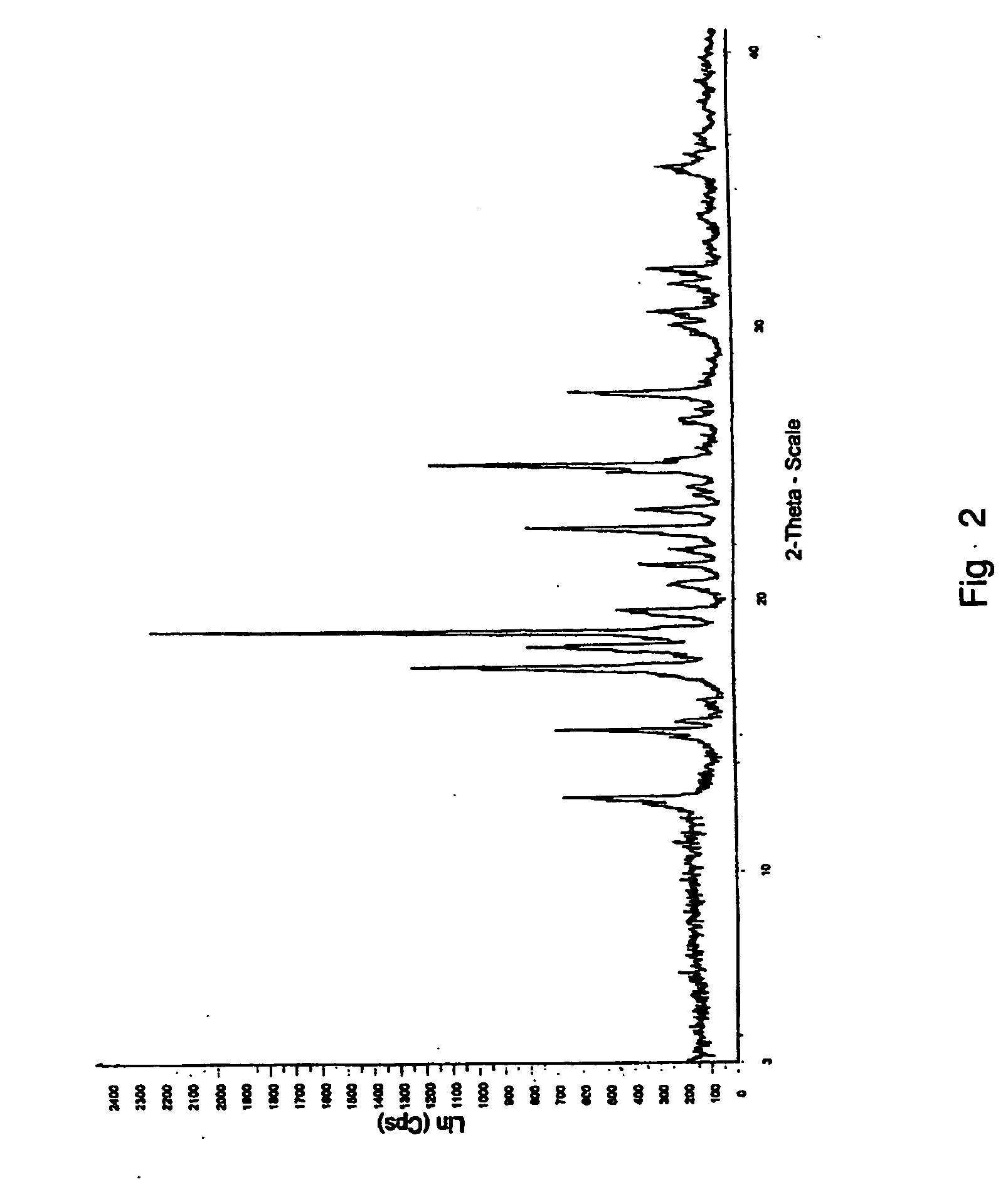
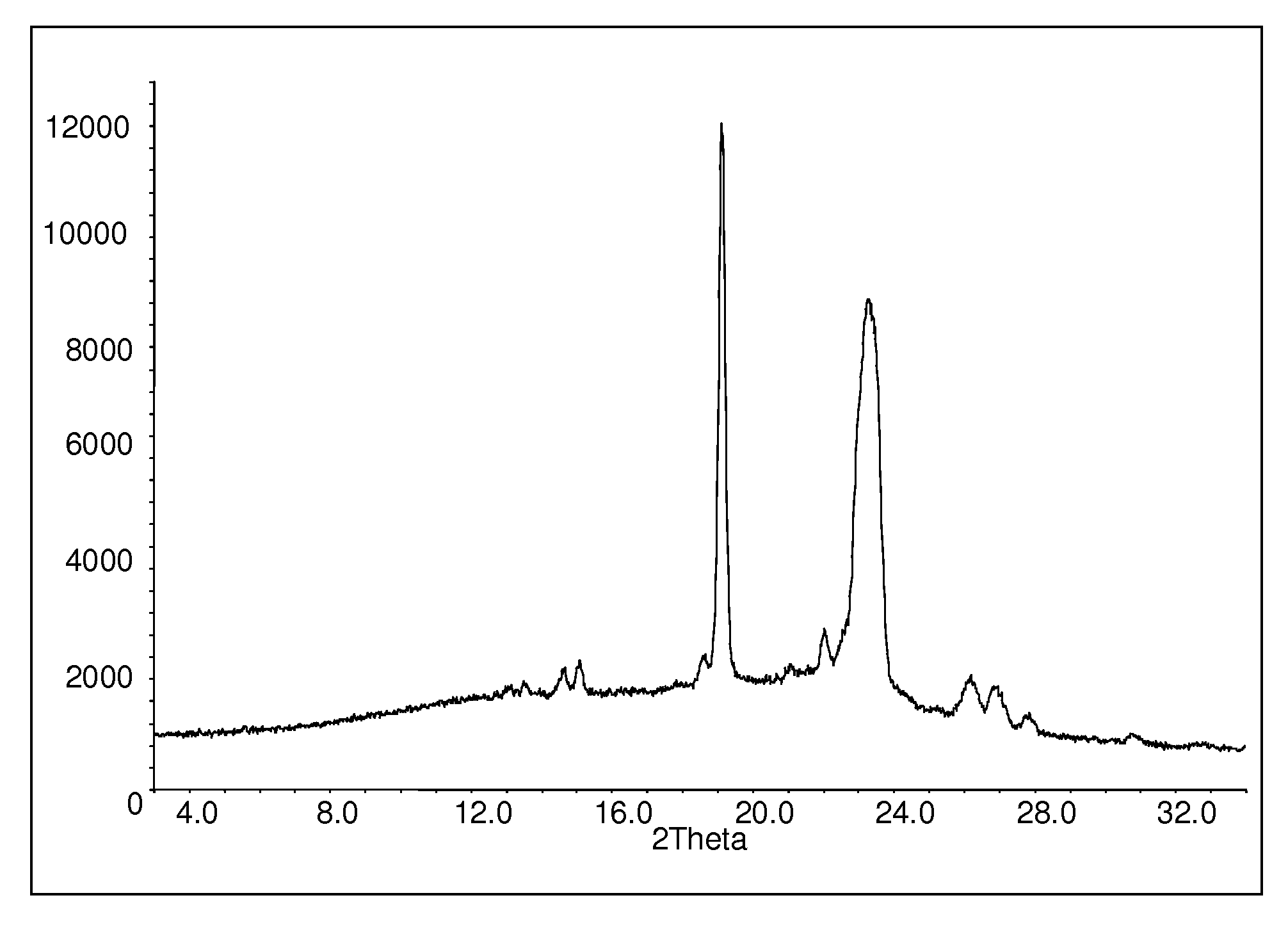
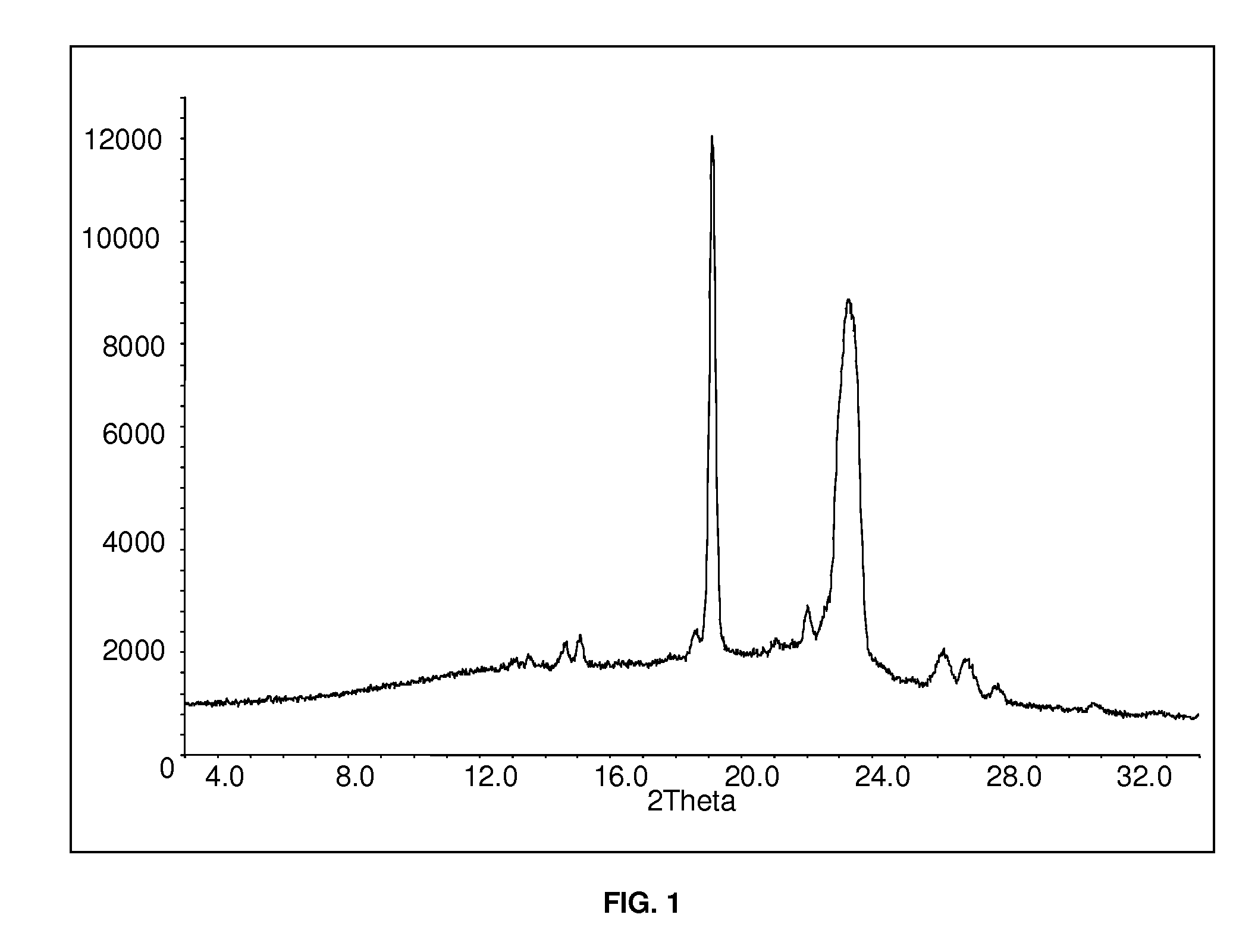
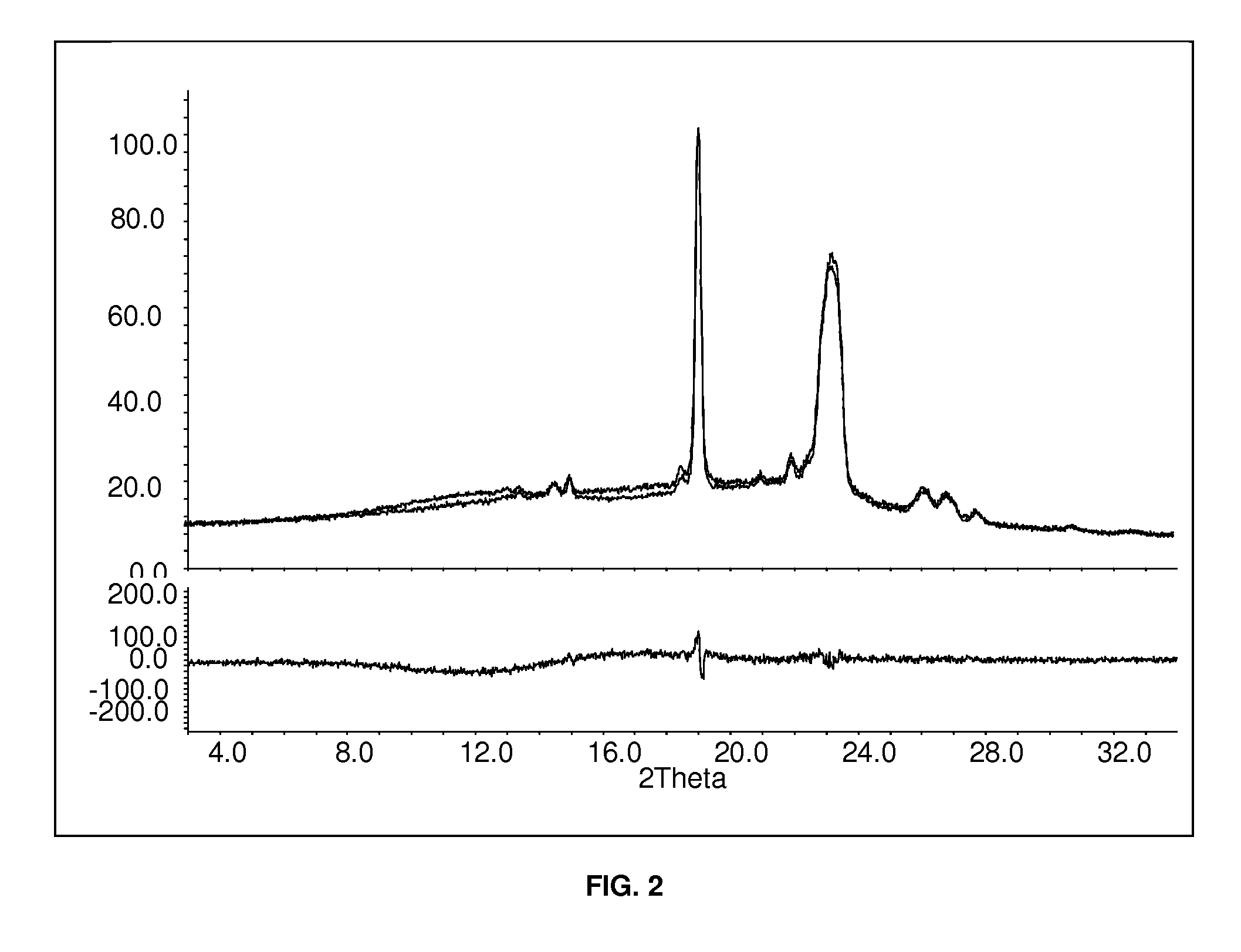
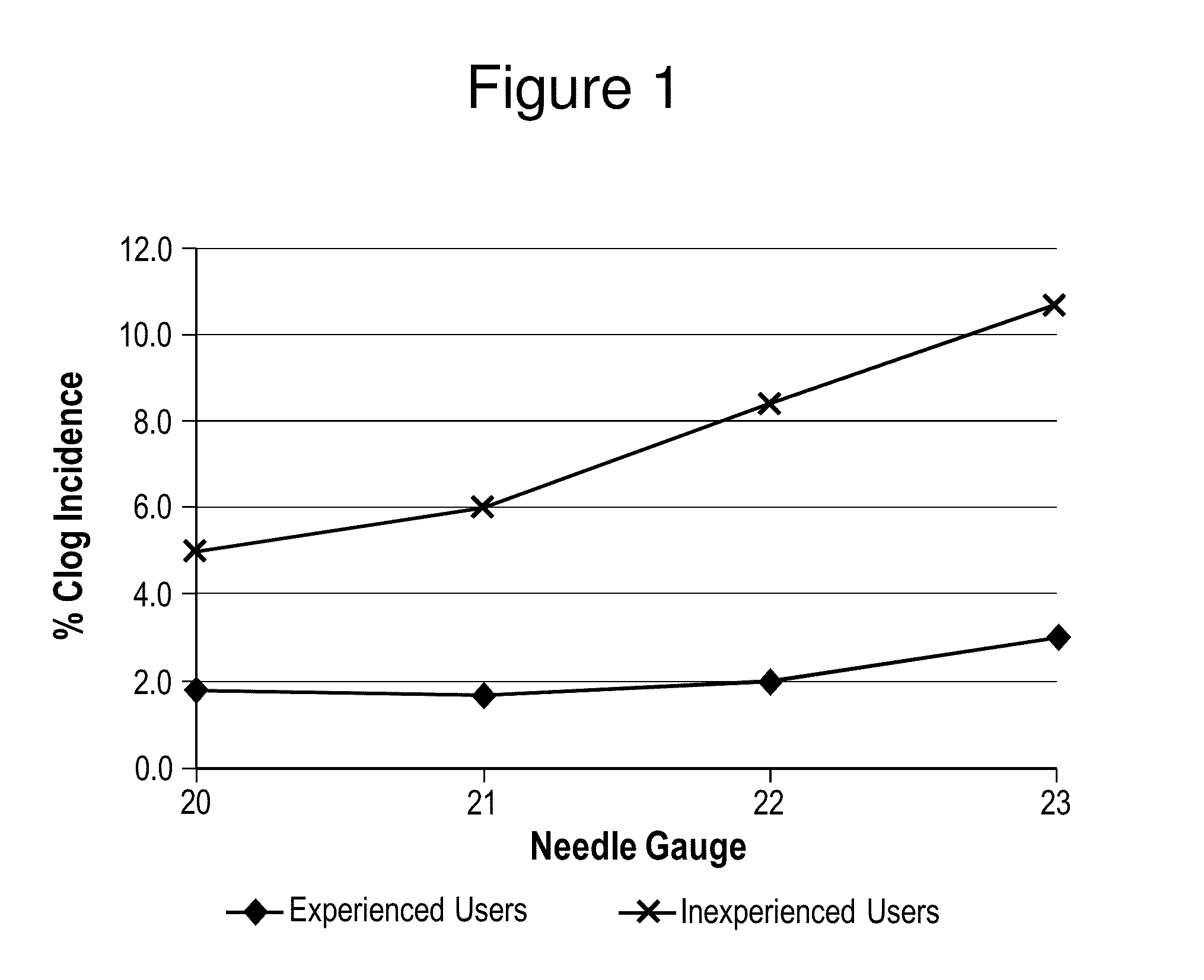
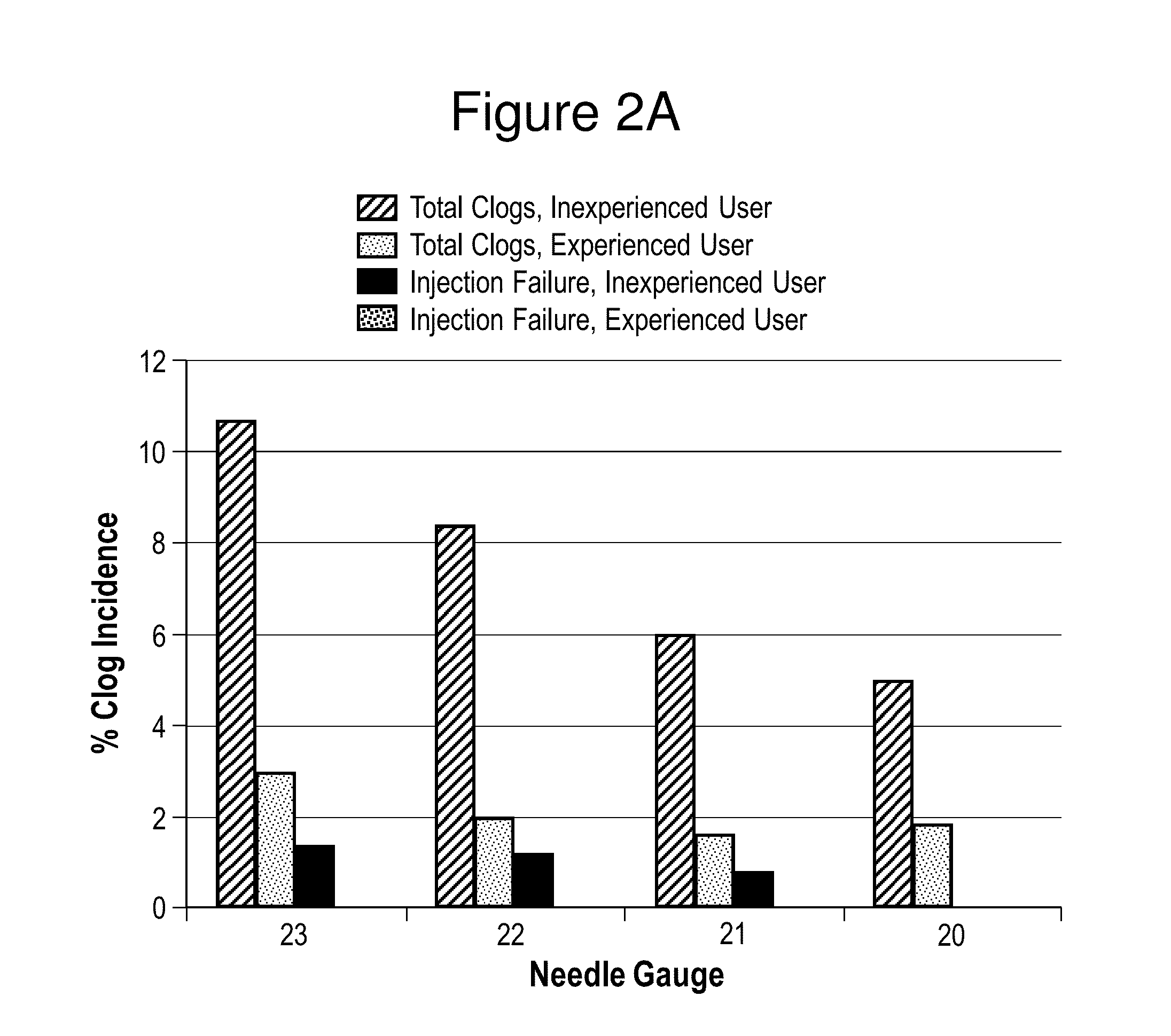
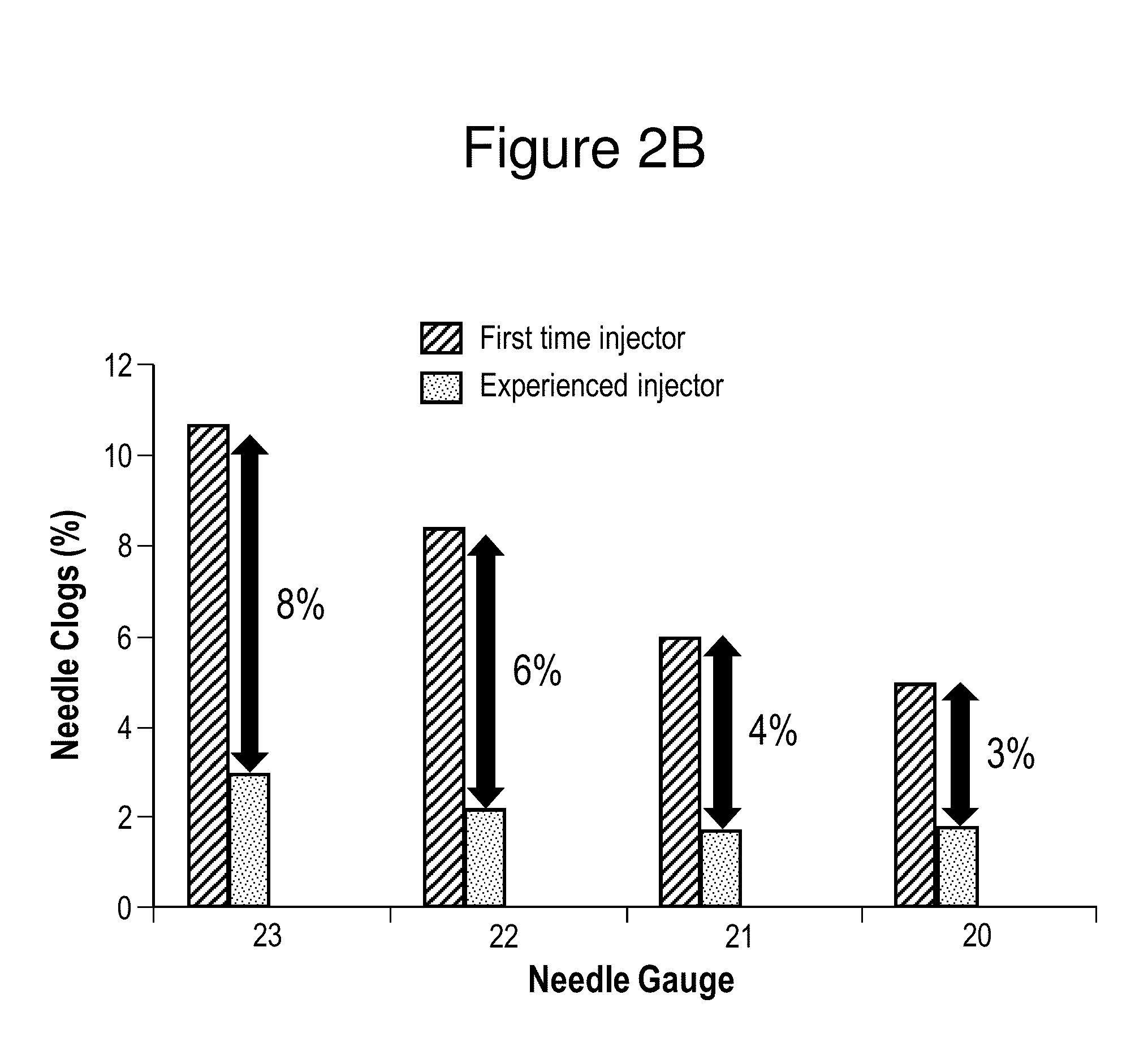
Patents
Literature
289 results about "Aripiprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain mental/mood disorders (such as schizophrenia, depression).
