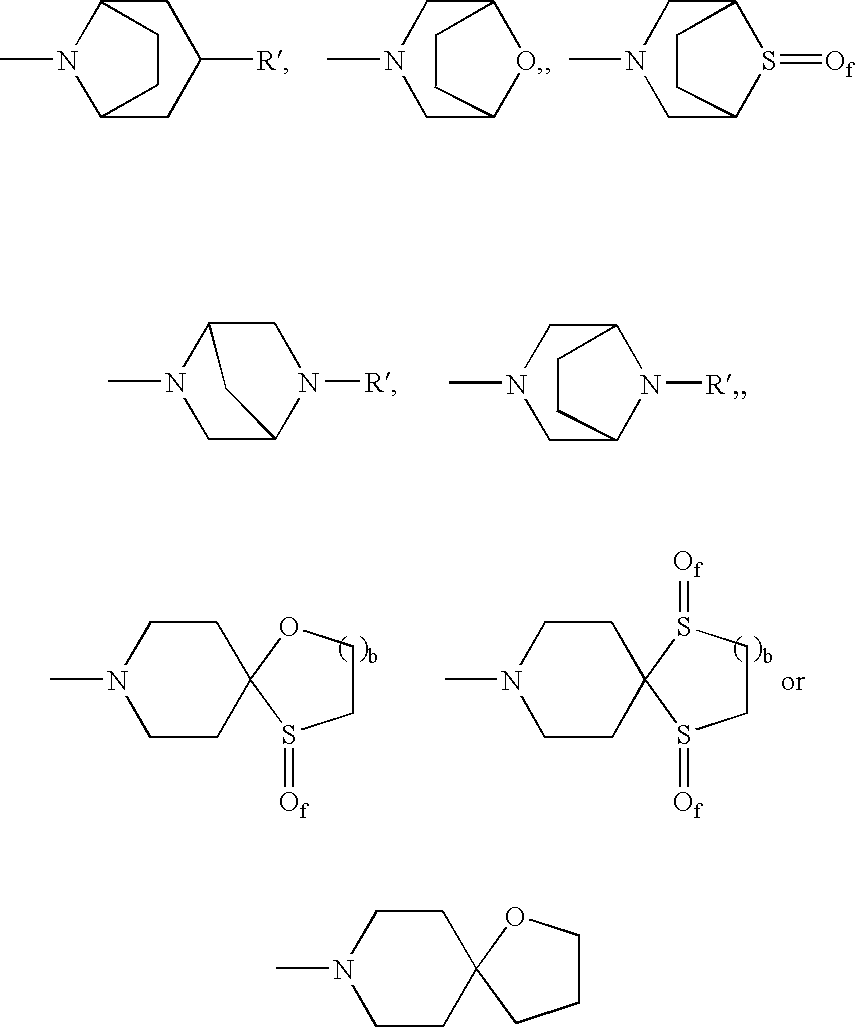
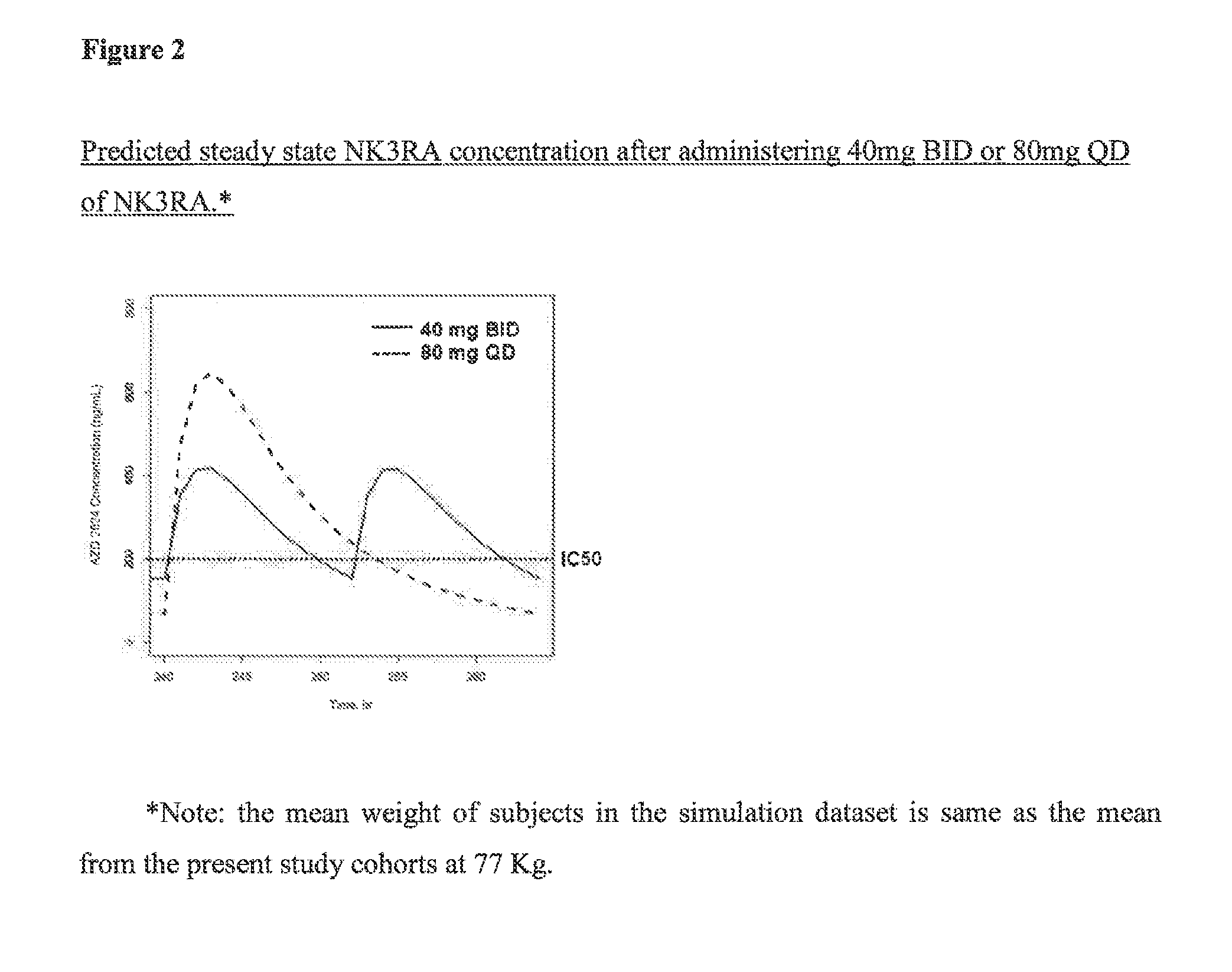
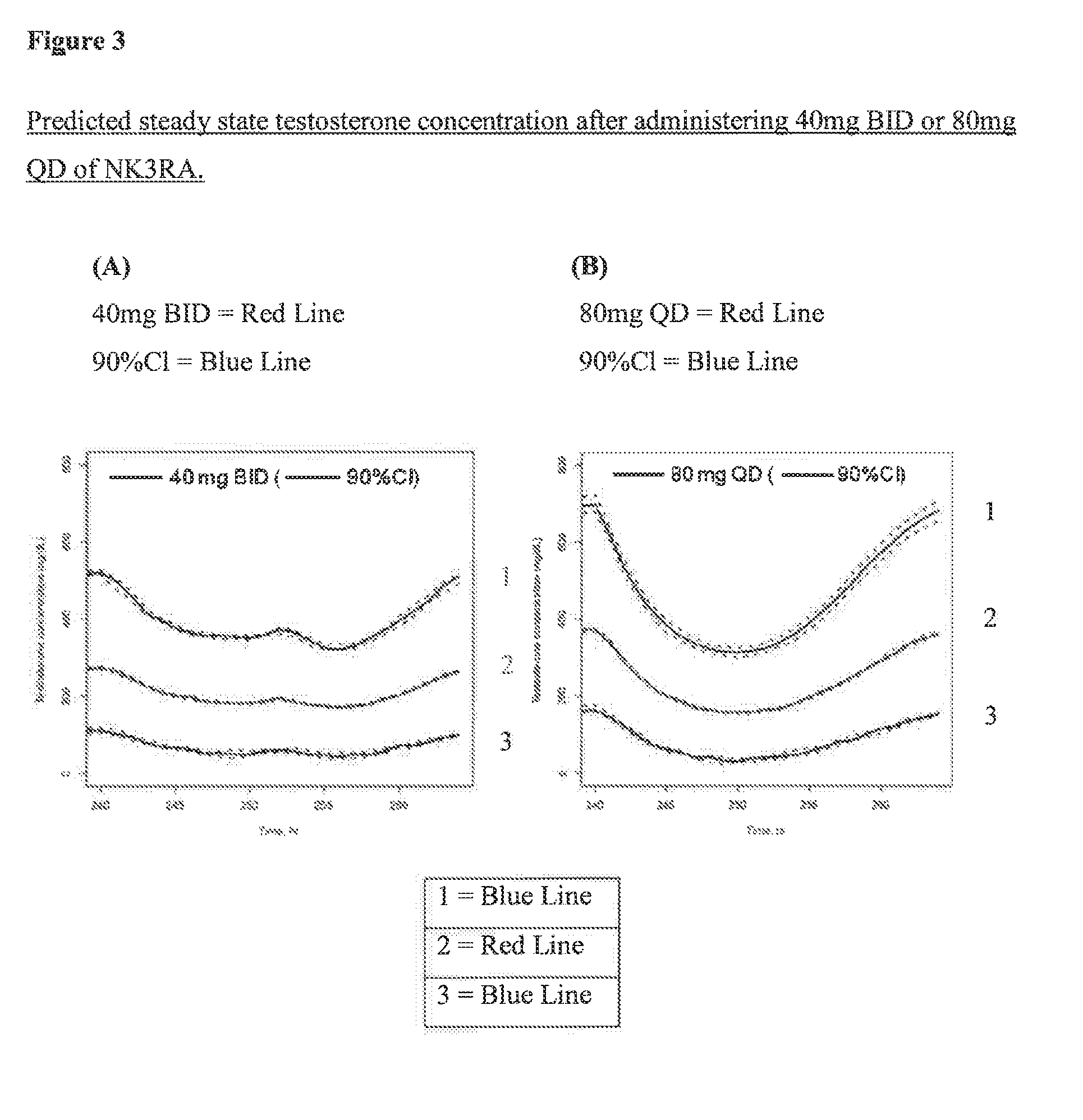
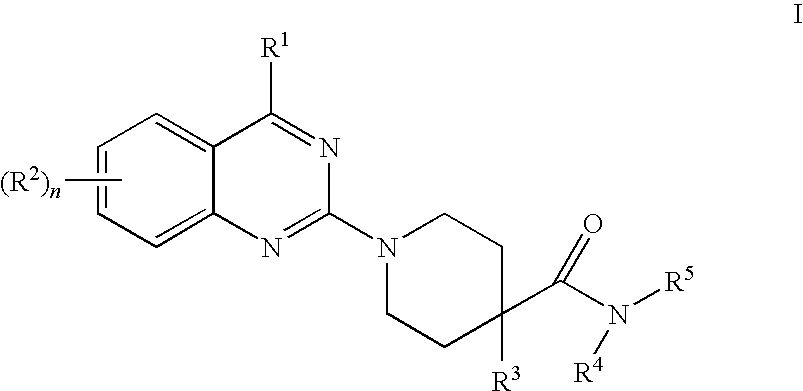
Patents
Literature
46 results about "Nk3 receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
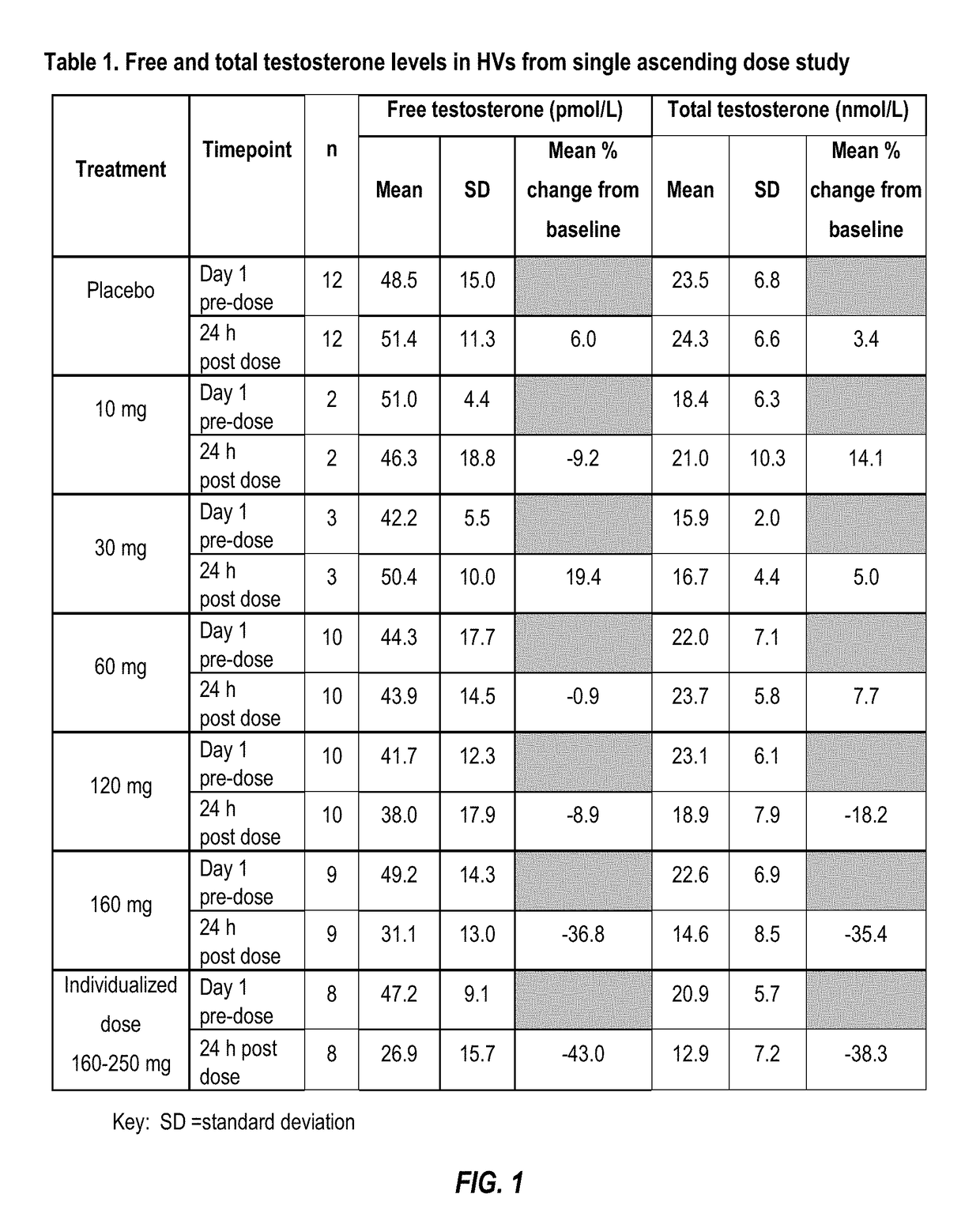
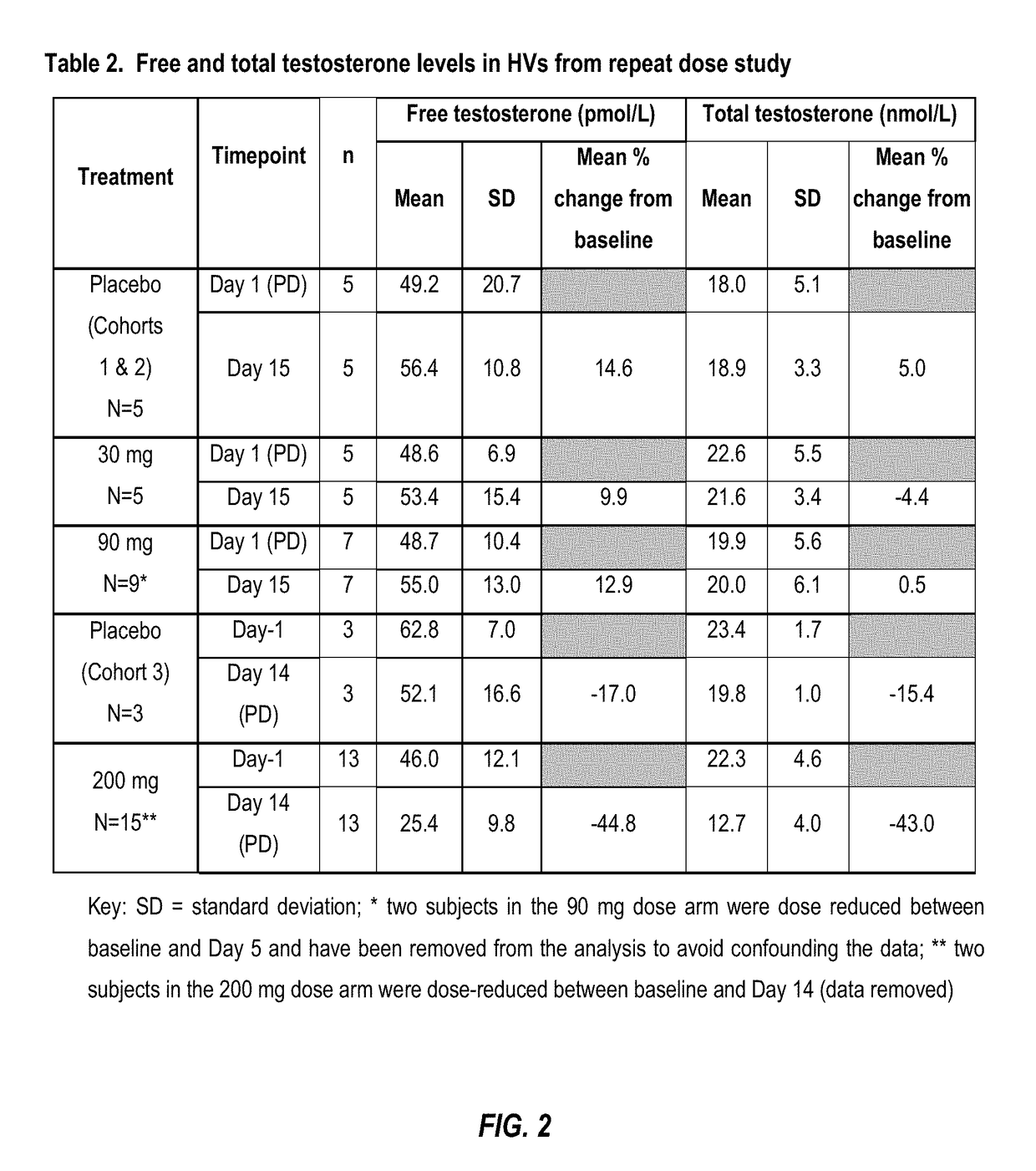
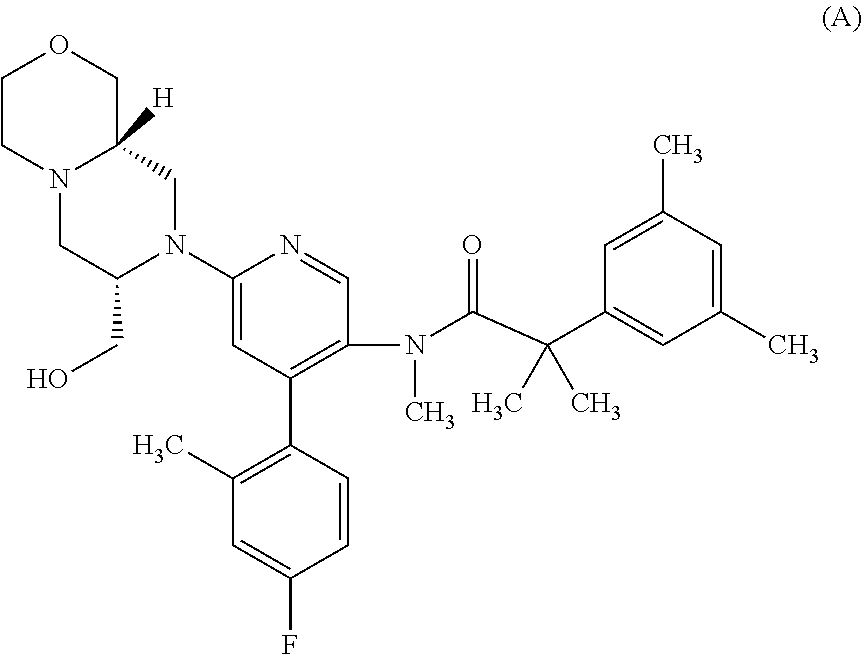
NK 3 Receptors. The neurokinin 3 (NK3) receptor is a member of the tachykinin family of G-protein-coupled receptors which also includes NK1 and NK2 receptors. The NK 3 receptor is predominantly expressed in the CNS (including the hippocampus, hypothalamus and substantia nigra).
