Patents
Literature
56 results about "PSYCHIC DISORDER" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Psychotic disorders are severe mental disorders that cause abnormal thinking and perceptions. People with psychoses lose touch with reality. Two of the main symptoms are delusions and hallucinations.
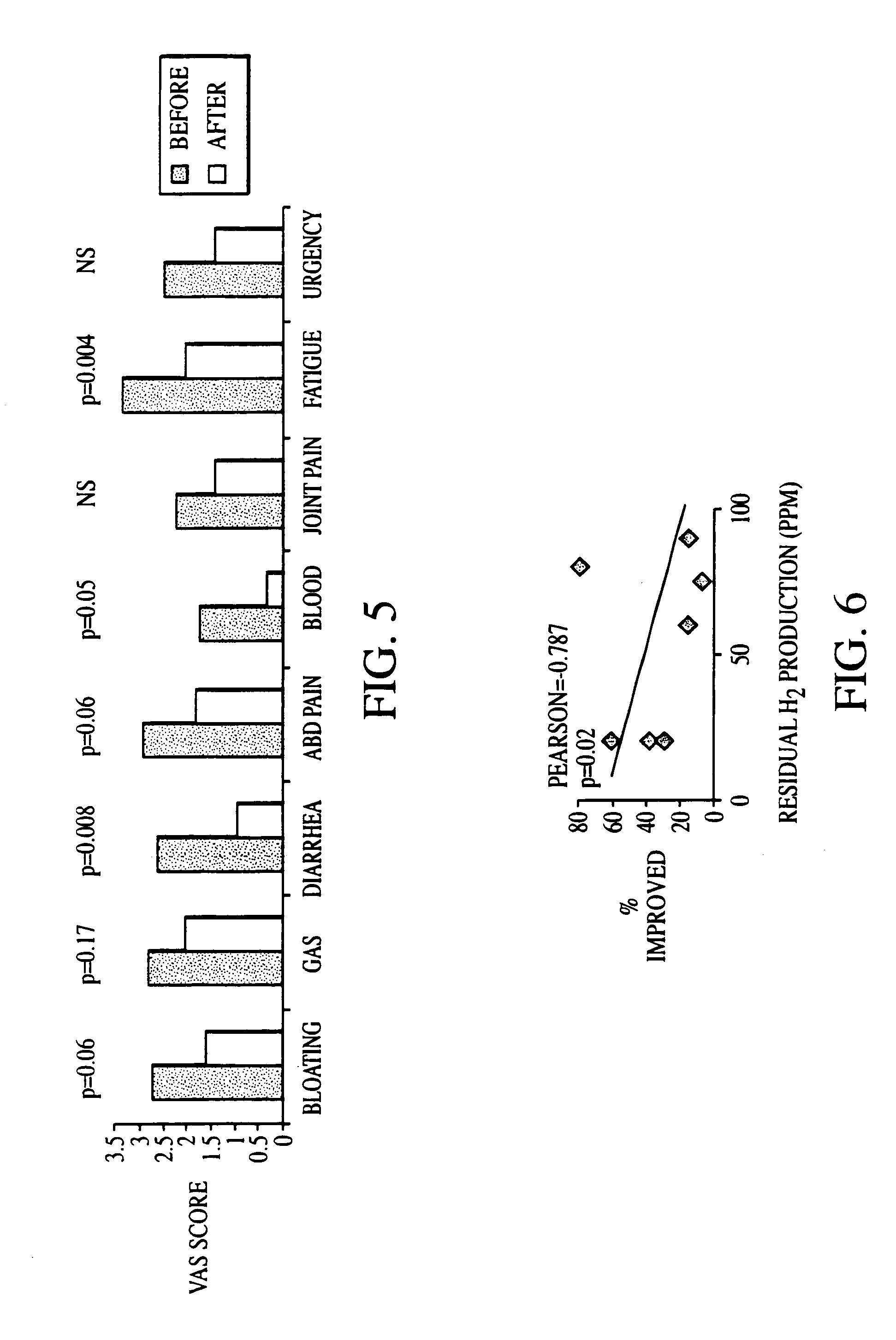
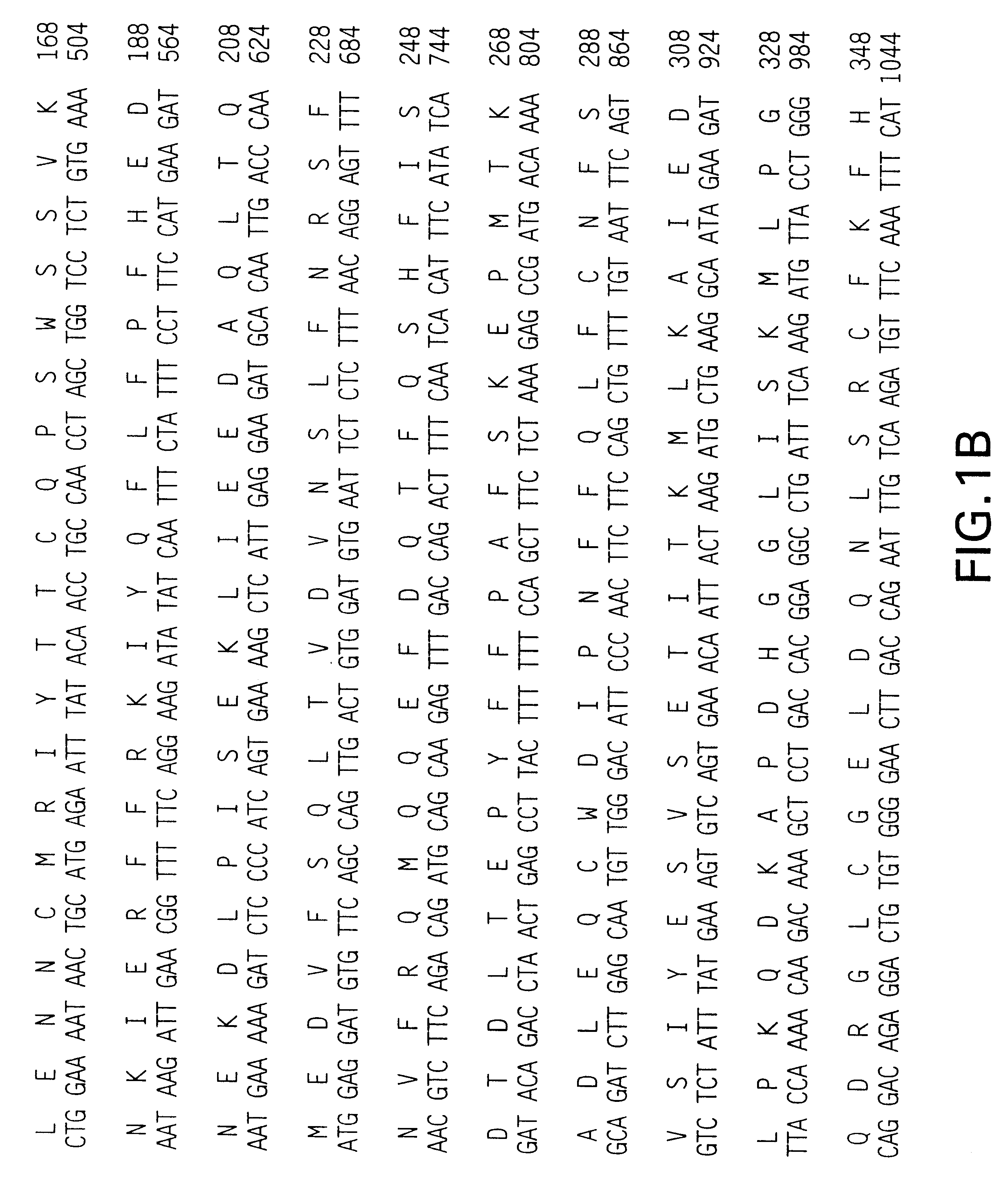
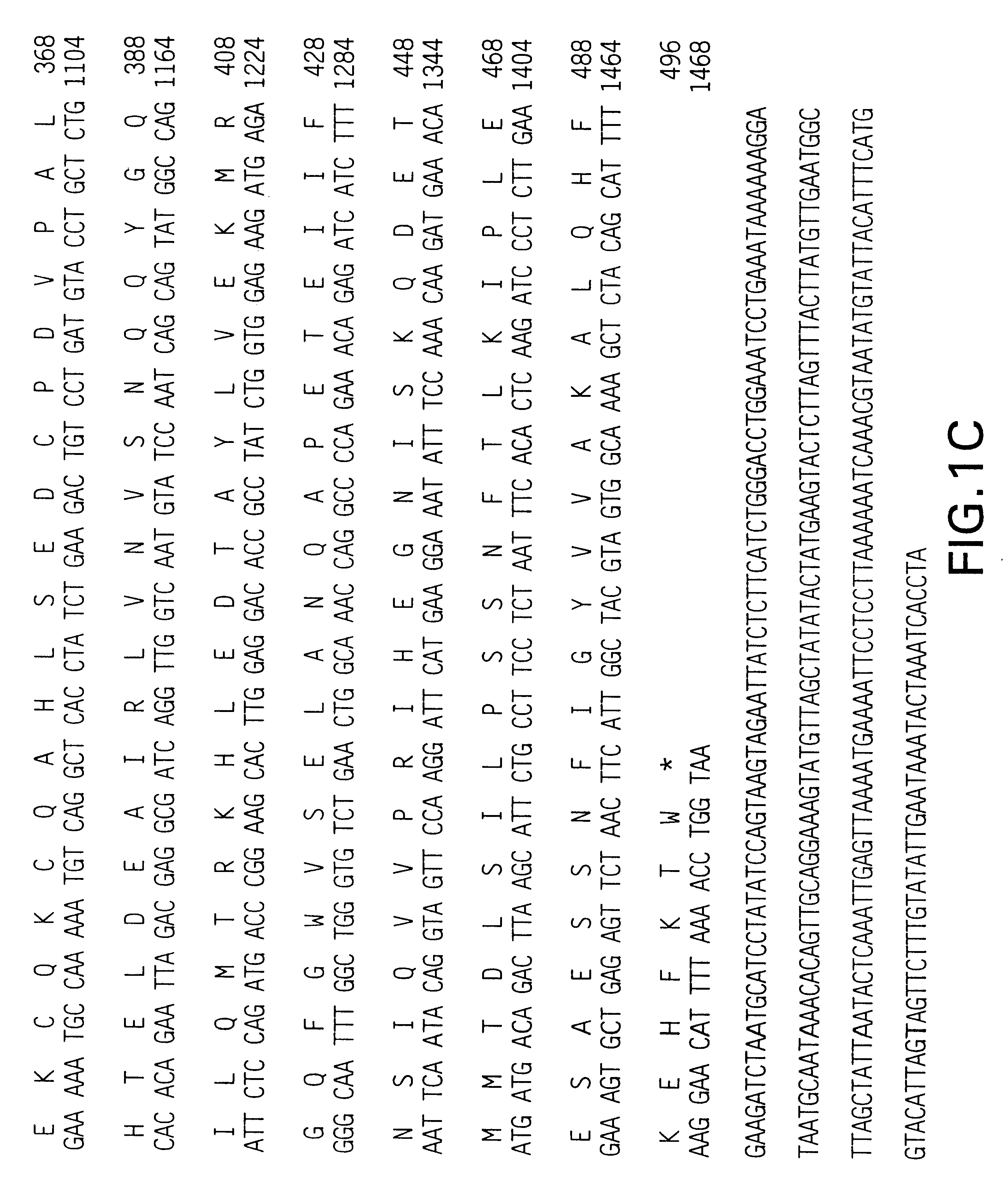
Methods of diagnosing and treating small intestinal bacterial overgrowth (SIBO) and SIBO-related conditions
InactiveUS7048906B2Prevent further growthReduced magnitudeAntibacterial agentsCompounds screening/testingImmunologic disordersPhysiology
Disclosed is a method of treating small intestinal bacterial overgrowth (SIBO) or a SIBO-caused condition in a human subject. SIBO-caused conditions include irritable bowel syndrome, fibromyalgia, chronic pelvic pain syndrome, chronic fatigue syndrome, depression, impaired mentation, impaired memory, halitosis, tinnitus, sugar craving, autism, attention deficit / hyperactivity disorder, drug sensitivity, an autoimmune disease, and Crohn's disease. Also disclosed are a method of screening for the abnormally likely presence of SIBO in a human subject and a method of detecting SIBO in a human subject. A method of determining the relative severity of SIBO or a SIBO-caused condition in a human subject, in whom small intestinal bacterial overgrowth (SIBO) has been detected, is also disclosed.
Owner:CEDARS SINAI MEDICAL CENT
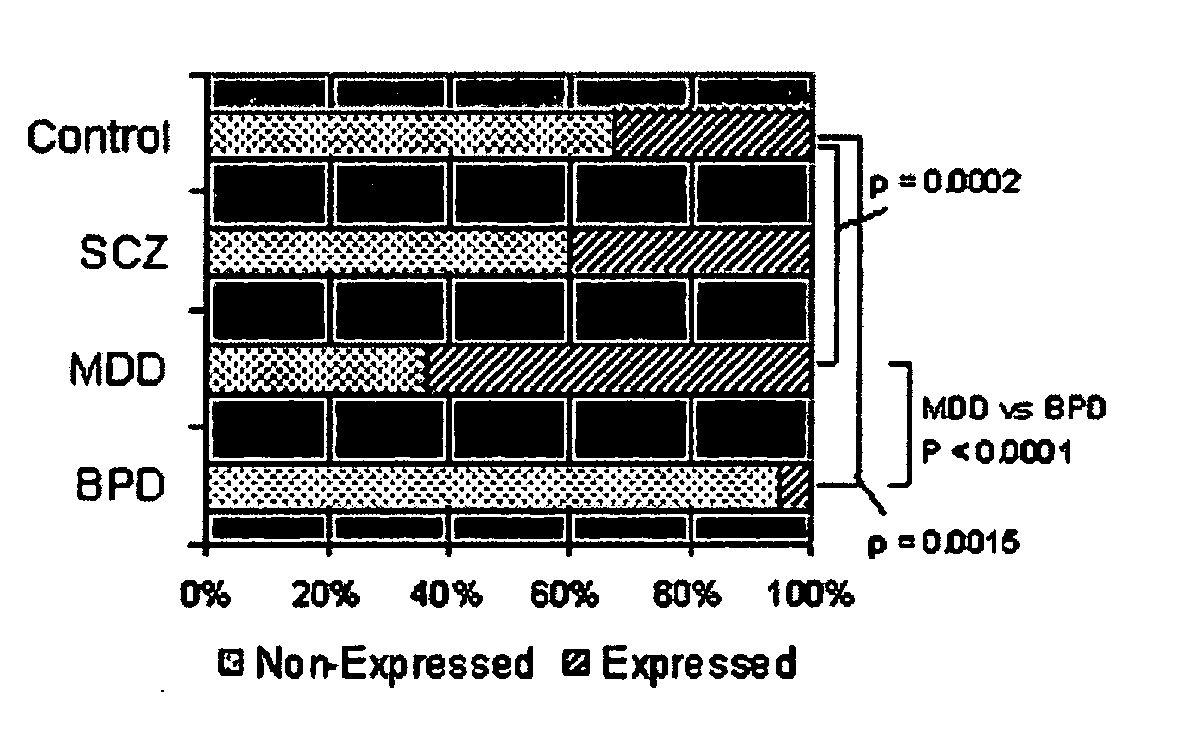
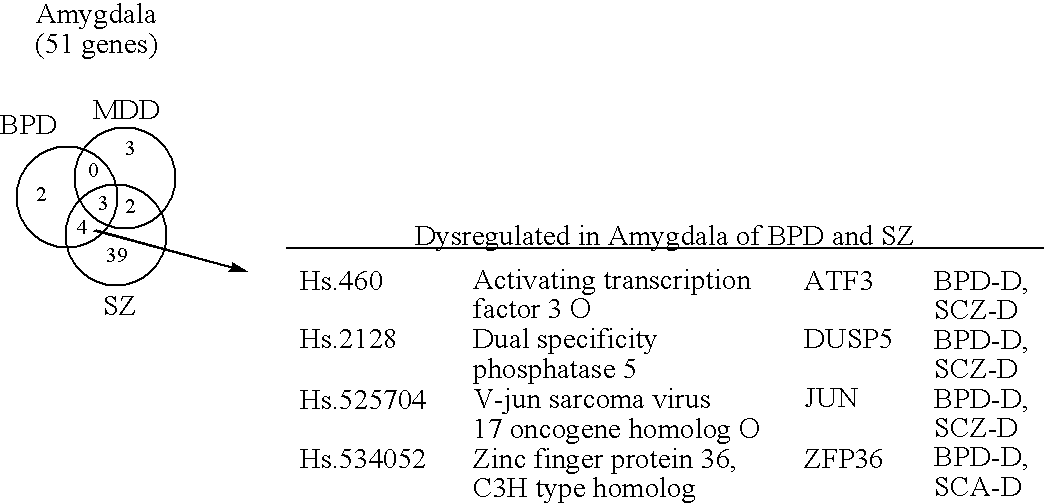
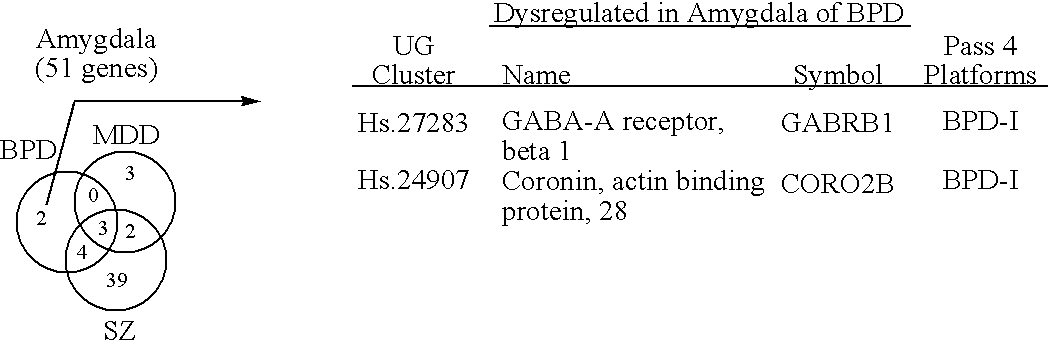
Compositions and methods for diagnosing and treating neuropsychiatric disorders
ActiveUS20060257903A1High expressionReduce expressionCompound screeningNervous disorderBipolar mood disorderPSYCHIC DISORDER
The present invention provides methods for diagnosing mental disorders (e.g., psychotic disorders such as schizophrenia and mood disorders such as major depression disorder and bipolar disorder). The invention also provides methods of identifying modulators of such mental disorders as well as methods of using these modulators to treat patients suffering from such mental disorders.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
AMPA Receptor Antagonists for Epilepsy, Mental Disorders or Deficits in Sensory Organ
The invention provides methods for treating epilepsy, mental disorders and / or deficits in sensory organ by administering to patients therapeutically effective amounts of AMPA receptor antagonists in combination with one or more other active ingredients useful for treating epilepsy, mental disorders and / or deficits in sensory organ. The invention also provides pharmaceutical combinations, kits, and pharmaceutical compositions comprising therapeutically effective amounts of AMPA receptor antagonists, and optionally, one or more other active ingredients that are useful for treating epilepsy, mental disorders and / or deficits in sensory organ.