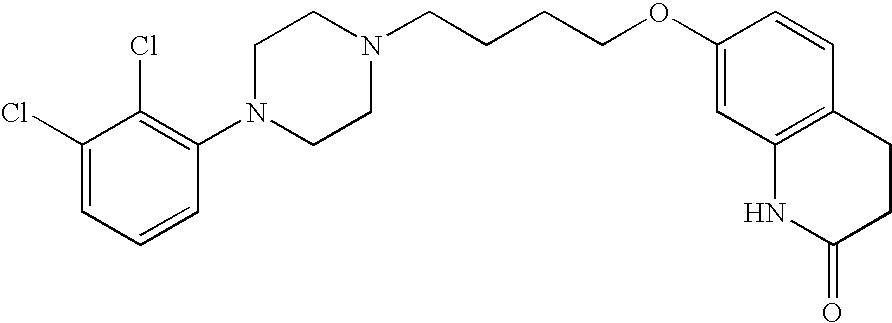
Aripiprazole complex formulation and method
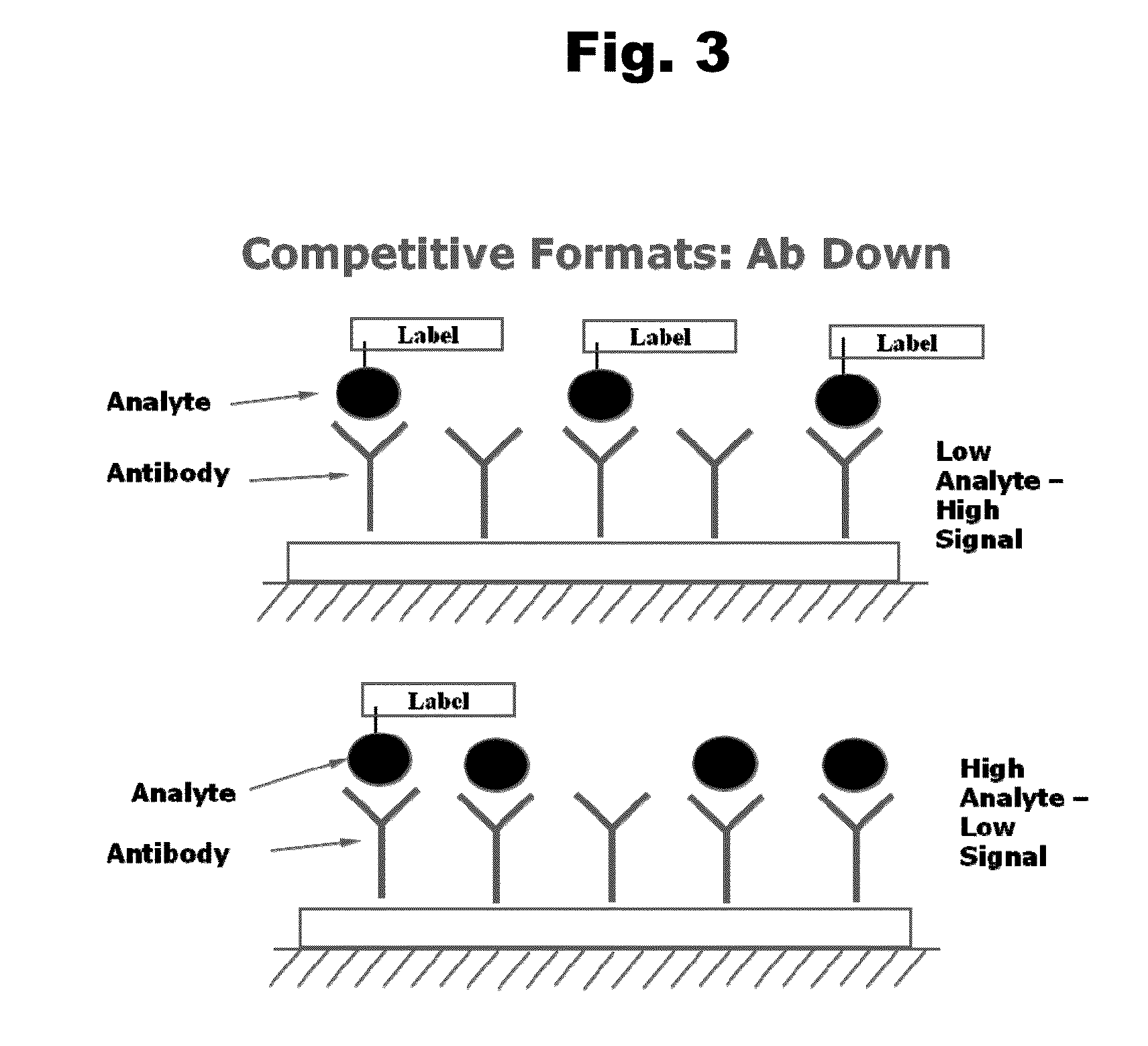
An aripiprazole formulation is provided which includes the antipsychotic agent aripiprazole in the form of an inclusion complex in a β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBECD), which in the form of an injectable produces reversible generally minimal to mild irritation at the intramuscular injection site. A method for minimizing or reducing irritation caused by aripiprazole at an intramuscular injection site and a method for treating schizophrenia employing the above formulation are also provided.
Owner:OTSUKA PHARM CO LTD
Methods for administering aripiprazole
InactiveUS20050032811A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
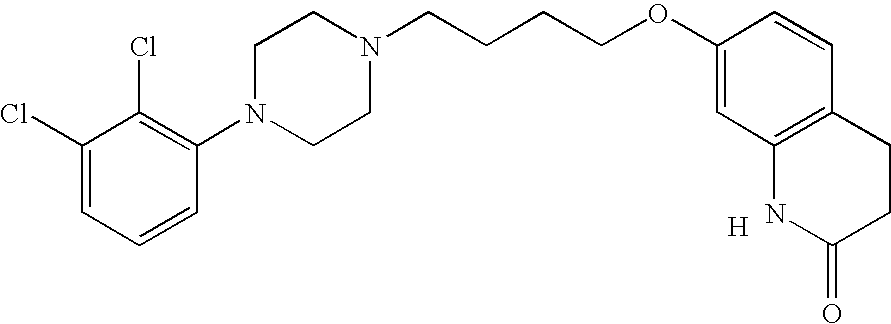
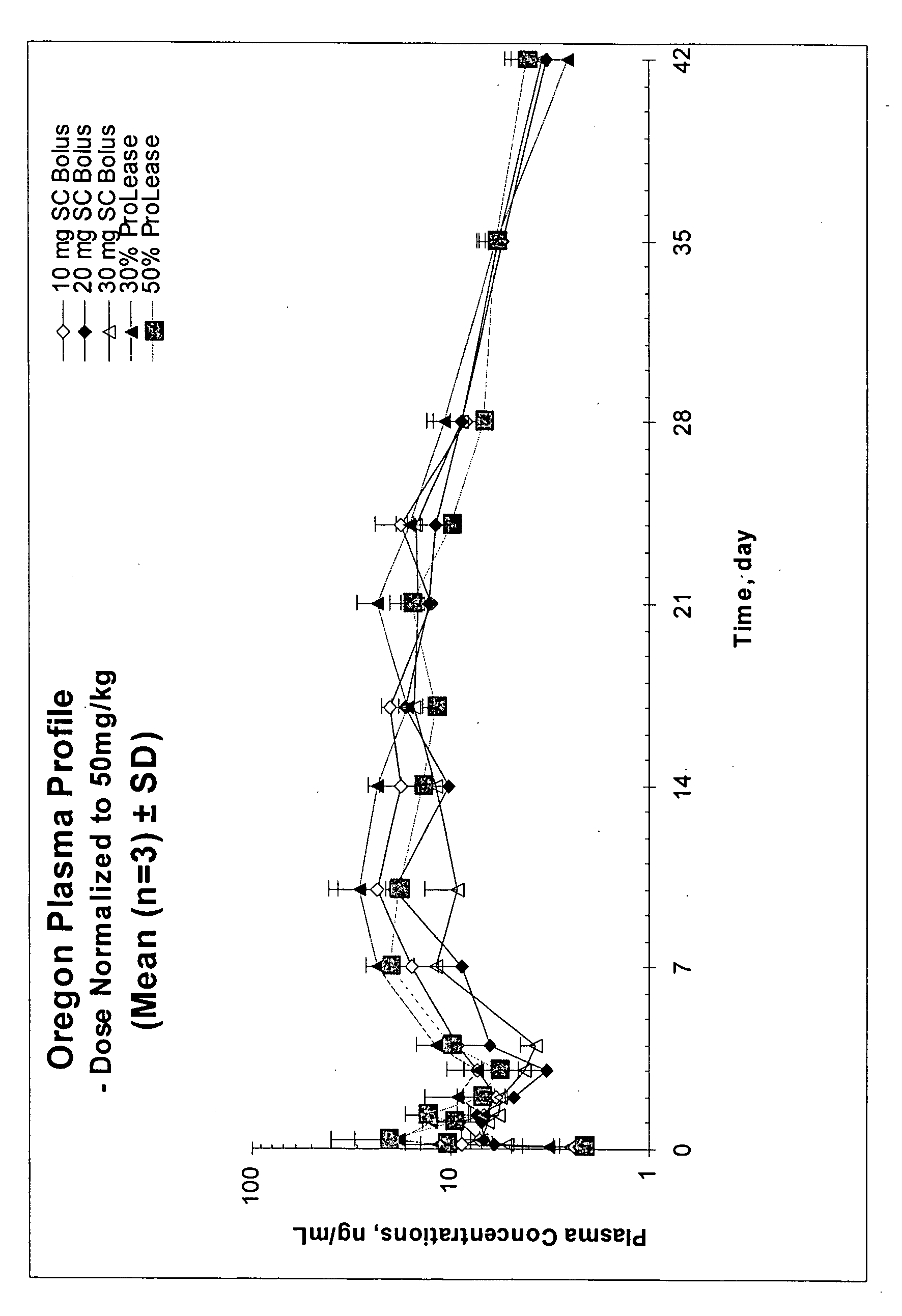
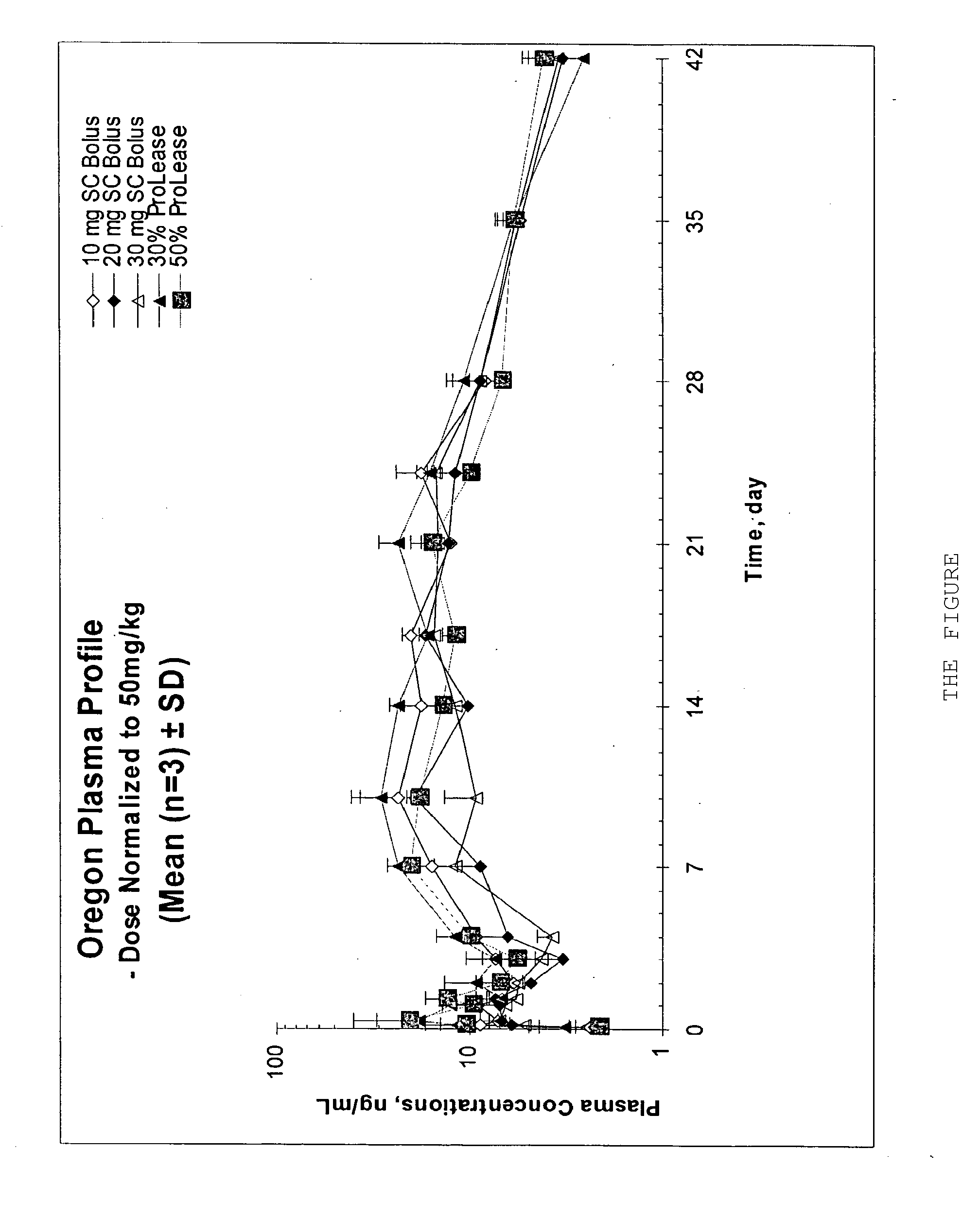
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:ALKERMES INC
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
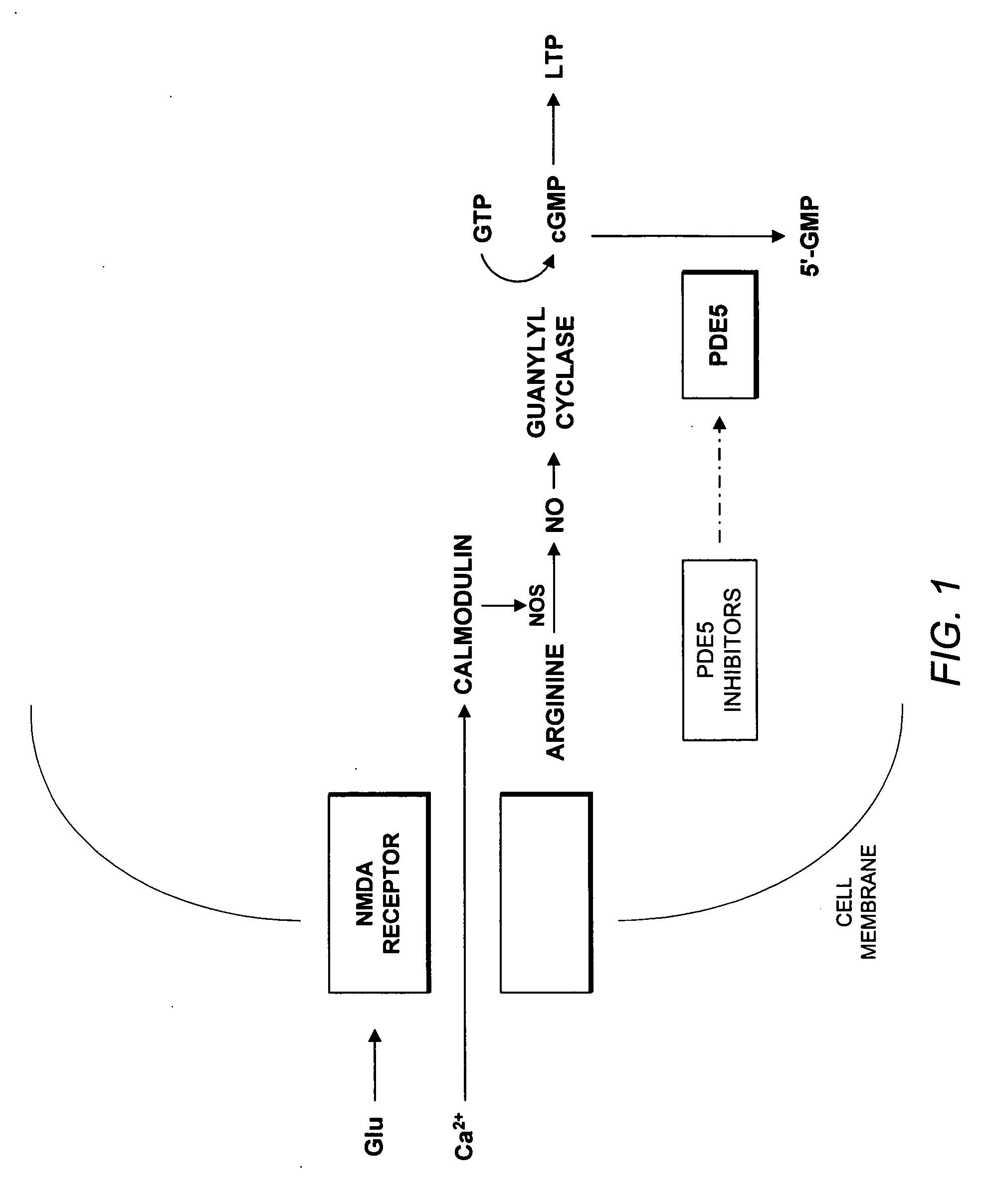
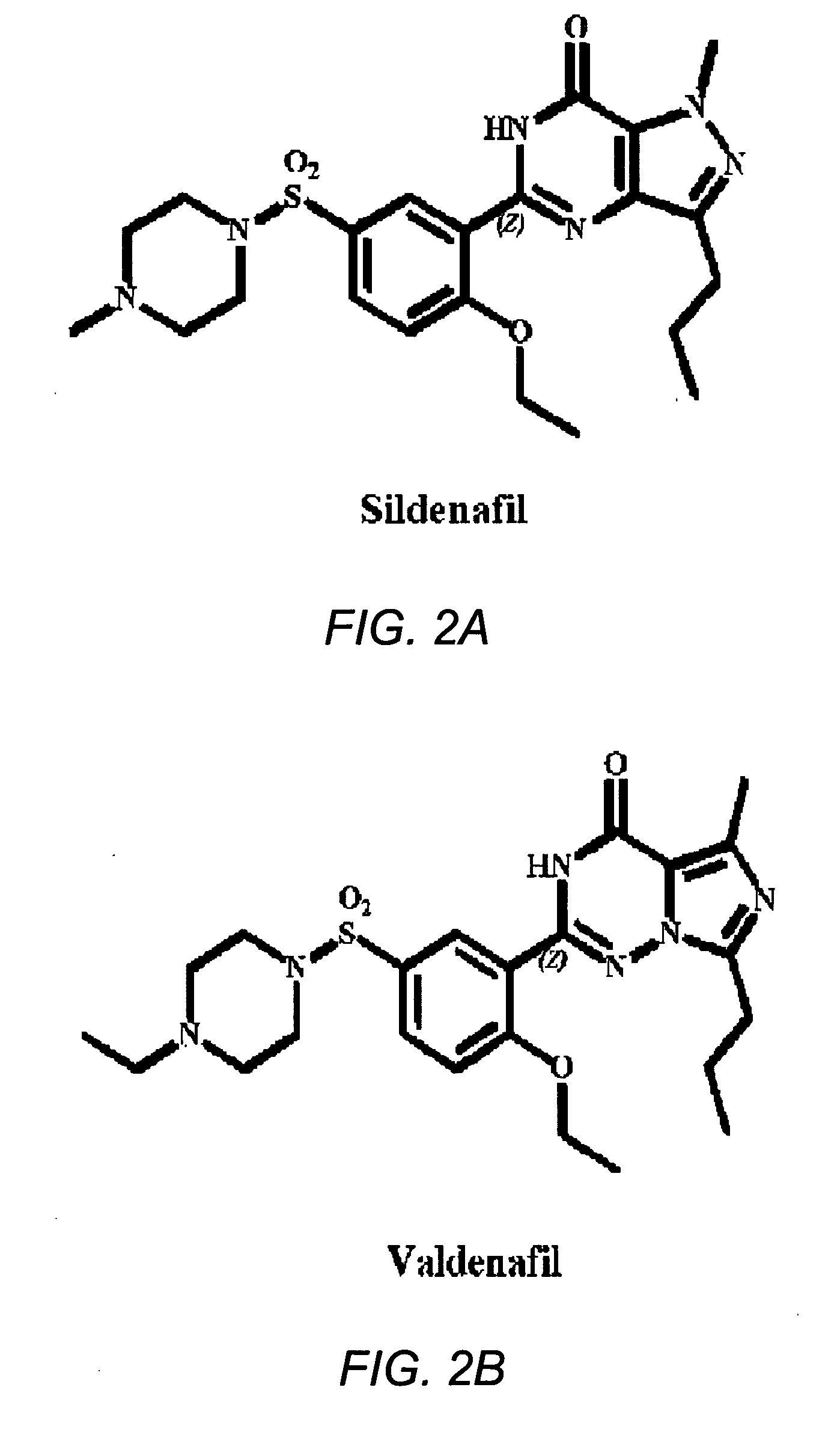
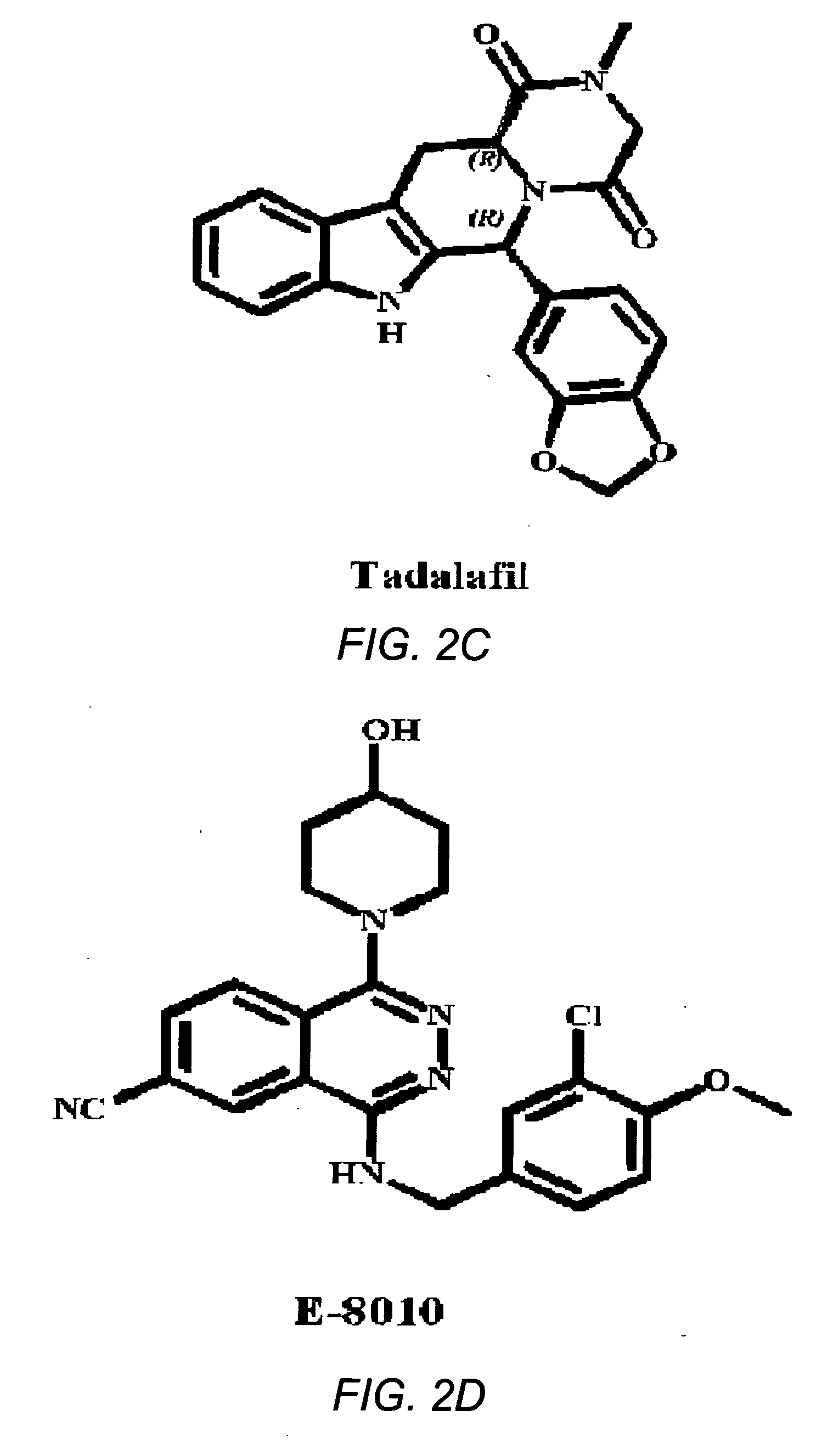
Use of phosphodiesterase 5 (PDE5) inhibitors in the treatment of schizophrenia
The use of phosphodiesterase 5 (PDE5) inhibitors for treatment of schizophrenia is described. Suitable PDE5 inhibitors for use for treatment of schizophrenia include sildenafil, vardenafil, tadalafil, E-8010, zaprinast, and E-4021. In one embodiment, for example, a method is described for treating schizophrenia in a patient which comprises treating the patient with an effective amount of a PDE5 inhibitor, or a pharmaceutically acceptable salt, solvate, or composition thereof. The PDE5 inhibitor may be administered orally. The PDE5 inhibitor may also be administered together with one or more conventional antipsychotic medications such as risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine, haloperidol, and fluphenazine.