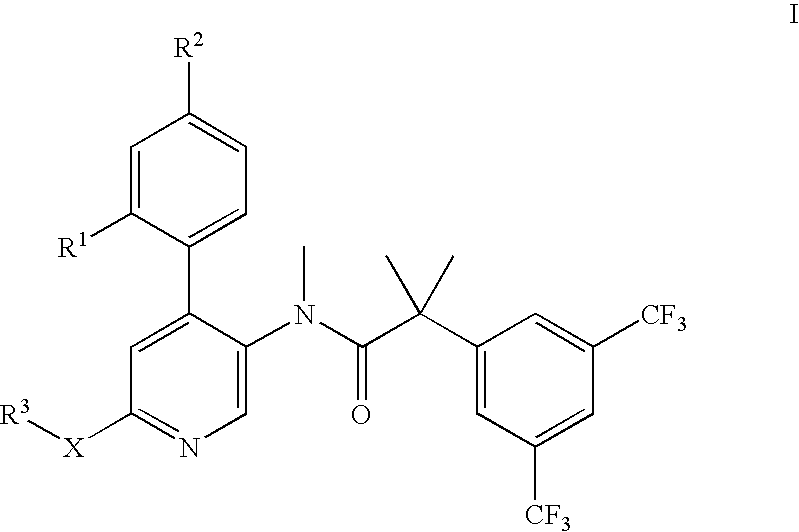
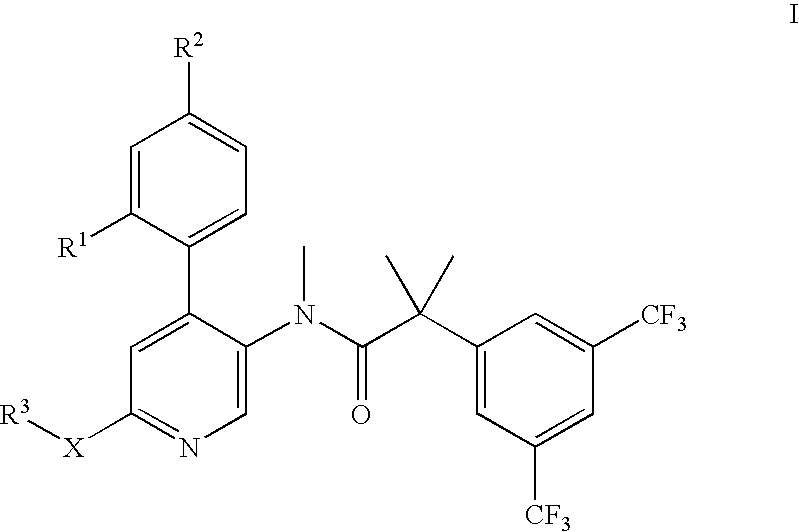
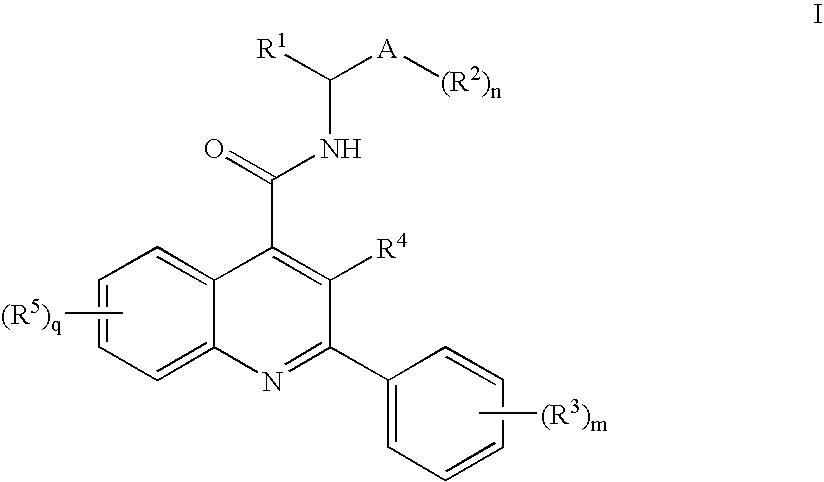
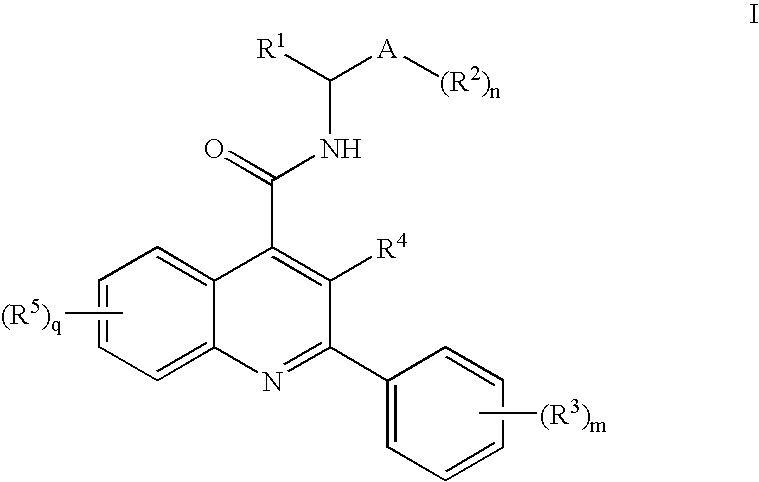
Dual NK1/NK3 receptor antagonists for the treatment of schizophrenia
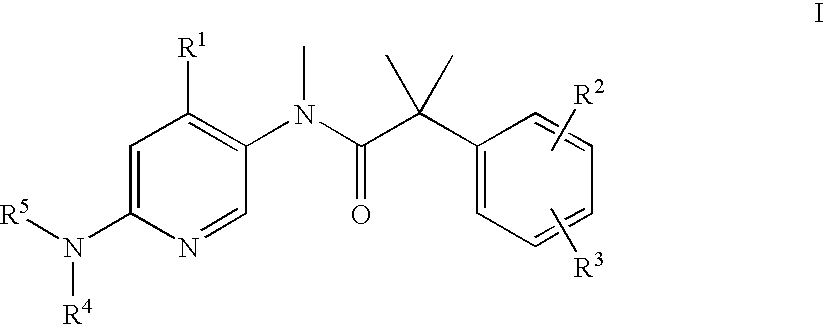
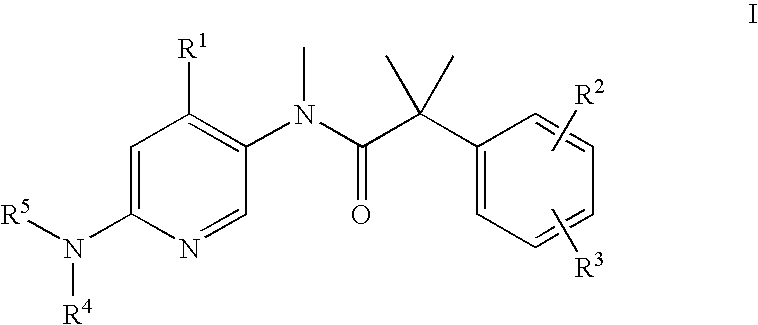
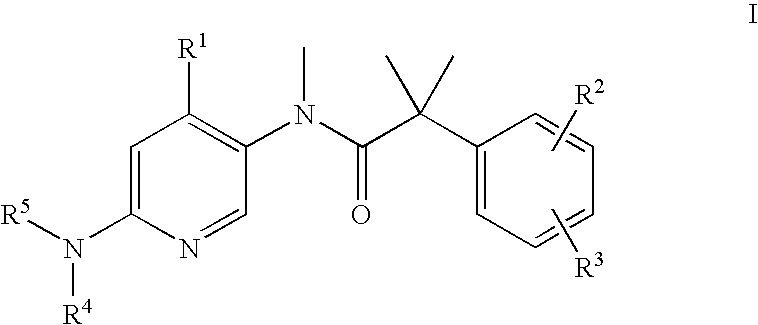
The present invention relates to a method of treating schizophrenia which comprises administering a therapeutically effective amount of a compound of formula I wherein R1, R2, and R3 are as defined in the specification or to pharmaceutically active acid-addition salts thereof.
