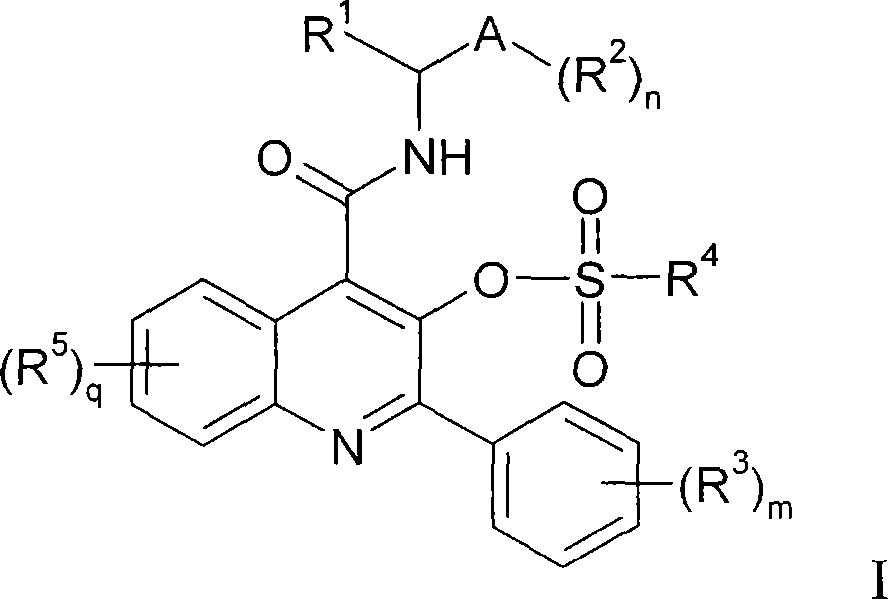
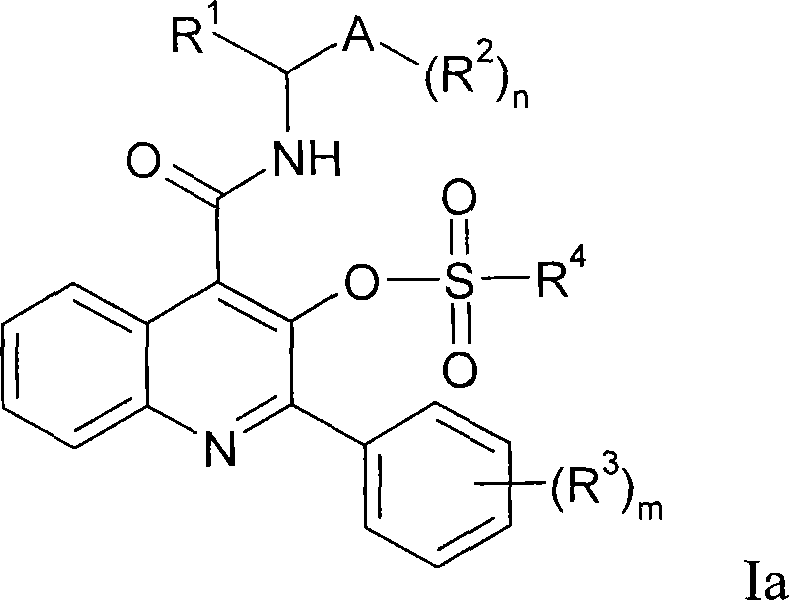
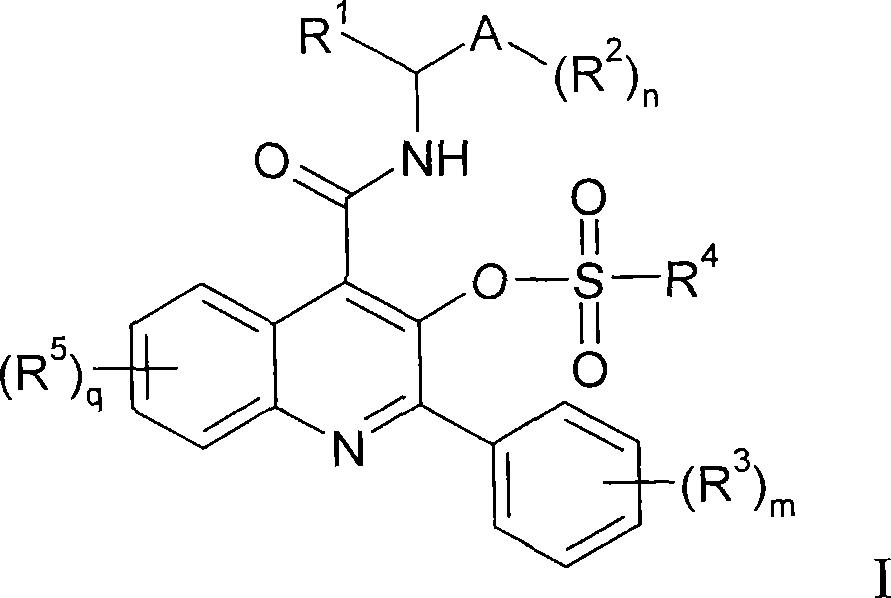
Quinoline 3-sulfonate esters as NK3 receptor modulators
A quinoline and precursor technology, applied in the field of quinoline derivatives, can solve problems such as limited evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] 2-Phenyl-4-({[(1S)-1-phenylpropyl]amino}carbonyl)quinolin-3-yl methanesulfonate:
[0142]
[0143] at room temperature, at N 2 Add triethylamine (14 μL, 0.104 mmol) to 3-hydroxy-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide (20 mg, 0.052 mmol) solution in dichloromethane (0.5 mL). The reaction mixture was cooled to 0 °C and methanesulfonyl chloride (5 μL, 0.062 mmol) was added. The reaction mixture was stirred at 0°C for 1 h, then diluted with dichloromethane, washed with aqueous citric acid (5%), NaHCO 3 saturated aqueous solution and brine. The organic layer was dried (Na 2 SO 4 ), filtration and concentration afforded the title compound (1) as a solid (19 mg, 79% yield). 1 H NMR (300MHz, CDCl 3 )δ0.98(t, J=7.5Hz, 3H), 1.91-2.00(m, 1H), 2.10-2.19(m, 1H), 2.48(s, 3H), 5.20(dt, J=8.0, 8.0Hz , 1H), 6.75(bd, J=8.0Hz, 1H), 7.30-7.44(m, 5H), 7.49-7.59(m, 4H), 7.76(dd, J=8.4, 8.4Hz, 1H), 7.81( d, J=8.4Hz, 1H), 7.87(d, J=7.8Hz, 2H), 8.16(d, J=8.4Hz, 1H)....