Owner:SHARY CIRCLE
Methods for Administering Aripiprazole
ActiveUS20090143403A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:OTSUKA PHARM CO LTD
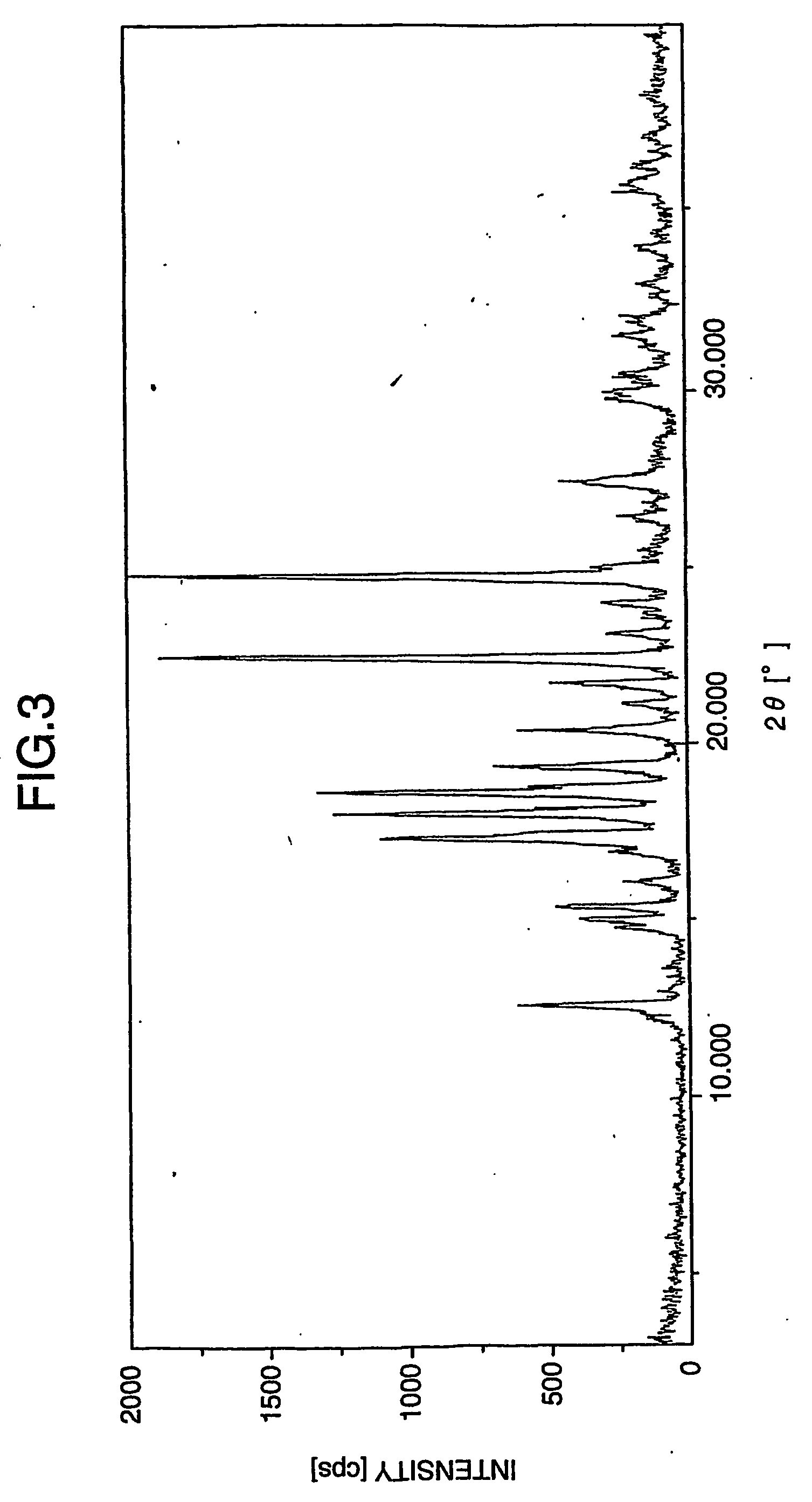
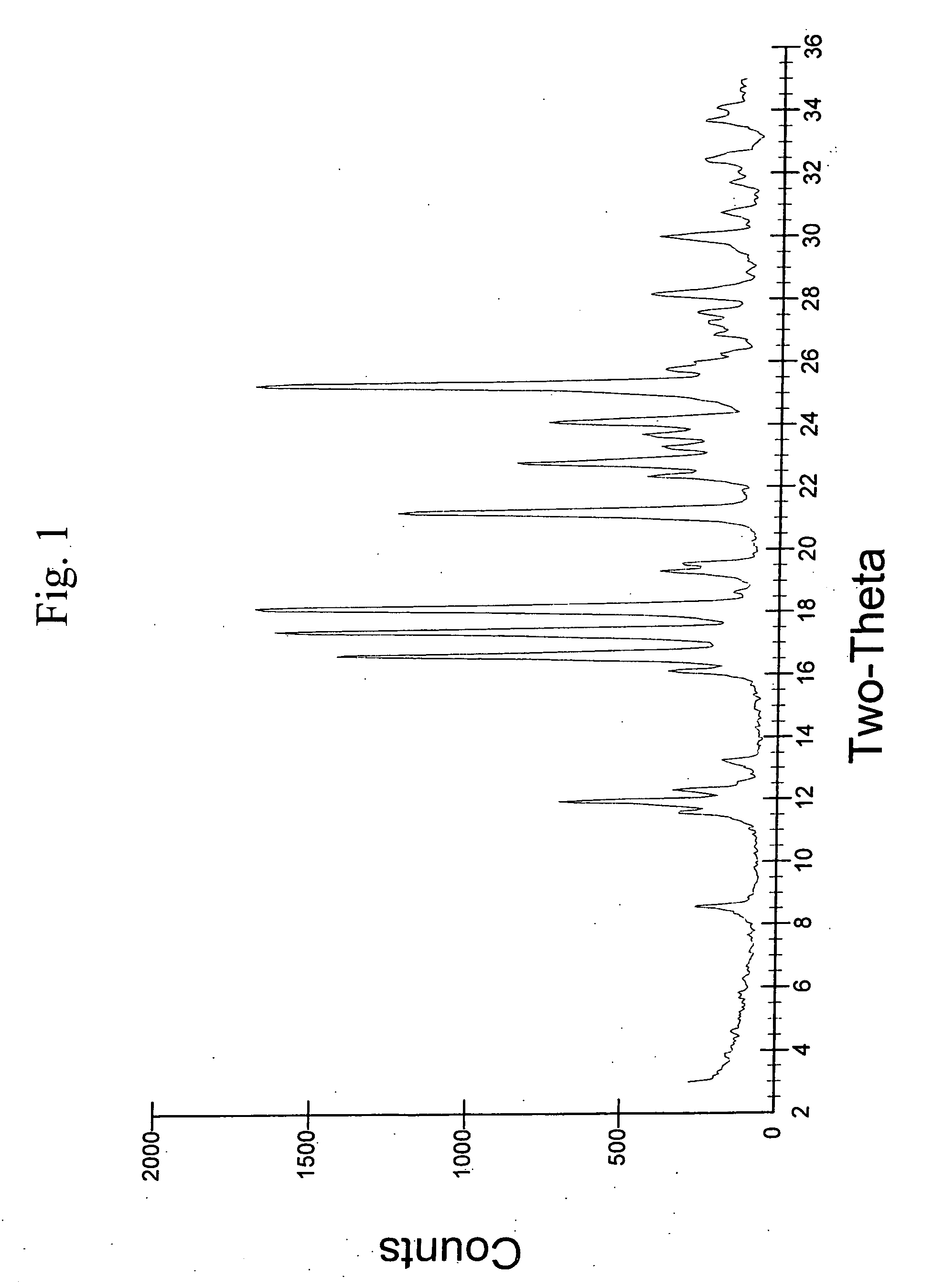
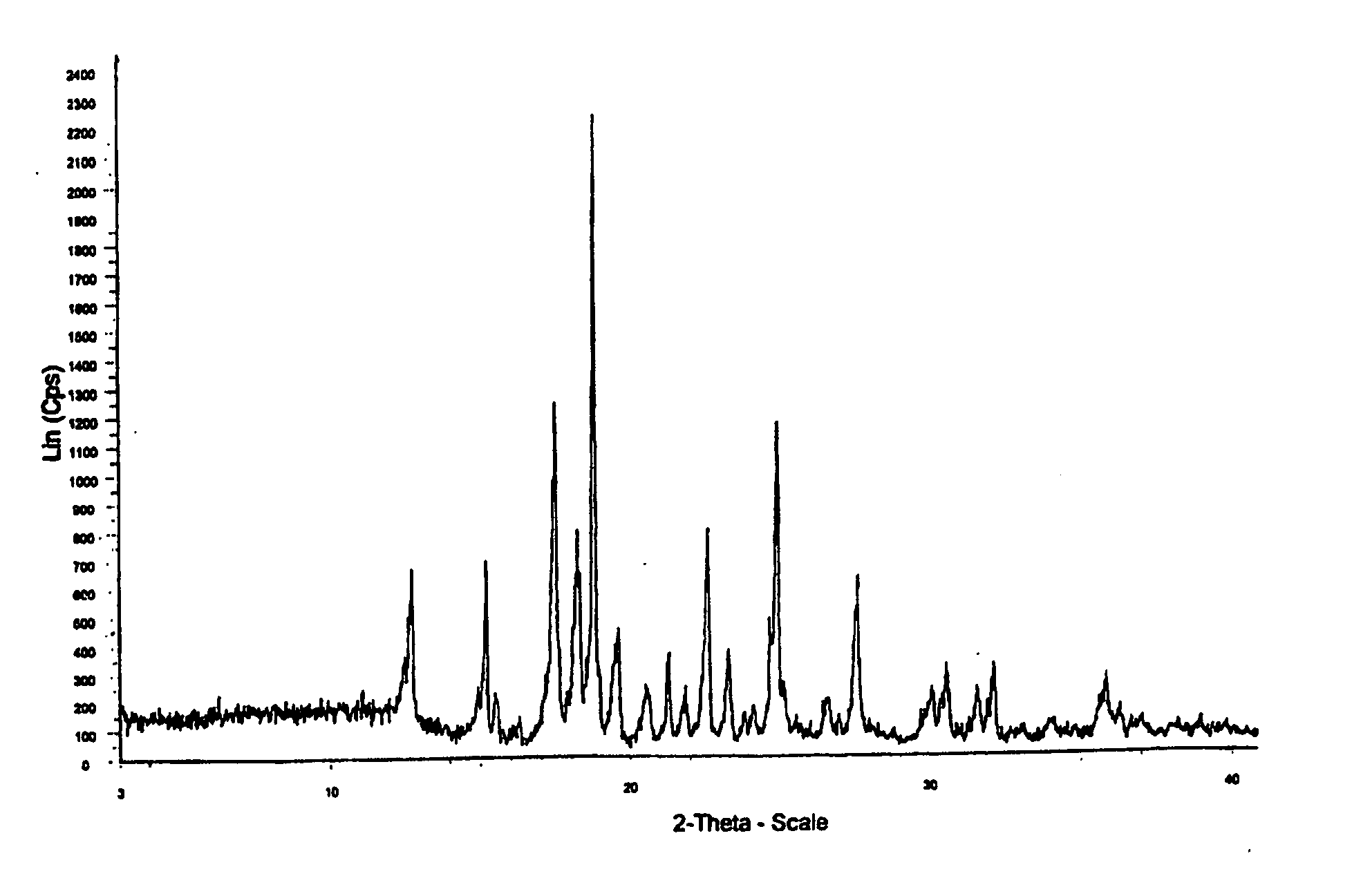
Crystalline aripiprazole salts and processes for preparation and purification thereof
InactiveUS20060223820A1Improve propertiesHigh yieldOrganic active ingredientsOrganic chemistry methodsCarboxylic acidAripiprazole
Provided are novel crystalline carboxylic acid salts of aripiprazole, methods of using such salts, and processes for producing such salts.
Owner:CHEMAGIS
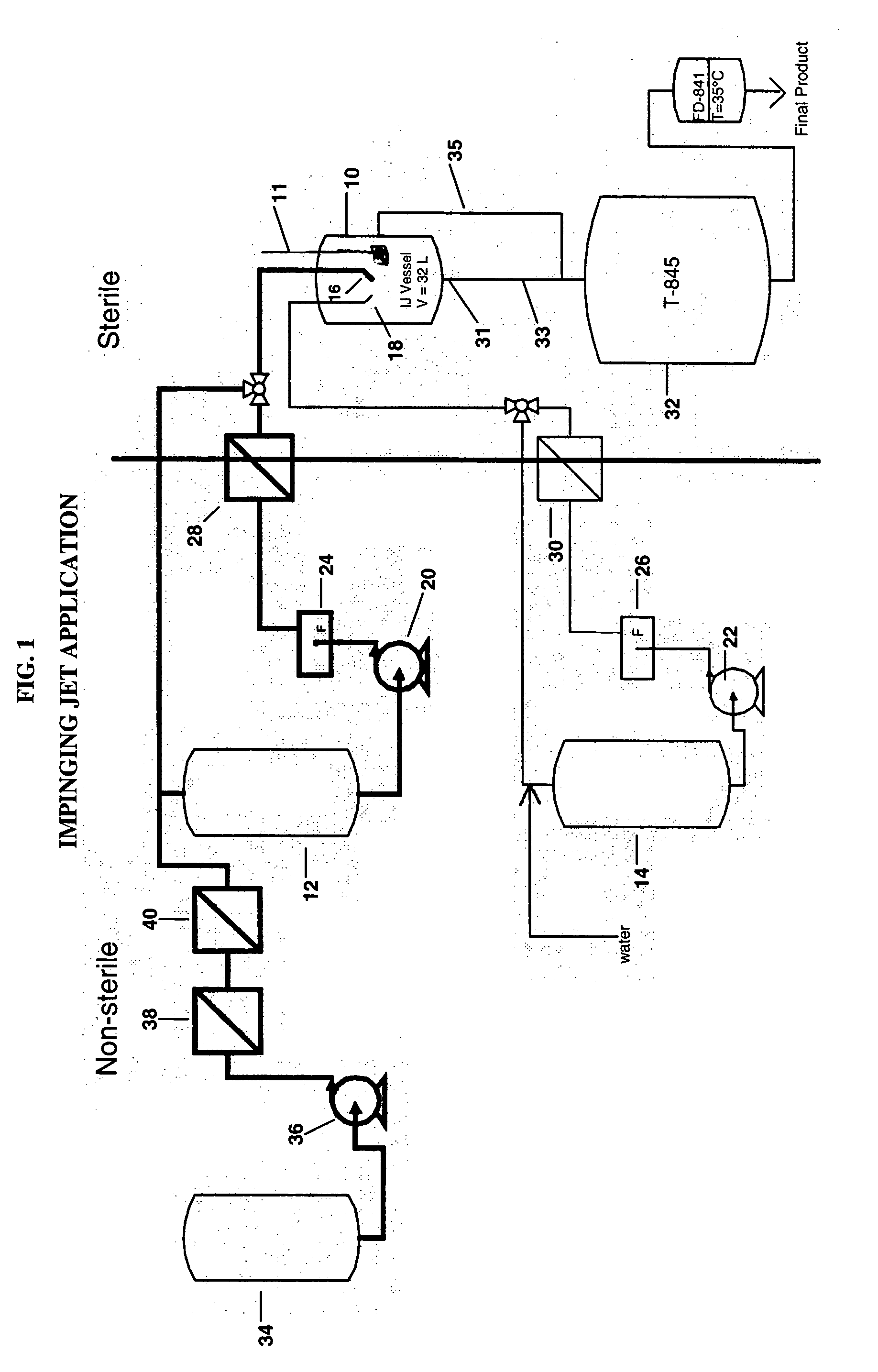
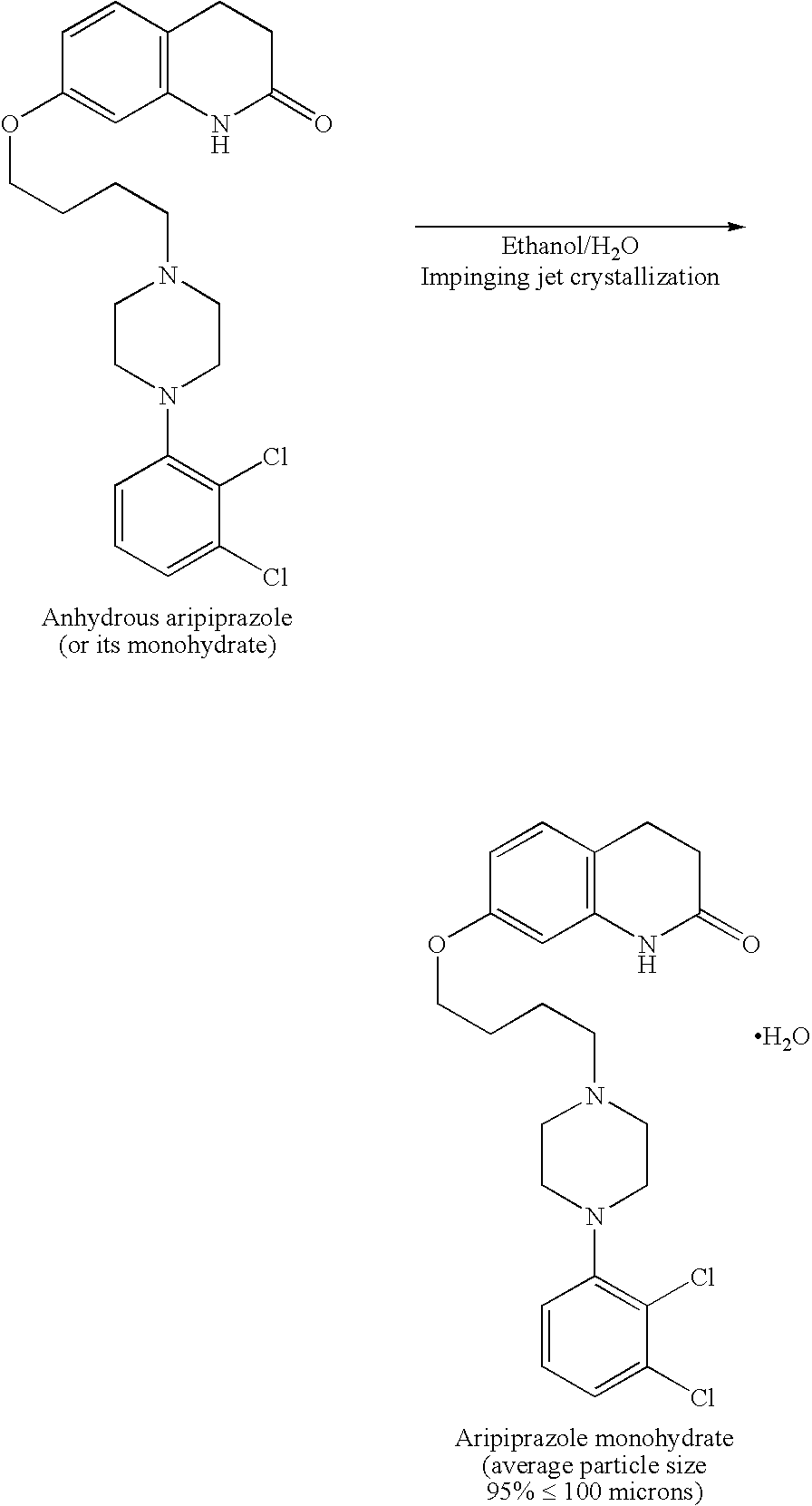
Process for making sterile aripiprazole of desired mean particle size
InactiveUS20050152981A1Enable formationHigh strengthOrganic active ingredientsPowder deliveryIntramuscular injectionFreeze-drying