Owner:F HOFFMANN LA ROCHE INC
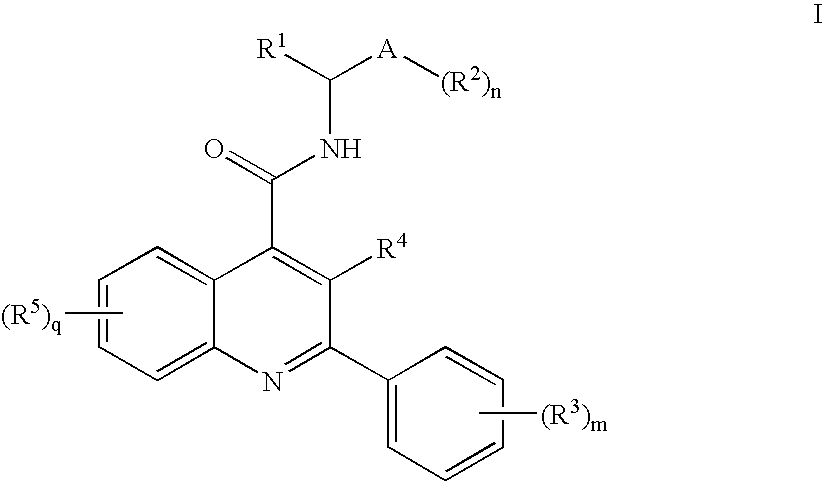
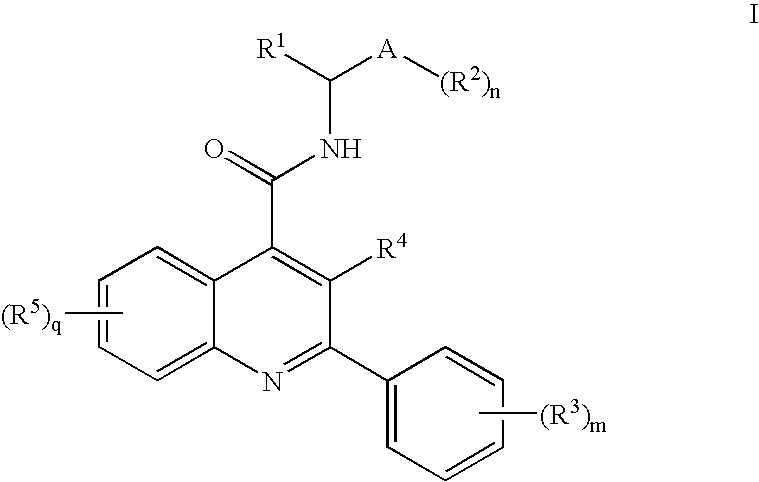
Dual NK1/NK3 receptor antagonists
The present invention provides a method for the treatment of schizophrenia which comprises administering a compound of formulawherein the substituents are as described herein or a pharmaceutically active acid-addition salt thereof. In particular, the invention provides methods for treating both positive and negative symptoms of schizophrenia through dual inhibition of NK1 and NK3 receptors. The invention also provides novel compounds with formula I and methods for preparing compounds of the invention.
Owner:F HOFFMANN LA ROCHE INC
Dual NK1/NK3 receptor antagonists
The present invention provides a method for the treatment of schizophrenia which comprises administering a compound of formula wherein the substituents are as described herein or a pharmaceutically active acid-addition salt thereof. In particular, the invention provides methods for treating both positive and negative symptoms of schizophrenia through dual inhibition of NK1 and NK3 receptors. The invention also provides novel compounds with formula I and methods for preparing compounds of the invention.
Owner:F HOFFMANN LA ROCHE & CO AG
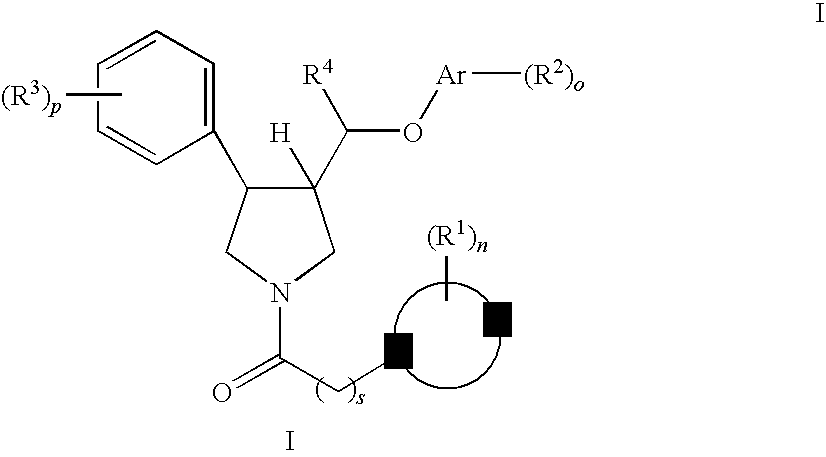
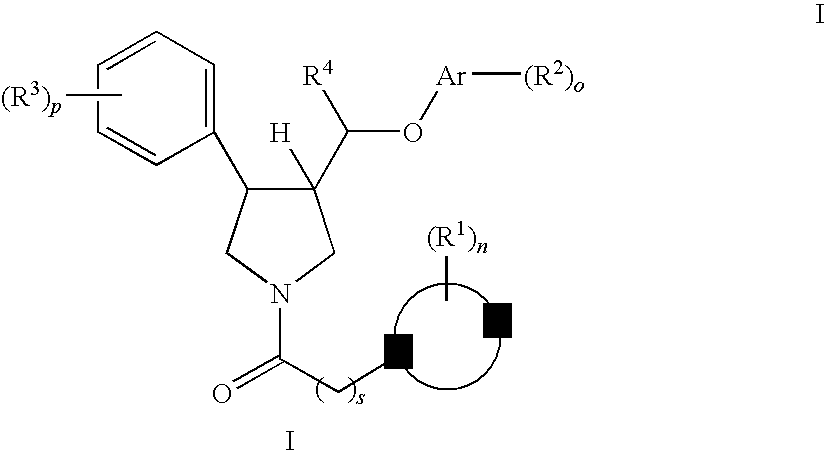
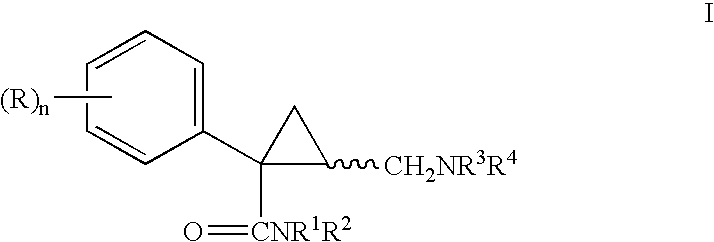
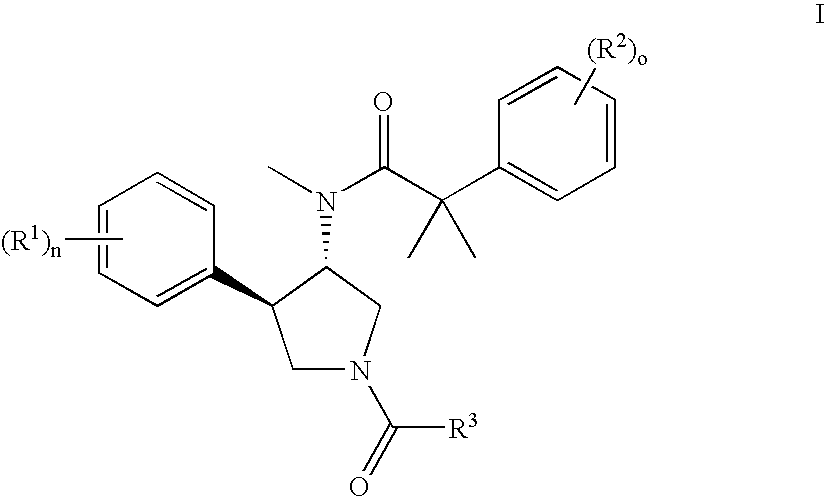
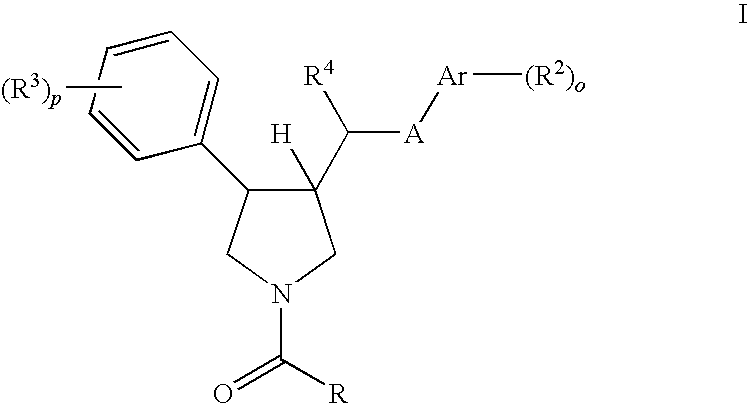
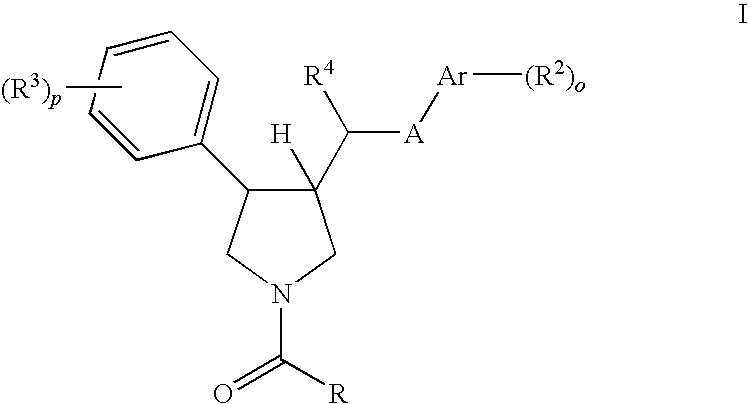
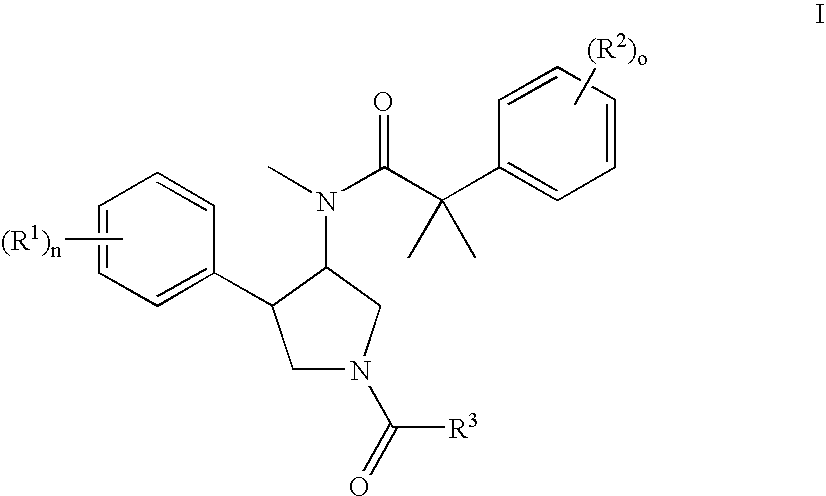
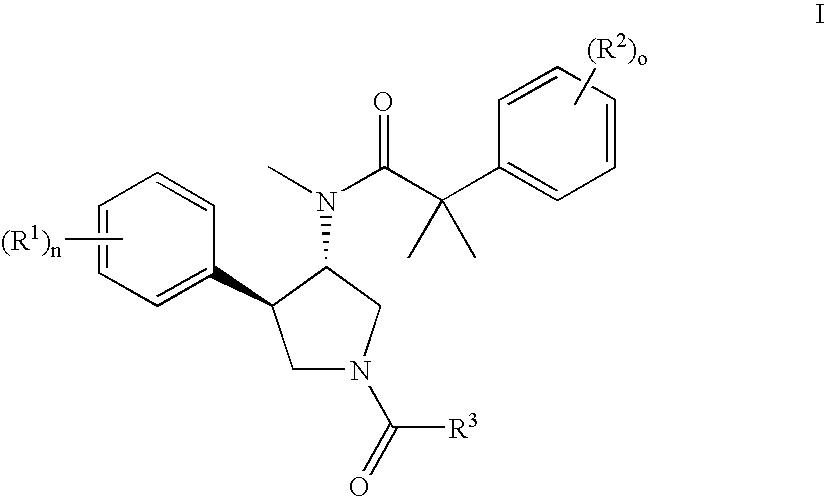