Embodiment 2-11
[0145] Examples 2-11 in the table below were prepared by using a method similar to that of Example 1, using the indicated sulfonyl chlorides to give the expected compounds.
[0146]
[0147]
[0148]
[0149]
[0150]
Embodiment 12
[0152] 2-(Dimethylamino)ethanesulfonic acid 2-phenyl-4-({[(1S)-1-phenylpropyl]amino}carbonyl)quinolin-3-yl ester:
[0153]
[0154] A solution of dimethylamine (1.0 mmol) in methanol (0.5 mL) was added to ethylenesulfonic acid 2-phenyl-4-({[(1S)-1-phenylpropyl]amino}carbonyl)quinoline-3- Ethyl ester (Example 10) (30 mg, 0.063 mmol). The reaction mixture was stirred for 4 hr at room temperature, concentrated, and passed directly through column chromatography (SiO 2 ) was purified using a gradient of 0-4% methanol in DCM. The title compound was isolated as a solid (18 mg, 56% yield). 1 H NMR (300MHz, CDCl 3 )δ0.97(t, J=7.3Hz, 3H), 1.89-1.99(m, 1H), 2.03-2.19(m, 1H), 2.08(s, 6H), 2.58-2.68(m, 3H), 2.78 -2.85(m, 1H), 5.19(dt, J=8.0, 8.0Hz, 1H), 6.79(bd, J=7.7Hz, 1H), 7.31-7.42(m, 5H), 7.46-7.56(m, 4H ), 7.71-7.79 (m, 2H), 7.84-7.87 (m, 2H), 8.14 (d, J=8.3Hz, 1H). MS ES+, m / z=518 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com