Patents
Literature
73 results about "Mirtazapine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
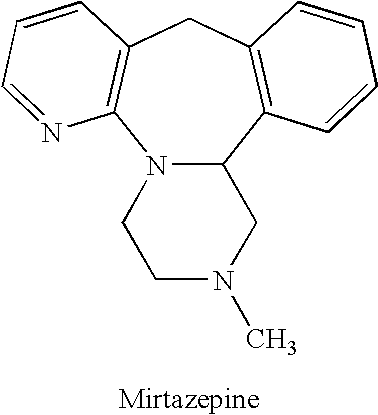
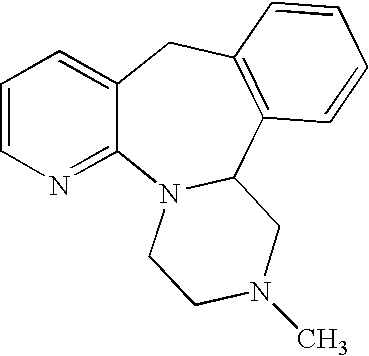
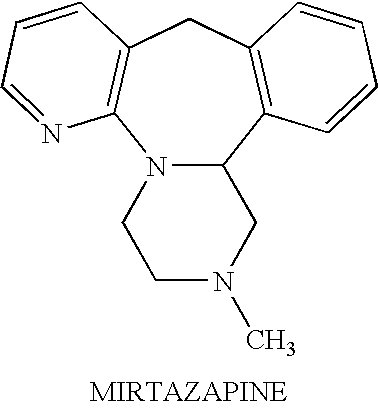
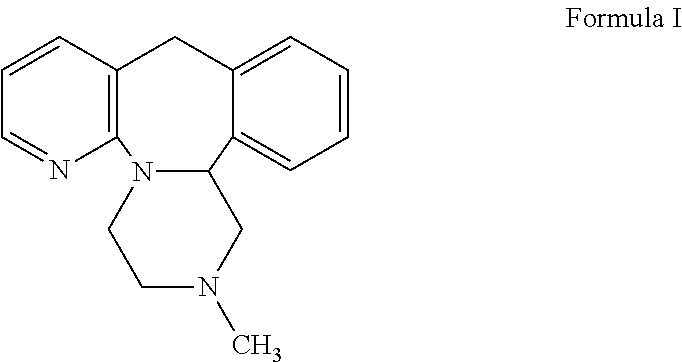
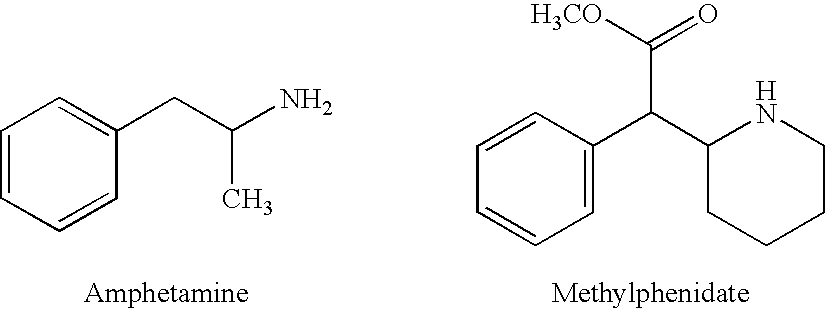
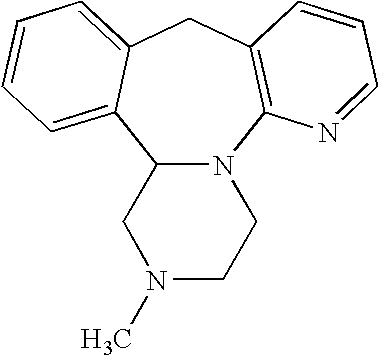
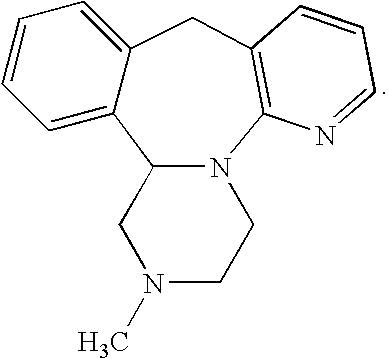
Mirtazapine is used to treat depression.
Methods for treating idiopathic hyperhidrosis and associated conditions
InactiveUS20040192754A1Reduced activityAlleviate and treat symptomBiocideAnimal repellantsAllosteric modulatorMianserin Hydrochloride
The subject invention provides methods for treating symptoms and / or conditions associated with idiopathic hyperhidrosis by using compounds that decrease the activity of serotonin 5-HT2C receptors. Compounds that can ameliorate symptoms of idiopathic hyperhidrosis and associated conditions include 5-HT2C receptor antagonists (i.e., ketanserin, ritanserin, mianserin, mesulergine, cyproheptadine, fluoxetine, mirtazapine, olanzapine, and ziprasidone) as well as 5-HT2C receptor modulators (i.e., inverse agonists, partial agonists, and allosteric modulators).
Owner:SHAPIRA NATHAN ANDREW +2
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
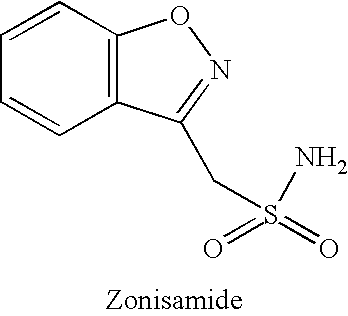
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
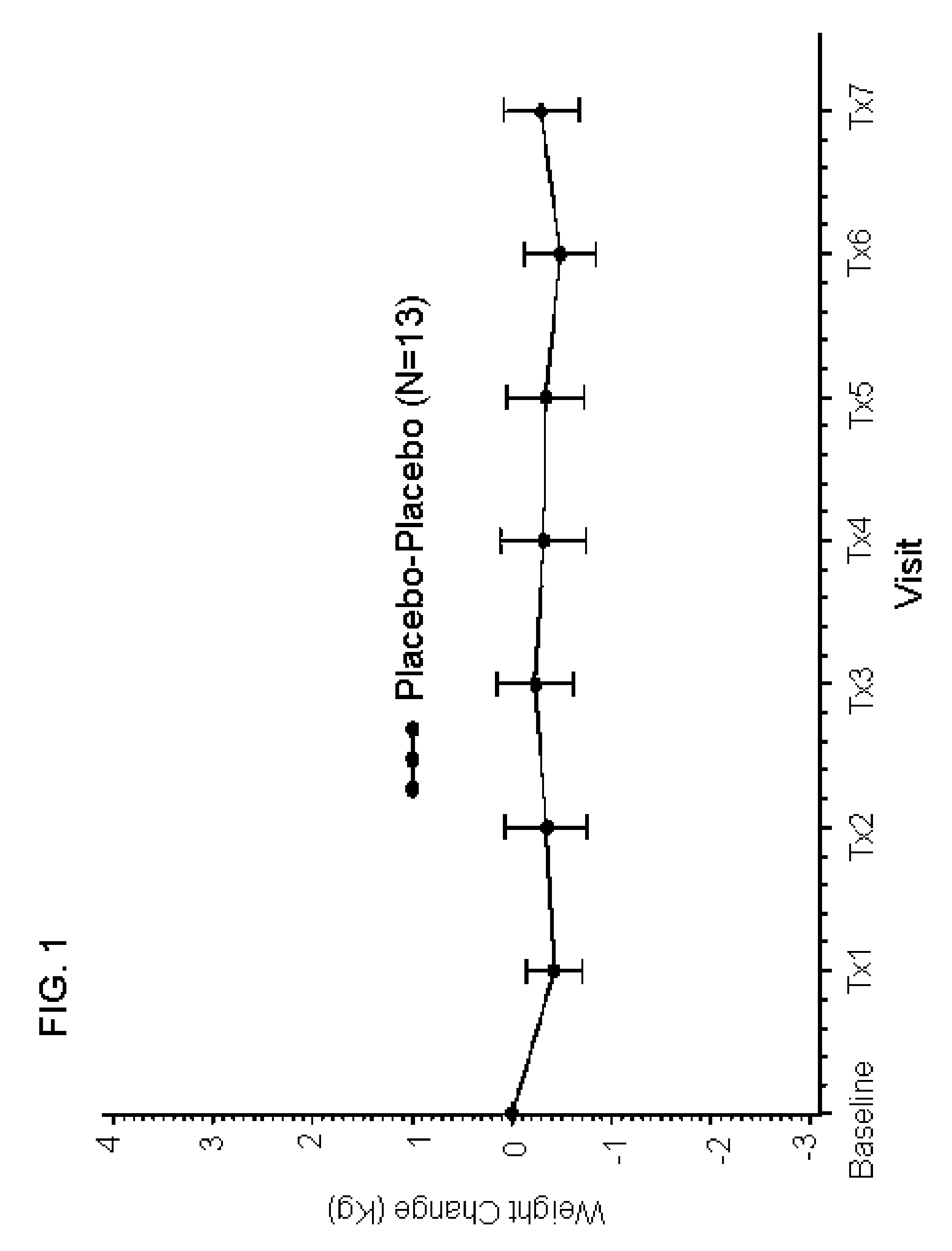
Compositions of an anticonvulsant and mirtazapine to prevent weight gain
ActiveUS20060079501A1Avoid weight gainGood curative effectBiocidePowder deliveryWeight gainPharmacology
Disclosed are pharmaceutical compositions comprising mirtazapine and an anticonvulsant drug. Also disclosed are methods of preventing weight gain associated with the administration of mirtazapine comprising identifying a patient to whom mirtazapine is to be administered and administering to said patient a pharmaceutical composition comprising mirtazapine and an anticonvulsant drug. Further disclosed are methods of increasing the efficacy of mirtazapine comprising identifying a patient to whom mirtazapine is to be administered and administering to said patient a pharmaceutical composition comprising mirtazapine and an anticonvulsant drug.
Owner:DUKE UNIV
Method for treating sleep-related breathing disorders
InactiveUS20060039866A1Excellent toneSuppressing REM sleepBiocideAerosol deliveryHypopneaPositive airway pressure
Compositions and methods for the treatment of sleep related breathing disorders are provided. Compositions include mirtazapine in combination with other active pharmaceutical ingredients, such as zonisamide, topiramate or modafinil. The treated sleep related breathing disorders include sleep apnea and sleep hypopnea. In some embodiments, the pharmaceutical compounds are used as adjuvant therapy with positive airway pressure (PAP) therapy, thereby lowering the pressure required to maintain airway patency during PAP therapy.
Owner:CYPRESS BIOSCI
Treatment of insomnia
The invention is directed to a method for the treatment of a patient suffering from insomnia. The claimed method comprises the administration of a compound selected from the group consisting of the pharmaceutically acceptable forms of dosage of mirtazapine, nortriptyline and mixtures thereof in dosages ranging from about 0.5 to about 10.0 milligrams.