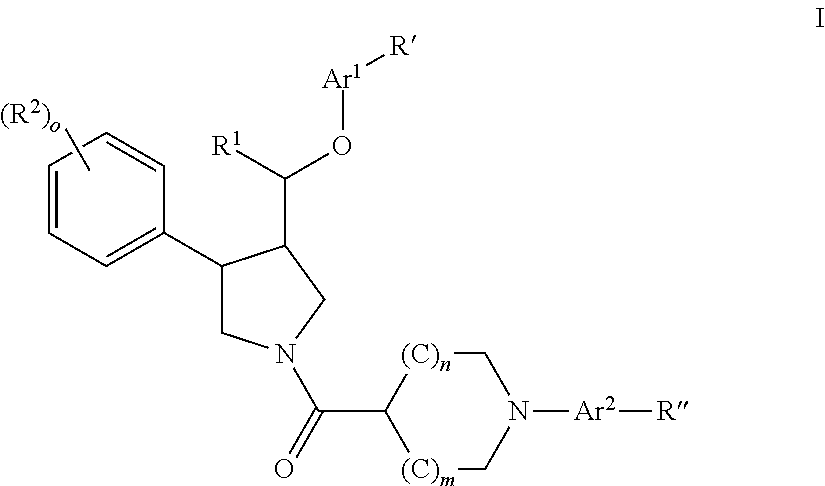
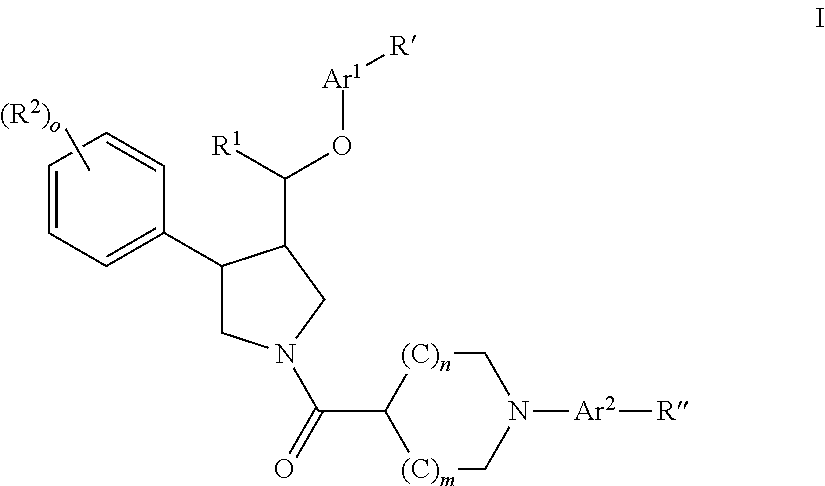
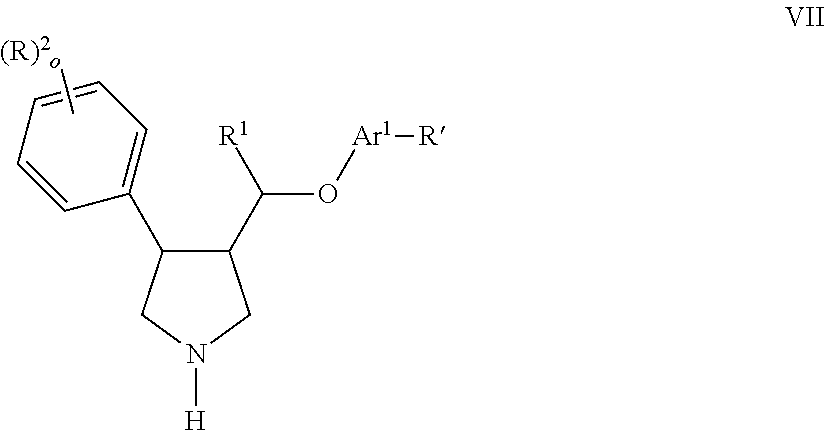
Pyrrolidine aryl-ether as NK3 receptor antagonists
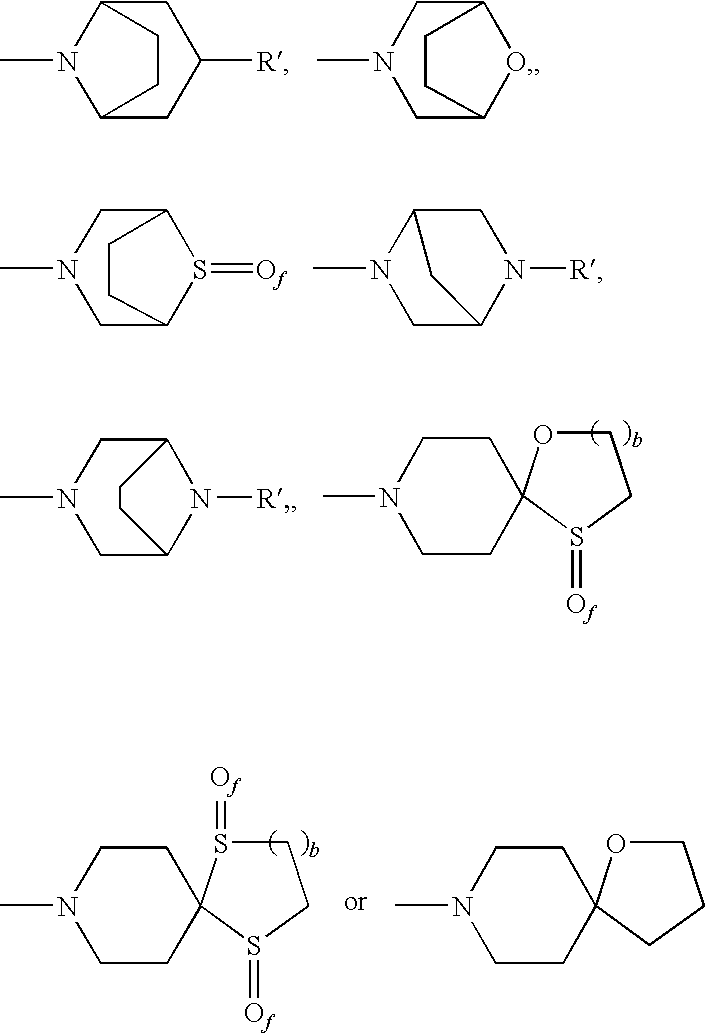
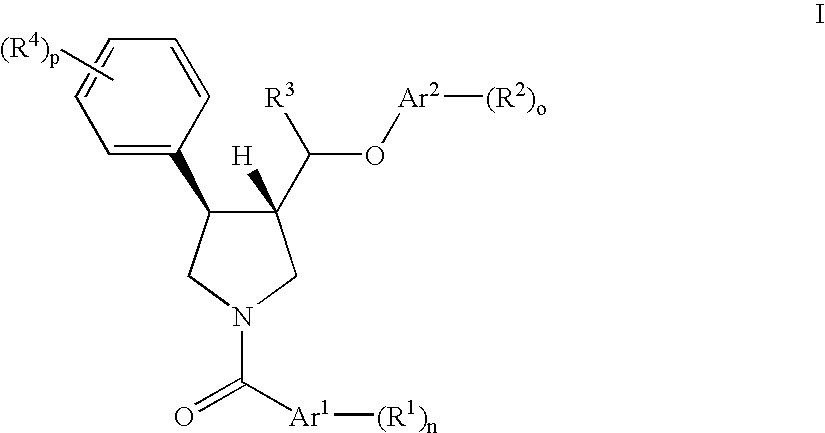
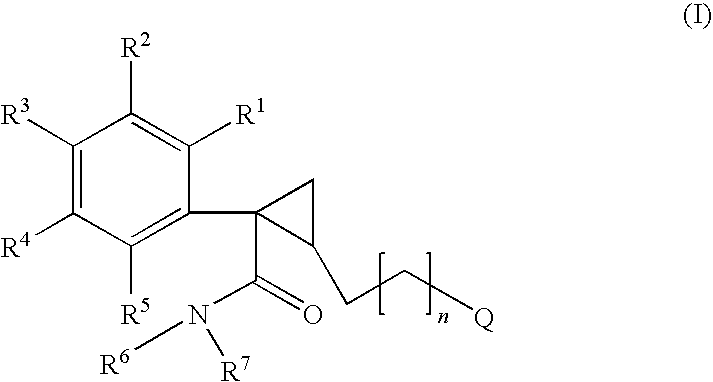
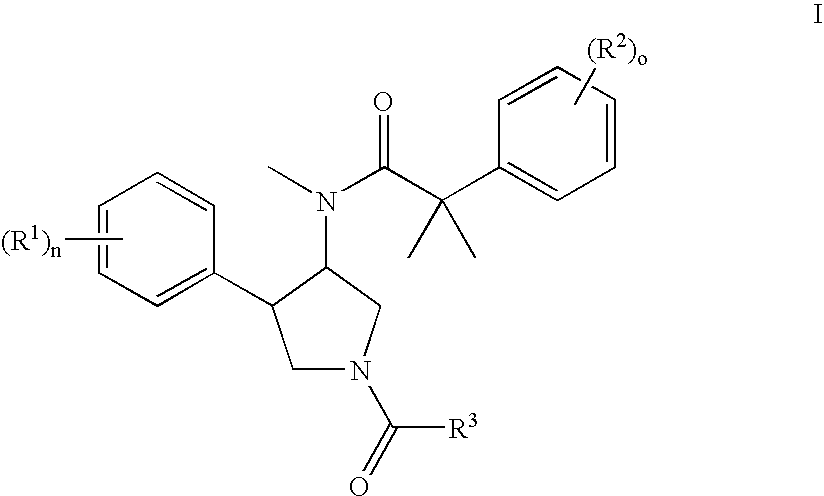
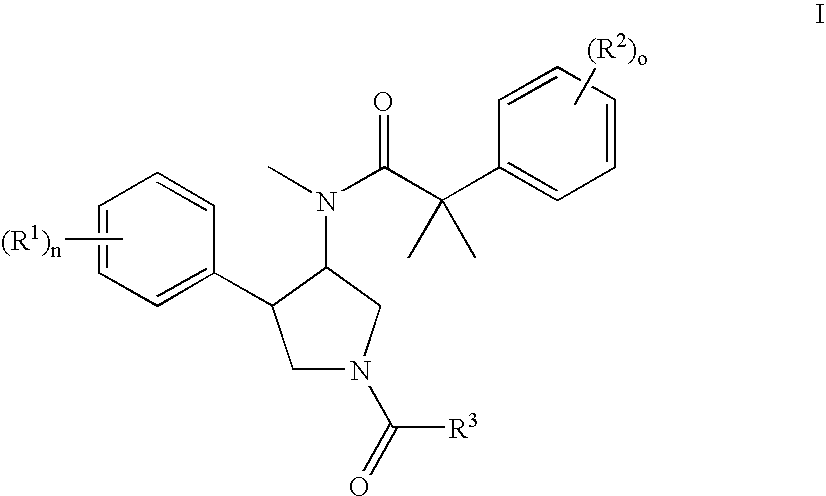
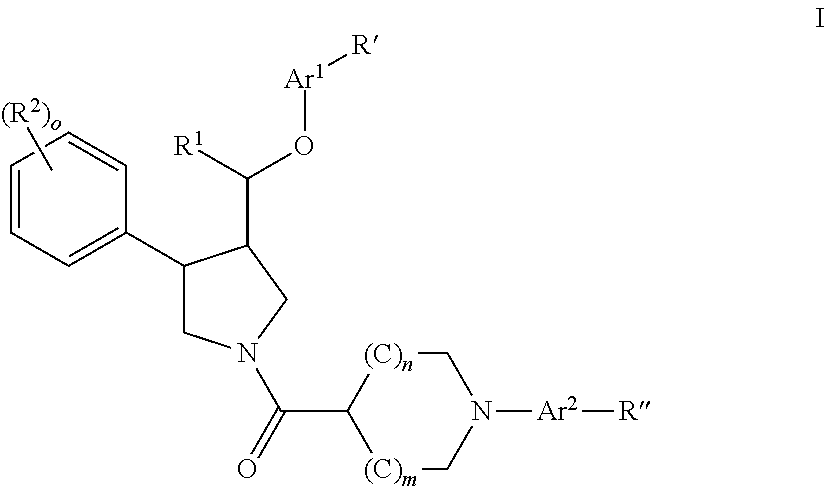
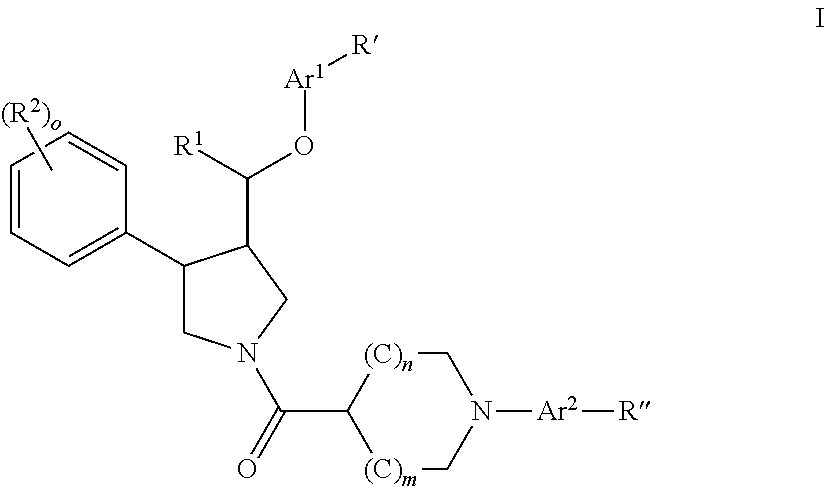
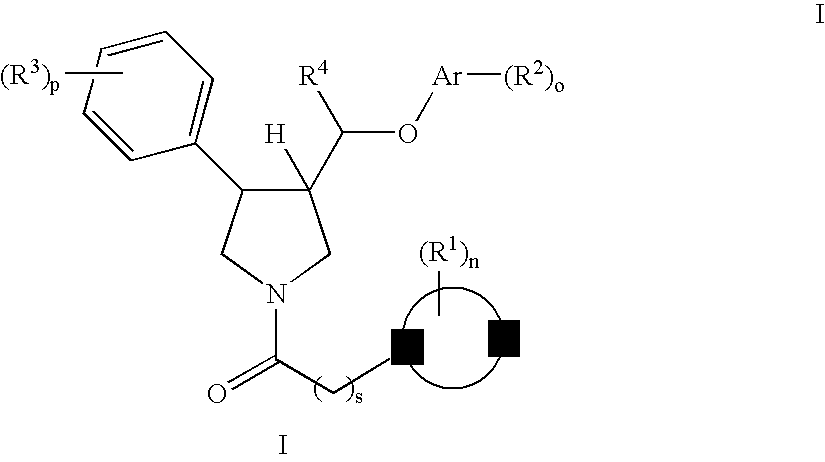
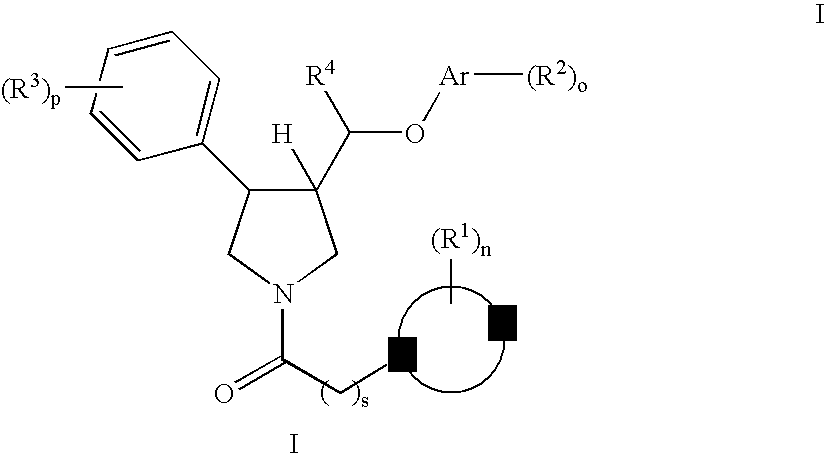
The invention relates to a compound of general formulawhereinAr, R1, R2, R3, R4, n, o, p, s, X andare as defined herein or to a pharmaceutically active salt thereof, including all stereoisomeric forms, individual diastereoisomers and enantiomers of the compound of formula (I) as well as racemic and non-racemic mixtures thereof. The compounds are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
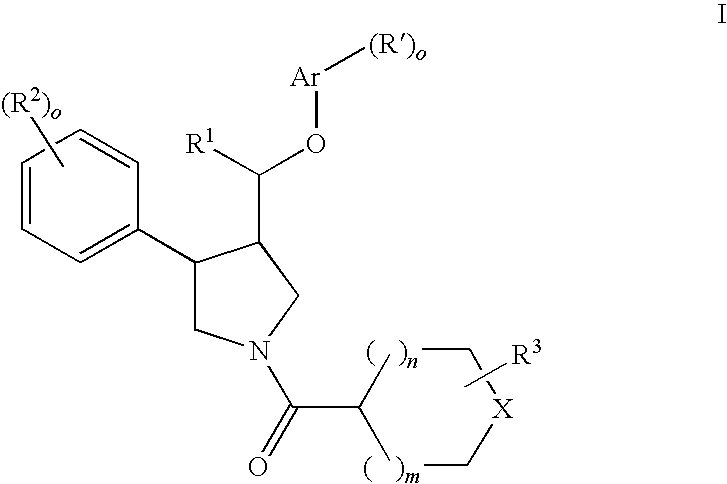
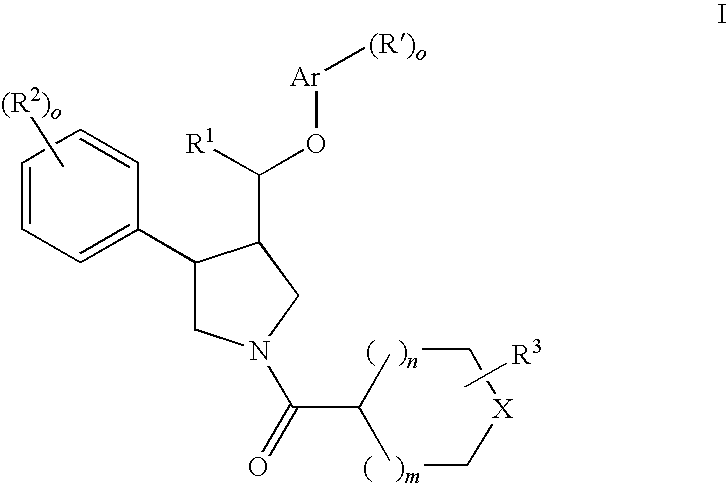
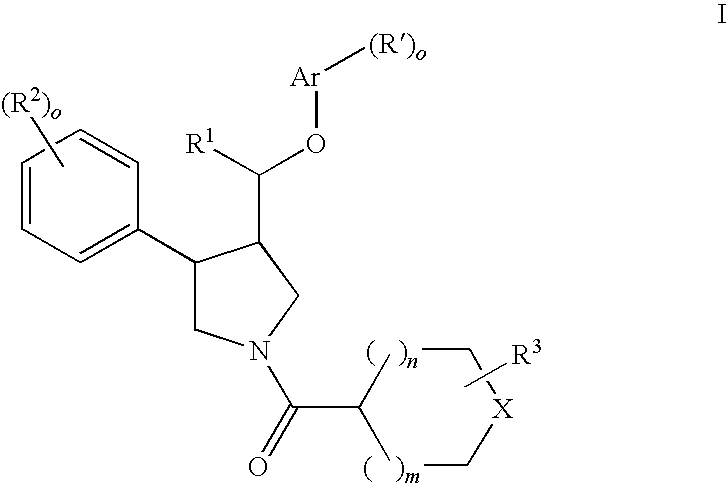
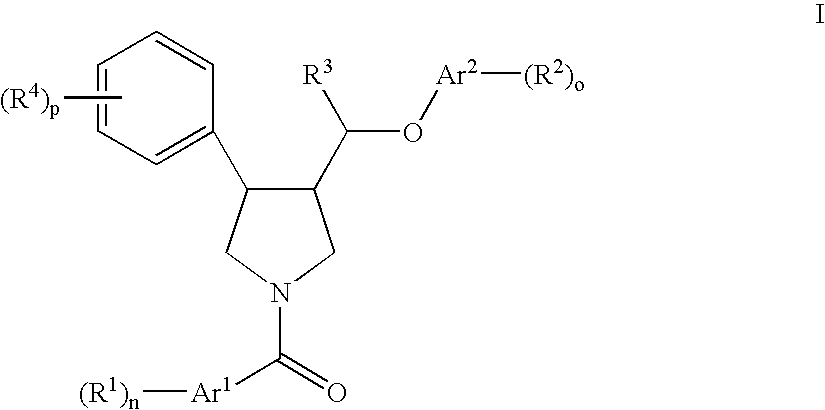
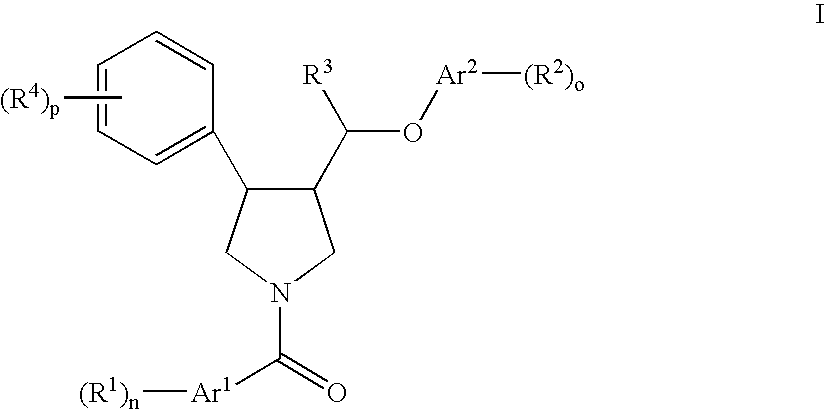
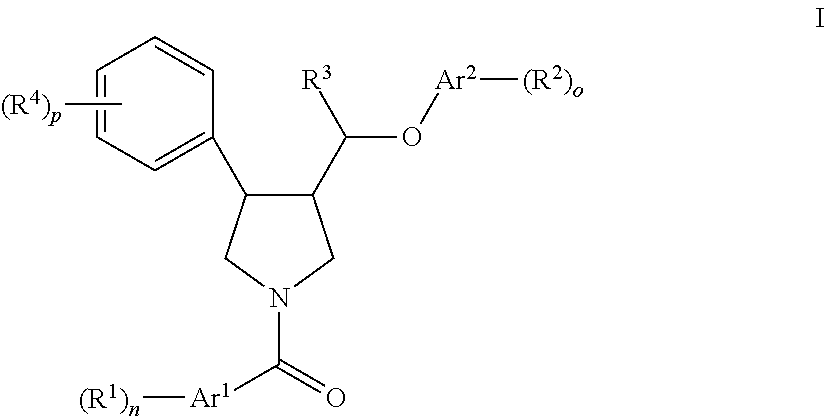
Pyrrolidine ether derivatives as NK3 receptor antagonists
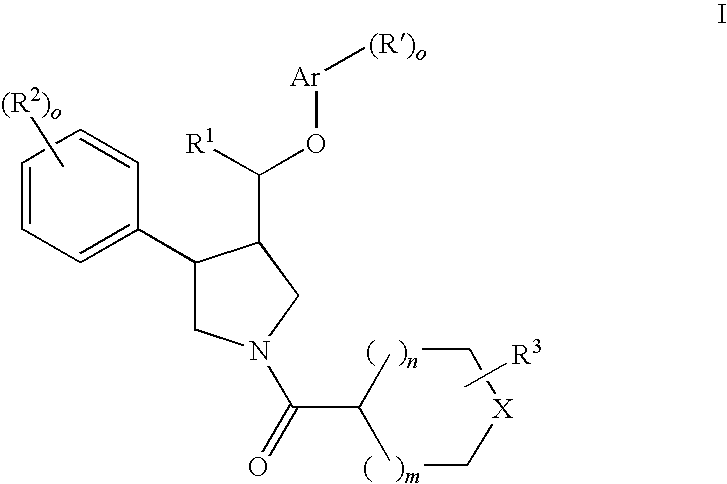
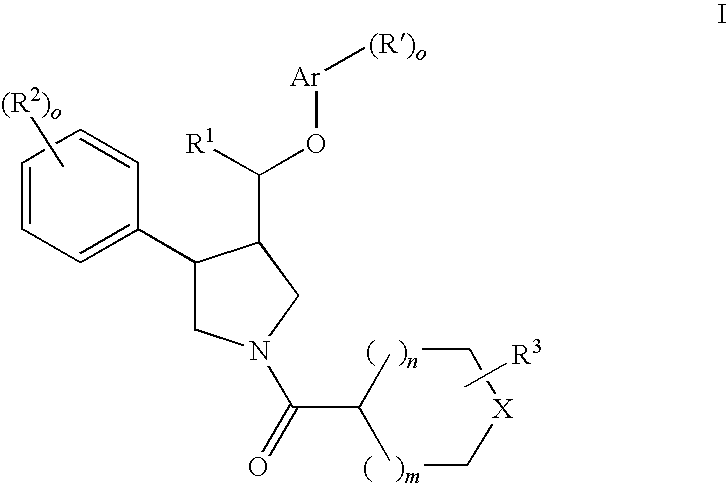
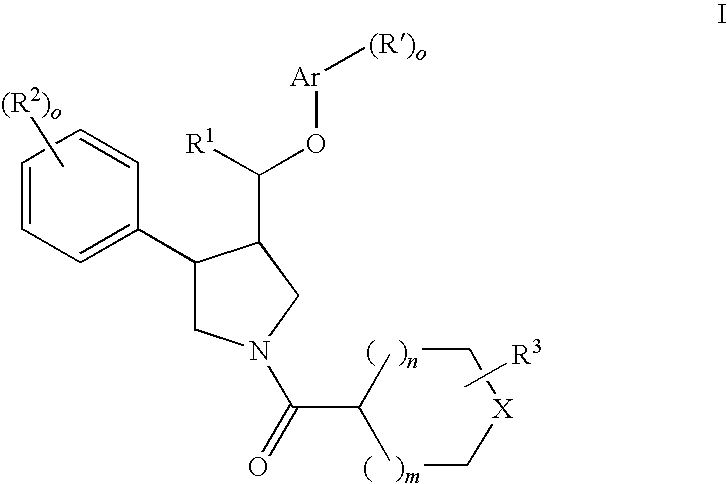
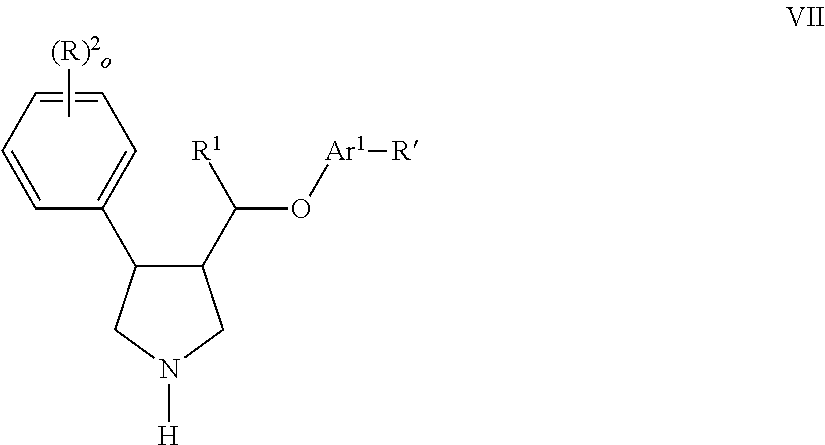
The present invention relates to compounds of formula Iwherein R1, R2, R3, R′, Ar, m, n, and o are as defined herein. The invention also relates to pharmaceutical compositions containing compounds of formula I and methods for the manufacture of such compounds and compositions. Compounds of the invention are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
