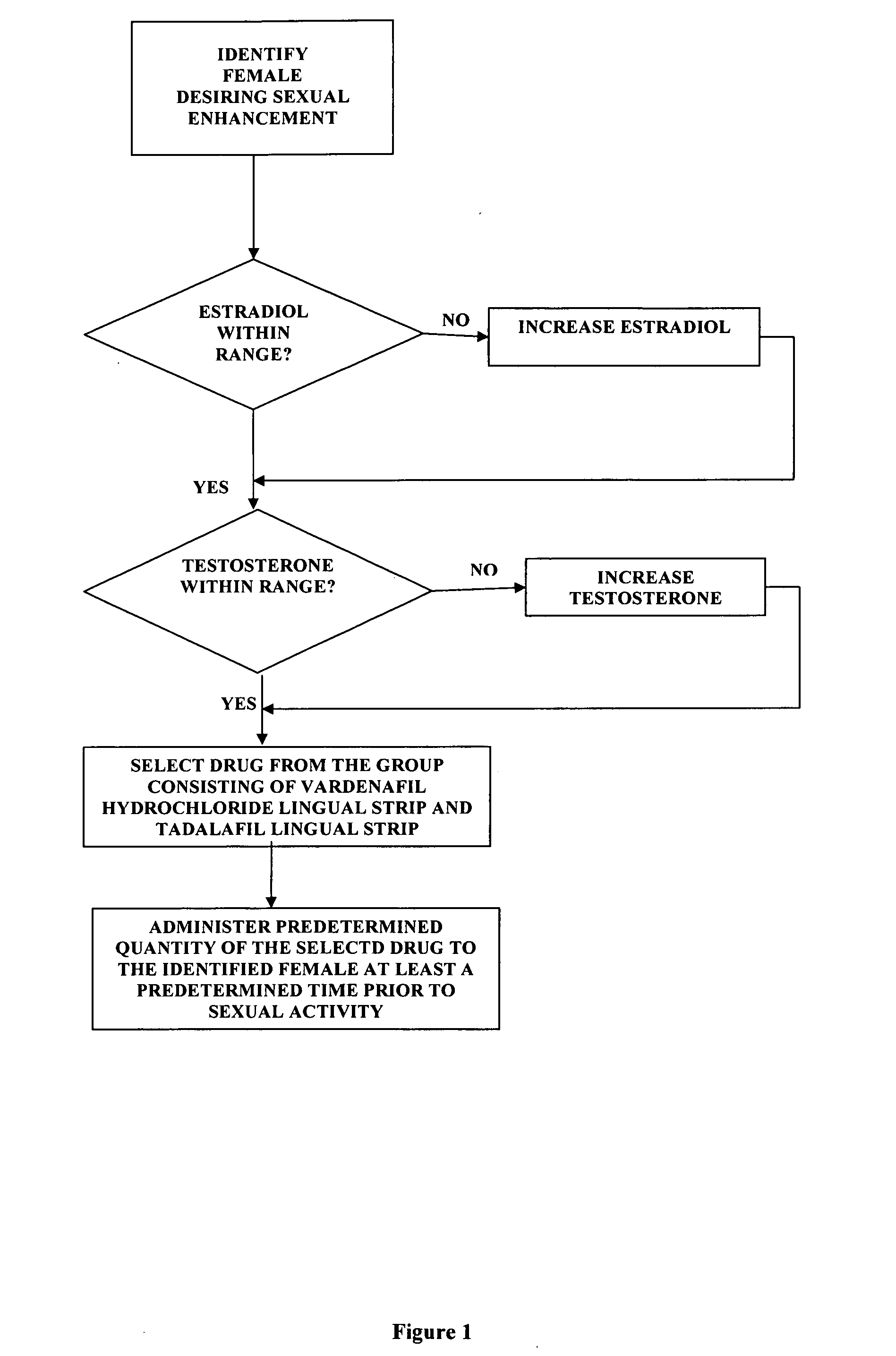
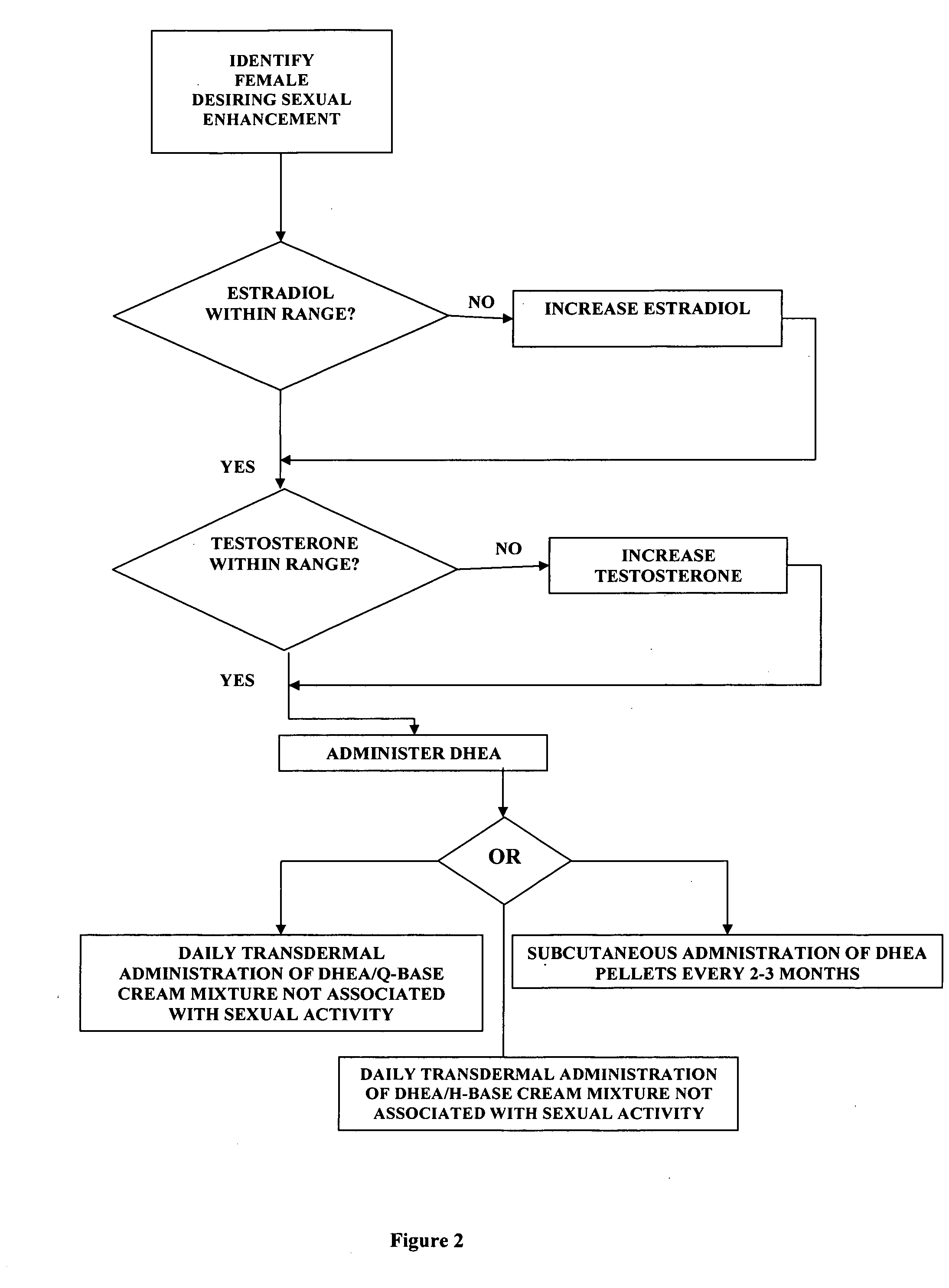
A method of sexual enhancement in women includes the steps of identifying a woman requesting sexual enhancement, assuring that the woman's blood includes estradiol within a first predetermined range and testosterone within a second predetermined range, and thereafter administrating a drug selected from the group consisting of vardenafil hydrochloride and tadalafil prior to sexual activity. The selected drug may be loaded into a starch strip which is then applied to the woman's tongue. Sexual enhancement in women can also be achieved by transdermal or subcutaneous application of the steroid hormone DHEA.
Owner:LES MEDECINS
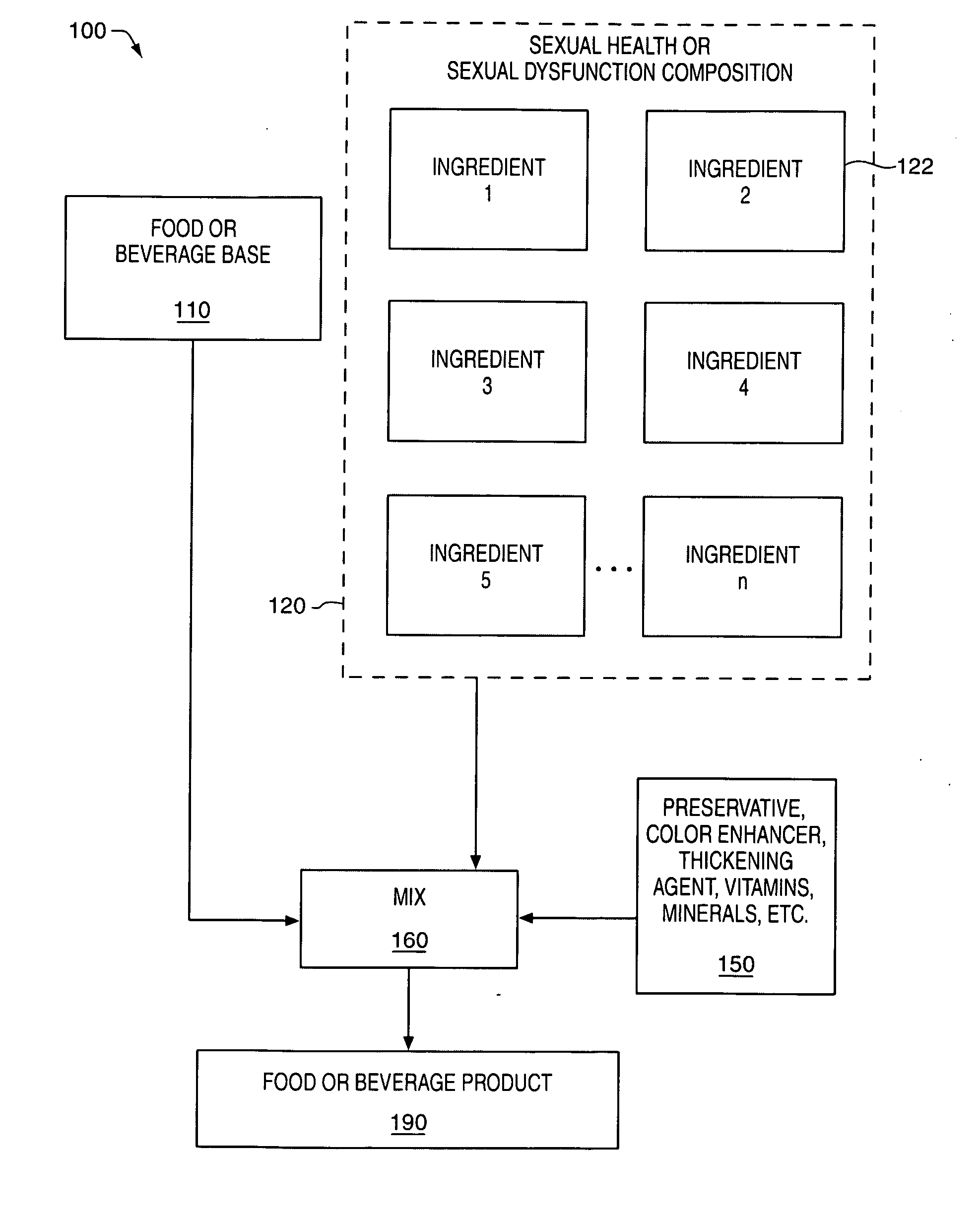
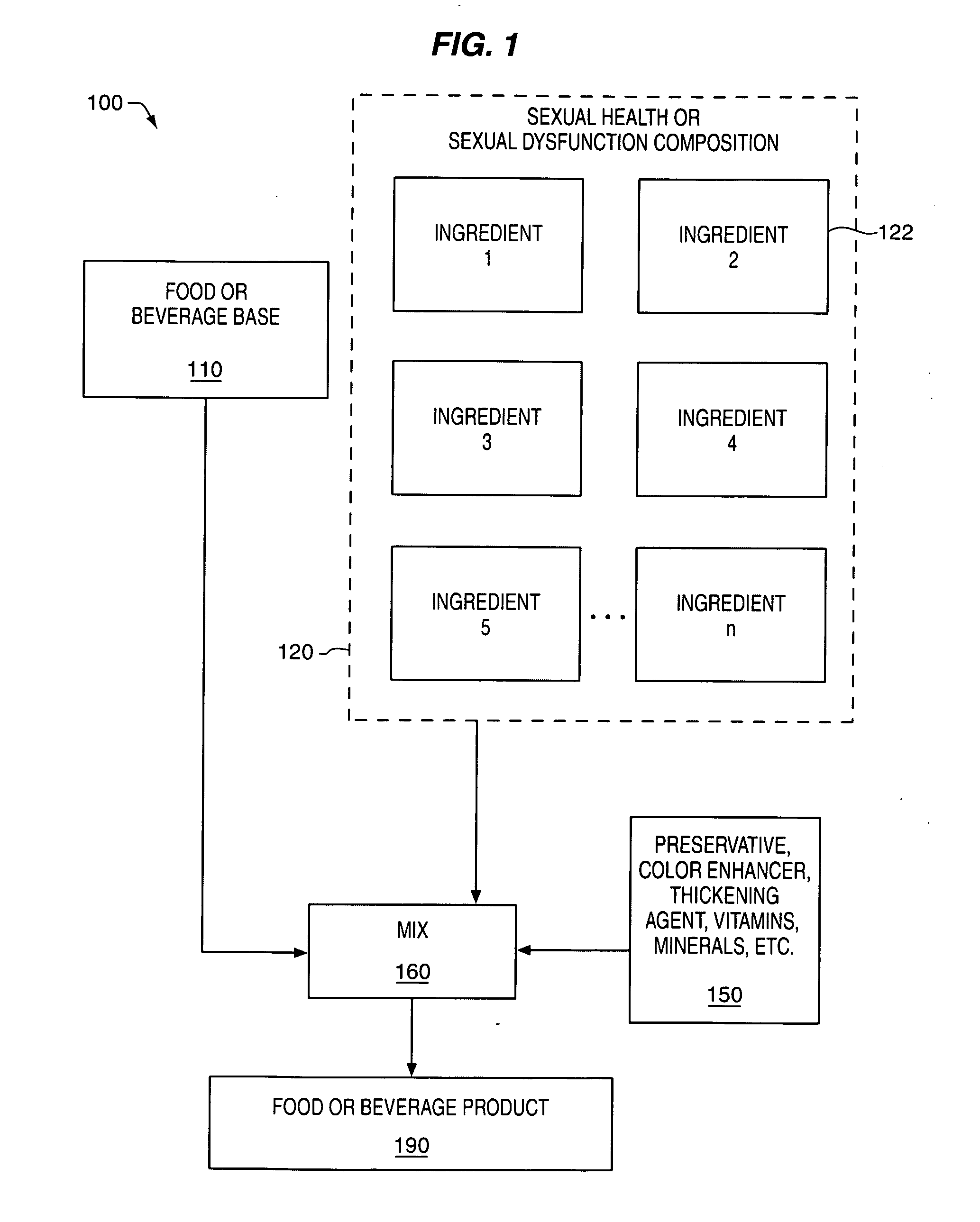
Delivery system and method for supporting and promoting healthy sexual function and prevention and treatment of sexual dysfunction
InactiveUS20060110478A1Increase in cGMPGood curative effectFood ingredient as antioxidantBiocideSexual functionVardenafil
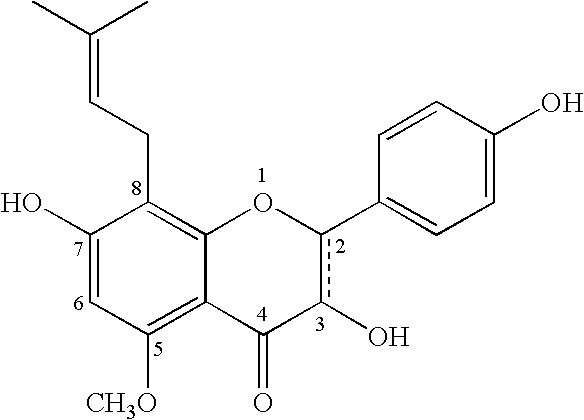
Improved delivery systems and delivery methods for supporting and promoting healthy sexual function, for preventing sexual dysfunction, or for treatment of sexual dysfunction. A compositions including one or more cGMP-specific PDE5 