A process is provided for making sterile aripiprazole having an average particle size less than 100 microns but preferably greater than 25 microns employing an impinging jet crystallization procedure. The resulting bulk aripiprazole of desired particle size may be used to form a sterile freeze-dried aripiprazole formulation, which upon constitution with water and intramuscular injection releases aripiprazole over a period of at least about one week and up to about eight weeks.
Owner:BRISTOL MYERS SQUIBB CO
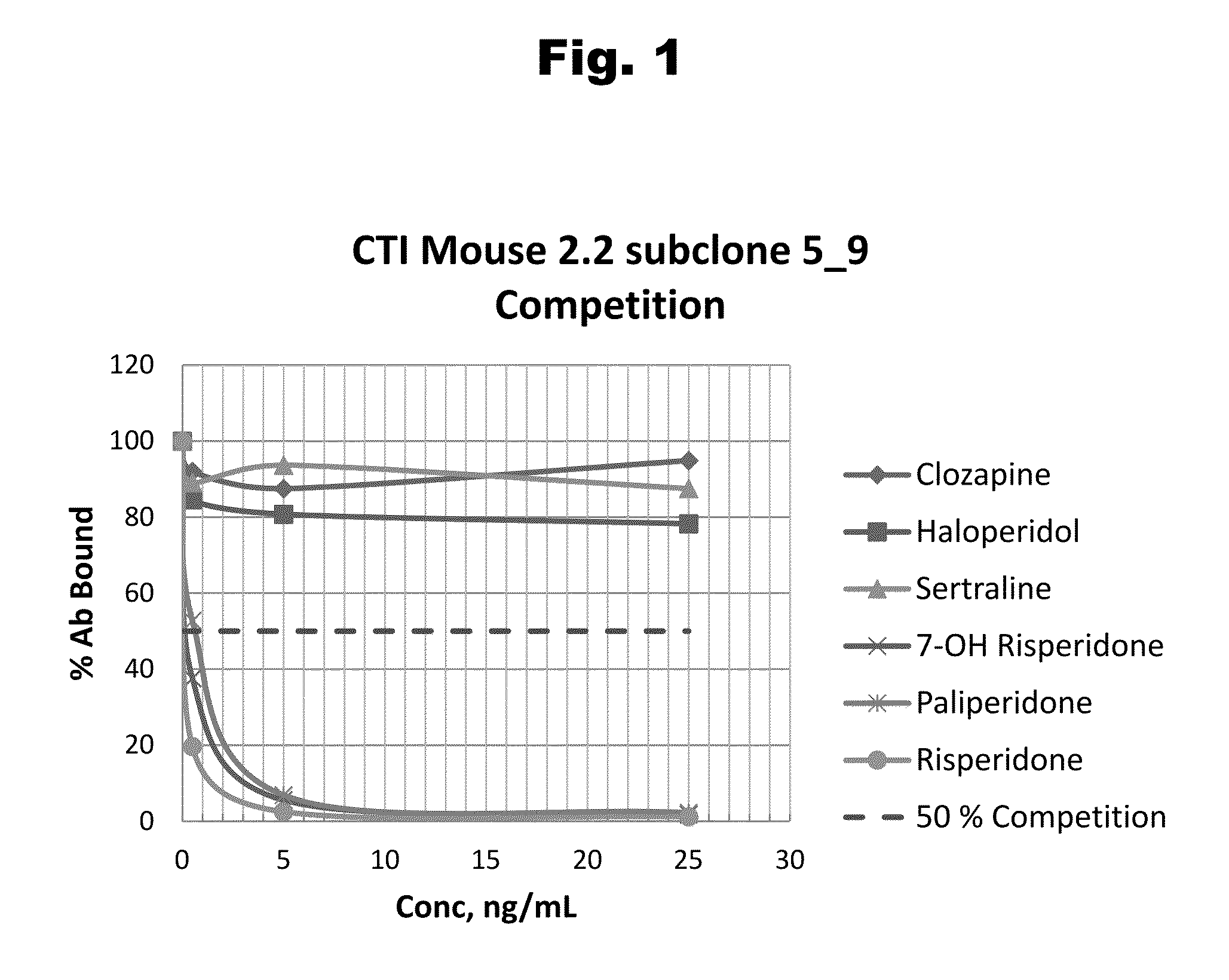
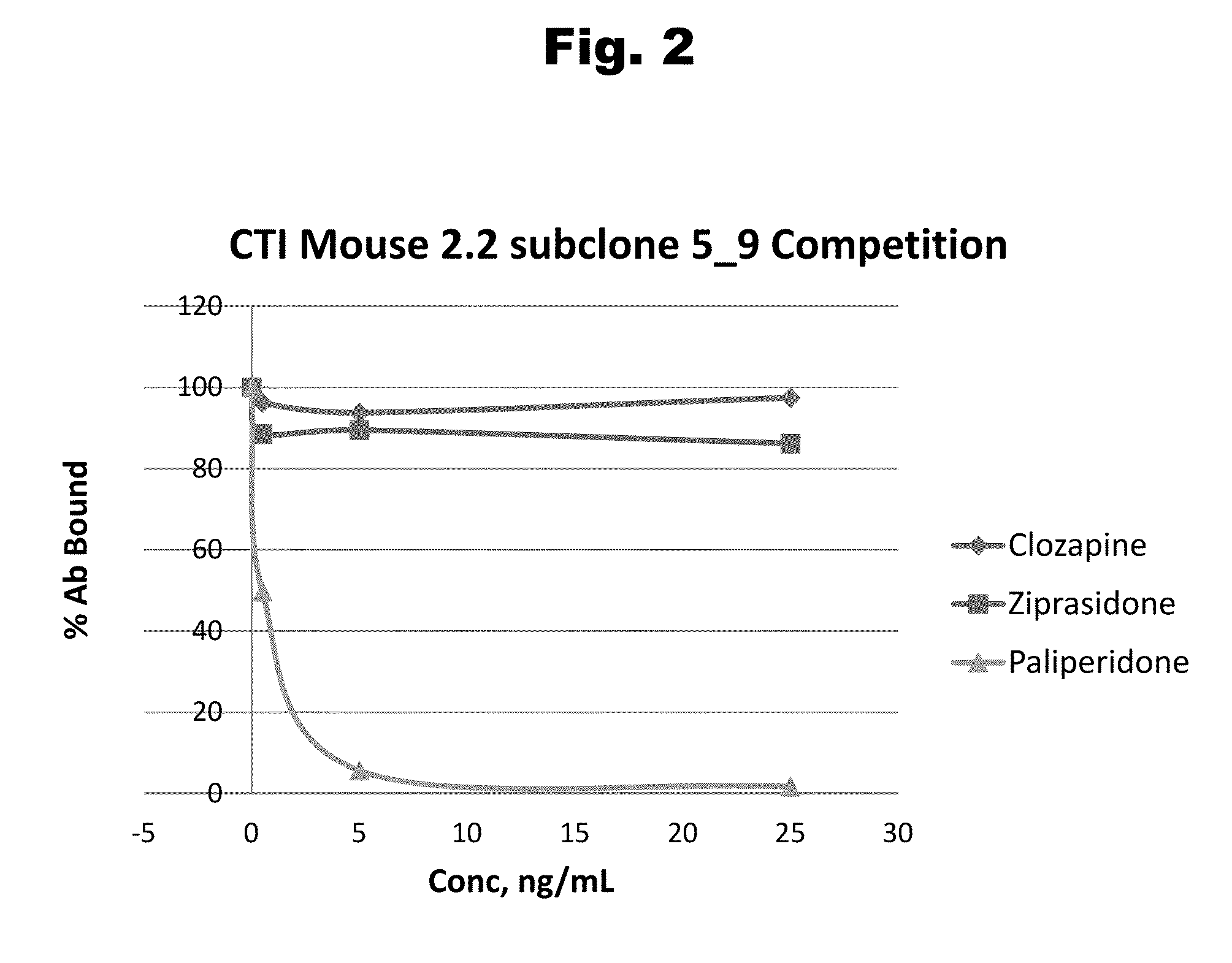
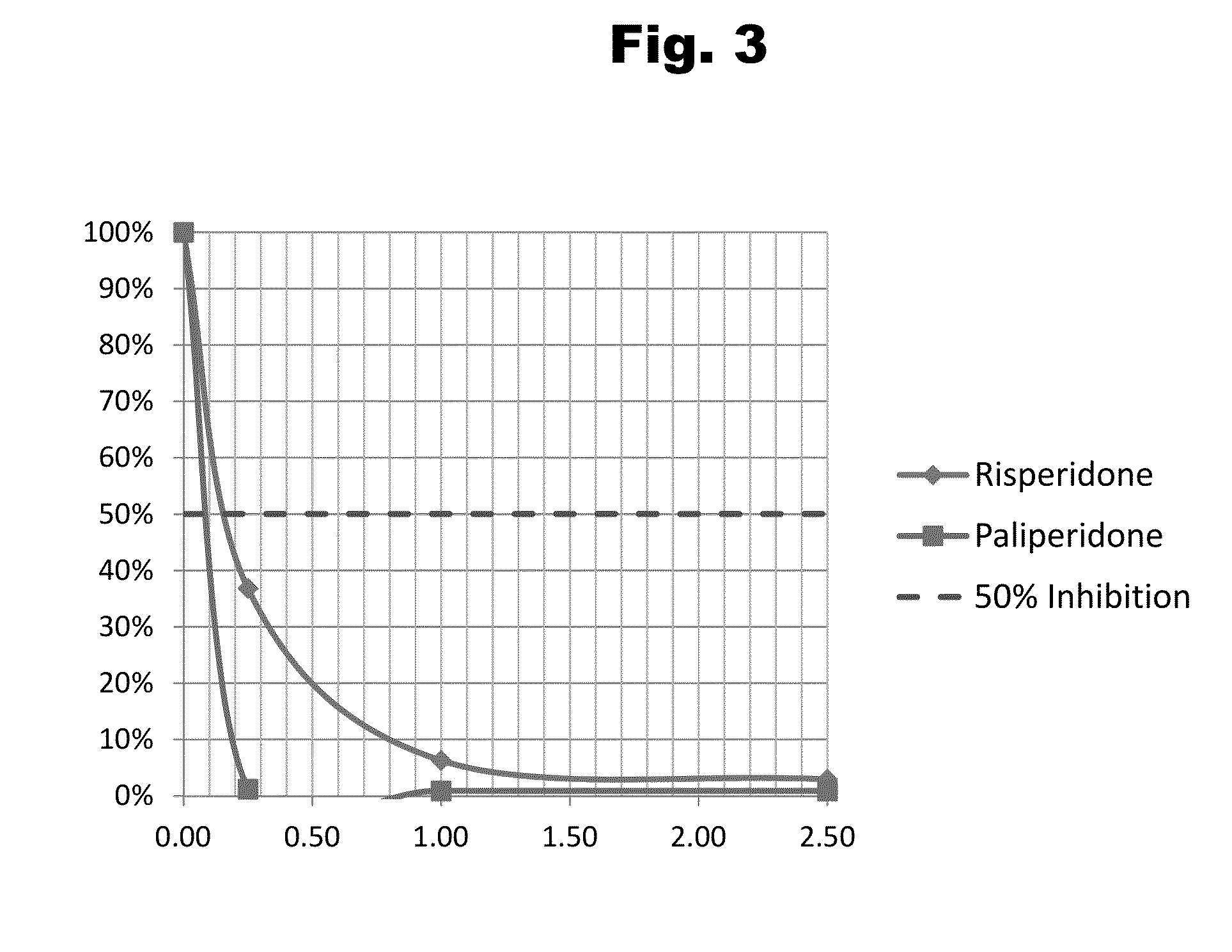
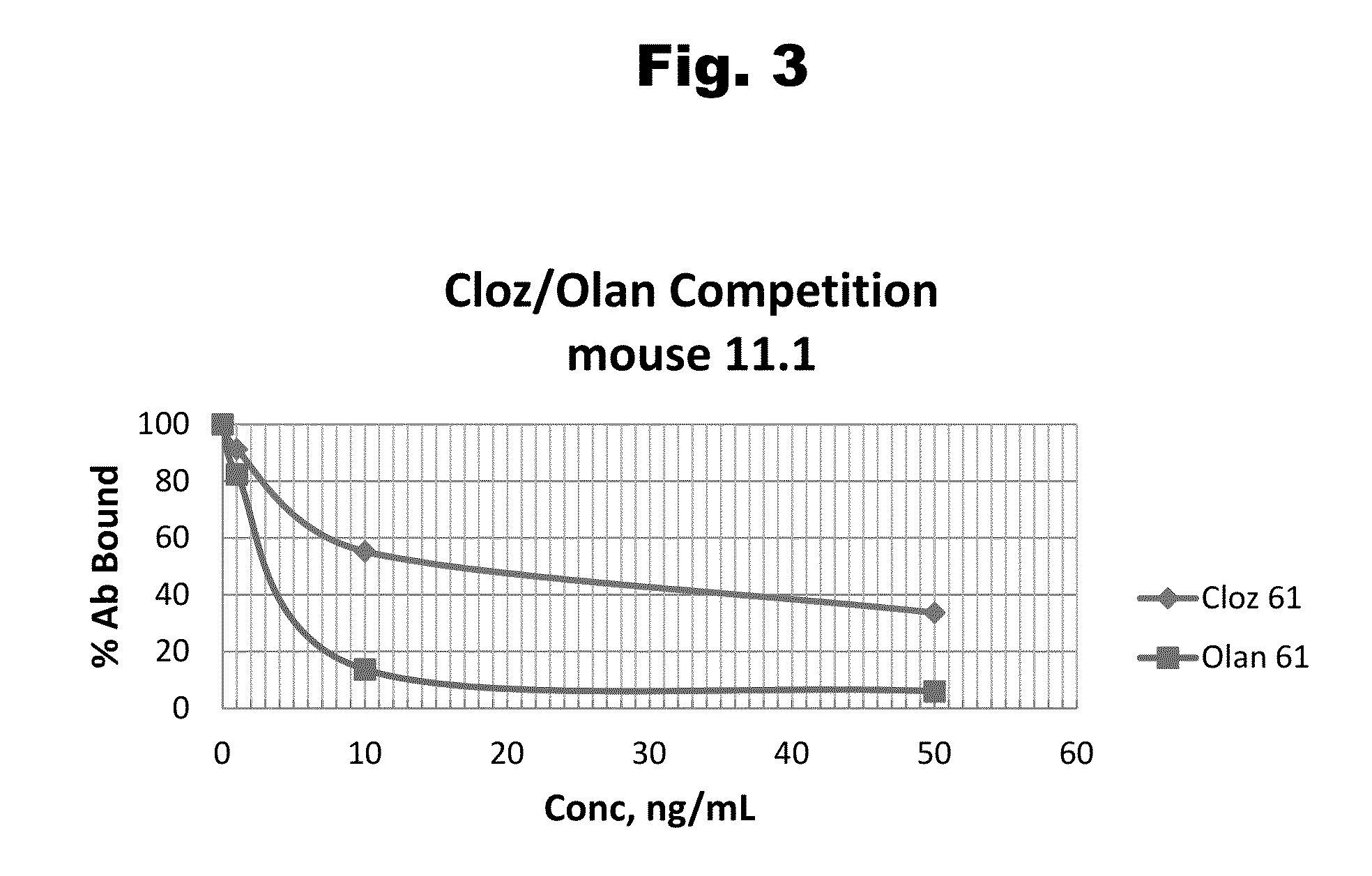
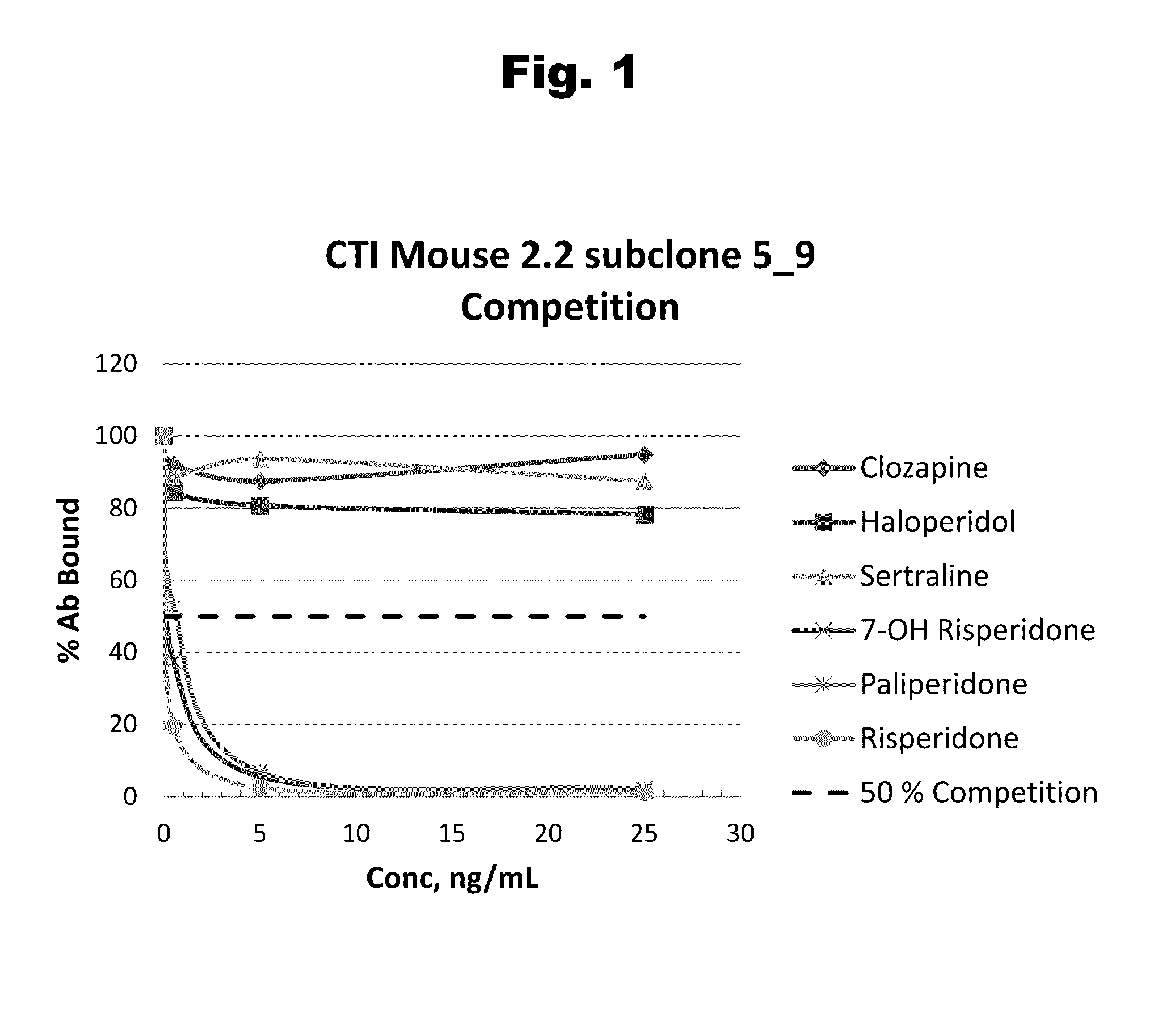
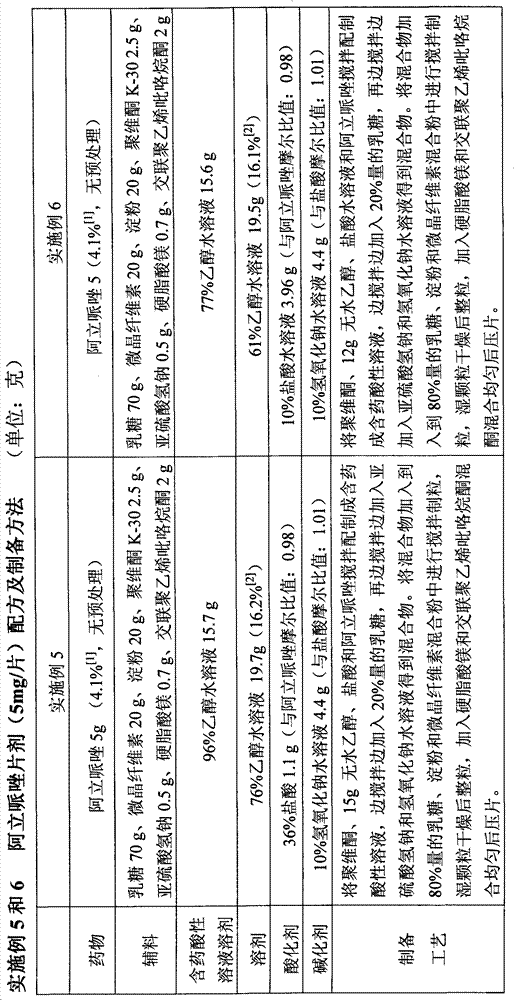
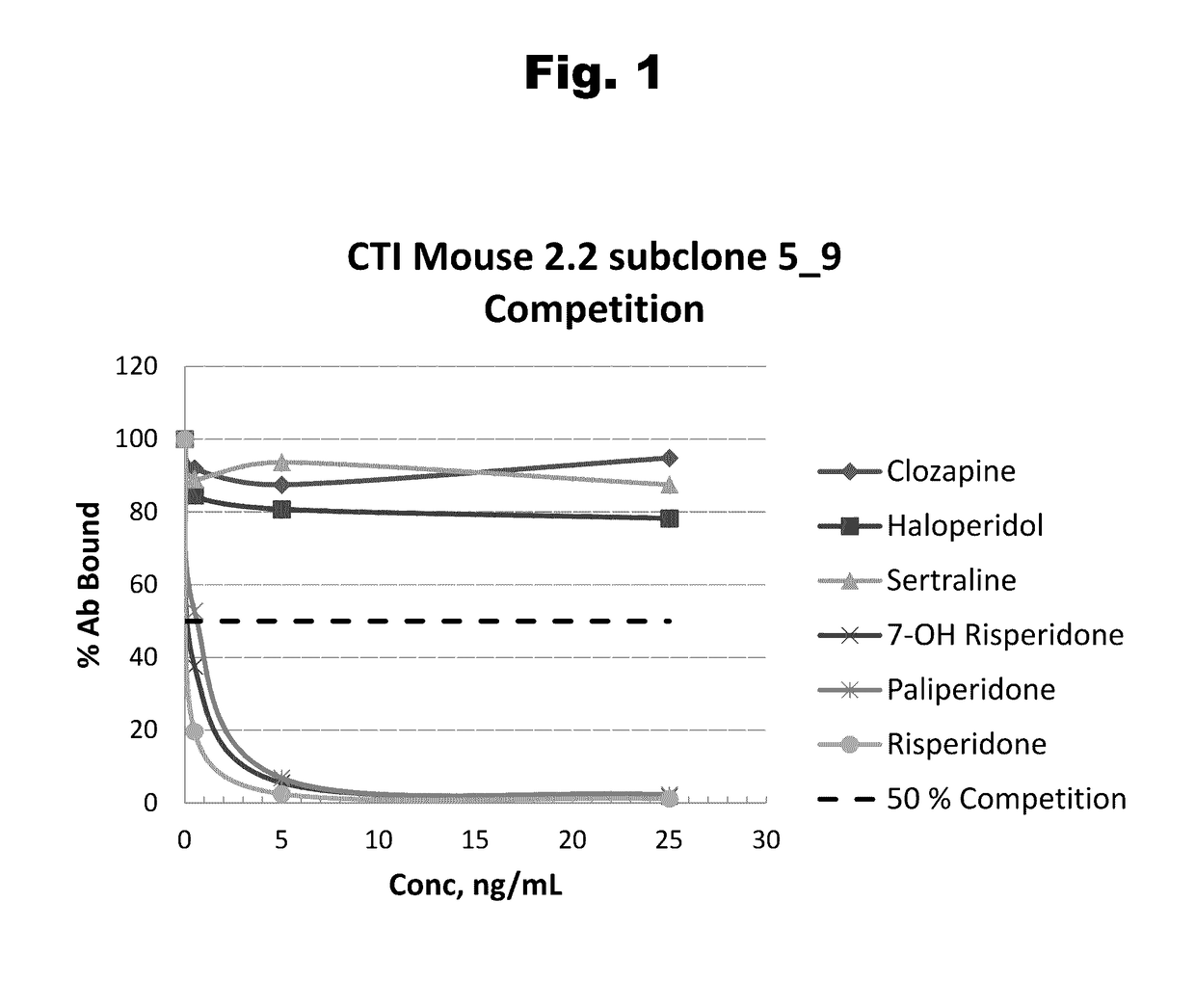
Antibodies to Risperidone Haptens and Use Thereof
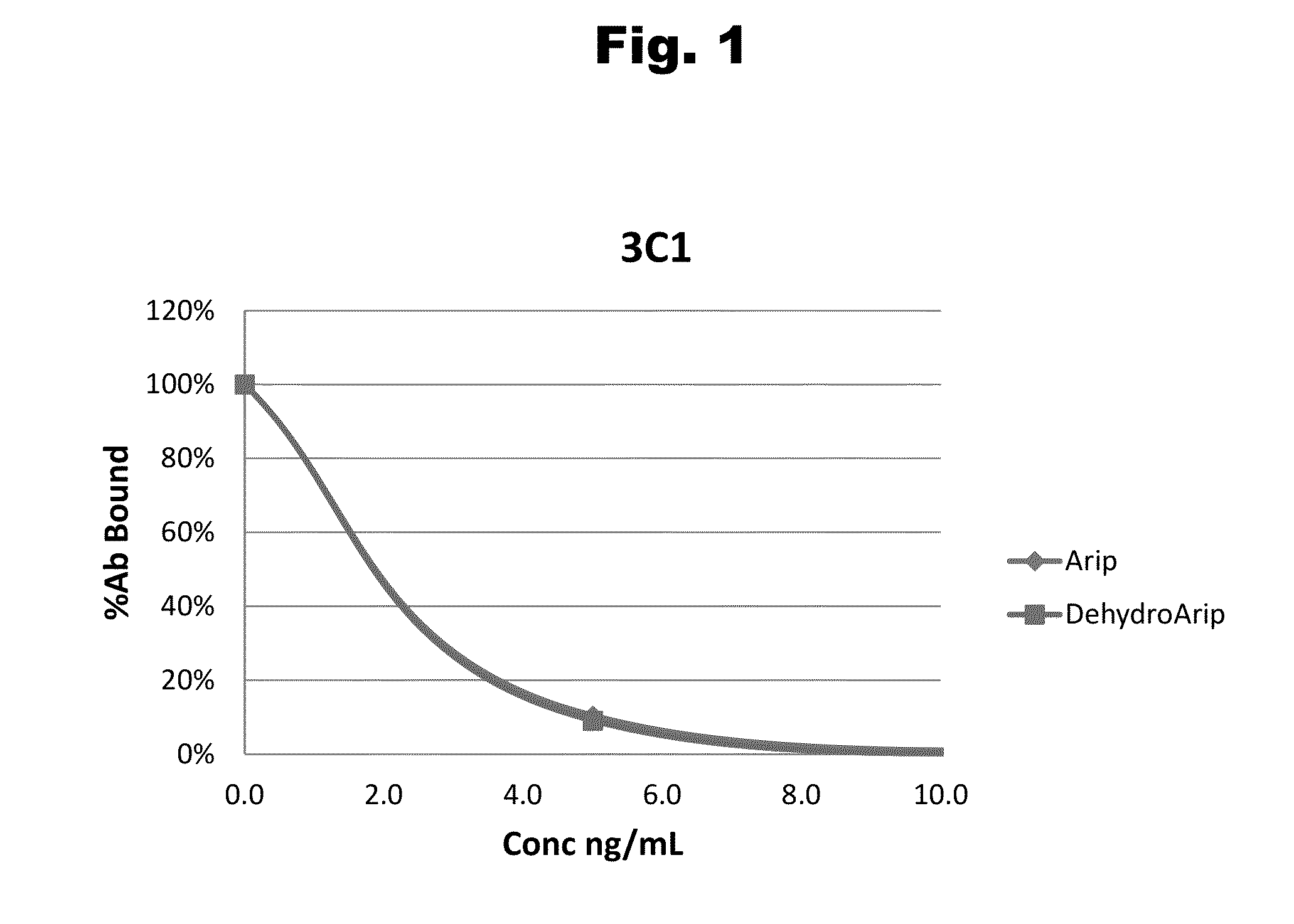
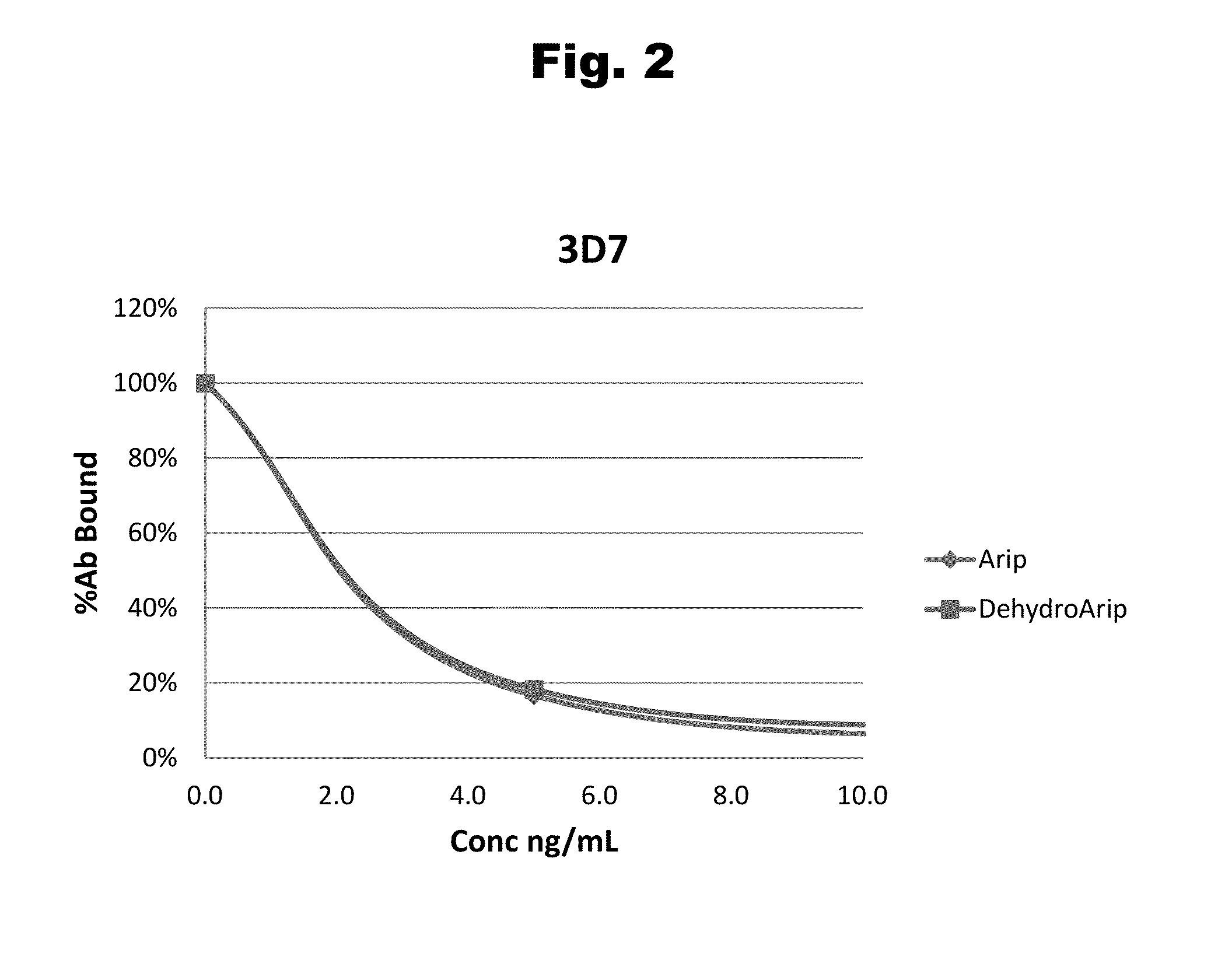
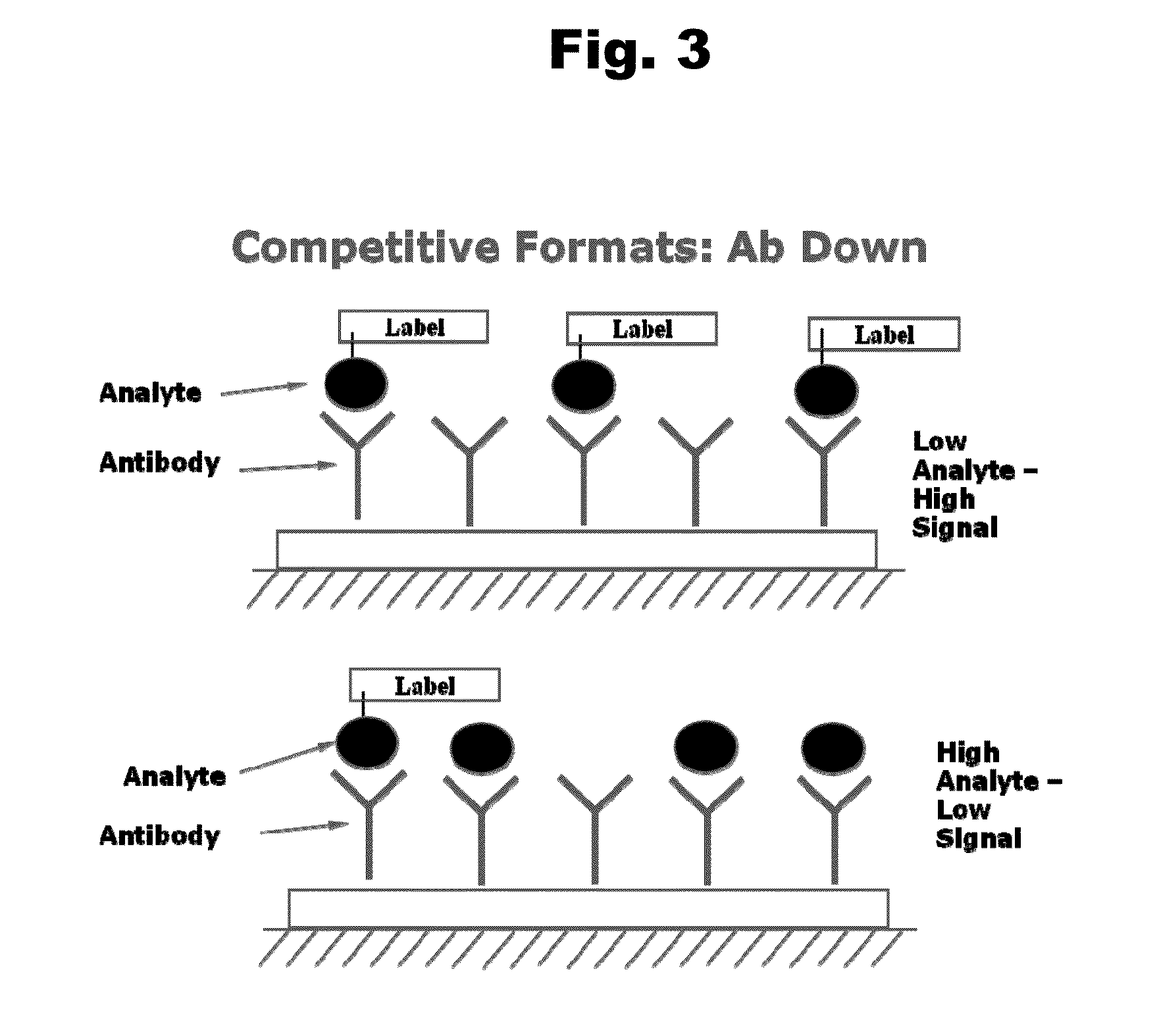
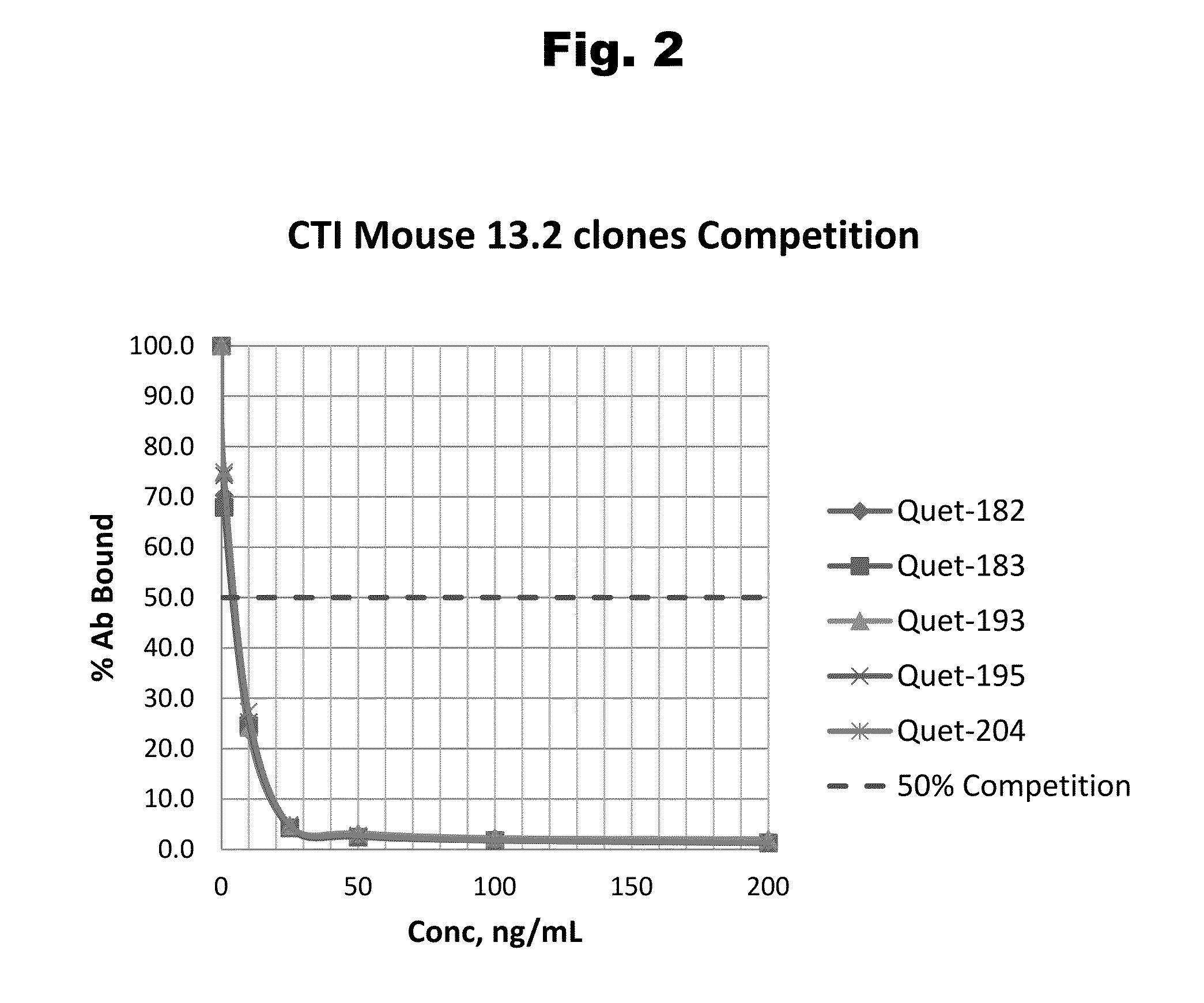
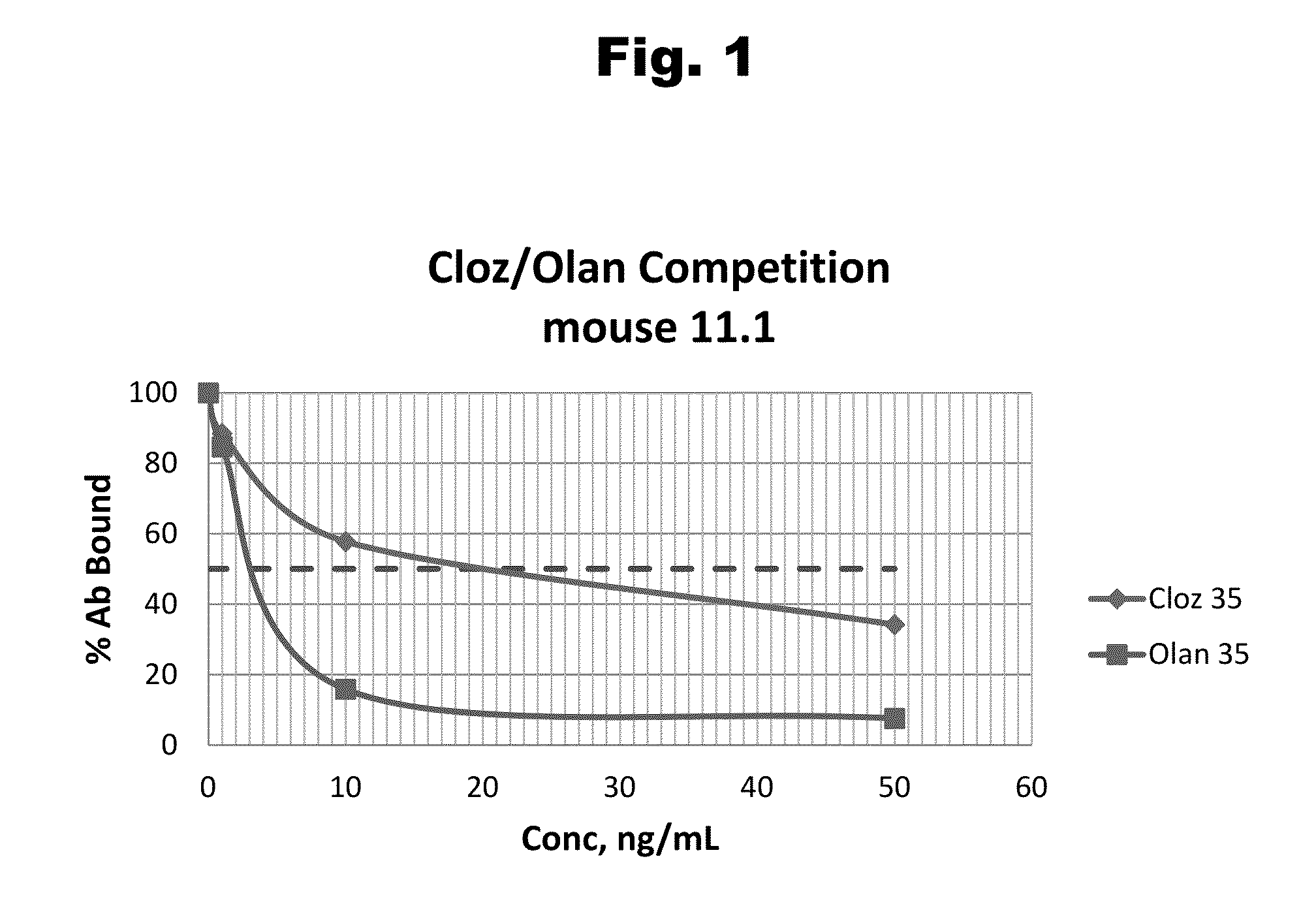
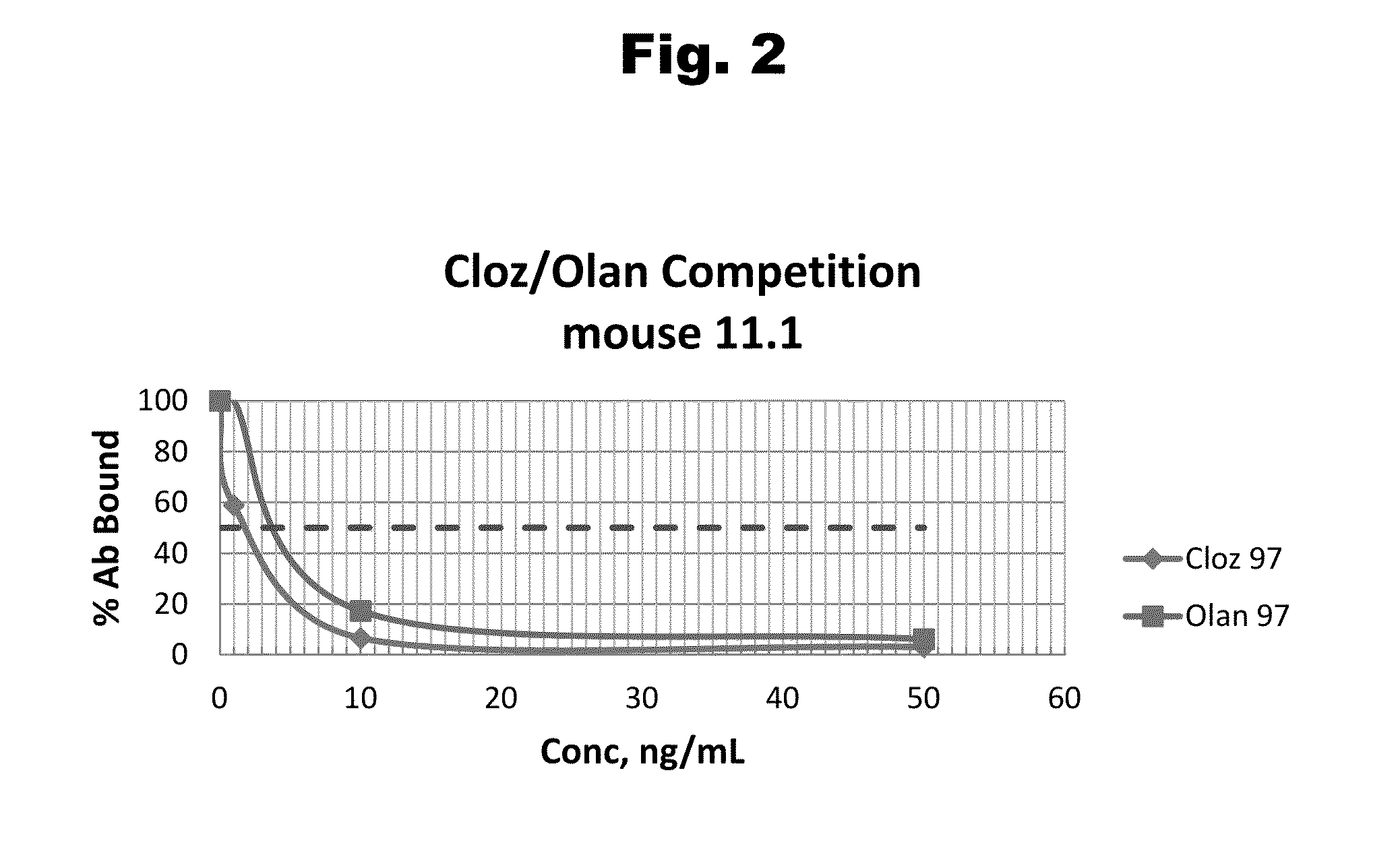
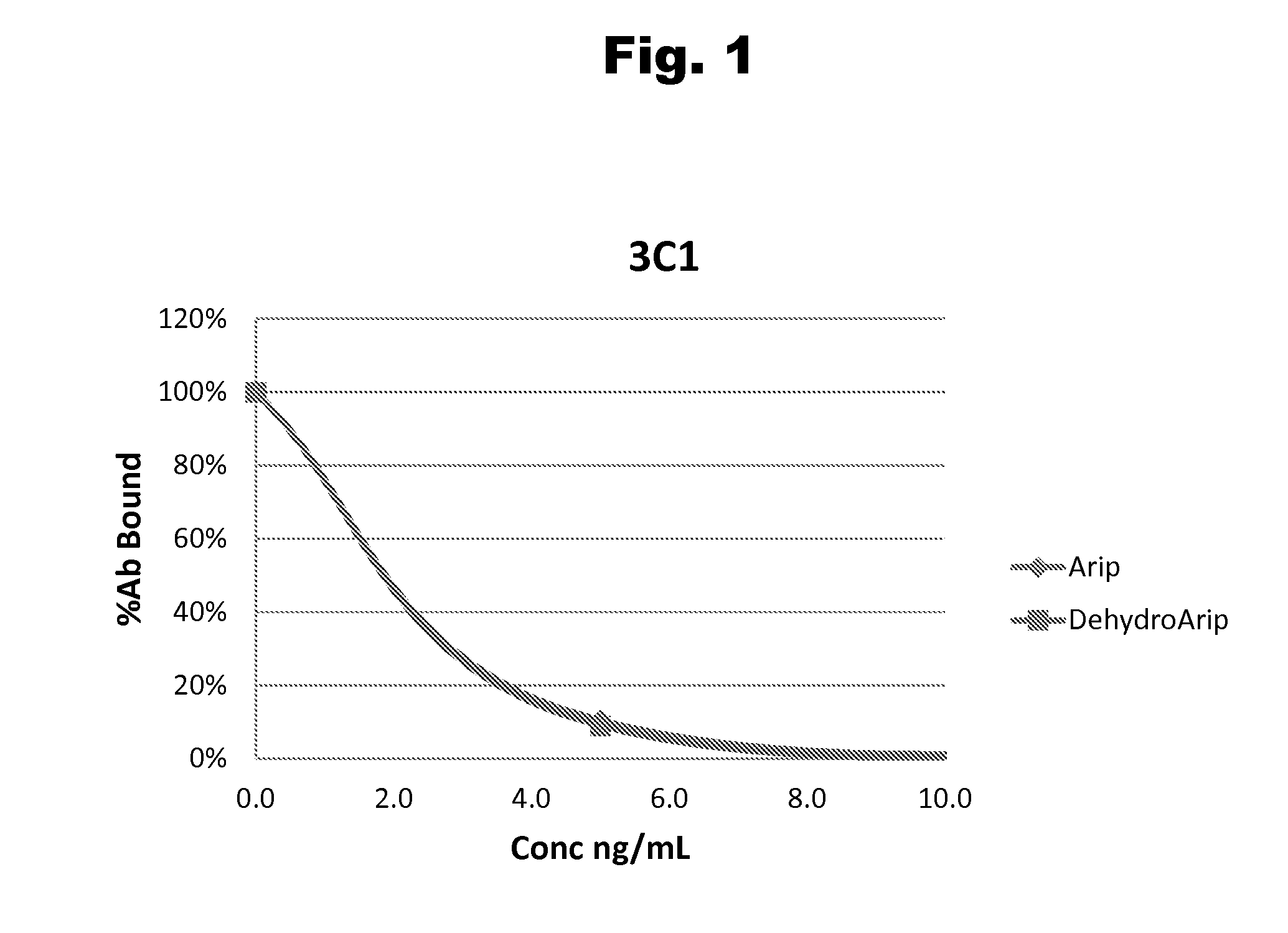