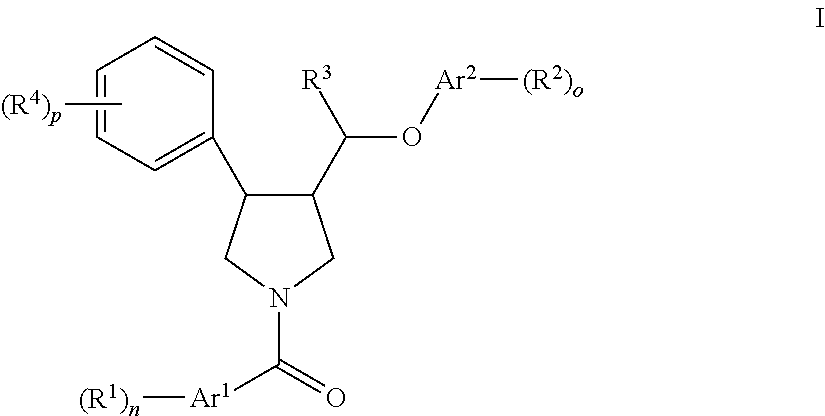
Pyrrolidine aryl-ether as nk3 receptor antagonists
The invention relates to a compound of formula IwhereinAr1,Ar2,R1,R2, R3, R4, n, o, p, and q are as defined herein and to a pharmaceutically active salt thereof, including all stereoisomeric forms, individual diastereoisomers and enantiomers of the compound of formula (I) as well as racemic and non-racemic mixtures thereof. The compounds are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
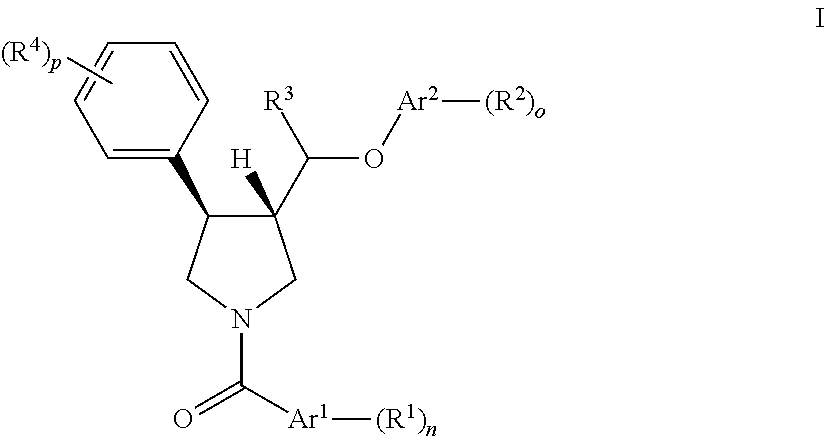
Pyrrolidine ether derivatives as nk3 receptor antagonists
The present invention relates to compounds of formula Iwherein R1, R2, R3, R′, Ar, m, n, and o are as defined herein. The invention also relates to pharmaceutical compositions containing compounds of formula I and methods for the manufacture of such compounds and compositions. Compounds of the invention are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
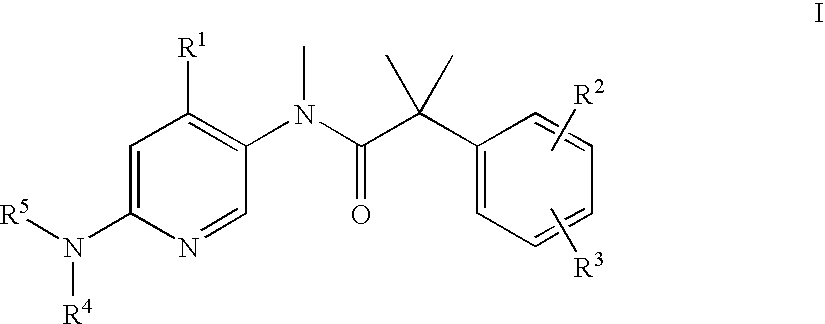
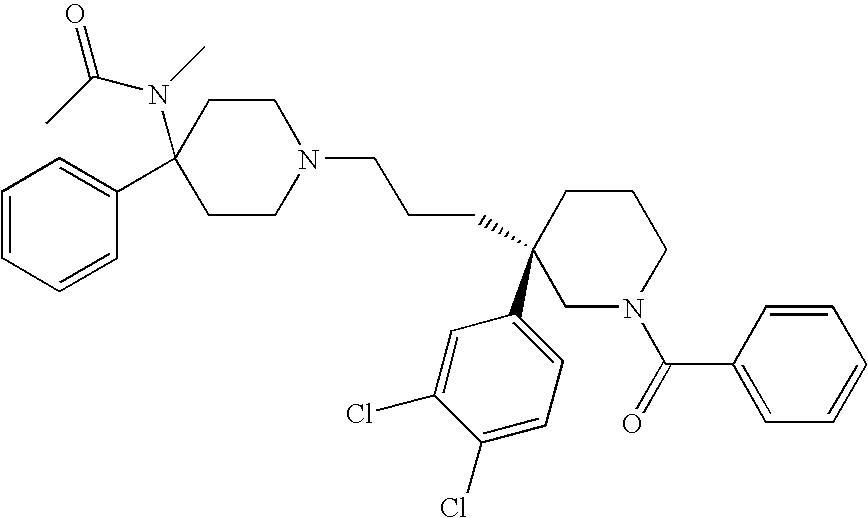
Pyrrolidine derivatives as dual NK1/NK3 receptor antagonists
The invention relates to pyrrolidine derivatives of formulawhereinR1,R2,R3,n, and o are defined in the specification and to pharmaceutically active acid-addition salts thereof. Compounds of formula I have a high affinity simultaneously to both the NK1 and the NK3 receptors (dual NK1 / NK3 receptor antagonists), useful in the treatment of schizophrenia.