Owner:PROMCOM ONE
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
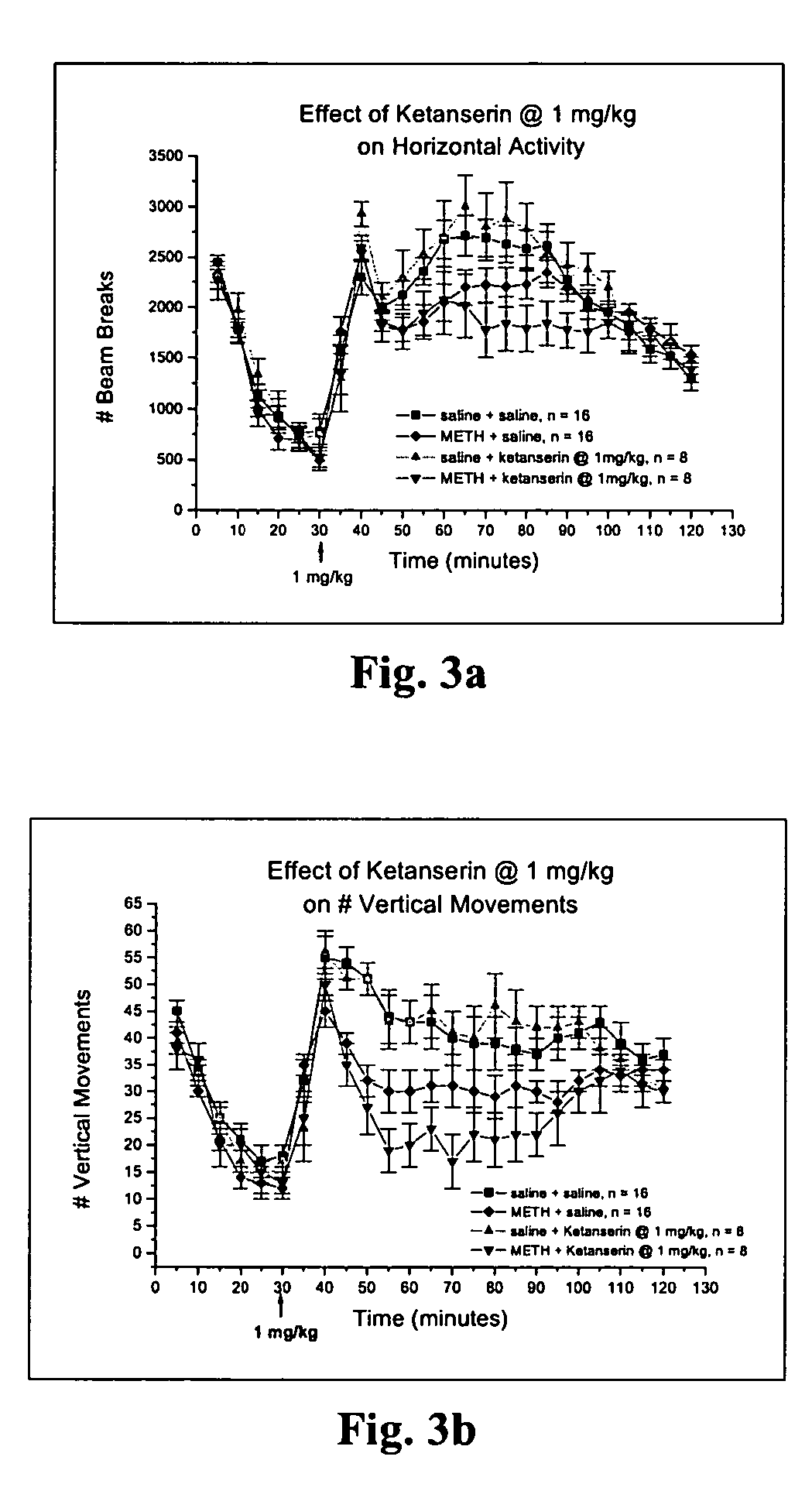
Treatment for methamphetamine addiction and reduction of methamphetamine use using serotonin antagonists
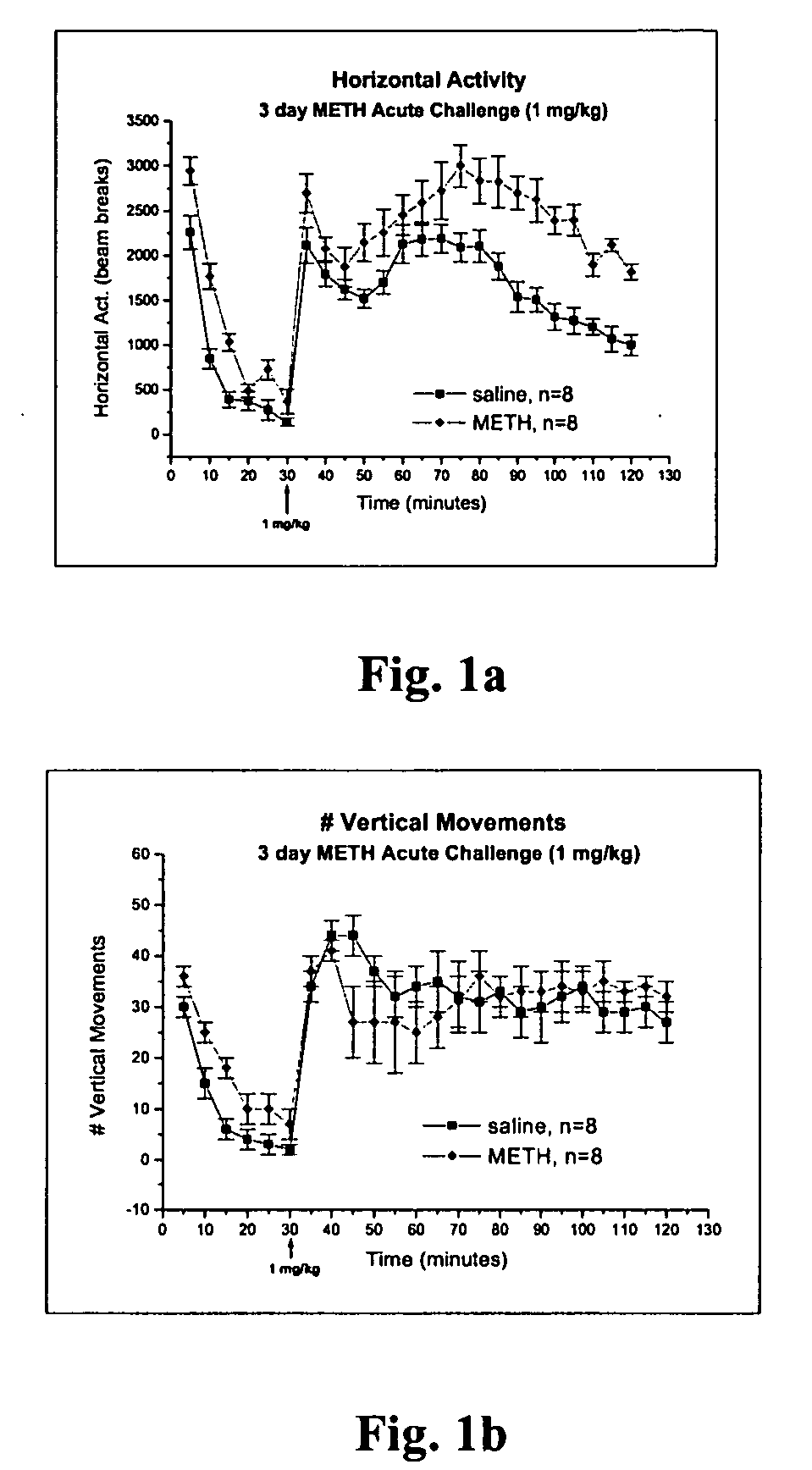
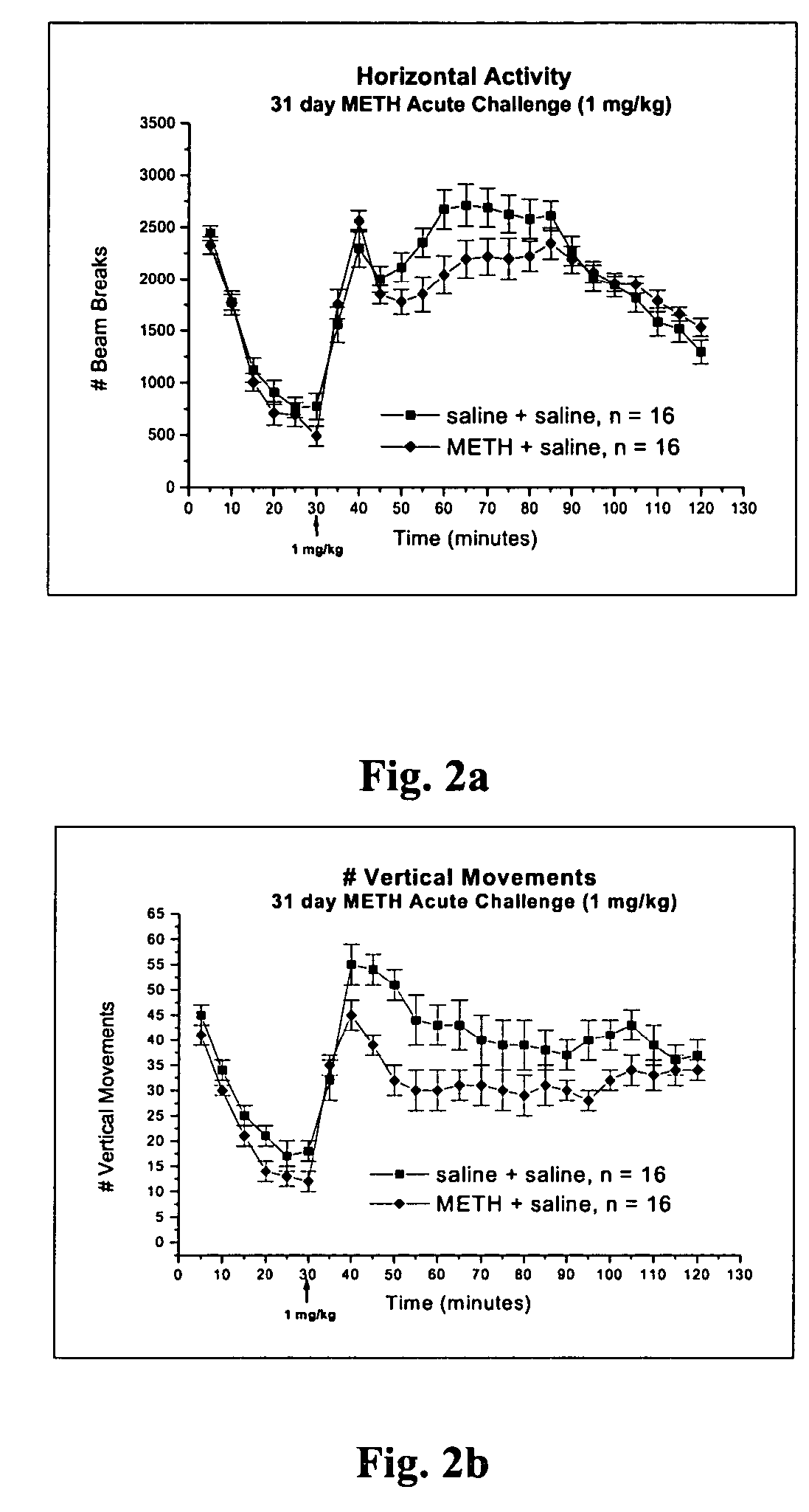
Methods for screening specific biological endpoints that can be utilized to identify potential therapeutic agents for METH addiction. In one aspect of the invention, the methods involve reversal of behavioral sensitization and / or conditioned place preference in an animal previously treated with METH in the presence of a known amount of a 5-HT2A / 2C antagonist or a selective 5-HT2C antagonist, and reversal of the electrophysiological endpoints in a METH-treated animal in the presence of a known amount of the 5-HT2A / 2C antagonist or the selective 5-HT2C antagonist. Therapeutic treatment methods for reversing the set of biological endpoints that change in the METH drug addict using mirtazapine, SDZ SER 082, and related serotonin antagonists are also provided. The methods of the invention may be utilized in the identification of potential new therapies for multiple drugs of abuse.
Owner:OMEROS CORP +1
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
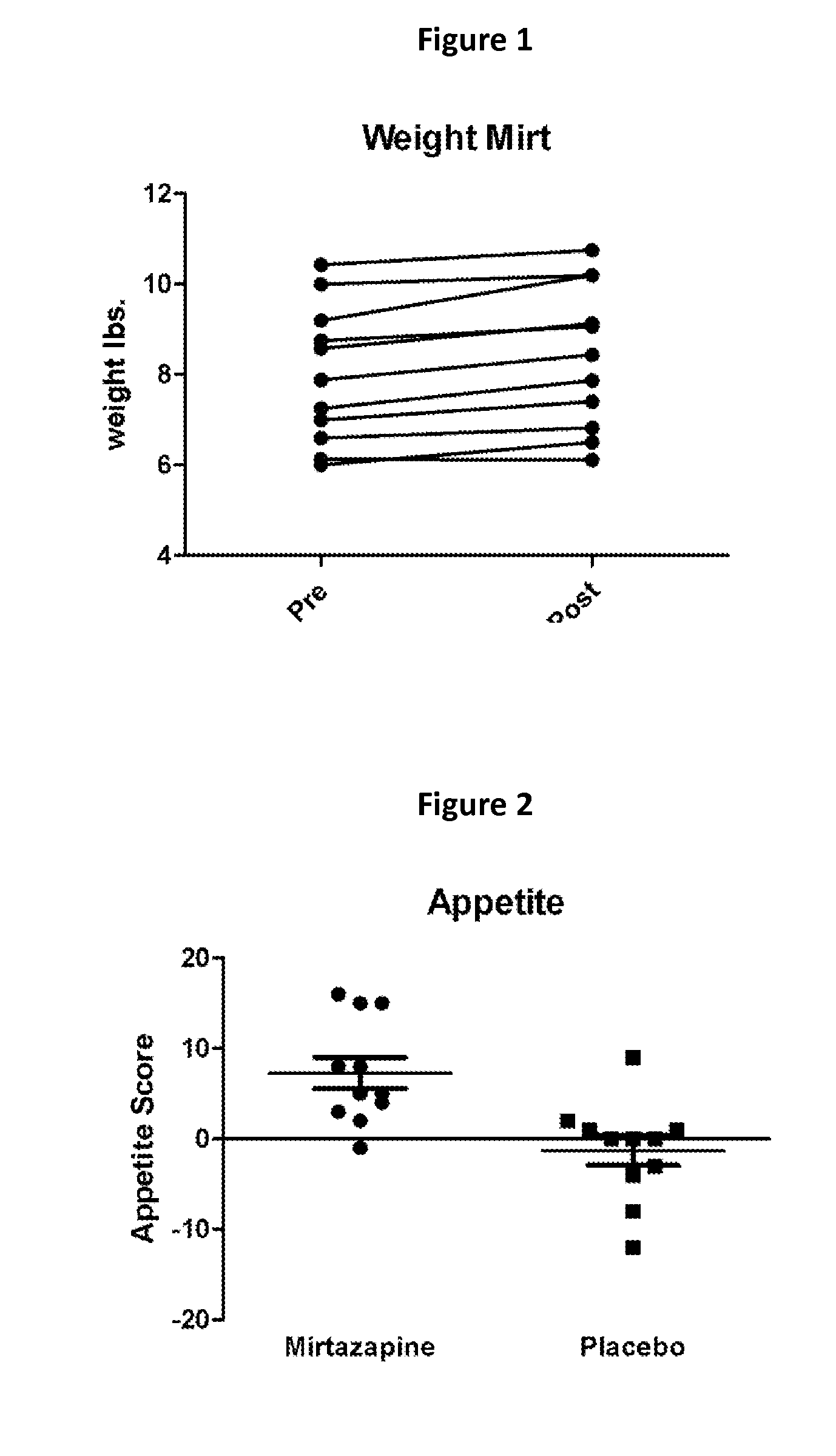
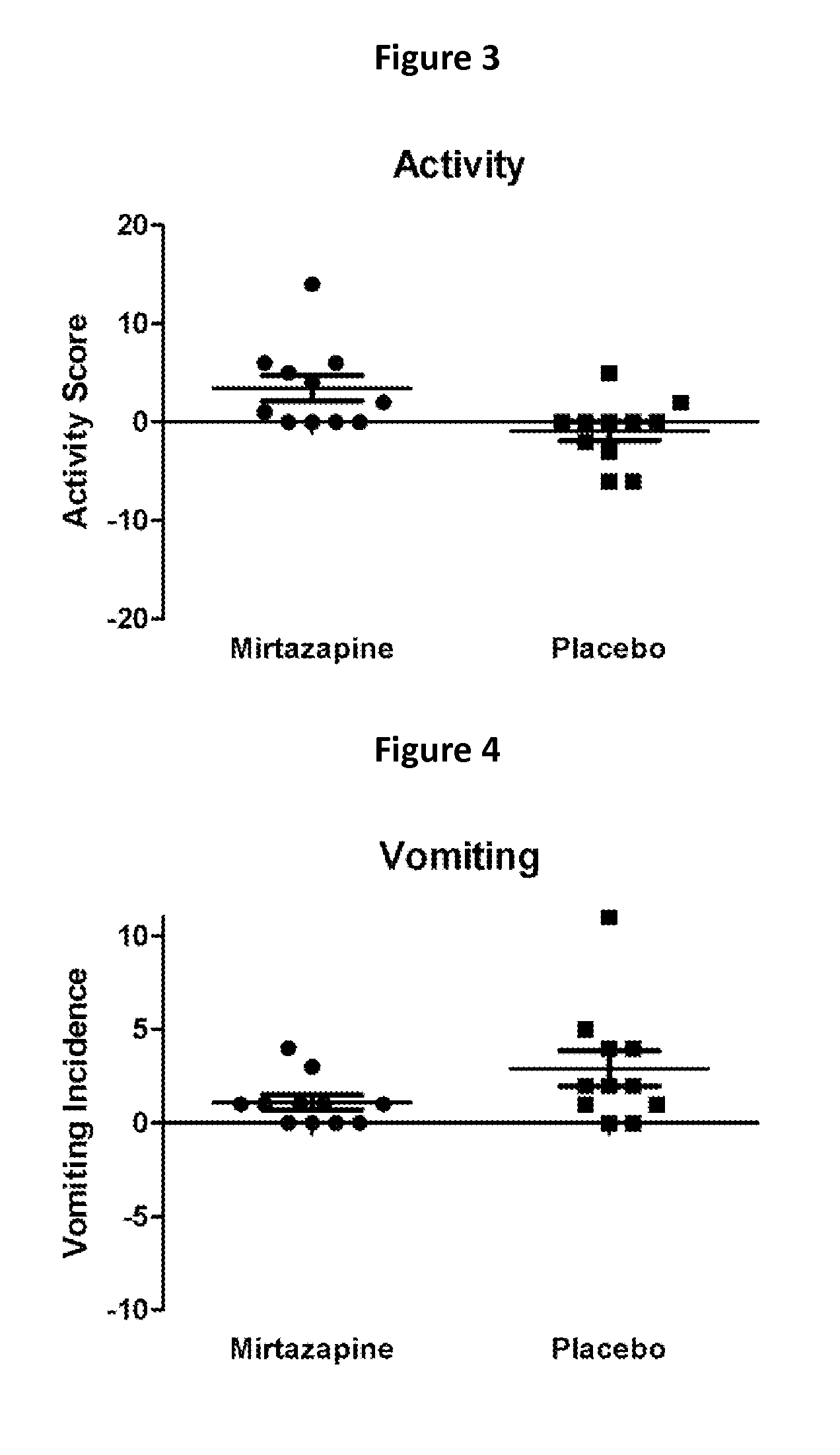
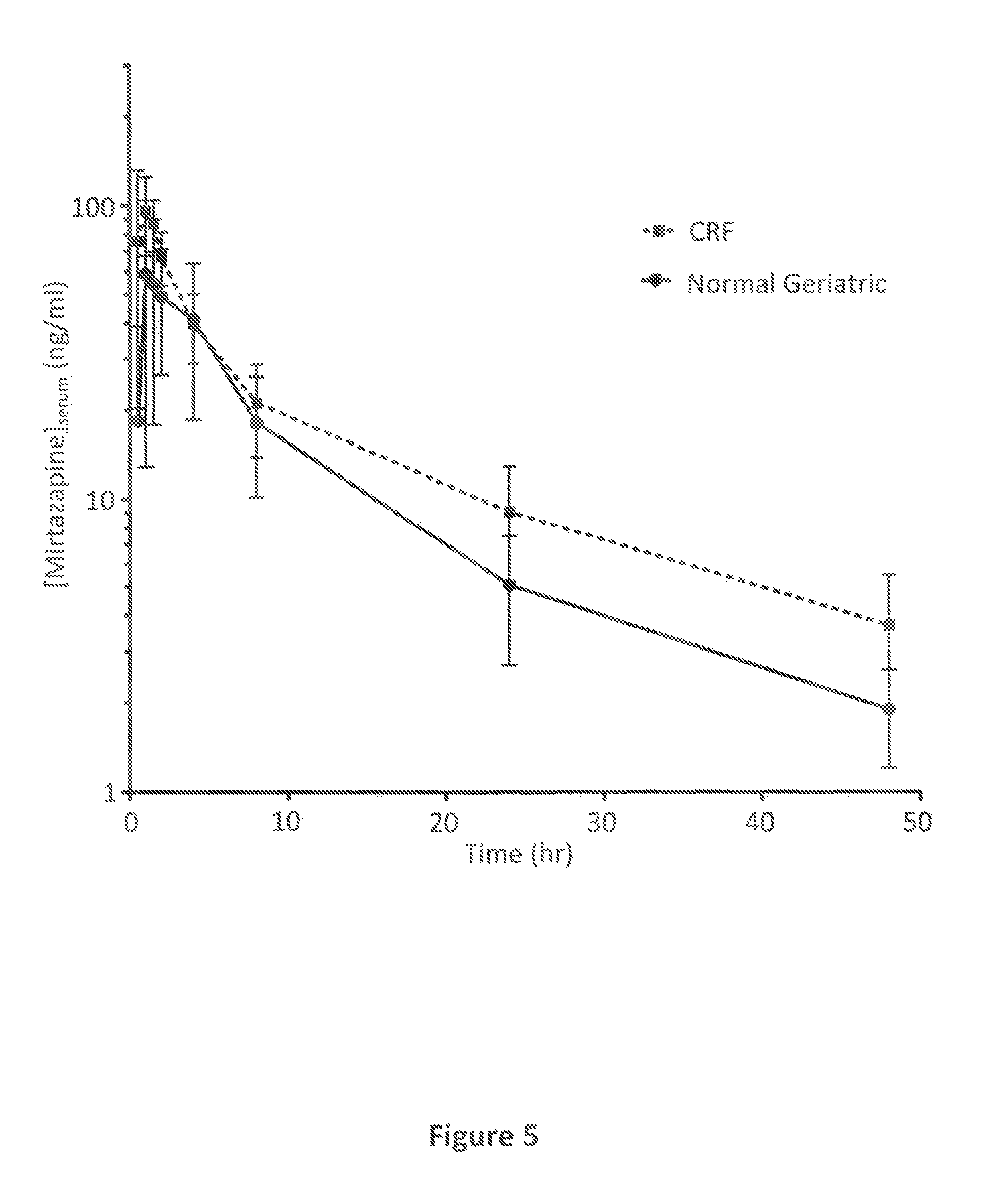
Mirtazapine as an Appetite Stimulant for Cats
Owner:COLORADO STATE UNIVERSITY
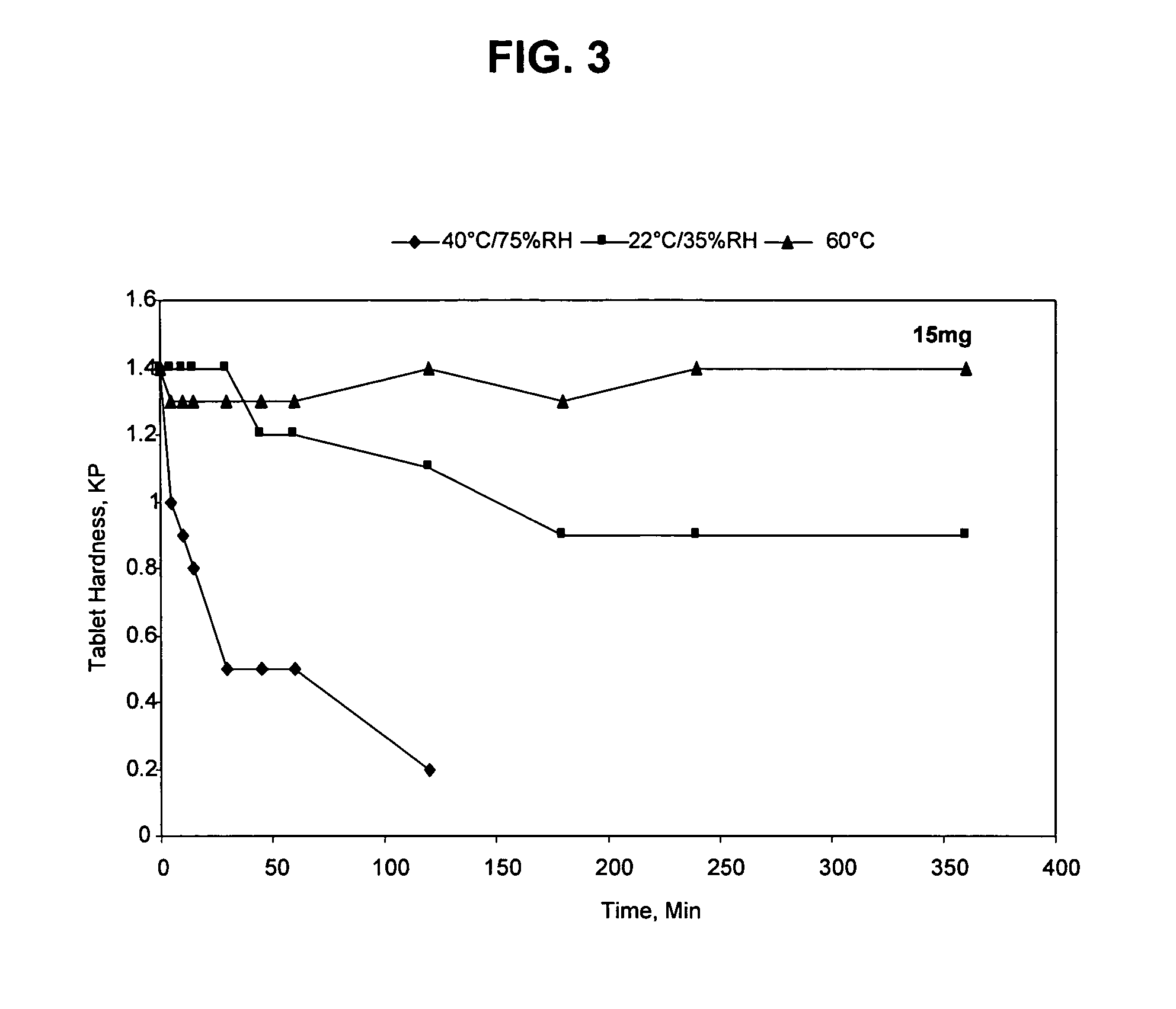
Pharmaceutical Compositions of Mirtazapine
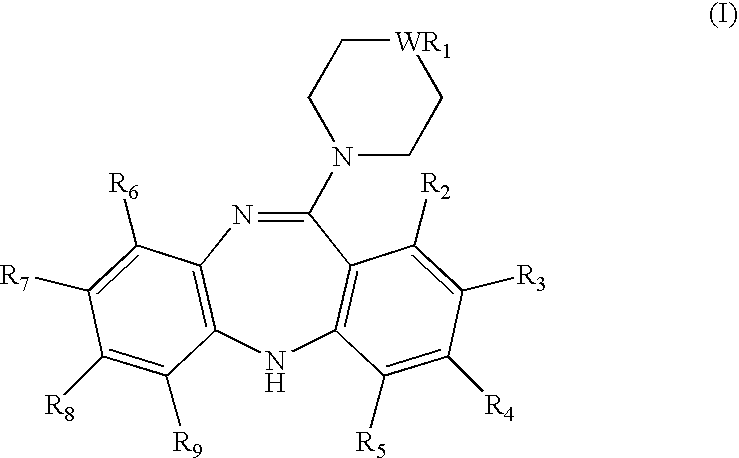
InactiveUS20070298107A1Solve the lack of hardnessGood reproducibilityBiocideNervous disorderOral medicationDosage form
The present invention relates to pharmaceutical compositions of mirtazapine or its pharmaceutically acceptable salts. More particularly, the present invention relates to solid unit dosage forms of anhydrous mirtazapine or its pharmaceutically acceptable salts suitable for oral administration. The present invention also relates to a process for the preparation of pharmaceutical compositions of mirtazapine or its pharmaceutically acceptable salts.
