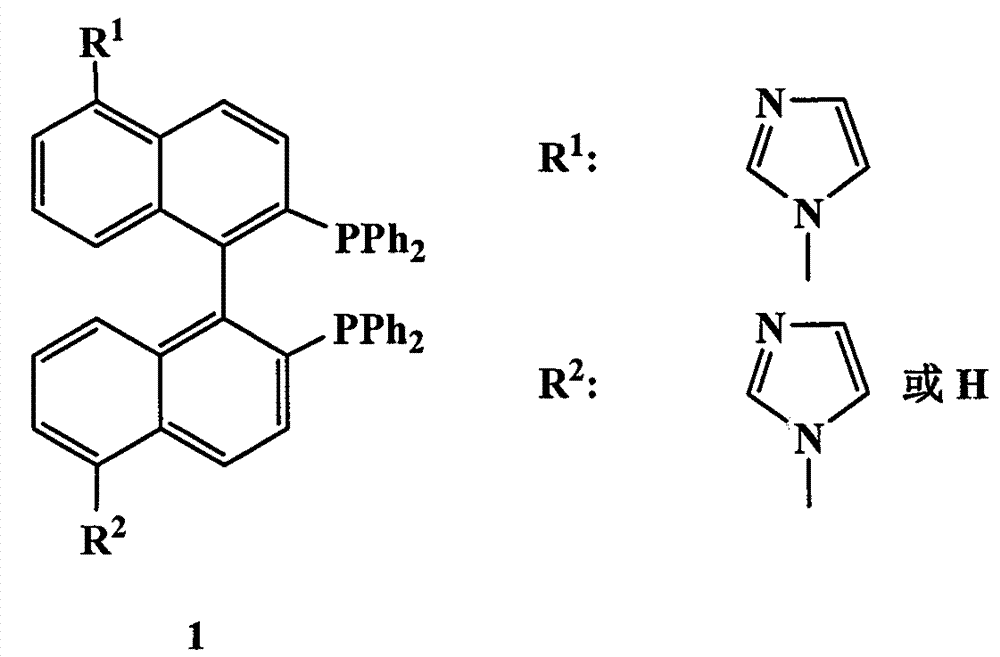
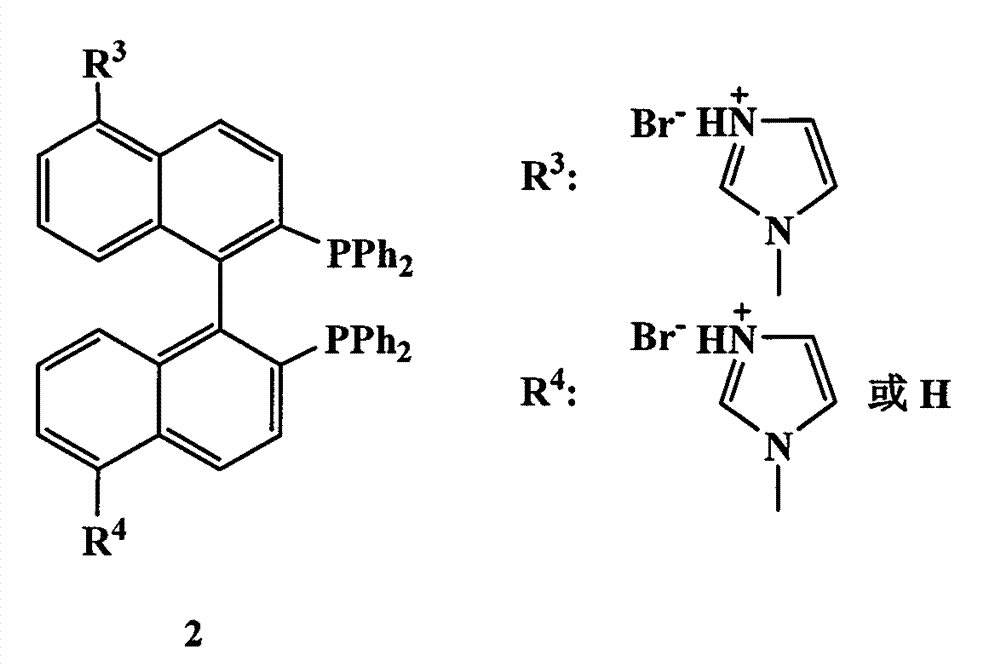
Chiral bisphosphine ligand and chiral catalyst and preparation and application methods
A technology of chiral bisphosphine ligands and chiral catalysts, which is applied in organic chemistry methods, chemical instruments and methods, and the preparation of organic compounds. It can solve problems that have not yet been seen, and achieve improved renewable performance and enhanced stability. , to overcome the effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
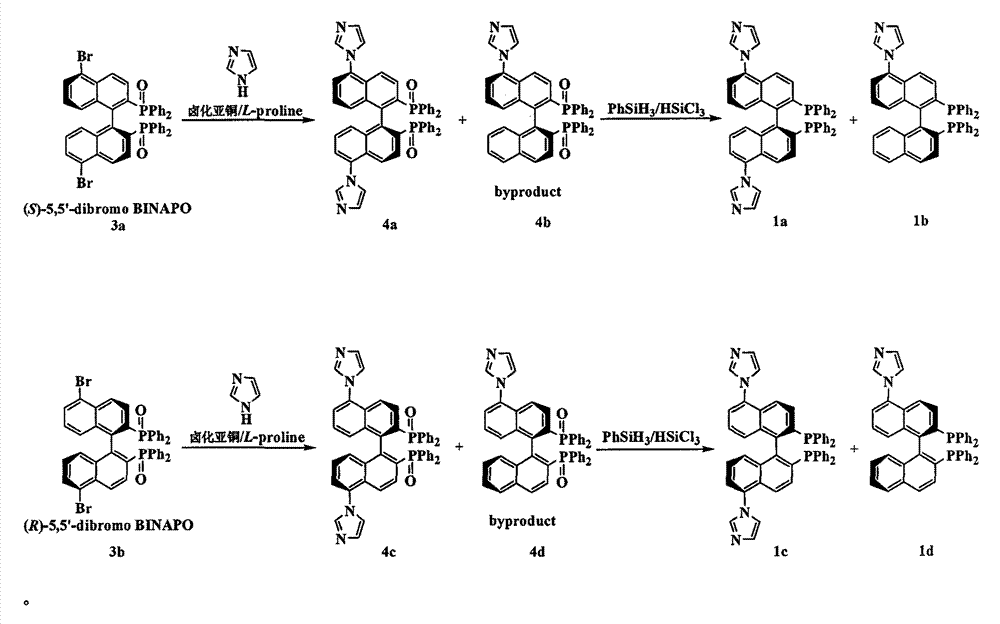
[0040] Embodiment 1, the synthesis of chiral bisphosphine ligand precursor 4a and 4b
[0041](S)-5,5'-dibromo-2,2'-bisdiphenylphosphine oxide-1,1'-binaphthyl 3a (3.6g, 4.4mmol), imidazole (6.1g, 89.0mmol), K 2 CO 3 (2.5g, 18.0mmol), a mixture of CuI (0.35g, 1.8mmol) and L-proline (0.42g, 3.6mmol) was dissolved in 70mL of DMSO, and reacted at 140°C under an argon atmosphere for 48 Hour. After cooling, it was filtered, ethyl acetate was added to the filtrate, the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the residual solid was separated by silica gel column chromatography to obtain white solid 4a (1.7 g, 49.1%) and by-product 4b (0.78 g).
[0042] Characterization data for 4a: [α] 25 D : -187.3 (c0.5, DMF); 1 HNMR (500.0MHz, CDCl 3 ): δ=6.97(dd, J=8.5, 7.5Hz, 2H), 7.02(d, J=8.5Hz, 2H), 7.22-7.45(m, 22H), 7.50(dd, J=11.5, 9.0Hz, 2H), 7.62-7.69(m, 6H), 7.82(s, 2H); 13 C NMR (...
Embodiment 2
[0045] Embodiment 2, the synthesis of chiral bisphosphine ligand precursor 4c and 4d
