Patents
Literature
104 results about "1-Methylimidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH₃C₃H₃N₂. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
Coumarin derivatives as well as preparation method and application thereof
InactiveCN103570701ANo significant fluorescence responseHigh fluorescence intensityOrganic chemistryBiological testingDiseaseFluorescence
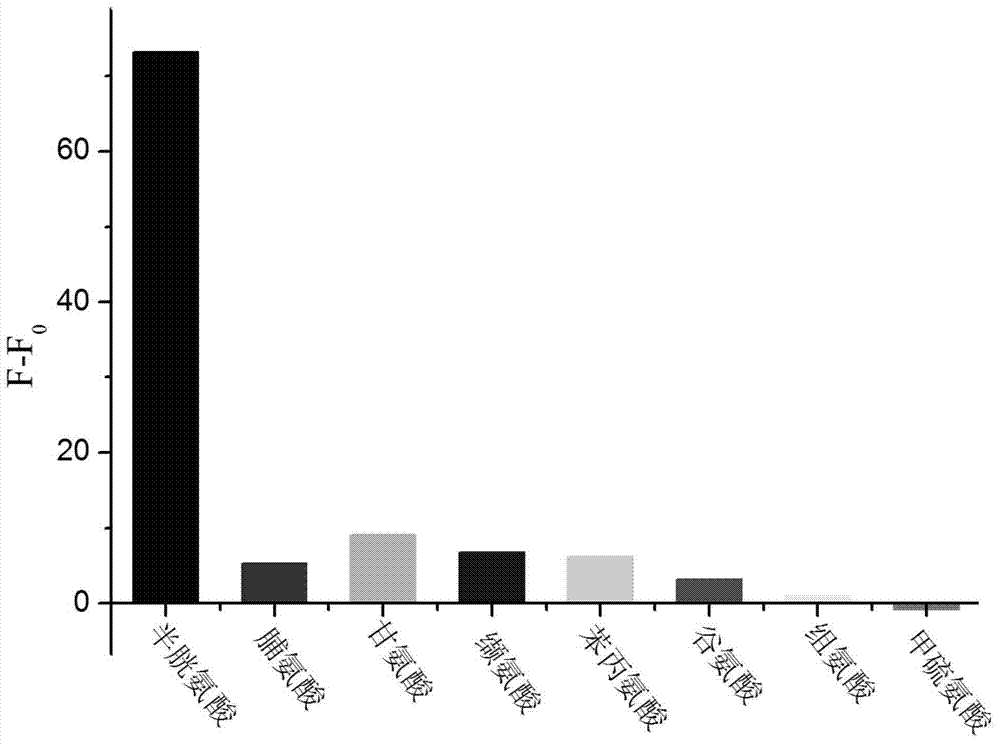
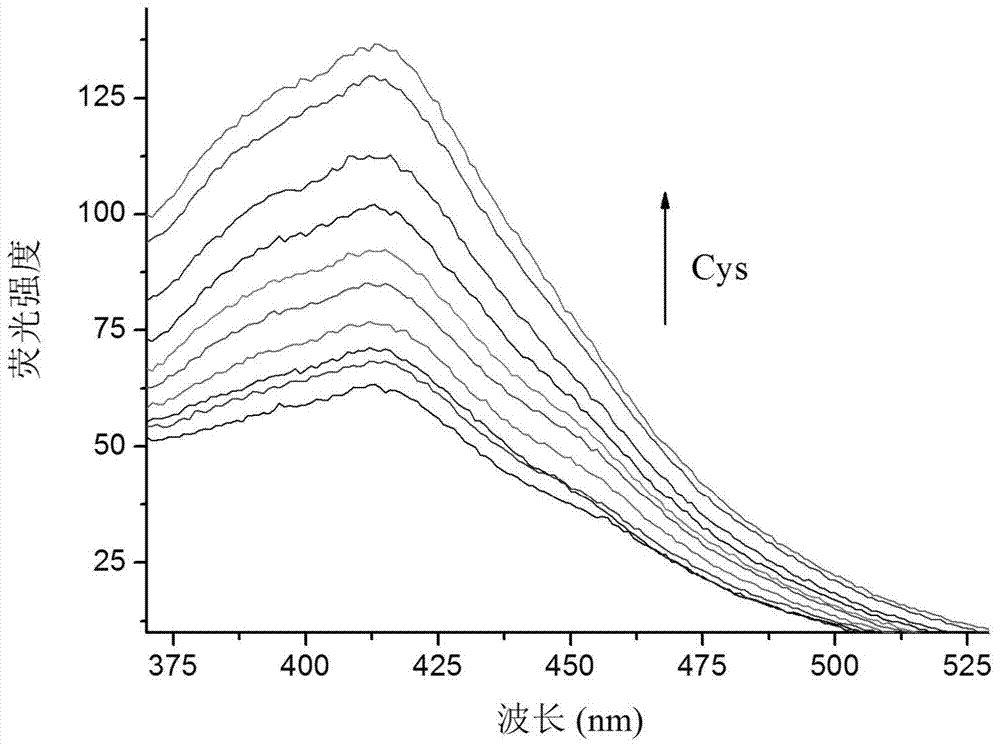
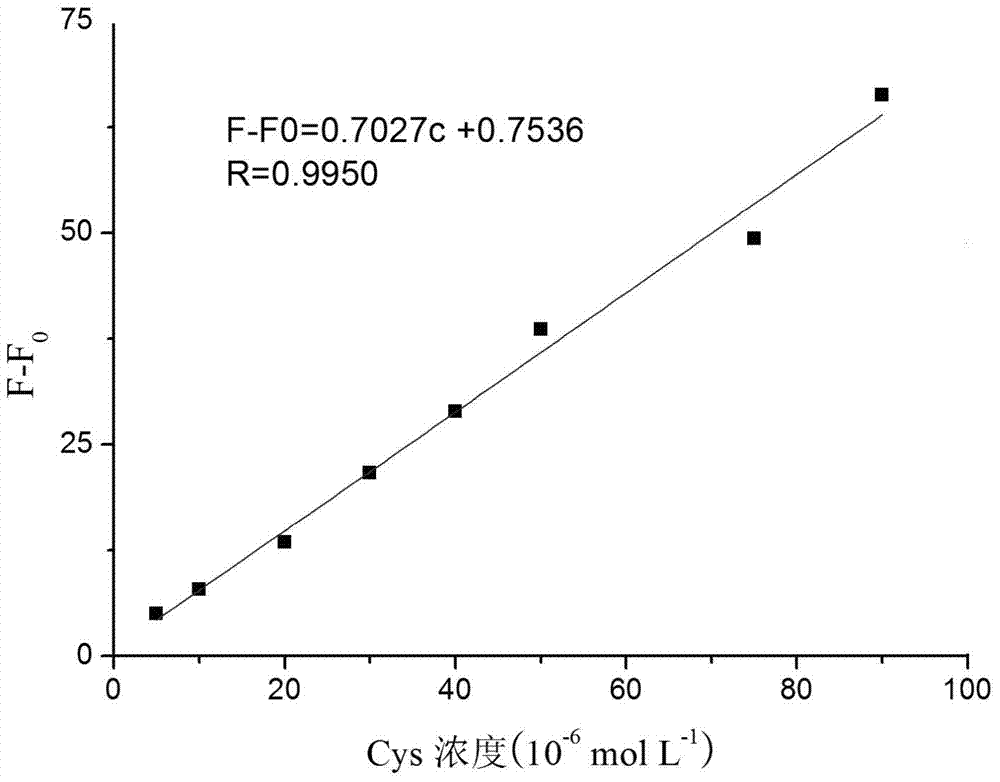
The invention provides coumarin derivatives as well as a preparation method and application thereof. The coumarin derivatives have the molecular formula: Cl3H6N2O4. The preparation method comprises the steps: firstly, preparing 4-chloromethyl-7-hydroxycoumarin, 4-hydroxymethyl-7-hydroxycoumarin and 7-hydroxycoumarin-4-formaldehyde; and then, adding the 7-hydroxycoumarin-4-formaldehyde into water, next, adding malononitrile and 1-methylimidazole, stirring at the room temperature, and filtering to obtain 2, 2-dicyano-3-(7-hydroxyl-4-coumarinyl)oxacyclopropane. The 2, 2-dicyano-3-(7-hydroxyl-4-coumarinyl)oxacyclopropane prepared by the preparation method can be used as a fluorescent probe for selectively detecting cysteine in an organism and can also be used for fluorescently marking cysteine in a cell so as to provide help for the diagnosis of related diseases in clinical medicals.