Owner:AUROBINDO PHARMA LTD
Tolerability of mirtazapine and a second active by using them in combination
A reduction in the side effects of treating with an agent having combined 5HT2 / 5HT3 and alpha-2 antagonistic activity is obtained by administering an agent having selective norepinephrine reuptake inhibitory or histamine H1 agonist activity. In some embodiments, the invention provides synergistic combinations of 5HT2 / 5HT3 antagonist / alpha-2 antagonist and selective norepinephrine reuptake inhibitor or histamine H1 agonist.
Owner:CYPRESS BIOSCI
Compositions of an anticonvulsant and mirtazapine to prevent weight gain
InactiveUS20100179129A1Good curative effectAvoid weight gainBiocideMetabolism disorderWeight gainAnticonvulsant
Owner:DUKE UNIV
Tolerability of mirtazapine and a second active agent by using them in combination
A reduction in the side effects of treating with an agent having combined 5HT2 / 5HT3 and alpha-2 antagonistic activity is obtained by administering an agent having histamine H1 receptor agonist activity. A combined dosage form comprising an agent having 5HT2 / 5HT3 and alpha-2 antagonistic activity and an agent having histamine H1 receptor agonist activity is presented. Some embodiments of the combined dosage form comprise an immediate release component comprising an agent having 5HT2 / 5HT3 and alpha-2 antagonistic activity and a delayed release component comprising an agent having histamine H1 receptor agonist activity. Some embodiments of the combined dosage form comprise a delayed release component comprising an agent having 5HT2 / 5HT3 and alpha-2 antagonistic activity and an immediate release component comprising an agent having histamine H1 receptor agonist activity. Methods of treatment and kits for administration are also provided.
