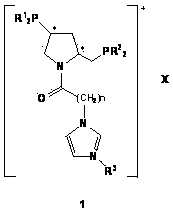
Imidazole ionic liquid supported chiral phosphine ligands and preparation method thereof
A technology of chiral bisphosphine ligands and ionic liquids, applied in the field of chemistry and chemical engineering, can solve the problems of catalytic activity and selectivity decline, catalyst loss, etc., and achieve the effect of high stereoselectivity and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
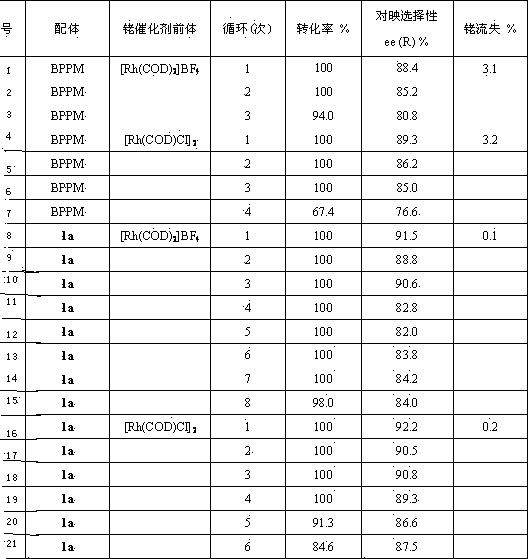
[0026] Synthesis of Chiral Bisphosphine Ligand 1a
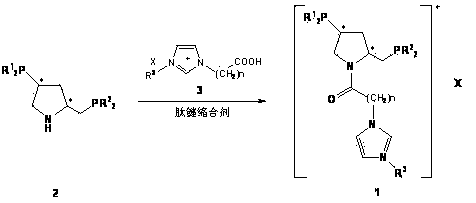
[0027] In a Schlenk bottle, (2S, 4S)-4-diphenylphosphino-2-diphenylphosphinomethylpyrrolidine ( 2a, 0.56g, 0.0012mmol), 1-methyl-3-carboxymethylimidazolium tetrafluoroborate ( 3a , 0.28g, 0.0012mmol) and DIC (0.16g, 0.0012mmol) were dissolved in 10mL of deoxygenated dichloromethane, stirred at room temperature for 10h under the protection of argon, and monitored the end of the reaction with TLC. The solvent was then removed under reduced pressure, and the residue was subjected to column chromatography (degassed silica gel, EtOAc / MeOH / H 2 O=15 / 6 / 2) were separated to obtain 0.48g white solid 5 , yield 58.5%. mp : 149~150℃; [α] 20 D : -34.8 ( c 0.25, MeOH); 1 H NMR (500.0 MHz, CD 3 CN): δ=8.30 (s, 1H, NC H =N), 7.32-7.54 (m, 21H, Ph-H and NC H =CHNCH 3 or NCH=C H NCH 3 ), 7.21 (t, J= 1.6 Hz, 1H, NCH=C H NCH 3 or NC H =CHNCH 3 ), 4.89 (d, J= 16.8Hz, 1H, COC H HN), 4.61 (d, J= 16.8Hz, 1H, COCH H N),...
Embodiment 2
[0029] Synthesis of Chiral Bisphosphine Ligand 1b
[0030] The preparation method is the same as in Example 1. Yield 55.3%; 1 H NMR (500.0 MHz, CD 3 CN): δ=8.38 (s, 1H, NC H =N), 7.32-7.55 (m, 21H, Ph-H and NC H =CHNCH 3 or NCH=C H NCH 3 ), 7.23 (t, J= 1.6 Hz, 1H, NCH=C H NCH 3 or NC H =CHNCH 3 ), 4.87 (d, J= 17.0Hz, 1H, COC H HN), 4.61 (d, J= 17.0Hz, 1H, COCH H N), 4.13 (m, 1H, CONC H ), 3.87 (s, 3H, NC H 3 ), 3.69 (t, J= 10.0 Hz, 1H, CONC H H), 3.41 (q, J= 10.0Hz, 1H, CONCH H ), 3.07 (m, 1H, Ph 2 PC H ), 2.95 (td, J =13.0Hz, J= 3.3Hz, 1H, Ph 2 PC H H), 2.21-2.26 (overlapped m, 2H, Ph 2 PCH H and Ph 2 PCHC H H), 1.89 (m, 1H, Ph 2 PCHCH H ); 13 C NMR (125.7MHz, CD 3 CN): Δ = 163.51, 139.94, 138.64, 138.11, 138.04, 137.55, 134.31, 133.75, 133.57, 130.30, 129.75, 129.53, 123.93, 51.85, 37.81, 35.8, 35.81, 35.81, 35.81, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 35.8, 3...
Embodiment 3
[0032] Synthesis of Chiral Bisphosphine Ligand 1c
[0033] The preparation method is the same as in Example 1. Yield 61.3%; 1 H NMR (500.0 MHz, CD 3 CN): δ=8.33 (s, 1H, NC H =N), 7.32-7.51 (m, 21H, Ph-H and NC H =CHNCH 3 or NCH=C H NCH 3 ), 7.22 (t, J= 1.6 Hz, 1H, NCH=C H NCH 3 or NC H =CHNCH 3 ), 4.88 (d, J= 16.8Hz, 1H, COC H HN), 4.62 (d, J= 16.8Hz, 1H, COCH H N), 4.13 (m, 1H, CONC H ), 3.89 (s, 3H, NC H 3 ), 3.66 (t, J =10.0Hz, 1H, CONC H H), 3.39 (q, J= 10.0Hz, 1H, CONCH H ), 3.07 (m, 1H, Ph 2 PC H ), 2.96 (td, J =13.4Hz, J= 3.3Hz, 1H, Ph 2 PC H H), 2.23-2.28 (overlapped m, 2H, Ph 2 PCH H and Ph 2 PCHC H H), 1.87 (m, 1H, Ph 2 PCHCH H ); 13 C NMR (125.7MHz, CD 3 CN): Δ = 164.21, 140.14, 138.52, 138.07, 138.02, 137.51, 134.30, 133.71, 133.54, 130.24, 129.71, 129.52, 123.94, 57.97, 51.07, 37.8.8, 36.8, 36.8, 36.8, 35.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8, 36.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com