Owner:SHANXI UNIV
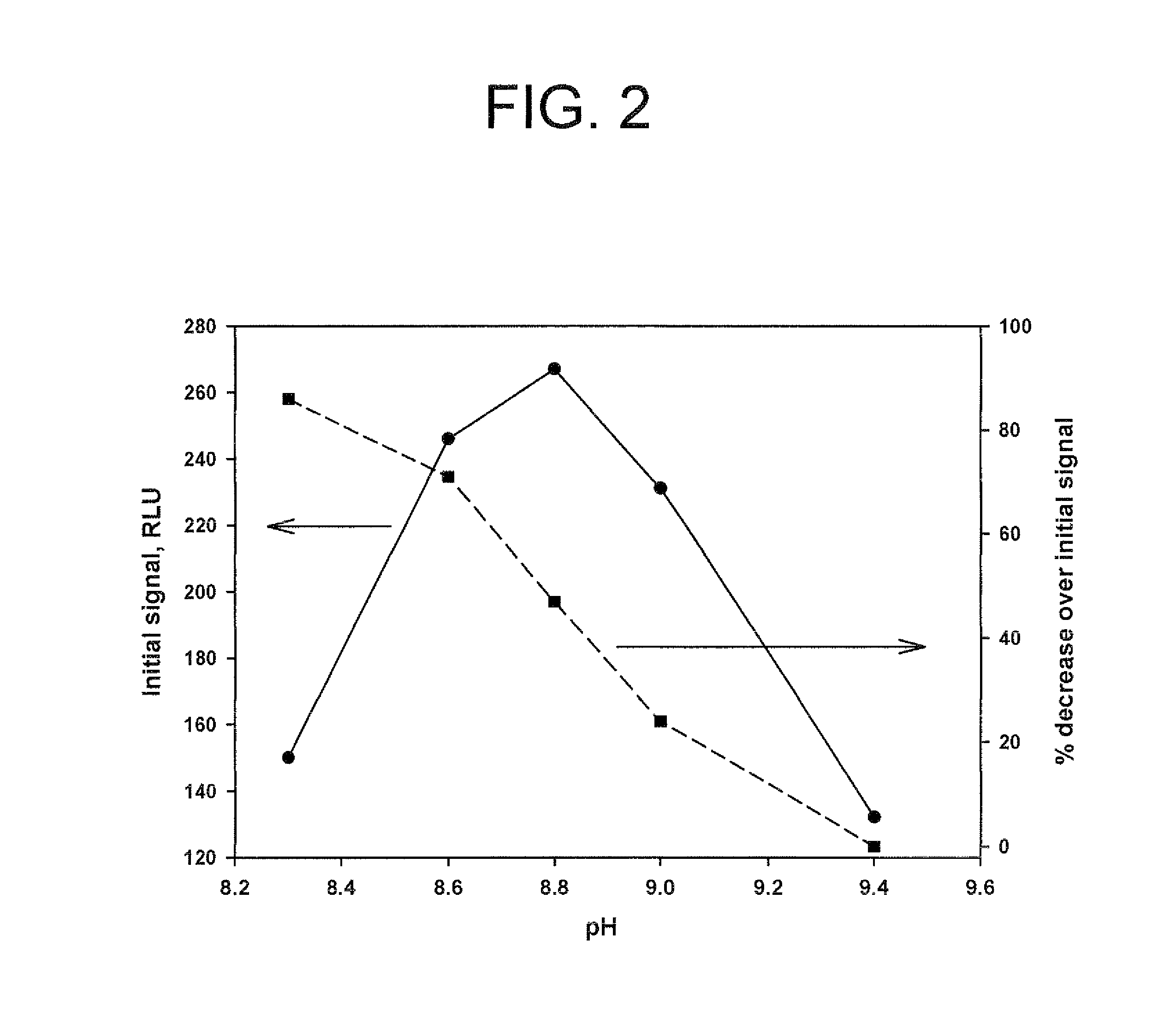
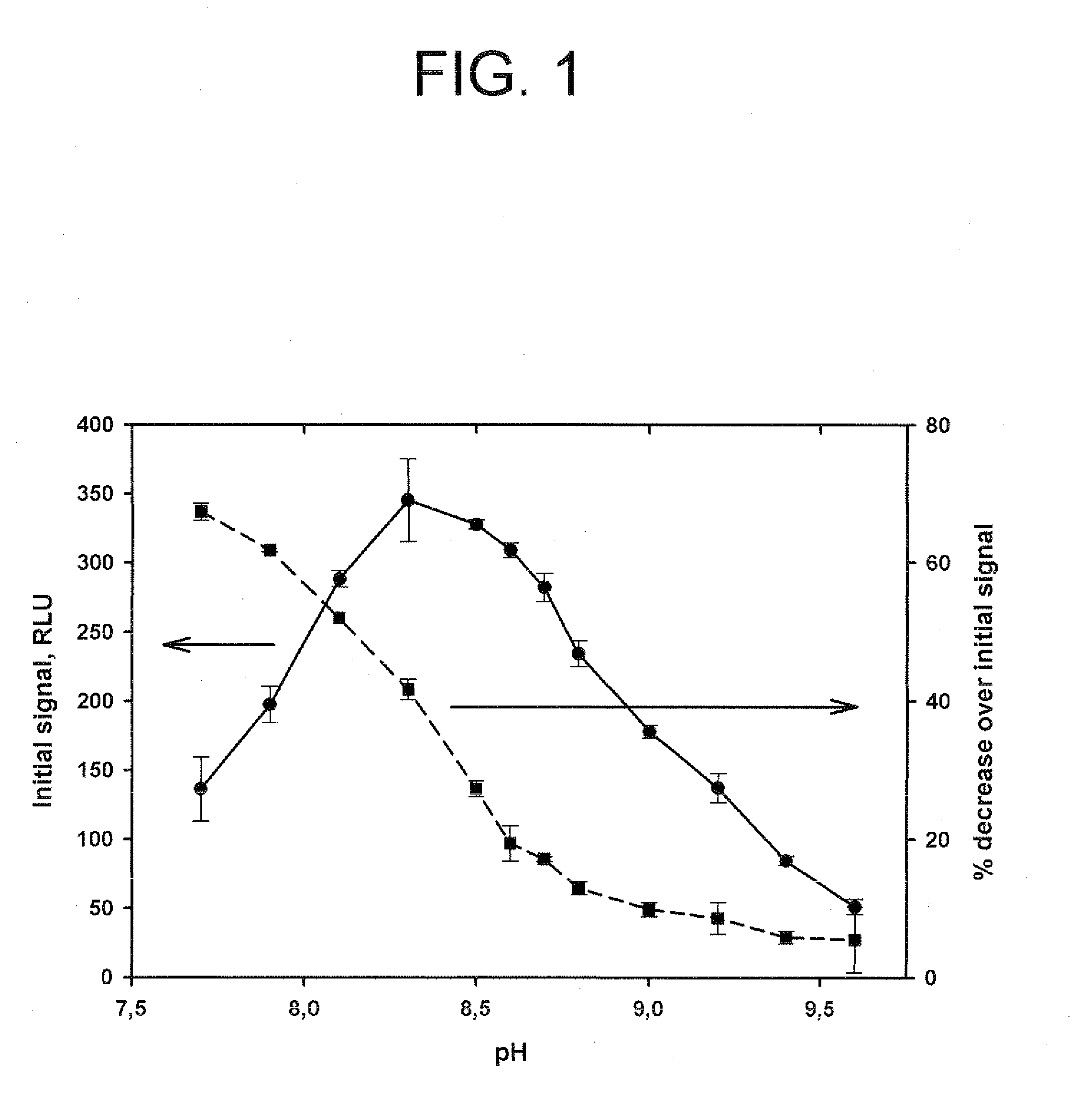
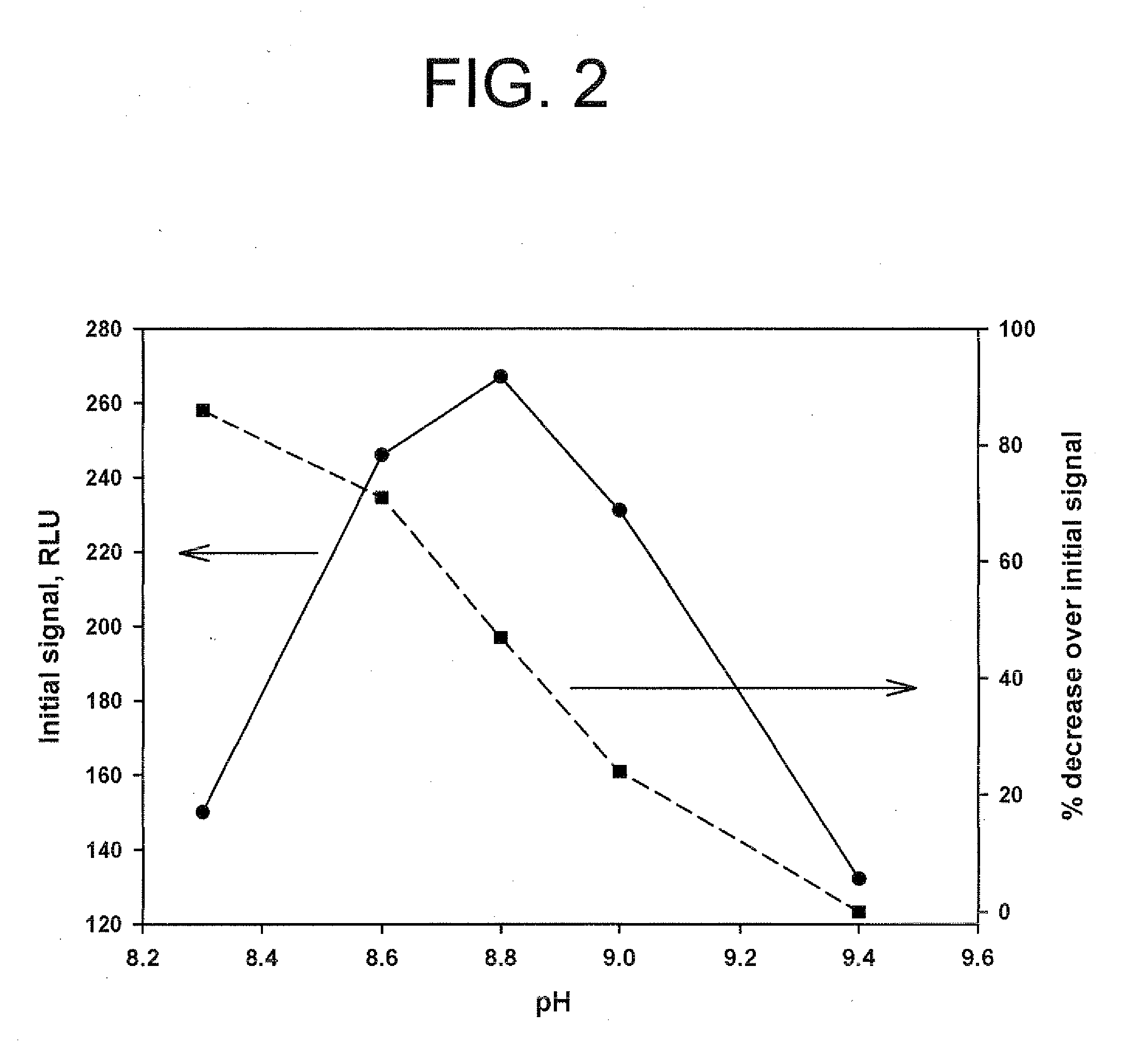
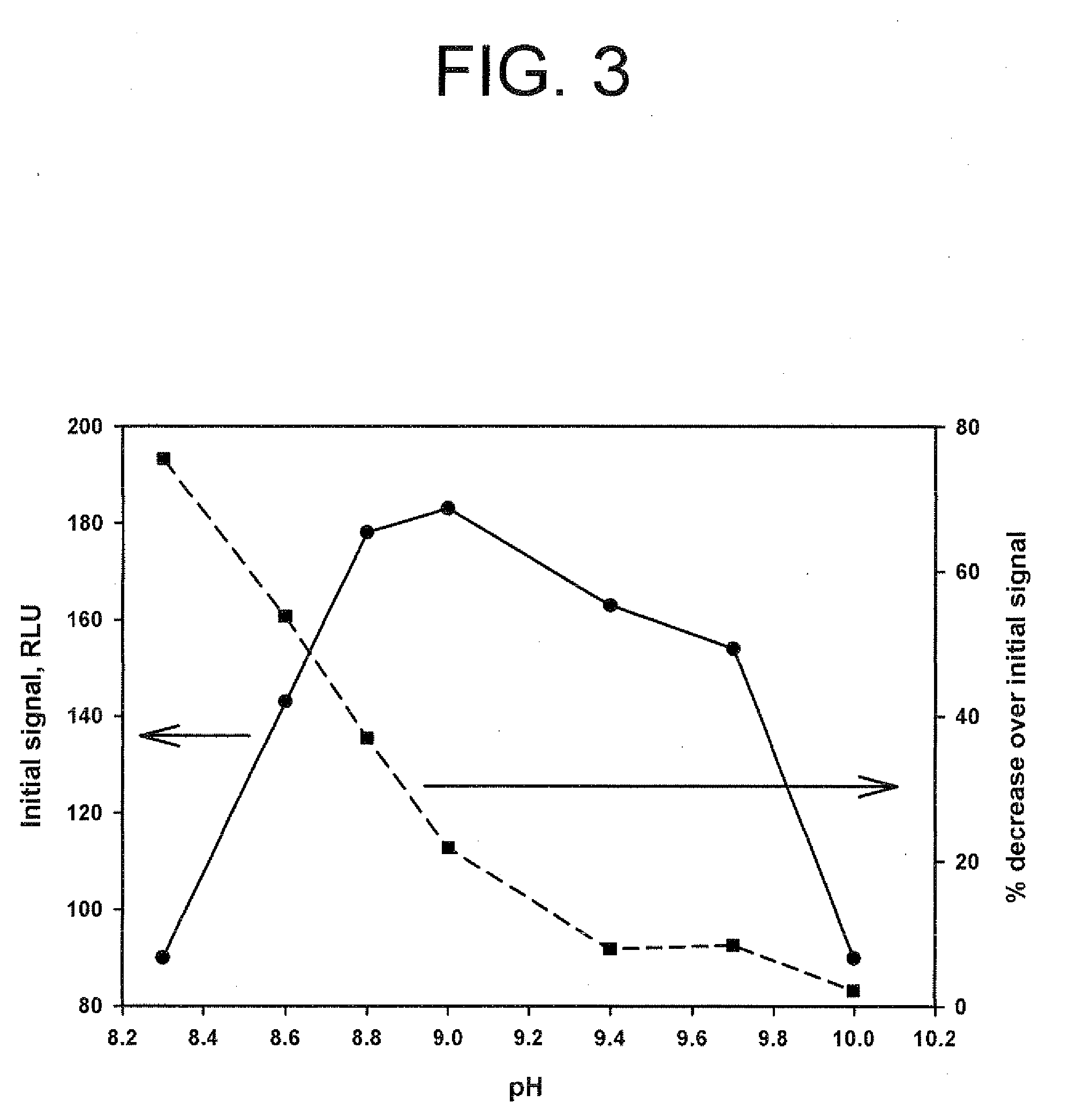
Method for increasing and regulating light emission from a chemiluminescent reaction
ActiveUS9040252B2Microbiological testing/measurementChemiluminescene/bioluminescencePeroxidase1-Methylimidazole
Method for increasing and regulating the emission of light from a chemiluminescent reaction including luminol, a peroxidase enzyme, an oxidant and an electron mediator (primary enhancer) through the use of an acylation catalyst (secondary enhancer) belonging to the class of N-azoles, i.e., a class of five-membered nitrogen heteroaromatic ring compounds containing at least one other atom of nitrogen. N-azoles, which are especially useful as secondary enhancers are imidazole, 1-methylimidazole, 1,2,3-triazole and 1,2,4-triazole. The invention also describes the use in diagnostic assays of chemiluminescent substrates containing said N-azoles, as secondary enhancers.
