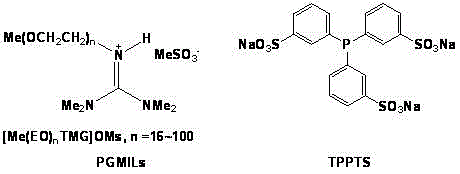Olefin two-phase hydroformylation method
A hydroformyl and olefin technology, applied in chemical instruments and methods, carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of activity and selectivity decline, olefin isomerization, catalyst loss And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Two-phase 1-octene hydroformylation of PGMILs
[0019] Under nitrogen or argon atmosphere, add TPPTS, Rh(acac)(CO) into a 60mL stainless steel autoclave 2 and [Me(EO) 16 TMG] OMs. Its ratio is: TPPTS / Rh(acac)(CO) 2 =10 / 1 (molar ratio), [Me(EO) 16 TMG]OMs / Rh(acac)(CO) 2 =1000 / 1 (mass ratio). Replace the air with nitrogen or argon 4 to 6 times, and then use synthesis gas (H 2 / CO=1:1) pressurize to 5.0MPa, the reaction temperature is 85°C, the reaction time is 24 hours, then lower to room temperature, vent the synthesis gas and open the kettle, add 1-octene and internal standard cyclohexyl under nitrogen or argon atmosphere alkanes in the ratio 1-octene / RhCl 3 ·3H 2 O=1000 / 1 (molar ratio). Then again with syngas (H 2 / CO=1:1) Pressurize to 5.0MPa, react at 85°C for 5 hours, then cool down to below 20°C, vent the synthesis gas and open the kettle, the lower PGMILs phase solidifies, the upper product aldehyde can be separated by decantation, or use normal After e...
Embodiment 2
[0021] Two-phase 1-decene hydroformylation of PGMILs
[0022] Under nitrogen or argon atmosphere, add TPPTS and RhCl to a 60mL stainless steel autoclave 3 ·3H 2 O and [Me(EO) 16 TMG] OMs. Its ratio is: TPPTS / RhCl 3 ·3H 2 O=10 / 1 (molar ratio), [Me(EO) 16 TMG]OMs / RhCl 3 ·3H 2 O=1000 / 1 (mass ratio). Replace the air with nitrogen or argon 4 to 6 times, and then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 85°C, reaction time 24 hours, then lowered to room temperature, vented synthesis gas and opened the kettle, added 1-decene and internal standard n-octyl under nitrogen or argon atmosphere alkanes in the ratio 1-decene / RhCl 3 ·3H 2 O=1000 / 1 (molar ratio). Then again with syngas (H 2 / CO=1:1) Pressurize to 5.0MPa, react at 85°C for 5 hours, then cool down to below 20°C, vent the synthesis gas and open the kettle, the lower PGMILs phase solidifies, the upper product aldehyde can be separated by decantation, or use normal After extraction ...
Embodiment 3
[0024] Two-phase 1-dodecene hydroformylation of PGMILs
[0025] Under nitrogen or argon atmosphere, add TPPTS and RhCl to a 60mL stainless steel autoclave 3 ·3H 2 O and [Me(EO) 16 TMG] OMs. Its ratio is: TPPTS / RhCl 3 ·3H 2 O=10 / 1 (molar ratio), [Me(EO) 16 TMG]OMs / RhCl 3 ·3H 2 O=1000 / 1 (mass ratio). Replace the air with nitrogen or argon 4 to 6 times, and then use synthesis gas (H 2 / CO=1:1) pressurize to 5.0MPa, reaction temperature 85°C, reaction time 24 hours, then lower to room temperature, vent the synthesis gas and open the kettle, add 1-dodecene and internal standard normal under nitrogen or argon atmosphere Decane in the ratio 1-dodecene / RhCl 3 ·3H 2 O=1000 / 1 (molar ratio). Then again with syngas (H 2 / CO=1:1) Pressurize to 5.0MPa, react at 85°C for 5 hours, then cool down to below 20°C, vent the synthesis gas and open the kettle, the lower PGMILs phase solidifies, the upper product aldehyde can be separated by decantation, or use normal After extraction w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com