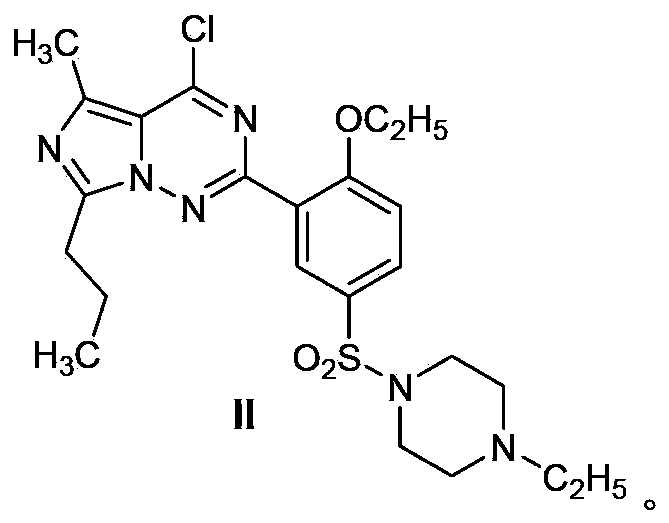
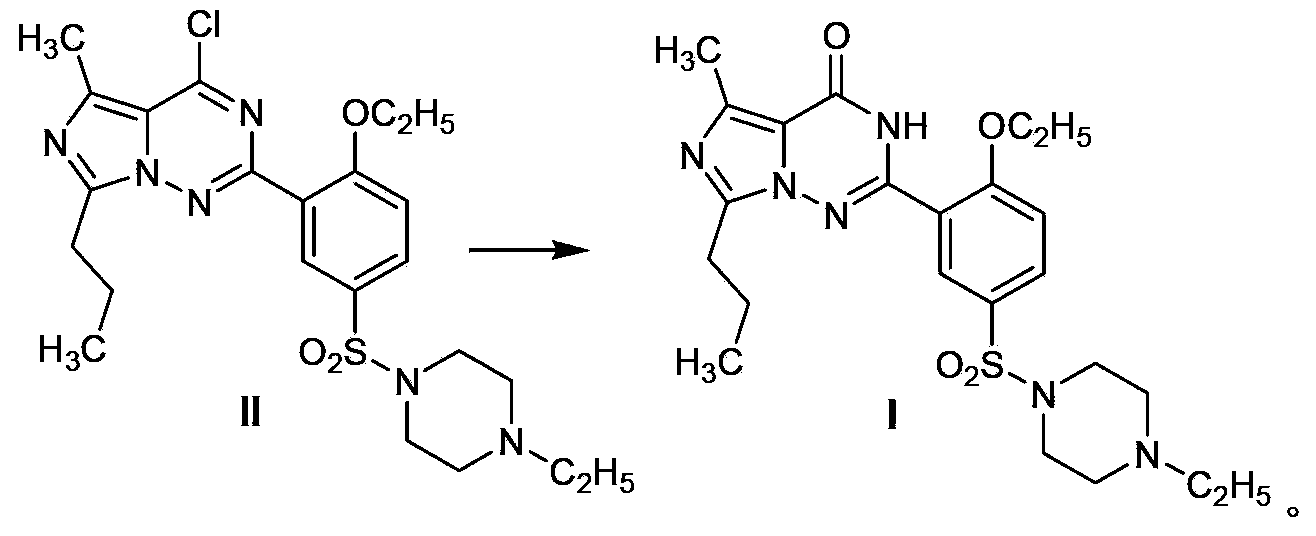
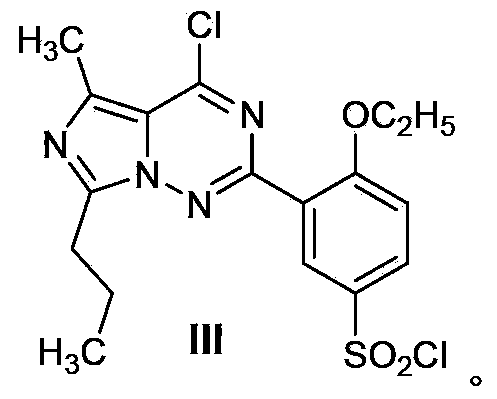
Process for preparing vardenafil and intermediates thereof
A compound and hydrolysis reaction technology, applied in the direction of organic chemistry, can solve the problems of low reaction yield and unsuitable reaction conditions for industrial scale-up, and achieve the effects of easy control of reaction conditions, reduction of side reactions and impurities, and improvement of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation of VI and IX involved in the invention can be prepared with reference to the method of patent WO9924433A1.
[0061] The compound shown in the structural formula IV in the invention can also be formed by the compound of the formula VII With the compound of formula VIII treated with hydrazine hydrate After the reaction, it can be treated with phosphorus oxychloride.
[0062] Another preparation method of the compound shown in the formula IV is: after the reaction of the compound shown in the formula VII and the compound shown in the formula VIII is completed, the solvent is evaporated, and POCl is added immediately 3 , PCl 3 , PCl 5 One or more of the reactions in the reaction, after the reaction is poured into crushed ice, extracted with an organic solvent.
[0063] The compound shown in VIII involved in the invention can be prepared by referring to the literature (J.Chem.Soc.Perkin Trans.1; EN; 1980; 1139-1146) by reacting o-ethoxybenzamidine and h...
Embodiment 1
[0068] Preparation 1: Preparation of 2-butyrylaminopropionic acid (X)
[0069] Add D, L-alanine (20.0g, 0.225mol) into a mixed solvent made of water (100mL) and acetone (50mL), and then add NaOH (22.5g, 0.225mol) to obtain a clear solution. , slowly added n-butyryl chloride (26.4 mL, 0.248 mol) dropwise. After the dropwise addition, keep the reaction below 5°C for 2 hours, remove the ice bath, distill off the acetone under reduced pressure, adjust the pH to 1.0 with 6mol / L hydrochloric acid, add butyl acetate (100mL×3), divide water and dry, evaporate Butyl acetate was removed to obtain a light yellow oil, and an appropriate amount of petroleum ether was added to precipitate a white solid, which was filtered with suction and dried to obtain product X (25.8 g), with a yield of 72%. 1 H NMR (CDCl 3 , 300MHz) δ: 0.94(3H, t), 1.44(3H, d), 1.65(2H, m), 2.22(2H, t), 4.57(2H, m), 6.37(1H, d), 6.62(1H , s).
[0070] Preparation 2: Preparation of 3-butyrylamino-2-oxo-butyric acid e...
Embodiment 2
[0083] Preparation 1: Preparation of 2-(2-ethoxyphenyl)-4-chloro-5-methyl-7-propylimidazol[5,1-f][1,2,4]-triazine (IV)
[0084] O-ethoxybenzamidine hydrochloride (VIII) (2.01g, 10mmol) was dissolved in ethanol (15mL), and 85% hydrazine hydrate (0.59mL, 10mmol) was slowly added dropwise under ice-cooling. After the addition was complete, React in an ice bath for 30 minutes, stir at room temperature for 1 hour, then add the ethanol (15 mL) solution of the product (2.58 g) prepared in Example 1, heat to reflux for 3 hours, filter, spin evaporate the solvent to obtain an oil, add POCl 3 (3mL), heated under reflux for 3 hours, TLC observed that the reaction was complete, evaporated the excess phosphorus oxychloride under reduced pressure, poured the residue into crushed ice slowly, extracted the product with dichloromethane (30mL) immediately, and washed the organic phase with saturated Wash with brine (20mL×2), dry over anhydrous sodium sulfate, distill off the solvent to an appro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com