Owner:CYANAGEN
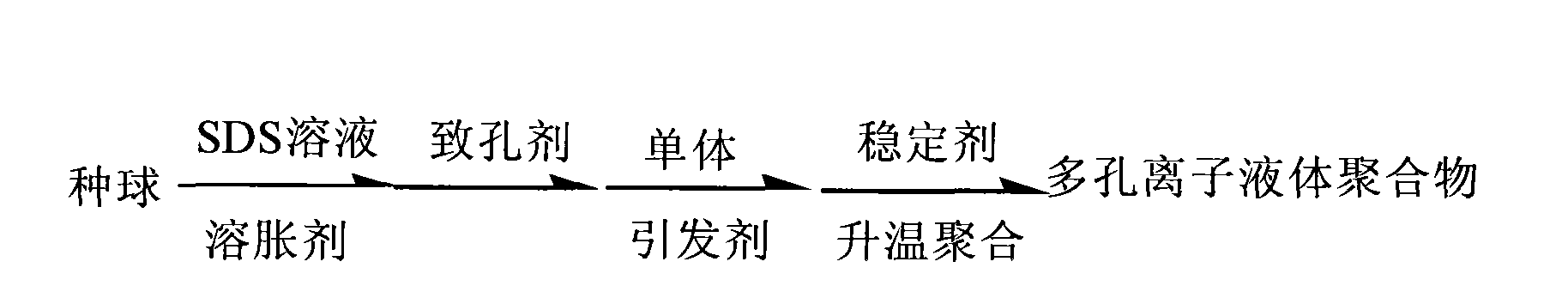
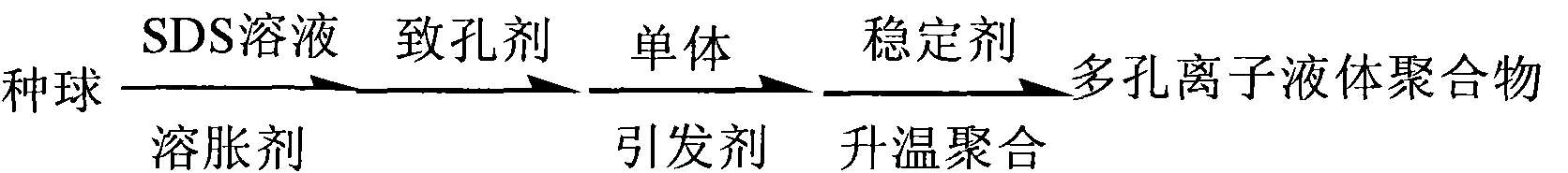
Synthesizing method of porous ion liquid polymer
InactiveCN101786988ANo pollutionIncrease concentrationOrganic chemistryTetrafluoroborateHigh absorption
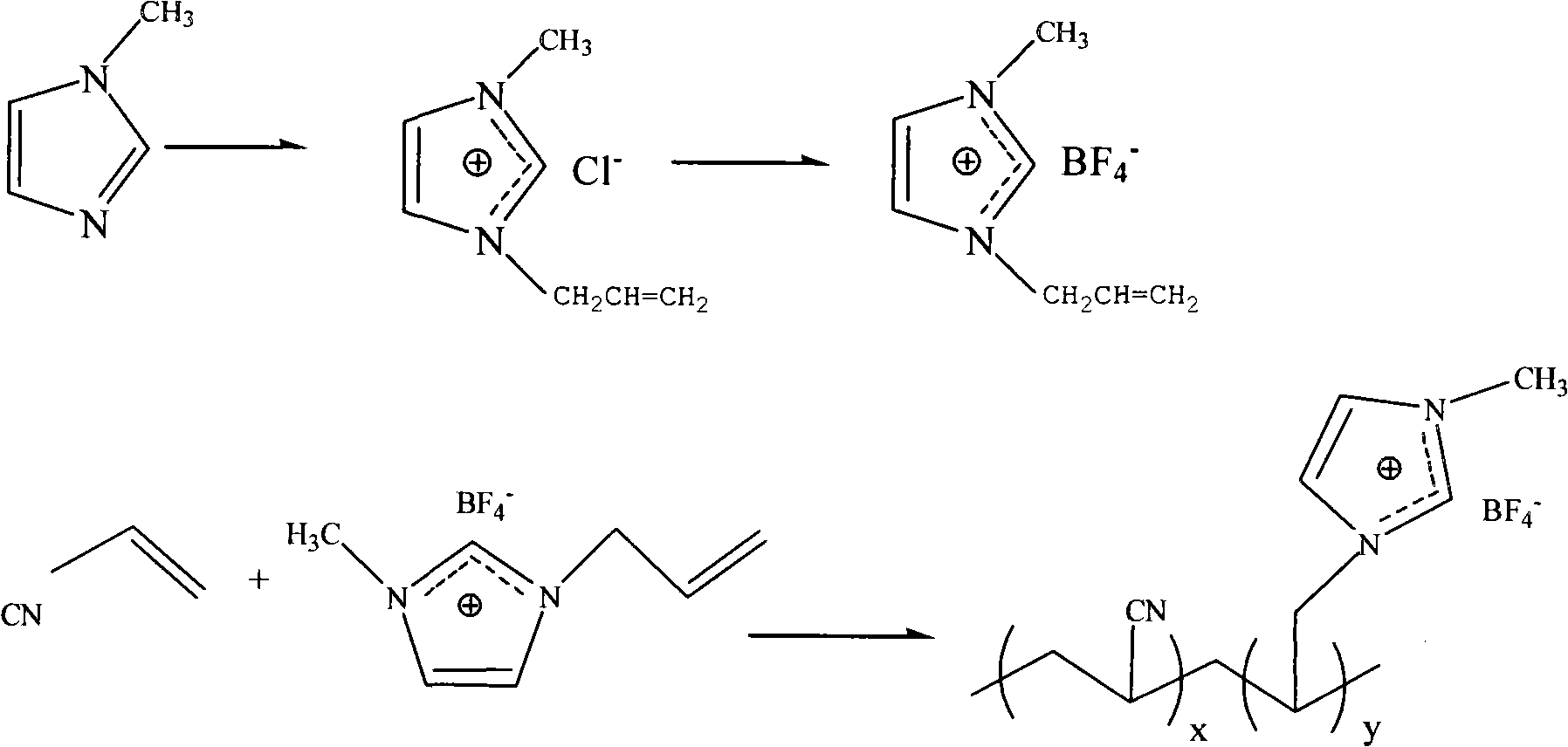
The invention relates to a synthesizing method of a porous ion liquid polymer, belonging to the field of synthesizing methods for separating and revering CO2 polymers. The synthesizing method of the porous ion liquid polymer comprises the following steps of: A, performing reaction of 1-methylimidazole and chloropropene to generate chlorinated 1-allyl-3-methylimidazole ion liquid; B, performing reaction of the chlorinated 1-allyl-3-methylimidazole ion liquid and fluoboric acid to generate 1-allyl-3-methylimidazole tetrafluoroborate ion liquid; C, performing reaction of the 1-allyl-3-methylimidazole tetrafluoroborate ion liquid with acrylonitrile to generate an ion liquid polymer; D, preparing the porous ion liquid polymer, i.e. adding sodium dodecyl benzene sulfonate, dibutyl phthalate and deionized water to carry out activation by taking the ion liquid polymer as a seedball, then respectively adding normal heptane, normal octane and toluene or cyclohexane to carry out swelling, then adding a monomer and benzoperoxide to carry out secondary swelling, and adding polyvinyl alcohol to carry out polymerization so as to obtain the porous ion liquid polymer. The invention has moderate reaction condition and short CO2 absorption time and high absorption amount in industrial application.
Owner:CHINA UNIV OF MINING & TECH
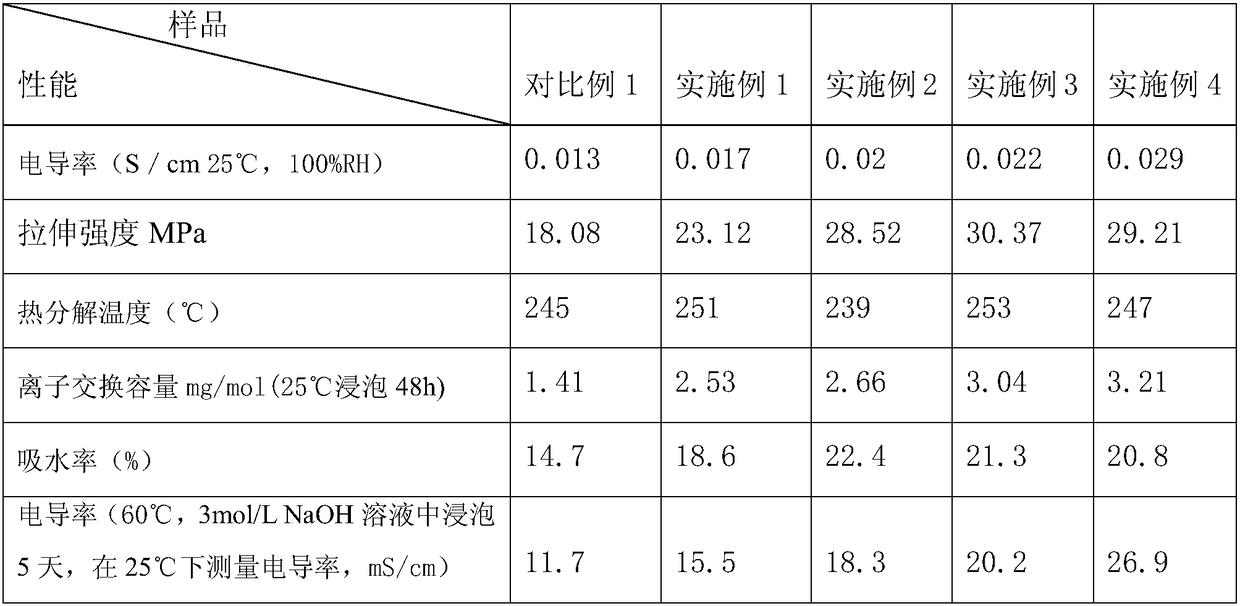
Crosslinking imidazole type polyether-ether-ketone anion-exchange membrane for fuel cell and preparation method thereof
ActiveCN108110290AGood dimensional stabilityImprove internal stabilityFinal product manufactureFuel cellsIonic conductance1-Methylimidazole
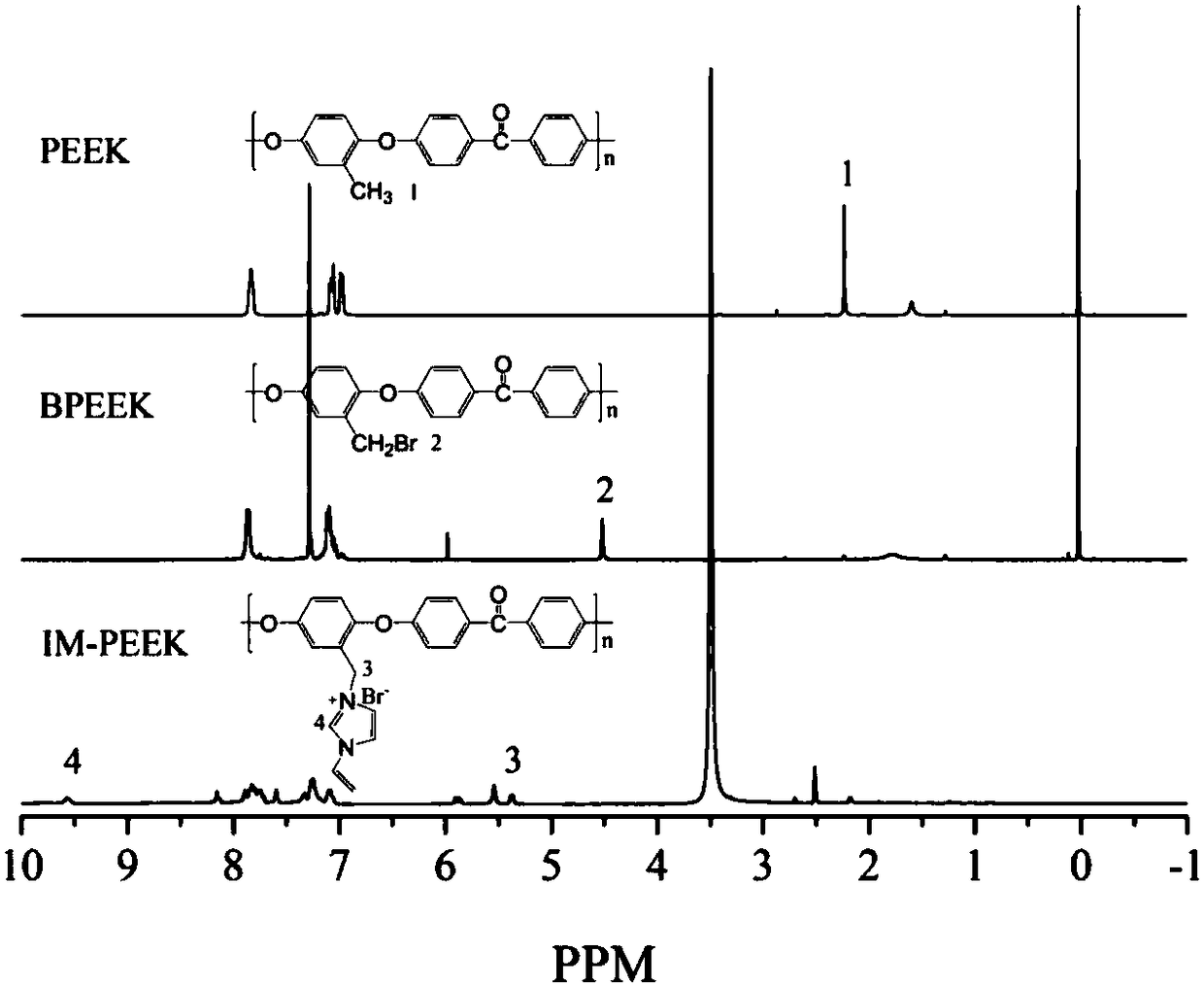
The invention provides a crosslinking imidazole type polyether-ether-ketone anion-exchange membrane for a fuel cell, and a preparation method thereof, which belong to the fields of high polymer chemistry and anion-exchange membrane fuel cells. A structural formula of the anion-exchange membrane is as shown in a formula I. The invention also provides the preparation method of the crosslinking imidazole type polyether-ether-ketone anion-exchange membrane for the fuel cell. The method comprises the steps of firstly preparing polyether-ether-ketone; then carrying out bromination substitution reaction on methyl on the polyether-ether-ketone, and obtaining brominated polyether-ether-ketone; adding 1-methylimidazole and 1-vinyl imidazole into a brominated polyether-ether-ketone solution, and obtaining a mixed solution; finally pouring the mixed solution onto a glass plate, drying, and obtaining a paved membrane which is the crosslinking imidazole type polyether-ether-ketone anion-exchange membrane for the fuel cell. The anion-exchange membrane provided by the invention has high ionic conductance, high alkali resistance, higher mechanical performance and higher relative selectivity; the preparation method provided by the invention is simple and low in cost.