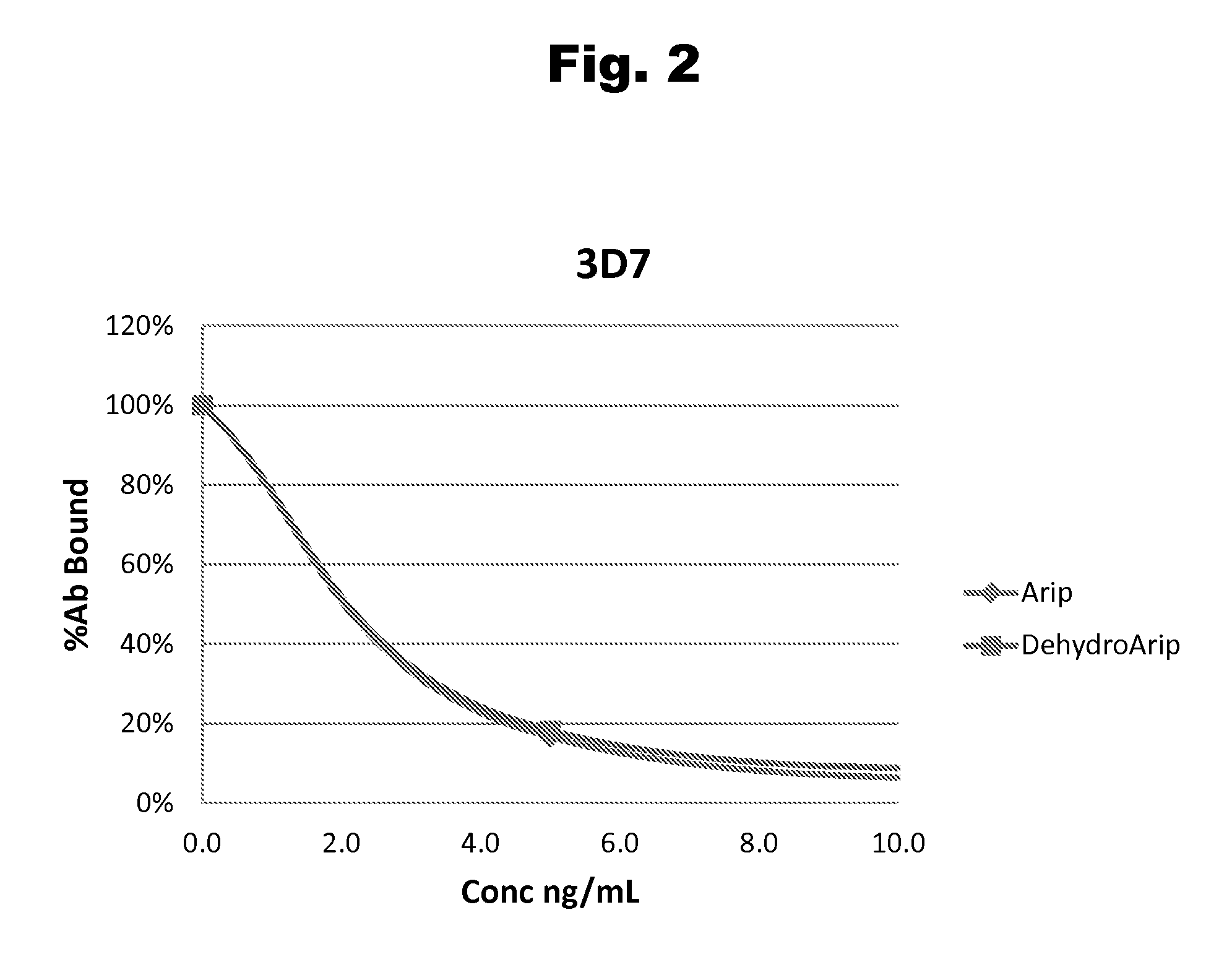
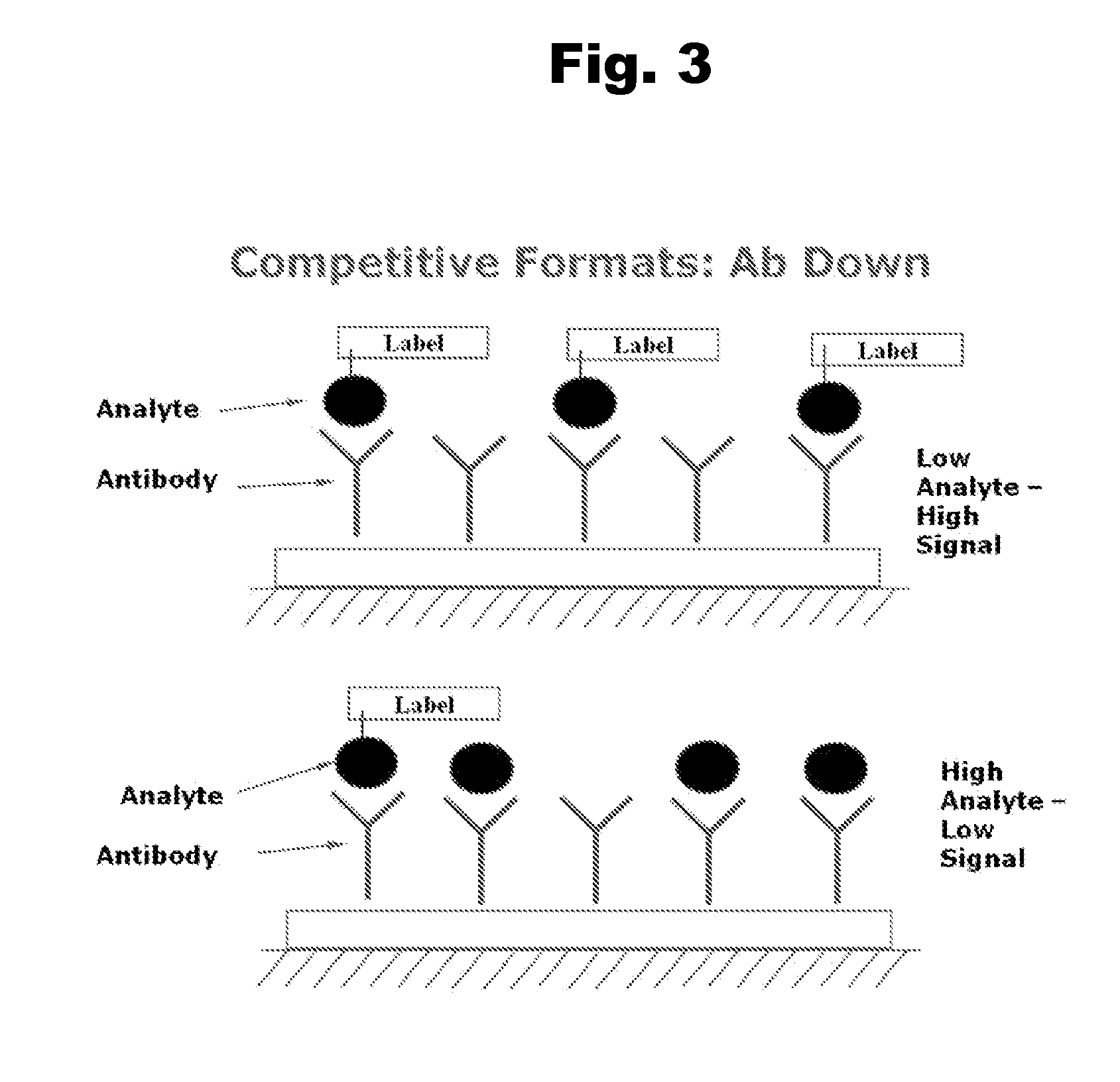
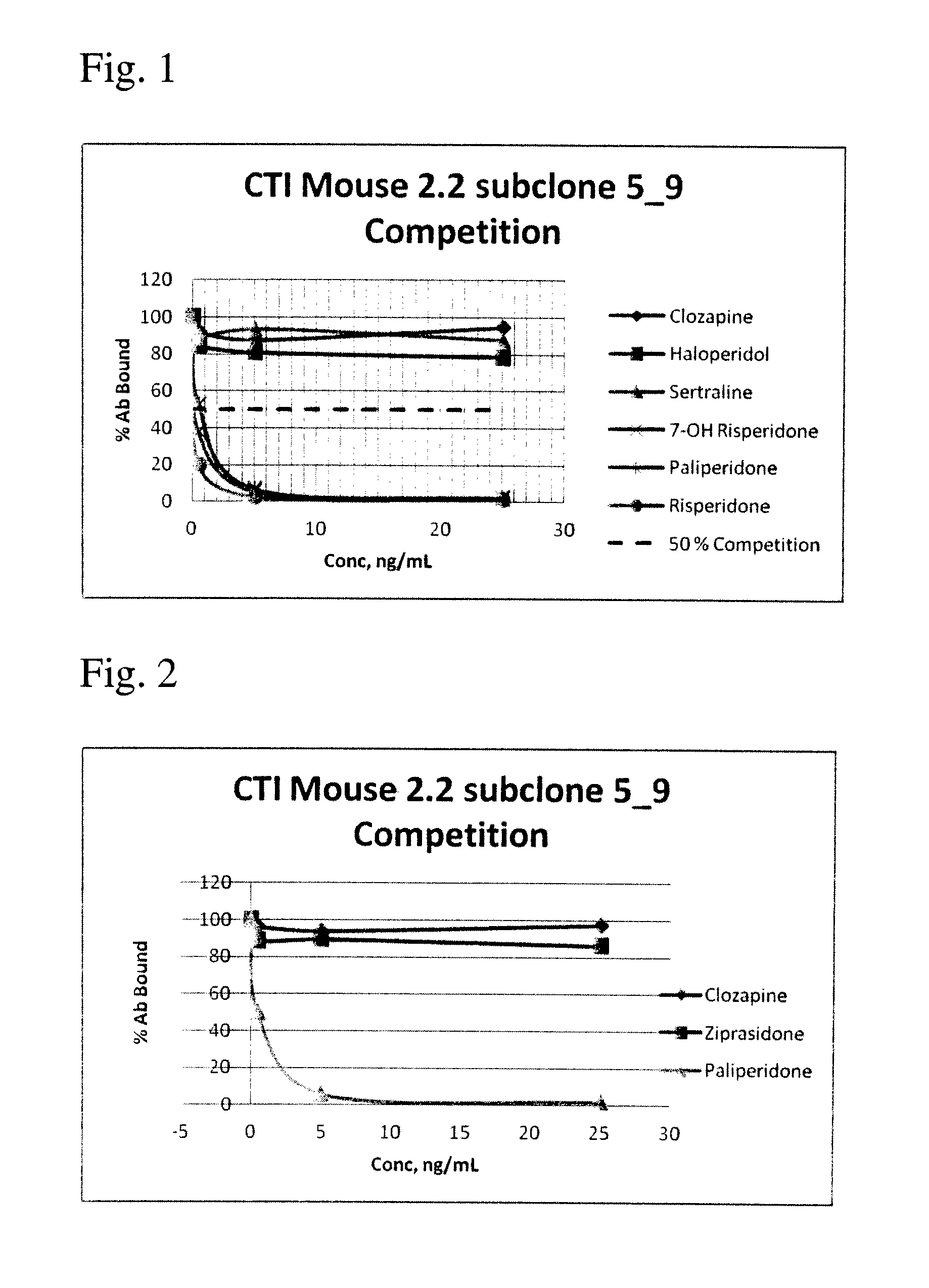
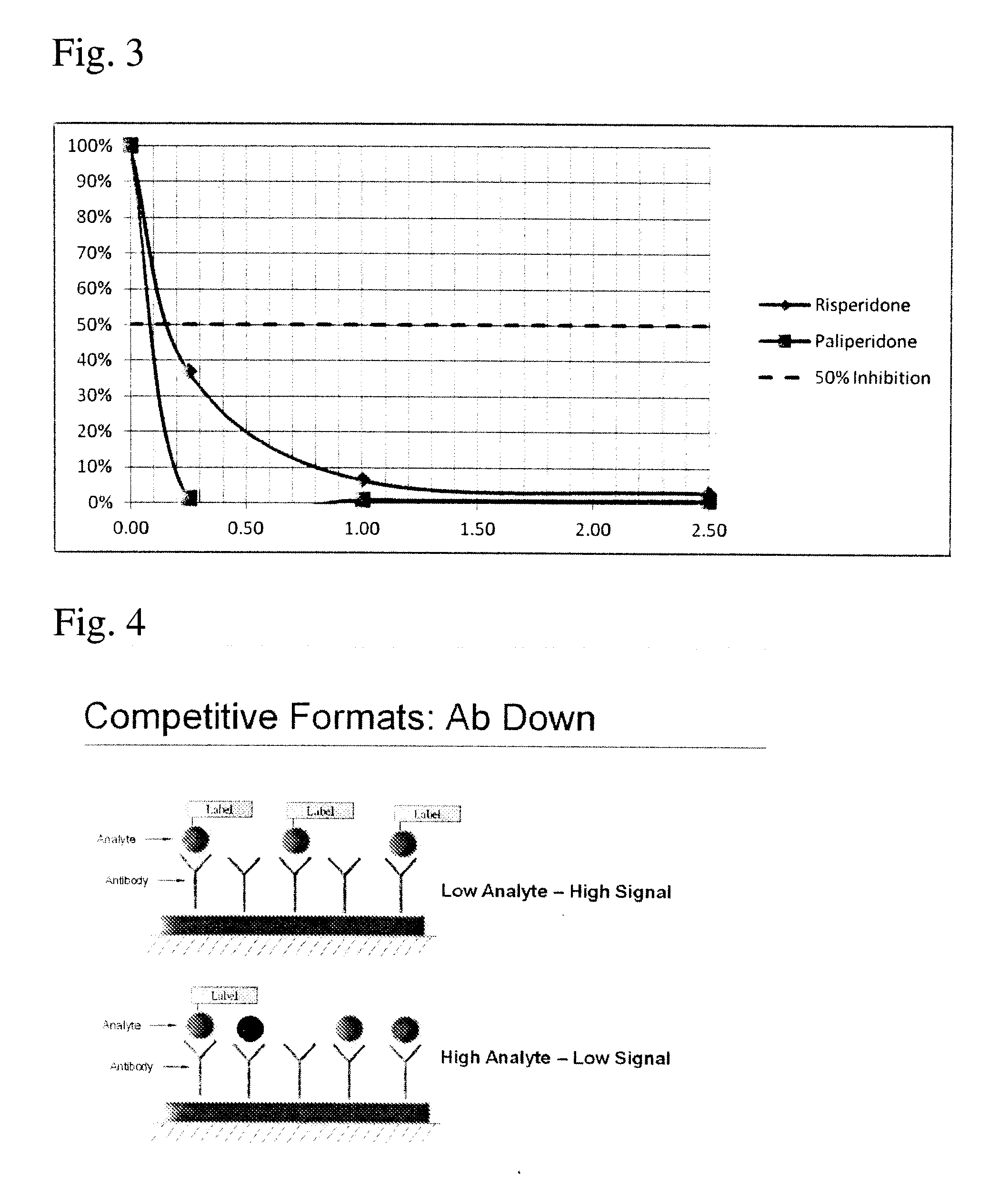
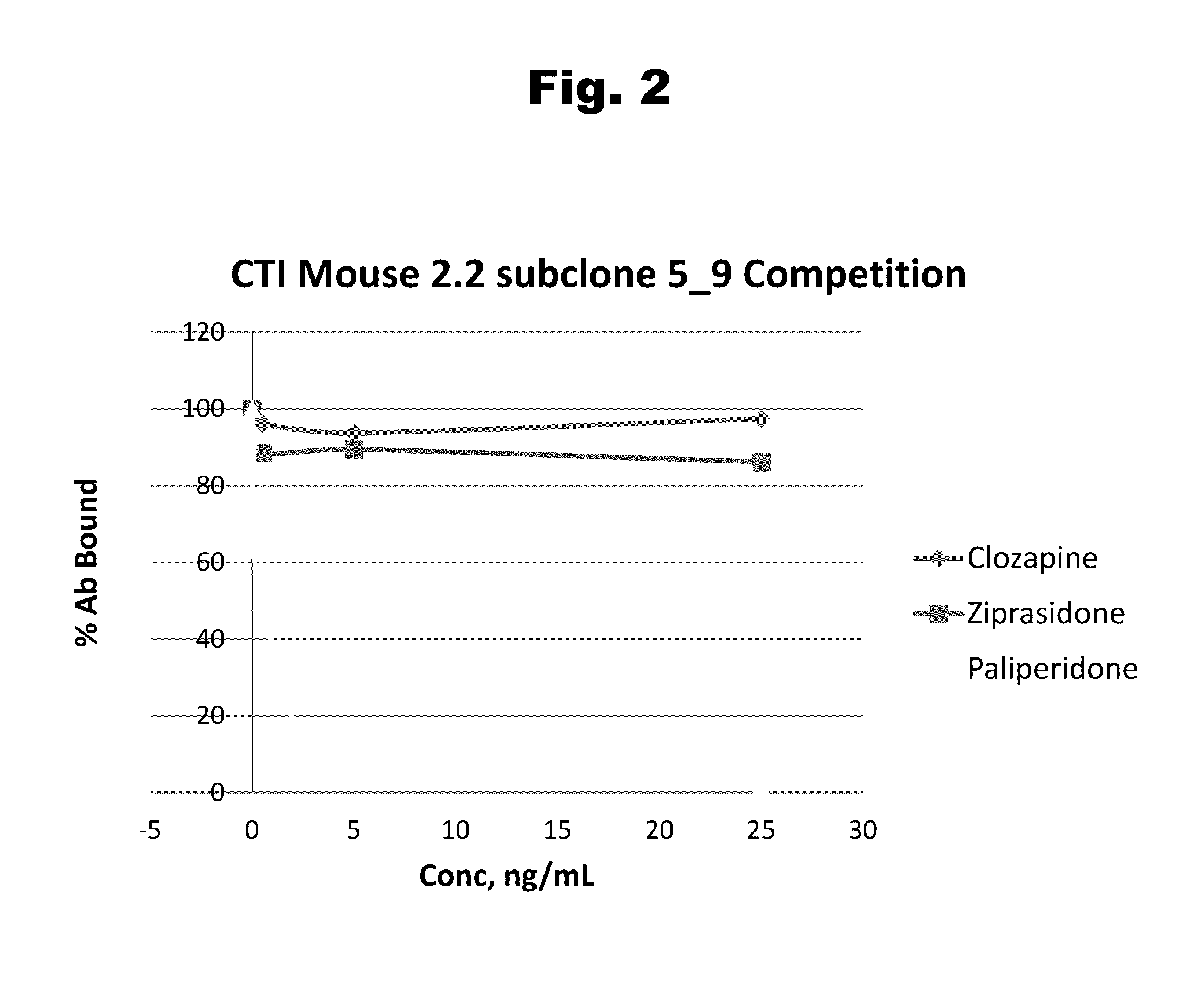
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Aripiprazole, olanzapine and haloperidol pamoate salts
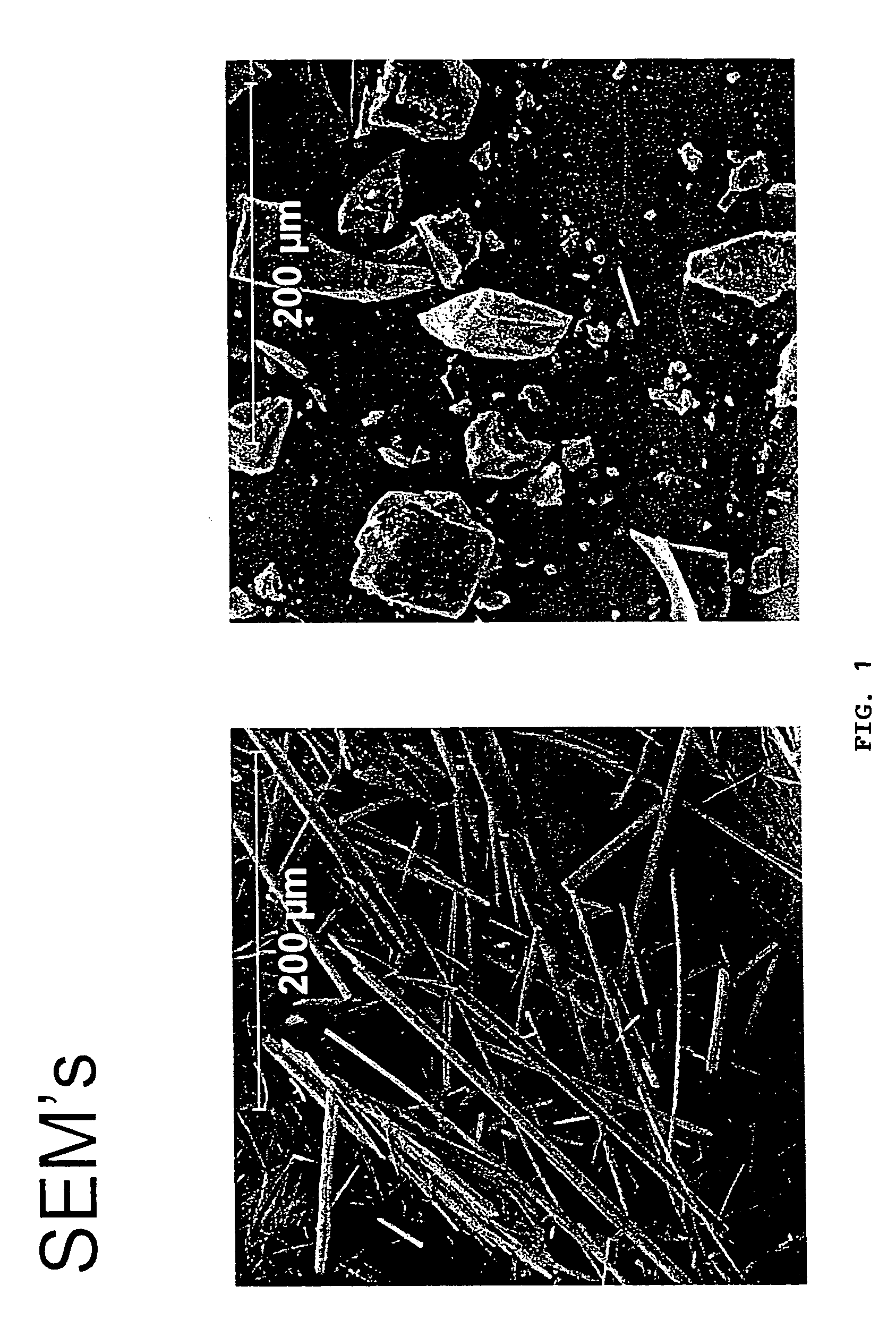
The invention relates to the discovery that pamoate salts of haloperidol and aripiprazole result in a good to superior long acting and / or extended release profile. Thus, in one aspect of the invention, the invention includes pamoate salts of haloperidol or aripiprazole. Preferably, the pamoate salt is characterized by a ratio of haloperidol to pamoate of 1:1 or 2:1. The pamoate salt can be crystalline, such as a needle or a dense crystal, such as described in the Figures.The invention further relates to methods of treating an individual in need thereof comprising administering a pharmaceutical composition comprising a pamoate salt of haloperidol and aripiprazole.