[0046] Using (R)-5,5'-dibromo-2,2'-bisdiphenylphosphine oxide-1,1'-binaphthalene 3b as the starting material, the operation process is the same as in Example 1. The details are as follows: (R)-5,5'-dibromo-2,2'-bisdiphenylphosphine oxide-1,1'-binaphthyl 3b (3.6g, 4.4mmol), imidazole (6.1g, 89.0mmol ), K 2 CO 3 (2.5g, 18.0mmol), a mixture of CuI (0.35g, 1.8mmol) and L-proline (0.42g, 3.6mmol) was dissolved in 70mL of DMSO, and reacted at 140°C under an argon atmosphere for 48 Hour. After cooling, it was filtered, ethyl acetate was added to the filtrate, the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the residual solid was separated by silica gel column chromatography to obtain white solid 4c (1.8 g) and by-product 4d (0.7 g).
[0047] Explanation: The main reason for using (R)-5,5'-dibromo-2,...
Embodiment 3
[0048] Embodiment 3, the synthesis of chiral bisphosphine ligand 1a
[0049] Under an argon atmosphere, a mixture of 4a (0.2 g, 0.26 mmol) and phenylsilane (1.5 mL, 12.2 mmol) was heated to 130 °C, and three portions of trichlorosilane (3 × 0.4 mL) were heated for 1 h and 3 h, respectively. and 15 hours were added, and the reaction continued to stir for 2 hours. After cooling, the volatile components were removed under reduced pressure to obtain a white solid. Washing with cyclohexane, filtering, and evaporating the solvent gave the chiral bisphosphine ligand 1a in quantitative yield. [α] 20 D : -90.4 (c 0.5, DMF); 1 H NMR (500.0MHz, CDCl 3 ): δ=6.91(d, J=8.5Hz, 2H), 6.97(t, J=7.6Hz, 2H), 6.99-7.25(m, 20H), 7.36(d, J=7.0Hz, 2H), 7.40 (s, 2H), 7.47(s, 2H), 7.53(d, J=8.5Hz, 2H), 7.58(d, J=8.5Hz, 2H), 8.23(s, 2H); 13 CNMR (125.7MHz, CDCl 3 ): δ=121.8, 124.6, 125.2, 128.4, 128.5, 128.6, 129.1, 129.3, 132.3, 132.7, 132.8, 132.9, 133.8, 134.3, 134.4, 134.5, 135.7, 136.4, 138...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com