Owner:OBECURE
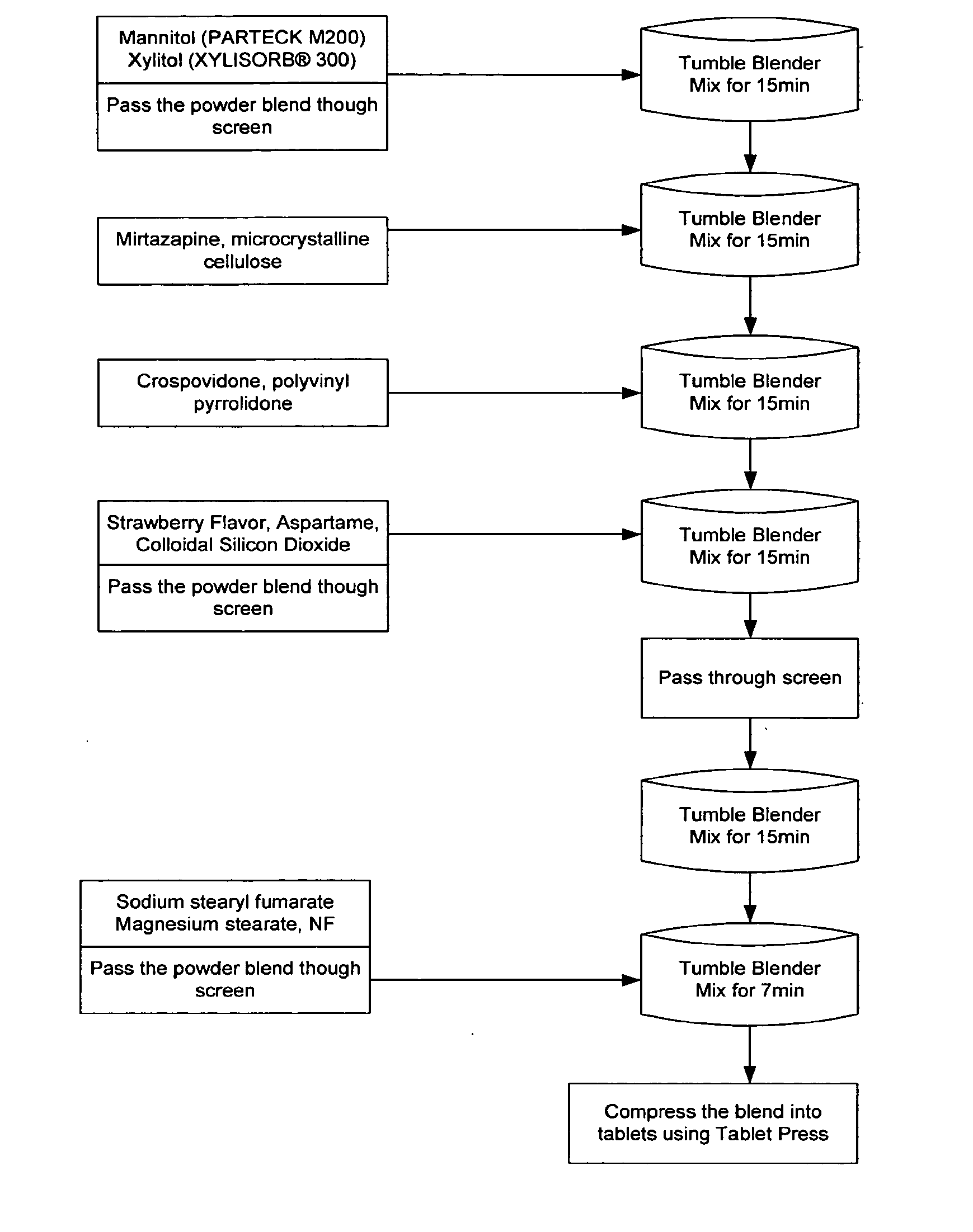
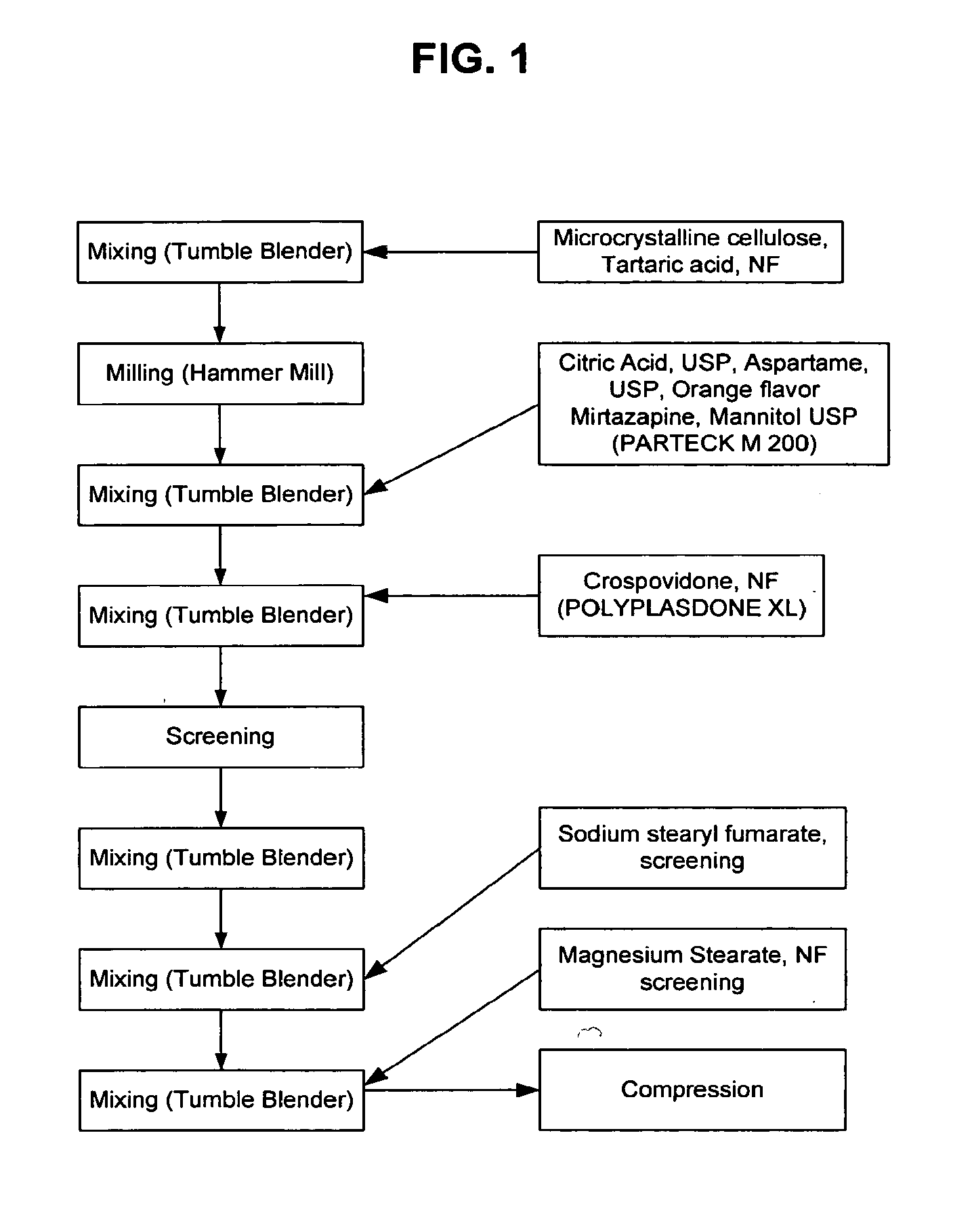
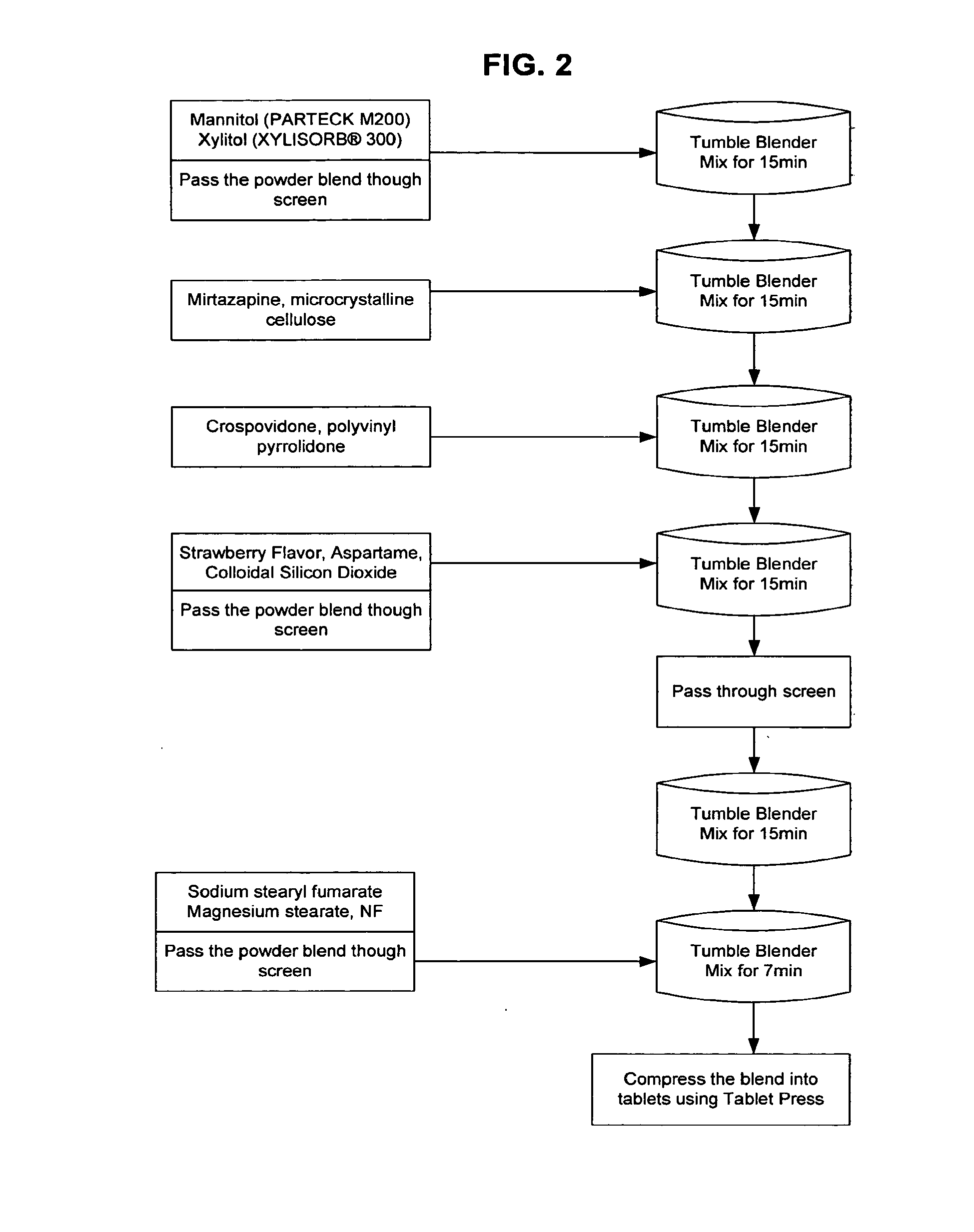
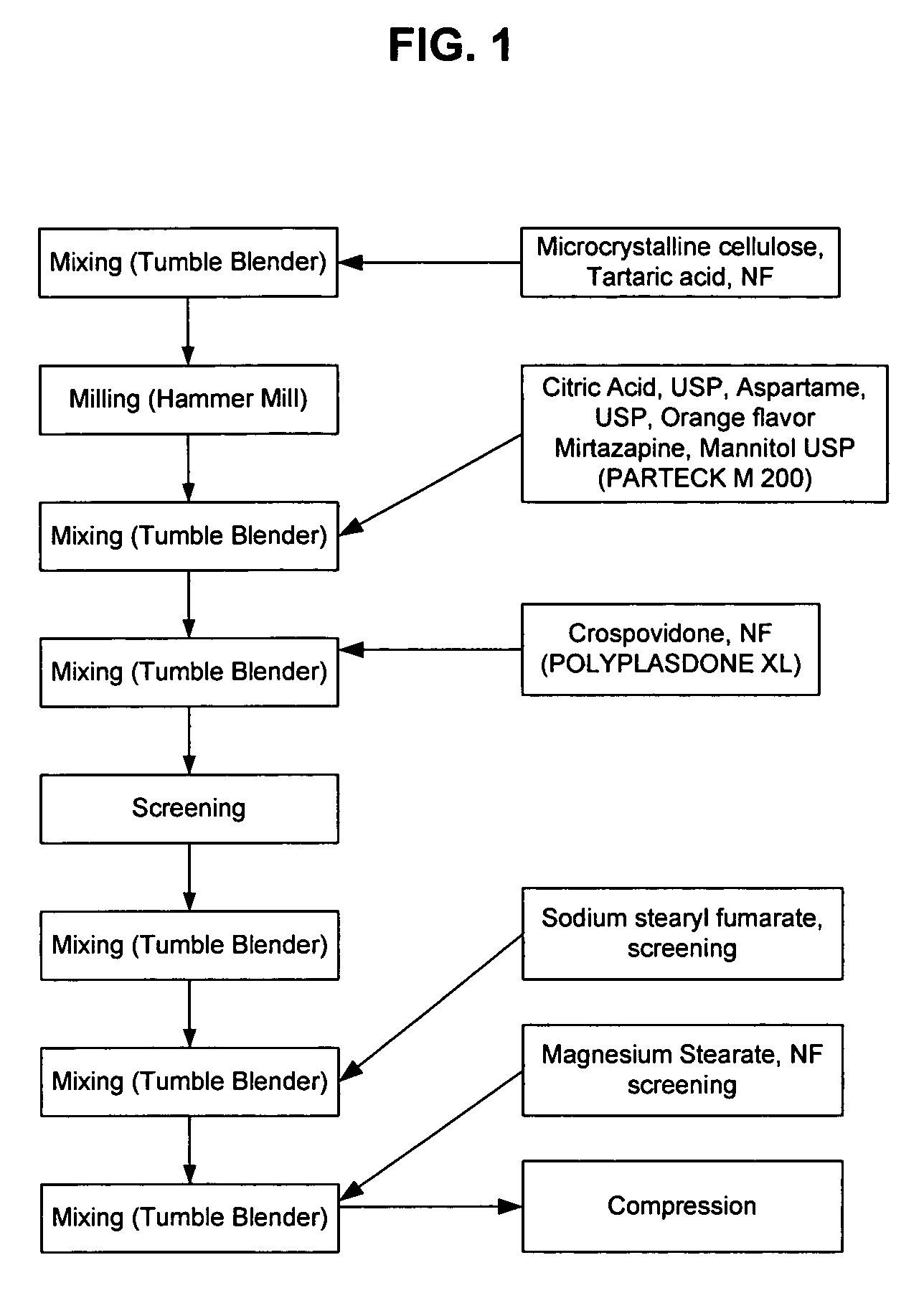
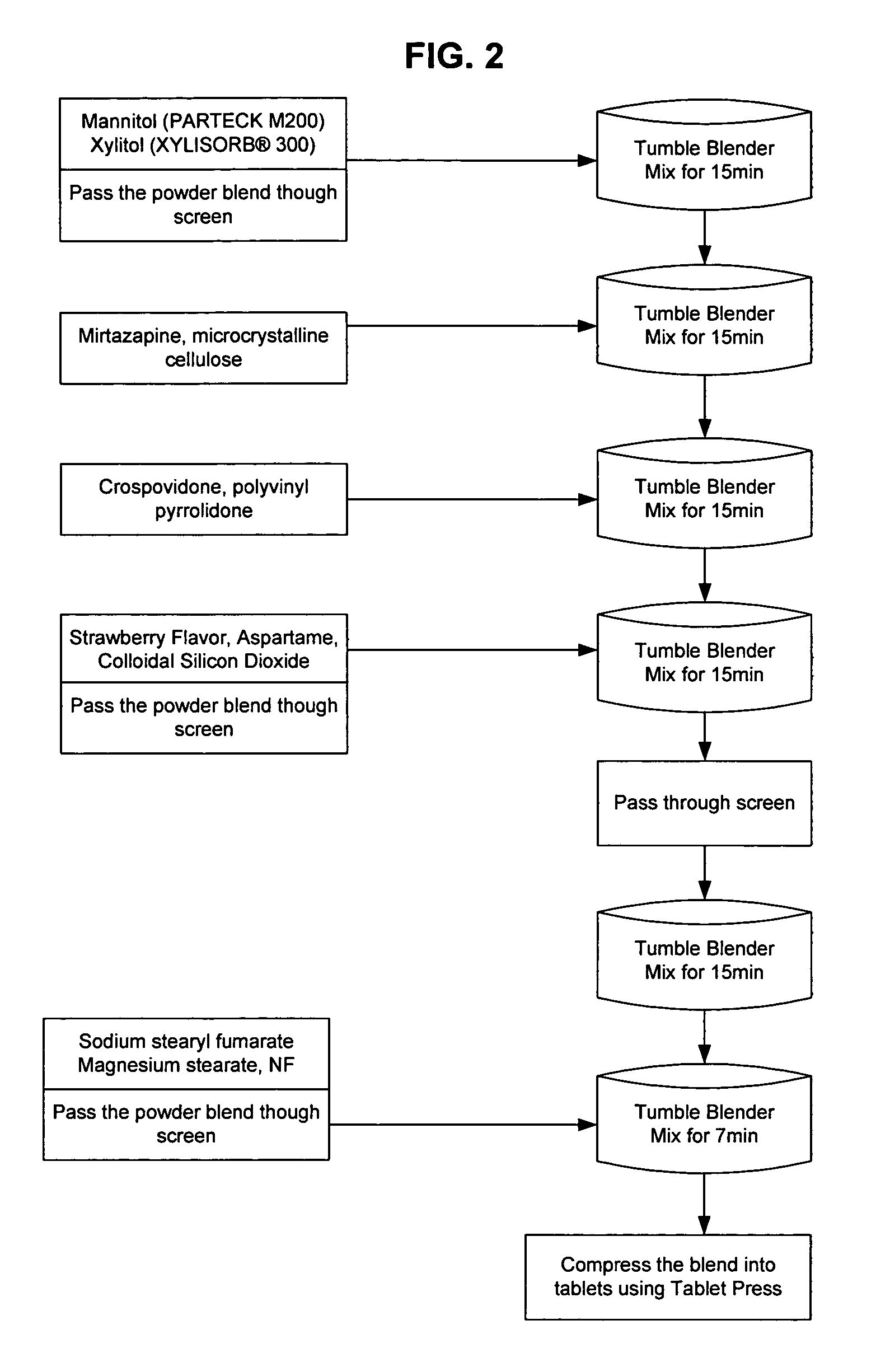
Orally disintegrating tablet formulations of mirtazapine and process for preparing the same
InactiveUS20110257159A1High mechanical strengthPleasant mouth-feelBiocideNervous disorderOrally disintegrating tabletSilicon dioxide
Silicon dioxide free orally disintegrating tablet formulations of mirtazapine or a pharmaceutically acceptable salt thereof having crospovidone and sodium stearyl fumarate and one or more pharmaceutically acceptable excipients and a process for preparing such a formulation.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Mirtazapine Solid Dosage Forms
InactiveUS20110046115A1More physically stableReduce humidityBiocideNervous disorderOrally disintegrating tabletDosage form
A non-effervescent, solid dosage form containing mirtazapine, which is used to form mirtazapine pharmaceutical tablets. The dosage form contains mirtazapine, a hydrophilic component, and at least one lubricant. In some embodiments, the dosage forms contain a salivating agent. Processes for producing mirtazapine orally disintegrating tablets are also provided.