inhibitors and / or dopaminergic agonists is administered in the form of a breath-care strip, mint or lozenge, or a food or beverage product. The cGMP-specific PDE5 inhibitor comprises an ingredient selected from the group consisting of sophoflavescenol, vardenafil, tadalafil, and sildenafil. The dopaminergic agonist comprises apomorphine. Vitex agnus-castus extract, and one or more of lipoic acid, L-Arginine, folic acid, trimethylglycine, policosanol, carnitine, biotin, and acetyl L-Carnitine may also be included in the delivery vehicle.
Owner:MCCLEARY EDWARD LARRY +2
Methods of female sexual enhancement
InactiveUS20060252734A1Enhanced female sexual pleasureImprove satisfactionOrganic active ingredientsBiocideVardenafilGynecology
Owner:LES MEDECINS
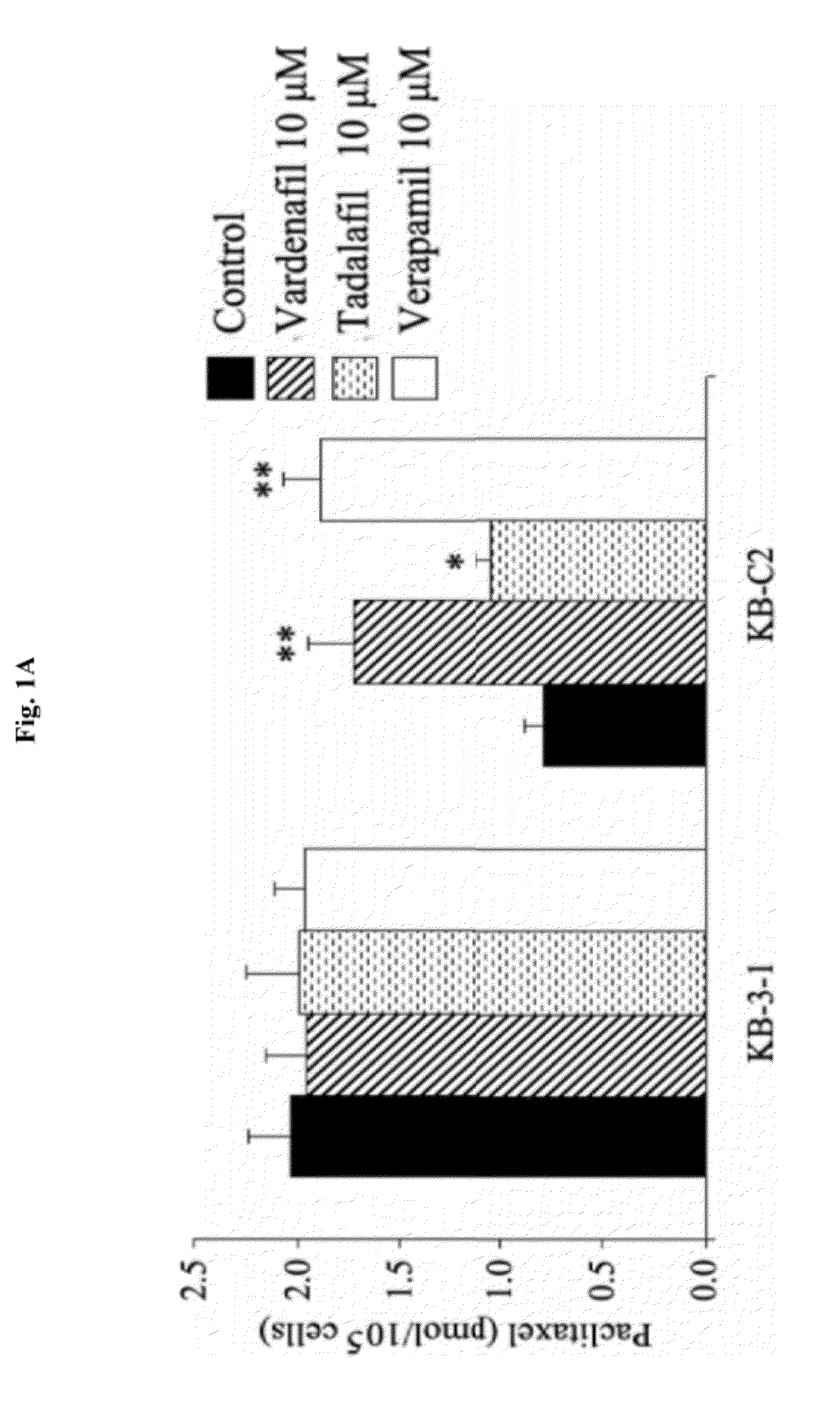
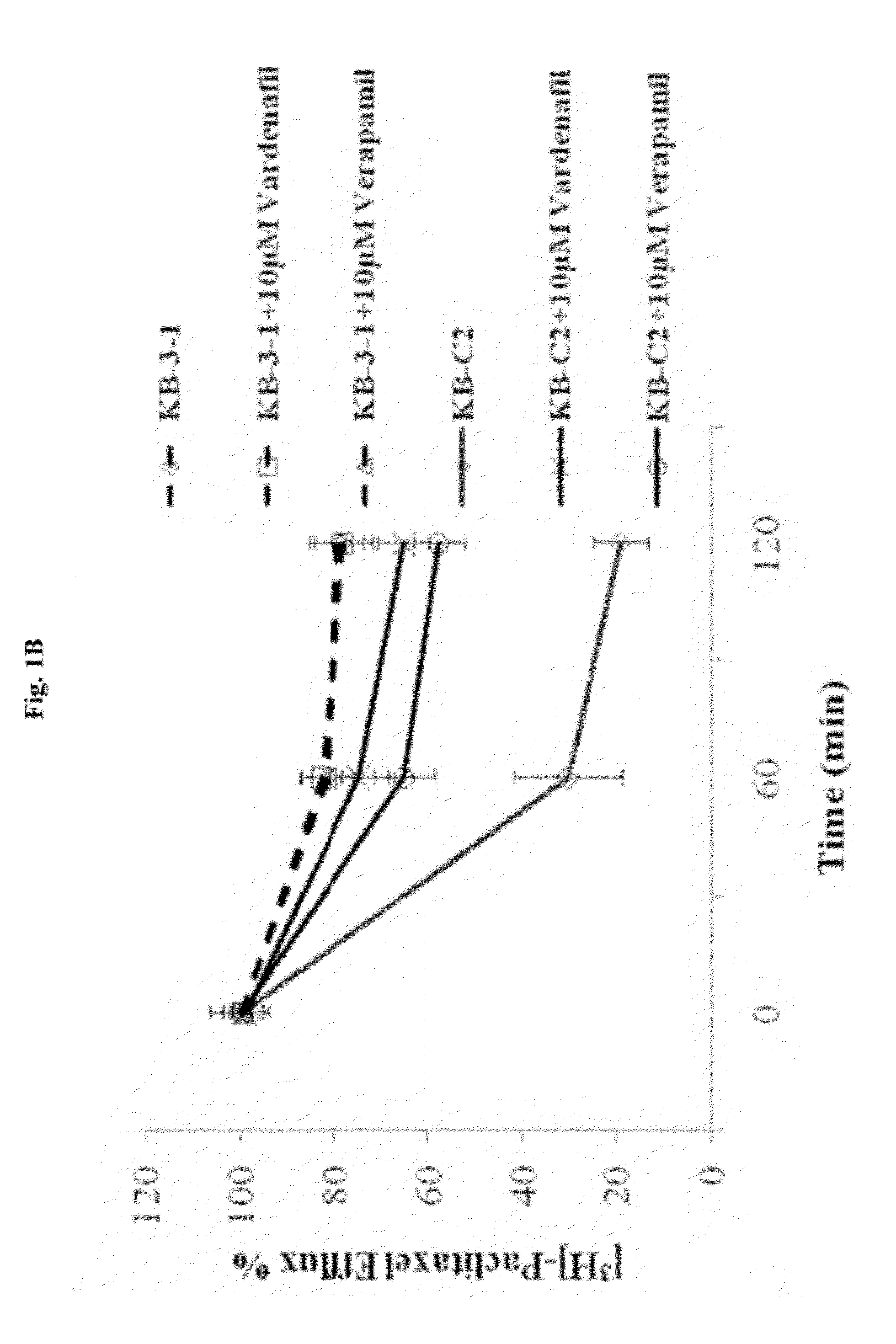
Use of phosphodiesterase inhibitors for treating multidrug resistance
InactiveUS20120252816A1Suppression problemInhibiting ABCG transporter activityBiocideAnimal repellantsVardenafilPde5 inhibition
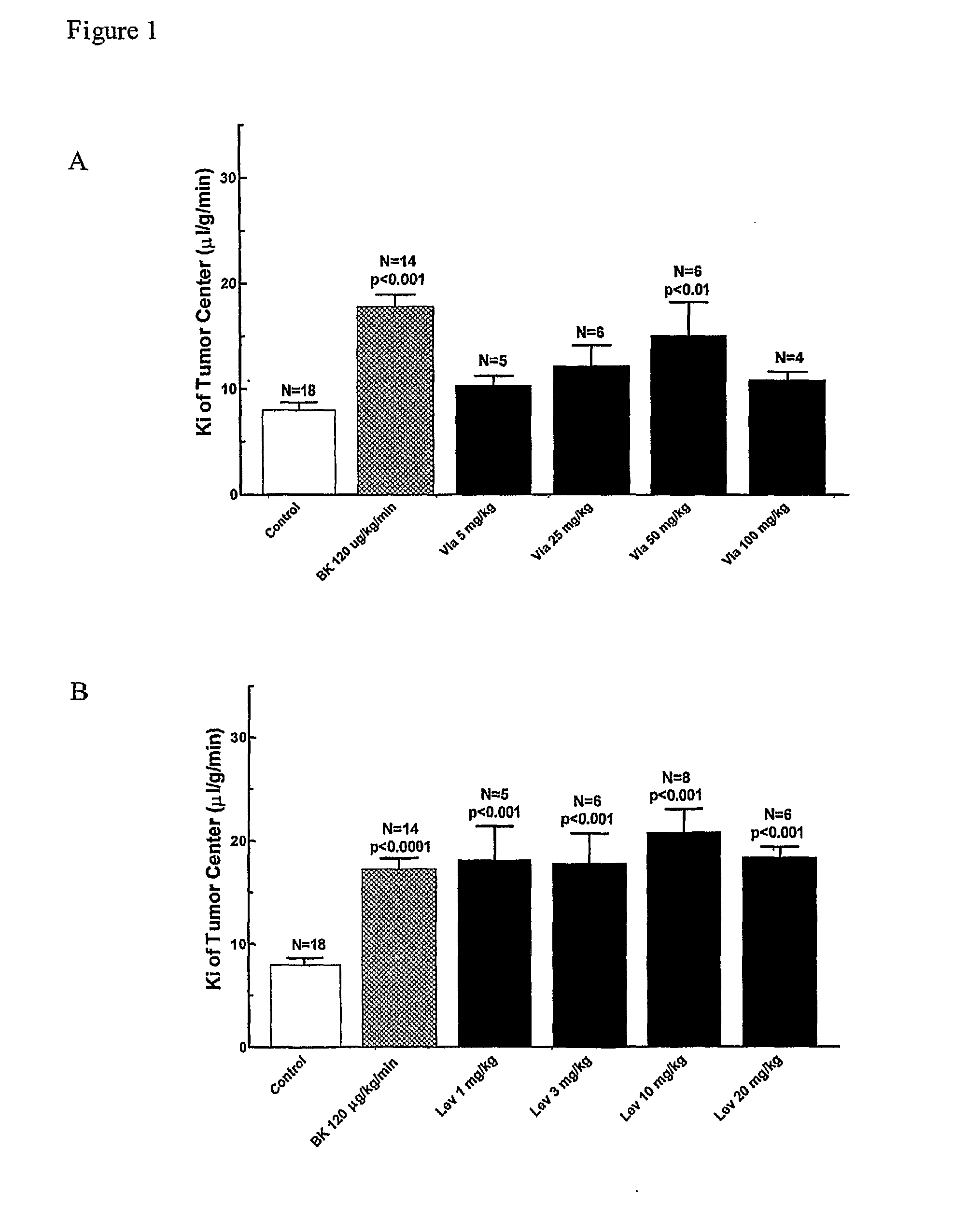
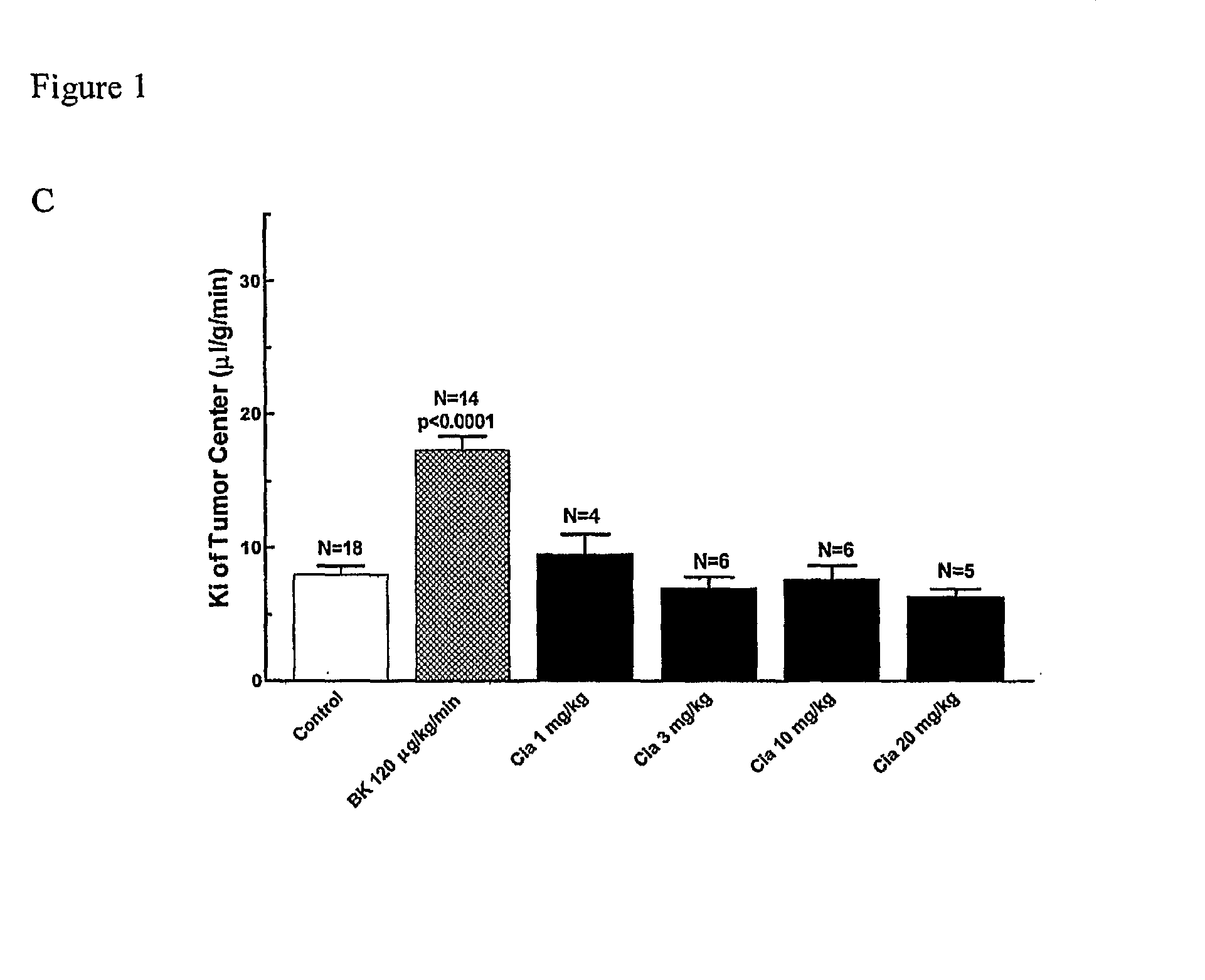
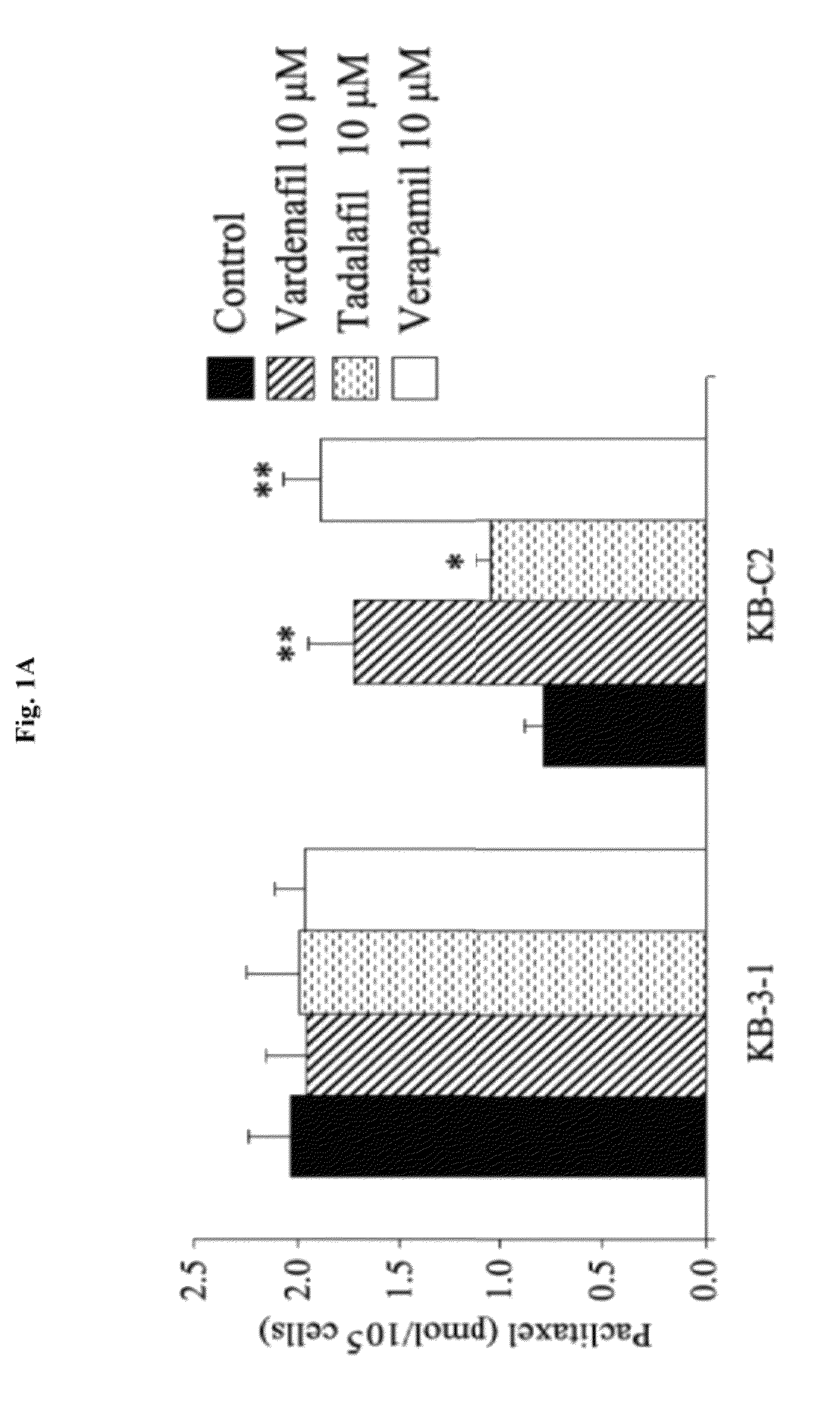
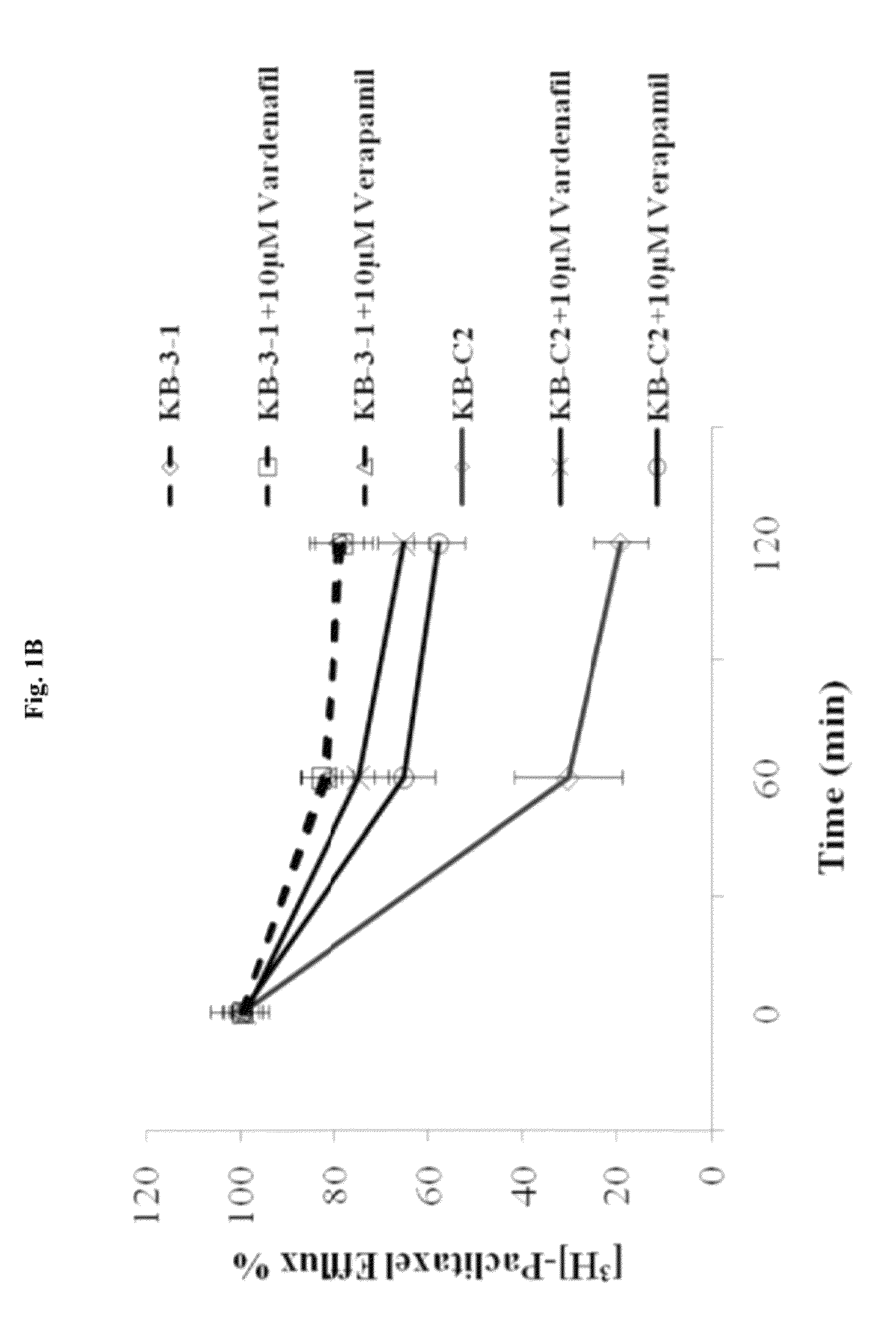
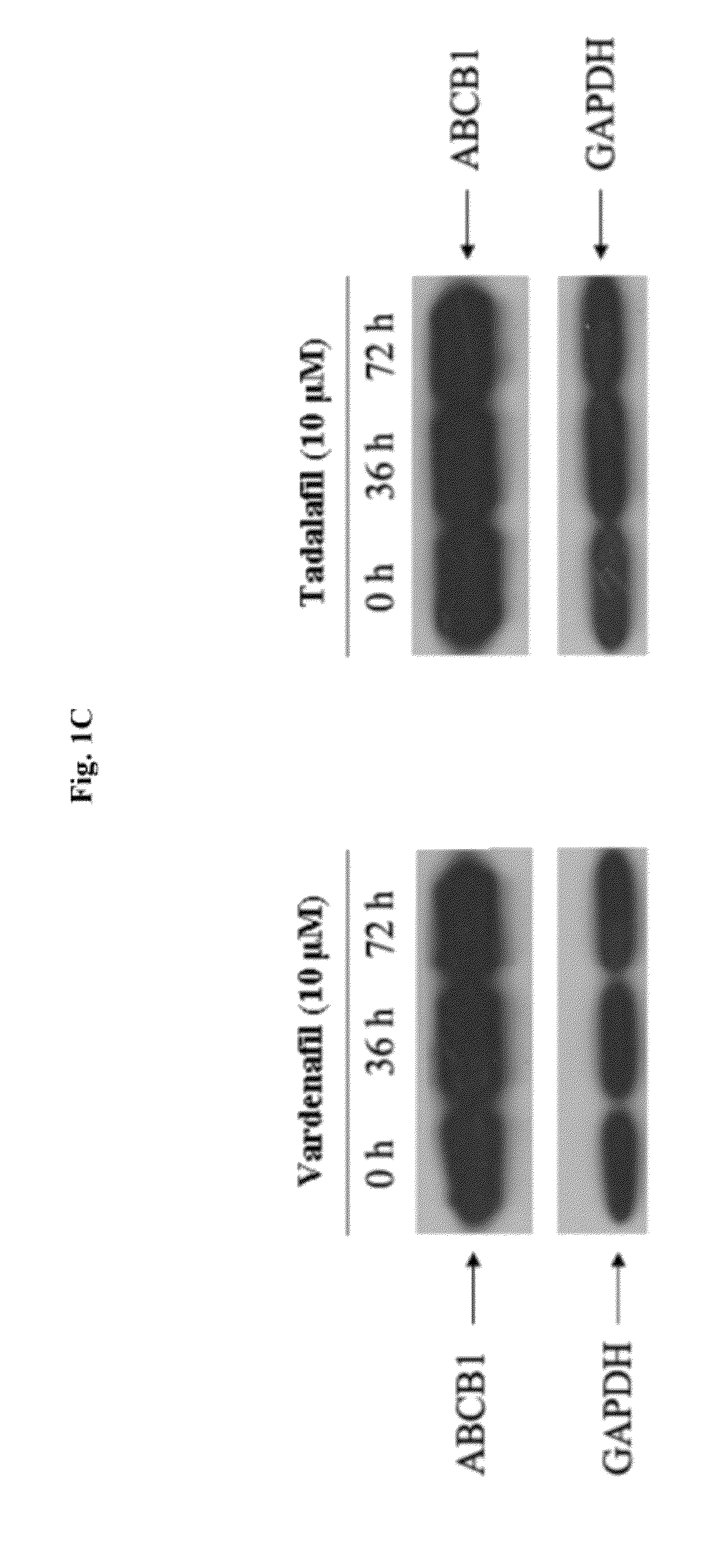
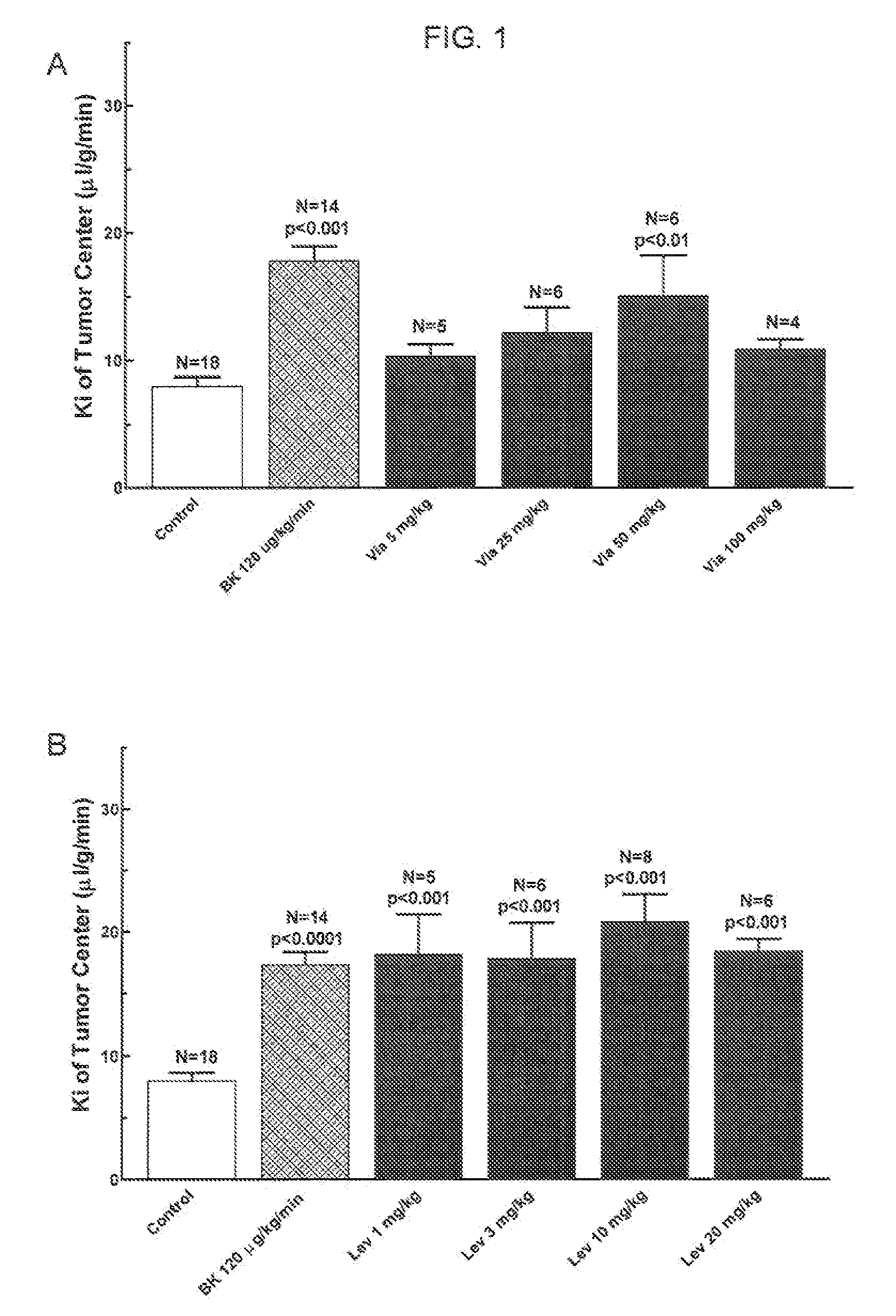
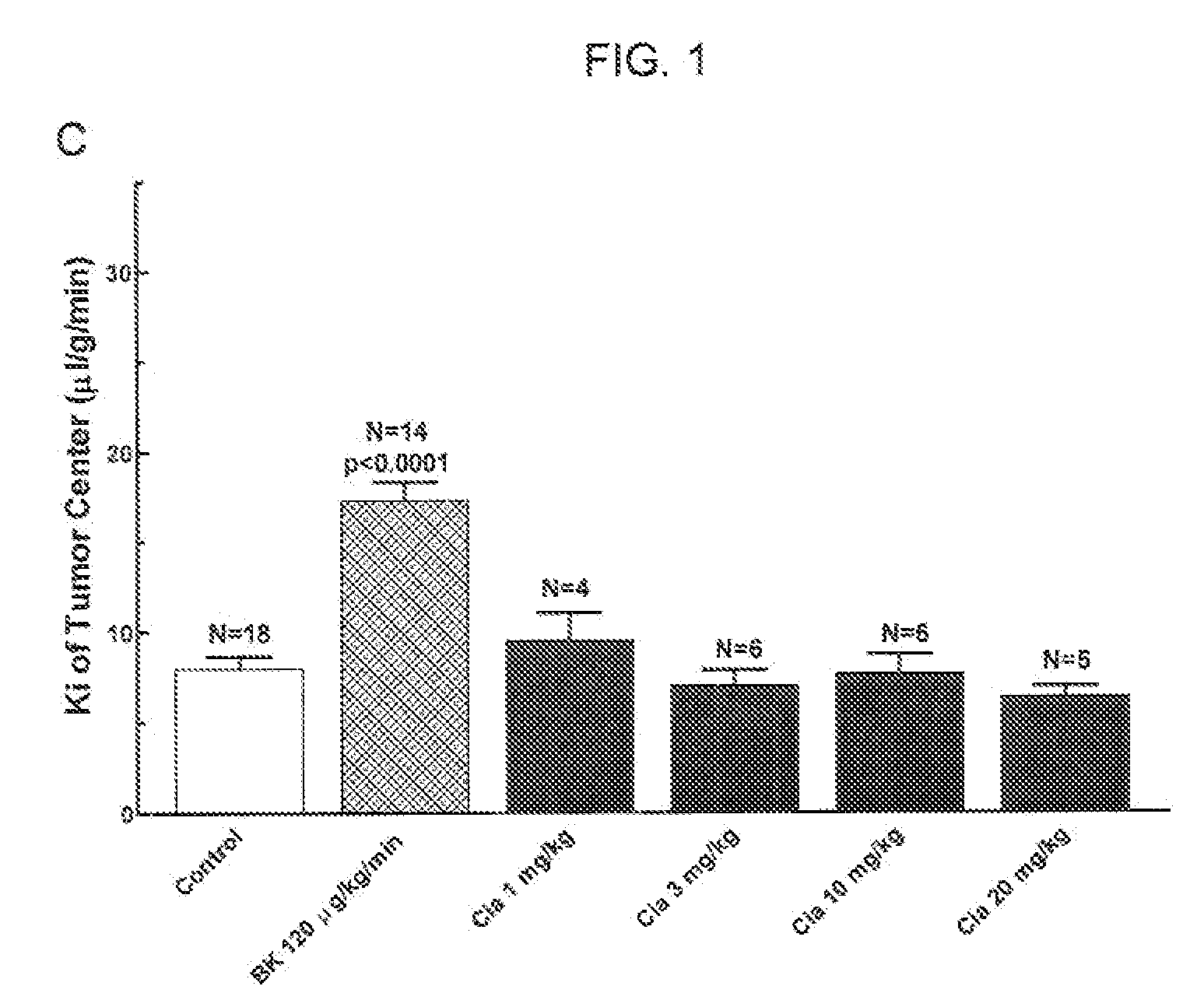