Owner:CHANGCHUN UNIV OF TECH
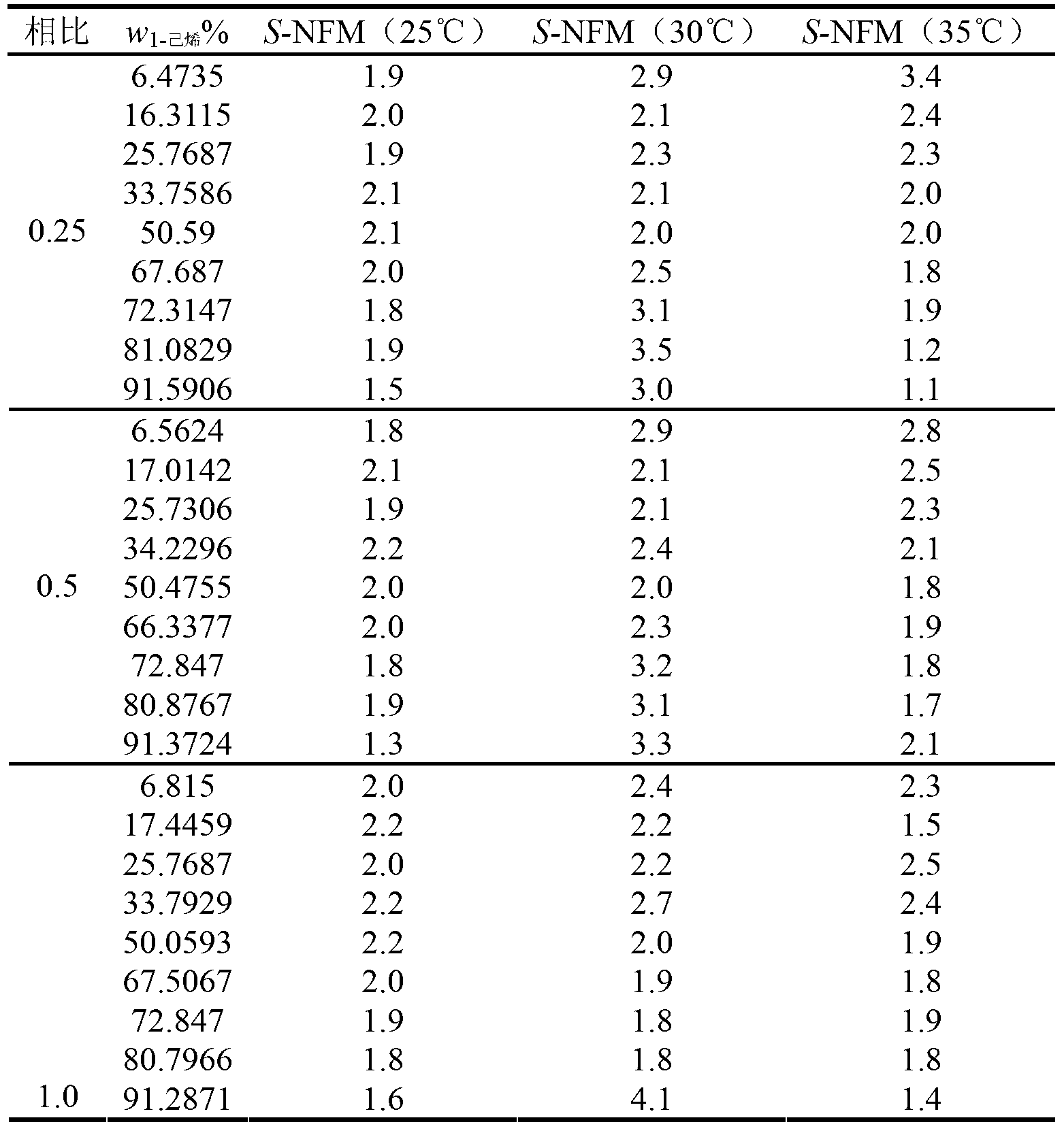
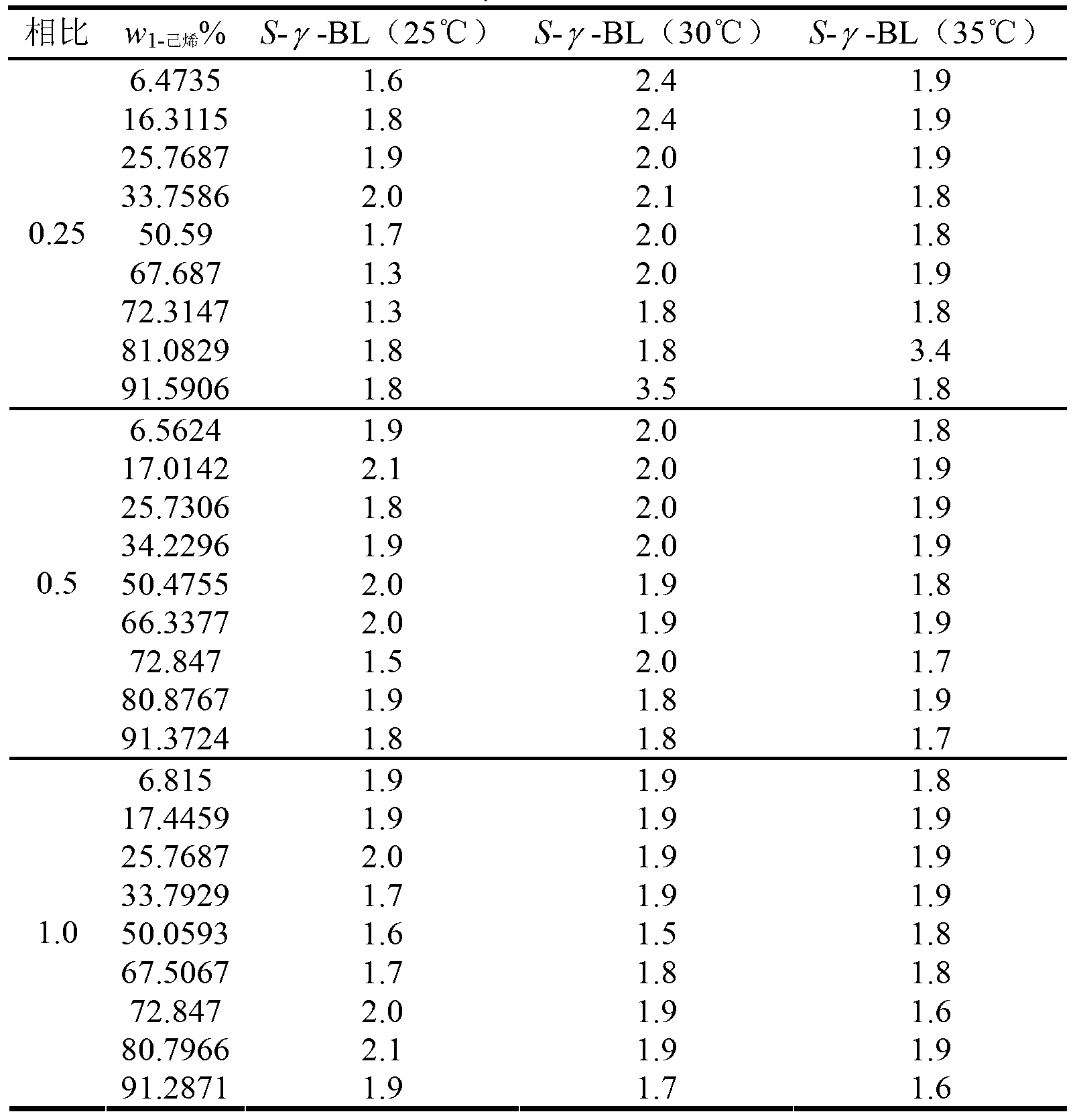
Extraction and separation method of alkane/olefin
The invention discloses an extraction and separation method of alkane / olefin and belongs to the technical field of separation of olefin. The extraction and separation method of alkane / olefin comprises the following steps of: sufficiently contacting and mixing a to-be-separated mixture containing carbon-isotope normal alkane and high-carbon alpha-olefin with an extracting agent; standing, layering and separating, wherein the high-carbon alpha-olefin enters an extracting phase, the carbon-isotope alkane enters the extracting phase for separating. The extracting agent is N-methylpyrrolidone (NMP), N-formyl morpholine (NFM), 1-methylimidazole(1-MI) or gamma-butyrolactone(gamma-BL); and the total volume ratio of the extracting agent to the to-be-separated mixture is 0.25, 0.5, 1.0 or 2.0. The extraction and separation method of the alkane / olefin is simple and environment-friendly.
Owner:BEIJING UNIV OF CHEM TECH
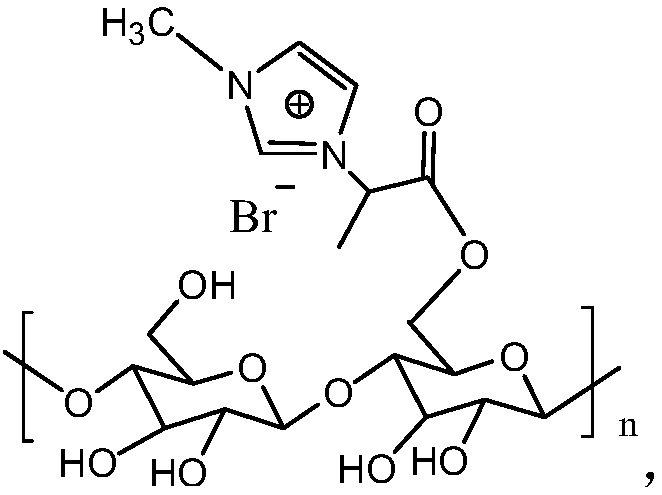
Ion liquid modified cellulose-based adsorbent as well as preparation method and application thereof
ActiveCN108393075AImprove adsorption capacitySolve the problem of low adsorption capacityOther chemical processesWater contaminantsWater bathsPropanoic acid
The invention discloses an ion liquid modified cellulose-based adsorbent as well as a preparation method and application thereof, and relates to the technical field of dye adsorption. The adsorbent can be degraded; the environment sustainable development is facilitated; the adsorbent can be widely applied to the printing and dying waste water treatment. The preparation method comprises the following steps of S1, adding cellulose, 2-bromo-propionic acid, p-toluenesulfonic acid and toluene into a container; connecting a water distributer on a container; S2, putting the container in an oil bath pot being 110 to 130 DEG C for constant temperature heating; performing condensing backflow for 7 to 10 hours; then, opening a side pipe piston of a water distributor to discharge out toluene-water mixed solution in the side pipe; evaporating out the toluene in the container to obtain a cellulose 2-bromopropionate coarse product; S3, performing post treatment on the cellulose and 2-bromopropionatecoarse product in the S2 to obtain dry cellulose 2-bromopropionate; S4, adding 1-methylimidazole and cellulose 2-bromopropionate dried in S3 into ethanol; performing water bath constant temperature stirring reaction for 24 to 48 hours at 40 to 70 DEG C; then, performing washing and drying to obtain the adsorbent. The method is simple; the used raw materials can be easily obtained.