Owner:WATSON LAB INC
Intra-oral disintegration compositions and process for producing same
InactiveCN101129341AIncrease dissolution ratePromote absorptionNervous disorderPill deliveryFiller ExcipientCurative effect
The invention relates to an orally disintegrating composition which comprises the following constituents (by weight percent): mirtazapine 1-10%, disintegrating agent 10-50%, filling agent 35-80%. The invention also relates to the process for preparing the orally disintegrating composition.
Owner:SHANGHAI INST OF PHARMA IND
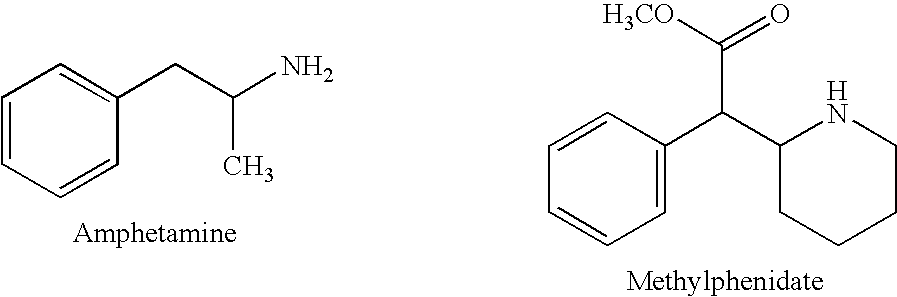
Synergistic effects of combined administration of mirtazapine and a stimulant compound
The invention discloses combination therapies and formulations of a stimulant (e.g., amphetamine) and mirtazapine and their methods of use.
Owner:KIRK RANDAL J
Mirtazapine tablet and preparation method thereof
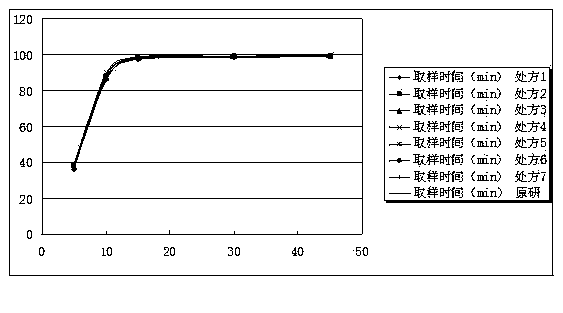
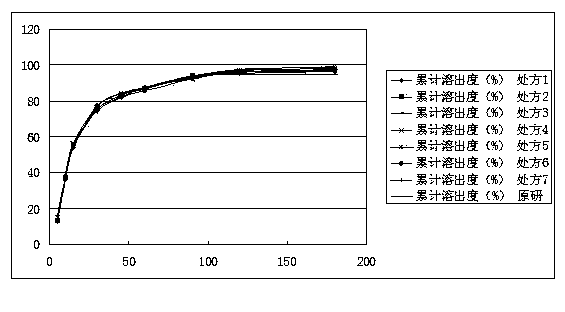
ActiveCN103520169AReduce manufacturing costQuality improvementNervous disorderPharmaceutical delivery mechanismCelluloseMagnesium stearate
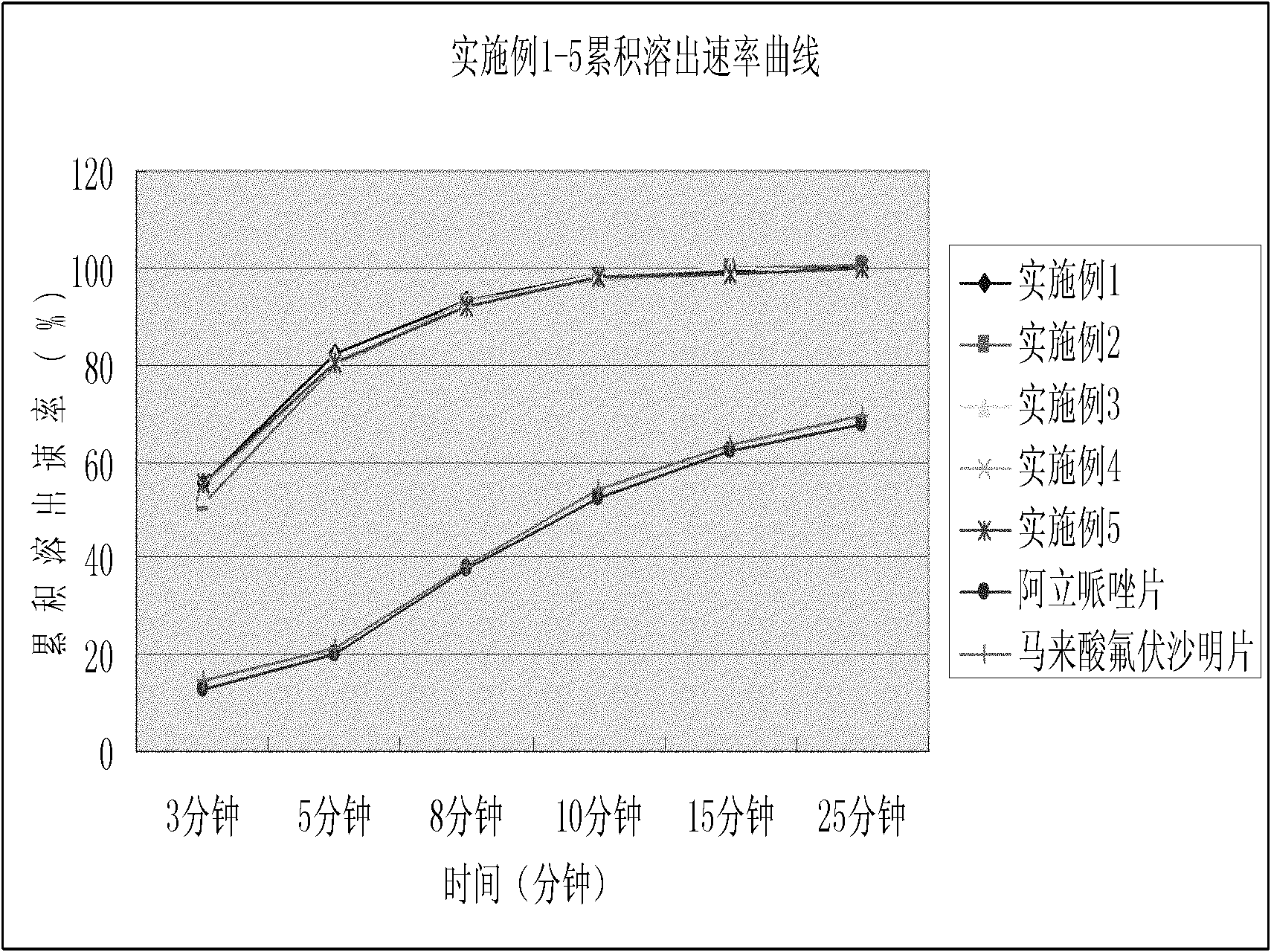
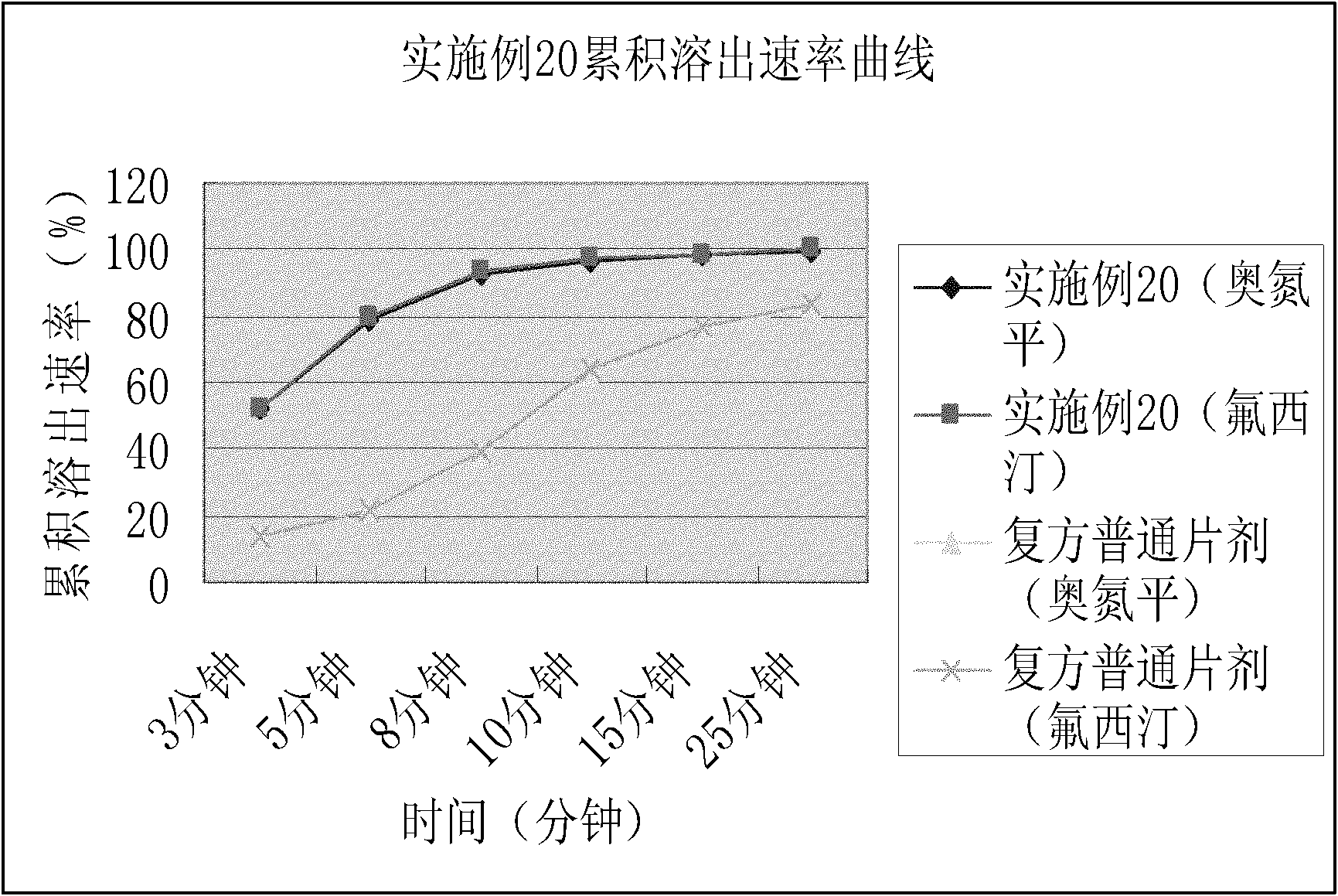
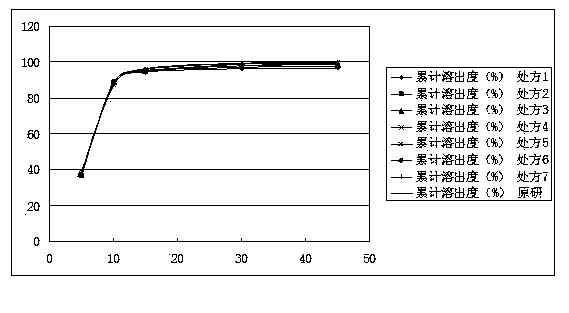
The invention relates to a mirtazapine tablet and a preparation method thereof. The mirtazapine tablet is prepared from a tablet core and a coating layer, wherein the tablet core contains 15-45mg of mirtazapine, 40-180mg of lactose, 70-200mg of starch, 8-25mg of hydroxy propyl cellulose, 0.75-3mg of magnesium stearate and 0.75-3mg of aerosol. The particle size range of the mirtazapine is 3.0-96.0 microns; the coating layer accounts for 2.0-3.0% of weight of the tablet core. The obtained mirtazapine tablet preparation disclosed by the invention is stable in quality, simple to prepare and operate, low in production cost, and applicable to industrial mass production. The mirtazapine tablet prepared by the invention simulates operation of the tablets in the stomach and intestines; an in vitro dissolution curve is determined; the mirtazapine tablet is good in consistency between batches, stable and controllable in quality, and consistent with the dissolution behaviors of an original development agent in four different mediums.