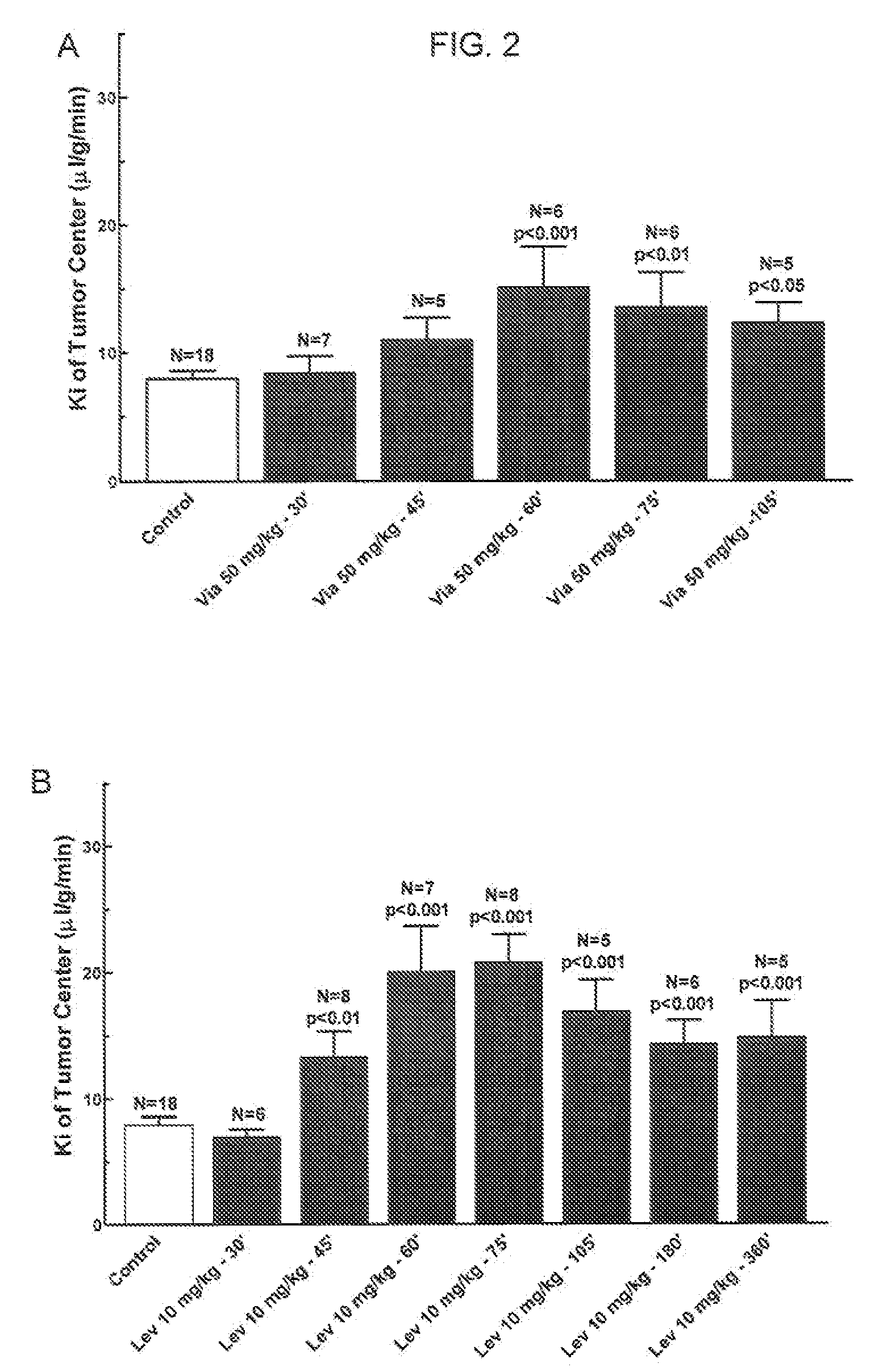
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
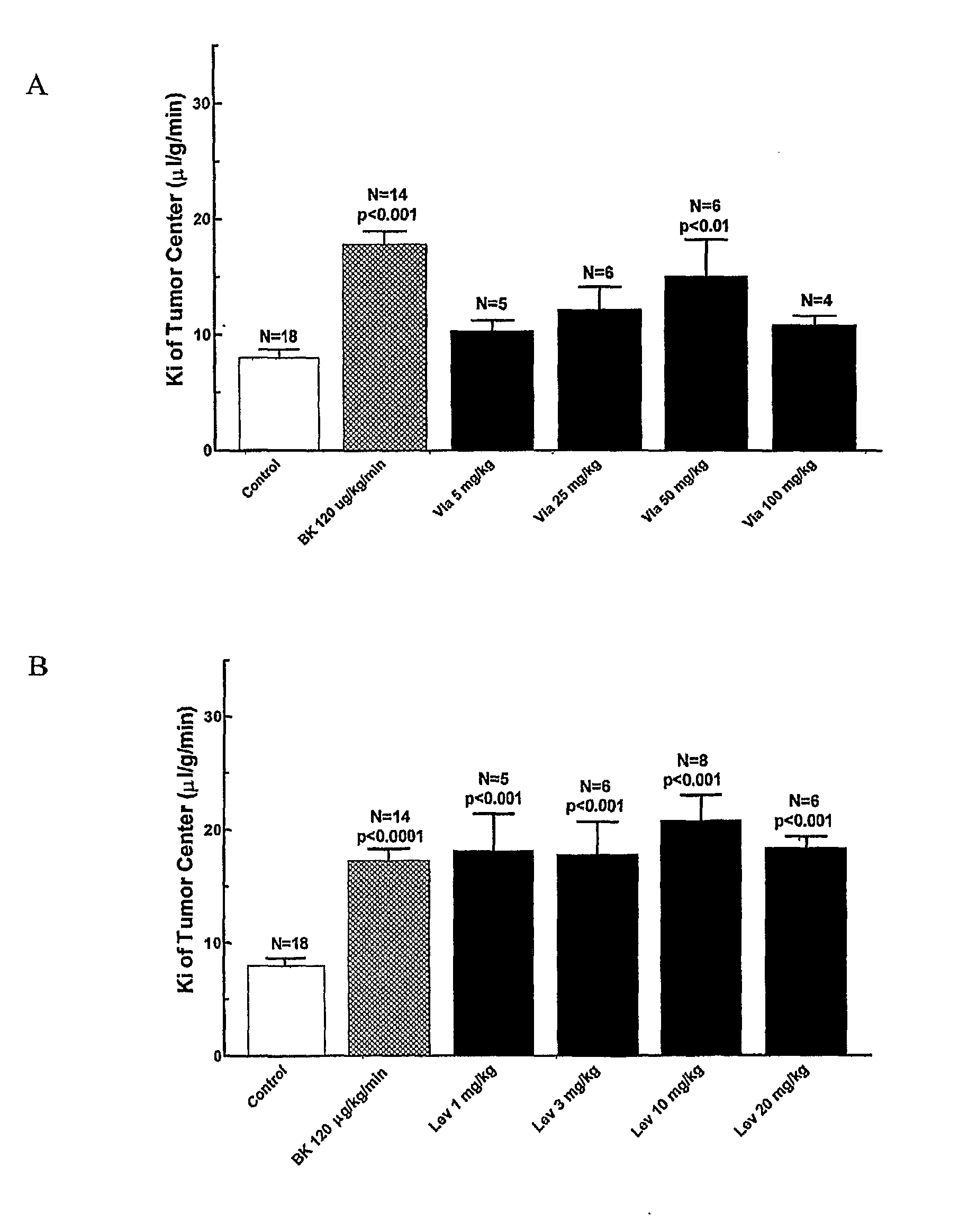
Use of Sildenafil, Vardenafil and Other 5-Phosphodiesterase Inhibitors to Enhance Permeability of the Abnormal Blood-Brain Barrier
InactiveUS20080188480A1Improve blood-brain barrier permeabilityImprove breathabilityBiocideNervous disorderVardenafilMedicine
This invention relates to compositions, methods and kits for enhancing the permeability of the blood-brain barrier. Particularly, compositions comprising 5-phosphodiesterase inhibitors, such as sildenafil, vardenafil, or tadalafil, when administered to a mammal, will selectively enhance the permeability of the blood-brain barrier in abnormal brain tissue. This selective enhancement allows for selective delivery of therapeutic agents to treat the abnormal brain tissue; for example, a brain tumor.
Owner:CEDARS SINAI MEDICAL CENT
Use of phosphodiesterase inhibitors for treating multidrug resistance
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
Herbal composition and method of use for promoting erections and treating erectile dysfunction in men
InactiveUS20060269623A1Avoid dysfunctionIncrease concentrationBiocideBryophyta medical ingredientsVardenafilSexual impotence