Owner:SHAANXI UNIV OF SCI & TECH
Derivatization method of qualitative or quantitative analysis for polyhydroxy compound
InactiveCN101531557ASolve problems with derivatization reaction conditionsAvoid the problem of poor choiceEsterified saccharide compoundsSugar derivativesAcetic anhydride1-Methylimidazole
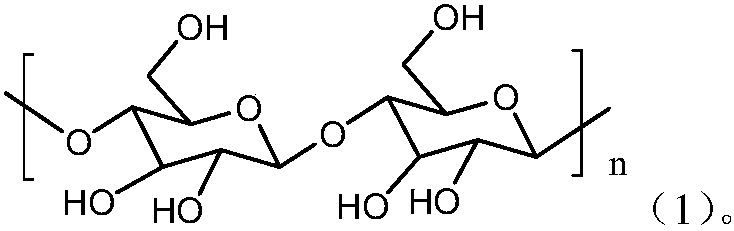
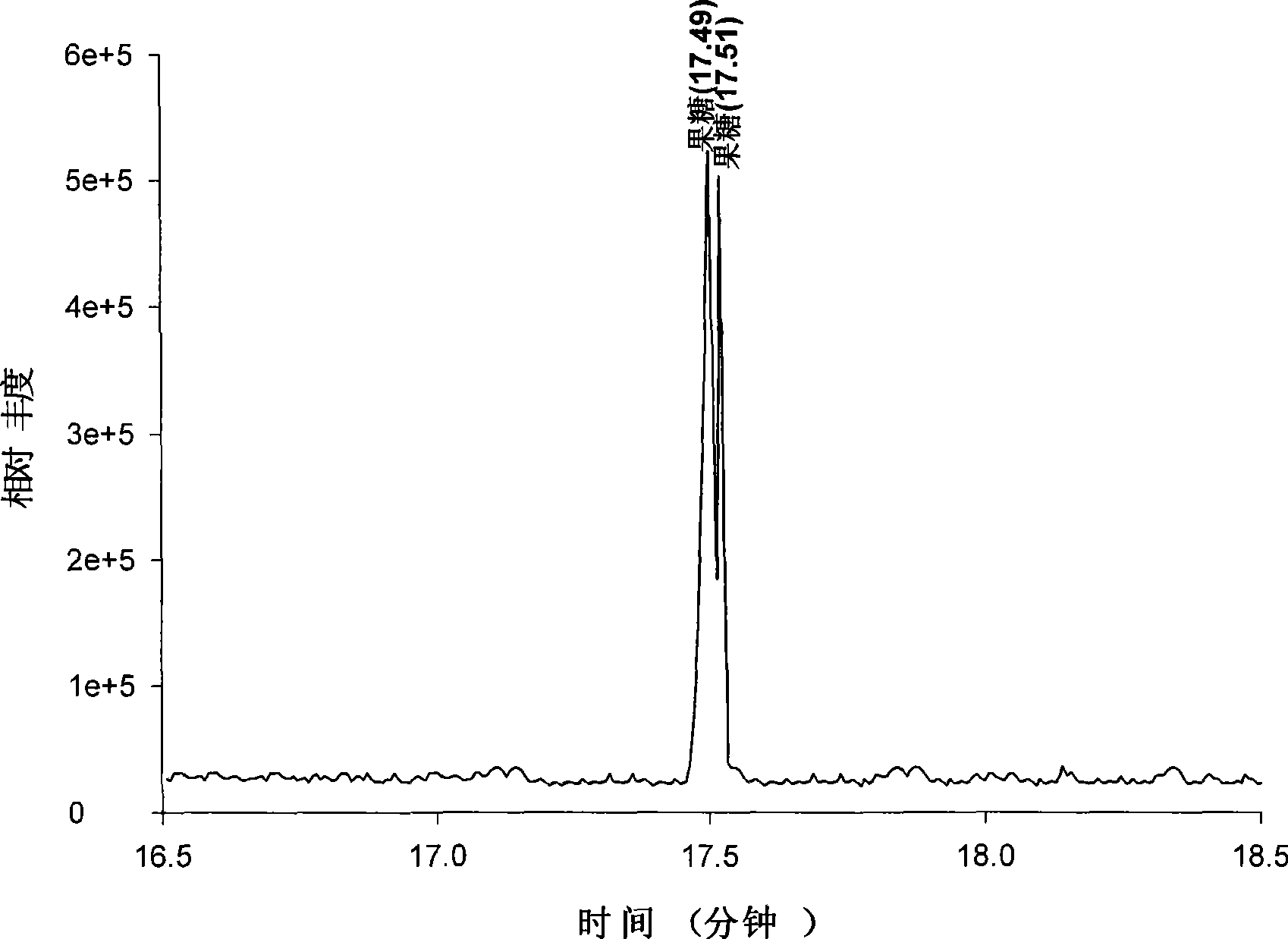
The invention discloses a derivatization method for polyhydroxy compound. In the method, a sugar-containing sample is disposed in a reaction system using 1-methylimidazole as catalyst and acetic anhydride as derivatization reagent to have acetylation derivatization reaction. The method in the invention can carry out acetylation derivatization on the basis of maintaining native conformation of sugar, single derivatization product is obtained after derivatization, and qualitative or quantitative analysis can be accurately carried out on various simple sugars.
Owner:BEIJING FORESTRY UNIVERSITY
Reverse transcriptase assay kit, use thereof and method for analysis of RT activity in biological samples
InactiveUS6849406B1Easy to useSensitive for RT activityMicrobiological testing/measurementBiological testingReverse transcriptasePolystyrene
A reverse transcriptase (RT) assay kit for analysis of RT activity in biological samples is described. The kit comprises solid phase bound prA and / or pdA template(s) obtainable by contacting a polystyrene-based solid phase with a 1-methylimidazole-containing coupling solution, and RT-type adapted assay components selected from a buffer, divalent metal ion, chelator, polyamine, RNase inhibitor, reducing agent, salt, stabilizing agent, and detergent, and deoxynucleotide triphosphate, primer, protective agent and concentrated washing buffer, and optionally lyophilized reference enzyme(s), and further optionally lyophilized alkaline phosphatase conjugated anti-BrdU monoclonal antibody, alkaline phosphatase substrate buffer and alkaline phosphatase substrate, and written instructions for use of the assay kit. Further, a method and a use of the assay kit for the qualitative and quantitative analysis of RT activity in a biological sample, optionally followed by evaluation of the status of a RT activity related disorder or disease based on the result of the analysis of the RT activity, are disclosed.
Owner:CAVIDI TECH AB
Modified silicon dioxide nanoparticles, and preparation method and applications thereof
ActiveCN106732384AHigh adsorption rateSave raw materialsOther chemical processesAlkali metal oxides/hydroxidesSorbent1-Methylimidazole
The invention relates to modified silicon dioxide nanoparticles, and a preparation method and applications thereof, and belongs to the technical field of nano material preparation. The preparation method comprises following steps: firstly, nano silicon dioxide particles and 3-chloropropyltriethoxysilane are added into an absolute ethyl alcohol solution, reflux reaction is carried out, and an obtained solid substance 1 is marked as COTS-SNPs; the COTS-SNPs is added into a 4,4-bipyridine solution, vinyl chloride, methylbenzene, and CuCl are added successively, reflux reaction is carried out, and an obtained solid substance 2 is marked as VC-SNPs; the obtained VC-SNPs is added into an absolute ethyl alcohol solution, triethylamine and 1-methylimidazole are added, reflux reaction is carried out, and an obtained solid substance 3, which is the modified silicon dioxide nanoparticles, is collected, and is marked as MDZ-SNPs. The obtained modified silicon dioxide nanoparticles can be taken as an adsorbent to absorbing palladium ions in solution, are cheap, are convenient to synthesis, can be easily separated with aqueous solution, possesses high adsorptivity, and can be recycled.
Owner:KUNMING UNIV OF SCI & TECH
Ion liquid catalyst for synthesizing diethyl carbonate by urea alcoholysis and preparation method thereof
InactiveCN102909076ARaw materials are easy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperature1-Methylimidazole
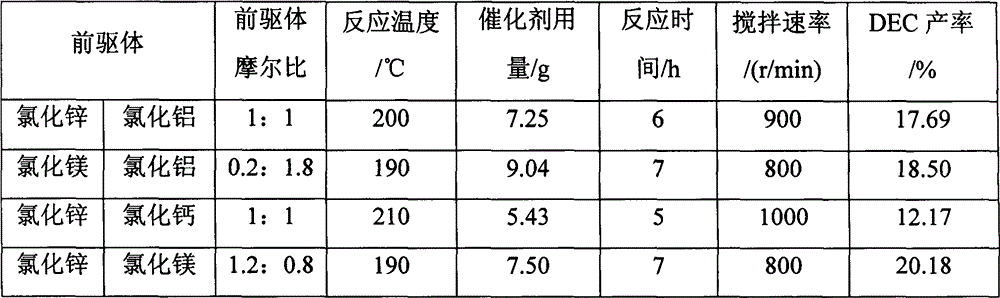
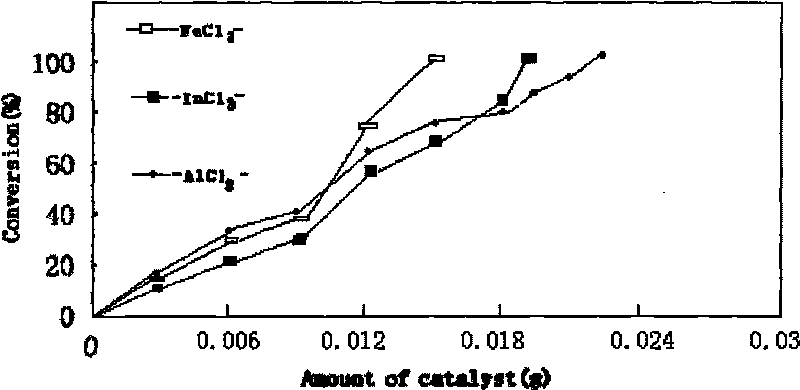
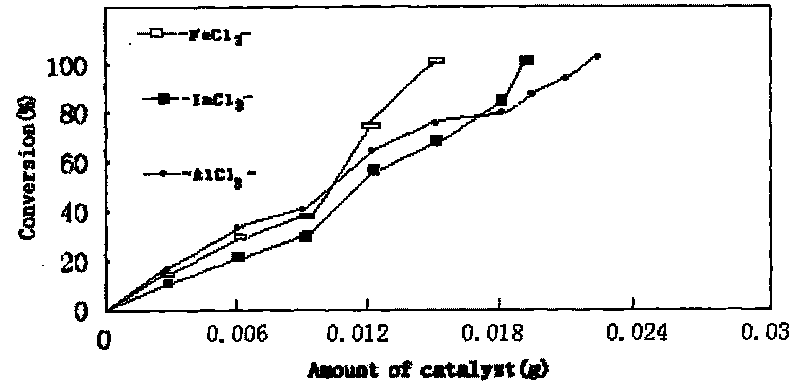
The invention provides a process for synthesizing diethyl carbonate by urea alcoholysis through an ion liquid catalyst. The catalyst is imidazole metal salts ion liquid. The method comprises the steps of adding absolute ethyl alcohol, urea and the catalyst into a high-pressure reaction kettle, wherein the agitation speed is 700-1000 r / min, the mol ratio of the absolute ethyl alcohol to the urea ranges from 5: 1 to 15: 1; the reaction temperature is 160-230 DEG C, the reaction time is 4-10 hours and the use amount of the ion liquid catalyst is 1-10% of the mass of raw materials; cooling to room temperature and separating to obtain a product; enabling 1-methylimidazole and n-chlorobutane (in a mol ratio of 1: 1) to react to synthesize an intermediate chlorinated 1-butyl-3-methylimidazole (BMIMCl); and further enabling the BMIMCl to react with a metal chloride to obtain a product. The added metal chloride is Lewis acid of zinc chloride, magnesium chloride, aluminum chloride, lead chloride, calcium chloride and the like, and is prepared by adding one or more of the Lewis acid to react according to a certain ratio. The method has the advantages of green environmental friendliness, high repeated utilization rate, simple process, no corrosion, selectivity of close to 100%, high yield (which is close to 30% to the greatest extent) and the like.
Owner:JIANGNAN UNIV
Method for preparing chitosan immobilized Lewis acidic ionic liquid and DCPP synthesized by using chitosan immobilized Lewis acidic ionic liquid in presence of high-efficient catalyst
InactiveCN101695673ACheap and easy to getMild responseGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSilanesEvaporation
The invention relates to a method for preparing chitosan immobilized Lewis acidic ionic liquid and DCPP synthesized by using chitosan immobilized Lewis acidic ionic liquid in presence of high-efficient catalyst. The method is characterized by comprising the following steps: performing refluxing reaction on 1-methylimidazole and Y-chloropropyl trimethoxy silane in a mol ratio of 1:1 at a temperature of 70 DEG C for 70 hours; then performing reaction in a mol ratio of Lewis acid anhydrous ferric chloride to a prepared product of 2:1 at a temperature of 70 DEG C for 24 hours; dispersing the obtained ionic liquid containing ferricyanide and chitosan which is 2 times of the ionic liquid in mass in acetonitrile to reflux for 20 hours; performing rotary evaporation to remove a solvent; and performing soxhlet extraction on the obtained solid with methylene dichloride for 30 hours to obtain a chitosan immobilized acidic ionic liquid catalyst PmimCl-FeCl3-CS. Anhydrous aluminum chloride and indium chloride are used for replacing anhydrous ferric chloride and serve as a Lewis acid in turn to obtain PmimCl-AlCl3-CS and PmimCl-InCl3-CS respectively. The method has the advantages of low-price and readily available reaction raw materials, mild reaction and simple condition.
