Glycyrrhetinic acid derivative and vardenafil compound chewable tablet
A technology of glycyrrhetinic acid and vardenafil, which is applied in the direction of drug combinations, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve the problem that the anti-tumor activity of the compound is not obvious, so as to improve the anti-ED effect, improve the therapeutic effect, The effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1~4
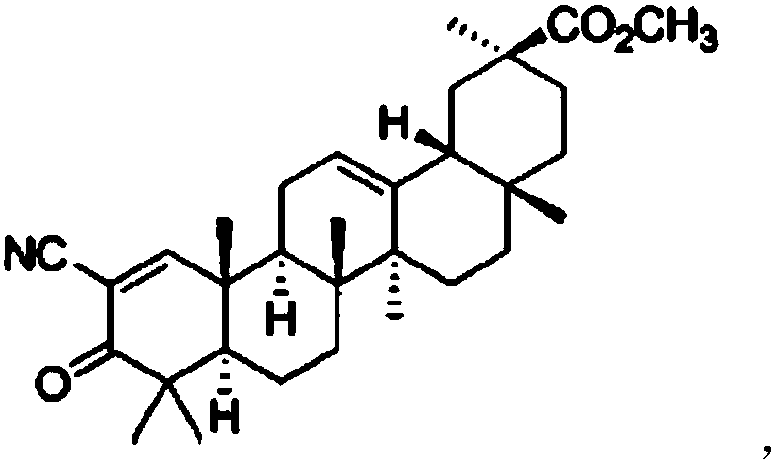
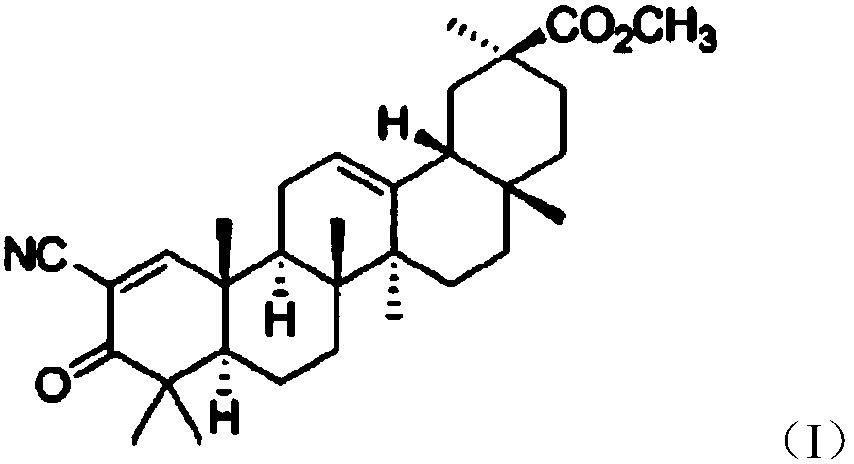
[0015] Preparation examples 1-4, compound chewable tablets of vardenafil and 2-methoxy-3-oxo-18β-oleanane-1,12-diene-30-acid (referred to as compound (I))
[0016]
[0017] The chemical formula of compound (I) is as follows
[0018]
[0019] The synthesis method of compound (I) is as described in CN201110131028.5.