Owner:F HOFFMANN LA ROCHE INC
Pyrrolidine aryl-ether as NK3 receptor antagonists
Owner:F HOFFMANN LA ROCHE & CO AG
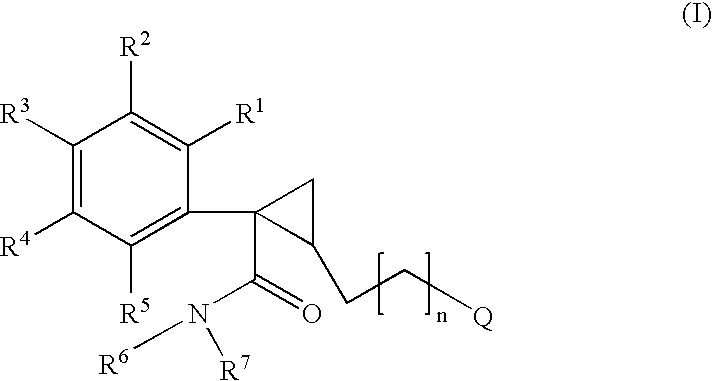
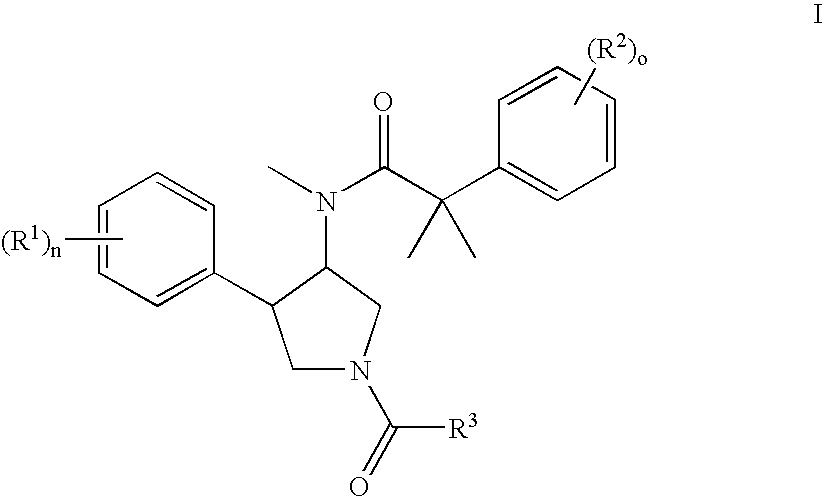
Pyrrolidine derivatives as nk3 receptor antagonists
The present invention relates to a compounds of formula Iwherein A, Ar, R, R2, R3, R4, p, and o are as defined in the specification and claims or to a pharmaceutically active salt thereof. The present compounds are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, bipolar disorders, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE INC
Pyrrolidine derivatives as dual nk1/nk3 receptor antagonists
InactiveUS20080275021A1High affinityValuable therapeutic propertyBiocideNervous disorderNk3 receptorPharmaceutical drug
The invention relates to pyrrolidine derivatives of formulawhereinR1,R2,R3,n, and o are defined in the specification and to pharmaceutically active acid-addition salts thereof. Compounds of formula I have a high affinity simultaneously to both the NK1 and the NK3 receptors (dual NK1 / NK3 receptor antagonists), useful in the treatment of schizophrenia.
Owner:F HOFFMANN LA ROCHE INC
Uses of dual NK1/NK3 receptor antagonists for treating sex-hormone diseases
Owner:KANDY THERAPEUTICS LTD
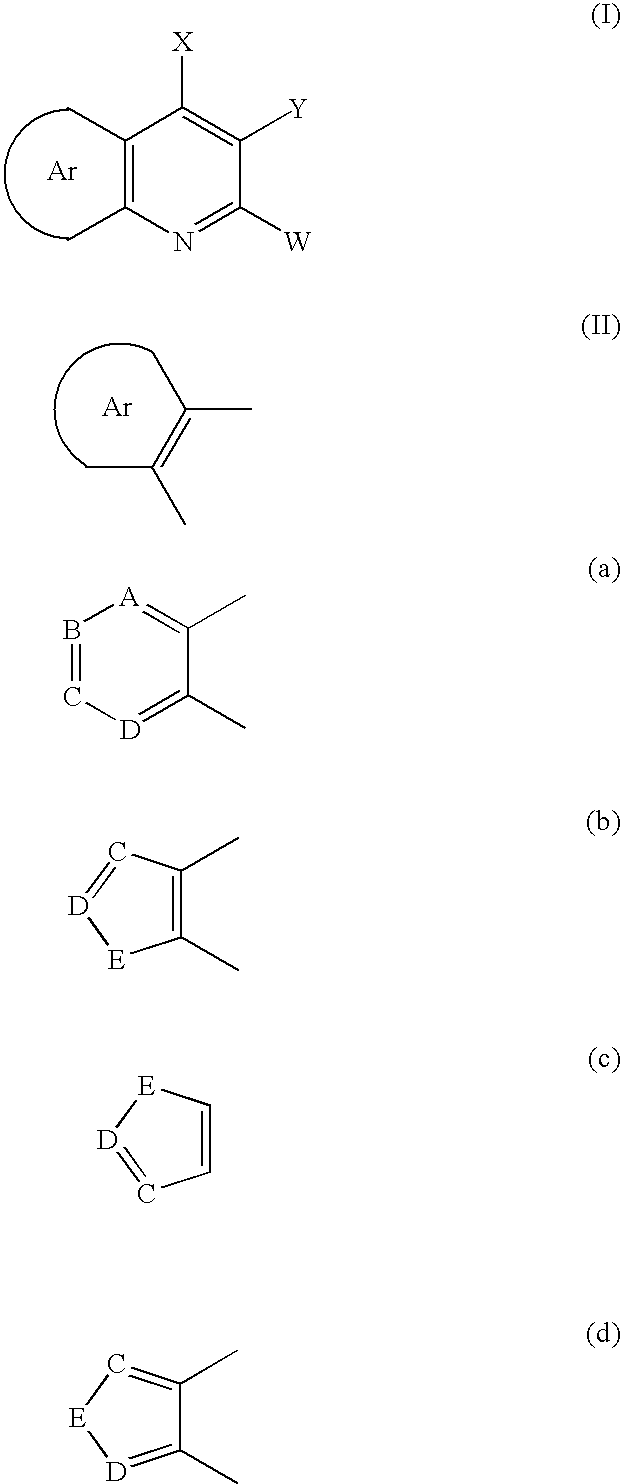
Aryl fused 2,4-disubstituted pyridines: NK3 receptor ligands
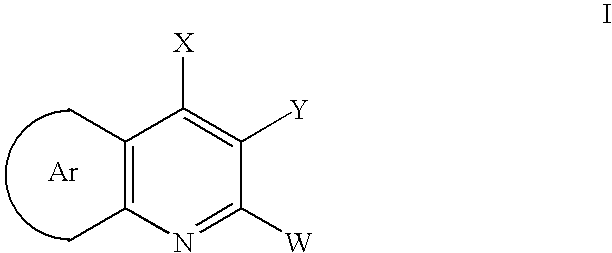
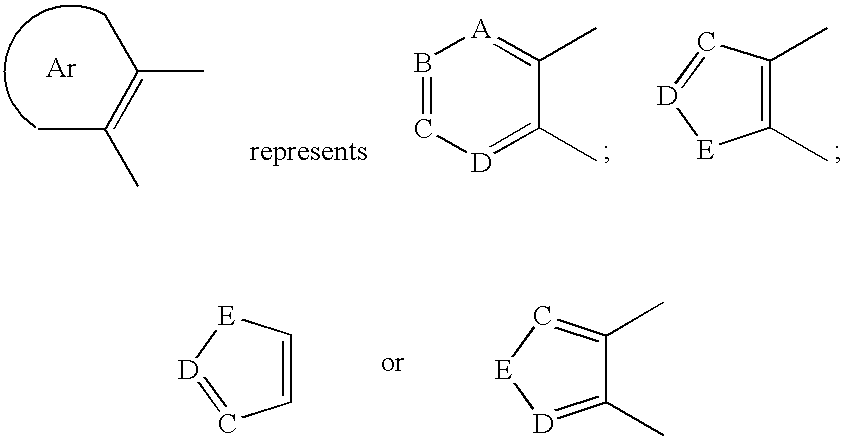
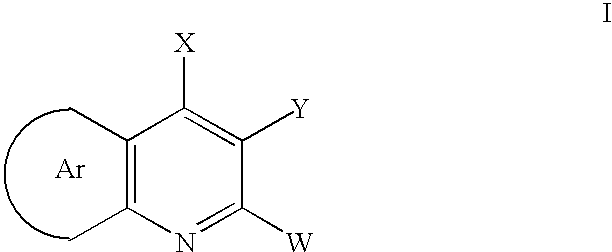
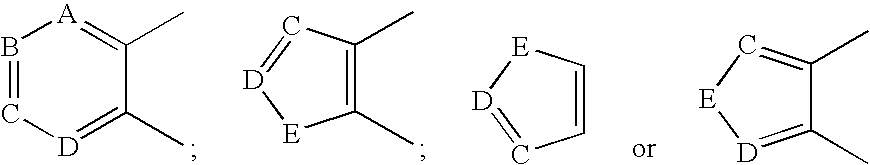
Disclosed are compounds of formula (I) or pharmaceutically acceptable non-toxic salts or pharmaceutically acceptable solvates thereof wherein: (II) represents (a), (b), (c) or (d) and W, X, Y, A, B, C, D, E are variables as described herein. These compounds are highly selective agonists or antagonists at NK3 receptors or prodrugs thereof. The novel tachykinin NK-3 receptor antagonists contained in this invention have potential utility in the treatment of a broad array of disorders and diseases of the central nervous system (CNS) and periphery in mammals in which activation of NK-3 receptors is of importance.