Owner:EISIA R&D MANAGEMENT CO LTD
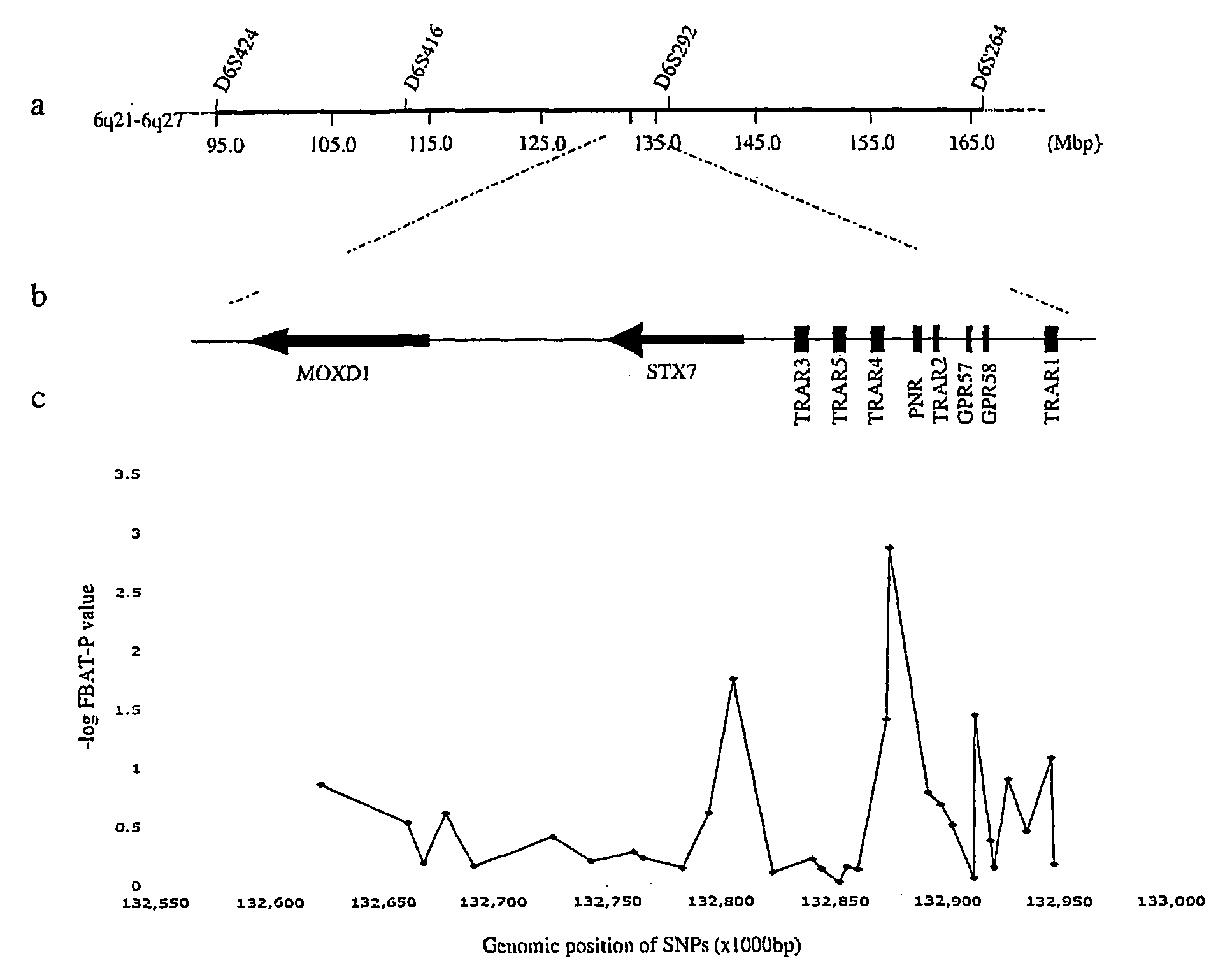
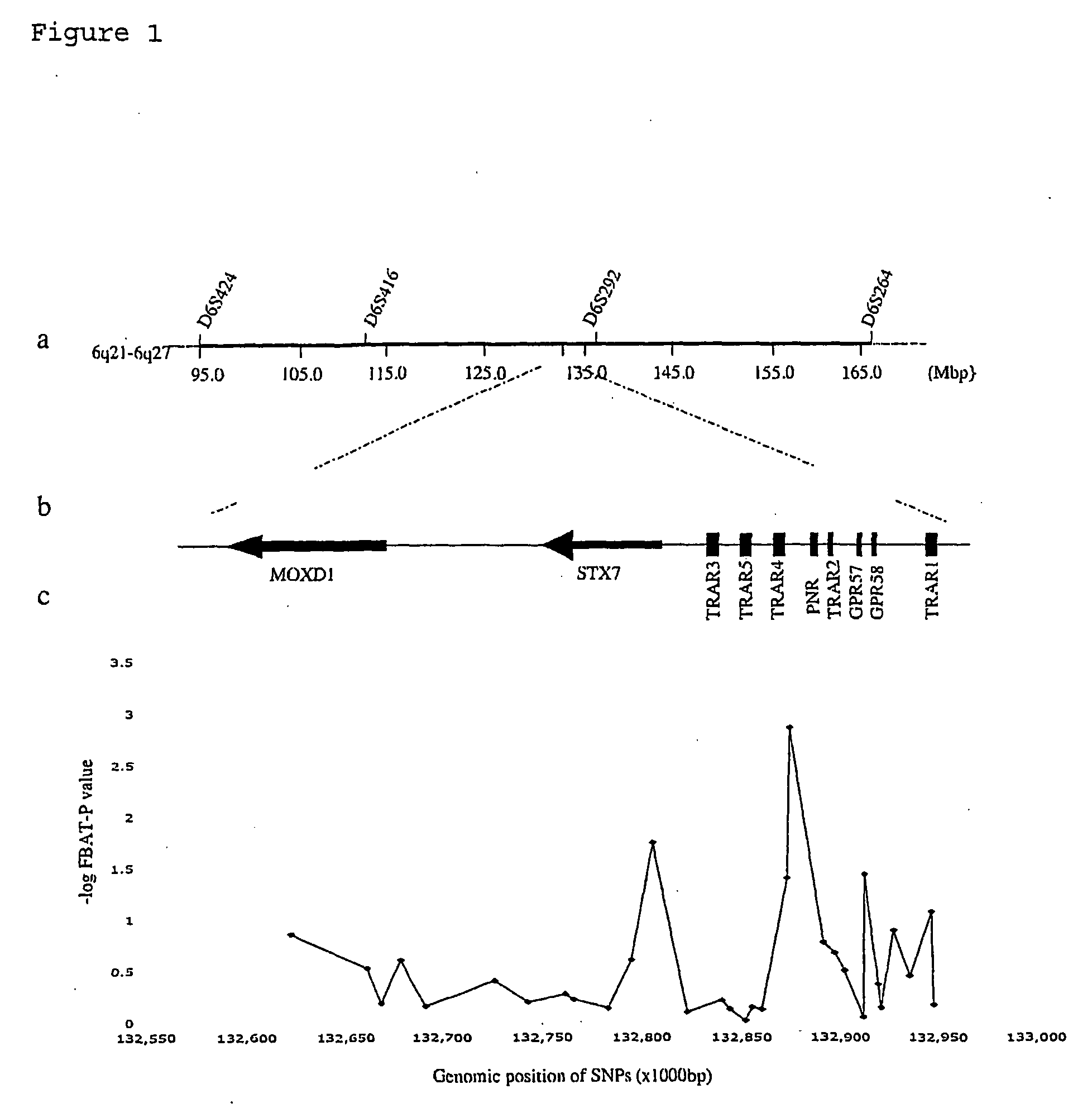
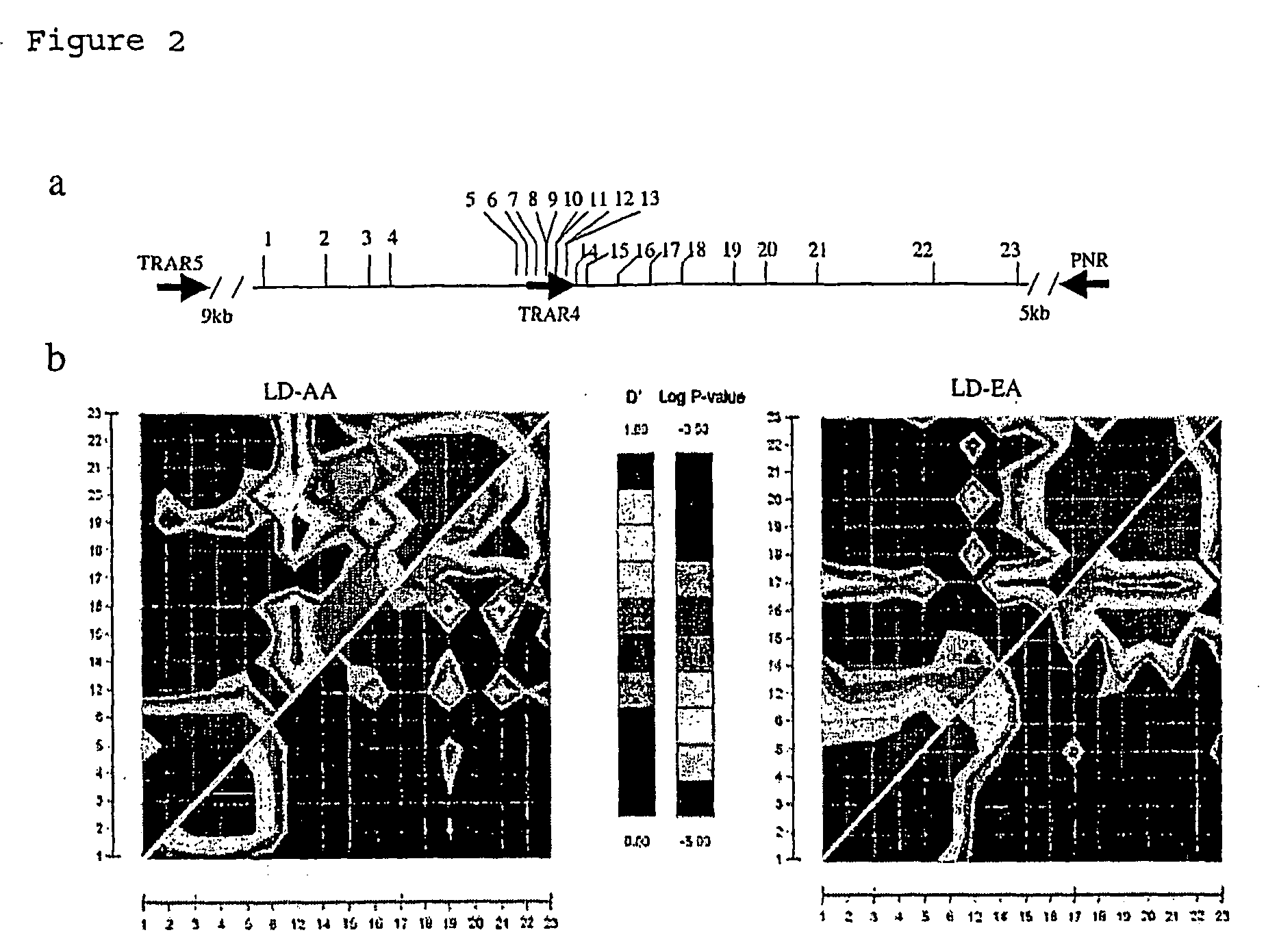
Schizophrenia, Schizoaffective Disorder and Bipolar Disorder Susceptibility Gene Mutation and Applications to Their Diagnosis and Treatment
InactiveUS20080268436A1Increased riskReduce riskSugar derivativesMicrobiological testing/measurementBipolar mood disorderPSYCHIC DISORDER
The present invention provides the identification of a number of SNPs that are associated schizophrenia, schizoaffective disorder, bipolar disorder and related mental disorders which were found to be strongly linked to individuals with the disease. The invention provides SNP locations on human chromosome 6, as well as methods of making PCR primers and assays for detecting the SNPs in tested individuals.
Owner:EVANSTON NORTHWESTERN HEALTHCARE RES INST
Drug for treating senile postoperative acute mental disorders
InactiveCN103463495AEffective treatmentClear curative effectHeavy metal active ingredientsNervous disorderValeriana jatamansiGleditsia triacanthos
The invention relates to a drug for treating senile postoperative acute mental disorders. The drug is prepared from the following substances in proportion by weight: common alstonia bark leaves, herba selaginellae, ophicslcite ophicalcitum, fagopyrum cymosum, fiveleaf akebia fruits, euryale ferox, pummelo peel, celosia cristata, stalactite, folium polygoni tnctorii, seeds of Chinese dodder, grand torreya seeds, litchi, dyosma difformis, eucommia ulmoides, exocarpium benincasae, peucedanum decursivum maxim, tuber fleeceflower stems, chingma abutilon seeds, longan pulp, semen lepidii, soybean germinated, biotite schist and mica, valeriana jatamansi jones, saxifraga stolonifera, spina gleditsiae, folium turpiniae, Chinese honey locust, glechoma longituba, nodular branches of pine, adonis, impatiens balsamina, caulis bambusae in taeniam, desert asparagus, murraya paniculata, saururus chinensis, string beans, leaves of purpleflower holly, veronicastrum herb, Chinese starjasmine stems, abalone, lysionotius pauciflorus maxim, radix ampelopsis, radix scrophulariae, microcos paniculata, dayflowers, fructus piperis longi, corn stigmas, huperzia serrata and amethyst. The drug for treating senile postoperative acute mental disorders disclosed by the invention is prepared from natural medicines, has a clear curative effect, can be used for effectively treating the senile postoperative acute mental disorders, and therefore, the drug is very suitable for clinical application.
Owner:陈军
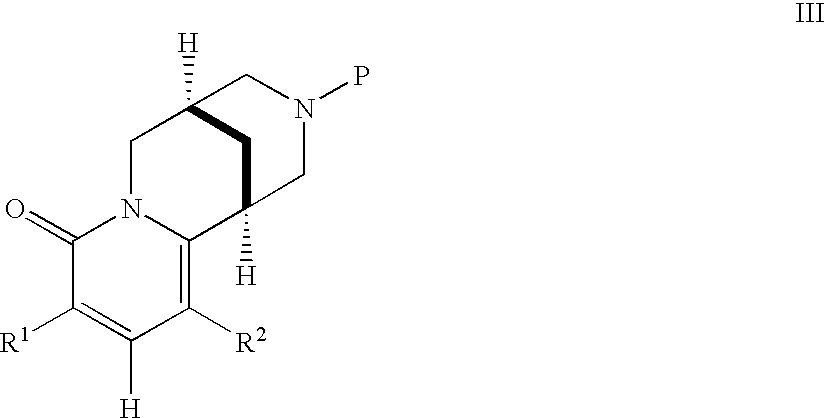
Pyridone-fused azabicyclic- or cytisine derivatives, their preparation and their use in addiction therapy
InactiveUS6630467B2Reduce addictionLessing of tobacco useBiocideNervous disorderUlcerative colitisAzabicyclo Compounds
Owner:PFIZER INC
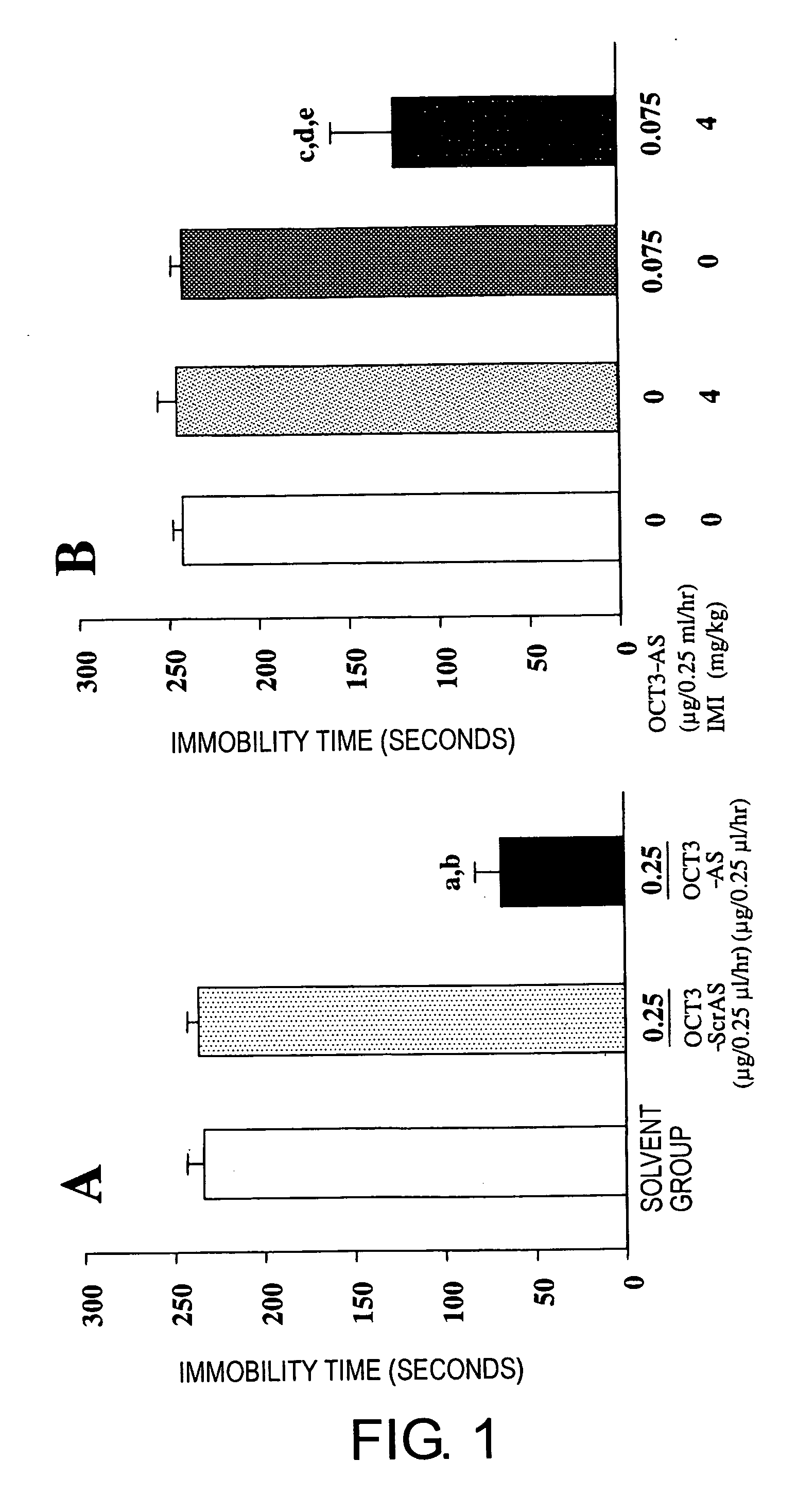
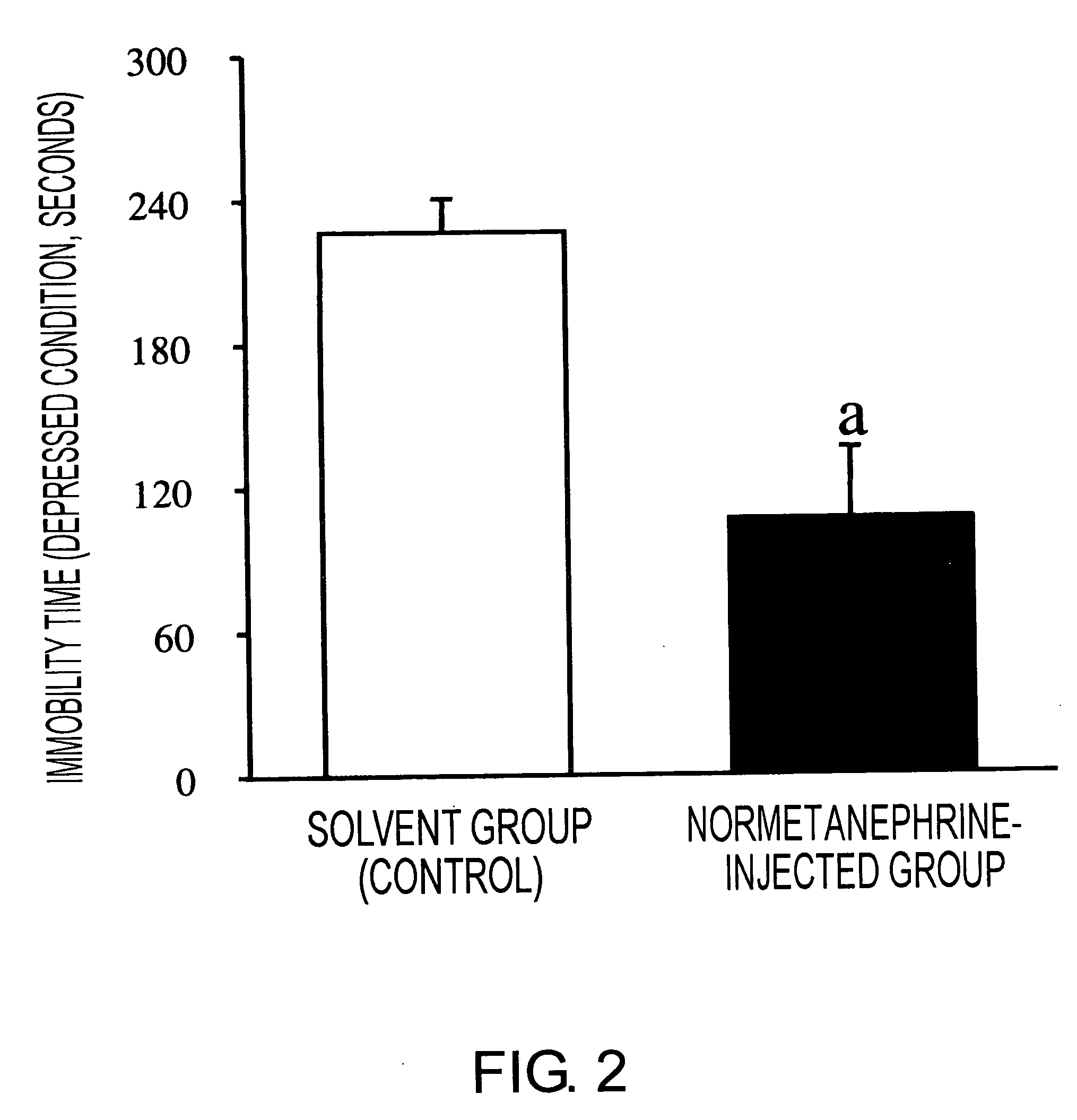
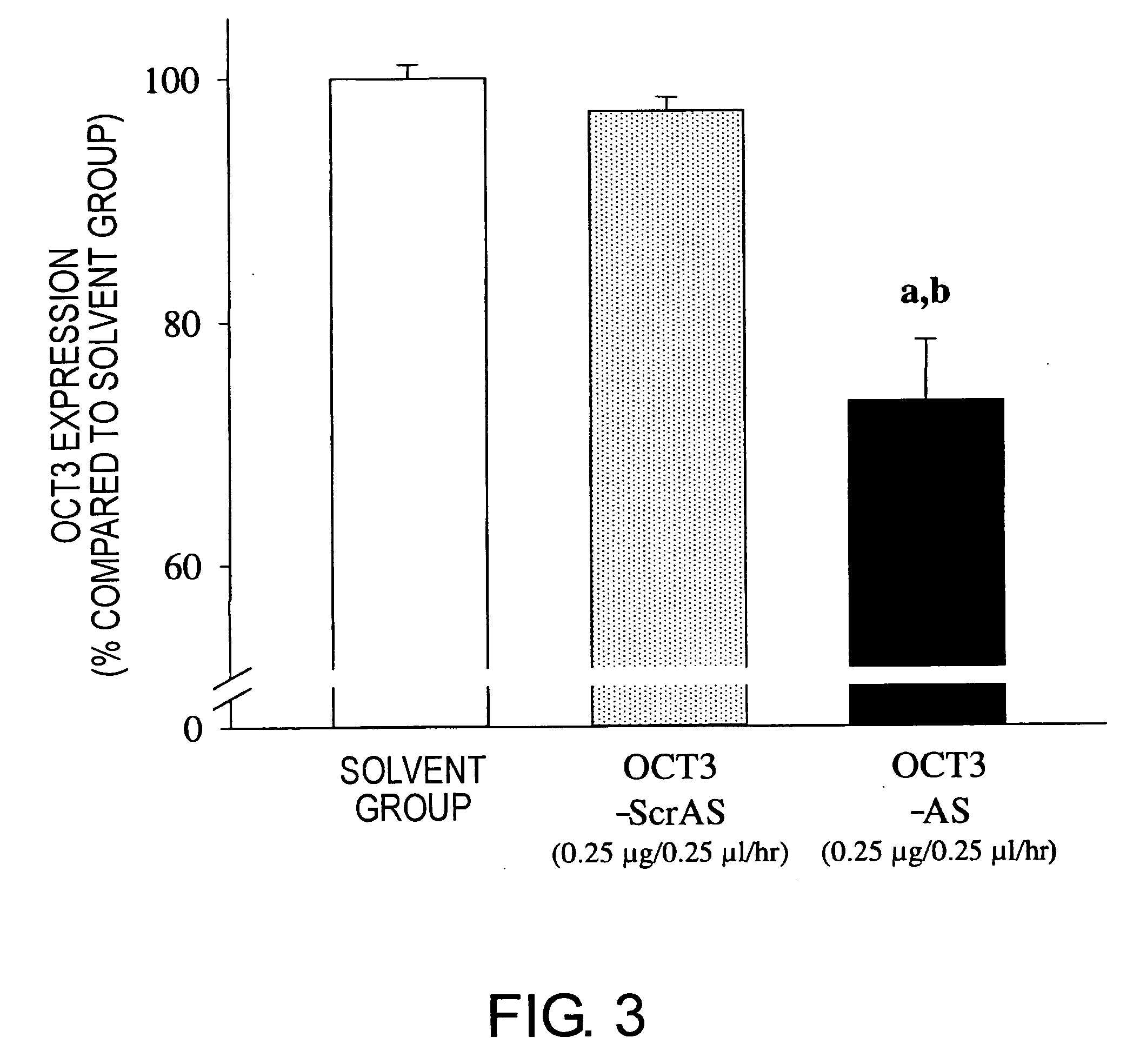
Methods of using molecules related to organic cation transporter 3 (oct3) for treating depression, anxiety neuroses, drug dependencies, and other similar mental disorders
InactiveUS20070136828A1Suppressing OCT gene expressionGood effectOrganic active ingredientsNervous disorderOrganic cation transport proteinsPSYCHIC DISORDER
Mice in which OCT3 expression is suppressed were successfully constructed by administering antisense against OCT3 gene into the brain. Mice in which OCT3 expression is suppressed display phenotypes related to mental disorders such as anti-depression and anti-anxiety, and therefore the mice can be applied to the screening of therapeutic agents for mental disorders. In addition, substances suppressing the OCT3 expression or function were shown to be in fact effective as therapeutic agents for depression and anxiety neuroses.