Owner:NANCHANG HANGKONG UNIVERSITY
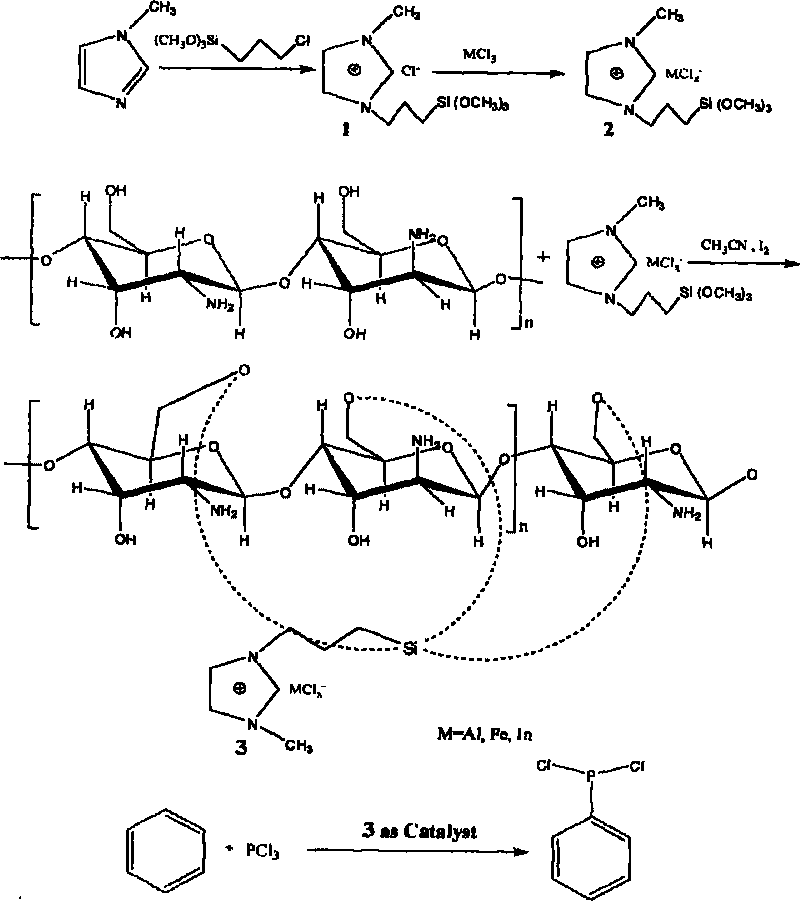
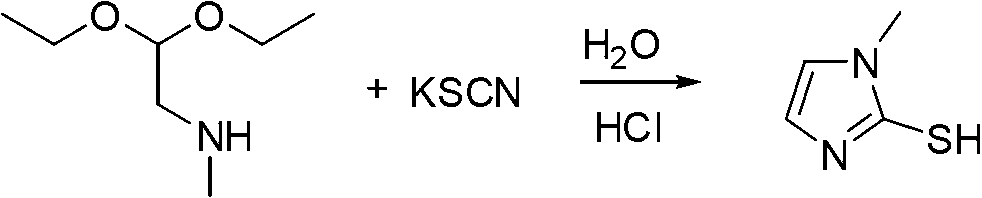
Industrialization production method of 2-mercapto-1-methylimidazole
Owner:XUZHOU B&C CHEM CO LTD
Silane cross-linked polyaryletherketone anion exchange membrane and preparation method thereof
The invention relates to a silane cross-linked polyaryletherketone anion exchange membrane and a preparation method thereof. The preparation method comprises the following steps: 1) preparing polyaryletherketone polymer powder; 2) dissolving the polyaryletherketone polymer powder, N-bromosuccinimide and benzoyl peroxide into chloroform; heating and refluxing for 12 to 24h under nitrogen gas; thenpouring a reaction solution into acetone and washing to obtain brominated polyaryletherketone; 3) dissolving the brominated polyaryletherketone into N,N-dimethylacetylamide into a brominated polyaryletherketone solution; adding 1-methylimidazole and carrying out substitution reaction to obtain a pre-polymer solution; then adding 3-(2-iminazole-1-linyl)propyltriethoxysilane into the pre-polymer solution and carrying out crosslinking reaction; carrying out membrane formation by utilizing sol-gel method, so as to obtain the silane cross-linked polyaryletherketone anion exchange membrane. The anion exchange membrane provided by the invention has the advantages of high electrical conductivity, strong alkali resistance, low water absorption, good size stability, great tensile strength and good comprehensive performance.
Owner:WUHAN UNIV OF TECH
Grit powder coating with high impact resistance and method for preparing grit powder coating
ActiveCN105754449AImprove impact resistanceImprove ductilityPowdery paintsEpoxy resin coatingsEthylenediaminePolyetherimide
The invention provides grit powder coating with high impact resistance.The grit powder coating comprises, by weight, 30-60 parts of epoxy resin, 20-30 parts of alkyd resin, 20-30 parts of organic silicon modified resin, 10-20 parts of fluorocarbon resin, 10-20 parts of polyetherimide, 1-10 parts of ethylenediamine, 1-10 parts of isophorone diamine, 1-10 parts of 1-methylimidazole, 1-10 parts of N-diglycidyl ether, 1-5 parts of sodium lignin sulfonate, 1-8 parts of polyethylene glycol, 1-5 parts of nanometer titanium dioxide, 1-5 parts of gas-phase silicon dioxide, 1-5 parts of light calcium carbonate, 1-3 parts of silane coupling agents, 1-3 parts of dimethyl siloxane, 1-3 parts of dibutyltin dilaurate, 1-3 parts of diatomaceous earth and 1-4 parts of grit agents.The grit powder coating and the method have the advantage that the grid powder coating is excellent in impact resistance under the condition of high film thicknesses (higher than 100 micrometers).
Owner:ZHEJIANG HUACAI NEW MATERIAL CO LTD
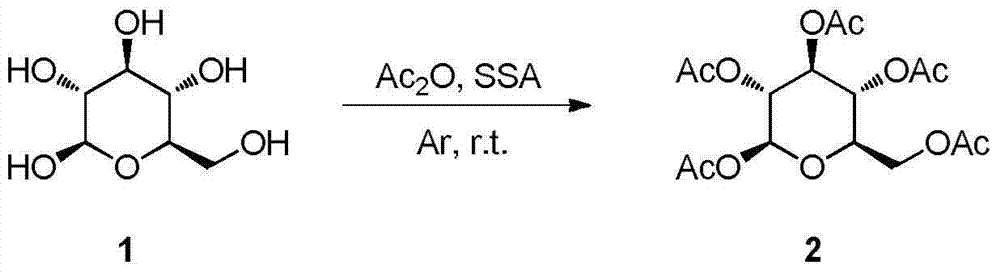
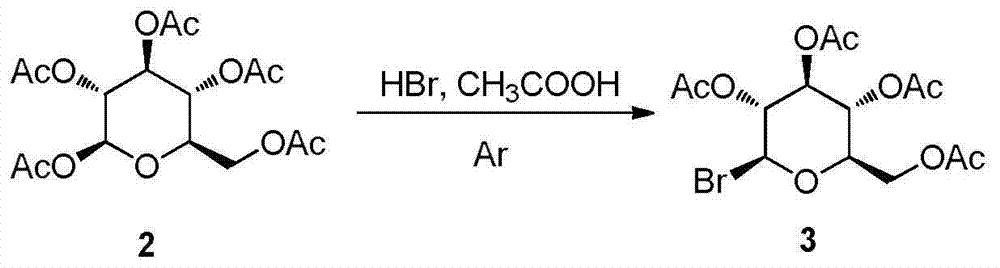
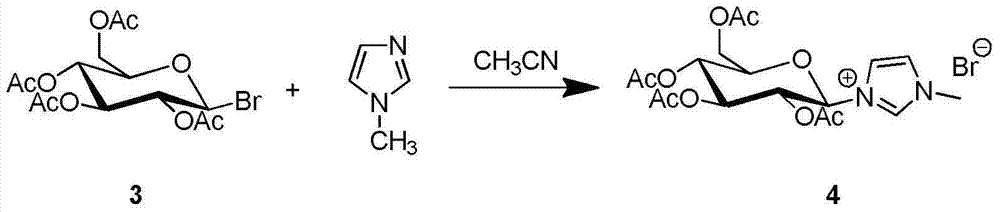
Synthesis and uses of beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate
ActiveCN104761601AEasy accessHigh catalytic activitySugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisChlorosulfuric acid
The invention relates to synthesis and uses of beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate and belongs to hydrosulfate chemical synthesis by multi-component reactions and uses of the hydrosulfate. The synthesis includes: subjecting glucose 1 that is adopted as a raw material to acetylation to obtain a compound 2; performing bromination by adopting the compound 2 as a raw material to obtain a compound 3; reacting the compound 3 with 1-methylimidazole to obtain a compound 4; hydrolyzing the compound 4 under alkaline conditions to obtain a target compound 5; and reacting the compound 5 with chlorosulfonic acid to obtain a target product 6 that is the beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate. A five-component reaction of 2 equivalents of an aromatic aldehyde, 2 equivalents of an arylamine and 1 equivalent of ethyl acetoacetate is performed by adopting the target product 6 as a catalyst at 40 DEG C under mild conditions to synthesize an ethyl 4-phenylamino-1-phenyl-1,2,5,6-tetrahydro-2,6-diphenylpyridine-3-carboxylate derivative. The synthesis and the uses are characterized in that: (1) the product 10 is obtained rapidly with a high yield; (2) reaction conditions are mild, operation is simple, reaction time is short, the yield is high and after-treatment is simple and convenient; and (3) the synthesis is green, economical and efficient.
Owner:江西省德兴市百勤异VC钠有限公司
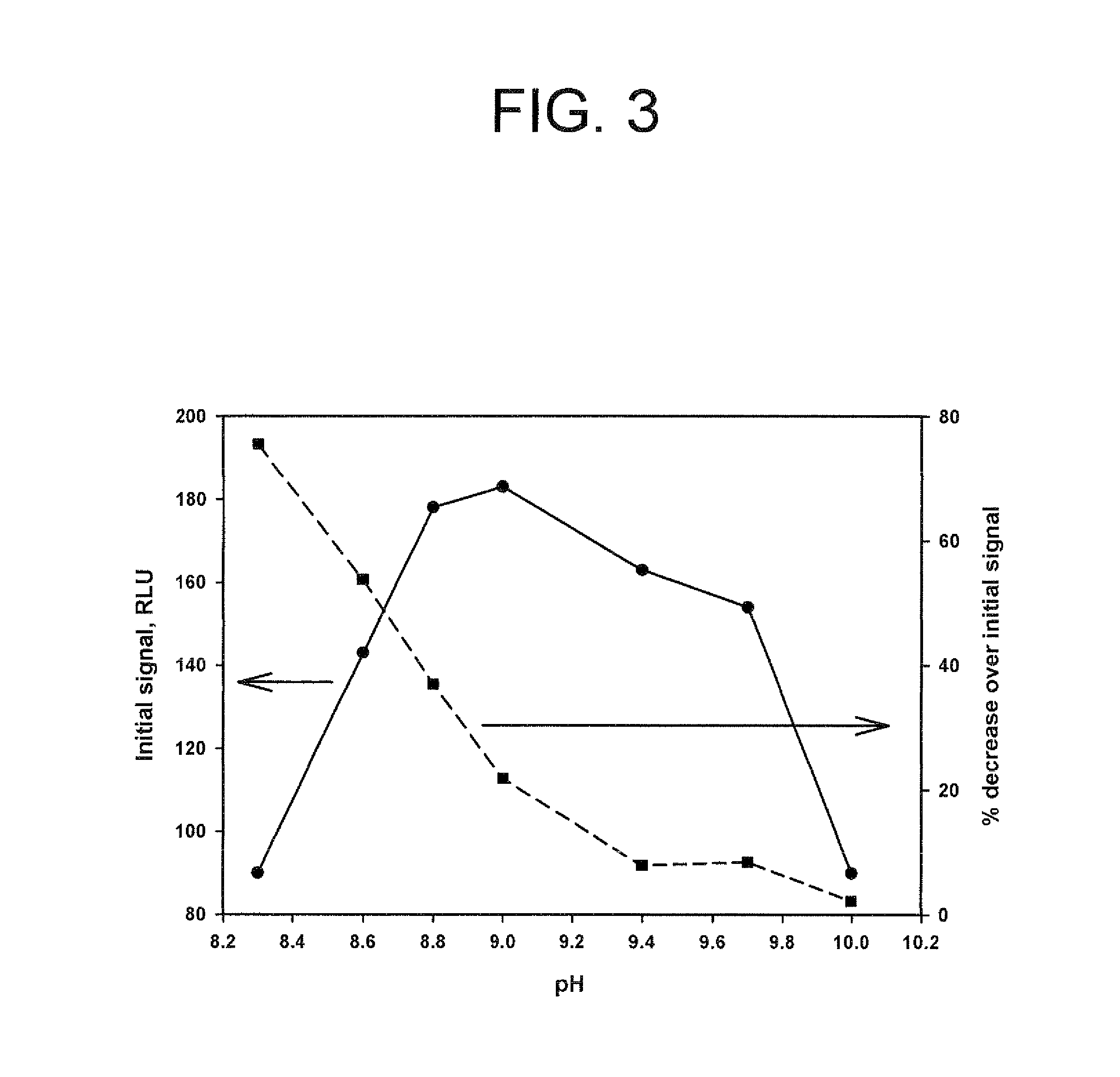
Method for increasing and regulating light emission from a chemiluminescent reaction
ActiveUS20120009603A1Increasing and regulating light emissionMicrobiological testing/measurementChemiluminescene/bioluminescencePeroxidaseLuminol
Method for increasing and regulating the emission of light from a chemiluminescent reaction including luminol, a peroxidase enzyme, an oxidant and an electron mediator (primary enhancer) through the use of an acylation catalyst (secondary enhancer) belonging to the class of N-azoles., i.e., a class of five-membered nitrogen heteroaromatic ring compounds containing at least one other atom of nitrogen. N-azoles, which are especially useful as secondary enhancers are imidazole, 1-methylimidazole, 1,2,3-triazole and 1,2,4-triazole. The invention also describes the use in diagnostic assays of chemiluminescent substrates containing said N-azoles, as secondary enhancers.