Owner:NEUROGEN
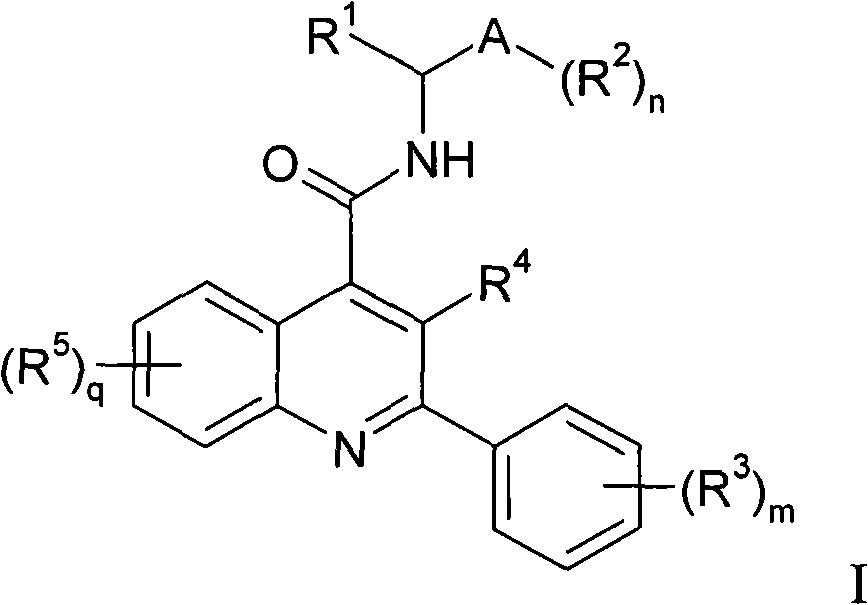
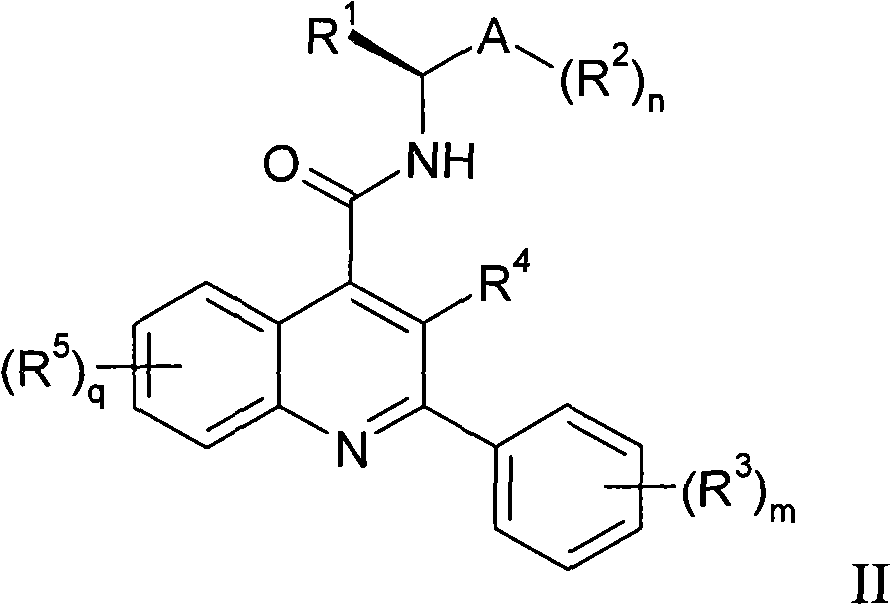
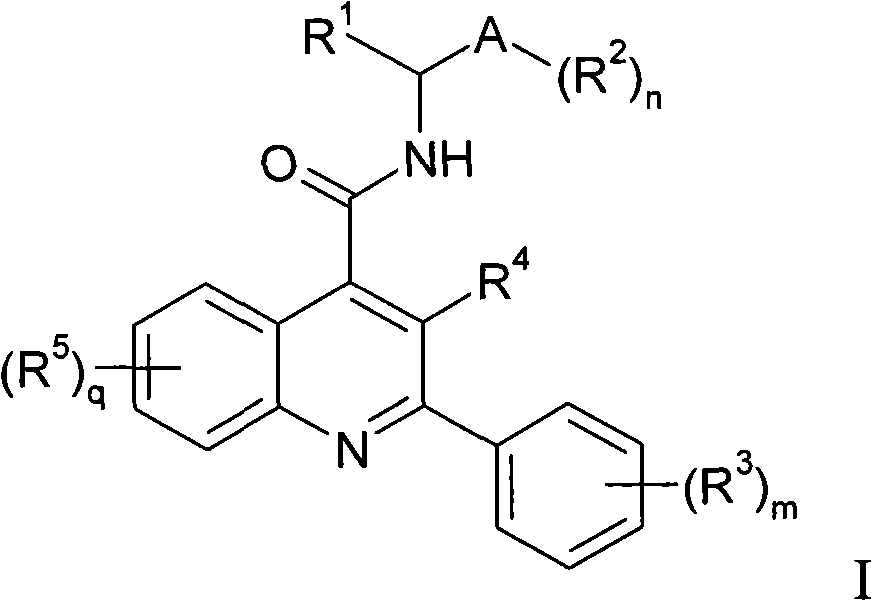
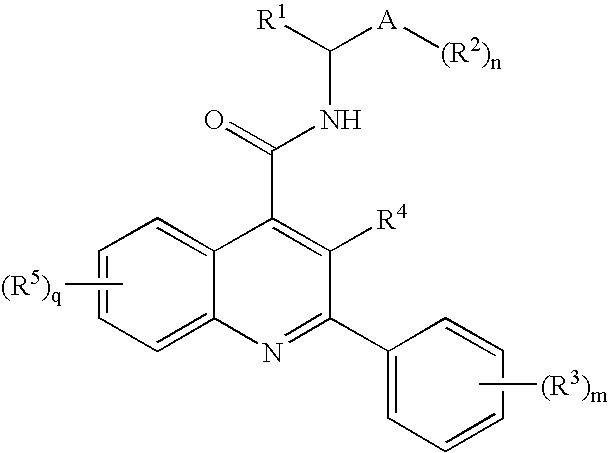
Alkylpyridyl quinolines as NK3 receptor modulators
InactiveCN101282961AImprove solubilityPromote absorptionOrganic active ingredientsNervous disorderNk3 receptorQuinoline
Owner:ASTRAZENECA AB
Oxopyridyl Quinoline Amides as Nk3 Receptor Modulators
Owner:ASTRAZENECA AB
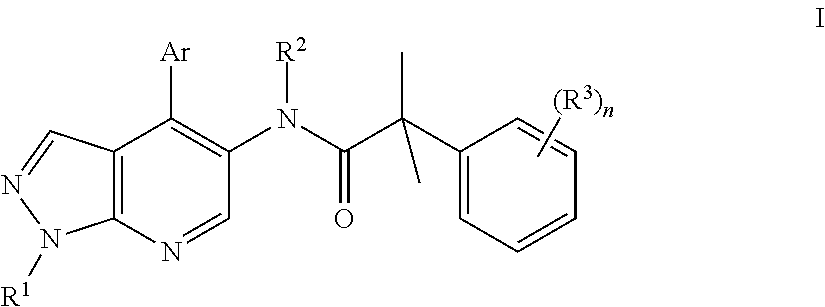
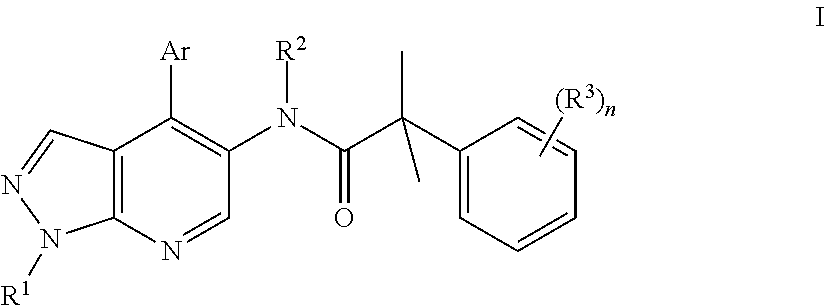
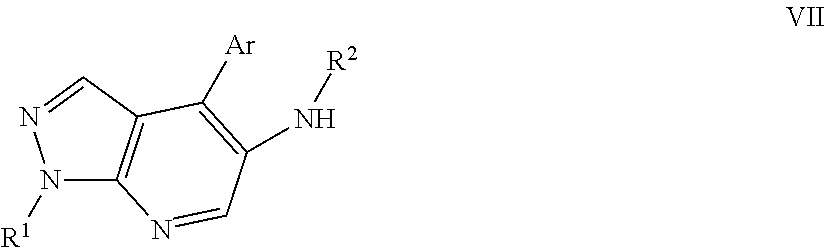
Pyrrazolopyridine compounds as dual nk1/nk3 receptor antagonists
InactiveUS20110257402A1Organic active ingredientsNervous disorderNk3 receptorNK1 receptor antagonist
The present invention relates to a compound of formula Iwherein R1, R2, R3, Ar, and n are as defined herein or to a pharmaceutically active acid addition salt. Compounds of formula I show a high affinity simultaneously to both the NK1 and the NK3 receptors (dual NK1 / NK3 receptor antagonists), useful in the treatment of schizophrenia.
Owner:F HOFFMANN LA ROCHE & CO AG
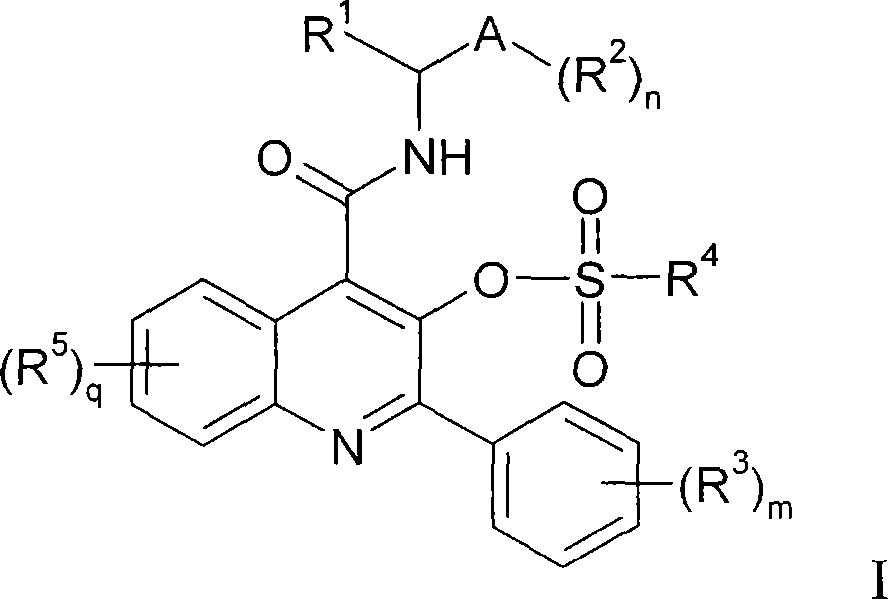
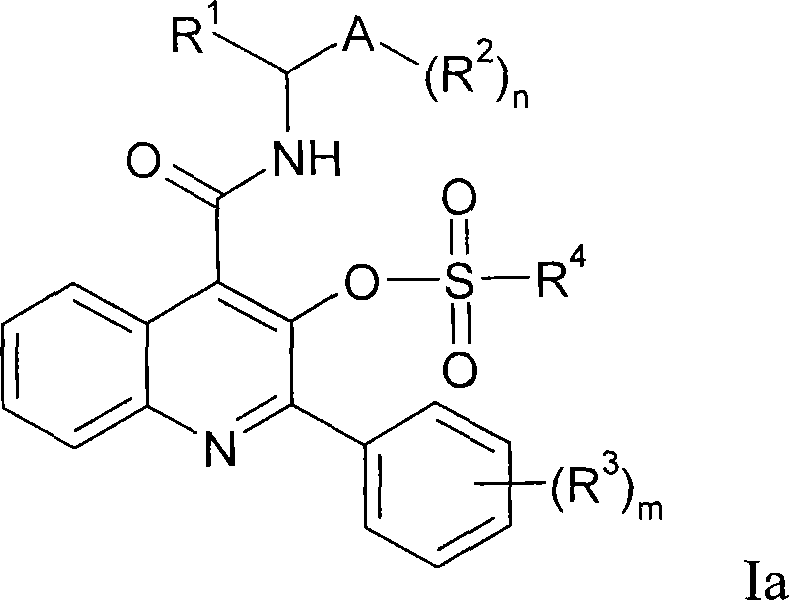
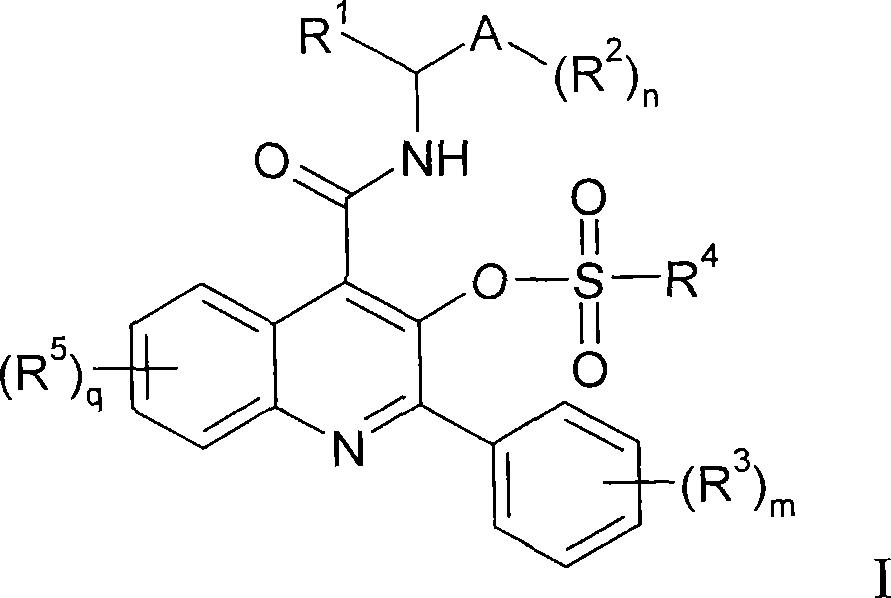
Quinoline 3-sulfonate esters as NK3 receptor modulators
Owner:ASTRAZENECA AB
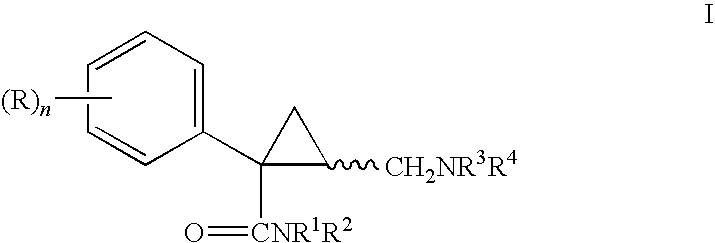
Pyrrolidines as NK3 receptor antagonists
Owner:F HOFFMANN LA ROCHE & CO AG
Alkylpyridyl Quinolines as Nk3 Receptor Modulators
Owner:ASTRAZENECA AB
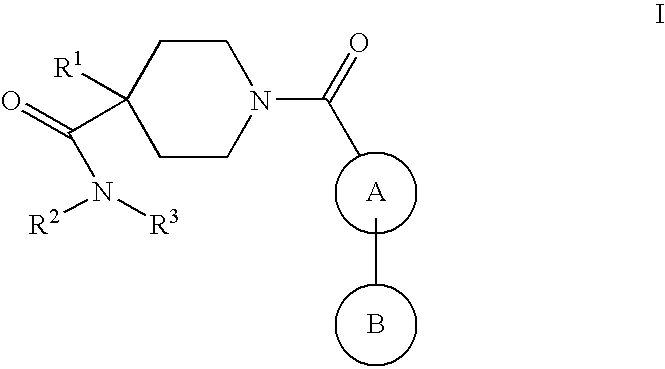
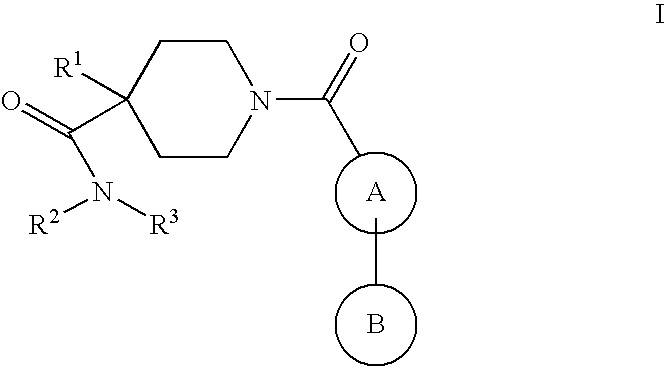
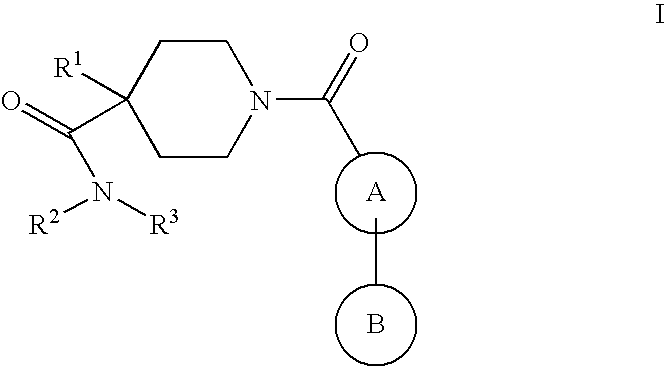
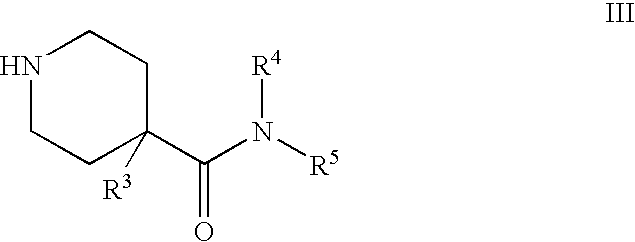
Piperidine derivatives as nk3 receptor antagonists
The present application relates to compounds of formulawherein the definitions are as described herein. The present compounds are high potential NK-3 receptor antagonists that are useful for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE INC
Pyrrolidines as nk3 receptor antagonists
The present invention relates to a compounds of formula Iwherein R1, R2, Ar1, Ar2, R′, R″, m, n, and o are defined in the specification or to a pharmaceutically active salt, racemic mixture, enantiomer, optical isomer or to tautomeric form thereof. The present compounds are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
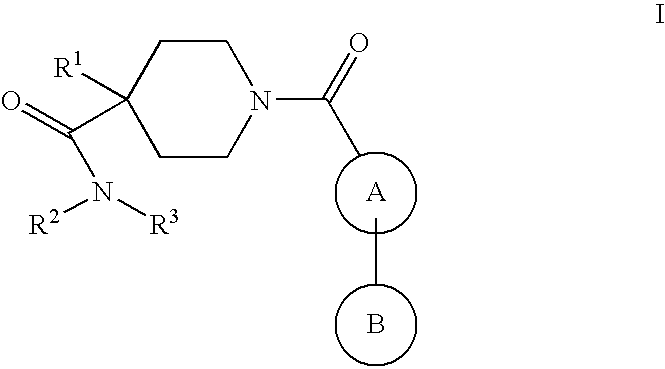
Piperidine derivatives as NK3 receptor antagonists
The present application relates to compounds of formulawherein the definitions are as described herein. The present compounds are high potential NK-3 receptor antagonists that are useful for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE INC
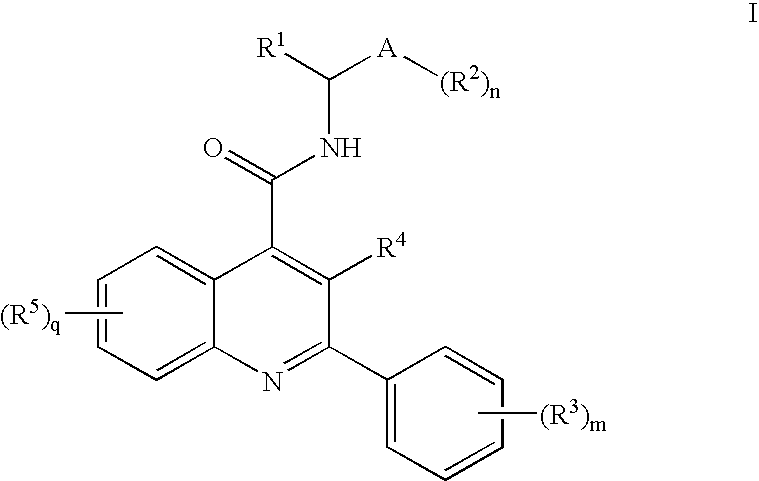
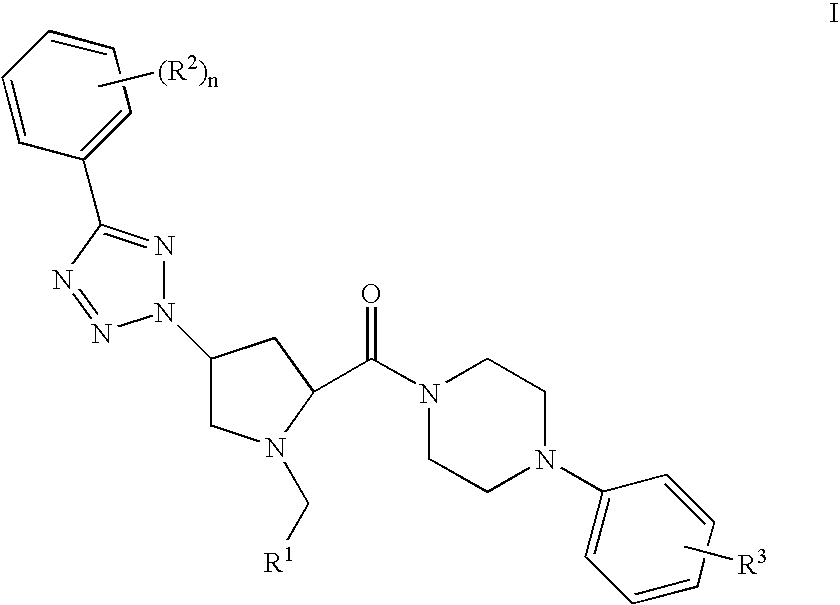
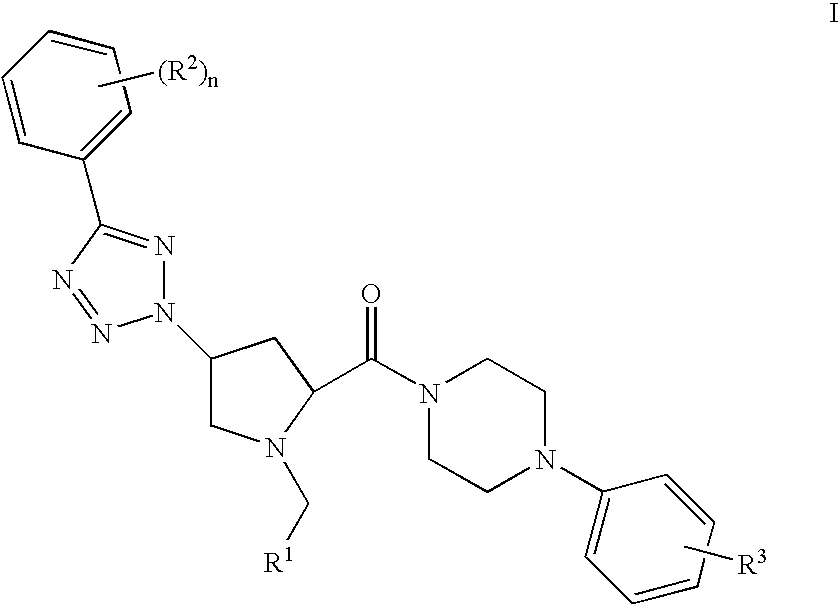
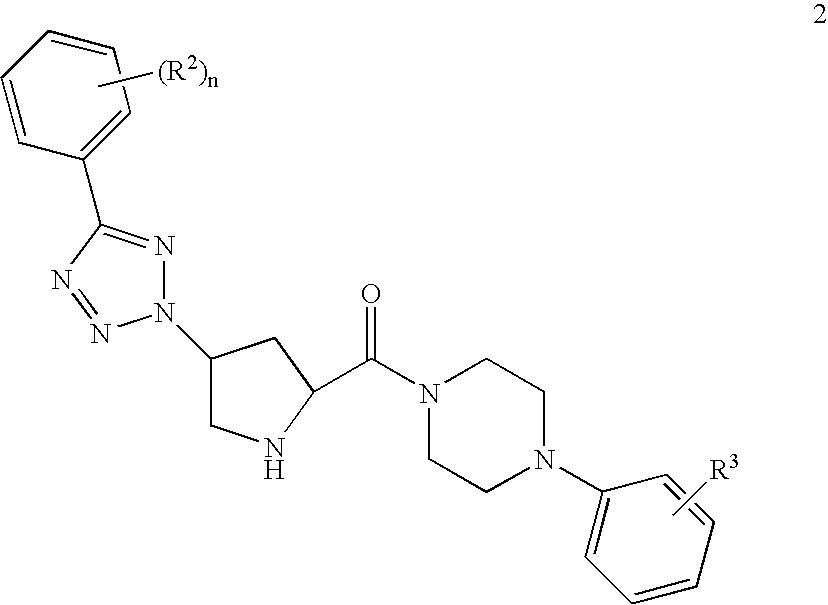