Owner:BIOSTATION
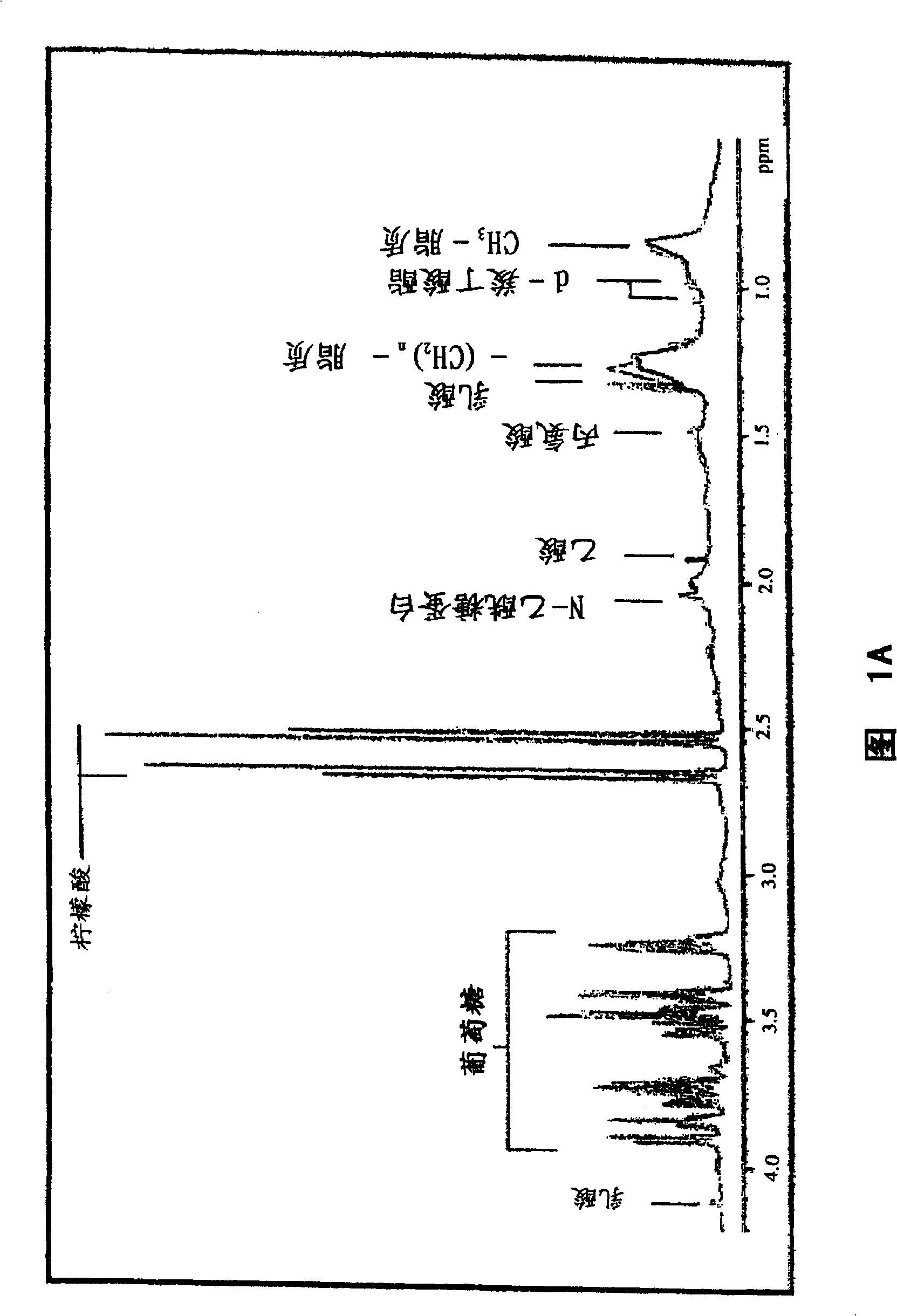
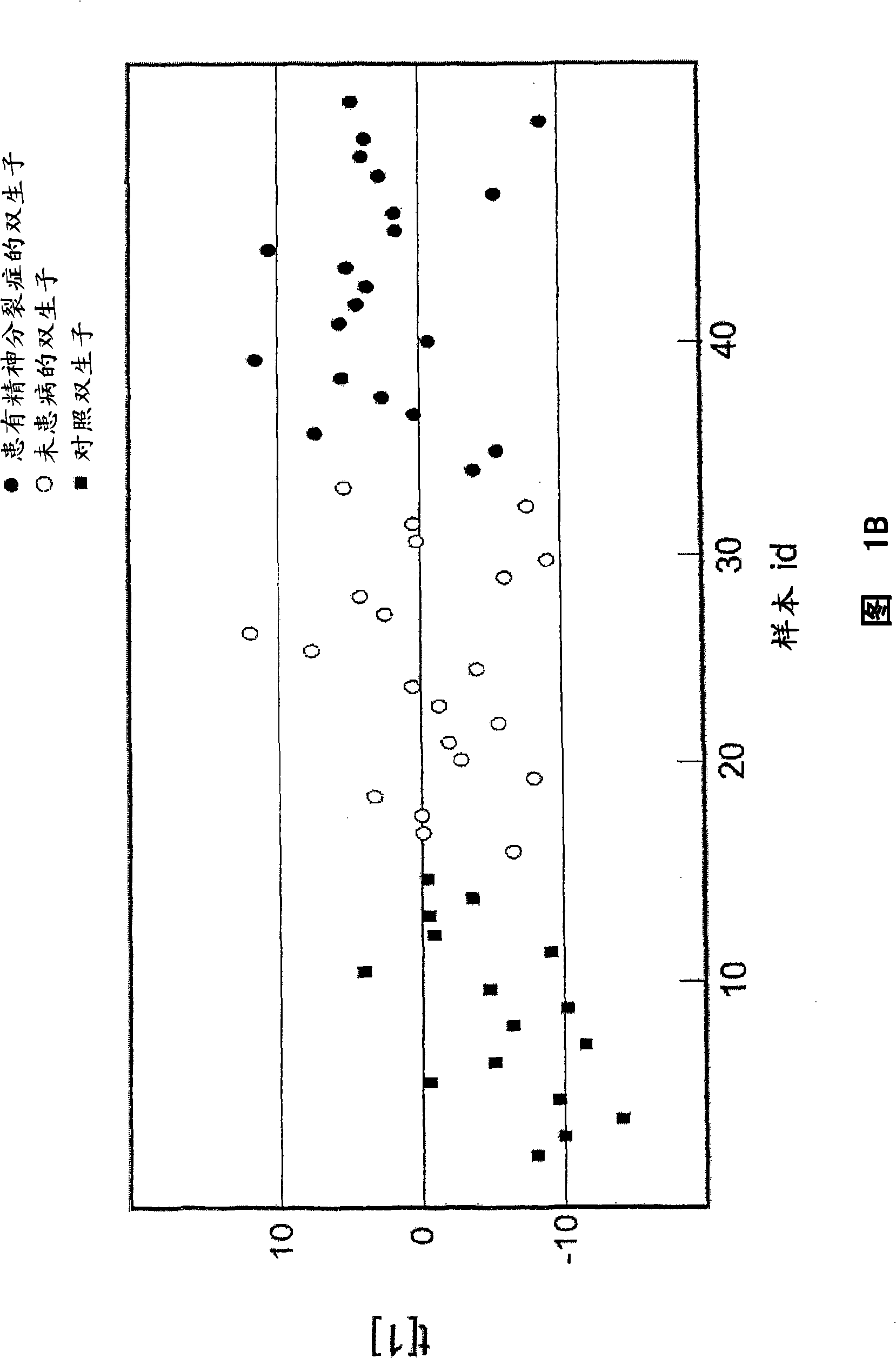
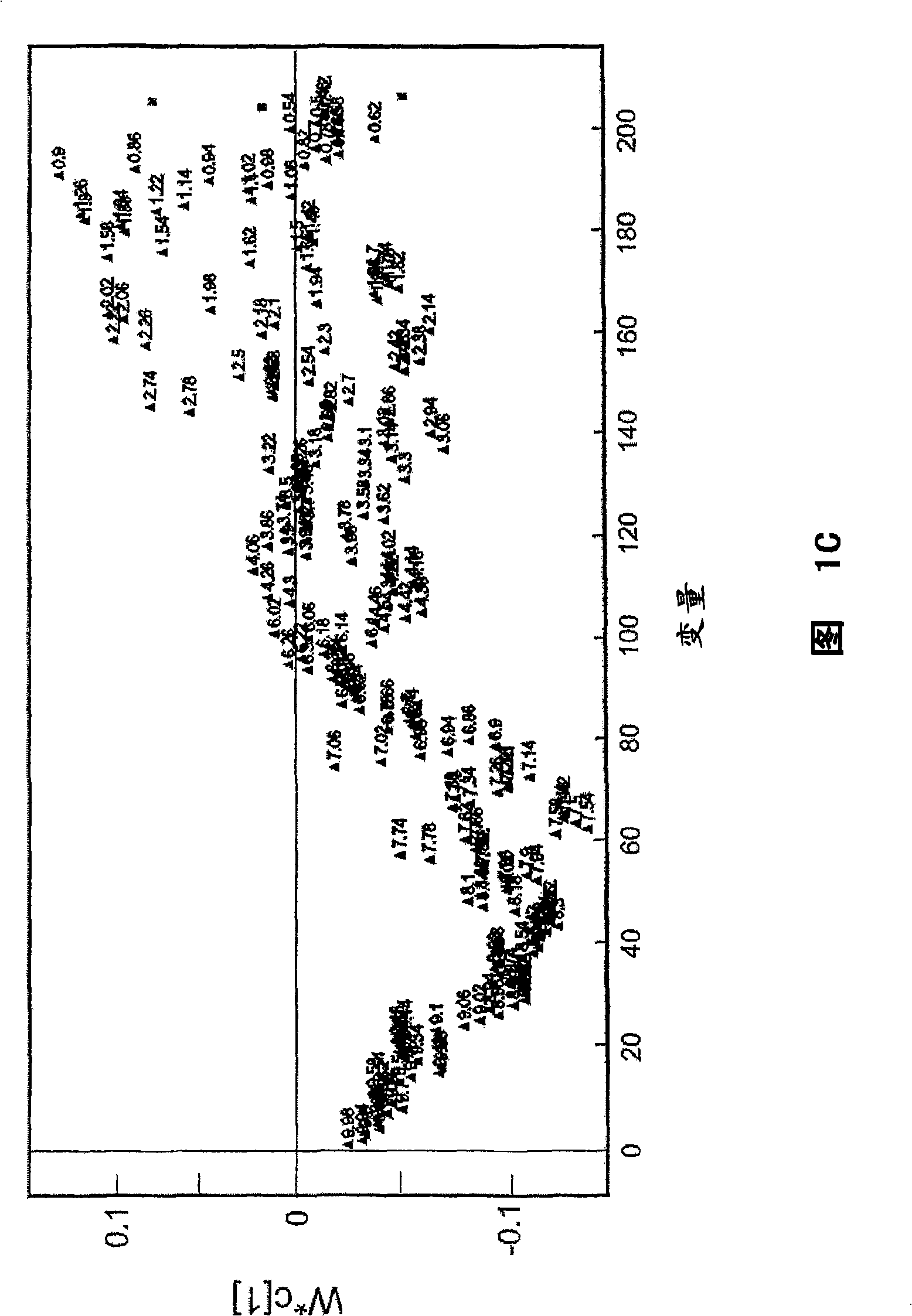
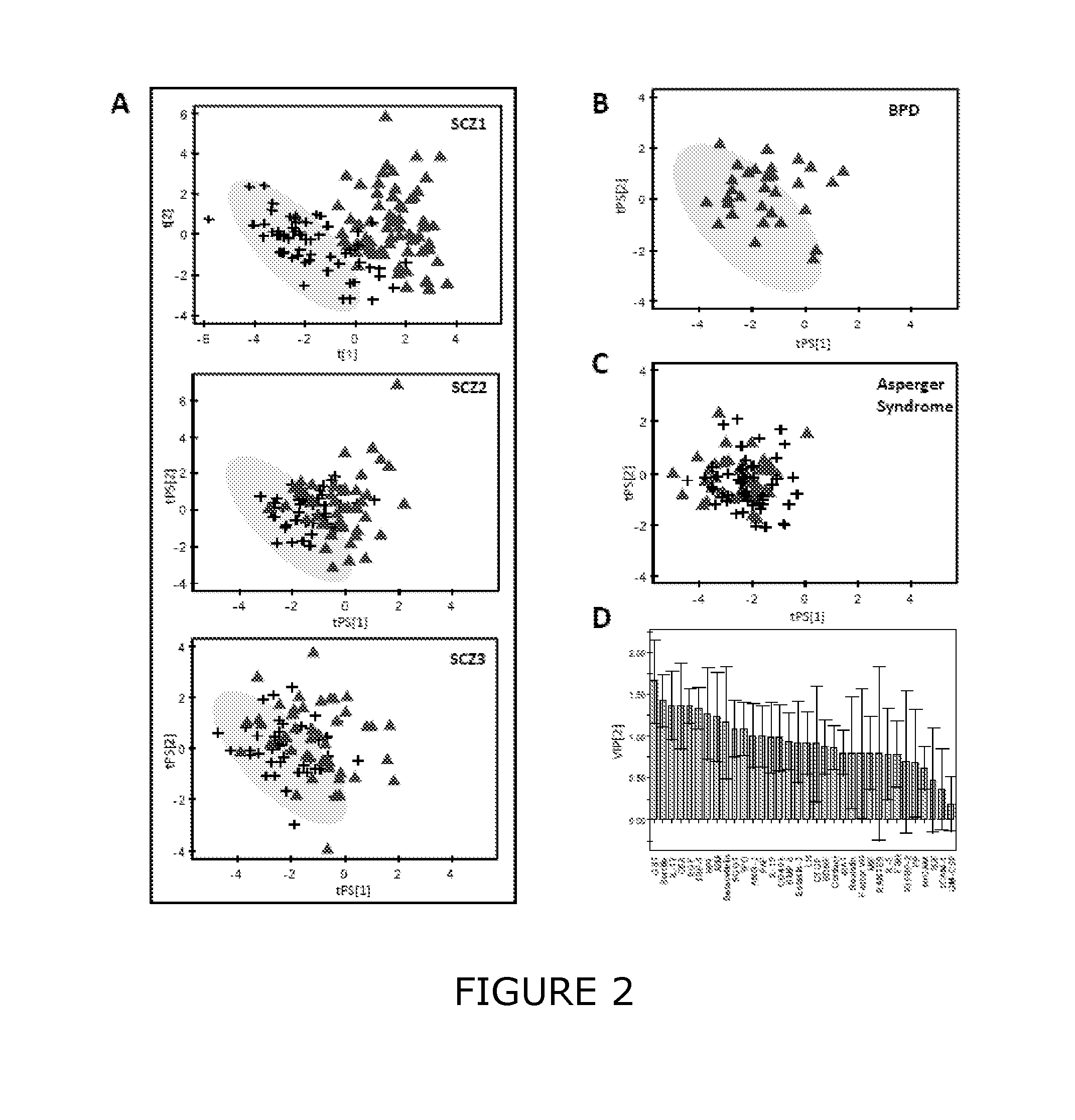
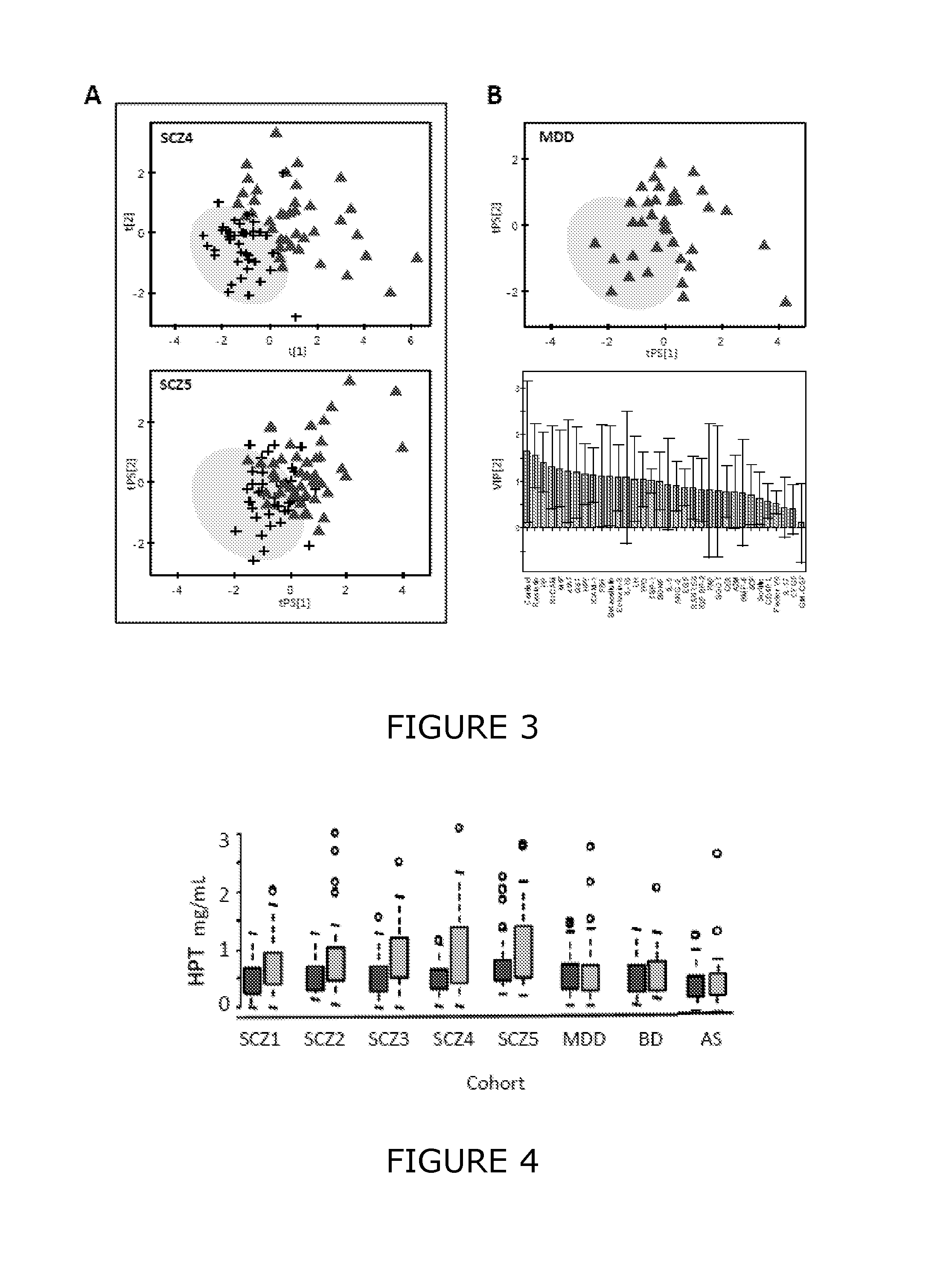
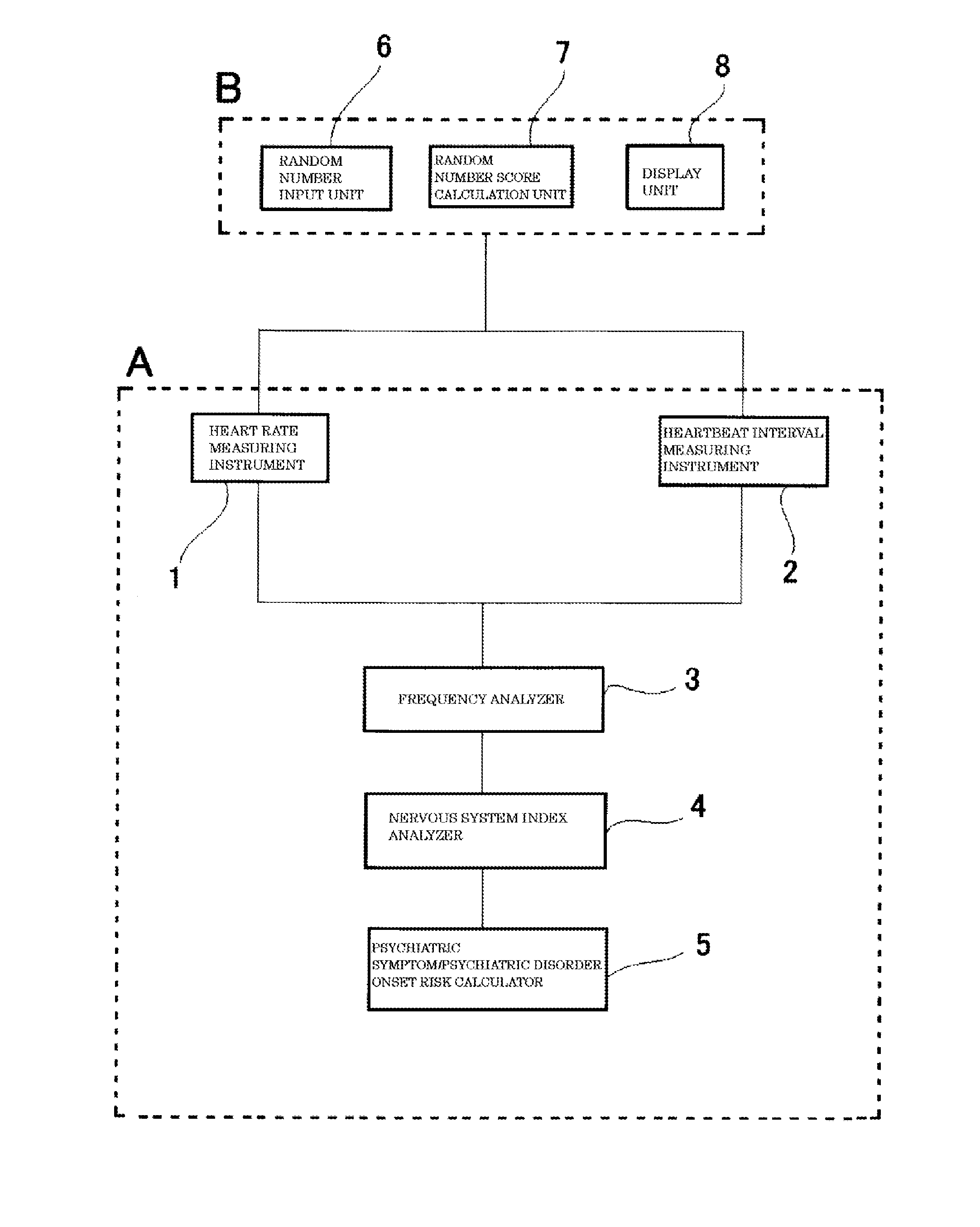
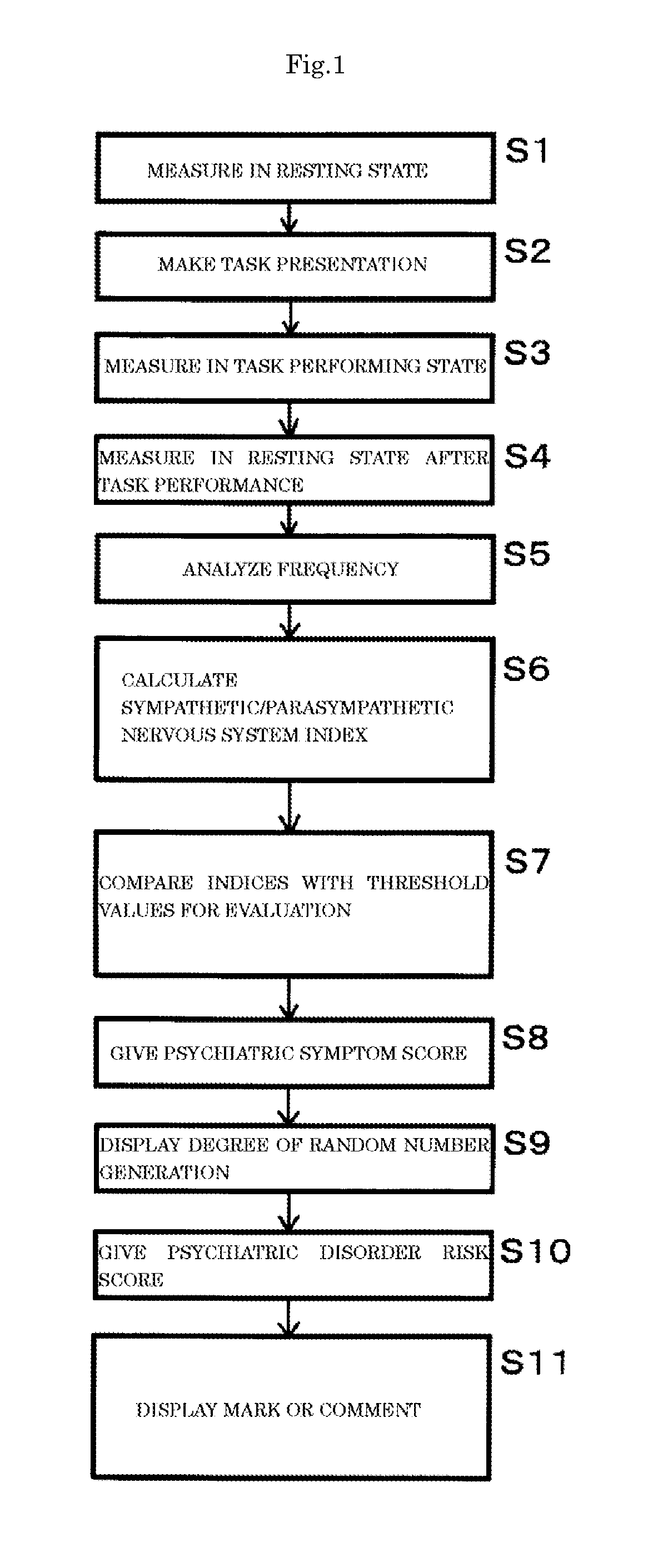
Methods and biomarkers for diagnosing and monitoring psychotic disorders such as schizophrenia
InactiveCN101356446ALittle side effectsShorten the timeMagnetic measurementsBipolar mood disorderMulti analyte
The invention relates to methods of diagnosing or monitoring a psychotic disorder in a subject comprising providing a test biological sample from the subject, performing spectral analysis on said test biological sample to provide one or more spectra, and, comparing the one or more spectra with one or more control spectra. The invention also relates to methods for diagnosing or monitoring psychotic disorders such as schizophrenic or bipolar disorders, comprising measuring the level of one or more biomarkers present in a biological sample taken from a test subject, said biomarkers being selected from the group consisting of transthyretin, ApoA1, VLDL, LDL and aromatic species such as plasma proteins. The invention also relates to sensors, biosensors, multi-analyte panels, arrays, assays and kits for performing methods of the invention.