This invention is directed toward herbal compositions and methods of treatment for prevention or treatment of erectile dysfunction disorders and ameliorating symptoms thereof and as a preventative measure against erectile dysfunction. The methods comprise administering a therapeutically effective composition of matter comprising the following herbal and other components: Herba cynomorii, Rhizhomnas atractylodis macrocephalae, Radix rehmannia glutinosea longui, Herba epimedii, Fructus lycii, Fructus schisandrae chinensis, Radix poloygoni multiflor, Cortex cinnamonia cassiae, Fructus amoni, and Radix ginseng. The components of the invention interact synergistically once consumed to remedy or prevent erectile dysfunction when taken as prescribed. The invention is completely herbal in composition and does not contain any hormones, morphine, or any of the compositions of matter know by trade names Viagra, Cialis, Sildenafil or Vardenafil. Side benefits of taking this dietary supplement include increased energy, improved immune system strength, and improved erectile activity in men without erectile dysfunction.
Owner:SWAAB PIERRE
Methods of female sexual enhancement
InactiveUS20080119445A1Enhanced female sexual pleasureImprove satisfactionOrganic active ingredientsSexual disorderVardenafilGynecology
A method of sexual enhancement in women includes the steps of identifying a woman requesting sexual enhancement, assuring that the woman's blood includes estradiol within a first predetermined range and testosterone within a second predetermined range, and thereafter administrating a drug selected from the group consisting of vardenafil hydrochloride and tadalafil prior to sexual activity. The selected drug may be loaded into a starch strip which is then applied to the woman's tongue. Sexual enhancement in women can also be achieved by transdermal or subcutaneous application of the hormone DHEA.
Owner:LES MEDECINS
Use of 5-phosphodiesterase inhibitors to enhance the permeability of the blood-brain barrier of abnormal brain tissue and the blood-tumor barrier
This invention relates to methods and kits for enhancing the permeability of the blood-brain barrier of abnormal brain tissue or the blood-tumor barrier. Particularly, methods comprising the administration of 5-phosphodiesterase inhibitors, such as sildenafil and vardenafil, to selectively enhance the permeability of the blood-brain barrier of abnormal brain tissue or the blood-tumor barrier are described. This selective enhancement allows for selective delivery of therapeutic agents or imaging to treat the abnormal brain tissue or a tumor, including brain tumors and non-central nervous system tumors.