Prolinamide-tetrazole derivatives as nk3 receptor antagonists
The present invention relates to a compound of formula IwhereinR1, R2, R3, and n are as defined herein or to a pharmaceutically acceptable acid addition salt thereof. These compounds are NK3 receptor antagonists, useful for the treatment of such disorders as depression, pain, bipolar disorders, psychosis, Parkinson's disease, schizophrenia, anxiety, and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE INC
Pyrrolidine aryl-ether as nk3 receptor antagonists
The invention relates to a compound of general formulawhereinAr, R1, R2, R3, R4, n, o, p, s, X andare as defined herein or to a pharmaceutically active salt thereof, including all stereoisomeric forms, individual diastereoisomers and enantiomers of the compound of formula (I) as well as racemic and non-racemic mixtures thereof. The compounds are high potential NK-3 receptor antagonists for the treatment of depression, pain, psychosis, Parkinson's disease, schizophrenia, anxiety and attention deficit hyperactivity disorder (ADHD).
Owner:F HOFFMANN LA ROCHE & CO AG
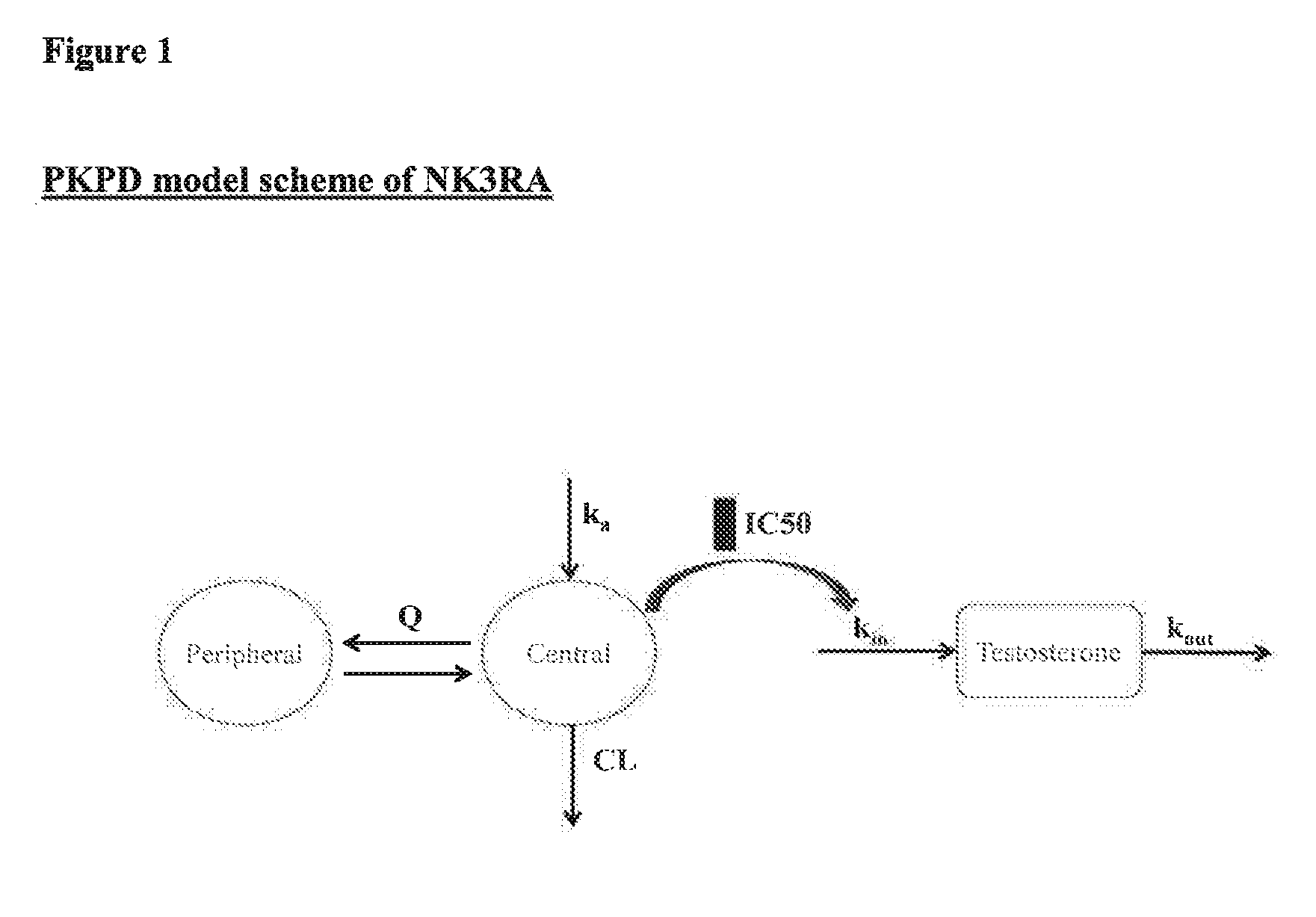
NK3 receptor antagonist compound (NK3RA) for use in a method for the treatment of polycystic ovary syndrome (PCOS)
Owner:ASTRAZENECA AB
Quinazoline derivatives as NK3 receptor antagonists
Owner:F HOFFMANN LA ROCHE INC
Synthetic peptide NK3R-A2 based on NK3 receptor and application thereof
ActiveCN106008672AHigh affinityImprove generation effectPeptide/protein ingredientsPeptide preparation methodsSide effectNk3 receptor
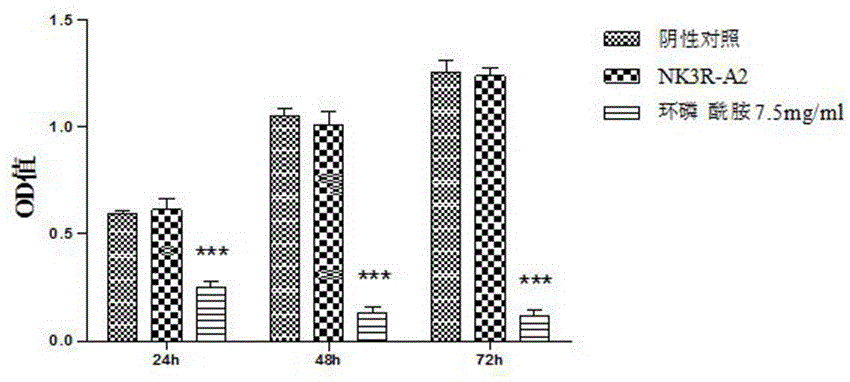
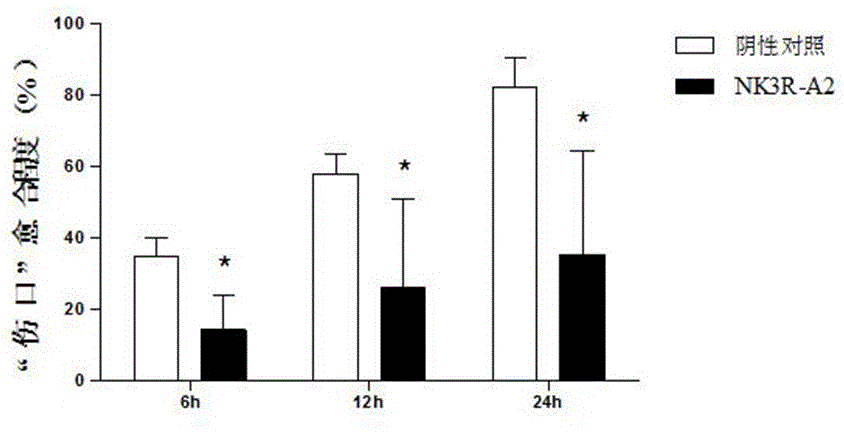
The invention belongs to the technical field of tumor treatment, and particularly relates to a synthetic peptide NK3R-A2 based on an NK3 receptor. The synthetic peptide NK3R-A2 based on the NK3 receptor has the tumor angiogenesis resisting effect. The molecular weight of the synthetic peptide is 1536.70 Da, and the specific sequence of the synthetic peptide is CNGRCGGDFF(MeF)GLM-NH2. The synthetic peptide is prepared through an Fmoc solid-phase polypeptide synthesis method and can be used for preparing anti-tumor preparations. Through in-vitro cell viability experiments, cell scratch experiments and cell migration experiments, the angiogenesis resisting effect of the synthetic peptide is verified; meanwhile, through further chicken chorioallantoic membrane carrier experiments and euangiotic mice S180 transplantation tumor model experiments on the synthetic peptide, it is verified that the synthetic peptide is obvious in tumor inhibiting effect, has the tumor angiogenesis resisting effect and has no obvious toxic or side effect; the synthetic peptide has good medical application prospects and also provides a targeting sequence with great potential for tumor treatment.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com