Owner:PSYNOVA NEUROTECH LTD
Biomarkers
ActiveUS20140200151A1Promoting and of suppressing generationPromoting and suppressing generationPeptide librariesLibrary screeningClinical psychologyBiomarker (petroleum)
The invention relates to a method of differential diagnosis of schizophrenia or other psychotic disorder from a further psychiatric disorder.
Owner:CAMBRIDGE ENTERPRISE LTD
Tetrahydroindolone and purine derivatives linked to arylpiperazines
InactiveUS20030114463A1Improve cognitive functionImproved therapeutic propertyBiocideOrganic chemistryPurinePurine derivative
Pharmaceutical composite compositions comprising tetrahydroindolones linked to arylpiperazines and derivatives thereof are disclosed. Specifically, composite compositions useful in treating anti-psychotic disorders are disclosed. The composite compositions disclosed herein can effectively ameliorate symptoms and treat psychotic disorders without causing a decrease in cognitive function. Generally, the composite compounds consist of two moieties, moiety A and B in which a tetrahydroindolone comprises a moiety A linked through a linker L to a moiety B, where B is an arylpiperazinyl moiety. The composite compound provides anti-psychotic actively by interaction with GABA, seratoninne and dopamine receptors. The composite molecules with the combined activities will provide treat psychiatric and neurological diseases without cognitive impairment.
Owner:SPECTRUM PHARMA INC
Compositions and Methods for Treating Mental Disorders
The present invention relates, generally, to methods and compositions for detecting or treating mental disorders, such as schizophrenia or bipolar disorder. The present invention more particularly discloses the identification of human genes that can be used for the diagnosis, prevention and treatment of schizophrenia, bipolar disorder and related disorders, as well as for the screening of therapeutically active drugs to treat said disorders. The invention further discloses specific polymorphisms or alleles of the KCNQ3 gene that are related to schizophrenia or bipolar disorder, as well as diagnostic tools and kits based on these markers. The invention can be used in the diagnosis of or predisposition to, detection, prevention and / or treatment of schizophrenia, bipolar disorder and related disorders.
Owner:ARES TRADING SA
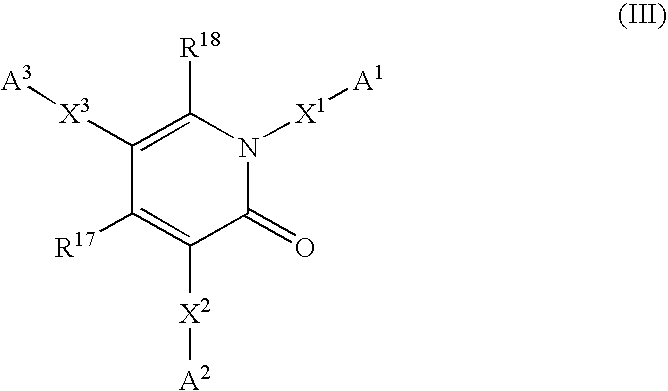
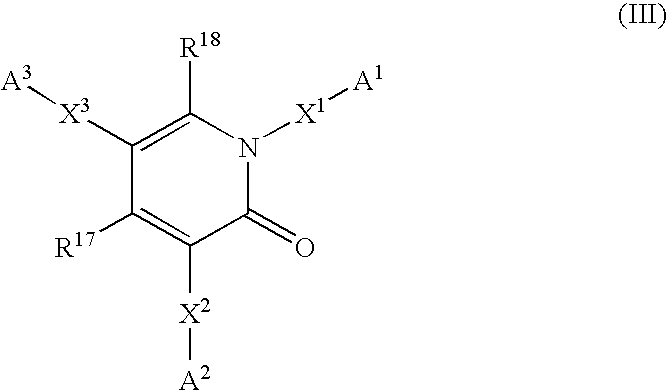
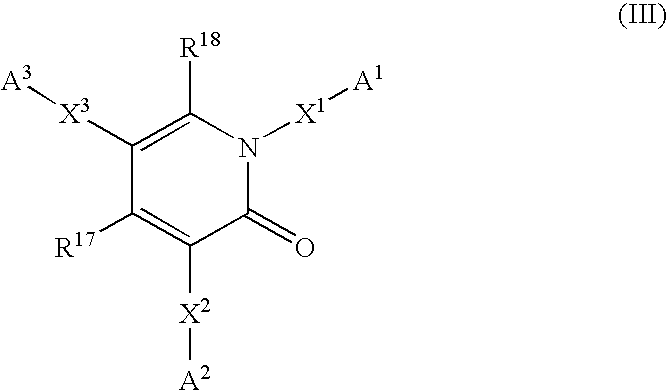
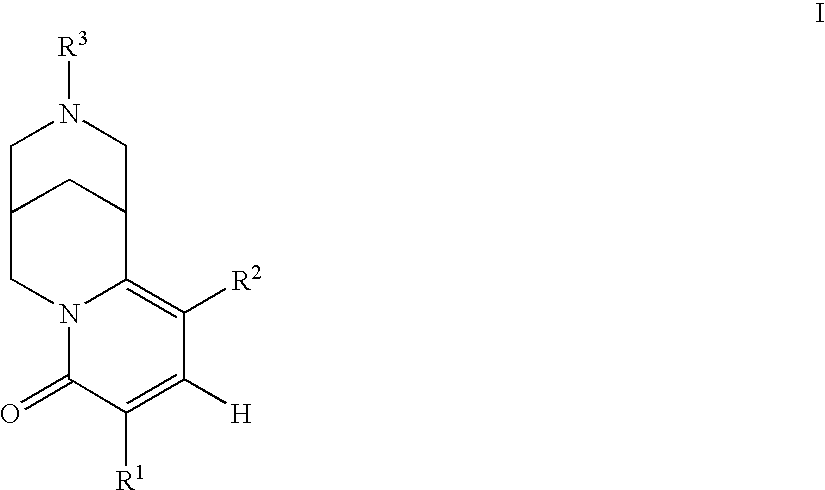
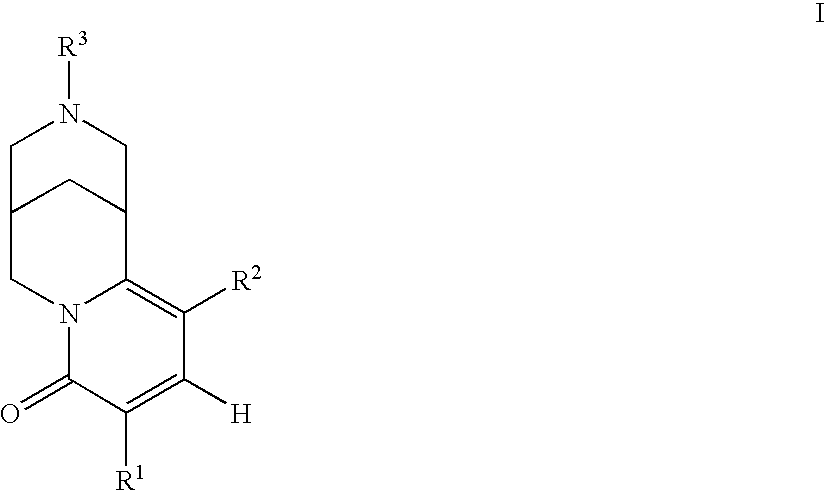
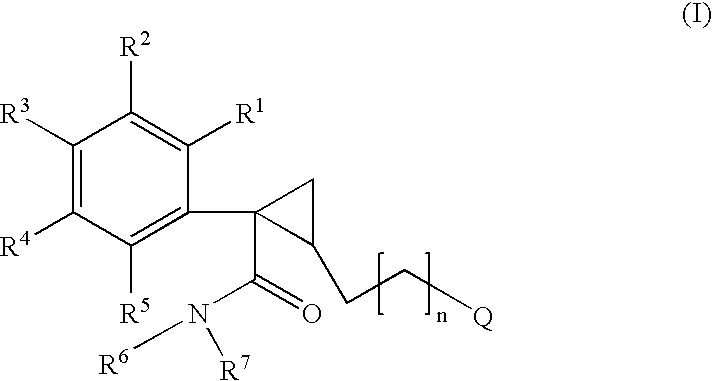
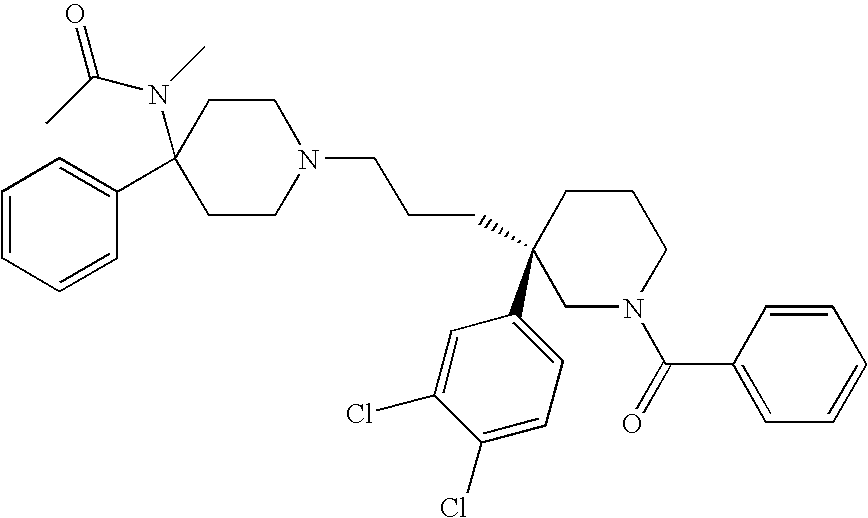
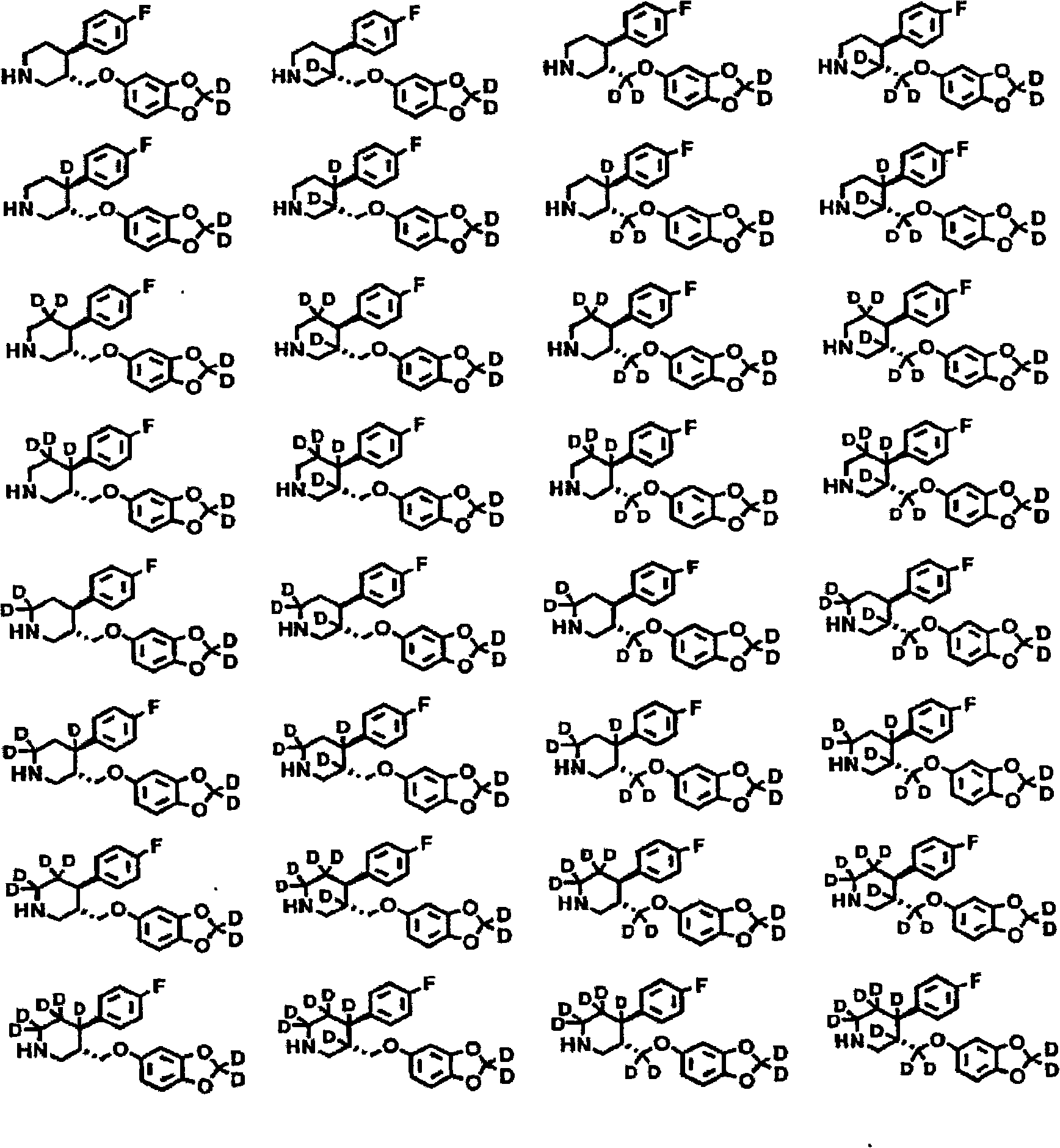
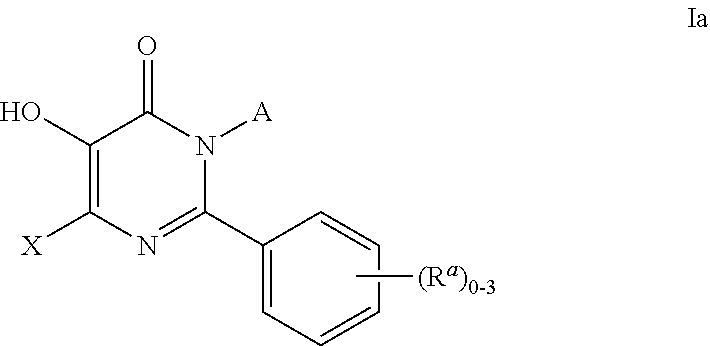
6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor
The present invention is directed to 6,7-dihydro-5H-pyrrolo[3,4-b]pyridine-5-one compounds which are allosteric modulators of the M4 muscarinic acetylcholine receptor. The present invention is also directed to uses of the compounds described herein in the potential treatment or prevention of neurological and psychiatric disorders and diseases in which M4 muscarinic acetylcholine receptors are involved. The present invention is also directed to compositions comprising these compounds. The present invention is also directed to uses of these compositions in the potential prevention or treatment of such diseases in which M4 muscarinic acetylcholine receptors are involved.
Owner:MERCK SHARP & DOHME LLC +1
Compositions and methods for diagnosing and treating mental disorders
InactiveUS20110224144A1High expressionReduce expressionOrganic active ingredientsNervous disorderPSYCHIC DISORDERSchizophrenia
The present invention provides methods for diagnosing mental disorders (e.g., psychotic disorders such as schizophrenia). The invention also provides methods of identifying modulators of such mental disorders as well as methods of using these modulators to treat patients suffering from such mental disorders.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for diagnosing and treating chromosome-18p related disorders
InactiveUS6342351B1Affect safetyAffect efficacyFungiNervous disorderSchizo-affective typeGenetic Screening (procedure)
The present invention relates of the mammalian HKNG1 gene, a gene associated with bipolar affective disorder (BAD) in humans. The invention relates, in particular, to methods for the diagnostic evaluation, genetic testing and prognosis of HKNG1 neuropsychiatric disorders including schizophrenia, attention deficit disorder, a schizoaffective disorder, a bipolar affective disorder or a unipolar affective disorder.