Owner:CEDARS SINAI MEDICAL CENT
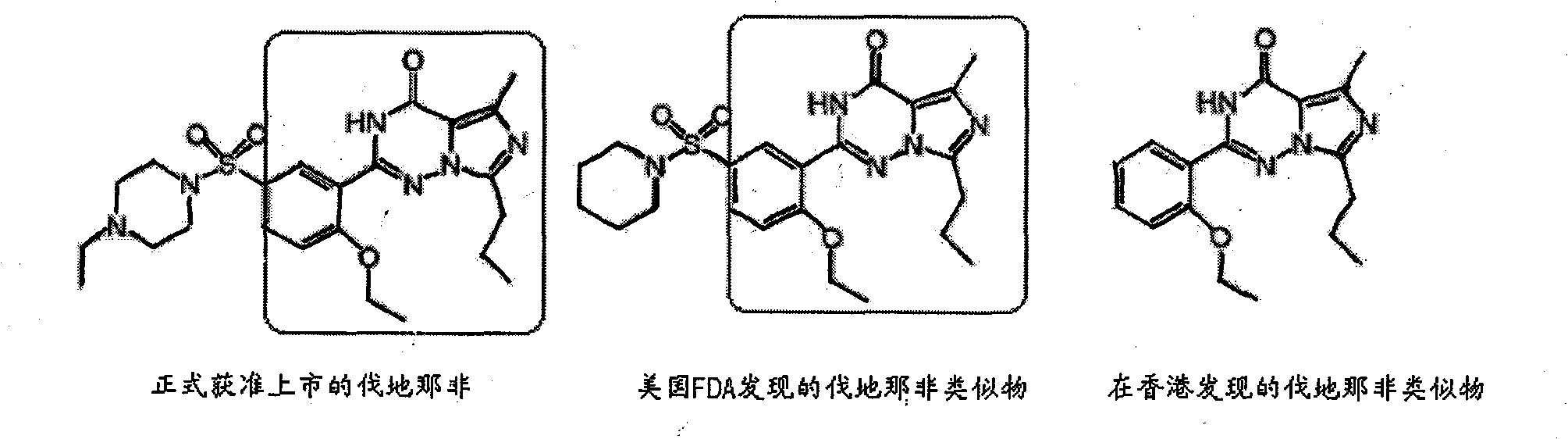
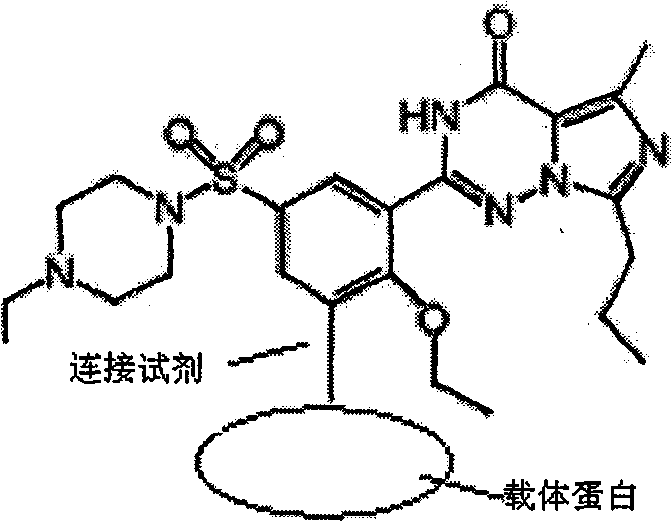
Preparation method for monoclonal antibody specifically binding vardenafil and analog thereof
The invention relates to a preparation method for a monoclonal antibody specifically binding vardenafil and an analog thereof. The preparation method comprises the steps of: synthesizing artificial immunizing antigens and artificial testing antigens, screening mice under immune state and the monoclonal antibody, and conducting structural specificity identification of the monoclonal antibody, specifically, mixing M-BSA with the vardenafil, regulating pH, adding a glutaraldehyde coupling agent, slowly stirring with a magnetic stirrer for reaction, dialyzing with distilled water, freezing and drying under vacuum condition, and synthesizing the artificial immunizing antigens; mixing M-OVA with the vardenafil, regulating pH, adding a glutaraldehyde coupling agent, slowly stirring with a magnetic stirrer for reaction, dialyzing with distilled water, freezing and drying under vacuum condition, and synthesizing the artificial testing antigens; and using a competitive inhibition ELISA method to detect the coasensual reaction between the monoclonal antibody and the vardenafil and piperidenafil and identifying the monoclonal antibody which has high coasensual reaction rate and can specifically bind the vardenafil and the analog thereof. The affinity equilibrium constant of the monoclonal antibody prepared by the method on the vardenafil and the analog thereof is higher than 10M.
Owner:NANCHANG UNIV
Method of female sexual enhancement
InactiveUS20060167022A1Enhancement of sexual and sexual satisfactionOvercome problemsOrganic active ingredientsBiocideVardenafilGynecology
A method of sexual enhancement in women includes the steps of identifying a woman requesting sexual enhancement, assuring that the woman's blood includes estradiol within a first predetermined range and testosterone within a second predetermined range, and thereafter administrating a drug selected from the group consisting of vardenafil hydrochloride and tadalafil prior to sexual activity. The selected drug is loaded into a starch strip which is then applied to the woman's tongue.
Owner:LES MEDECINS
Pde5 inhibitor powder formulations and methods relating thereto
Novel dry powder compositions comprising and methods relating thereto are provided. The dry powder compositions comprise PDE5 inhibitors, such as vardenafil, or pharmaceutically acceptable salts or esters thereof. The dry powder compositions may optionally include an carrier / excipient. The concentration of active agent may be at least about 2% by weight. Methods of aerosolizing the dry powder compositions and using them to treat various diseases are also disclosed.
Owner:RESPIRA THERAPEUTICS INC
Formulations of phosphodiesterase 5 inhibitors and methods of use
InactiveUS20060035905A1Improve concentrationImprove solubilityOrganic active ingredientsLyophilised deliveryVardenafilPhosphodiesterase 5 inhibitor
The present invention provides compositions and delivery methods to enhance treatment for sexual dysfunction or hypofunction through the delivery of phosphodiesterase 5 inhibitors to a mammal. Phosphodiesterase 5 inhibitors are one example of a compound class used for this indication. Examples of compounds in this class include taldalafil and vardenafil and sildenafil. Furthermore, the present invention provides compositions and delivery methods to enhance the sildenafil concentration in solution, suspension and gel formulations and methods of parenteral, intradermal, sublingual, intranasal, and buccal sildenafil delivery.
Owner:BECTON DICKINSON & CO
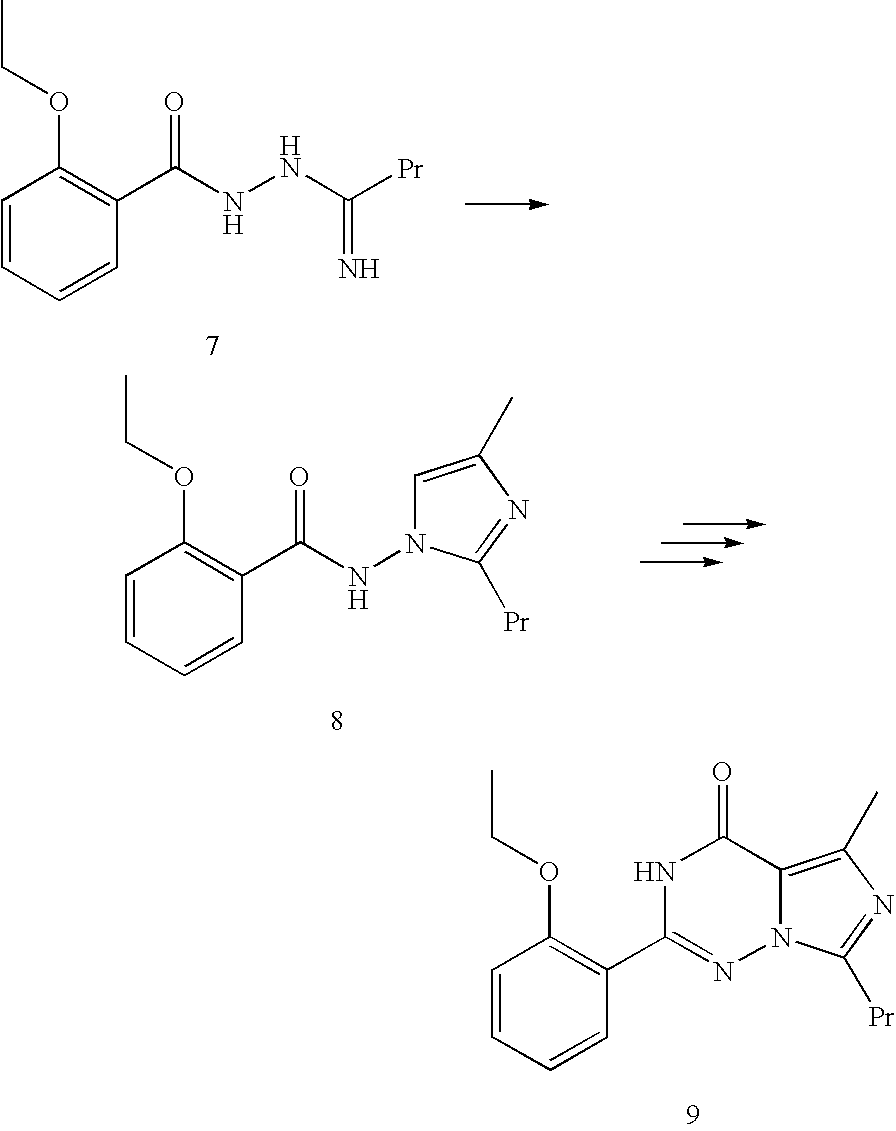
N-butyramide, the preparation method and use thereof
Disclosed are N-{1-[3-(2-ethoxy-5-(4-ethylpiperazinyl)sulfonylphenyl)-4,5-dihydro-5-oxo-1,2,4-triazin-6-yl]ethyl}butyramide (which is represented by formula III), its preparation method, intermediates during preparation procedure, preparation method for such intermediates and a method for preparing vardenafil from the compound. In the method for preparing vardenafil, a chloro-sulfonation reaction carries out in the early stage of the preparation procedure.
Owner:TOPHARMAN SHANGHAI
Medicinal composition containing vardenafil, and preparation method thereof
InactiveCN104971066AEasy to takeSexual disorderAnhydride/acid/halide active ingredientsVardenafilPharmaceutical medicine
The invention discloses a medicinal composition containing vardenafil, and a preparation method thereof, and belongs to the pharmaceutical field. The medicinal composition is used for treating male sexual disfunction. Vardenafil is reasonably combined with a trace element zinc, and pharmaceutically acceptable auxiliary materials, such as a humectant, a film forming material, a filler, a sweetener, a disintegrating agent, an adhesive, a lubricant and a stabilizer are added to prepare an oral pellicle, a chewing tablet, an oral solution and various oral dosage forms for clinic application.
