Slow-release composition of aripiprazole and derivative thereof and preparation method thereof
A sustained-release composition, aripiprazole technology, applied in the field of insoluble/slightly soluble drug sustained-release composition and its preparation, can solve problems such as injury and side effects of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
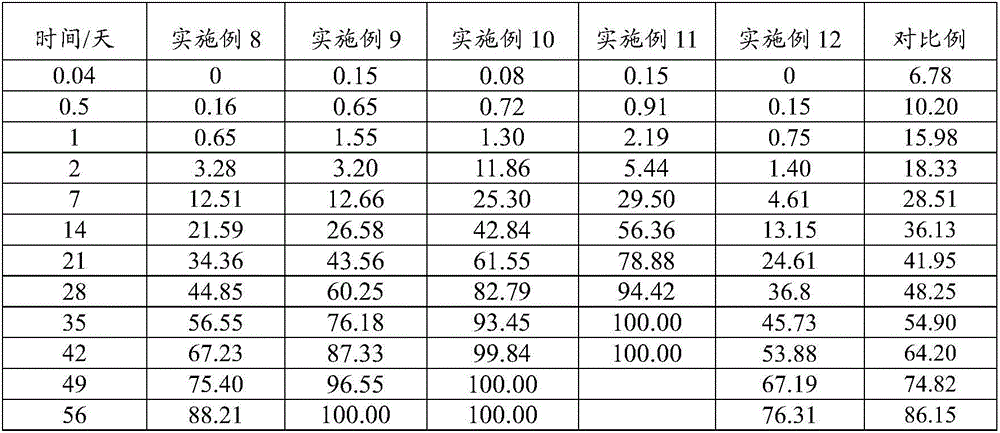
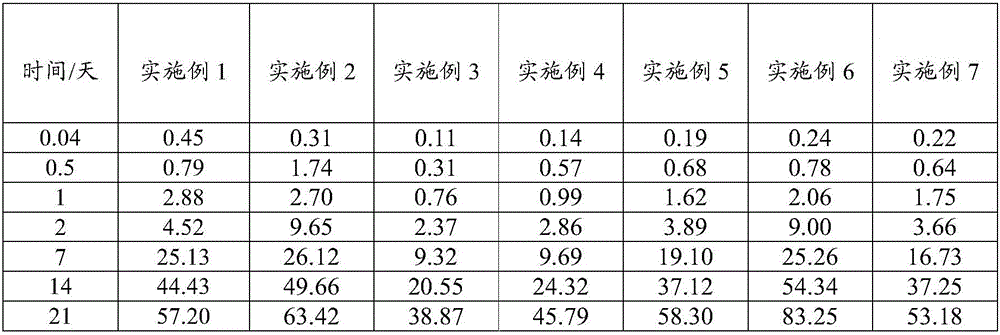
[0071] An embodiment of the sustained-release composition of aripiprazole and its derivatives of the present invention, the non-solvent preparation raw material of the sustained-release composition described in this embodiment contains the following components in mass percentage: lauroyl arabine 55% of ripiprazole and 45% of a poorly water-soluble polymer PLGA (mol ratio of lactide to glycolide: 85:15, weight average molecular weight of 50 kDa, viscosity of 0.44 dL / g, and carboxyl end).
[0072] The sustained-release composition described in this example is prepared by the following preparation method:
[0073] (1) dissolving the poorly water-soluble polymer in dichloromethane, the mass percentage of the poorly water-soluble polymer and dichloromethane is 7%, then adding lauroyl aripiprazole, dissolving evenly, forming an internal phase;
[0074] (2) PVA is dissolved in water to form an external phase, and the mass percentage of the PVA in the external phase is 0.1%;
[0075]...
Embodiment 2
[0078]An embodiment of the sustained-release composition of aripiprazole and its derivatives of the present invention, the non-solvent preparation raw material of the sustained-release composition described in this embodiment contains the following components in mass percentage: aripiprazole 60% and 40% of water insoluble polymer; Wherein, described water insoluble polymer is made of PLGA (the molar ratio of lactide and glycolide is 80:20, weight average molecular weight is 75kDa, viscosity is 0.63dL / g , with an ester group end) and PLA (with an ester group end, the weight average molecular weight of PLA is 80kDa, and the viscosity is 0.65dL / g), and the mass ratio of PLGA to PLA is 3:1.
[0079] The sustained-release composition described in this example is prepared by the following preparation method:
[0080] (1) dissolving the poorly water-soluble polymer in chloroform, the mass percentage of the poorly water-soluble polymer and chloroform being 5%, then adding aripiprazole...
Embodiment 3
[0085] An embodiment of the sustained-release composition of aripiprazole and its derivatives of the present invention, the non-solvent preparation raw material of the sustained-release composition described in this embodiment contains the following components in mass percentage: aripiprazole 45%, water insoluble polymer 55%; Wherein, described water insoluble polymer is PLGA (the molar ratio of lactide and glycolide is 95:5, weight average molecular weight is 25kDa, viscosity is 0.28dL / g , with ester-terminals) and PLGA (lactide-to-glycolide molar ratio of 85:15, weight-average molecular weight of 25 kDa, viscosity of 0.29 dL / g, with ester-terminals), PLGA (lactide The molar ratio to glycolide is 95:5, the weight-average molecular weight is 25kDa, the viscosity is 0.28dL / g, and has an ester terminal) and PLGA (the molar ratio of lactide to glycolide is 85:15, and the weight-average Molecular weight is 25kDa, viscosity is 0.29dL / g, has ester group end) mass ratio is 30:25.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com