Owner:MILLENNIUM PHARMA INC +1
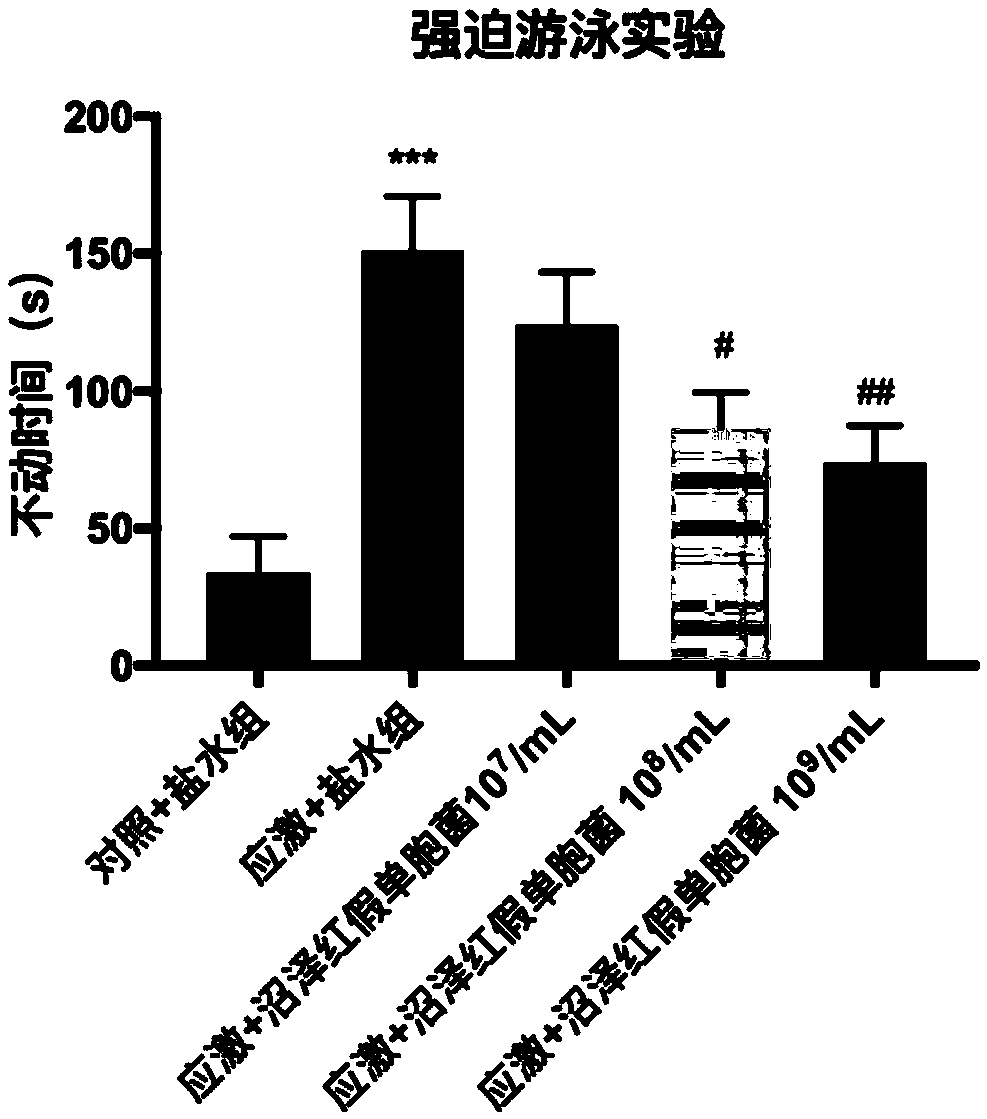
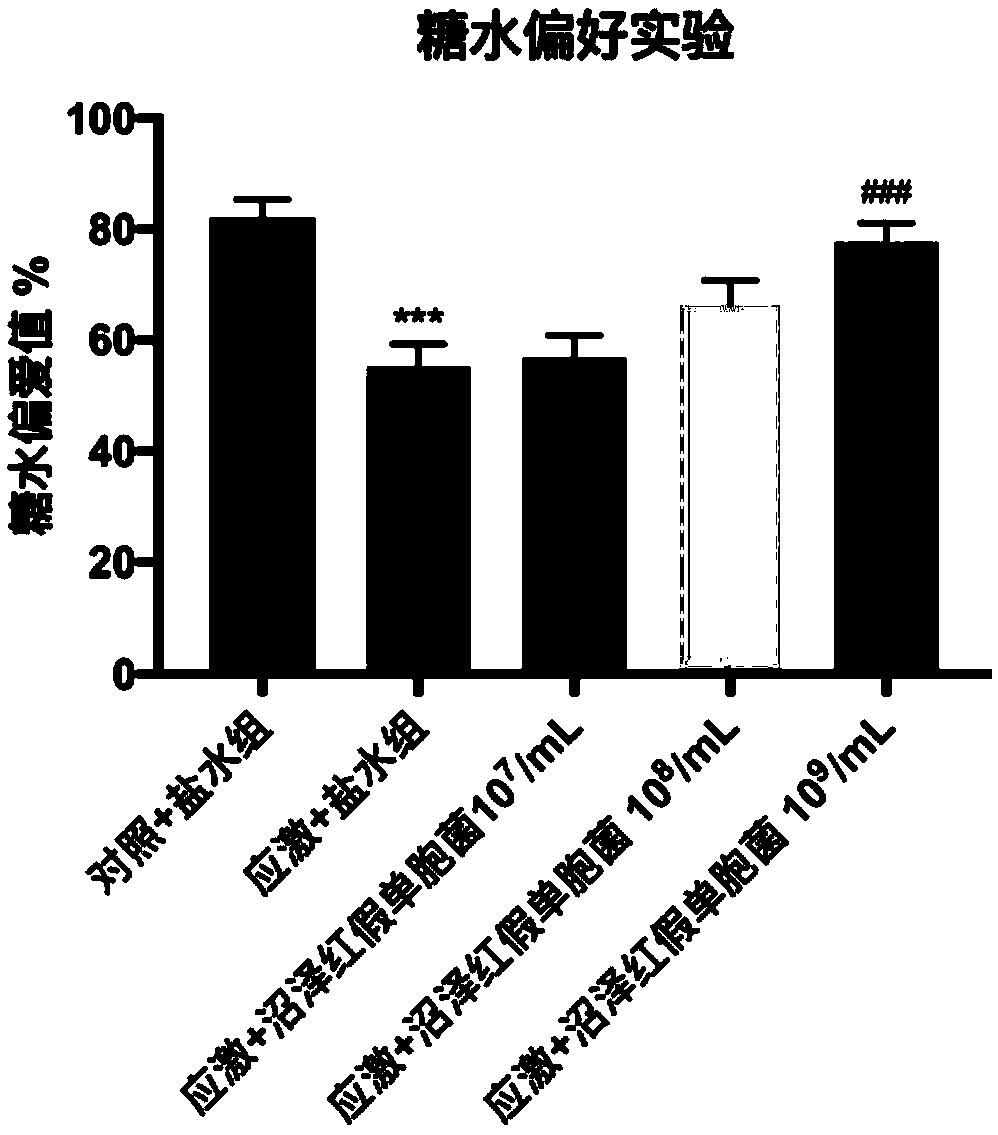
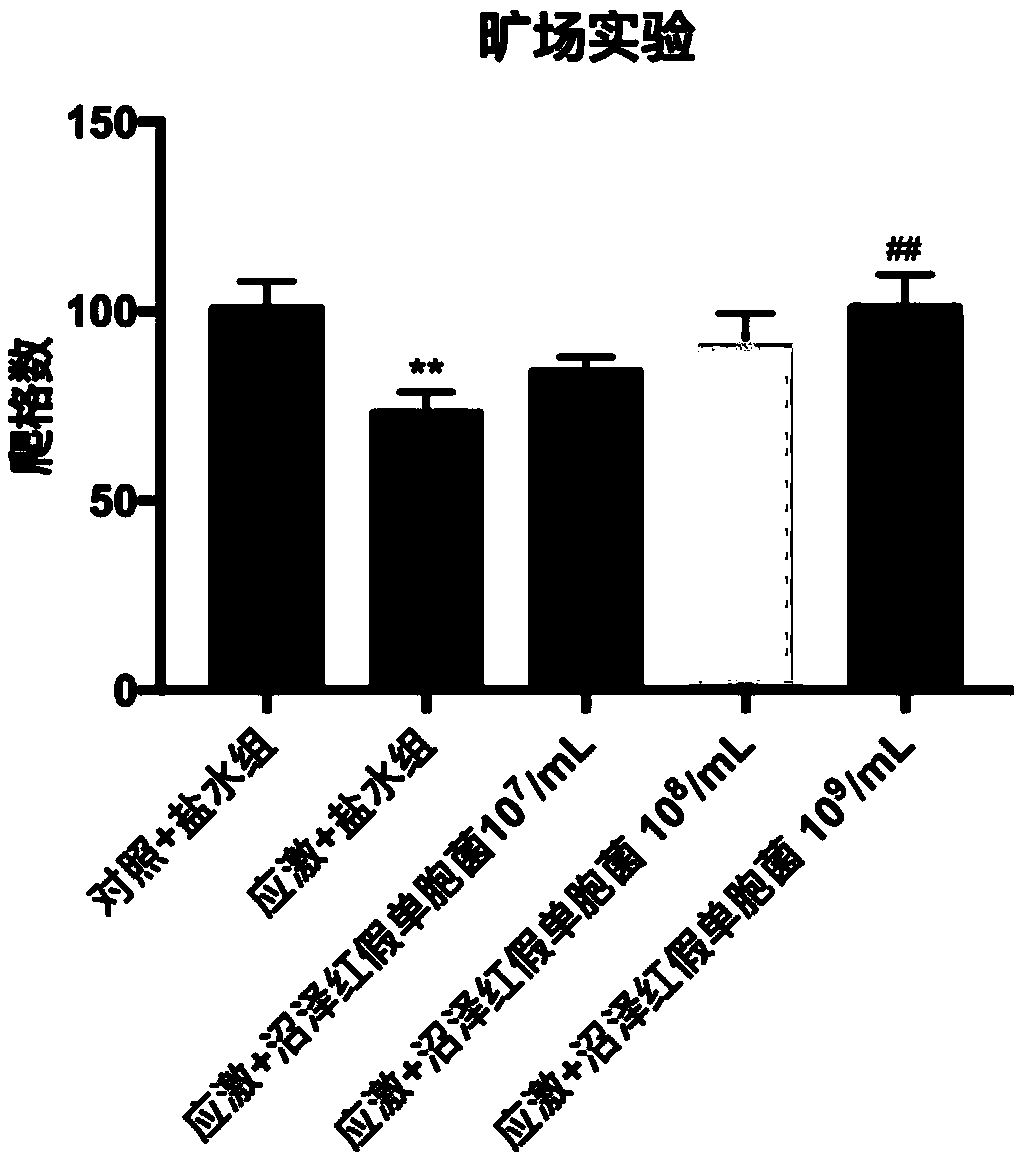
A biological preparation for preventing and treating mental disorder based on photosynthetic bacteria and application thereof
ActiveCN109172612ANo adverse reactionNervous disorderUnknown materialsBiological agentTherapeutic effect
The invention relates to a biological preparation for preventing and treating mental disorder based on photosynthetic bacteria. The preparation belongs to a probiotic agent capable of preventing and treating mental disorders such as depression and / or anxiety disorder, and has both preventive and therapeutic effects on the occurrence of mental disorders such as depression and / or anxiety disorder, and has no adverse reaction. The present invention has developed novel approaches to the prevention and treatment of mental disorders, such as depression and / or anxiety disorders.
Owner:UNIV OF SCI & TECH BEIJING +1
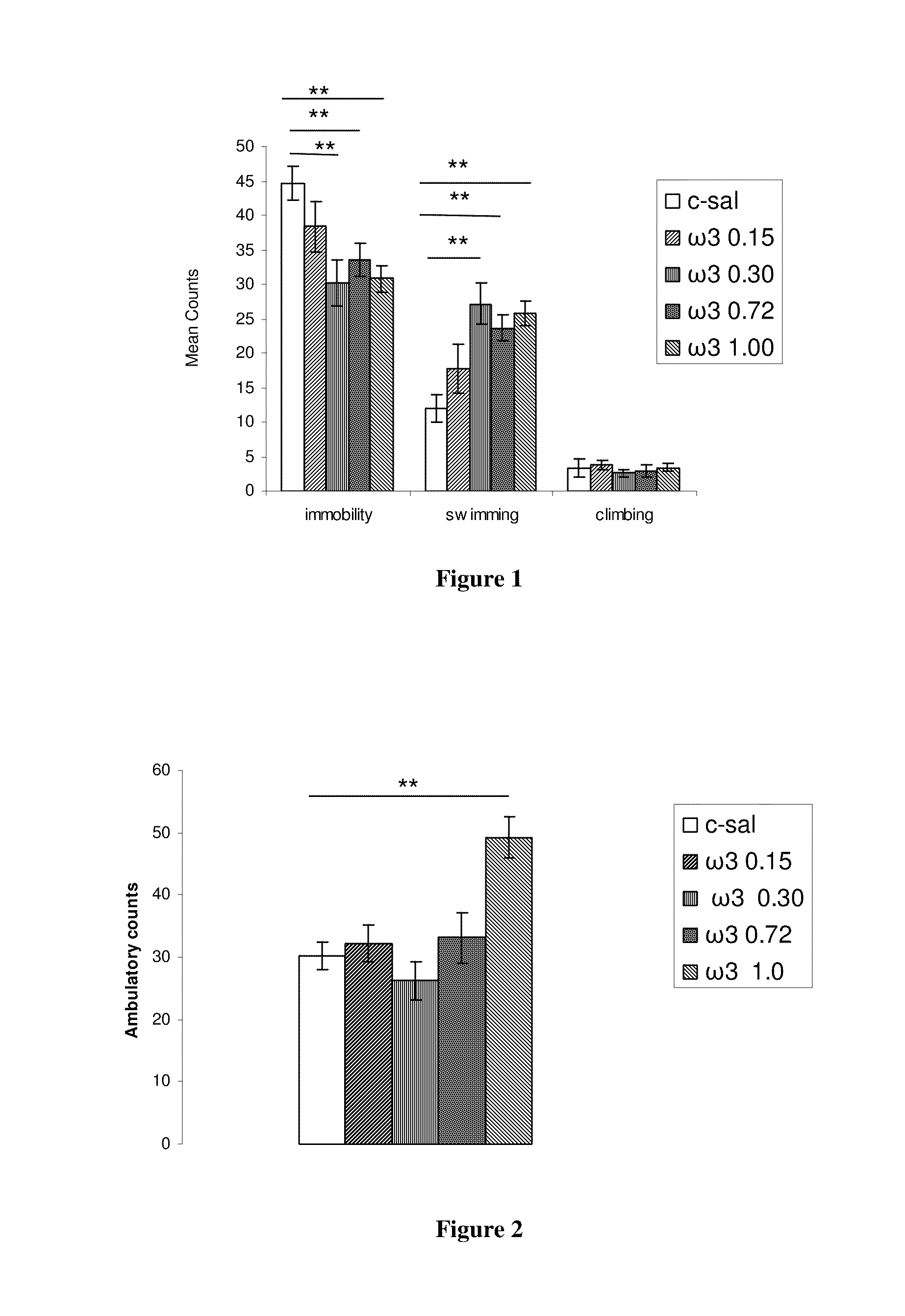
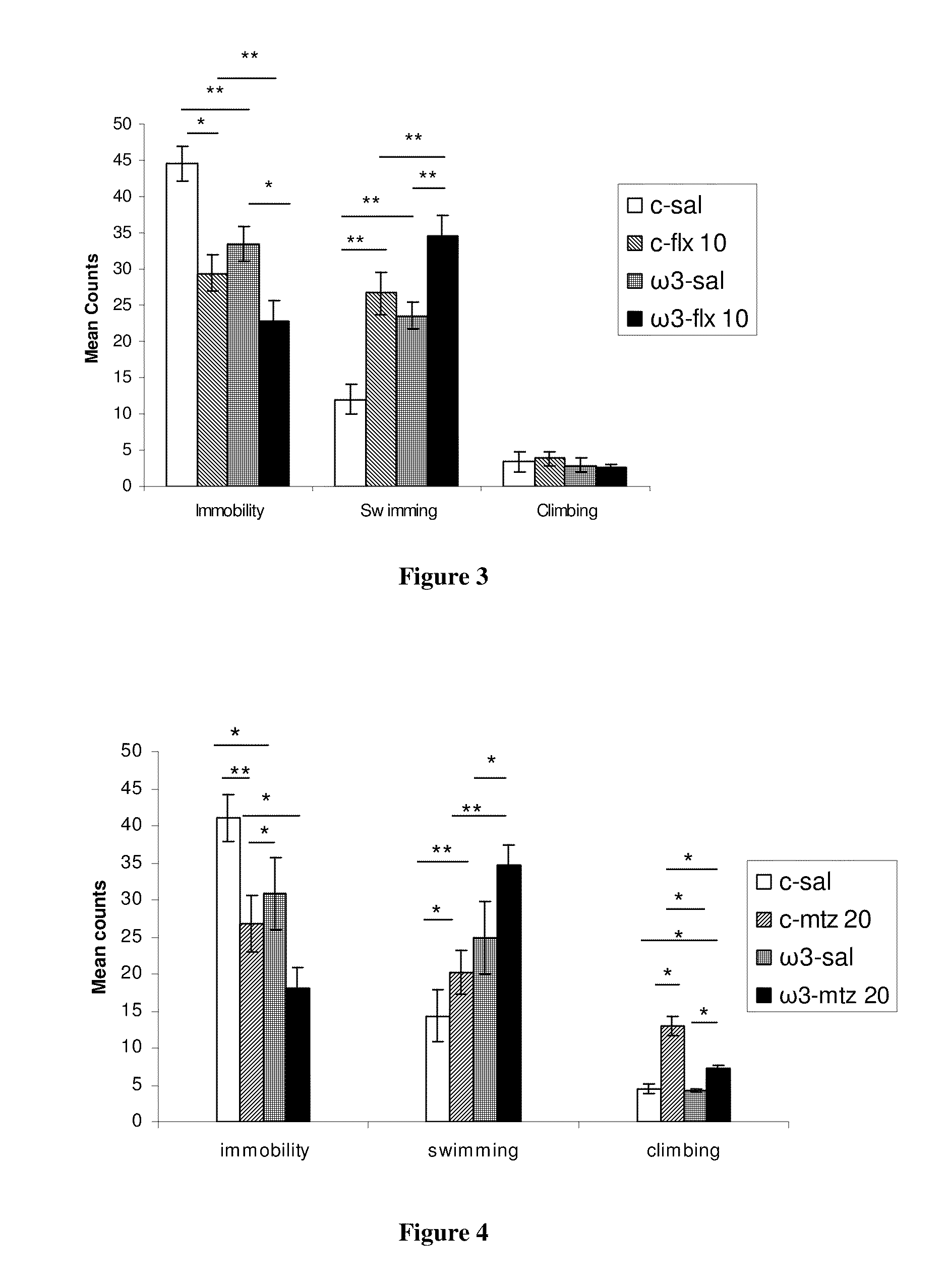
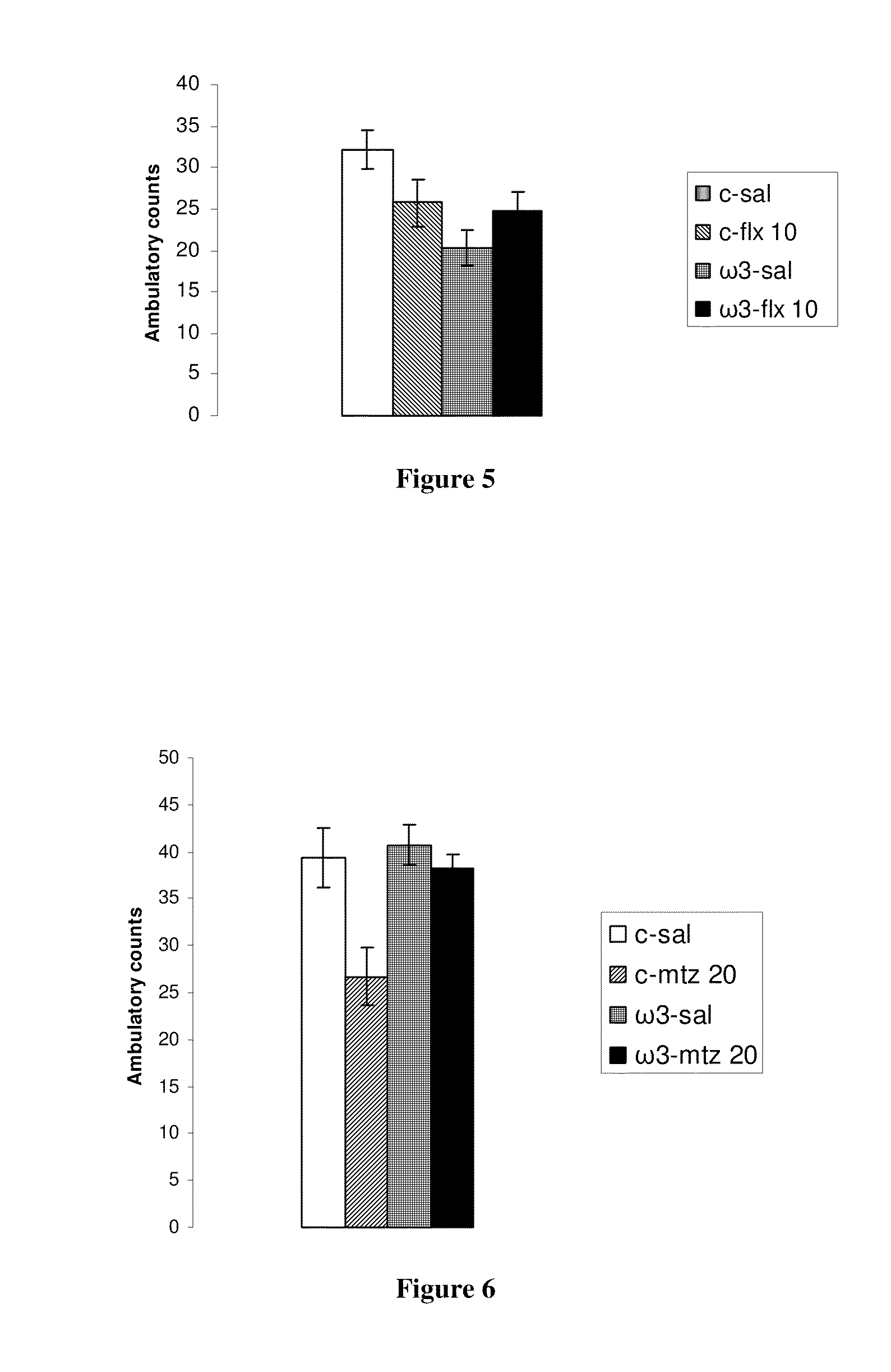
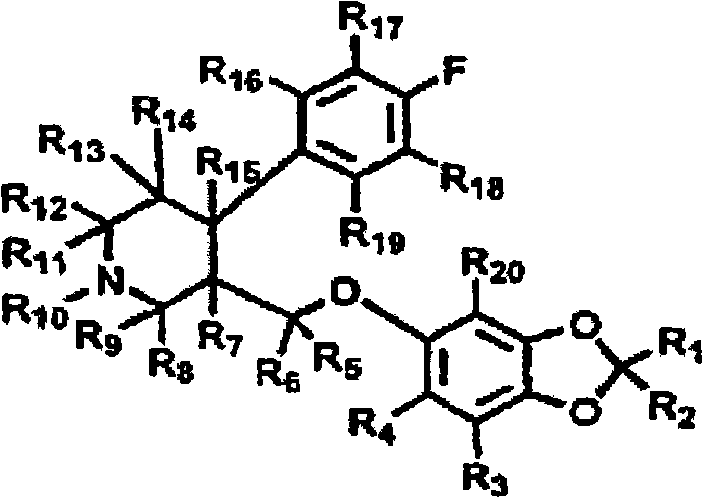
Method for the treatment of psychic disorders
Method for the treatment of psychiatric disorders, comprising administering to a subject in need thereof an amount of fatty acids and a suboptimal dose of at least one antidepressant. Wherein the fatty acid may be omega-3 (ω3), for example the docohexaenoic acid (DHA) and the eicosapentaenoic acid (EPA). The omega-3 fatty acid may be administered orally, in amounts that may be variable, for example in amounts between 0.15 and 1.00 g / kg / day. The antidepressant may be any antidepressant, preferably the antidepressant is fluoxetine or mirtazapine in sub-optimal doses.