Owner:天津市聚星康华医药科技有限公司
Drugs with improved pharmacokinetic properties
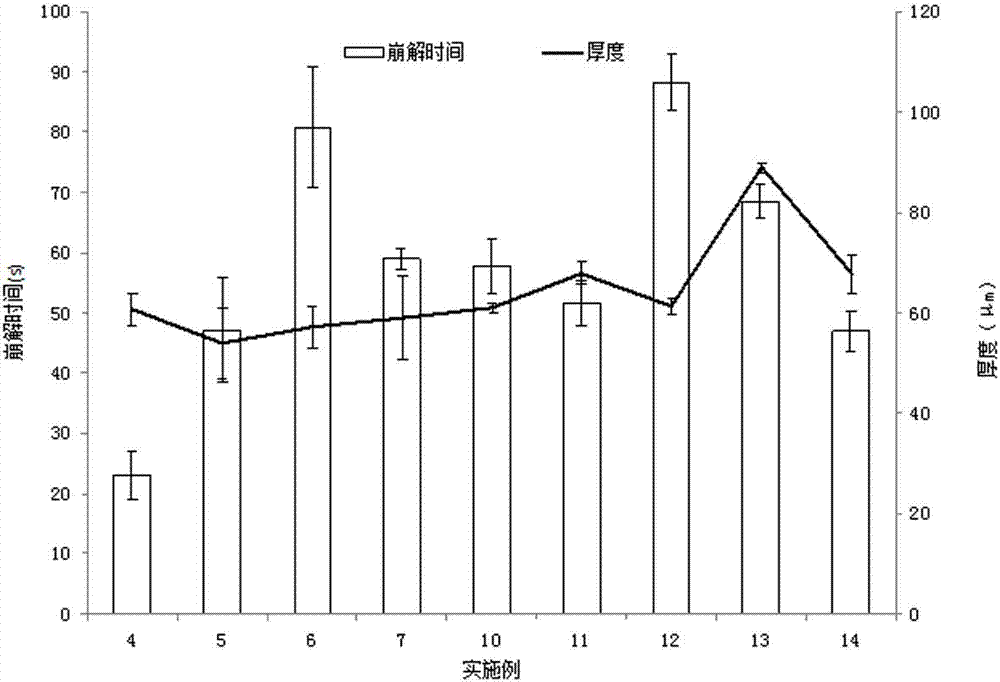
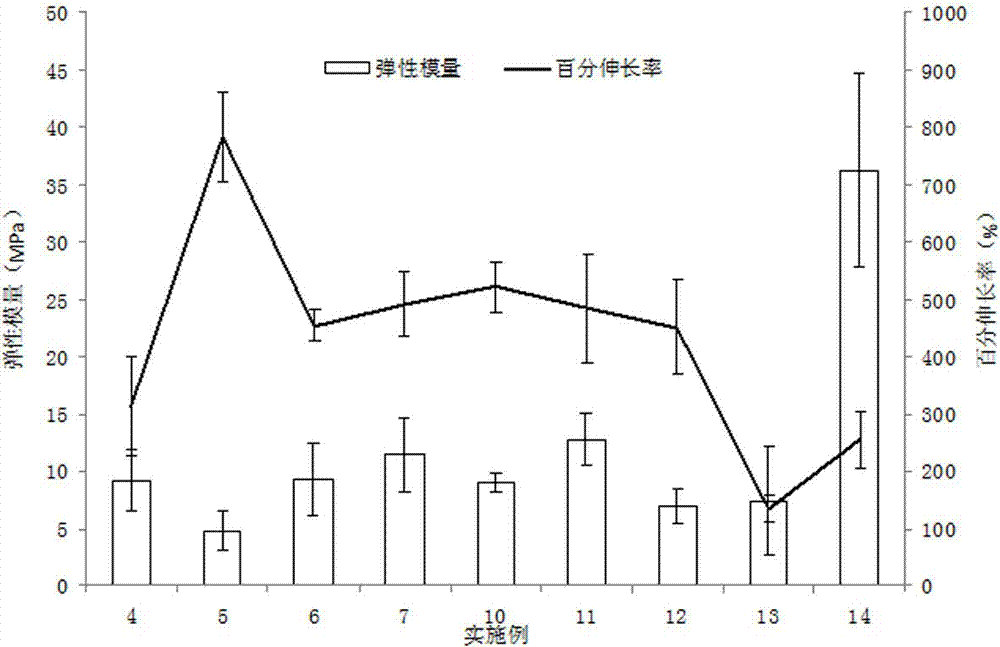
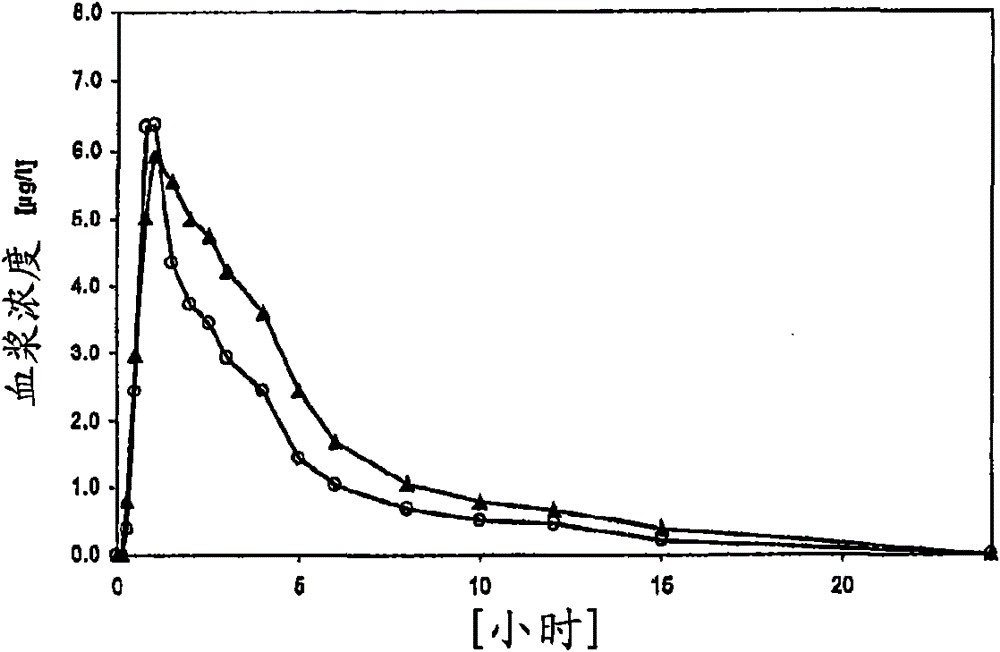
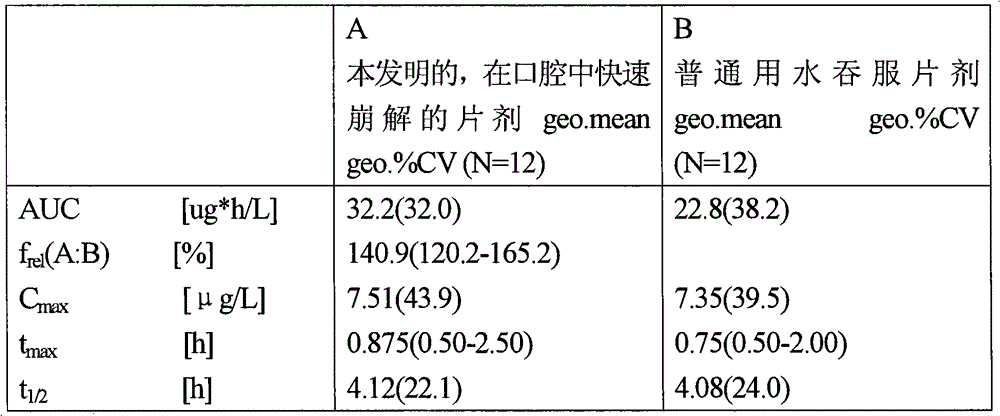
The application relates to a novel vardenafil pharmaceutical preparation, which disintegrates rapidly in the oral cavity, improves bioavailability, and causes a plateau-like plasma concentration change process, and a preparation method thereof.
Owner:BAYER IP GMBH
Vardenafil dropping pill and its preparing method
InactiveCN1813757ARapid dissolutionHigh dissolution ratePill deliverySexual disorderVardenafilMedicine
The present invention relates to a kind of vardenafil dripping pill and its preparation process. It is made up by using vardenafil and dripping pill matrix through a certain preparation process. Said vardernafil dripping pills can be used for curing male erection dysfunction, its bioavailability is high and medicine stability is good.
Owner:陈茜
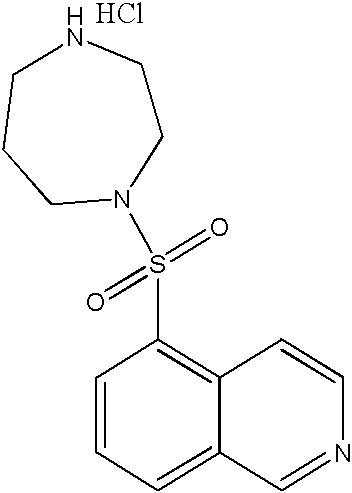
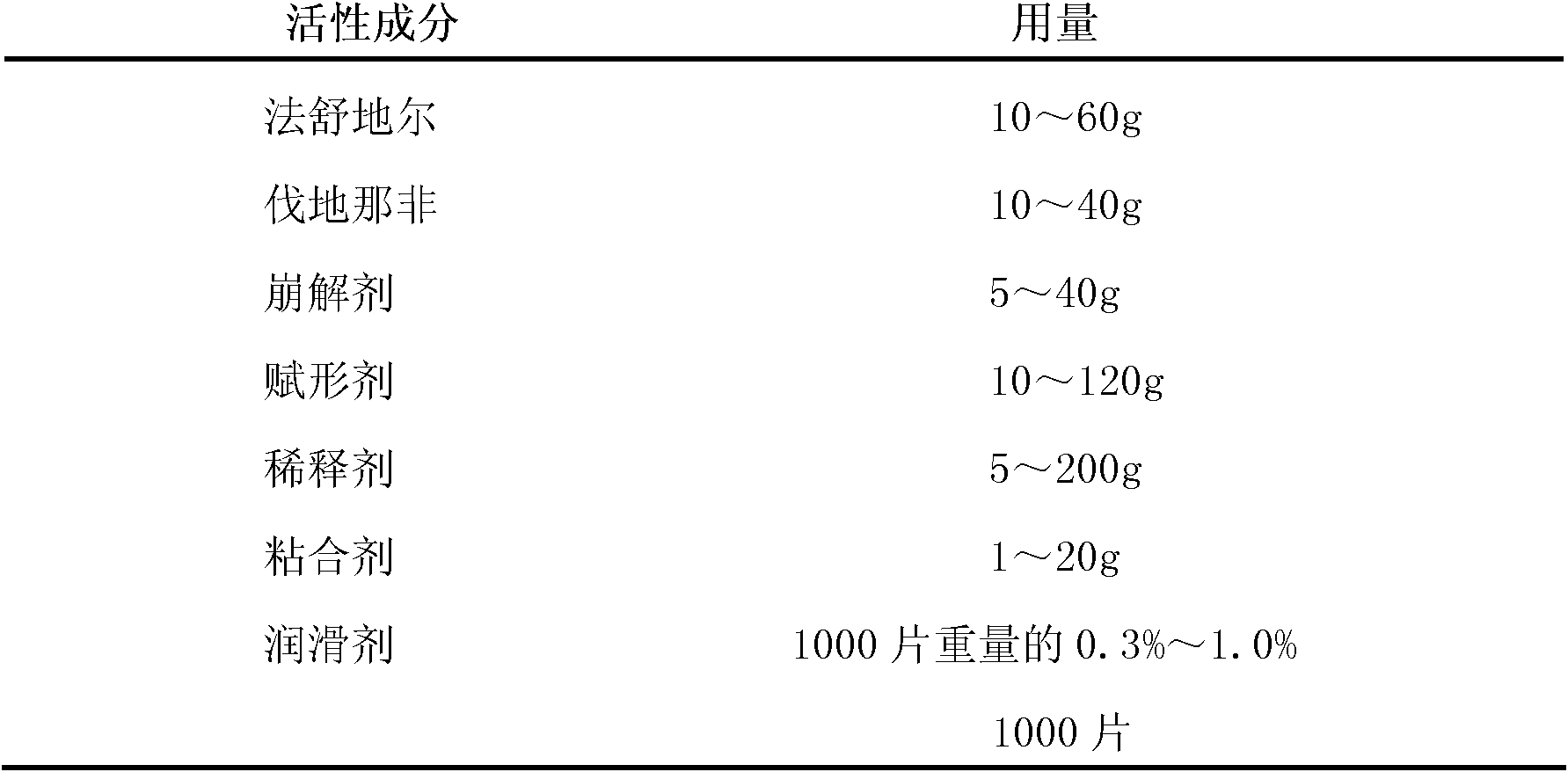
Fasudil and Vardenafil compound preparation and preparation method thereof
Owner:TIANJIN CHASE SUN PHARM CO LTD
Oral quick-dissolving film preparation and preparation method thereof
InactiveCN102824333BImprove disintegration time limitSolve the shortcomings of taking waterPharmaceutical non-active ingredientsSexual disorderVardenafilTadalafil
The invention discloses an oral quick-dissolving film preparation and a preparation method thereof. The oral quick-dissolving film comprises the following components in percentage by weight: 20 to 40 percent of medicinal active component, 40 to 75 percent of water-soluble film forming material, 10 to 25 percent of plasticizer, 0 to 25 percent of disintegrating agent, and 0.1 to 8 percent of water, wherein the medicinal active component is one of sildenafil, tadalafil, vardenafil or salts thereof. According to the oral quick-dissolving film preparation, the disintegration time limited of the film preparation can be remarkably accelerated, the problem that most oral solid preparations should be taken with water can be solved, medicine taking time cannot be delayed under a condition without water, and the taking compliance of a patient can be improved.
Owner:SUZHOU UNIV
Preparation method of vardenafil injection
InactiveCN103479570APharmaceutical delivery mechanismPharmaceutical non-active ingredientsVardenafilPh buffering
The invention discloses a preparation method of a vardenafil injection. In the preparation method, the vardenafil injection is prepared from vardenafil hydrochloride and a pH buffering agent through terminal sterilization. The preparation method of the vardenafil injection is characterized in that the content of 5-hydroxymethyl furfural can be effectively controlled to ensure the safety of clinical use.
Owner:NANJING QIHE MEDICINE SCI & TECH
Preparation method of vardenafil
The invention discloses a preparation method of vardenafil. The preparation method is characterized by comprising the following process steps: (1), slowly adding chlorosulfonic acid in an organic solution at a temperature of -0.5-10 DEG C, after a reaction system is stabilized, adding SM1, after naturally raising the temperature to room temperature, continuously reacting for 3-12 hours, diluting a reaction solution with icy dichloromethane, slowly pouring in brine ice, separating an organic phase, and drying with anhydrous sodium sulfate; (2), slowly dropping N-ethylpiperazine in a product obtained in the step 1 at a temperature of -0.5-1.5 DEG C, naturally raising the temperature to room temperature, continuously reacting for 3-8 hours, after reaction is ended, processing a reaction solution to obtain a product, and re-crystallizing the product. The preparation method is simple in operation, high in product yield, low in cost and less in consumption of chlorosulfonic acid.
Owner:LIAONING HUANREN PHARMA
Pde5 inhibitor powder formulations and methods relating thereto
Novel dry powder compositions comprising and methods relating thereto are provided. The dry powder compositions comprise PDE5 inhibitors, such as vardenafil, or pharmaceutically acceptable salts or esters thereof. The dry powder compositions may optionally include an carrier / excipient. The concentration of active agent may be at least about 2% by weight. Methods of aerosolizing the dry powder compositions and using them to treat various diseases are also disclosed.
Owner:RESPIRA THERAPEUTICS INC
Process for preparing vardenafil and intermediates thereof
The invention provides a process for preparing vardenafil and its intermediates. The process is novel. The invention also provides new intermediates for preparing vardenafil and their preparations. The process for preparing vardenafil decreased side reactions in the methods of the prior art and other steps, improved yield and is easy operated, thus has excellent industrial applicability.