Owner:CYANAGEN
Imidazoline polyether-ether-ketone/sulfonated polyether-ether-ketone blended membrane as well as preparation method and application thereof
ActiveCN104559047AHigh ion exchange capacityImprove ionic conductivityCell electrodesCross-linkIon exchange
The invention discloses imidazoline polyether-ether-ketone / sulfonated polyether-ether-ketone blended membrane. The blended membrane has the thickness of 80-160 microns and consists of imidazoline polyether-ether-ketone and sulfonated polyether-ether-ketone, wherein the mass fraction of sulfonated polyether-ether-ketone is 0.07-0.25, the degree of sulfonation of sulfonated polyether-ether-ketone is 47%-67%, and the modification degree of imidazoline polyether-ether-ketone is 94%-200%. According to the blended membrane, chloromethylated polyether-ether-ketone with the degree of sulfonation of 94%-200% is taken as a high-polymer raw material, sulfonated polyether-ether-ketone is taken as a cross-linking agent, mixed liquid of 1-methylimidazole and N,N-diethyl formamide is taken as a solvent; in a membrane forming process, imidazoline polyether-ether-ketone is generated by virtue of in-situ reaction of chloromethylated polyether-ether-ketone and 1-methylimidazole. The prepared blended membrane has very high ion exchange capacity and ionic conductivity; furthermore, the raw materials are easily available, and the preparation process is easy, convenient and controllable.
Owner:TIANJIN UNIV
Synthetic method of imidazole acidic ionic liquid
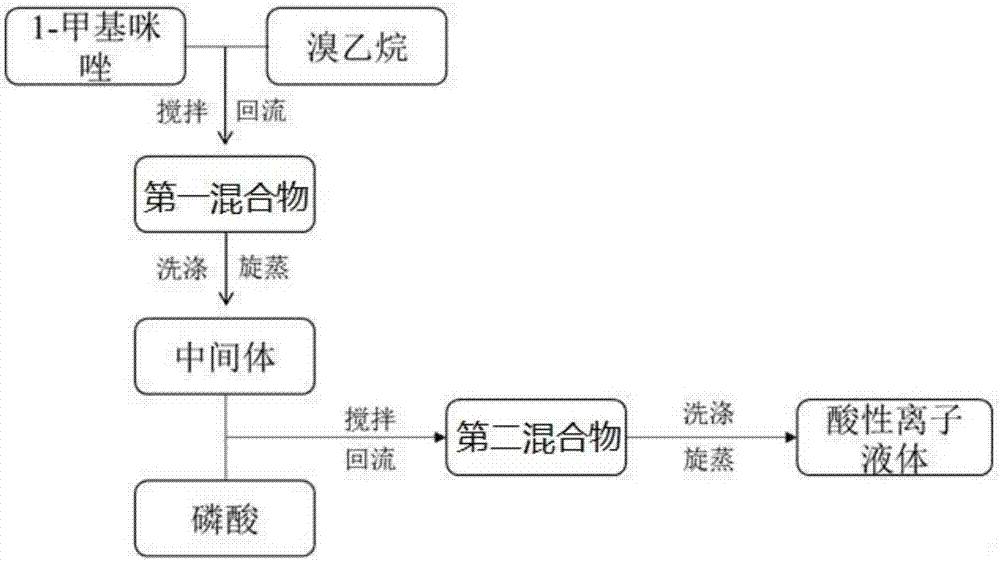
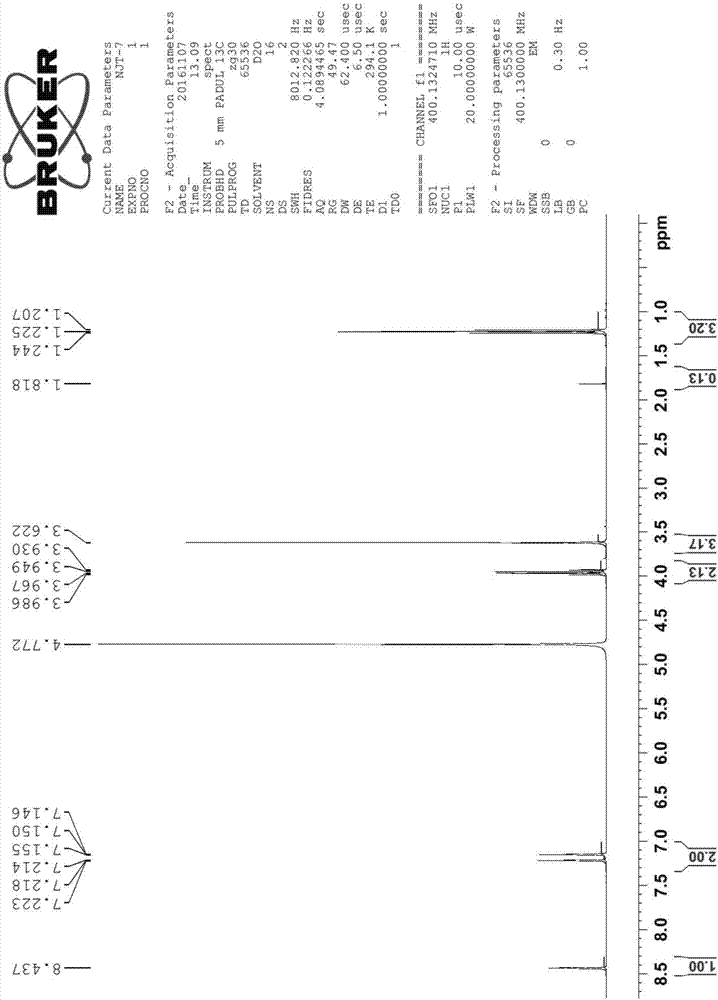
InactiveCN106946787AThe synthesis method is simpleImprove catalytic performanceOrganic chemistryChemical recyclingIce waterBromine
The invention discloses a synthetic method of an imidazole acidic ionic liquid. The method comprises the following steps: (1) taking 1-methylimidazole, heating the 1-methylimidazole to 65-85 DEG C, dropise adding bromoethane under a stirring condition, carrying out stirring refluxing at 65-85 DEG C for 9-15 h to obtain a first mixture, washing the first mixture, and drying the first mixture in a vacuum drying box to obtain an intermediate; and (2) taking the intermediate obtained in step (1), placing the intermediate in ice water bath, dropwise adding a phosphoric acid solution under a stirring condition at 0-5 DEG C, carrying out stirring refluxing at 20-30 DEG C for 1-4 h after the dropwise addition ends in order to obtain a second mixture, and carrying out washing, rotary evaporation and drying on the second mixture to obtain the imidazole acidic ionic liquid. The method has the advantages of simplicity and easiness in operation, and the imidazole acidic ionic liquid has the advantages of high catalytic performance, repeated use, easiness in separation from reactants, easiness in recycling, and convenience in use.
Owner:NANJING UNIV OF TECH
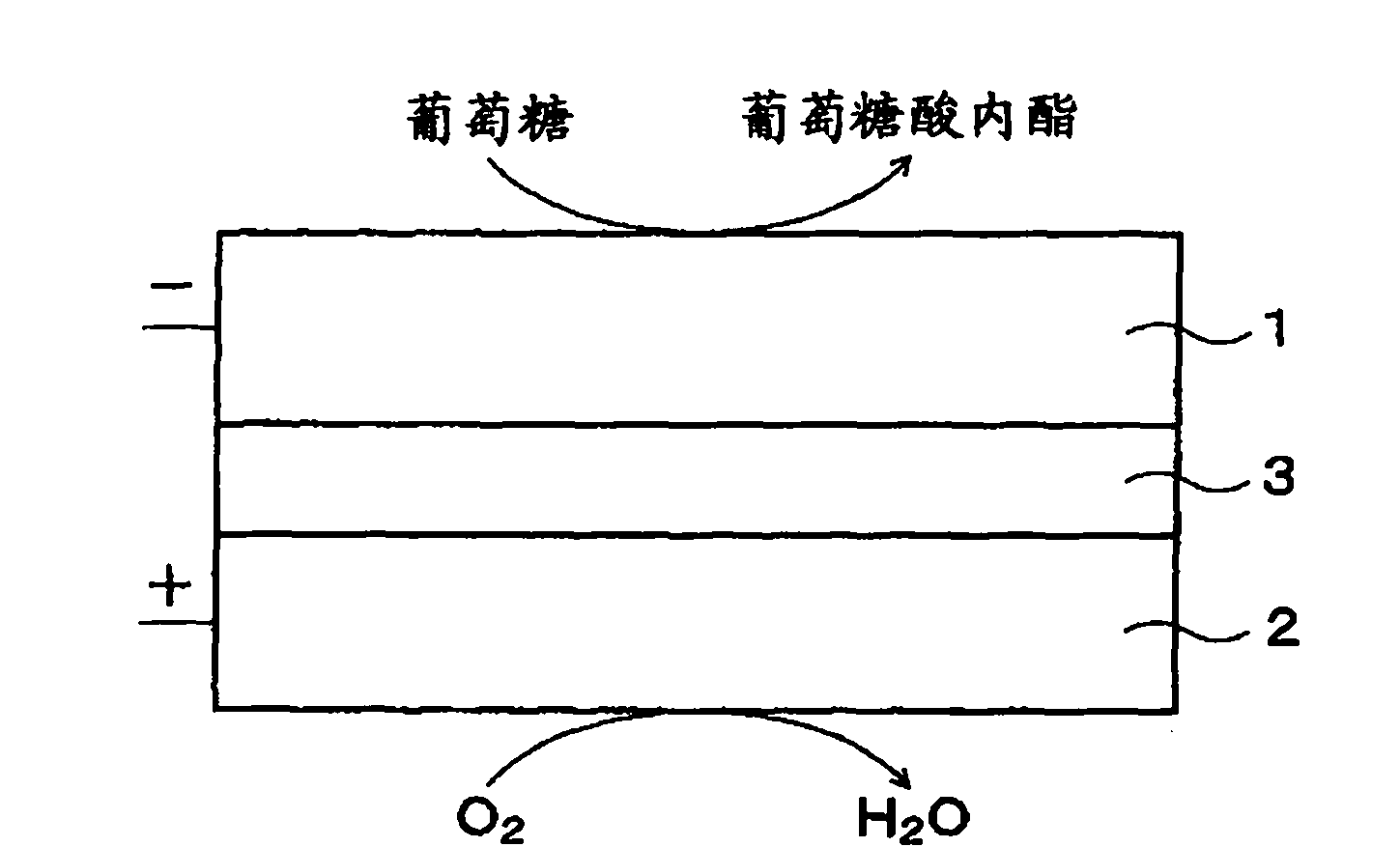
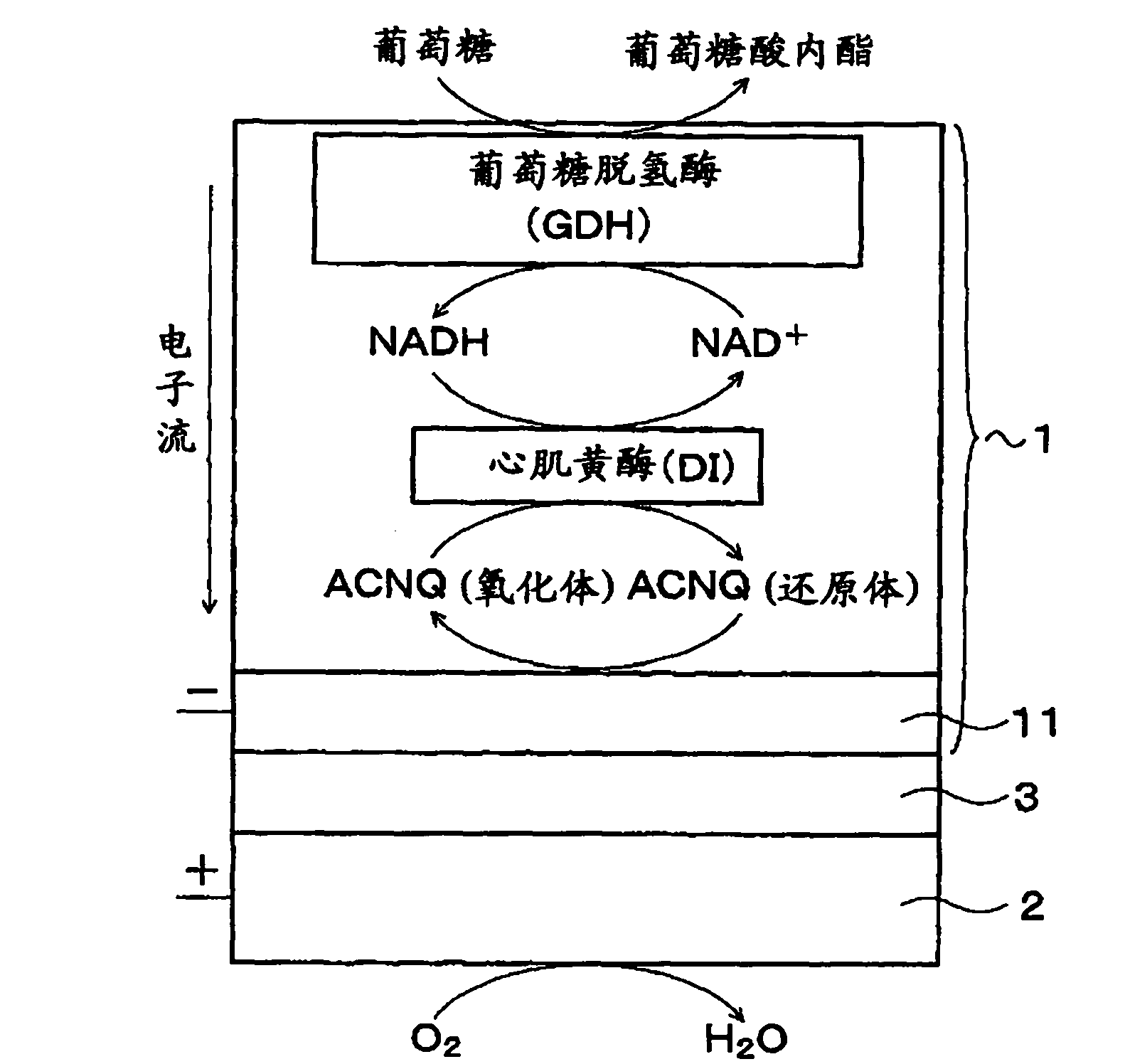
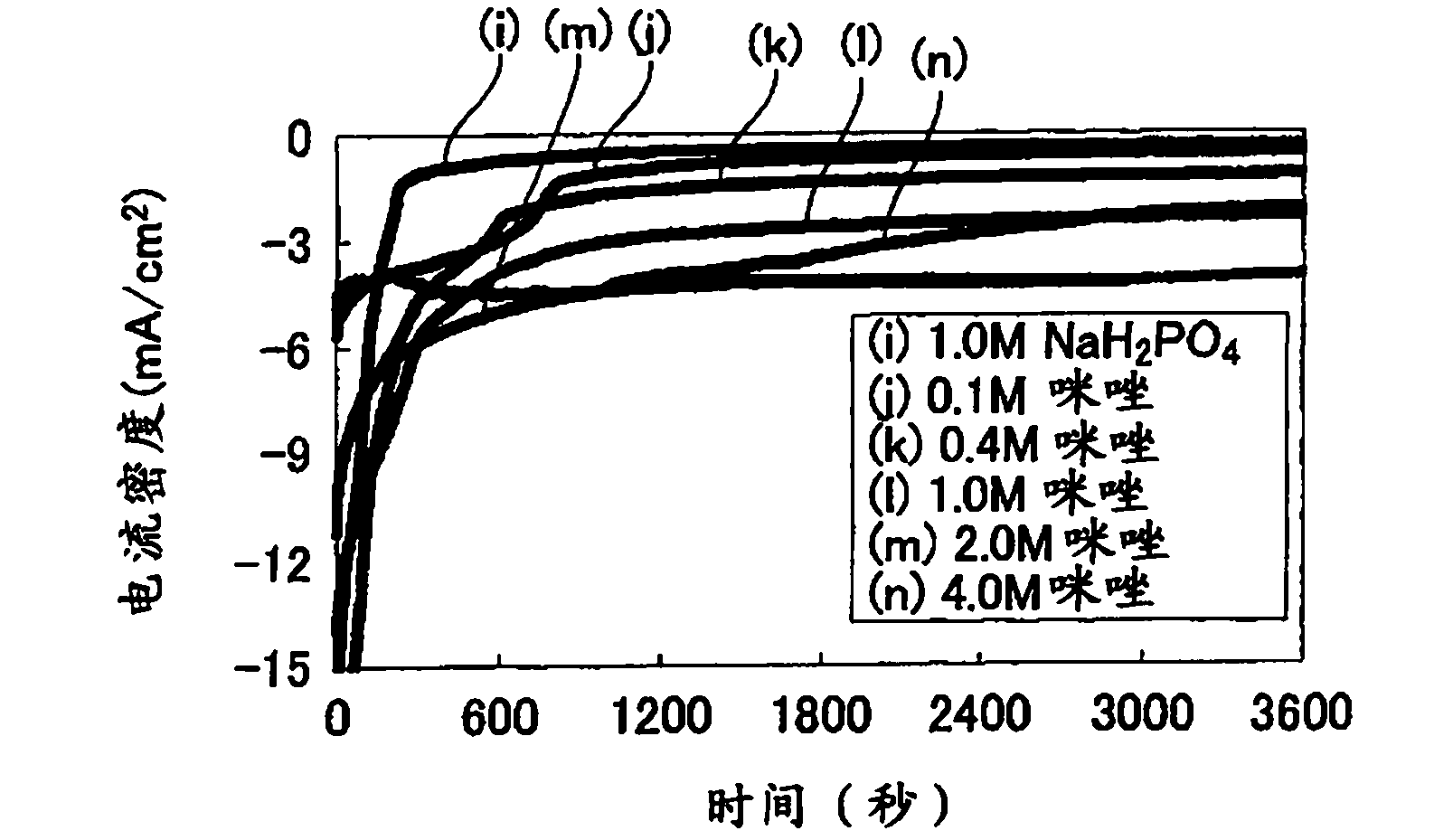
Fuel cell, electronic device and buffer solution for fuel cell
InactiveCN102171875ASufficient buffer capacityIncrease enzyme activityMicrobiological testing/measurementCell electrodesPhosphoric acid1-Methylimidazole
Provided is a fuel cell having an excellent performance, wherein sufficient buffering power can be obtained during high output operation when an enzyme is immobilized on a positive electrode and / or a negative electrode, and the enzyme can exert intrinsic abilities thereof sufficiently. Specifically disclosed is a biofuel cell having a structure wherein a positive electrode 2 and a negative electrode 1 are arranged opposite to each other with an electrolyte layer 3 containing a buffering substance interposed therebetween, and an enzyme is immobilized on the positive electrode 2 and / or the negative electrode 1. The electrolyte layer 3 contains, as a buffering substance, a compound having an imidazole ring, and at least one acid selected from the group consisting of acetic acid, phosphoric acid and sulfuric acid is added thereto. Imidazole, 1-methylimidazole, 2-methylimidazole, 4-methylimidazole, 2-ethylimidazole or the like is used as the compound having an imidazole ring.
Owner:SONY CORP
Crystal form of 5-hydroxy-1-methylimidazolidin-2,4-dione