Owner:CONSEJO NAT DE INVESTIGACIONES CIENTIFICAS Y +1
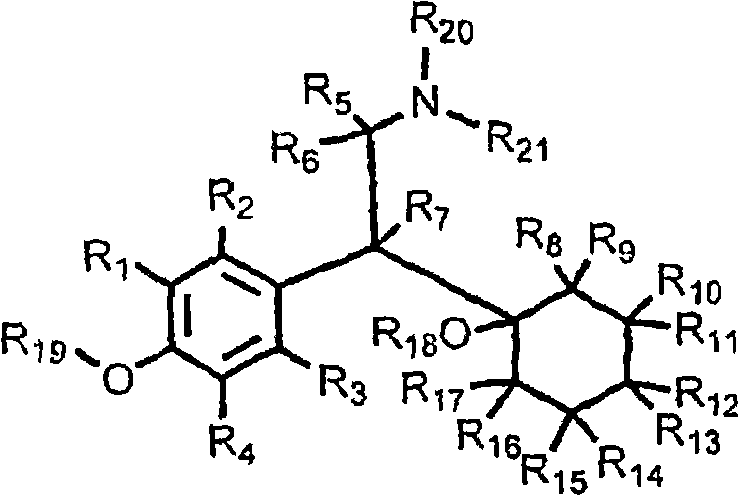
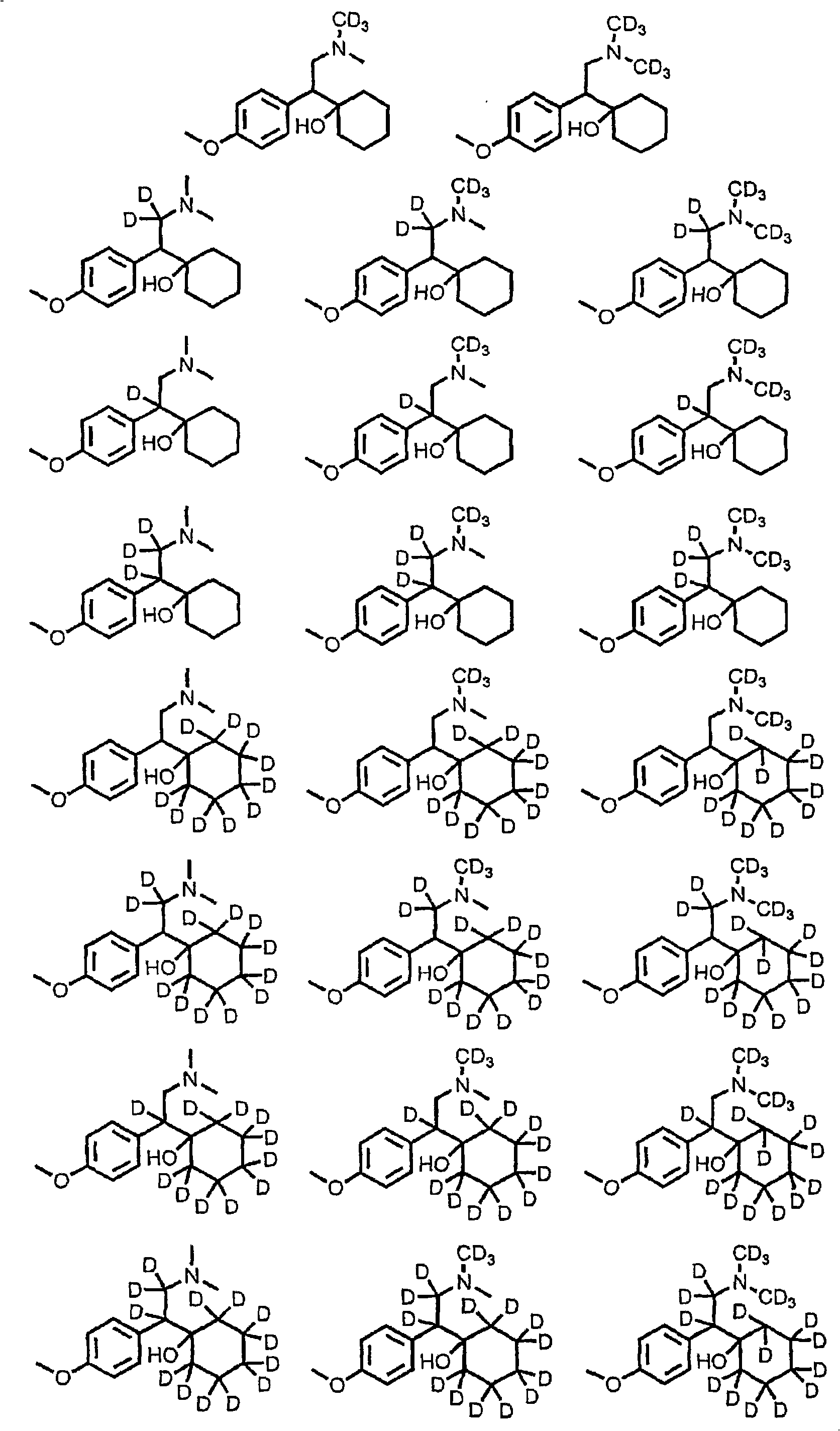
Substituted phenylpiperidines with serotoninergic activity and enhanced therapeutic properties
InactiveCN101360742AOrganic active ingredientsNervous disorderCompulsive disordersDiabetic retinopathy
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive- compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
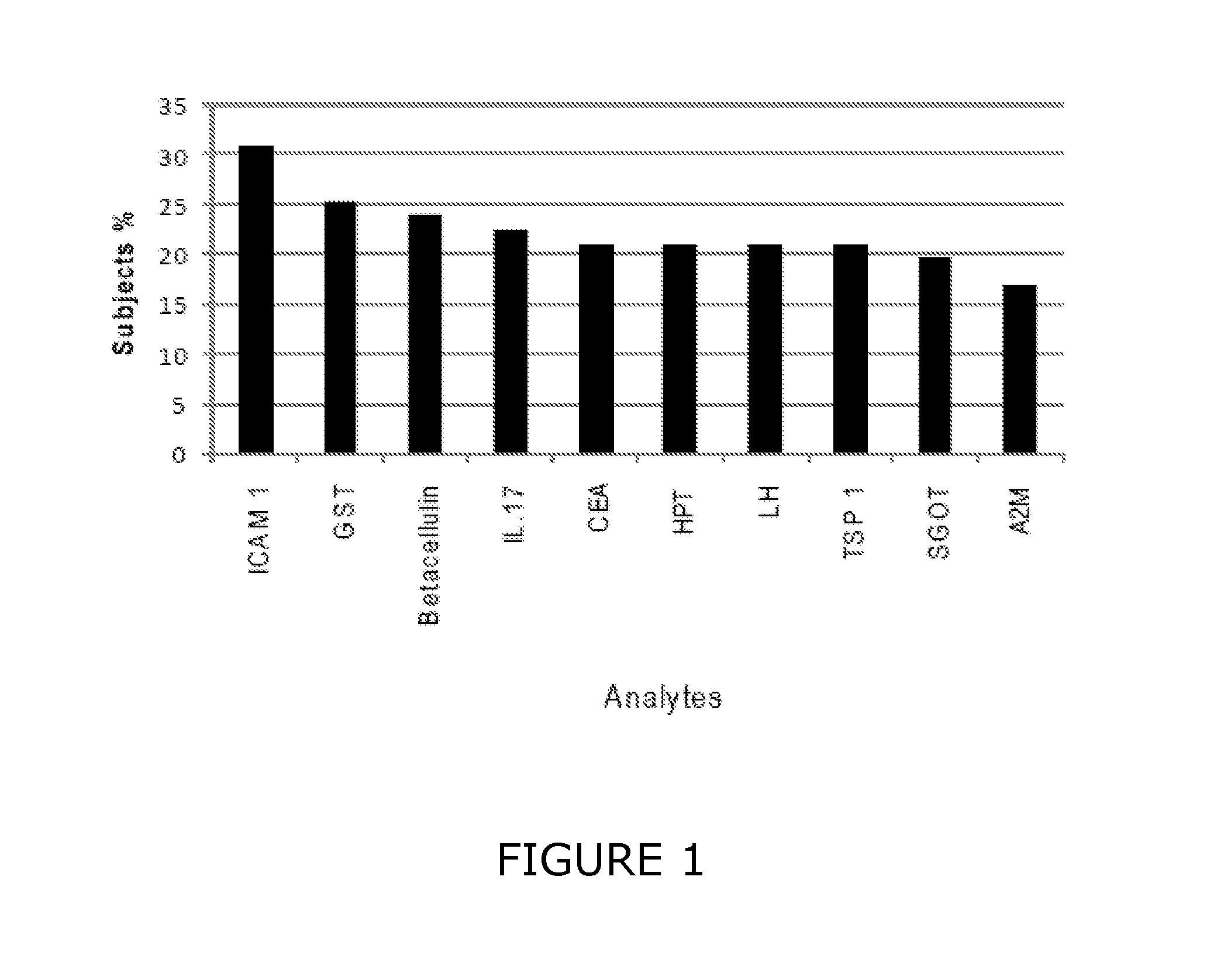
Methods to Monitor, Diagnose and Identify Biomarkers for Psychotic Disorders
InactiveUS20090286238A1Improve isolationHigh purityMicrobiological testing/measurementDisease diagnosisClinical psychologyT cell
A stimulated or non-stimulated T-cell sample can be used to diagnose or monitor a psychotic disorder, to identify a biomarker, or as to test a considerate as a potential therapeutic agent.
Owner:CAMBRIDGE ENTERPRISE LTD
Inhibitors of catechol o-methyl transferase and their use in the treatment of psychotic disorders
The present invention relates to 4-pyridinone compounds which are inhibitors of catechol O-methyltransferase (COMT), and are useful in the treatment and prevention of neurological and psychiatric disorders and diseases in which COMT enzyme is involved. The present invention also relates to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which COMT is involved.
Owner:MERCK SHARP & DOHME CORP
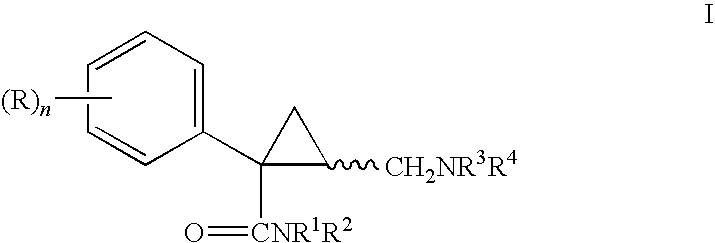
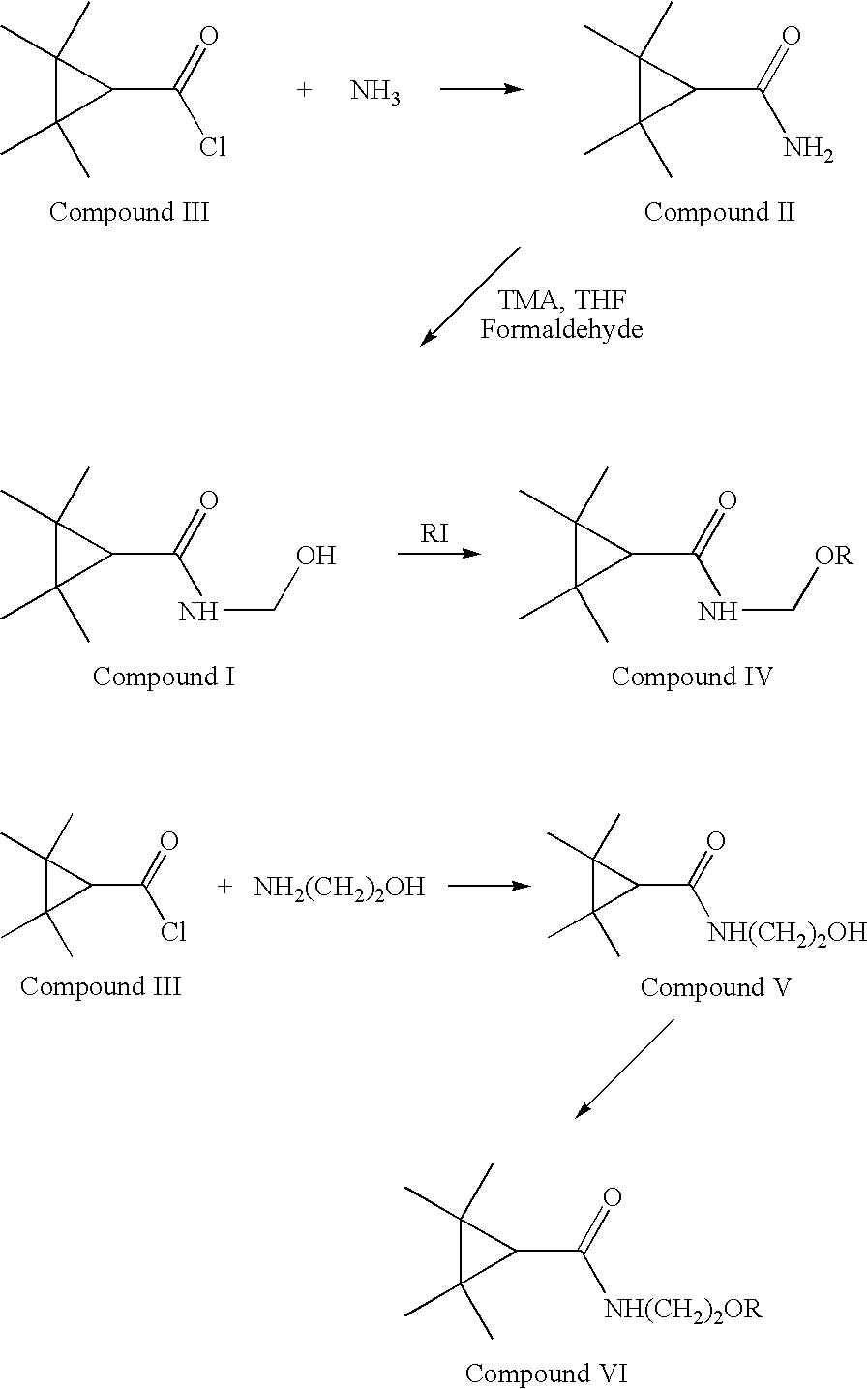
Derivatives and pharmaceutical compositions of n-hydroxyalkyl tetramethylcyclopropane-carboxamide, having anti-epiletic, neurological, and CNS activity, and method for their preparation
New derivatives of N-Hydroxyalkyl-tetramethylcyclopropane carboxamide, pharmaceutical compositions thereof, methods for their preparation, and use thereof for the treatment of epilepsy, neurological, affective and psychotic disorders and for the treatment of pain and migraine.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
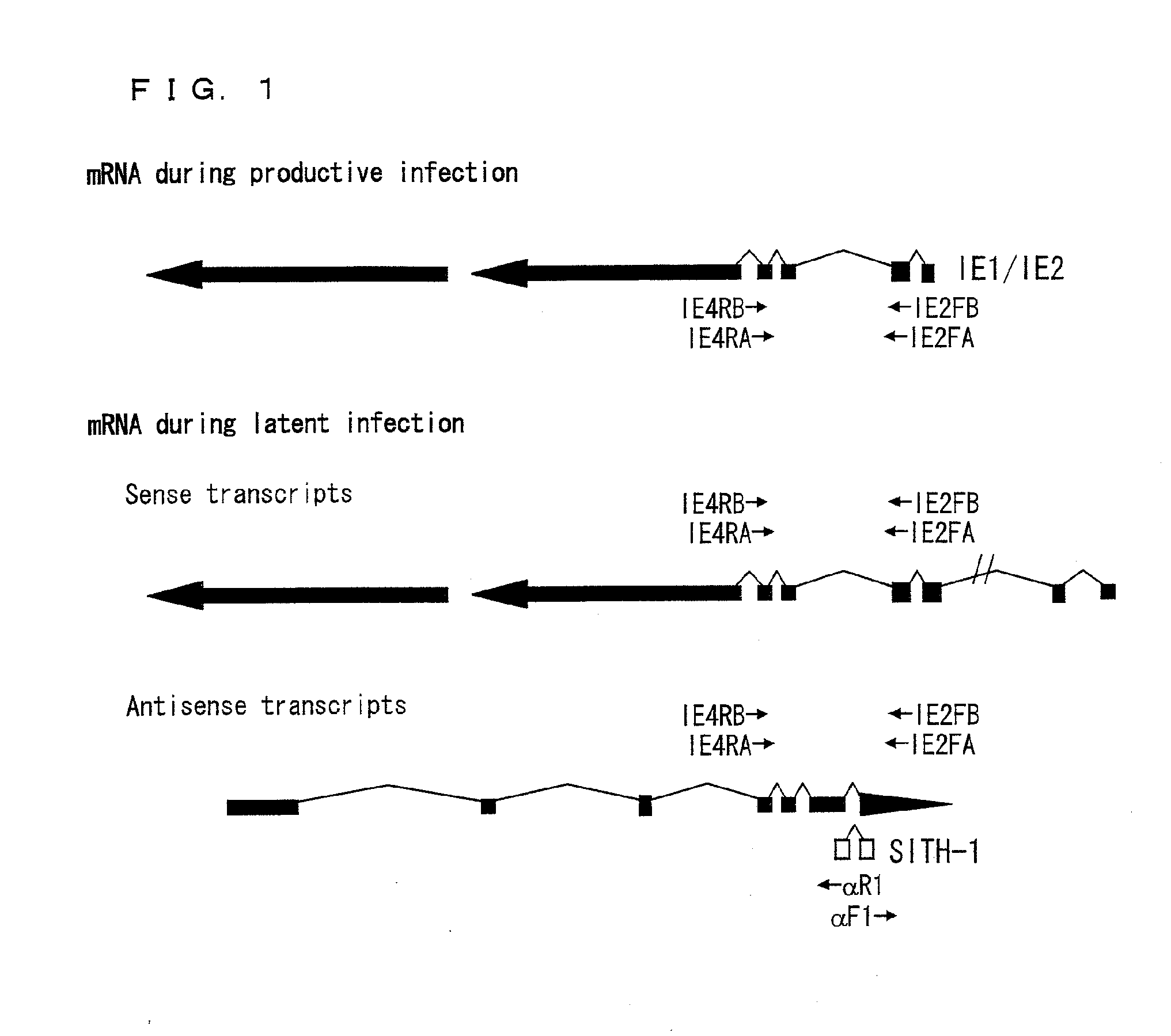
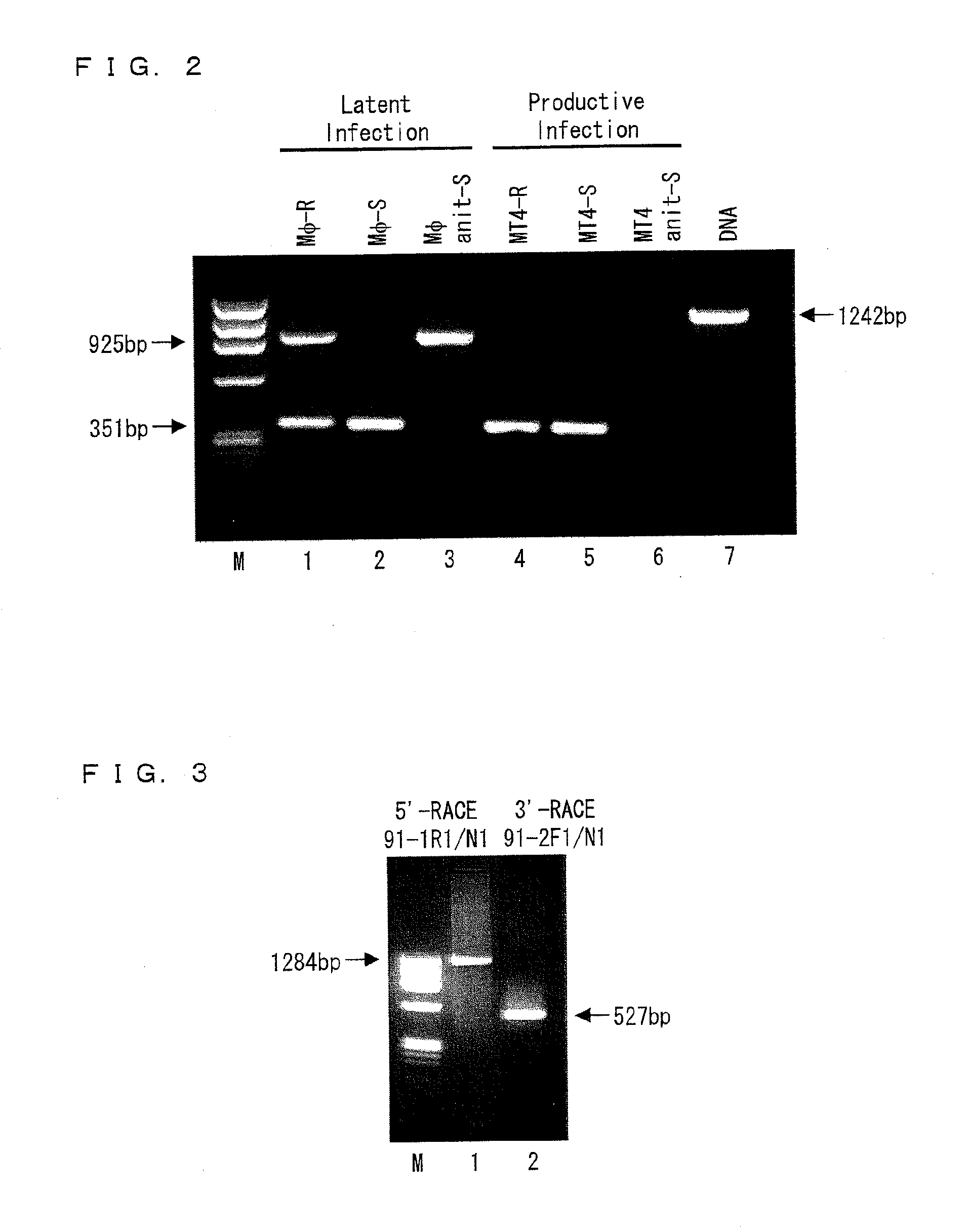
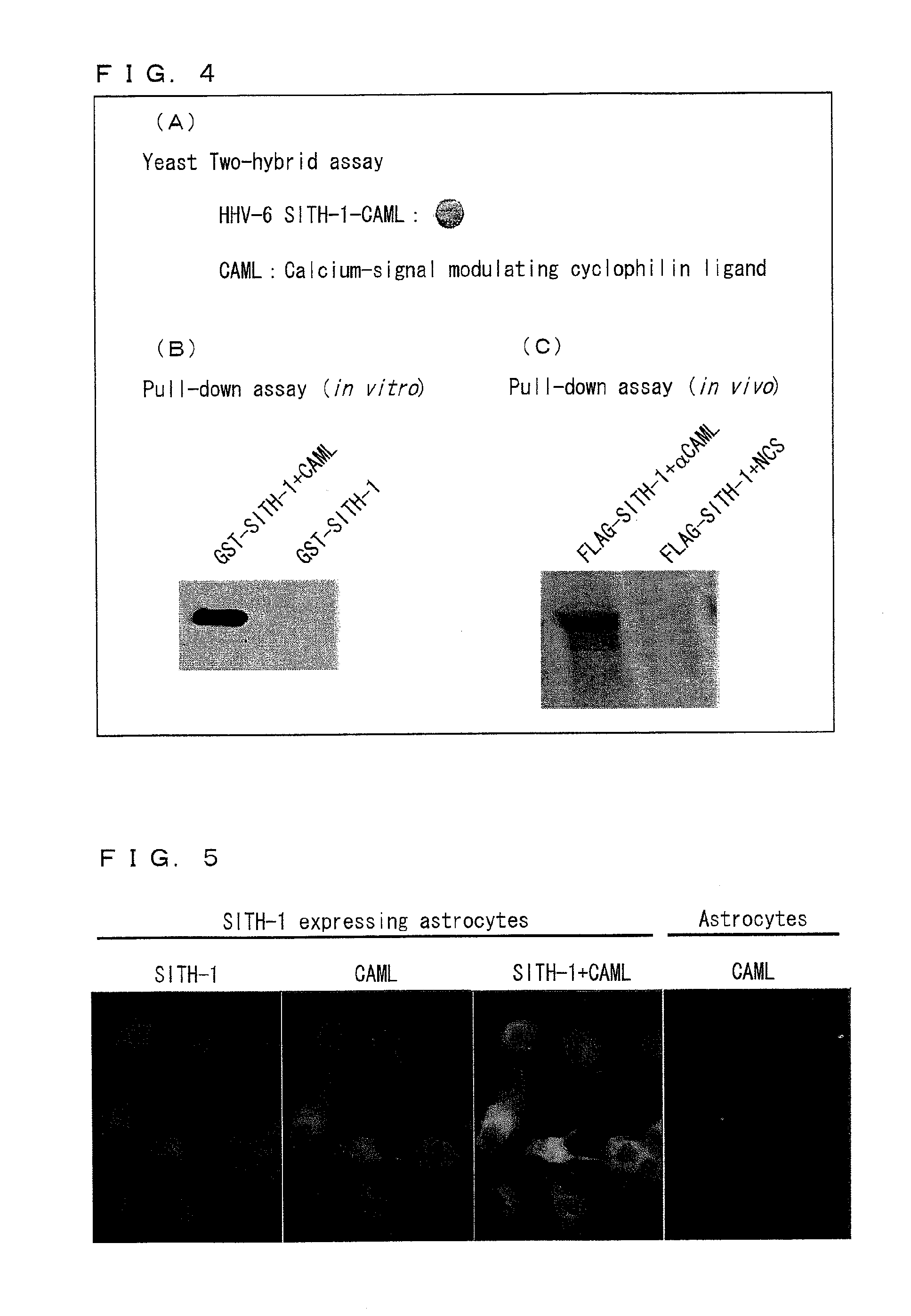
Factor involved in latent infection with herpesvirus, and use thereof
InactiveUS20130217044A1Regulate latent infectionVirus peptidesDisease diagnosisPSYCHIC DISORDERBiology
Owner:JAPAN TOBACCO INC +1
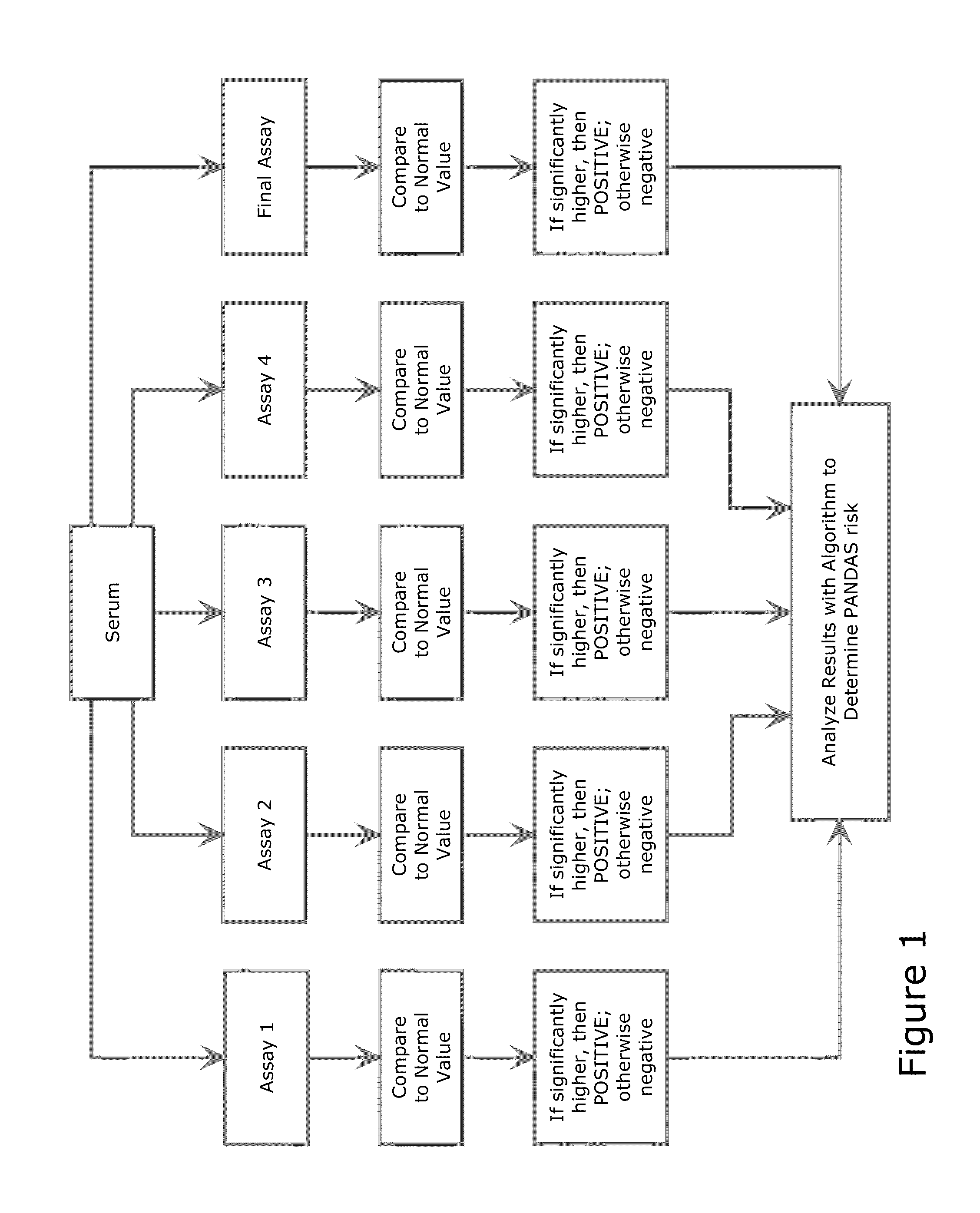
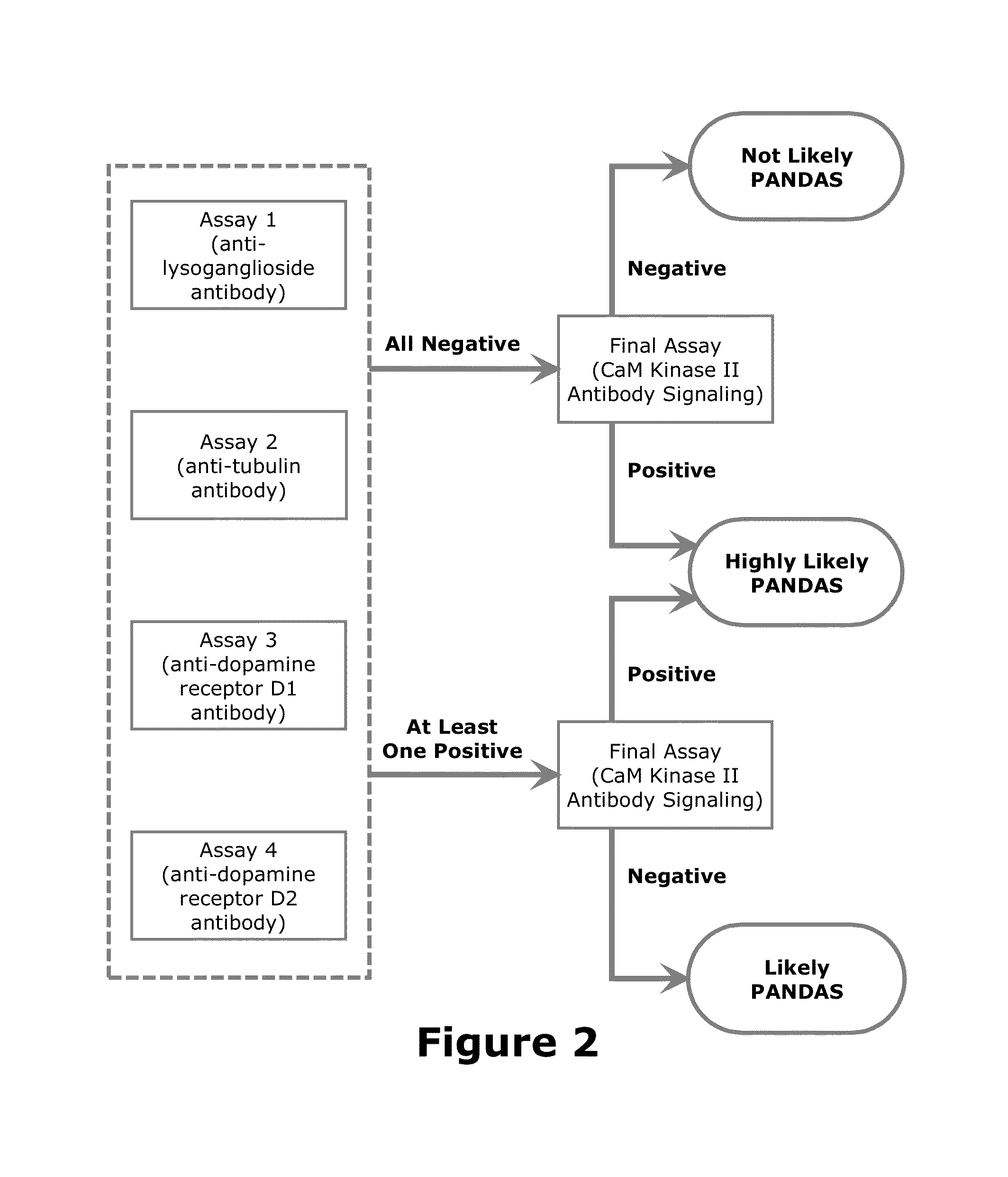
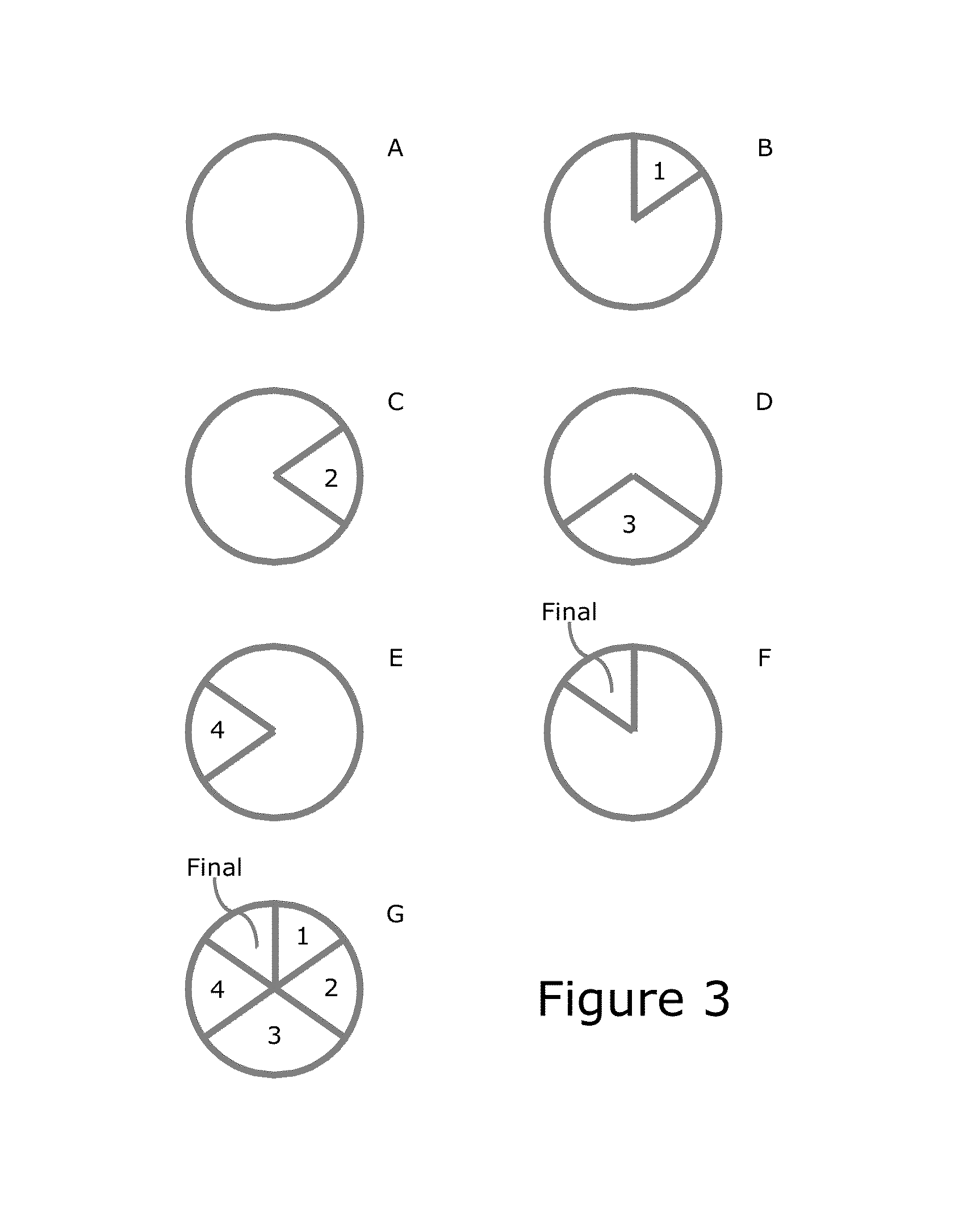
Diagnostic Method for Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococci Infection (PANDAS)
InactiveUS20140271678A1Convenient and accurate diagnosticReliable diagnosisAntibody ingredientsDisease diagnosisPresent methodCalmodulin-dependent protein kinase activity
