Process for making sterile aripiprazole of desired mean particle size
a technology of aripiprazole and aripiprazole, which is applied in the field of making sterile aripiprazole of desired particle size distribution and mean particle size, and can solve the problems of undesirable batch aripiprazole milling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
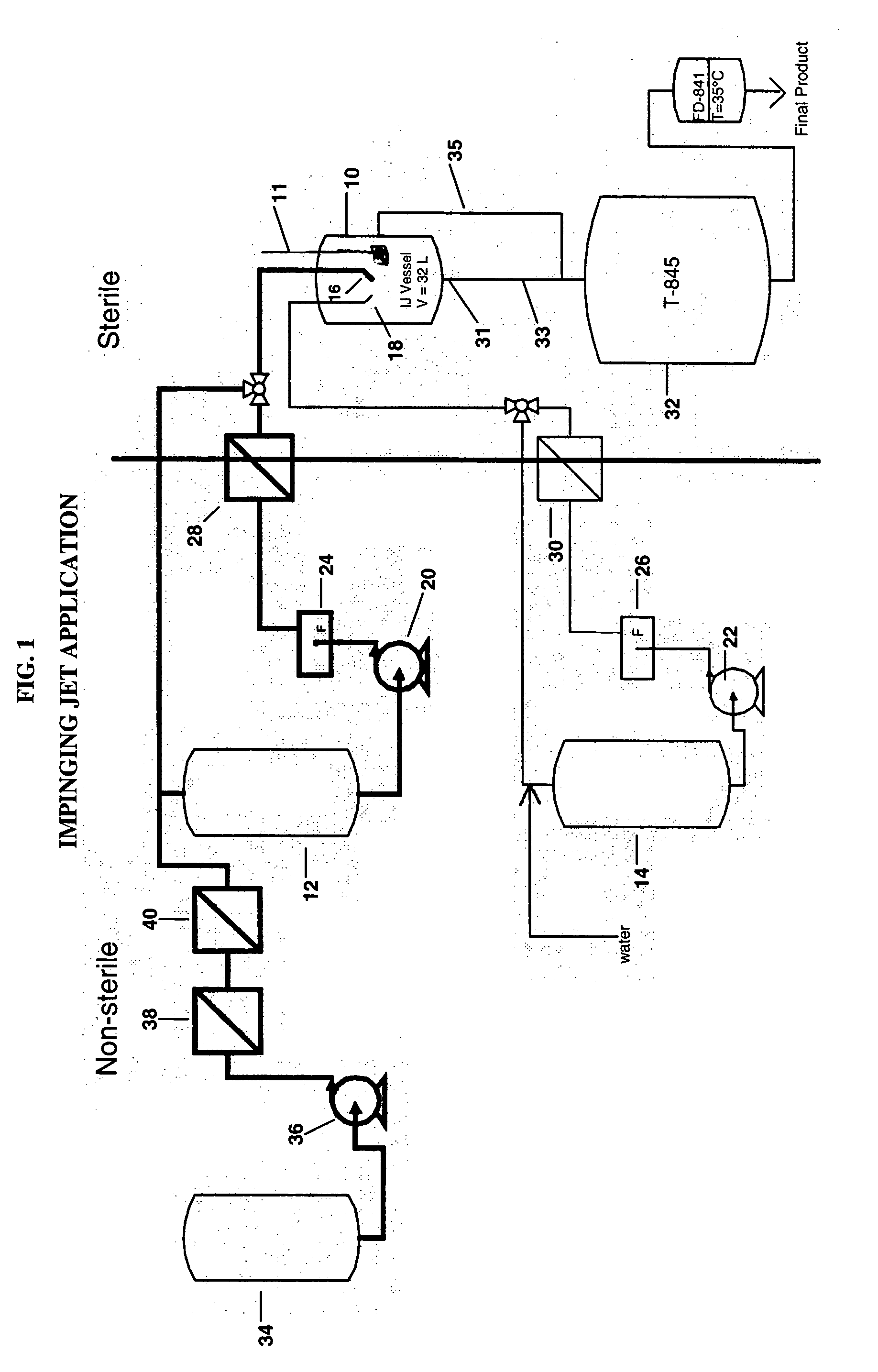
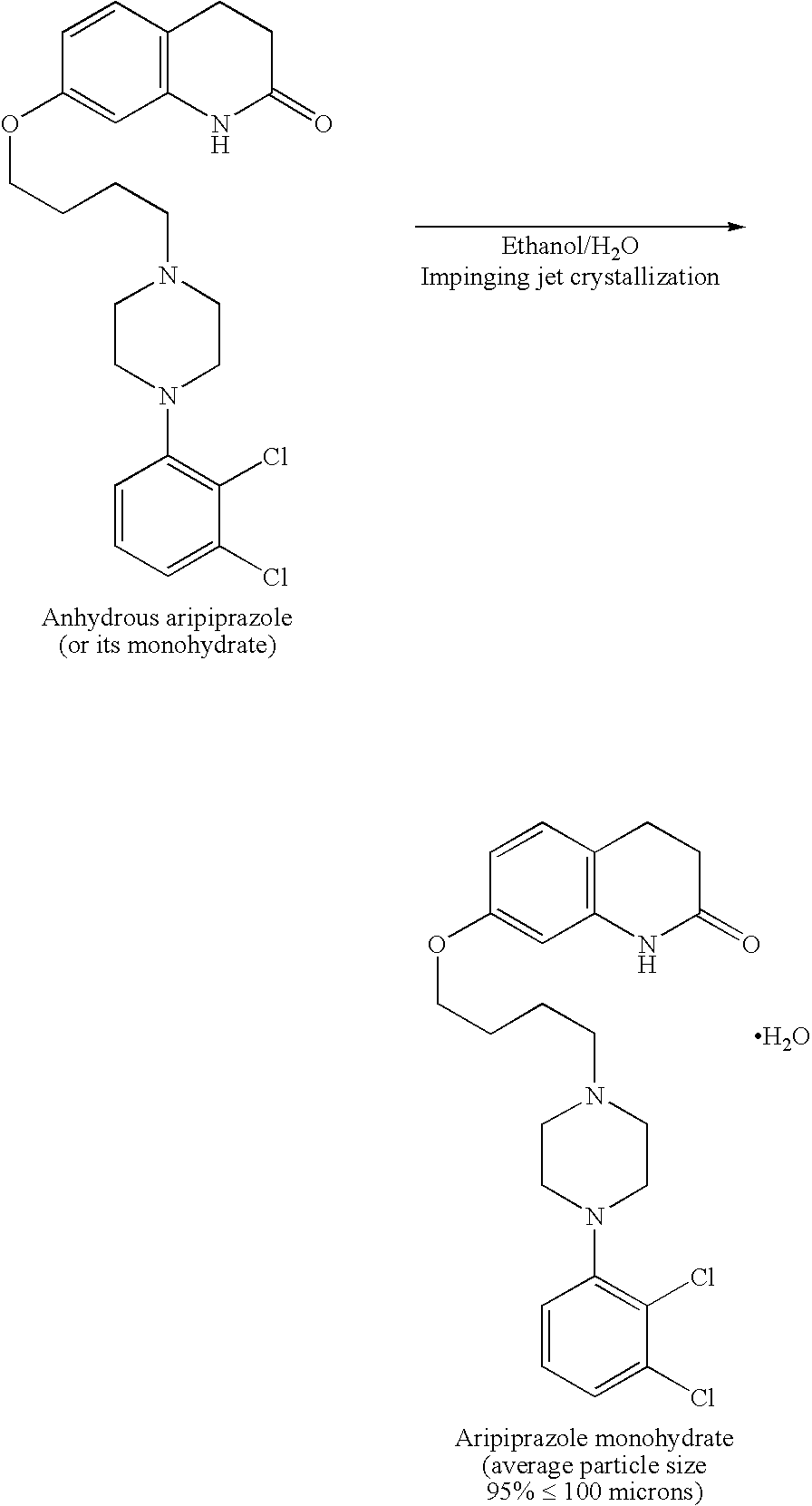
[0067] Sterile bulk active pharmaceutical ingredient (API) aripiprazole was prepared using impinging crystallization with sonication employing an apparatus set up as shown in the attached Figure.