[0020] The preparation method of the chewable tablet in the preparation example is as follows:
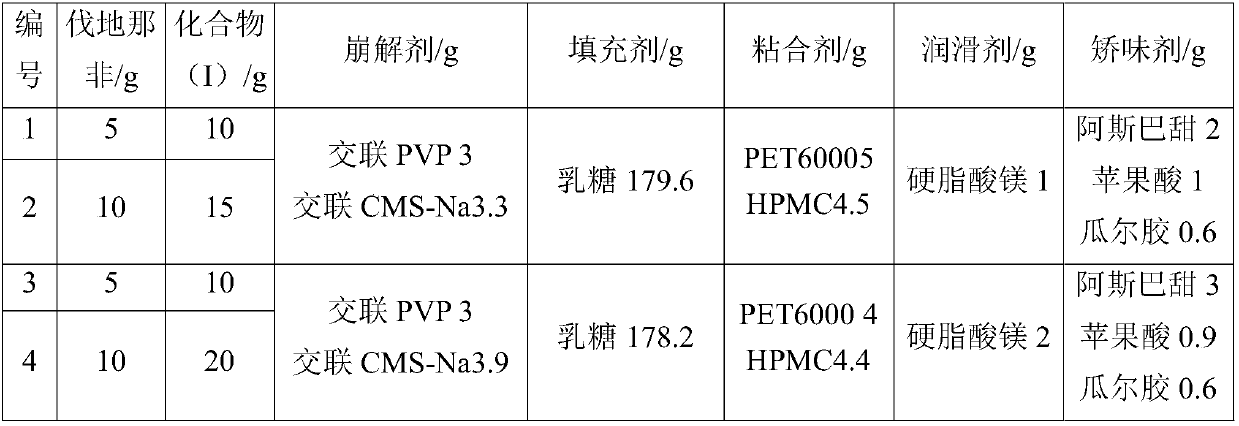
[0021] (1) Accurately weigh part of the raw and auxiliary materials (principal drug, binder, filler and disintegrating agent) according to the prescription ratio, pass through a 100-mesh sieve, mix thoroughly, add 50% ethanol in an appropriate amount to prepare a soft material, and use 24 Granulate with a mesh sieve, dry at 60°C, and granulate with a 24-mesh sieve.
[0022] (2) Add flavoring agent and lubricant to the above-mentioned sized granules, mix well, adjust tablet weight, and compress.
Embodiment 1
[0023] Pharmacological embodiment 1PDEs selectivity test
[0024] PDE6 is extracted from bovine retina, PDE3 and PDE5 are extracted from rabbit platelets, PDE4 is extracted from male SD rat kidney, PDE1 is extracted from male SD rat brain cortex, and PDE2 is extracted from beagle dog adrenal gland
[0025] PDEs activity test method
[0026] Vardenafil and compound (I) were prepared with DMSO concentration as a stock solution of 100mM, and then diluted with purified water in the ratio shown in the table below Mixed solution, the concentration of the mixed solution of experimental groups 1 to 7 was 10mM, 1mM, 100μM, 10μM, 1μM, 100nM, 10nM, 1nM, 0.1nM in terms of vardenafil, and the solutions of experimental groups 8 and 9 were respectively vardenafil Nafil and compound (I) stock solutions were diluted with purified water to solutions with concentrations of 10 mM, 1 mM, 100 μM, 10 μM, 1 μM, 100 nM, 10 nM, 1 nM, and 0.1 nM.
[0027]
[0028] According to different enzymes, ch...
Embodiment 2
[0037] Pharmacological Example 2 In Vivo Drug Efficacy Experiment
[0038] 2.1. Animal grouping
[0039] Male mature rabbits with a body weight of 3.5-4.5 kg were randomly divided into groups of 10. The grouping and administration conditions are shown in the table below:
[0040] Group No
Drug administration
1
(Blank control) Physiological saline 4ml / kg
2
(Positive control) Vardenafil 10mg / kg
3
Vardenafil 5mg / kg+ Compound (I) 5mg / kg
4
Vardenafil 5mgkg+ Compound (I) 7.5mg / kg
5
Vardenafil 5mg / kg+ Compound (I) 10mg / kg
6
Vardenafil 5mg / kg+ Compound (I) 12mg / kg
[0041] 2.3 Test method
[0042] SNP (sodium nitroprusside) as an exogenous NO donor was injected into the marginal ear vein, the administration volume was 1 ml / kg, and the SNP concentration of the injection was 0.1 μmol / L.
[0043] After weighing the rabbits, carry out intragastric administration according to the dosage plan in 2.1 above with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com