Owner:SHANDONG LUYAO PHARMA
Method for the treatment of psychic disorders
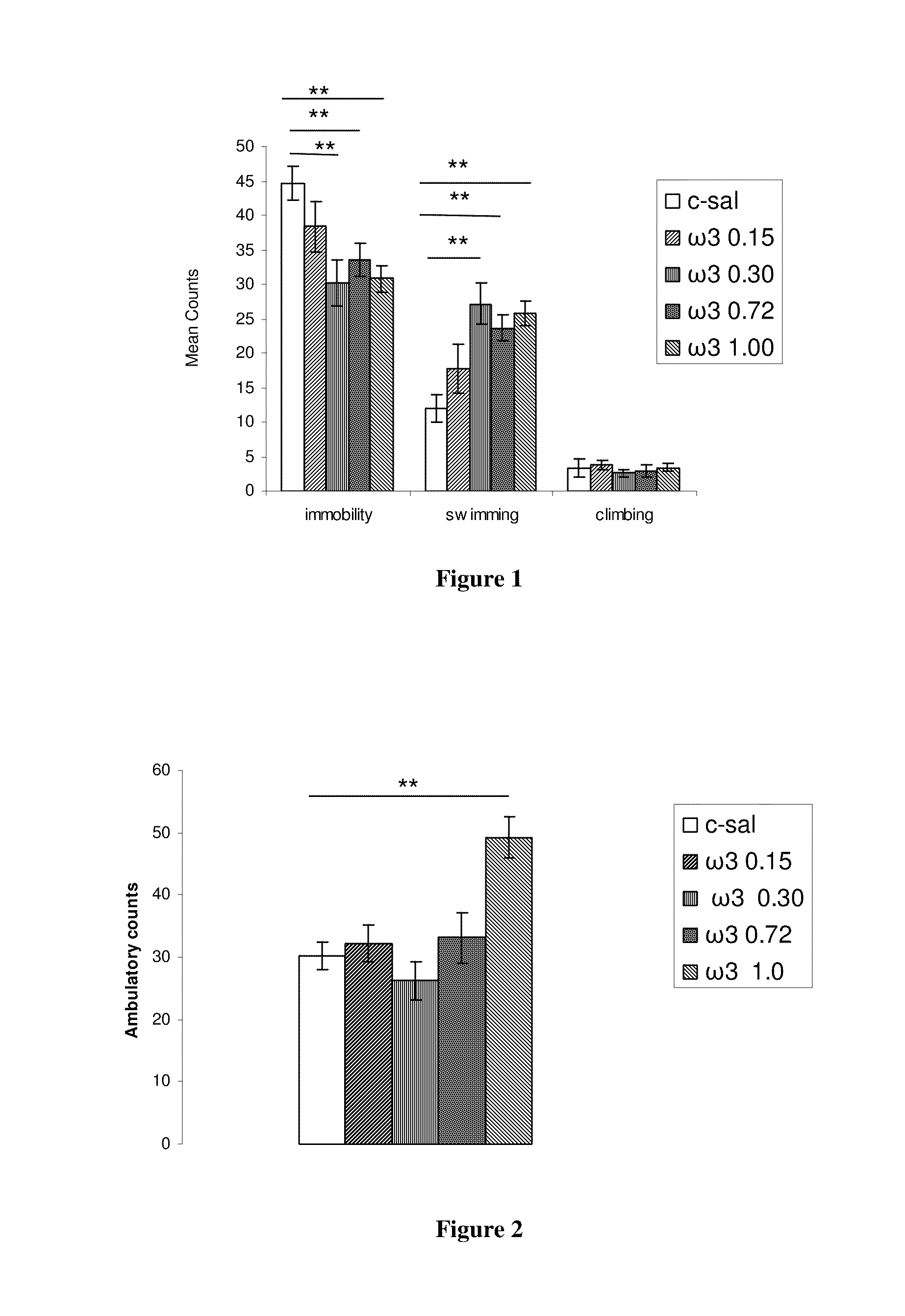
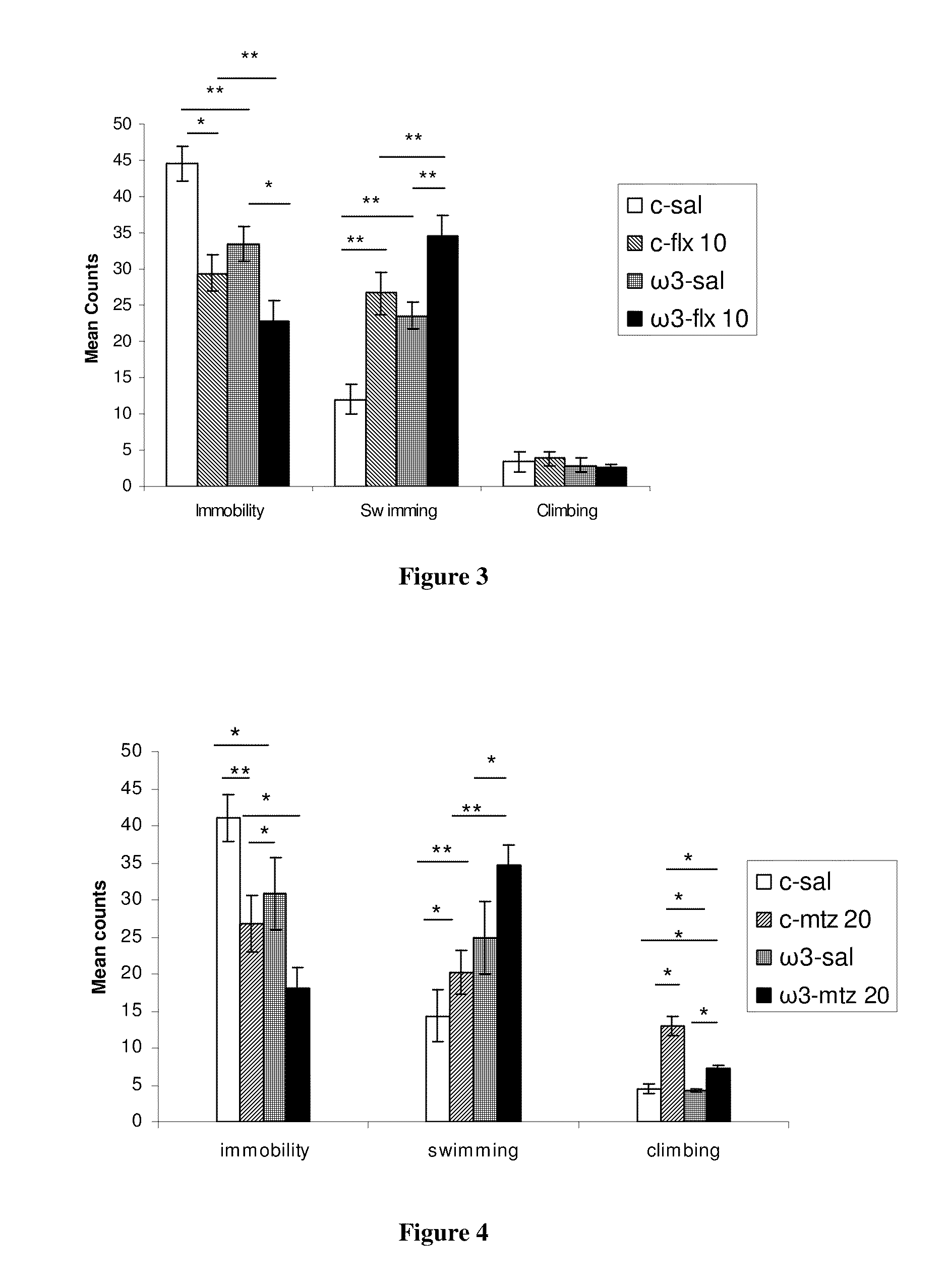
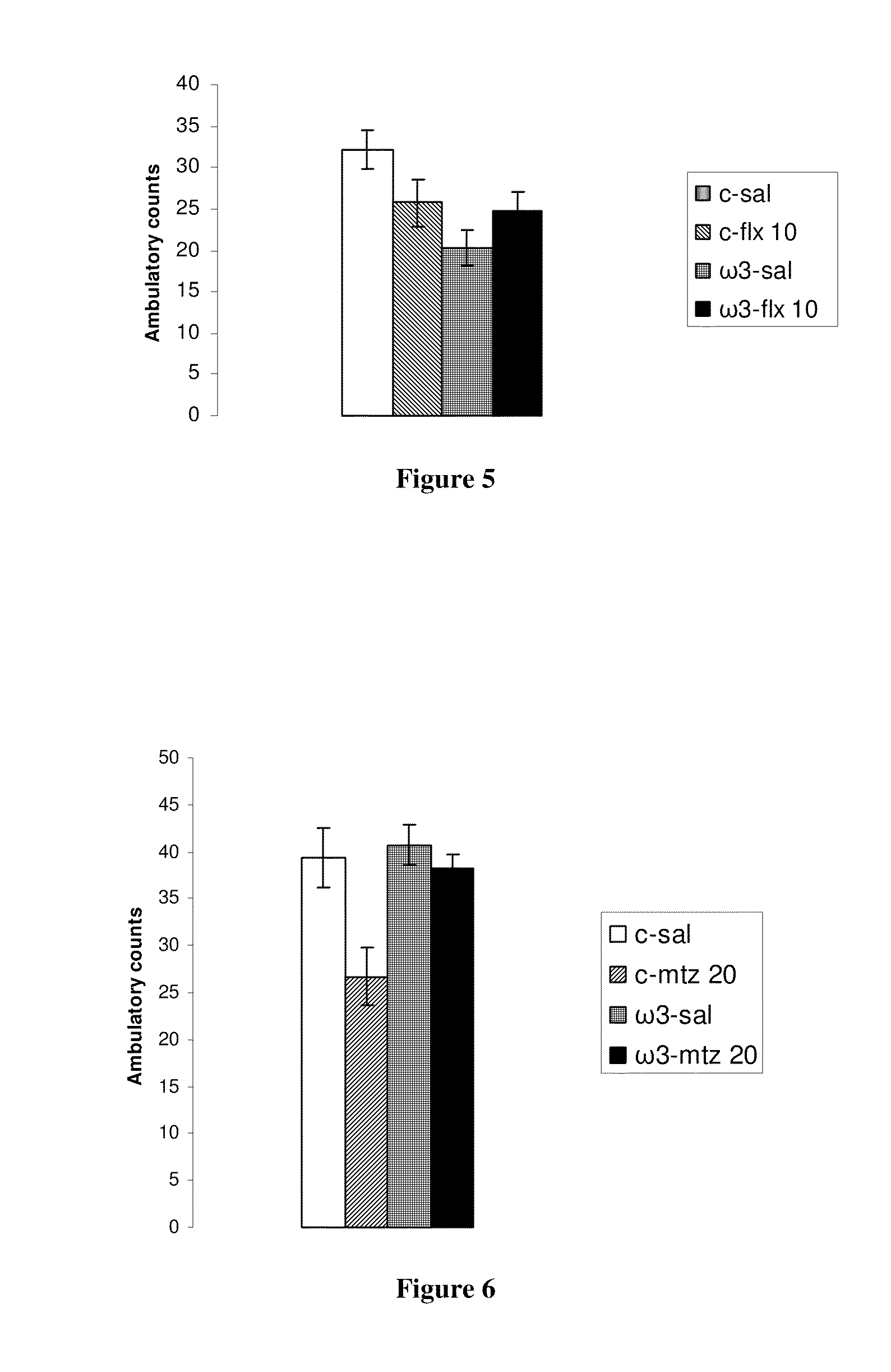
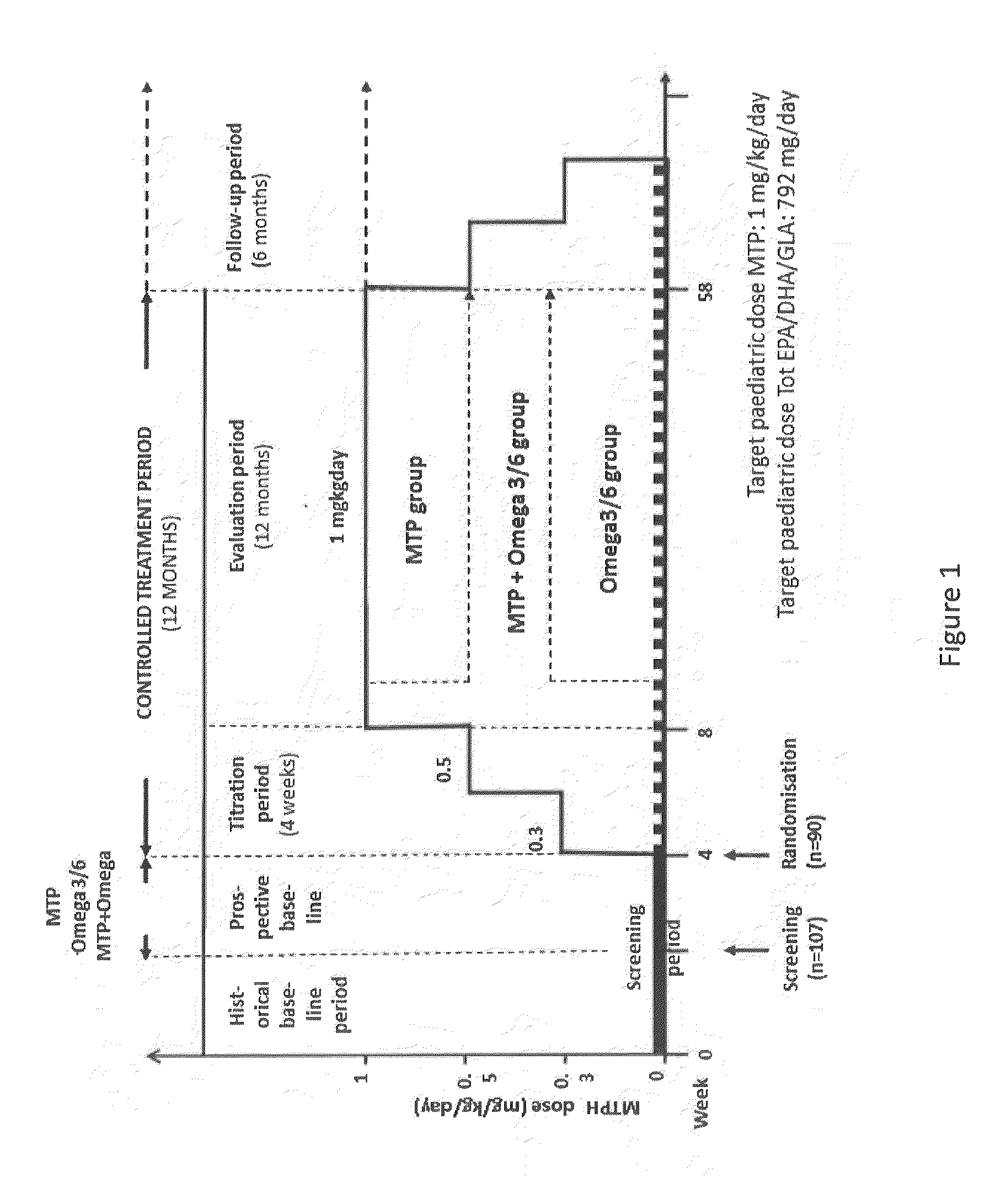
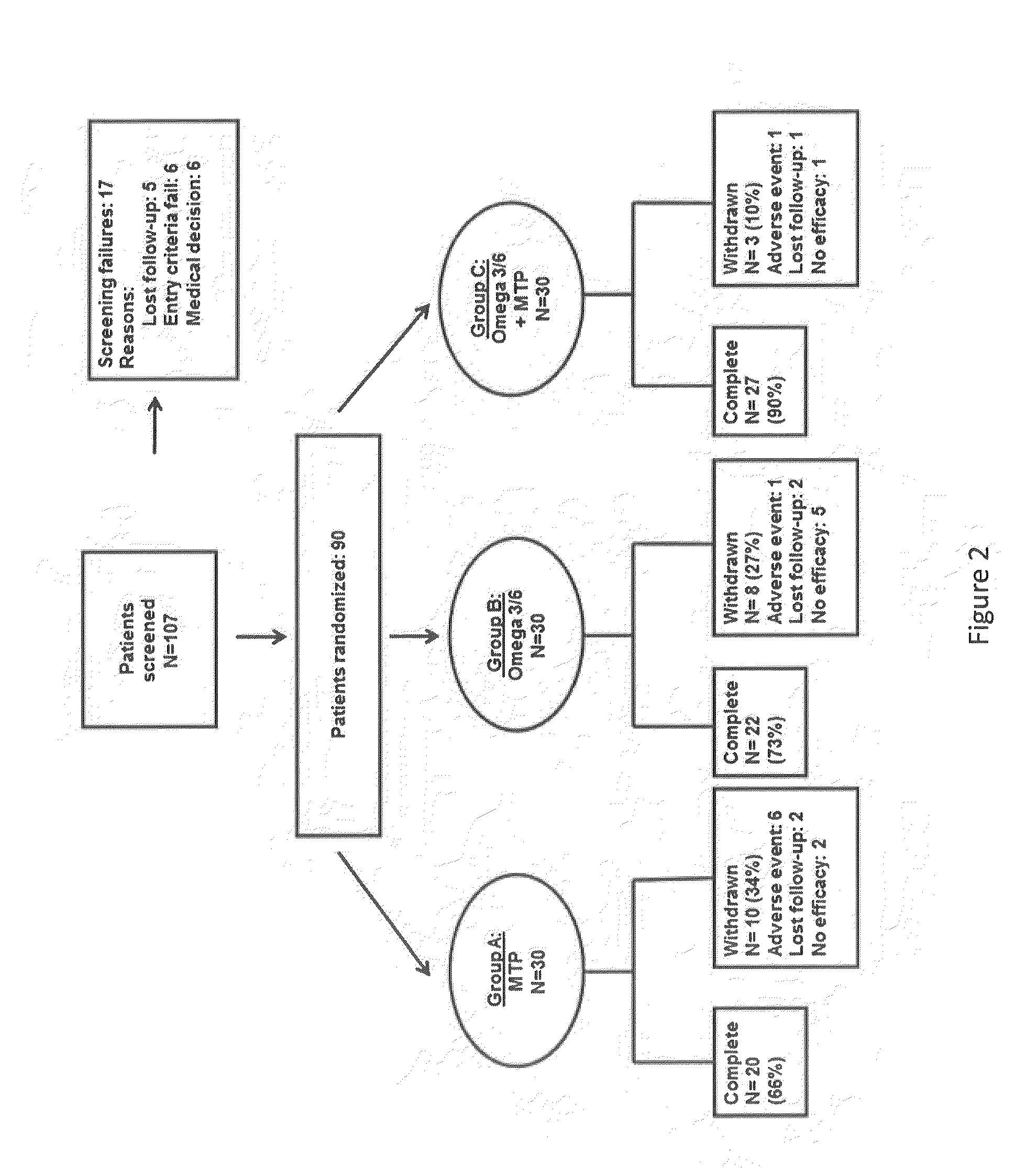
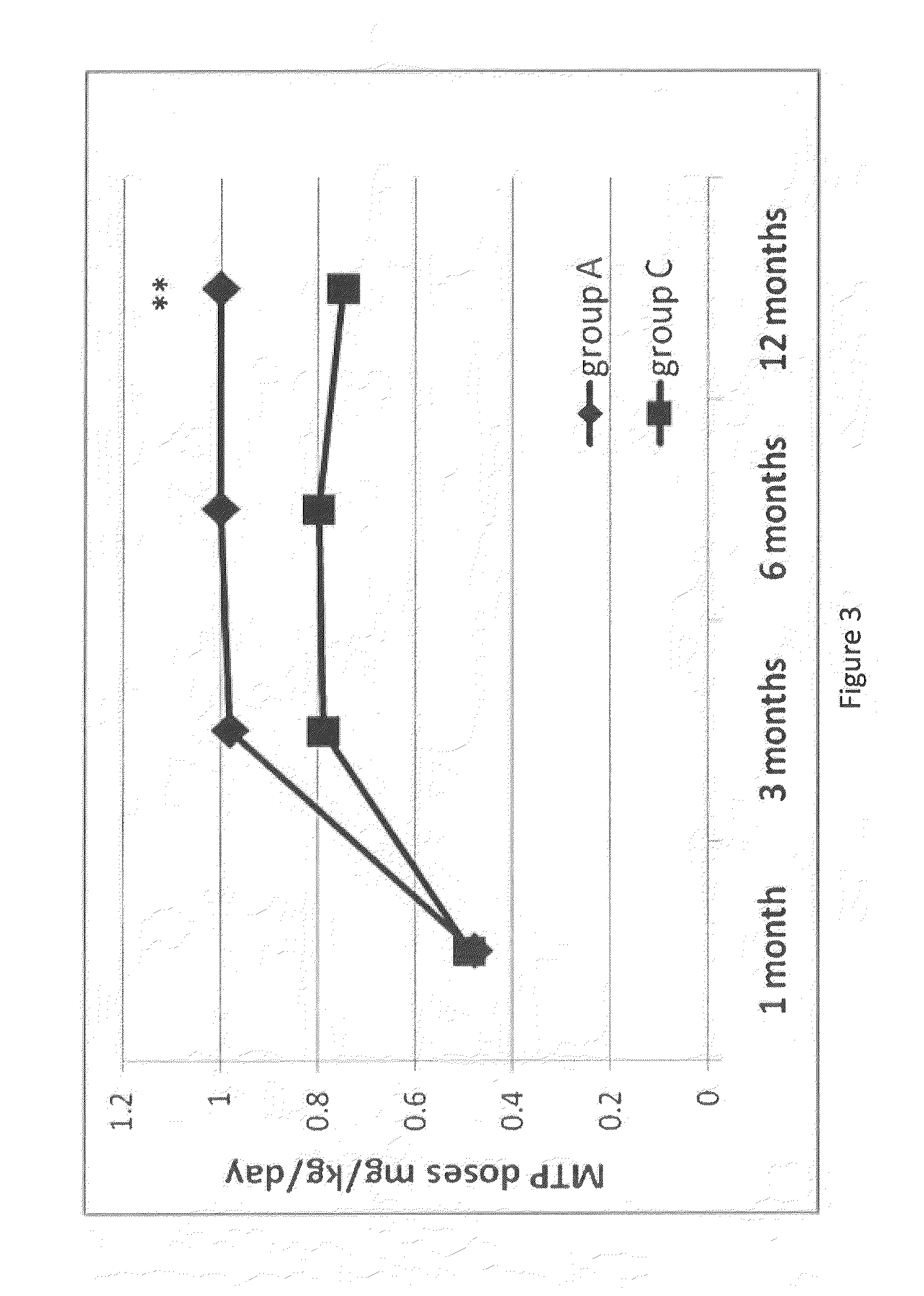
Method for the treatment of psychiatric disorders, comprising administering to a subject in need thereof an amount of fatty acids and a suboptimal dose of at least one antidepressant. Wherein the fatty acid may be omega-3 (ω3), for example the docohexaenoic acid (DHA) and the eicosapentaenoic acid (EPA). The omega-3 fatty acid may be administered orally, in amounts that may be variable, for example in amounts between 0.15 and 1.00 g / kg / day. The antidepressant may be any antidepressant, preferably the antidepressant is fluoxetine or mirtazapine in sub-optimal doses.
Owner:CONSEJO NAT DE INVESTIGACIONES CIENTIFICAS Y +1
Mirtazapine solid dosage forms
InactiveUS7838029B1Easy to manufactureImprove moisture resistanceNervous disorderOrganic chemistryOrally disintegrating tabletSolid Dose Form
A non-effervescent, solid dosage form containing mirtazapine, which is used to form mirtazapine pharmaceutical tablets. The dosage form contains mirtazapine, a hydrophilic component, and at least one lubricant. In some embodiments, the dosage forms contain a salivating agent. Processes for producing mirtazapine orally disintegrating tablets are also provided.