Owner:OTSUKA PHARM CO LTD
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
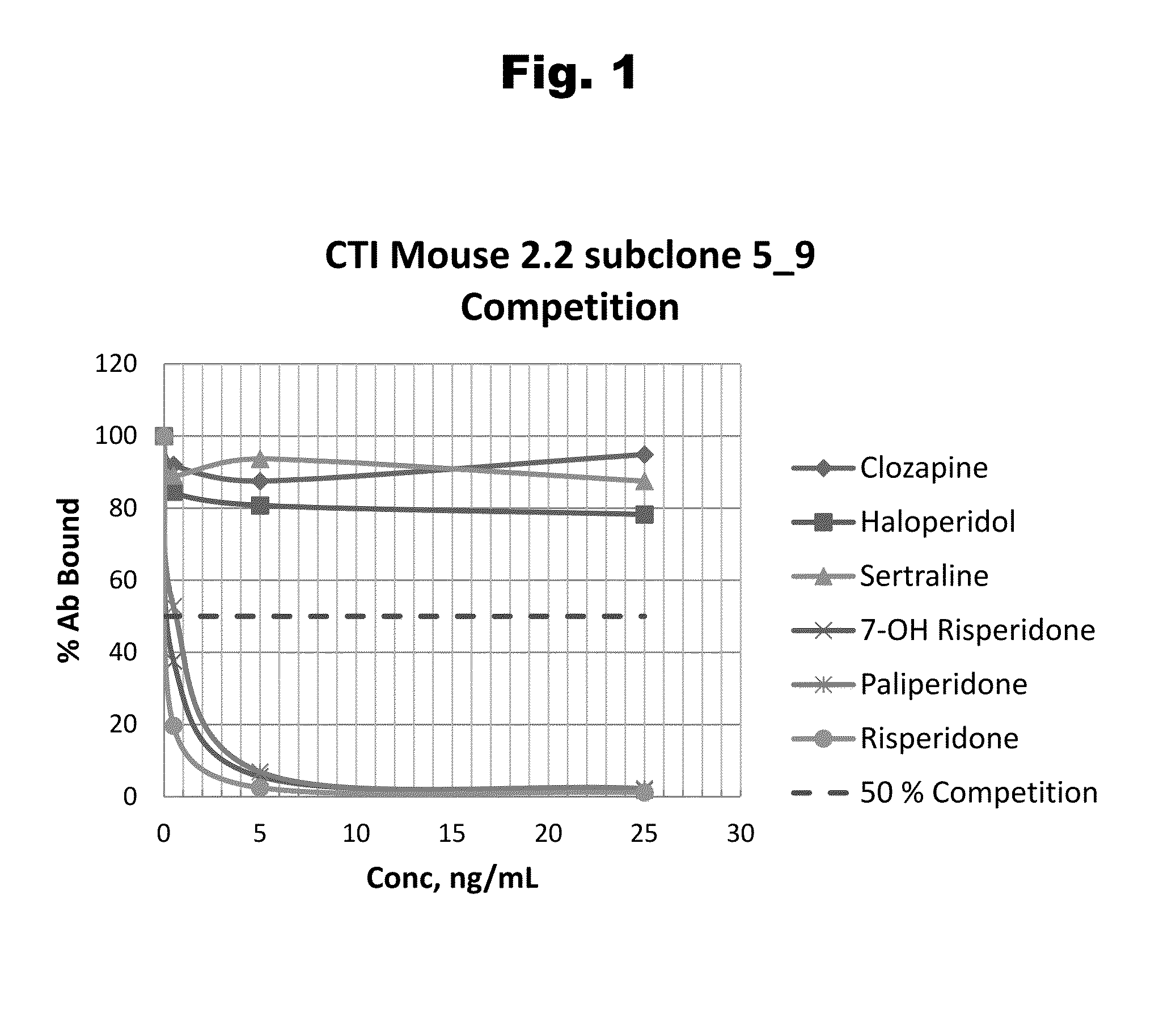
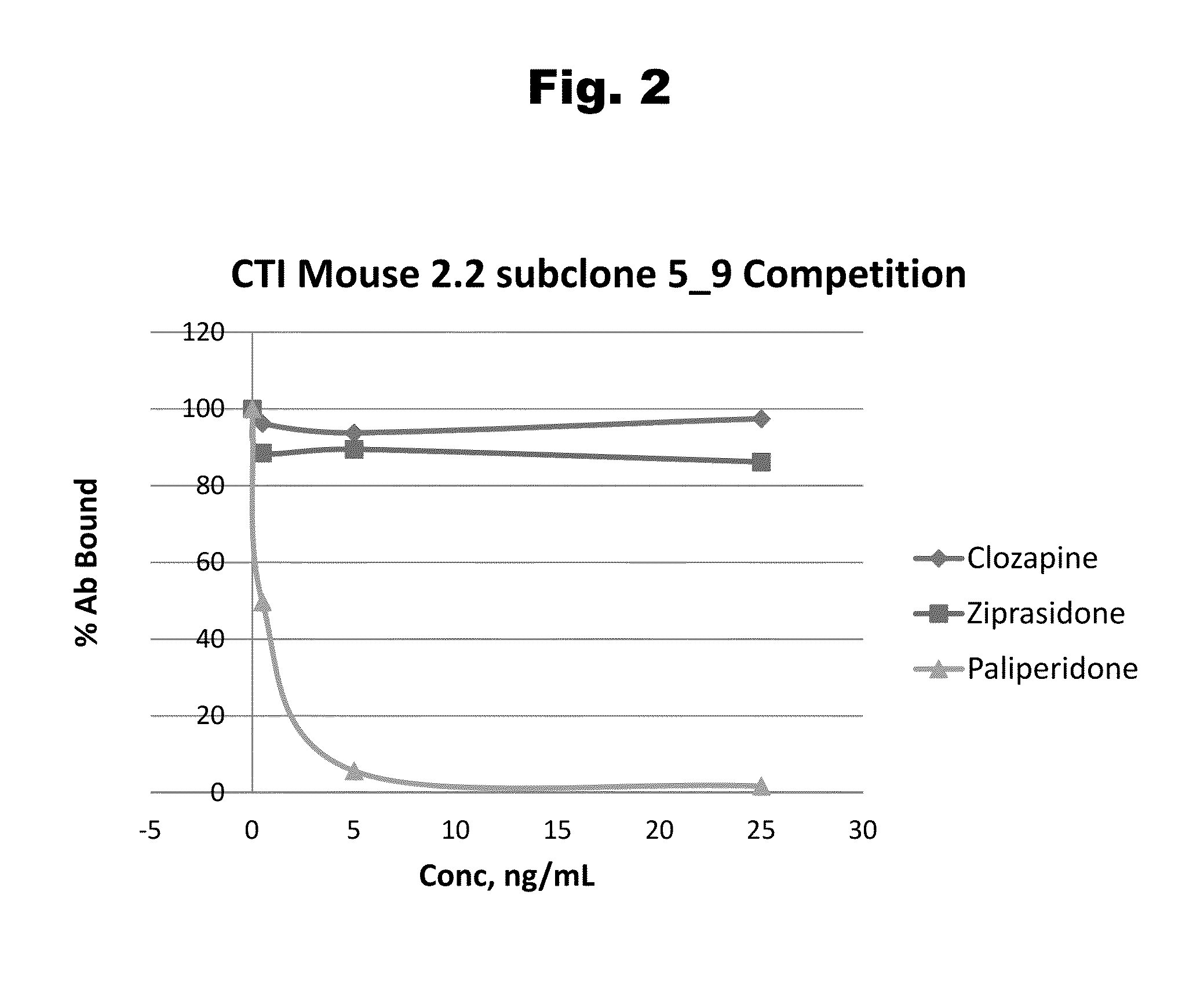
Antibodies to Aripiprazole Haptens and Use Thereof
ActiveUS20140057299A1Bioreactor/fermenter combinationsBiological substance pretreatmentsQuetiapineHapten
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
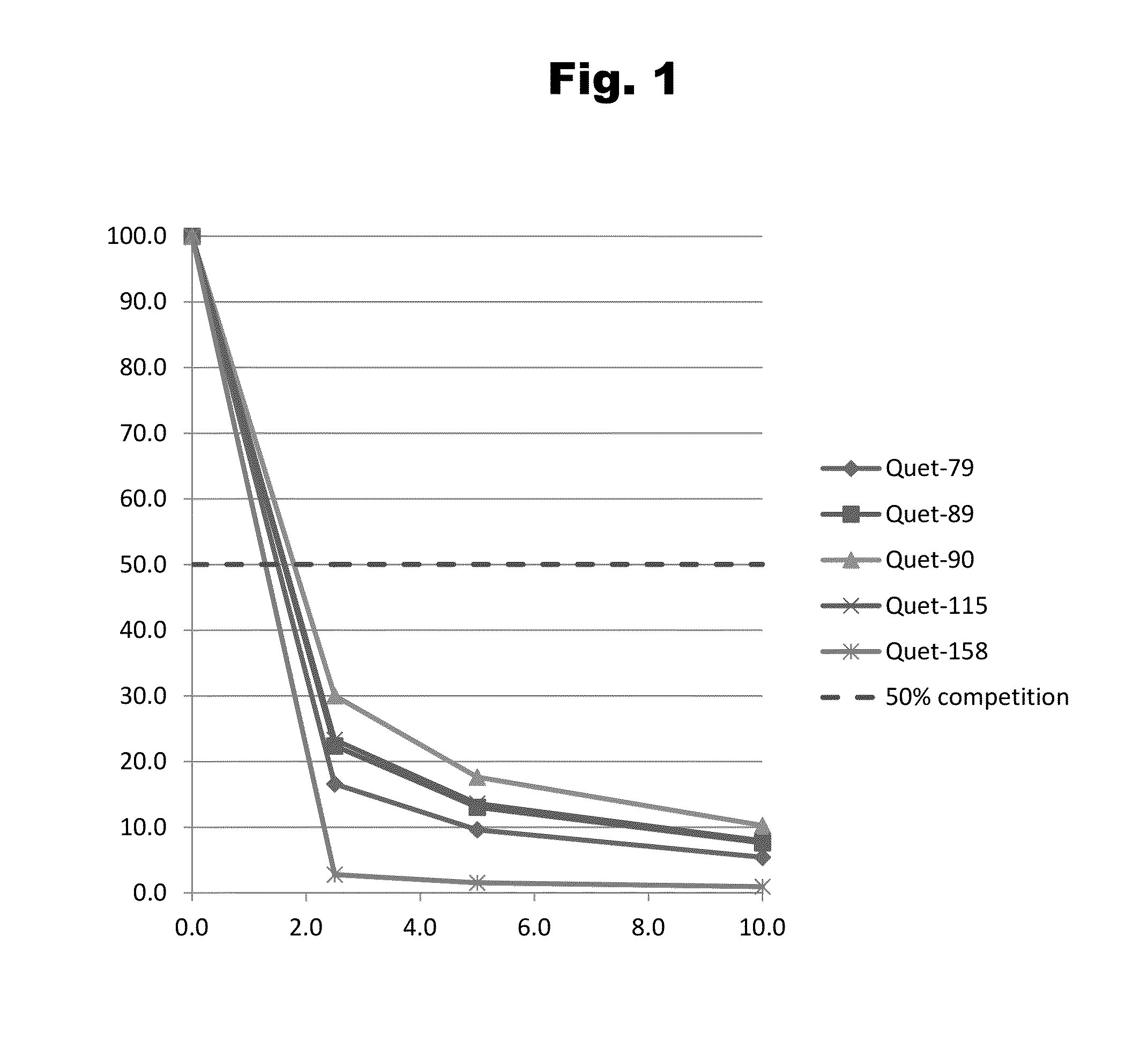
Antibodies to Quetiapine Haptens and Use Thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to Olanzapine and Use Thereof
Disclosed is an antibody which binds to olanzapine, which can be used to detect olanzapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of olanzapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
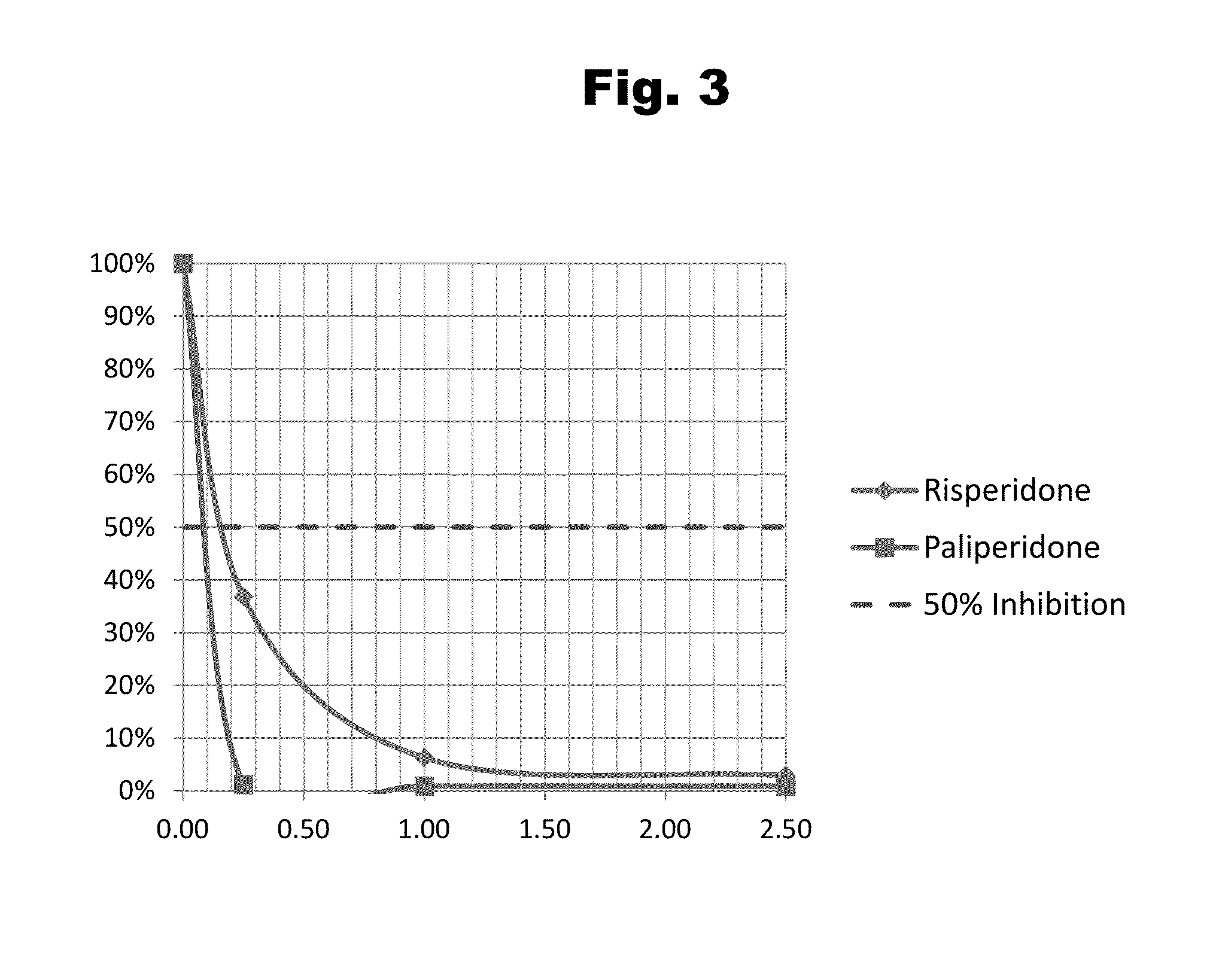
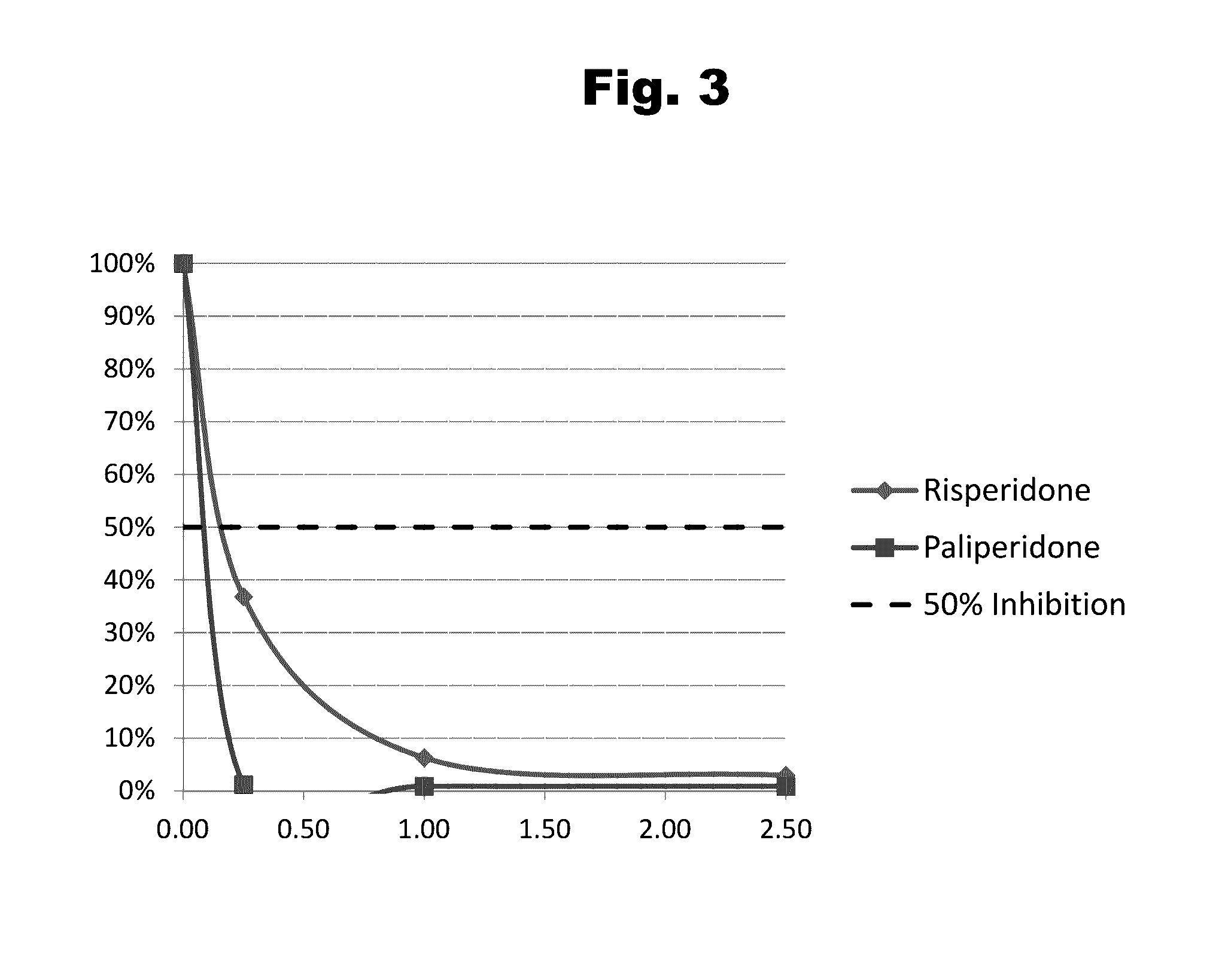
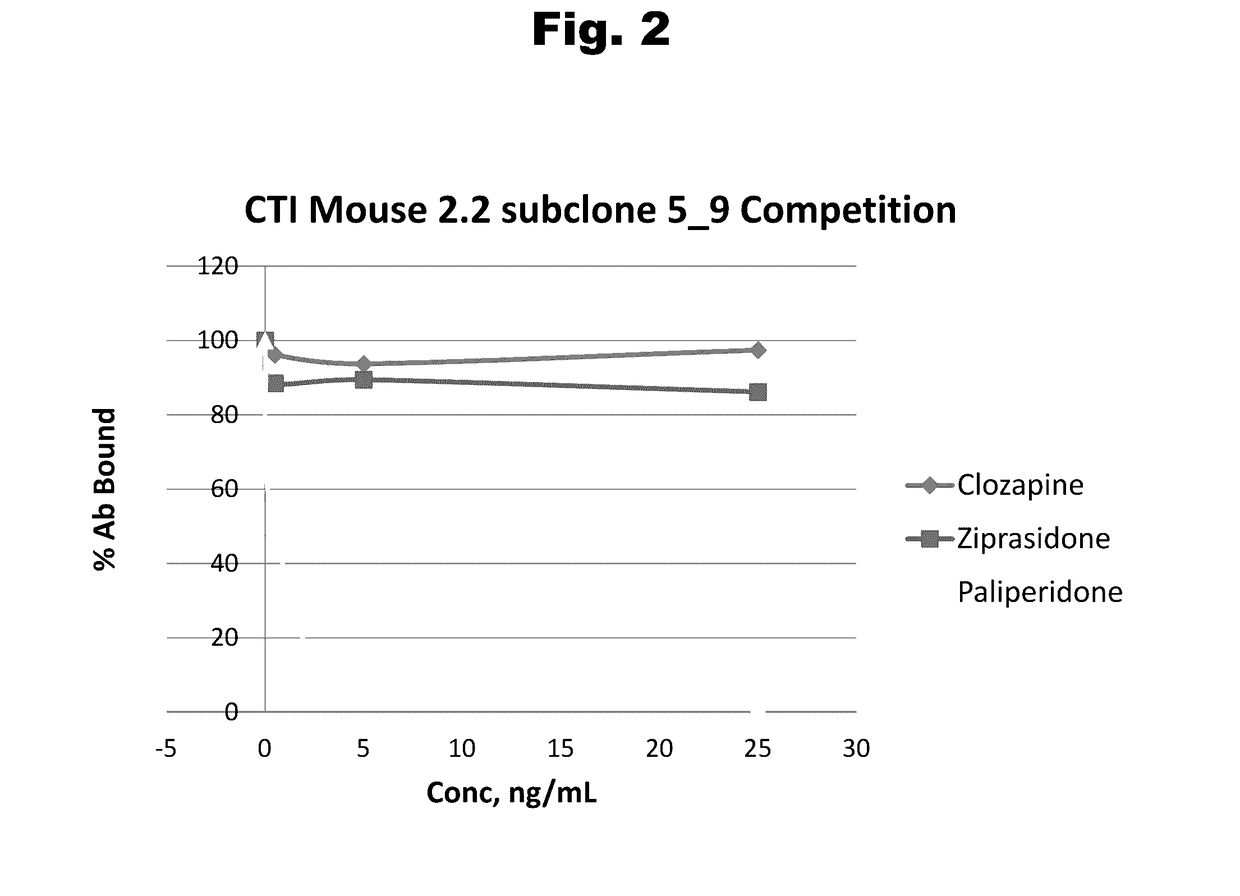
Antibodies to Paliperidone and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Haptens of aripiprazole
The invention relates to compounds of Formula I, wherein R1, R2, and R3 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from aripiprazole. The invention also relates to conjugates of an aripiprazole hapten and a protein.