The present invention provides a panel of at least five clinical analyses or tests (using serum samples) to determine the risk of pediatric acute-onset neuropsychiatric syndrome (PANS) and / or pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) in an individual. These include enzyme linked immunosorbent assays (ELISAs) to measure antibody titers against neuronal antigens present in the brain; the neuronal antigens include lysoganglioside, tubulin, dopamine receptor D1, dopamine receptor D2, serotonin receptor 5HT2A, and serotonin receptor 5HT2C. Antibody titers against at least four of these neuronal antigens are required in the present methods; preferably antibody tiers against all of these neuronal antigens are measured. A final assay is used to quantify calcium / calmodulin-dependent protein kinase activity using a neuronal cell line. The results of these analyses or tests are then combined using an algorithm to determine whether a PANS or PANDAS diagnosis is appropriate for the individual. Depending on the diagnosis, an appropriate treatment can be determined.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +2
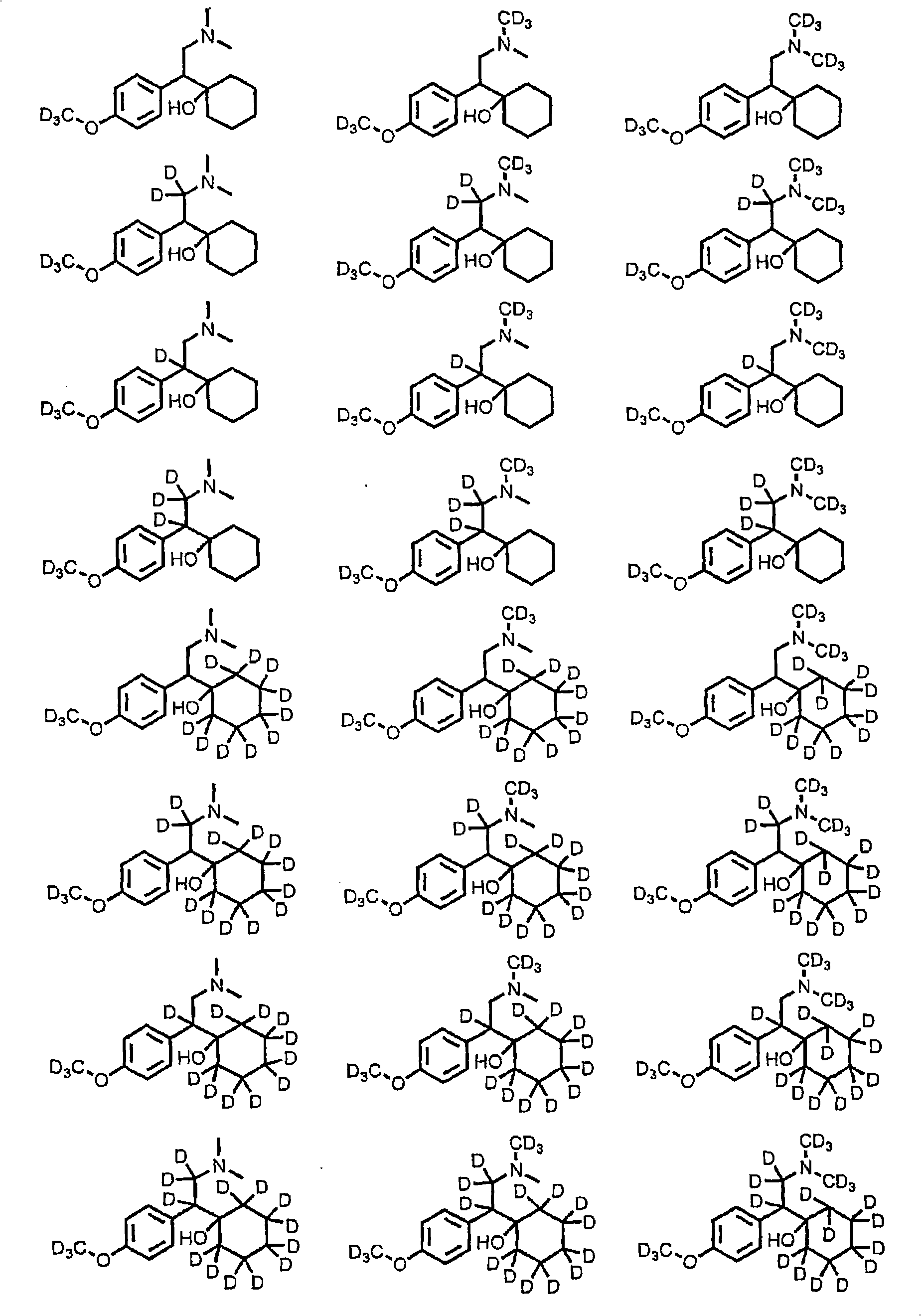
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:AUSPEX PHARMA INC
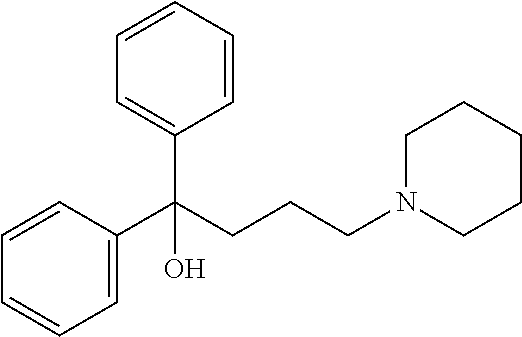
Method and pharmaceutical composition for treatment of mental disorders
InactiveUS20110065749A1Good effectLess side effectsBiocideNervous disorderAlcoholismsAttention deficits
A method of treating a mental disorder including administering to a patient in need thereof, an effective amount of a pharmaceutical composition including 1,1-diphenyl-4-piperidine-1-ylbuthan-1-ol or a pharmaceutically acceptable salt thereof. The mental disorder is depression, bipolar disorder, anxiety disorder, impulsive disorder, bulimia, panic disorder, social anxiety disorder, insomnia, attention deficit hyperactivity disorder (ADHD), schizophrenia, dementia, personality disorder, alcoholism, dissociative disorder, sleep apnea syndrome, or fibromyalgia. In a preferred embodiment, the mental disorder as a therapeutic target is fibromyalgia, chronic fatigue syndrome (CFS), or depression with pain, and the administration of the pharmaceutical composition is capable of reducing the pain caused by these diseases.
Owner:ACTIVE NETWORK LLC
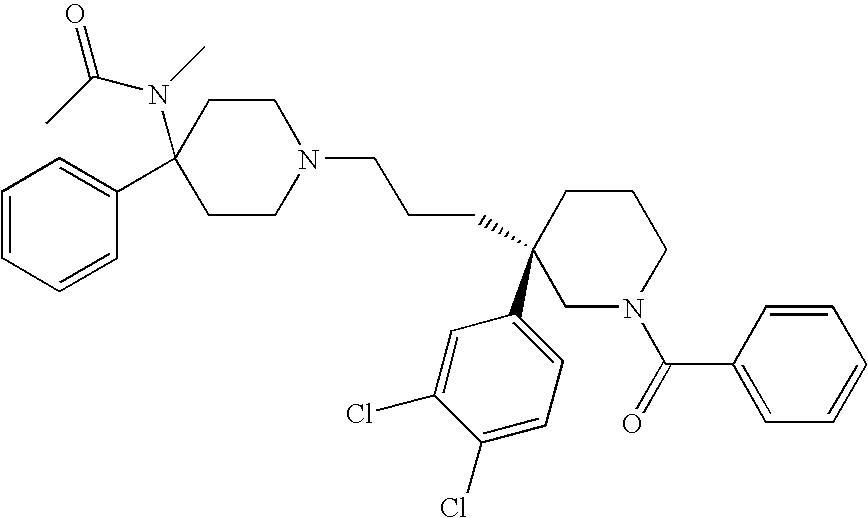
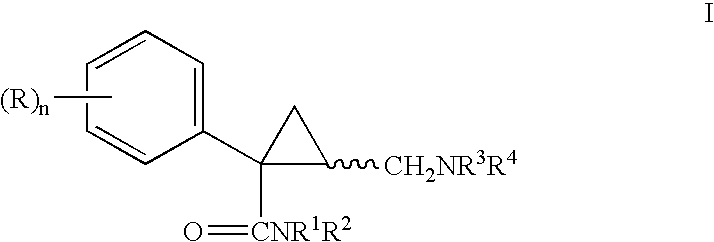
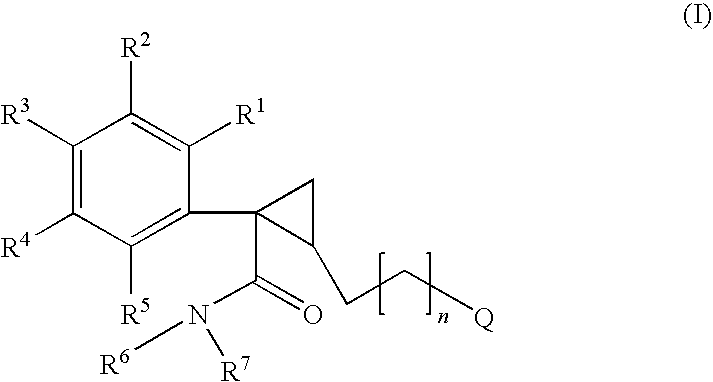
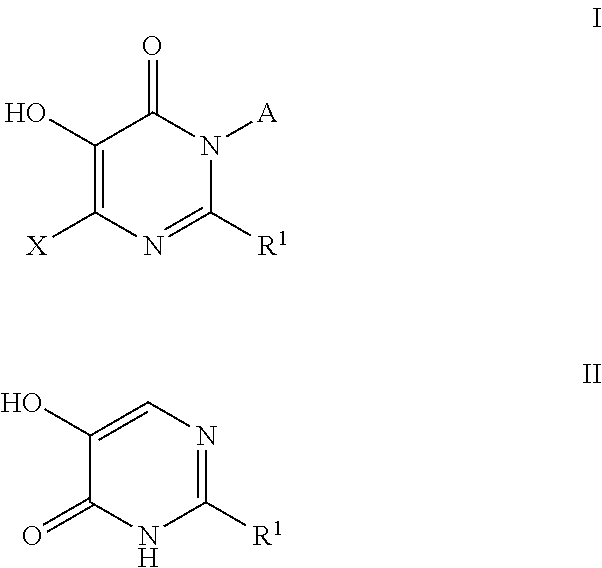
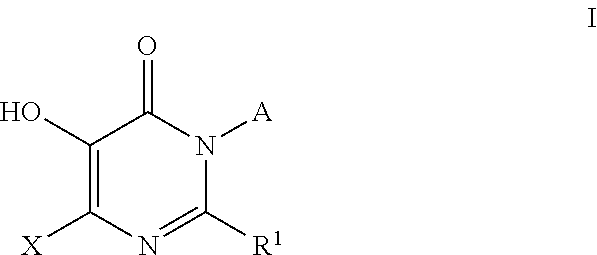
Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor
The present invention is directed to azabicyclo[4.1.0]heptane compounds which are allosteric modulators of the M4 muscarinic acetylcholine receptor. The present invention is also directed to uses of the compounds described herein in the potential treatment or prevention of neurological and psychiatric disorders and diseases in which M4 muscarinic acetylcholine receptors are involved. The present invention is also directed to compositions comprising these compounds. The present invention is also directed to uses of these compositions in the potential prevention or treatment of such diseases in which M4 muscarinic acetylcholine receptors are involved.