[0068] The following procedure was employed to form a sterile bulk aripiprazole.
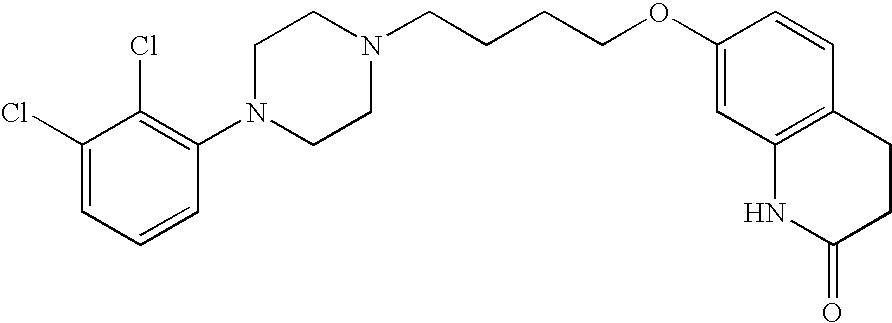
[0069] 1. Charge 100 g of aripiprazole in a 4 L flask 34.
[0070] 2. Add 2 L of 95% ethanol.
[0071] 3. Heat the suspension to 80° C. until it becomes a clear solution.
[0072] 4. Transfer the hot aripiprazole solution to a 2 L jacketed vessel 12 and maintain at 75-80° C.
[0073] 5. Charge 2 L of deionized (DI) water to a 2 L jacketed vessel 14.
[0074] 6. Cool the DI water to 2° C.
[0075] 7. Add 100 mL of 95% ethanol and 100 mL of DI water to the impinging vessel 10 and cool to 2° C.
[0076] 8. Initiate sonication (Sonication is provided by a 0.5 inch probe with 120 W power output employed as described in U.S. Pat. No. 6,302,958).
[0077] 9. Pump the aripiprazole solution through a 0.02 inch diameter nozzle 16 at 0.2...
example 2
[0083] Sterile bulk API aripiprazole was prepared using impinging jet crystallization and an apparatus set up as shown in the accompanying figure.
[0084] The following procedure was employed to form a sterile bulk aripiprazole:
[0085] 1. Suspend 100 g of aripiprazole in 2000 mL of 95% ethanol. Heat the suspension to 80° C. until it becomes a clear solution.
[0086] 2. Polish filter the aripiprazole solution into a holding vessel 12 and maintain at 80° C.
[0087] 3. Polish filter 2000 mL water to another holding vessel 14 and heat to 80° C.
[0088] 4. Pump the aripiprazole solution through a 0.02 inch diameter nozzle 16 at 0.25 kg / min and impinge it with the 30° C. water pumped at 0.25 kg / min through a 0.02 inch diameter nozzle 18 to form a crystal slurry which is collected in an impingement vessel 10.
[0089] 5. Agitate the newly formed crystal slurry in the impingement vessel 10 while continuously transferring it to a receiver 32 to maintain a constant volume in the impingement vessel ...
example 3
[0093] An aripiprazole injectable aqueous suspension (200 mg aripiprazole / 2 mL, 200 mg / vial) was prepared as follows.
[0094] The following ingredients were added to a 3L glass jacketed vessel maintained at 15° C. (±5° C.) to form a sterile primary suspension:
Aripiprazole (prepared by impinging jet 100 gcrystallization as described in Example 2):Carboxymethylcellulose, Sodium Salt 7L2P 9.0 gMannitol 45 gSodium Phosphate, Monobasic 0.8 gSodium Hydroxide Solution, 1Nq.s. to adjust pH to 7.0Water, USPq.s. to 1000 g
[0095] The sterile suspension was mixed at 500-1000 rpm for about 0.5 hour and then at 300-500 rpm for an additional 1 hour under 20 ″Hg (+5″Hg) vacuum.
[0096] 2.5 mL of the above suspension were aseptically filled into sterilized vials which were then aseptically partially stoppered with sterilized stoppers. The vials were aseptically transferred to a freeze dryer and lyophilized according to the following cycle:
[0097] (a) thermal treatment: freeze product at −40° C. over...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com