Drugs with improved pharmacokinetic properties
A technology of pharmaceutical preparation and trihydrate, which is applied in the field of new vardenafil pharmaceutical preparations, and can solve the problems of the decrease of vardenafil plasma concentration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
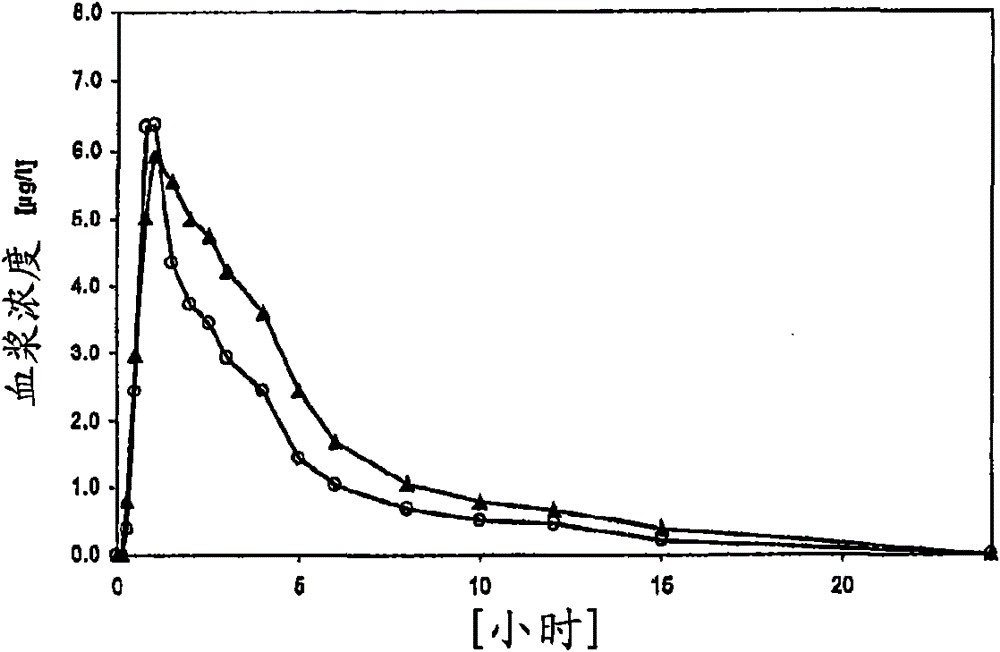
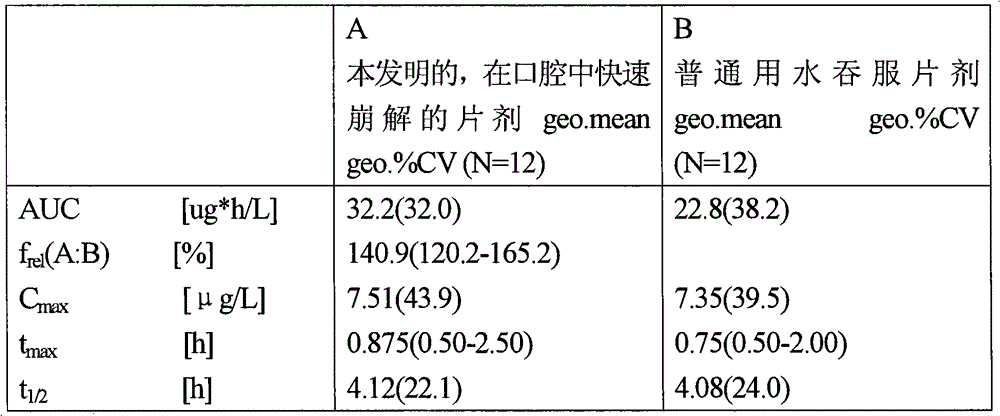
[0028] Demonstration of increased bioavailability in rapidly disintegrating tablets in the oral cavity according to the invention
[0029] Each of the 12 probands was administered oral rapidly disintegrating tablets consisting of the following: 11.85 mg of vardenafil hydrochloride trihydrate, 0.55 mg of yellow iron oxide, 0.075 mg of red iron oxide Iron, 0.75mg of apricot flavor, 0.125mg of neohesperidin dihydrochalcone, 2.50mg of aspartame, 0.625mg of highly dispersible silicon dioxide, 3.125mg of magnesium stearate and 105.4mg of At 25° C., about 10.4 mg of the active ingredient used is dissolved in 10 ml of physiological saline (corresponding to 8.8 mg of vardenafil), which is about 88% of the dose. At 37° C., in 900 ml of physiological saline, in a USP paddle stirring device with a rotating speed of 50 rpm, the release rate of the active ingredient within 5 minutes was 73%. Thereby, the solubility and dissolution rate criteria of the present invention are met. Compared ...
Embodiment 7
[0031] Demonstration of increased bioavailability in rapidly disintegrating tablets in the oral cavity according to the invention
[0032] Each of the 11 probands was administered oral rapidly disintegrating tablets consisting of 5.93 mg of vardenafil hydrochloride trihydrate, 0.352 mg of yellow iron oxide, 0.048 mg of red iron oxide Iron, 0.48mg of apricot flavor, 0.08mg of neohesperidin dihydrochalcone, 1.60mg of aspartame, 0.40mg of highly dispersible silicon dioxide, 2mg of magnesium stearate and 69.11mg of The active ingredient used is approximately 91% soluble in 10 ml of physiological saline at 25°C. The release rate of the active ingredient was 78% in 900 ml of physiological saline at 37° C. and in a USP paddle stirring apparatus at a speed of 50 rpm within 5 minutes. Thereby the solubility standard and dissolution rate standard of the present invention are realized. As a comparison, in the crossover approach, a conventional tablet swallowed with water, consisting o...
Embodiment 8
[0034] Demonstration of increased bioavailability in rapidly disintegrating tablets in the oral cavity according to the invention
[0035] The following ingredients were mixed in a plow mixer: 697 g of micronized vardenafil hydrochloride trihydrate, 500 g of a coloring material composed of 4.4% yellow iron oxide, 0.6% red iron oxide and 95% A premix consisting of 30g of apricot flavor, 5g of neohesperidin dihydrochalcone, 100g of aspartame and 3518g of The powder mixture was mixed with 25 g of highly disperse silicon dioxide in a drum mixer (Freifallmischer) and sieved through a 0.5 mm sieve. This mixture was then blended with 125 g of magnesium stearate in a tumbler mixer for 5 minutes. The final powder mixture was compressed on a tablet machine into round tablets weighing 170 mg, 8 mm in diameter and about 35N breaking strength. As a comparison, in the crossover approach, conventional tablets swallowed with water were administered, consisting of the following components:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com