Owner:MERCK SHARP & DOHME LLC
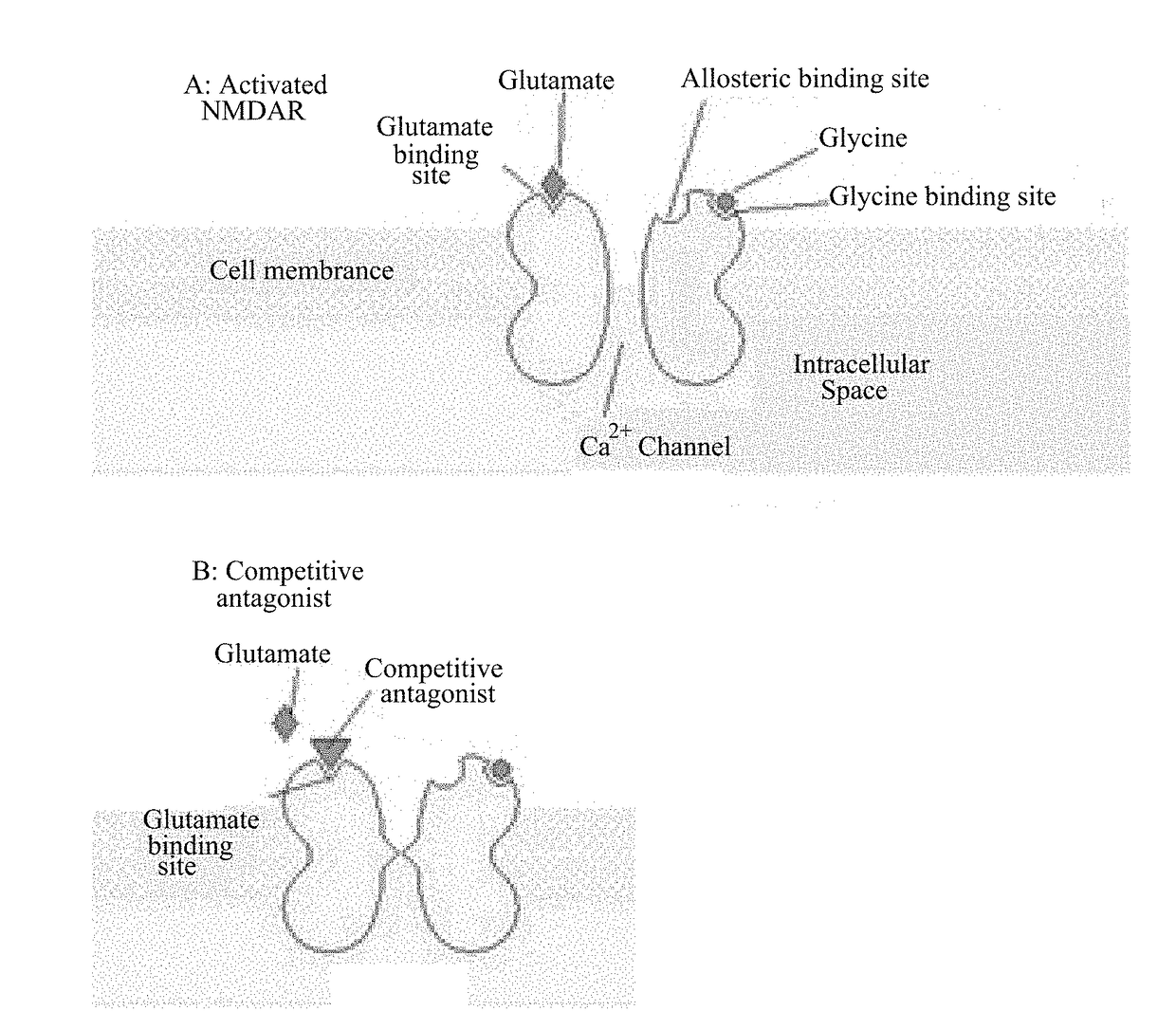
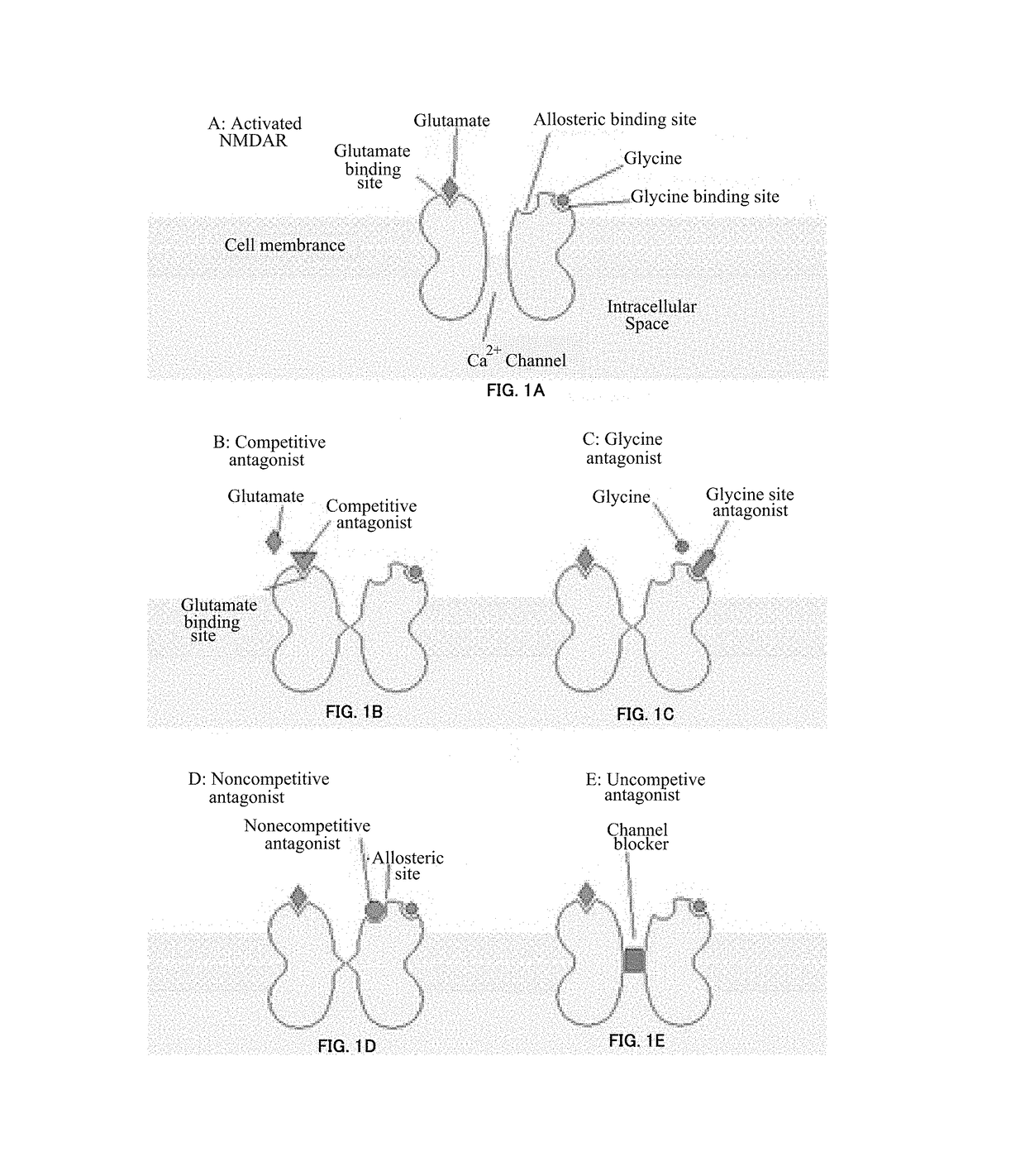
NMDA antagonists for the treatment of mental disorders with occurrence of aggressive and/or impulsive behavior
InactiveUS20180110742A1Improve latencyReduce generationOrganic active ingredientsNervous disorderPSYCHIC DISORDERPsychogenic disease
Disclosed is the use of N-Methyl-D-aspartate (NMDA) antagonists at sub-anesthetic doses for the treatment of motor dysfunction in mental or psychiatric disorders with occurrence of aggressive and / or impulsive behavior.
Owner:ICM INST DU CERVEAU & DE LA MOELLE EPINIERE +5
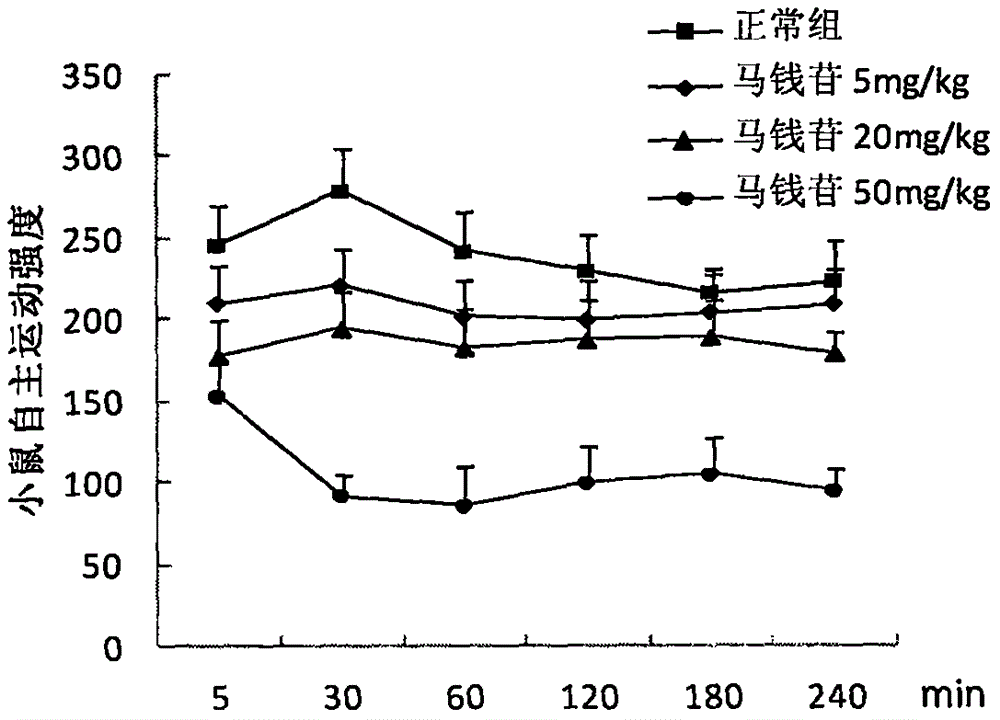
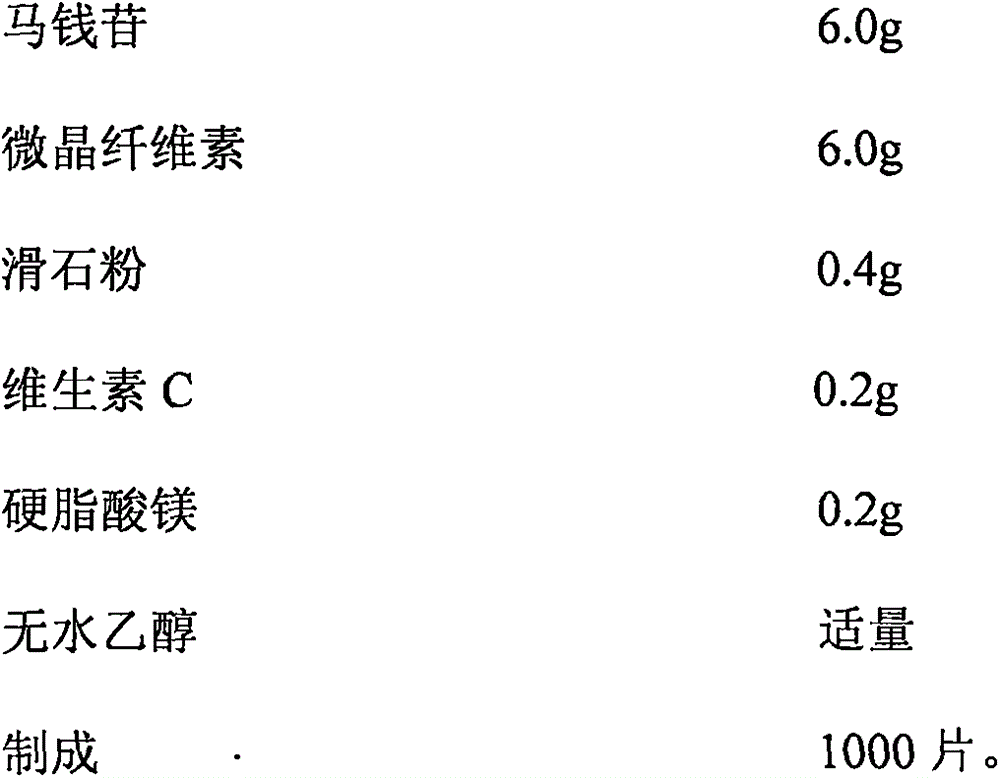
Application of loganin to preparation of drugs or health products for preventing and treating mental disturbance diseases such as depressive disorder and anxiety disorder
InactiveCN106176789ABroaden the scope of disease prevention and treatmentWide variety of sourcesOrganic active ingredientsNervous disorderDiseasePSYCHIC DISORDER
The invention belongs to the field of compound drugs, and relates to new drug application of loganin, particularly application of loganin to the preparation of drugs or health products for preventing and treating mental disturbance diseases such as depressive disorder and anxiety disorder. The loganin provided by the invention is one of main chemical components of Cornus officinalis which is a Cornaceae plant, the chemical formula of the loganin is C17H26O10, and the molecular weight of the loganin is 390.38; animal experiments prove that the loganin can reduce autonomous exercise intensity of a mouse, the number of rotational turns of a pulley poked by fore limbs of the mouse is obviously increased, the dead time of tail suspension of the mouse is shortened, and an antidepressant effect is shown; the loganin can further increase the time percentage of staying at the open arms of a maze of a stress rat and seek the frequency of arm opening, meanwhile, in a light and shade box shuttling experiment, the loganin can obviously increase the time that the mouse stays in a light box and the frequency of shuttling in the light box, and an obvious anxiolytic effect is shown. Above all, the loganin can be prepared into the drugs or health products for preventing and treating the mental disturbance diseases such as depressive disorder and anxiety disorder, and prepared dosage forms comprise granules, tablets, capsules, granules, powder, oral liquids, dropping pills, pellets or injections.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
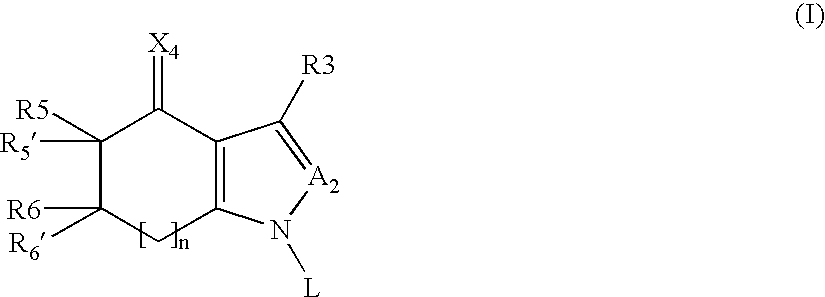
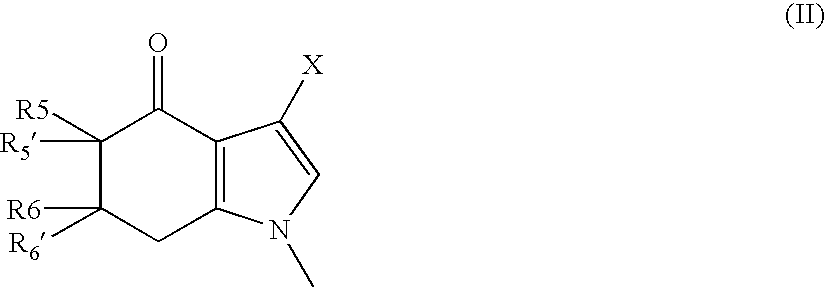
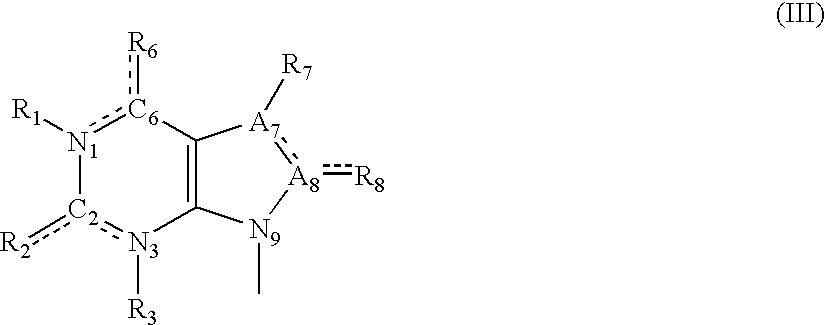
![6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor 6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/5ab30fb2-8fca-4493-8169-99a8cdaeecbf/US10329289-C00001.png)
![6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor 6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/5ab30fb2-8fca-4493-8169-99a8cdaeecbf/US10329289-C00002.png)
![6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor 6,7-dihydro-5H-pyrrolo[3,4-B]pyridin-5-one allosteric modulators of the M4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/5ab30fb2-8fca-4493-8169-99a8cdaeecbf/US10329289-C00003.png)
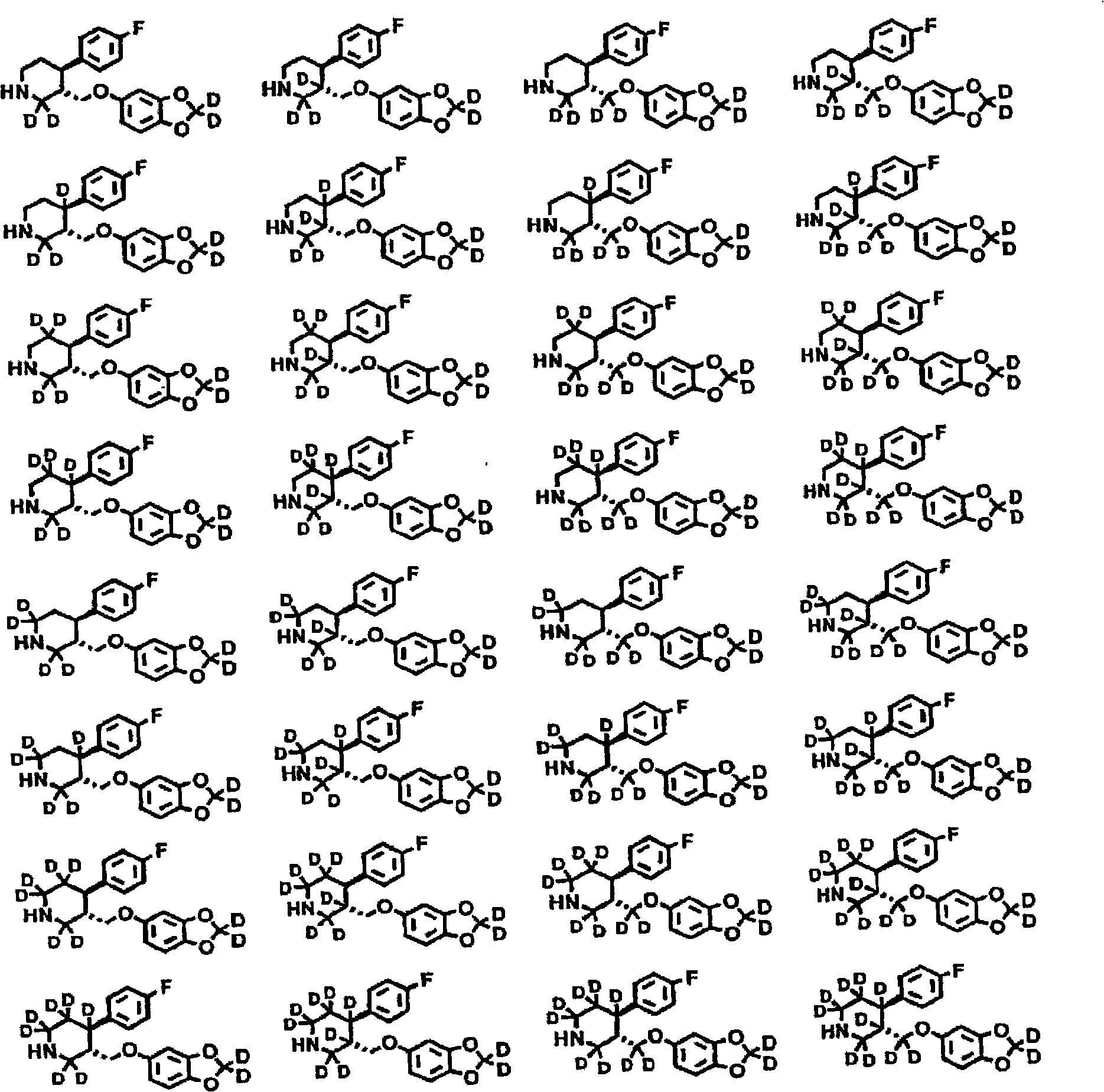
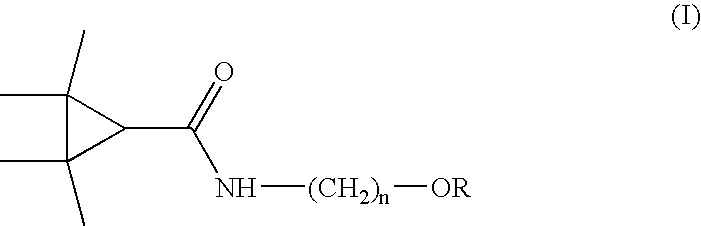
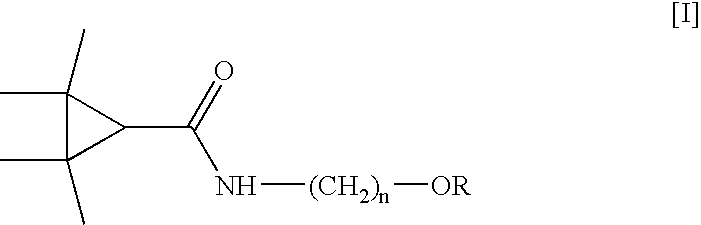
![Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/86396100-cd8a-449a-af2f-fda761481c5c/US20200095262A1-C00001.png)
![Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/86396100-cd8a-449a-af2f-fda761481c5c/US20200095262A1-C00002.png)
![Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor Azabicyclo[4.1.0]heptane allosteric modulators of the m4 muscarinic acetylcholine receptor](https://images-eureka.patsnap.com/patent_img/86396100-cd8a-449a-af2f-fda761481c5c/US20200095262A1-C00003.png)