Owner:WATSON LAB INC
Synergistic effects of combined administration of mirtazapine and a stimulant compound
InactiveUS20060100136A1Preventing and somnolenceBiocideNervous disorderStimulantCompound (substance)
The invention discloses combination therapies and formulations of a stimulant (e.g., amphetamine) and mirtazapine and their methods of use.
Owner:SHIRE PLC
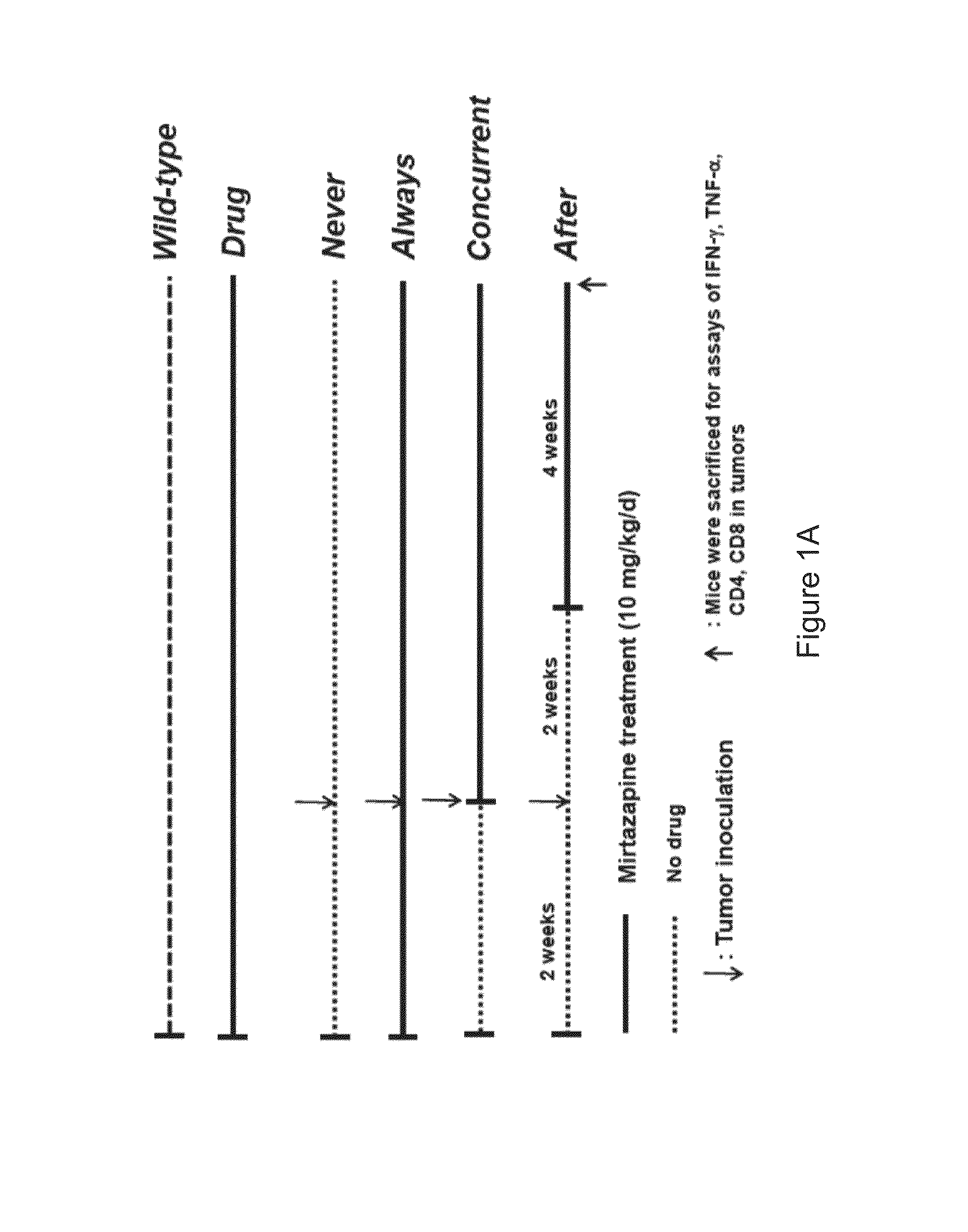
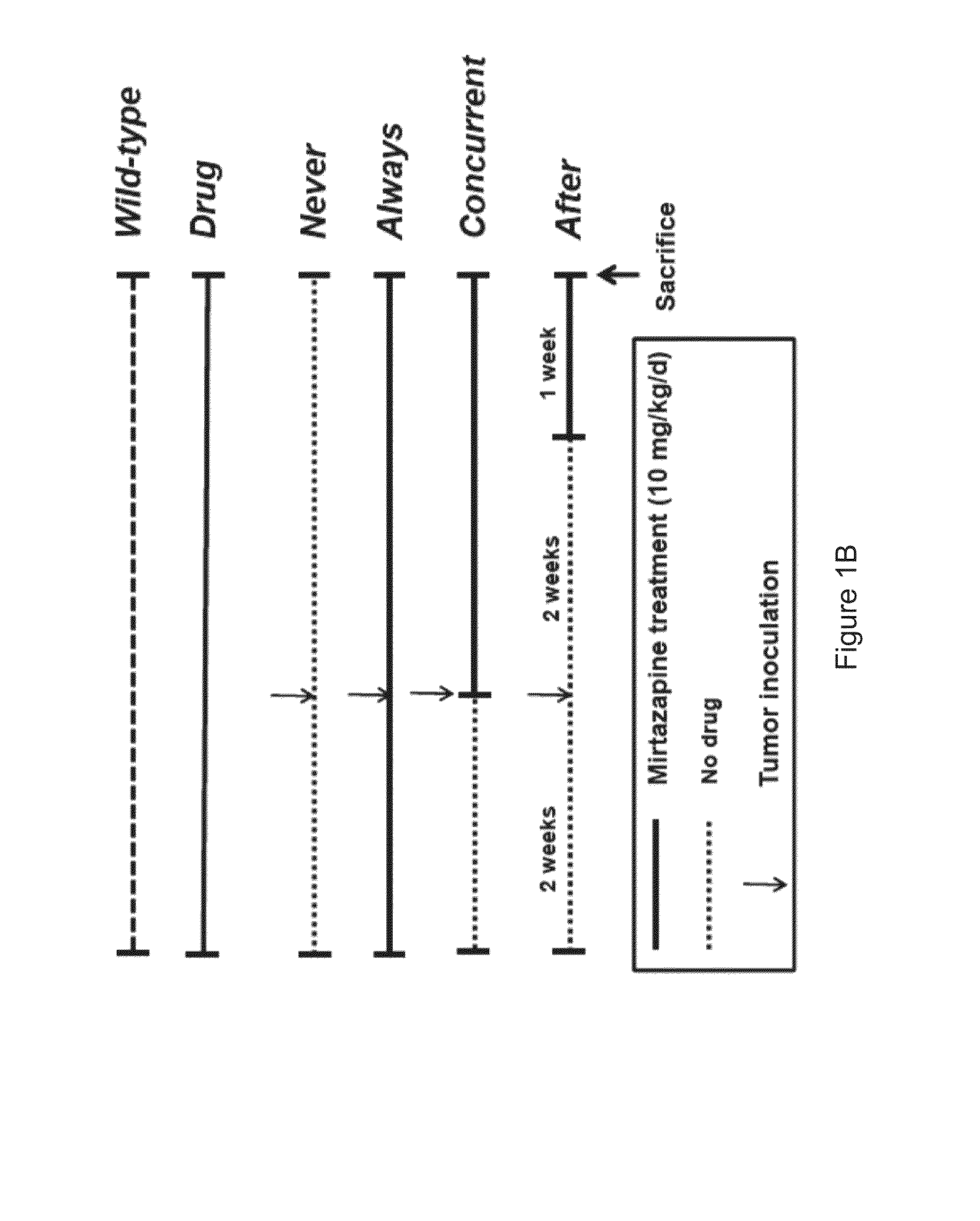
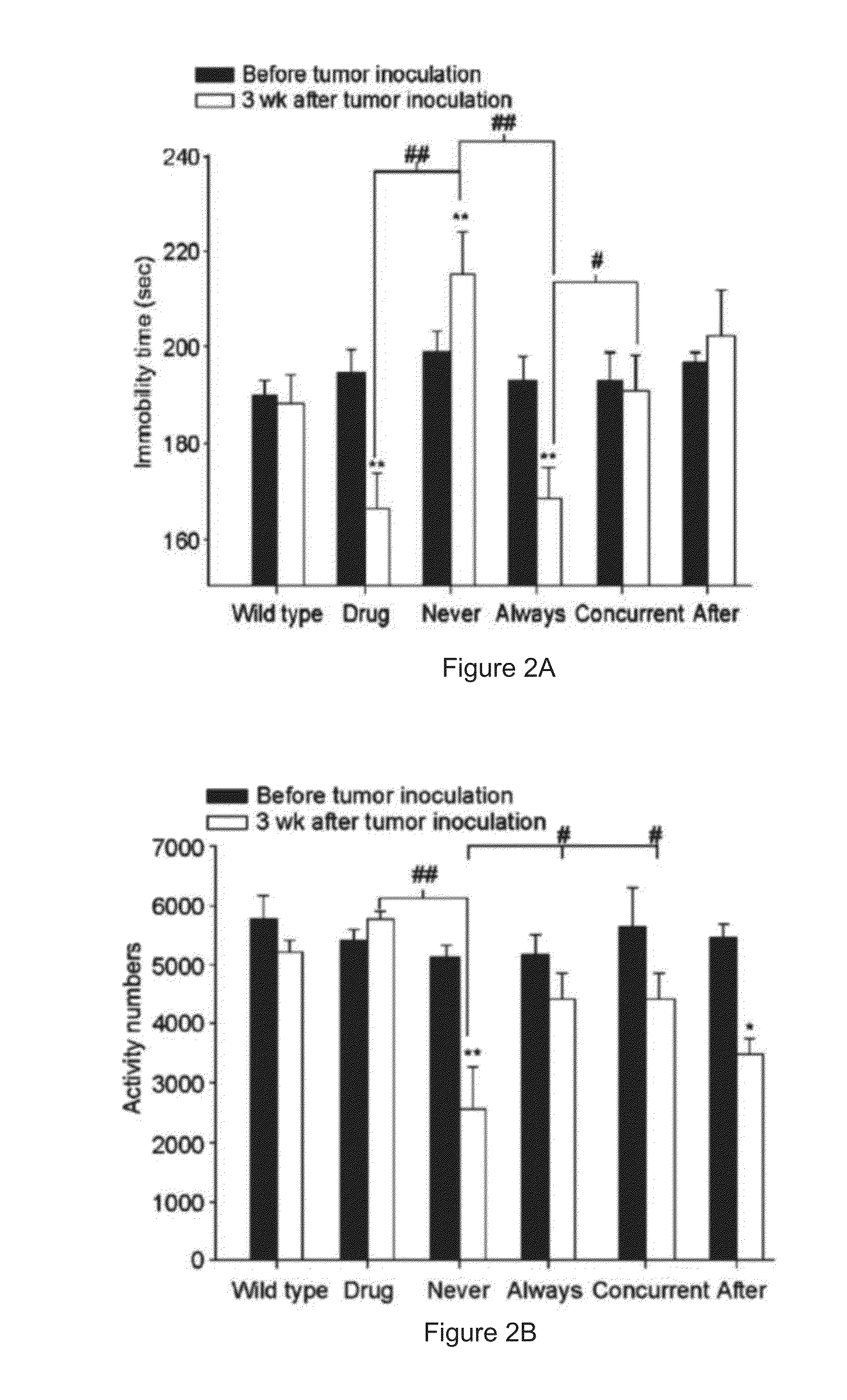
Method of using an antidepressant for increasing immunity of a subject and treating cancer
InactiveUS20140271727A1Increase volumeIncreases concentration of serotoninPharmaceutical non-active ingredientsHeterocyclic compound active ingredientsSerotoninTumor microenvironment
A method of increasing immunity of a subject by administering an antidepressant to the subject is disclosed in the present invention. Preferably, the antidepressant is mirtazapine. And further, another object of the present invention is related to a method of administering the abovementioned antidepressant to the subject for treating cancer. The present invention tries to find the mechanism of the tumor growth inhibition by mirtazapine, and it may be due to the alteration of the tumor microenvironment, which involves the activation of the immune response and the recovery of serotonin level.