Owner:JANSSEN PHARMA NV
Antibodies to Paliperidone Haptens and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, risperidone and paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
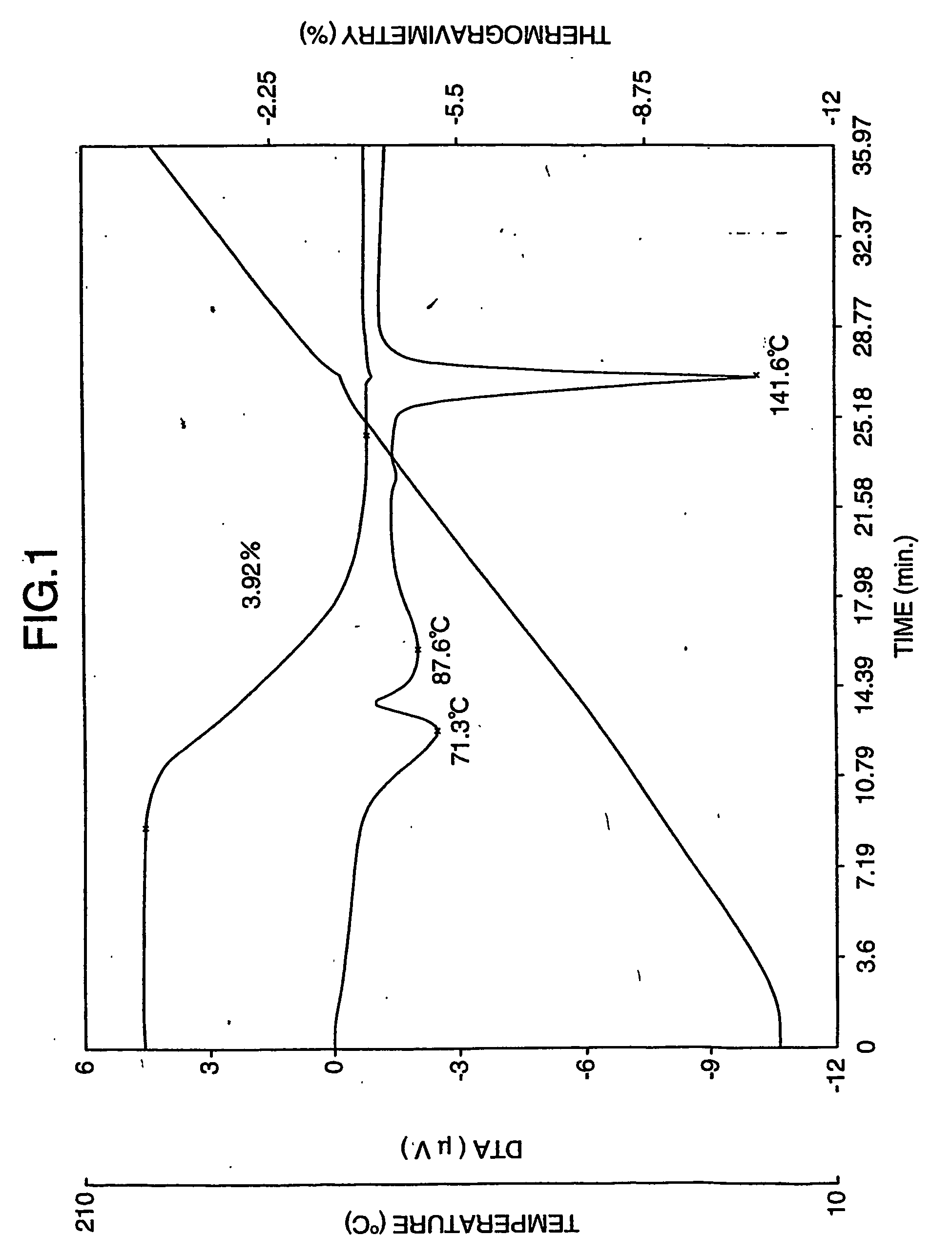
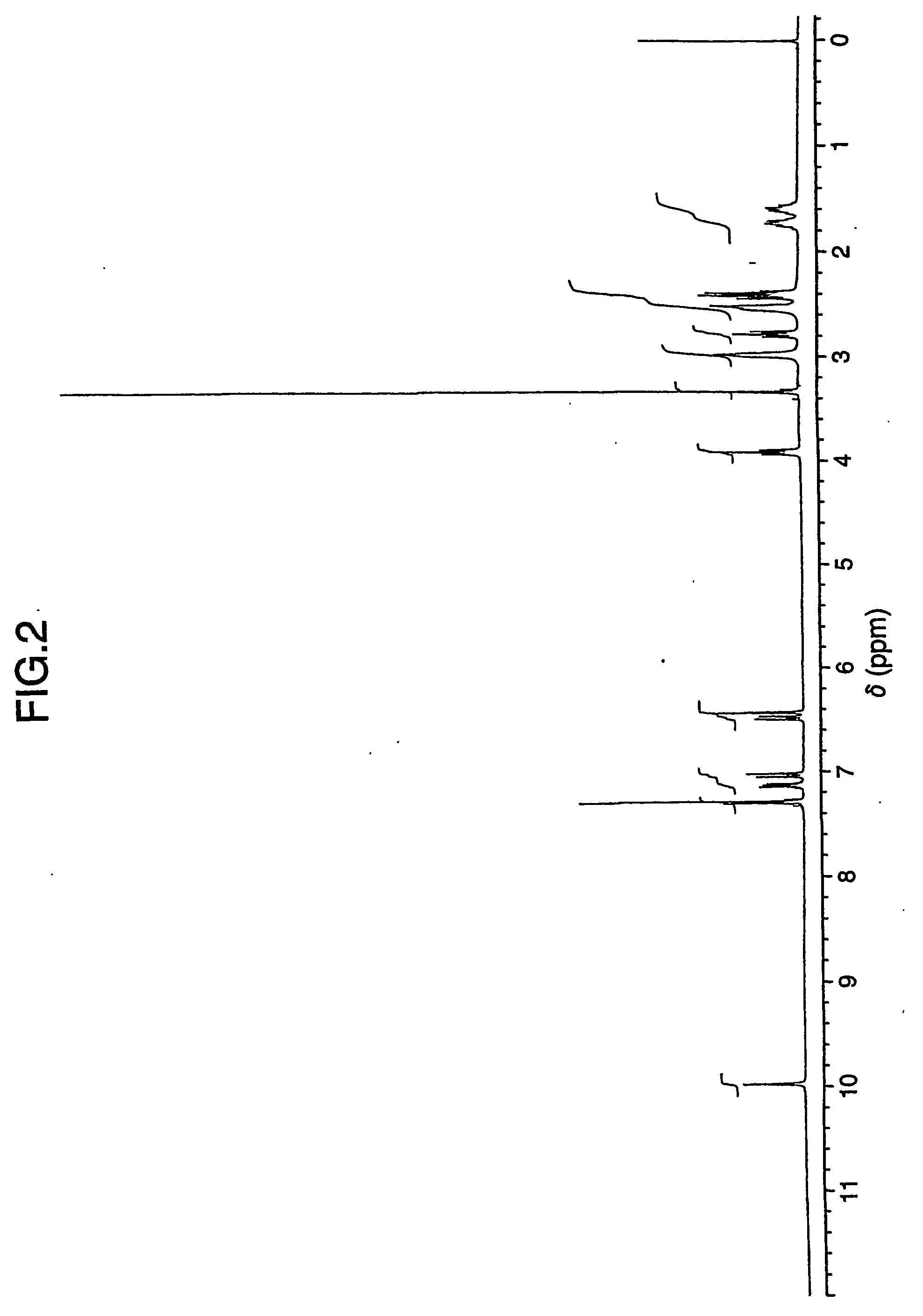
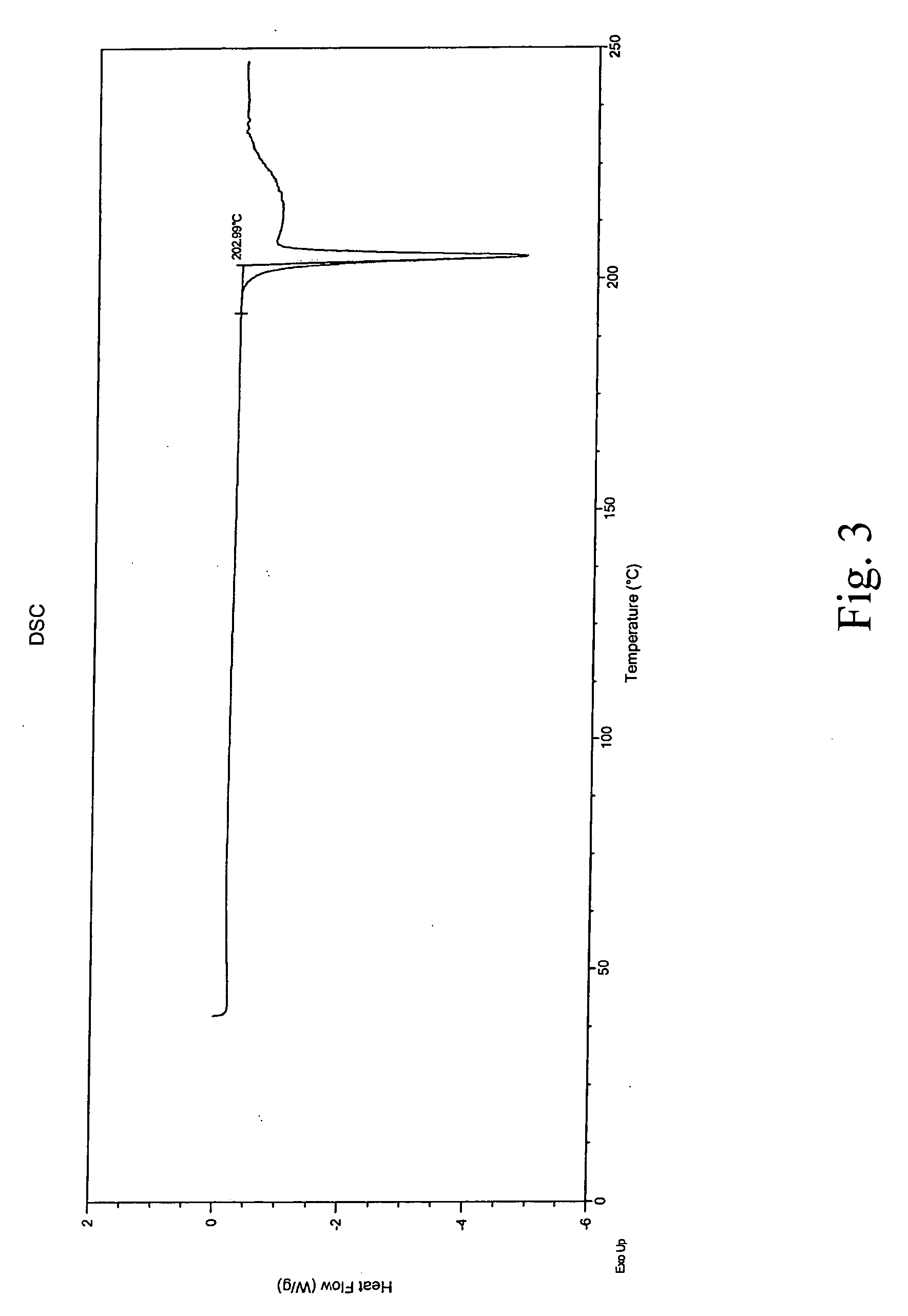
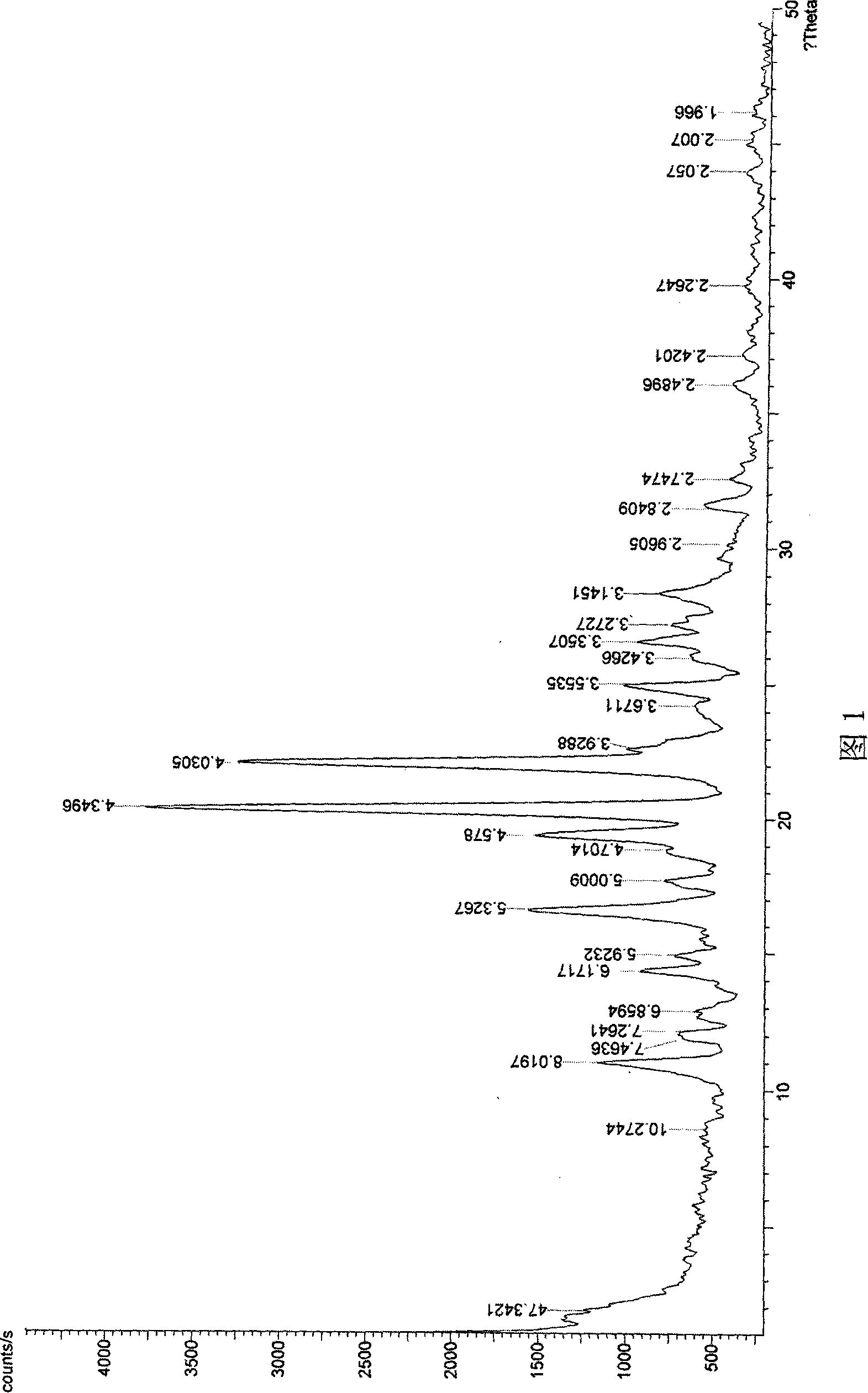
Process for preparing aripirazole hydrate
InactiveUS20050277650A1Avert costly hospitalizationUseful in treatmentOrganic active ingredientsOrganic chemistryOrganic solventAripiprazole
Aripriprazole hydrate is prepared by dissolving apripiprazole in a hydrous organic solvent at elevated temperature, adding seed crystals of aripiprazole hydrate to the solution, cooling the mixture, and isolating crystals of aripiprazole hydrate.
Owner:DR REDDYS LAB LTD +1
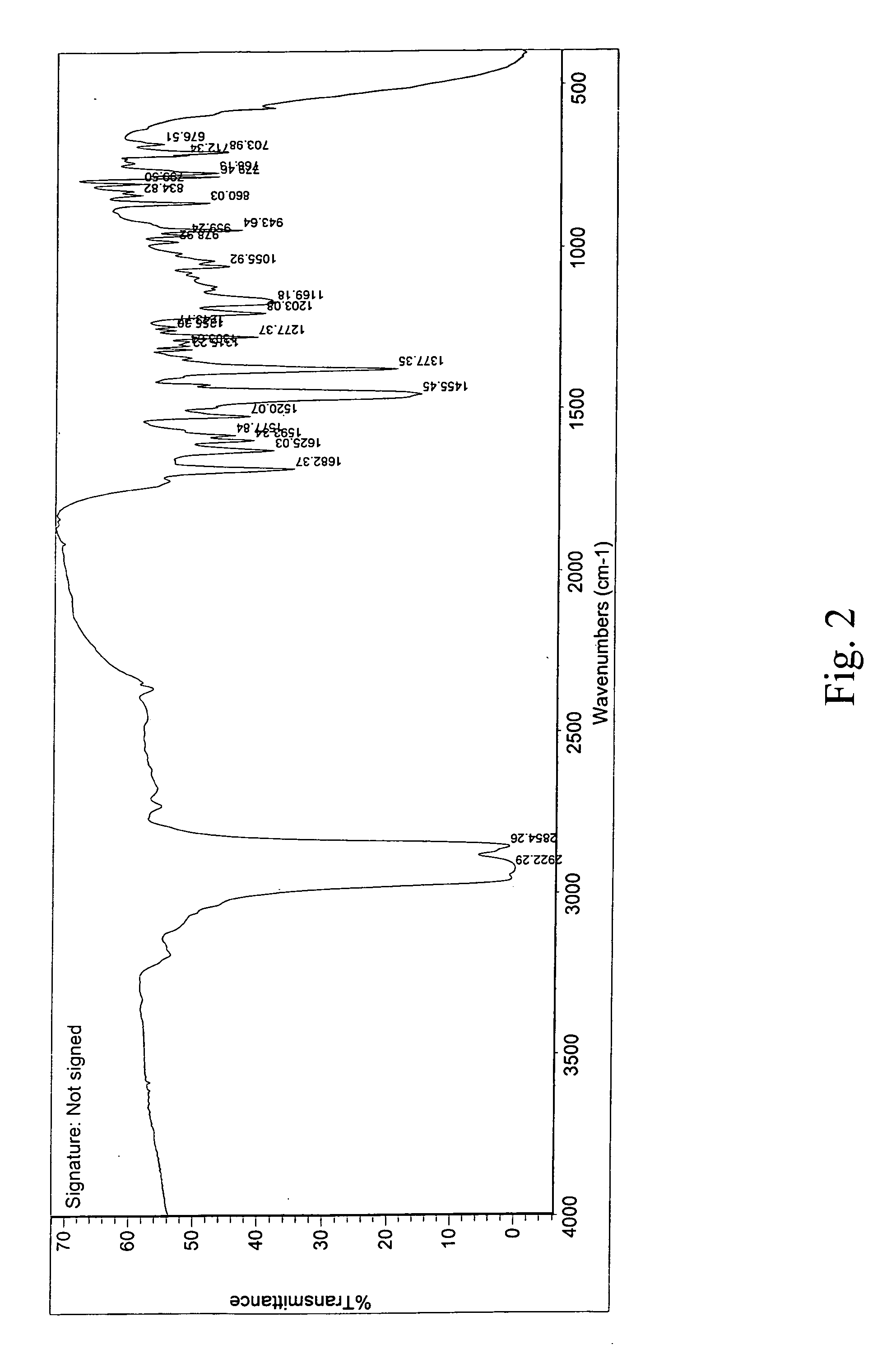
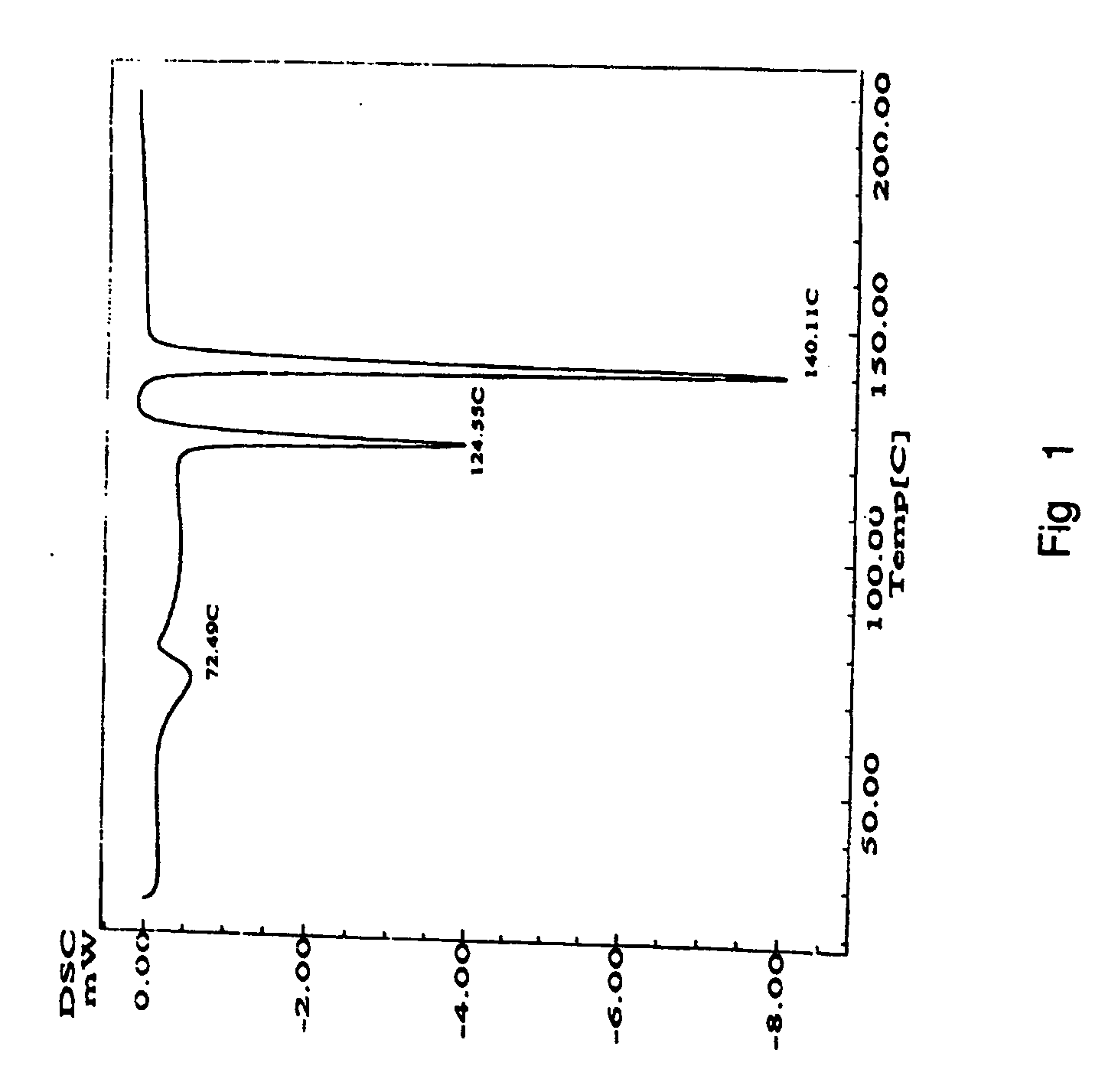
Amorphous Aripiprazole and Process for the Preparation thereof
InactiveUS20080280921A1Improve solubilityOrganic active ingredientsPowder deliveryPolymer scienceAripiprazole
Owner:HELM AG
Aripiprazole, olanzapine and haloperidol pamoate salts
The invention relates to the discovery that pamoate salts of haloperidol and aripiprazole result in a good to superior long acting and / or extended release profile. Thus, in one aspect of the invention, the invention includes pamoate salts of haloperidol or aripiprazole. Preferably, the pamoate salt is characterized by a ratio of haloperidol to pamoate of 1:1 or 2:1. The pamoate salt can be crystalline, such as a needle or a dense crystal, such as described in the Figures. The invention further relates to methods of treating an individual in need thereof comprising administering a pharmaceutical composition comprising a pamoate salt of haloperidol and aripiprazole.
Owner:ALKERMES INC
Long-acting non-water-carrier injection liquid and preparing method thereof
InactiveCN105012236AImprove physical stabilityGood chemical stabilityOrganic active ingredientsNervous disorderFreeze-dryingHigh pressure
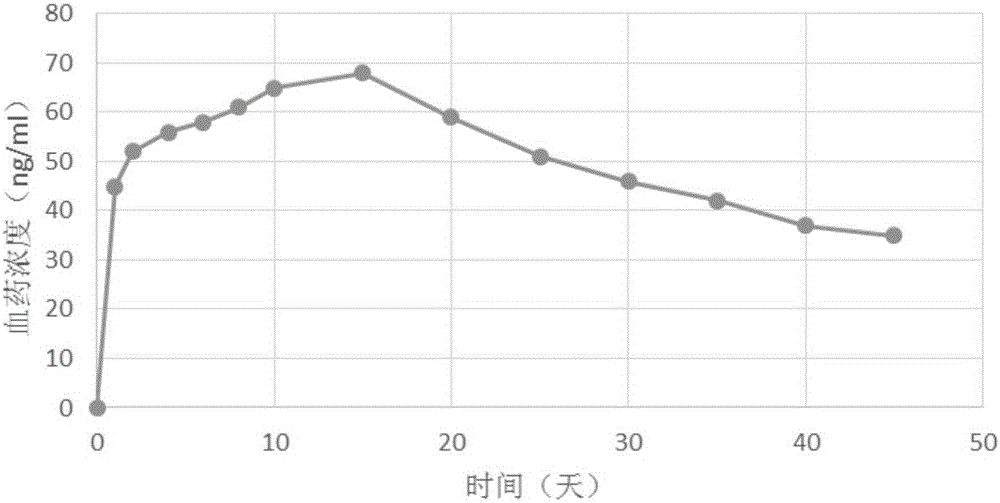
The invention discloses long-acting non-water-carrier injection liquid and a preparing method thereof. The long-acting non-water-carrier injection liquid comprises mental drugs and non-water carriers and can be continuously released within at least four weeks. The mental drugs are lauroyl aripiprazole or paliperidone palmitate. The preparing method is completed through high-pressure homogeneous steps. The preparation of the long-acting non-water-carrier injection liquid can be released slowly within at least four weeks. The continuously-releasing injection preparation is more stable, simple in preparation form, free of freeze drying, simple in preparation, good in economical effect and convenient to use.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
Slow-release composition of aripiprazole and derivative thereof and preparation method thereof
InactiveCN106727358AHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsNon solventWater insoluble
The invention discloses a slow-release composition of aripiprazole and derivative thereof and a preparation method thereof. The non-solvent form of the slow-release composition of aripiprazole and derivative thereof is prepared from raw materials including aripiprazole or aripiprazole derivative and water-insoluble polymer. The slow-release composition of aripiprazole and derivative thereof disclosed by the invention avoids obvious phenomenon of release lag or burst release after administration to realize a good slow-release property, and can maintain stable therapeutic plasma concentration in several weeks or longer time; and moreover, with relatively high stability, the slow-release composition still can maintain the slow release behavior after long-time storage.