Owner:TOPHARMAN SHANGHAI +1
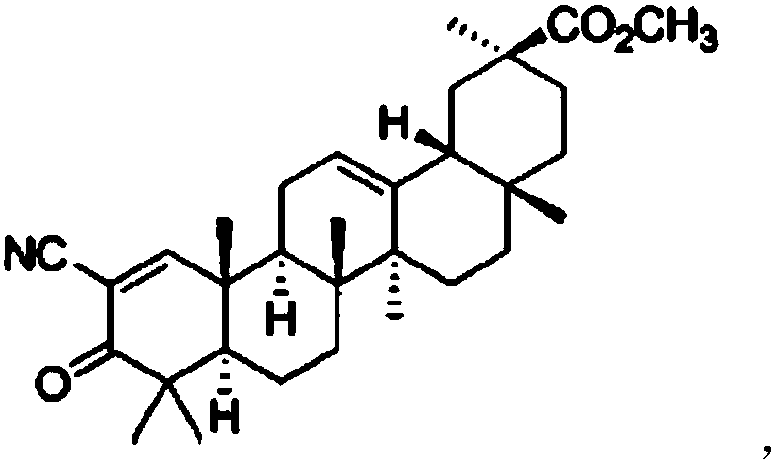
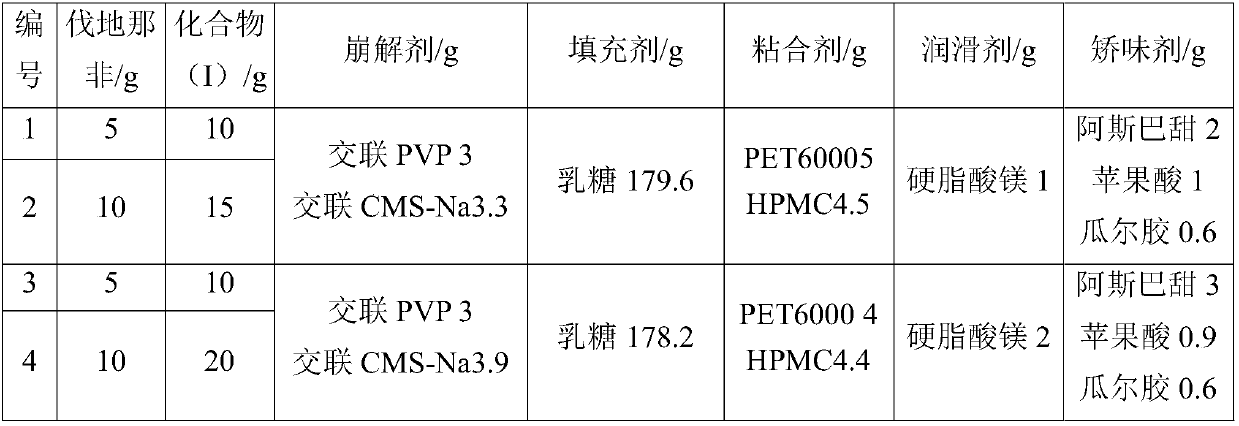
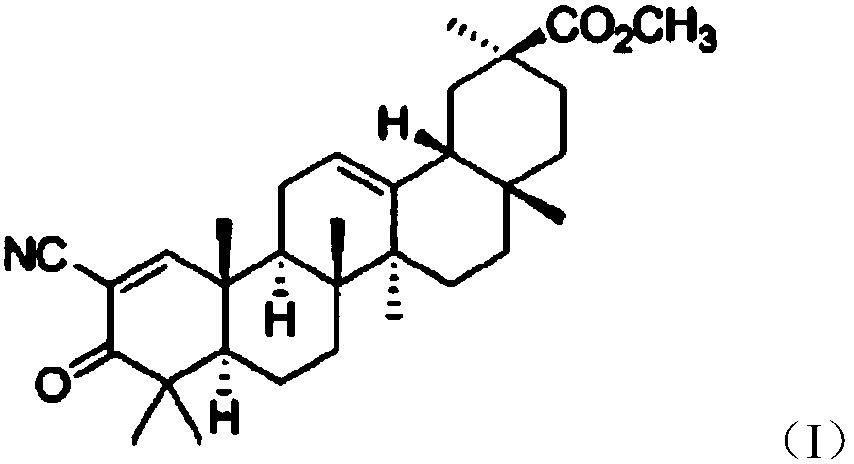
Glycyrrhetinic acid derivative and vardenafil compound chewable tablet
InactiveCN107595856AHigh selectivityReduce adverse reactionsPill deliveryPharmaceutical non-active ingredientsVardenafilMass ratio
The invention relates to a glycyrrhetinic acid derivative and vardenafil compound chewable tablet, which is characterized in that the chewable tablet is prepared from active ingredients and medical auxiliary materials applicable to the chewable tablet. The active ingredients are prepared from vardenafil and 2-cyano-3-oxo-18beta-oleanane-1,12-diene-30-methyl carbonate according to the mass ratio of1:(1.5 to 2); the medical auxiliary materials are prepared from 3 to 4 percent of disintegrants, 3 to 5 percent of bonding agents, 0.5 to 3 percent of flavoring agents, 0.5 to 1 percent of lubricating agents and the balance of filling agents (accounting for the total mass of the medical auxiliary materials).
Owner:陈有平
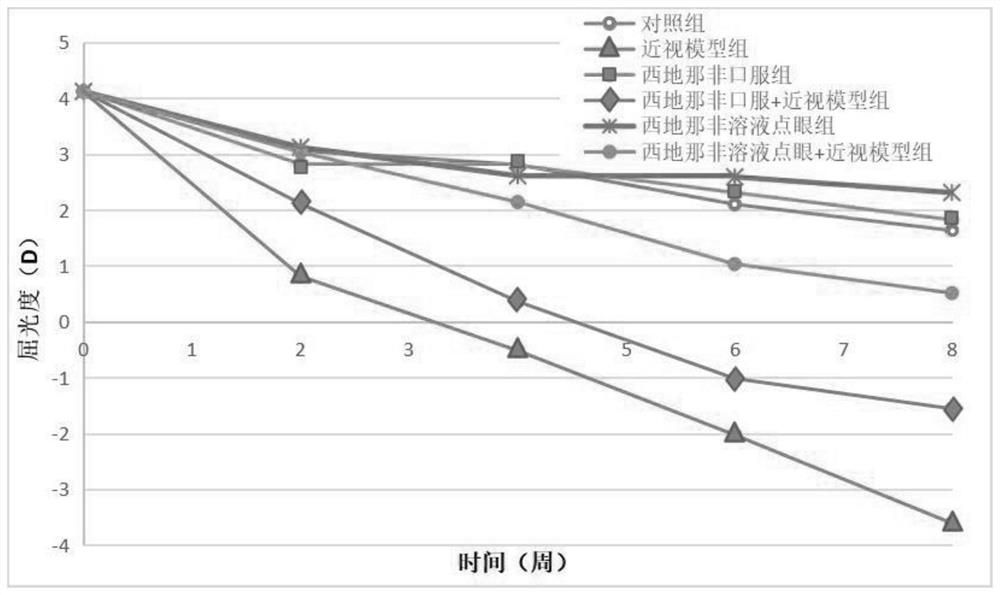
Application of sildenafil citrate/tadalafil/vardenafil in preparation of medicine for treating myopia
PendingCN114177182AReduce myopiaDelay myopia progressionSenses disorderHeterocyclic compound active ingredientsVardenafilTadalafil
The invention belongs to the technical field of new application of medicines, and particularly relates to application of sildenafil citrate / tadalafil / vardenafil in preparation of medicines for treating myopia. The application of sildenafil citrate, tadalafil and vardenafil in preparation of the medicine for treating myopia is the key protection content of the invention, and the medicine disclosed by the invention is a liquid medicine, such as eye drops. The medicine containing sildenafil, tadalafil and vardenafil for treating shortsightedness is also the key protection content of the invention. The medicine further comprises conventional auxiliary materials contained in the eye drops, such as preservatives, excipients and the like. The medicine sildenafil citrate, tadalafil and vardenafil provided by the invention have the beneficial effects that the medicine sildenafil citrate, tadalafil and vardenafil provided by the invention are applied to the treatment of myopia medicines, so that the blood supply of retina or choroid or ciliary muscle or sclera is effectively improved; or the axial length of the inner wall of the eyeball is reduced, or the adjusting force of eyes is enhanced, so that the purpose of treating myopia is achieved.
Owner:JINAN SANWEI MEDICAL INSTR CO LTD
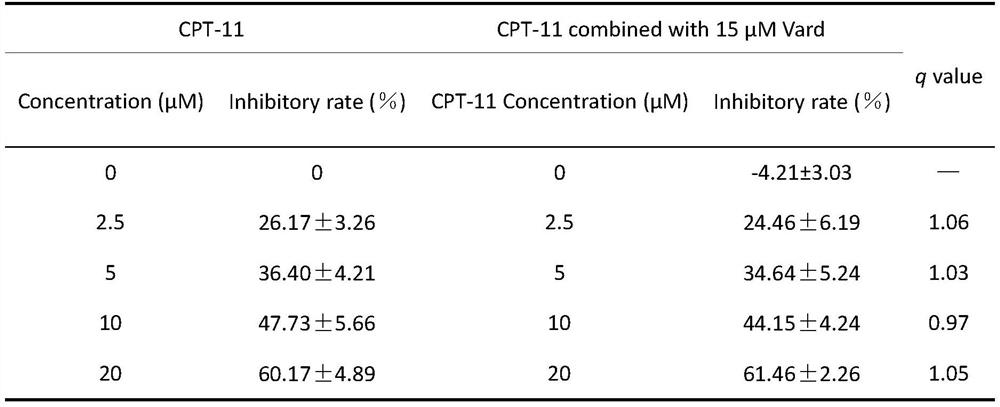
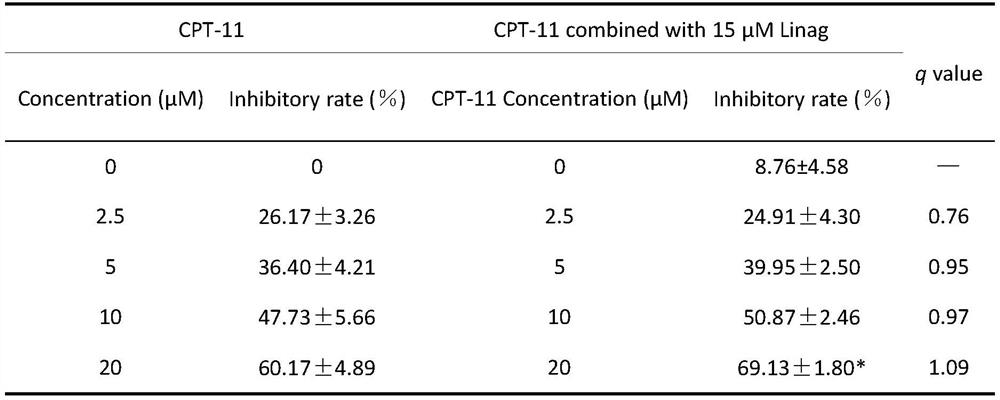
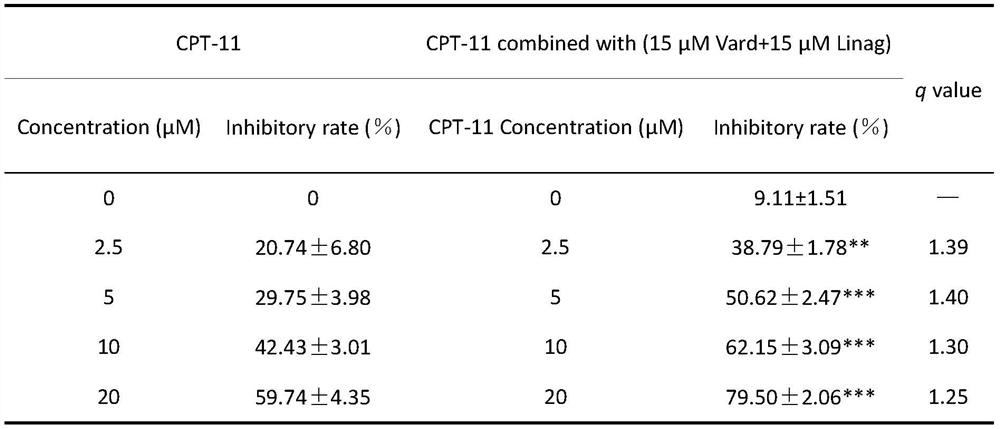
Composition and application of composition and irinotecan in preparation of antitumor drugs
PendingCN112773804AAntineoplastic agentsHeterocyclic compound active ingredientsVardenafilIrinotecan
The invention discloses a composition and application of the composition and irinotecan in preparation of antitumor drugs. The invention discovers that the composition of vardenafil and linagliptin has a synergistic enhancement effect on the anti-colon cancer activity of irinotecan, and vardenafil or linagliptin independently has no synergistic enhancement effect on the anti-colon cancer activity of irinotecan. Therefore, the composition of vardenafil and linagliptin can be combined with irinotecan to prepare the drugs for resisting colon cancer.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com