Owner:NATIONAL YANG MING UNIVERSITY
Method for detecting concentration of antidepressant drugs in serum by using ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting the concentration of antidepressant drugs in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antidepressant drugs comprise bupropion, agomelatine, hydroxybupropion, nortriptyline, o-desmethylvenlafaxine, mianserin, mirtazapine, venlafaxine, amitriptyline, doxepin, norfluoxetine hydrochloride, duloxetine, fluoxetine, fluvoxamine, citalopram, paroxetine, trazodone and vortioxetine. After a serum sample is pretreated, a to-be-detected substance is separated from a serum matrix by utilizing ultra-highperformance liquid chromatography, a calibration curve is established by utilizing a mass spectrum isotope internal standard quantitative method and taking a concentration ratio of the standard substance to an internal standard substance as an X axis and a peak area ratio of the standard substance to the internal standard substance as a Y axis, and the content of the drugs in serum is calculated.The method is high in sensitivity, high in specificity, accurate and simple in pretreatment process, separation and detection can be completed within 4.5 min, and the accuracy degree and precision basically meet the requirements.
Owner:南京品生医学检验实验室有限公司
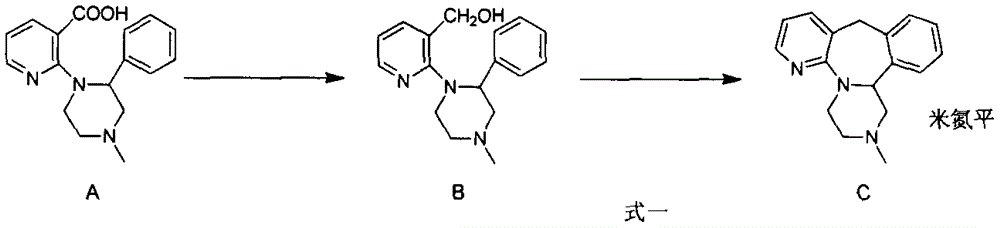
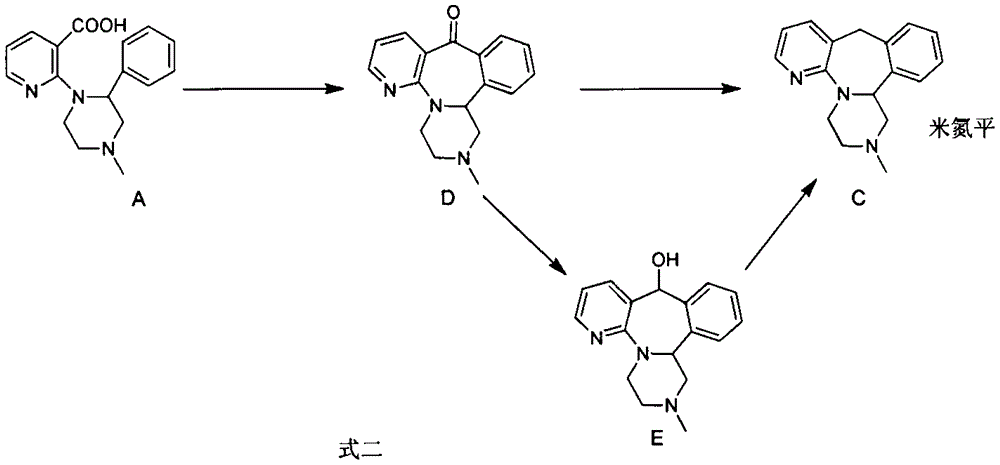
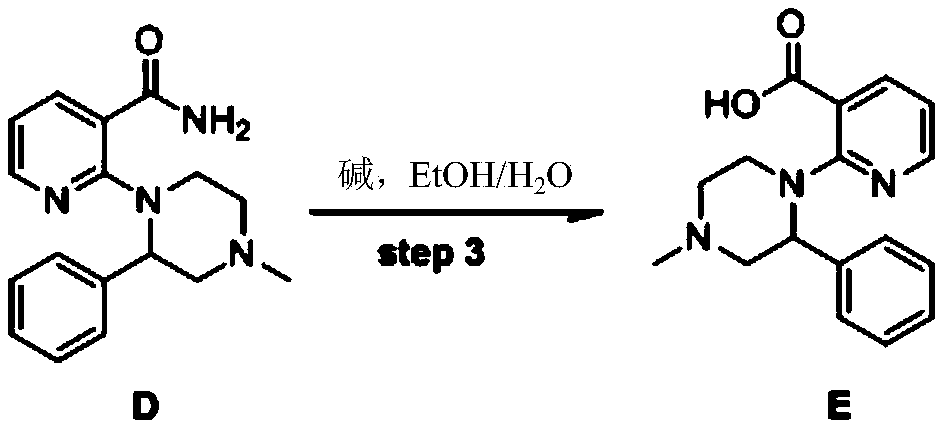
Method for preparing antidepressant mirtazapine
InactiveCN104356133AThe synthesis method is simplePractical synthetic methodOrganic chemistryKetoneCarboxylic acid
The invention discloses a method for preparing mirtazapine by using a ketone intermediate D to directly obtain the mirtazapine. The method comprises the following steps: carrying out a Friedel-Craft reaction on a carboxylic acid intermediate A under catalysis of an acylation reagent and Lewis acid to obtain ketone intermediate D, processing the ketone intermediate D to obtain a coarse product after the reaction, and implementing next reaction without further purification; through a Wolff-Kishner-Huangminglong reaction, reducing the ketone intermediate D into a target product, namely mirtazapine, post-processing the product and extracting coarse product from an aqueous phase, and recrystallizing through a mixed solvent of two or more components to obtain a high-purity product. The method disclosed by the invention is simple and convenient, safe and high in yield.
Owner:NANJING UNIV OF TECH
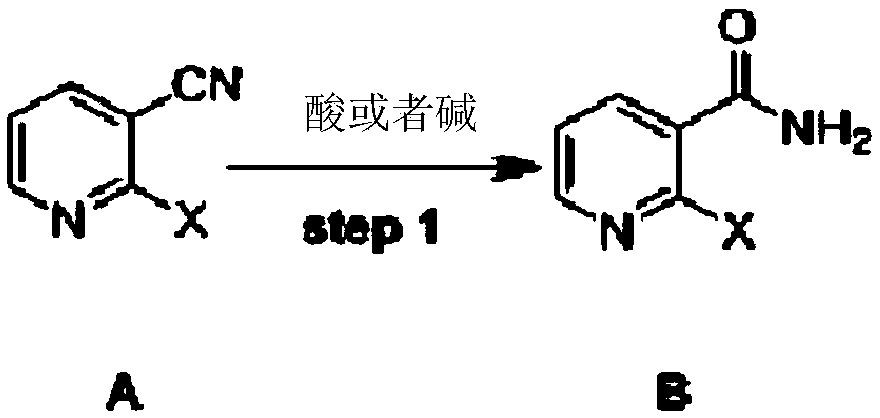
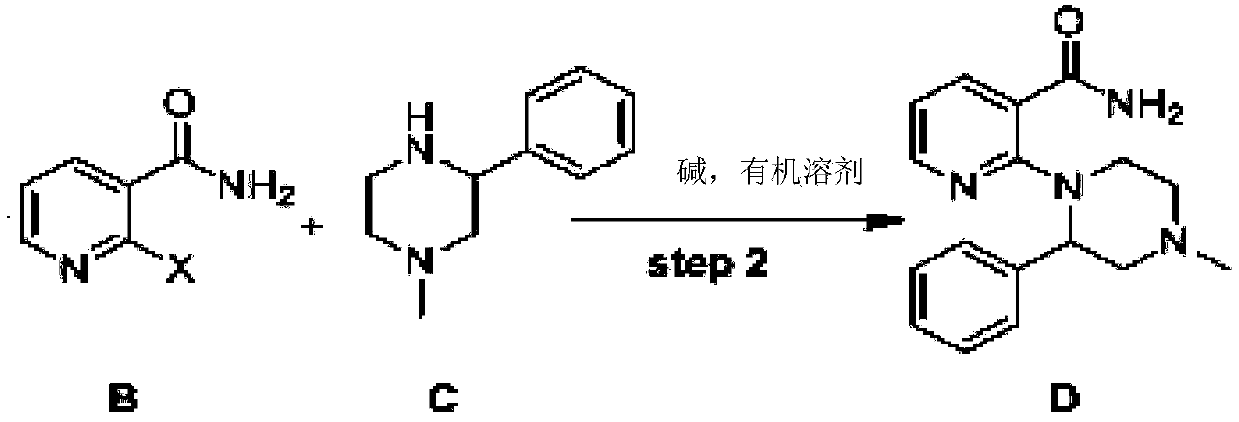
Method for synthesizing mirtazapine
The invention discloses a method for synthesizing mirtazapine. According to the method, 2-halogenated nicotinonitrile is used as an initial compound, and 2-chloronicotinamide, 2-(4-methyl-2phenyl-1-piperazinyl)nicotinamide, 2-(4-methyl-2phenyl-1-piperazinyl)nicotinic acid, 1-(3-hydroxymethylpyridyl-2-)-4-methyl-2-phenylpiperazine and other intermediate products are sequentially synthesized to prepare the mirtazapine. Against the defects in a current mirtazapine synthesizing method, the process is improved, a new synthesizing route is designed, and a preparation method which is economical and is easy for practical operation is provided to mirtazapine synthesis. The method is suitable for large-scale industrial production.
Owner:BEIJING MEDISAN TECH +2
Mirtazapine tablet and preparation method thereof
InactiveCN106943368ALow incidence of adverse reactionsRelease stabilityNervous disorderInorganic non-active ingredientsDrug release rateSide effect
The invention provides a mirtazapine tablet and a preparation method thereof. The mirtazapine tablet comprises a core and a coating layer. The core comprises 20 to 45 parts of mirtazapine, 120 to 240 parts of lactose, 40 to 80 parts of sodium carboxymethyl starch, 30 to 60 parts of microcrystalline cellulose, 15 to 30 parts of low-substituted hydroxypropylcellulose, 1 to 3 parts of magnesium stearate and 1 to 3 parts of fine powder silica gel. The coating layer is a polymer coating layer containing titanium dioxide and polyethylene glycol. The preparation method comprises 1) sieving the raw and auxiliary materials of the tablet core, 2) carrying out wet granulation on the core raw materials to obtain the core, and 3) coating the tablet core to obtain the mirtazapine tablet. The dissolution of the tablet has particle size dependence, the drug release rate is stable, and side effects caused by fluctuation of the drug release rate can be avoided.
Owner:HUAYI PHARMA ANHUI CO LTD
Method and composition for treating eating disorders
PendingUS20200046722A1Increase volumePrevent degradationHydroxy compound active ingredientsDigestive systemPhysiologyPharmacology
Method and composition for treating eating disorders in humans and veterinary animals by administering a composition including: (i) mirtazapine, (ii) a cannabis compound and (iii) a fat-soluble vitamin.
Owner:INDIA GLOBALIZATION CAPITAL
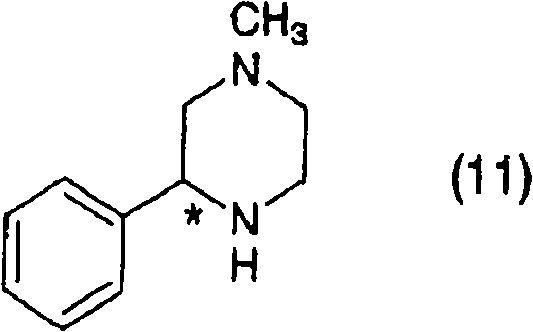
Process for producing optically active piperazine compound
InactiveCN101312955ACarbamic acid derivatives preparationOrganic compound preparationCompound aPhenylpiperazine
A process for producing optically active 1-methyl-3-phenylpiperazine, which is represented by the formula (11), or a salt thereof. It comprises the following steps (1) to (4) or comprises the following steps (5) to (7) and the step (4). Also provided is a process for producing optically active mirtazapine via that process.
Owner:SUMITOMO CHEM CO LTD
Novel omega-3 and omega-6 fatty acid compositions and uses thereof
InactiveUS20130295179A1Preventing functionsReducing secondary adverse eventsHeavy metal active ingredientsBiocideSertralineStimulant
Owner:TERREAUX CHRISTIAN +3
Mirtazapine-containing transdermally-absorbable skin-adhesive preparation
InactiveUS20150148758A1Inhibit expressionEasy to managePretreated surfacesAdhesive dressingsOrganic acidTransdermal patch
The purpose is to provide a mirtazapine-containing transdermal patch capable of suppressing deposition of a crystalline component derived from mirtazapine, deterioration in sense of use, and deterioration in adhesiveness to the skin. The transdermal patch contains a support, a drug-containing layer and a release liner, and the drug-containing layer contains mirtazapine and an organic acid.
Owner:YUTOKU PHARMA IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com