Owner:AC PHARMA CO LTD
Antibodies to paliperidone and use thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
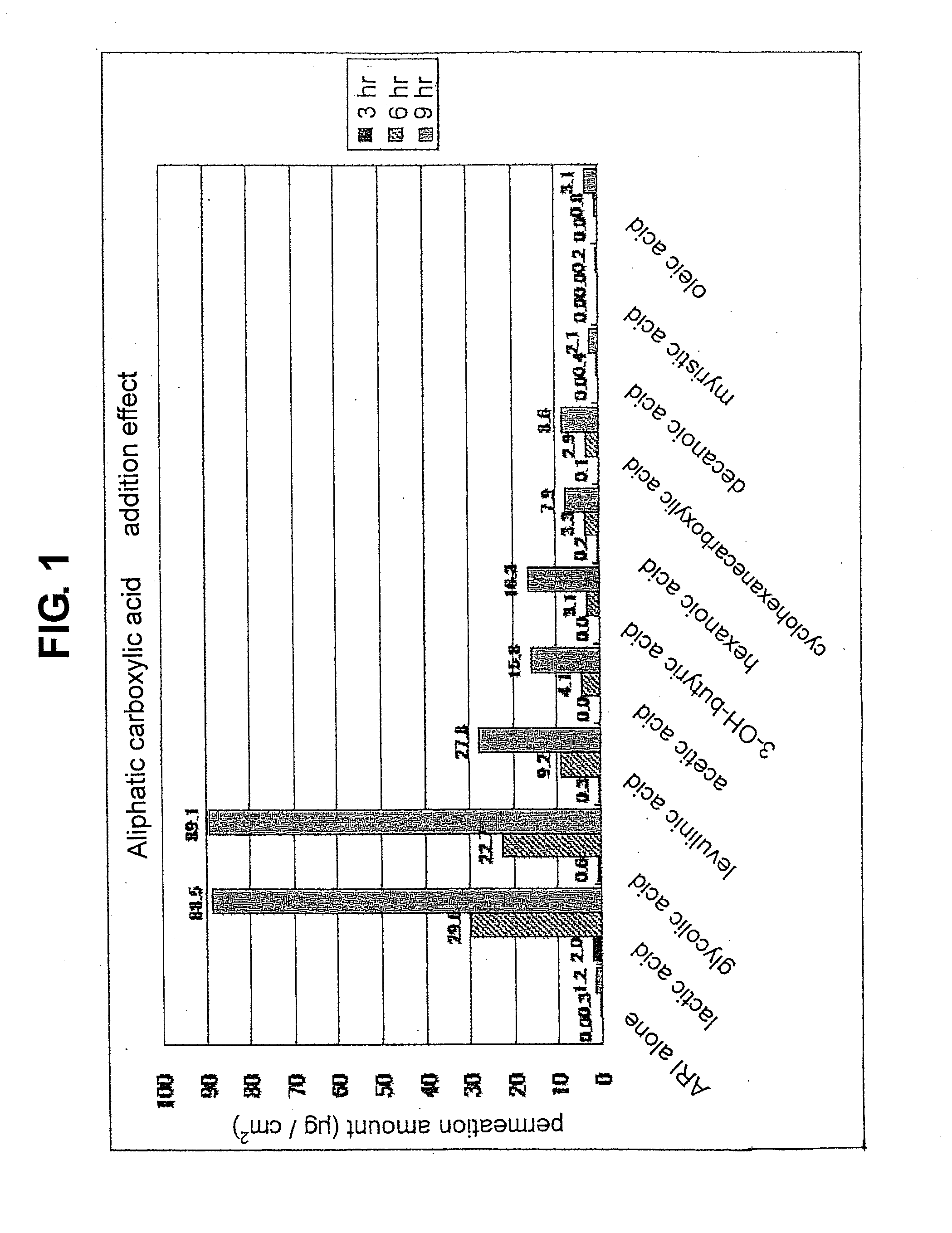
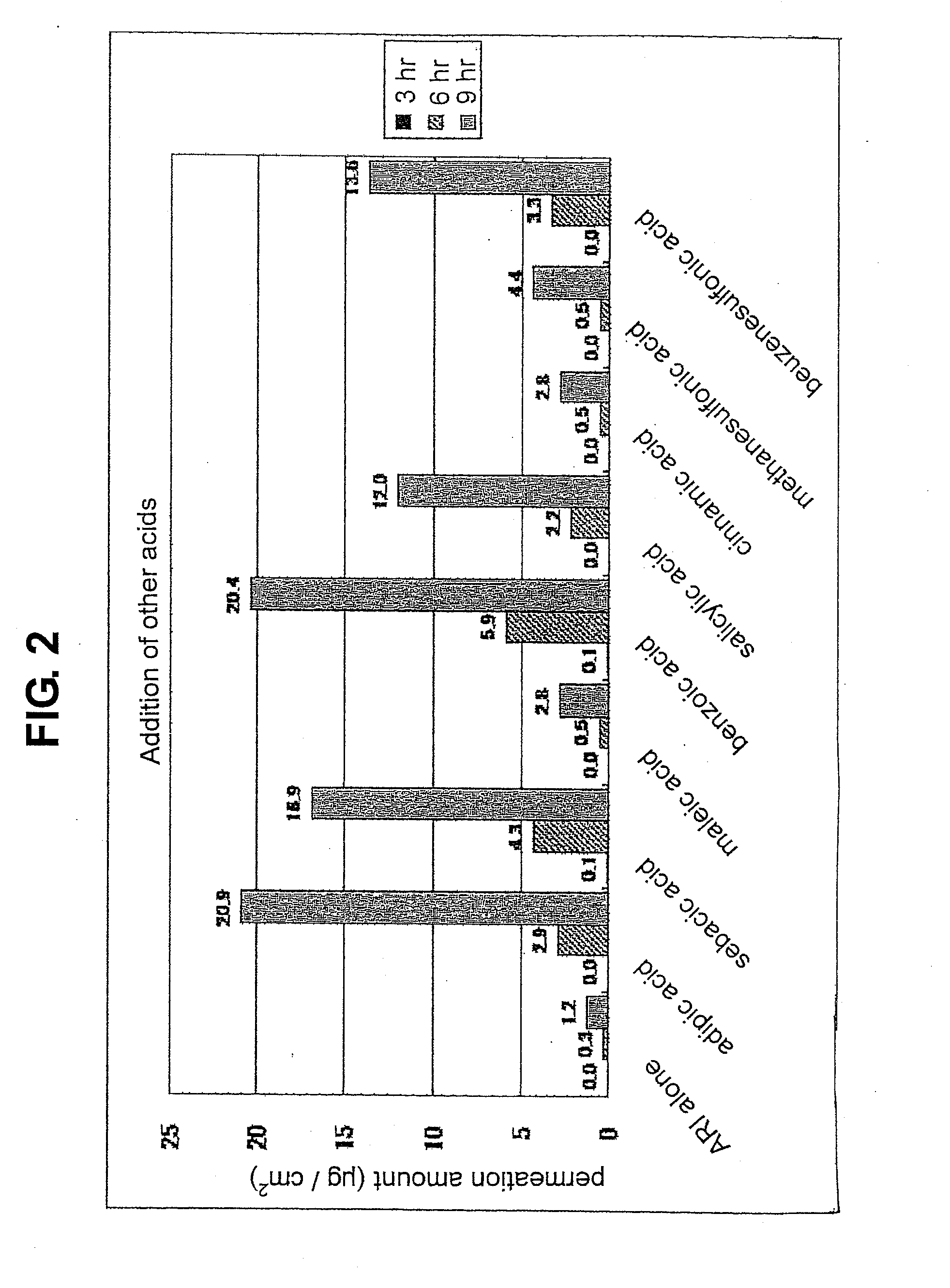
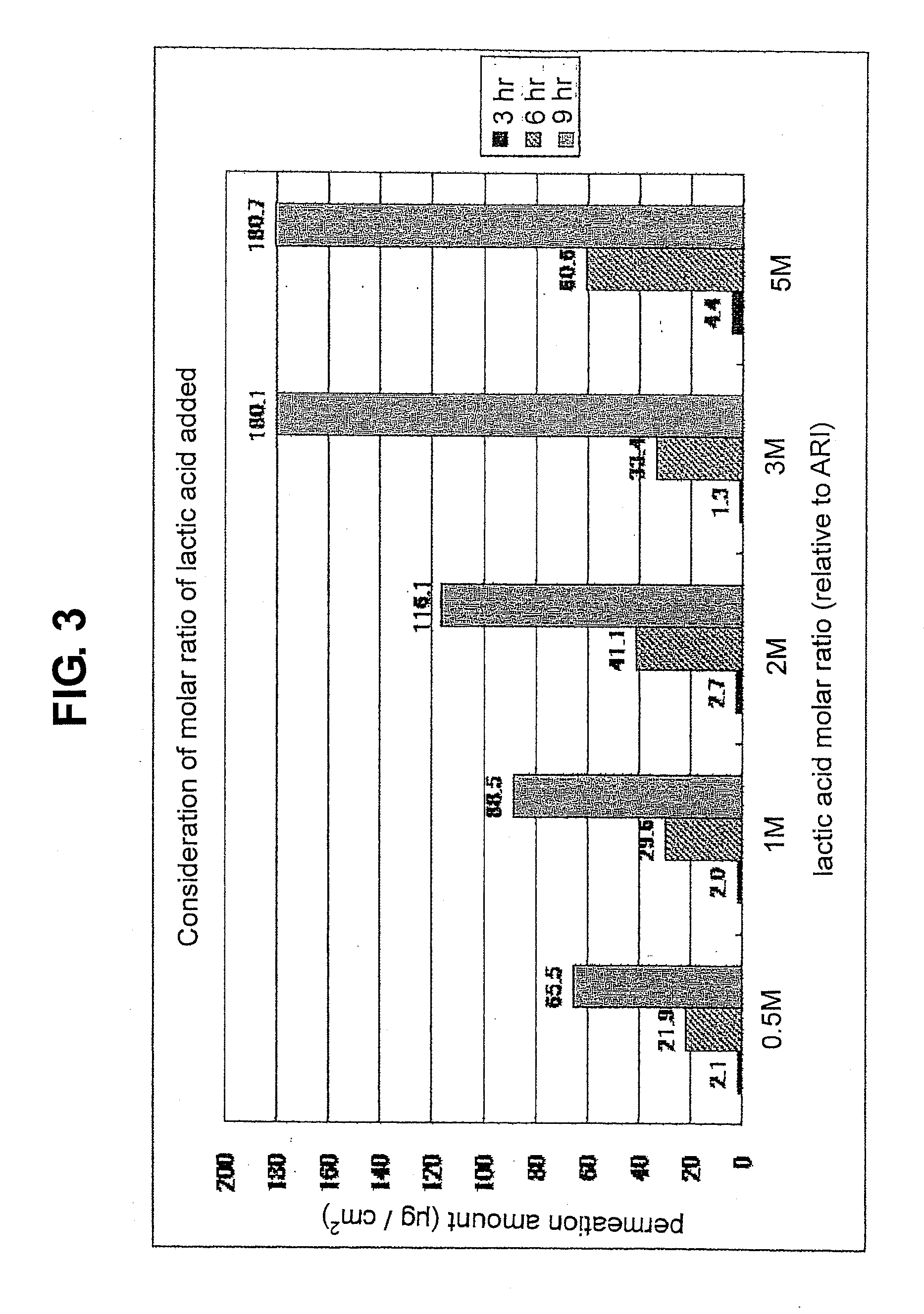
Composition for external application comprising aripiprazole and organic acid as active ingredients
InactiveUS20120184563A1Promote transdermal absorptionImprove stabilityOrganic active ingredientsNervous disorderOrganic acidExternal application
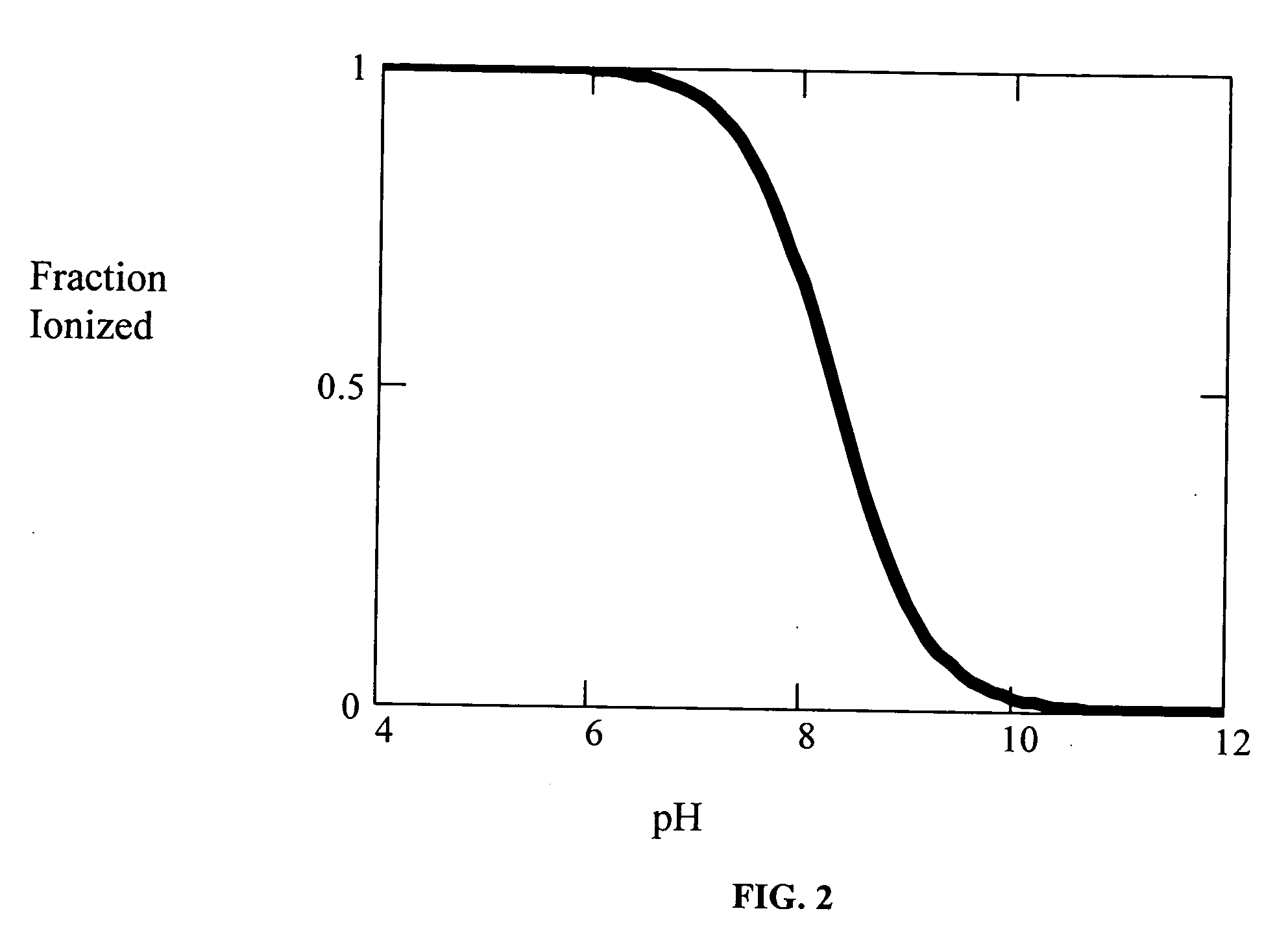
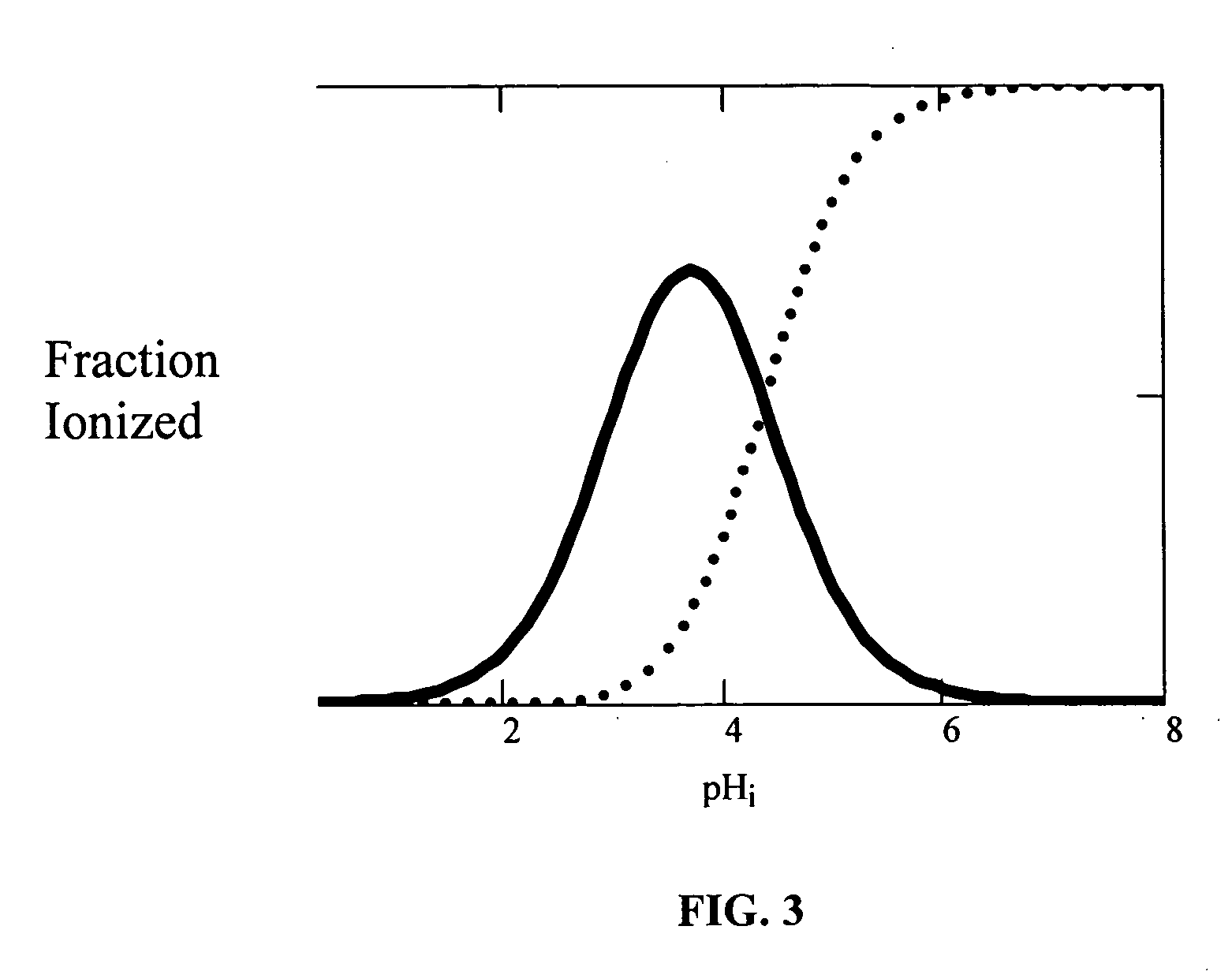
[Summary] An external preparation formulation superior in the transdermal absorbability has been desired as a new administration route of aripiprazole. Transdermal absorption of aripiprazole has been enabled for the first time by appropriately combining aripiprazole and an organic acid (particularly fatty acid with low lipophilicity). That is, it has been found that more superior transdermal absorbability can be achieved by forming a salt by using a compound showing lipophilicity within the range of −1.5-2, such as fatty acid and the like. It has been further found that the transdermal absorbability is remarkable improved by appropriately selecting the solvent composition. As a result, since a new dosage form of aripiprazole other than oral preparation has been developed, a new transdermal absorption preparation of aripiprazole can be provided.
Owner:MEDRX CO LTD
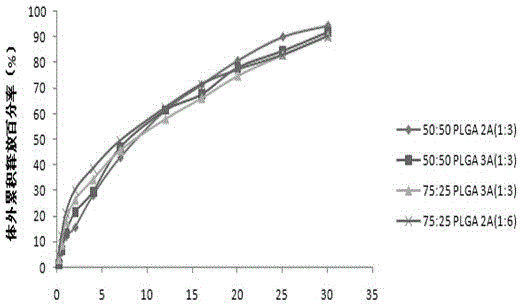
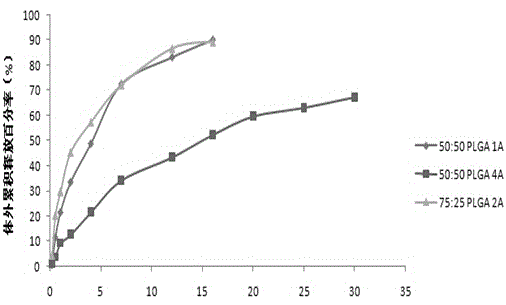
Aripiprazole sustained-release microspheres and preparation method thereof
ActiveCN105310997AImprove complianceGood treatment effectOrganic active ingredientsNervous disorderAcetic acidMicrosphere
The invention relates to aripiprazole sustained-release microspheres and a preparation method thereof. The sustained-release microspheres include aripiprazole and a bio-degradable pharmaceutical high-molecular material PLGA, wherein the ratio of lactic acid to hydroxyacetic acid in the PLGA is 75:50-25:50. The PLGA is 25000-35000 Dolton in molecular weight. The addition weight ratio of the aripiprazole to the PLGA is 1:1-20. The aripiprazole accounts for 3.01-21.09% of total weight of the microsphere. The aripiprazole sustained-release microspheres have high drug embedding rate, is high in drug loading capacity, is smooth and round in surface and can release more than 90% of the drug in 30 days.
Owner:CHONGQING PHARMA RES INST
Method for producing aripiprazole crystallite
The invention belongs to the chemical filed, in particular to an aripiprazole minicrystal preparation method, and comprises the steps as follow: aripiprazole rough products and ethanol or ethanol and non-alcohol solvent are mixed and heated to back flow into aripiprazole for full dissolution; water with low temperature is added into the solution; and the solution is cooled, and crystal is participated quickly; and then the solution is filtered under 30 to 130 DEG C and depressurized and dried for 1 to 20 hours, thereby obtaining the minicrystal. The gain diameter of the crystal ranges from 100 to 10 micrometers with the average grain diameter of 35 micrometers or smaller. The minicrystal obtained with the invention is suitable to be used for preparing oral solid preparation such as tablet, thereby improving the biological utilization degree of the minicrystal.
Owner:重庆凯林制药有限公司 +1
Methods for producing aripiprazole suspension and freeze-dried formulation
ActiveUS20100196486A1Good effectEasy to produceOrganic active ingredientsPowder deliveryFreeze-dryingEngineering
Disclosed are a method for producing an aripiprazole suspension, wherein the aripiprazole has a mean particle size of 1 to 10 μm, the method comprising the steps of: (a) combining bulk aripiprazole and a vehicle to form a primary suspension; (b) subjecting the primary suspension to first pulverization using e.g., a high shear pulverizing machine, a dispersion machine that applies shear force to a material to be processed, a colloid mill, an ultrasonic dispersion machine, or a high-pressure jet type emulsifying dispersion machine to form a secondary suspension; and (c) subjecting the secondary suspension to second pulverization using e.g., a high-pressure jet type emulsifying dispersion machine to form a sterile final suspension; and a method for producing a freeze-dried formulation from the aripiprazole suspension.
Owner:OTSUKA PHARM CO LTD
Solid oral medicine composition containing aripiprazole microcrystal
ActiveCN101066267AHigh dissolution rateUniform and stable contentOrganic active ingredientsNervous disorderDiseaseOral medicine
The present invention relates to one kind of solid oral medicine composition containing aripiprazole microcrystal. The solid oral medicine composition contains type I microcrystal of aripiprazole in the average grain size below 50 micron in 1-50 mg and pharmaceutically acceptable supplementary material. The medicine composition has obviously raised aripiprazole dissolution, and raised bioavailability and curative effect of aripiprazole. It is used in treating metal diseases, such as schizophrenia.
Owner:SHANGHAI ZHONGXI PHARMA +1
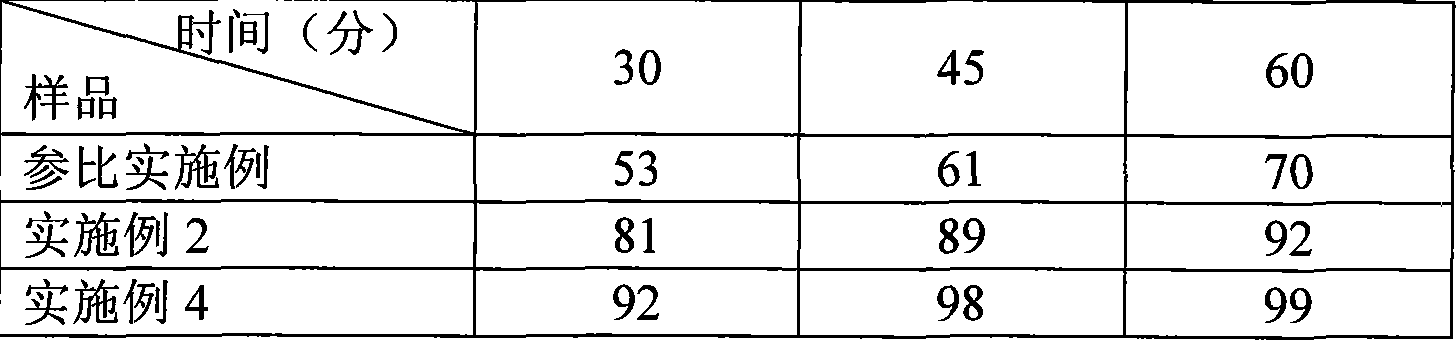
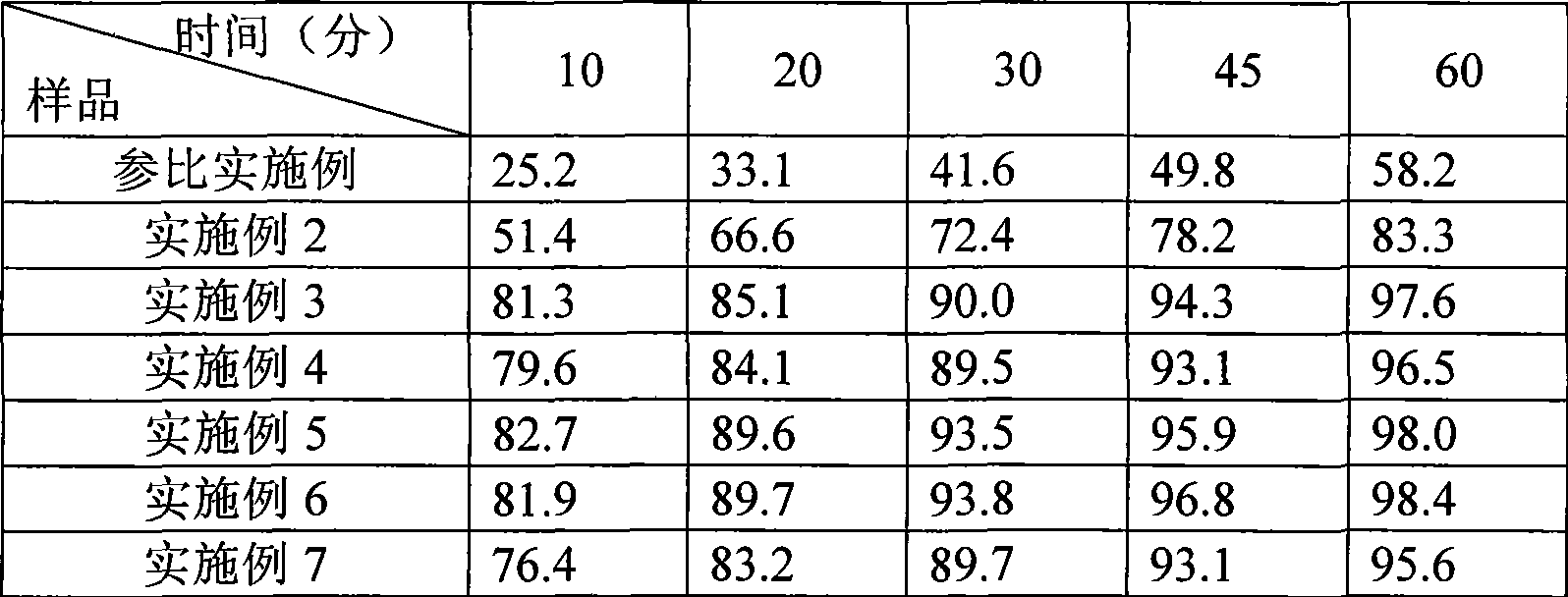
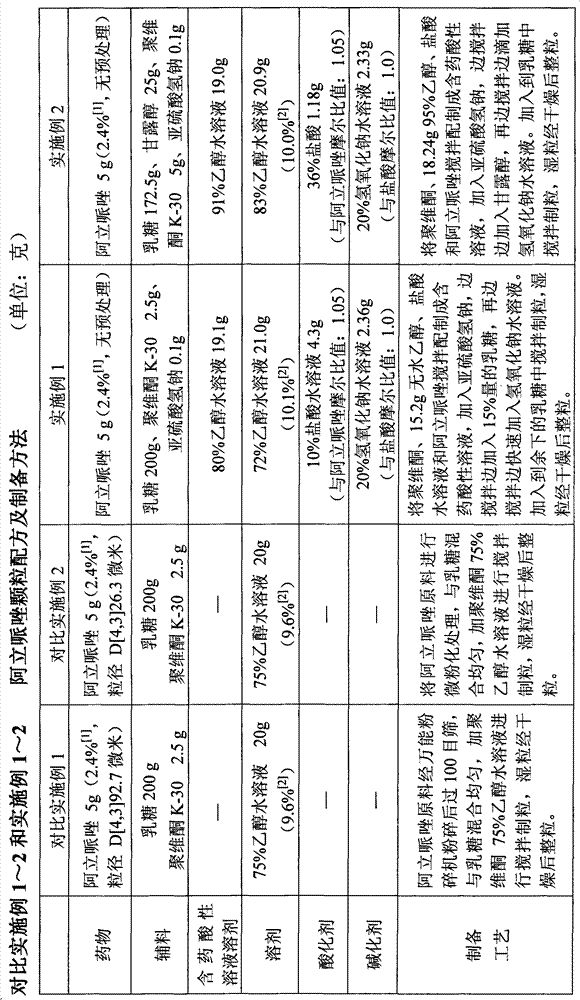
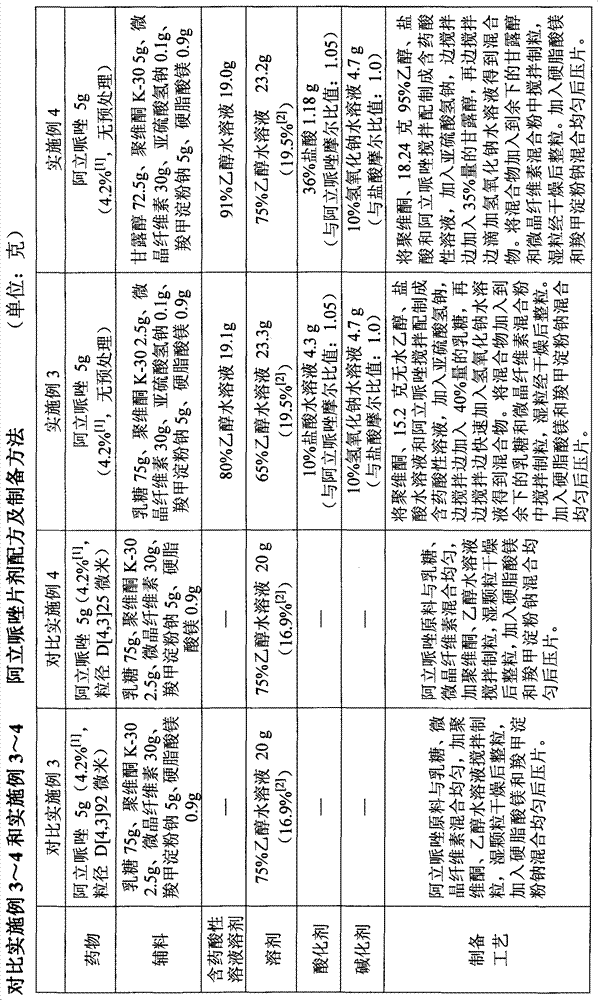
Aripiprazole pharmaceutic preparation and preparation method thereof
ActiveCN102846543ANo adverse effectsDrug effectOrganic active ingredientsNervous disorderPharmaceutical drugPharmaceutical Aids
The invention provides a preparation method of an aripiprazole pharmaceutic preparation, which comprises the following steps: dissolving aripiprazole in an acidic solution containing an acidifying agent to prepare a drug-containing acidic solution; then performing wet granulation or preparing a suspension by the drug-containing acidic solution, an alkalizer and an auxiliary material to obtain the aripiprazole pharmaceutic preparation; the auxiliary material comprises an anti-oxidant. The invention also provides an aripiprazole pharmaceutic preparation prepared by the method. The aripiprazole pharmaceutic preparation obtained by the preparation method of the invention has significantly reduced amounts of related materials, good dissolution property and stability, high bioavailability, less individual difference, and improved wetability and content uniformity of drugs which are difficult to dissolve. The method of the invention is simple in operation, and low in cost, requires no special equipment, and is easily applicable to industrial production; especially, the method eliminates the effect of raw material forms on preparation quality, and avoids defects of severe pollution, great loss, and severe potential safety hazard caused by aripiprazole pretreatment.
Owner:SHANGHAI ZHONGXI PHARMACEUTICAL CO LTD
Antibodies to risperidone and use thereof
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Aripiprazole formulations having increased injection speeds
ActiveUS20150265529A1Organic active ingredientsNervous disorderAntipsychotic MedicationsInjection rate
The present invention relates to pharmaceutical compositions comprising a compound of Formula (I) that are useful for the intramuscular delivery of antipsychotic drugs using rapid injection rates.
Owner:ALKERMES PHARMA IRELAND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com