InactiveUS7569701B2Easily soluble in waterImprove stabilityBiocideOrganic active ingredientsOrganic solvent1-Methylimidazole
Owner:NIPPON ZOKI PHARM CO LTD
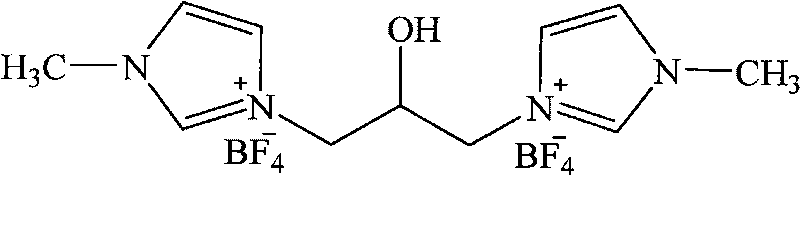
1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid and preparation method thereof
InactiveCN101759647AImprove electrochemical stabilityImprove thermal stabilityOrganic chemistryWater bathsTetrafluoroborate
The invention discloses ion liquid containing symmetrical functional groups, in particular to 1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid and a preparation method thereof. The 1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid has a general structure formula as follows. The preparation method of the 1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid comprises the following steps of: dissolving 1-methylimidazole into 2-3 times of alcohol; adding tetrafluoroboric acid with 0.9-1.1 times of molar weight under ice-water bath stirring at -5-10 DEG C; reacting for 30-90 minutes under the same condition, and then dripping 3-chlorine-propylene oxide with 0.8-1.0 times of molar weight to a reaction bulb; after a drying tube is prepared, placing the reaction bulb in a water bath of an experimental ultrasonic cleaner, and ultrasonically radiating (with the power of 300 W and the frequency of 40 KHz) and reacting for 3-12 hours under the condition of 15-70 DEG C; removing a solvent through rotation, decompression and evaporation; drying for 12 hours in vacuum at 80 DEG C and then obtaining an intermediate compound; then mixing the intermediate compound with the 1-methylimidazole with the molar ratio of 1:1.2-1:2.5; adding absolute alcohol with 2-3 times mount of total amount; then adding the tetrafluoroboric acid with 1.2-1.5 times of molar weight; reacting for 2-8 hours under the stirring condition at -5-10 DEG C; then increasing temperature to 10-60 DEG C, and reacting for 12-24 hours; removing the solvent through the rotation, the decompression and the evaporation; and drying for 12 hours in vacuum at 80 DEG C and then obtaining the pure1, 3-di-(1-methylimidazole)-2-propanol tetrafluoroborate ion liquid.
Owner:QINGDAO UNIV OF SCI & TECH
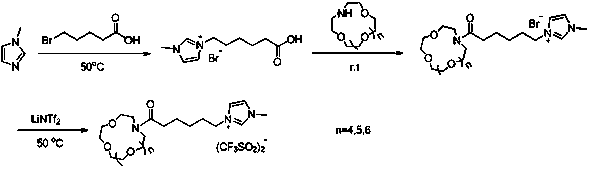
Synthesis method of aza-crown ether functionalized ionic liquid
PendingCN110396082AImprove stabilityHigh yieldOrganic chemistryPotassium hexafluorophosphateSynthesis methods
The invention discloses a synthesis method of aza-crown ether functionalized ionic liquid, and belongs to the technical field of organic synthesis and supermolecules. The invention aims to provide a synthetic method of hydrophobic crown ether functionalized ionic liquid. The method comprises the following steps of: 1) performing a reaction on 1-methylimidazole and bromoalkyl acid serving as raw materials to obtain 1-carboxyl-3-methylimidazole bromide; 2) carrying out a reaction with aza-crown ether to obtain 1-alkanoyl-aza-crown ether-3-methylimidazole bromide; and 3) dissolving the 1-alkanoyl-aza-crown ether-3-methylimidazole bromide and lithium / sodium / potassium bistrifluoromethanesulfonimide (or lithium / sodium / potassium hexafluorophosphate) in water for anion exchange, and finally washing and drying the products to obtain 1-alkanoyl-aza-crown ether-3-methylimidazole bistrifluoromethanesulfonimide / hexafluorophosphate ionic liquid. The synthesis method can effectively avoid the loss ofcrown ether molecules and improve the stability of the chelate. In addition, the anionic bistrifluoromethanesulfonimide salt of the ionic liquid is hydrophobic, so that the ionic liquid can be used for recycling metal ions in a water phase.
Owner:SHANXI UNIV
Nitric acid-free yellow fume-free aluminum alloy chemical polishing liquid and preparation method thereof
The invention discloses a nitric acid-free yellow fume-free aluminum alloy chemical polishing liquid and a preparation method thereof. The nitric acid-free yellow fume-free aluminum alloy chemical polishing liquid contains, by mass, 40 to 50 mL / L of citric acid, 15 to 30 mL / L of sulfonic acid, 45 to 65 mL / L of glacial acetic acid, 40 to 55 mL / L of a brightener, 5 to 10 g / L of a molybdate, 3 to 6 g / L of a tungstate, 1 to 3 g / L of 2-mercapto-1-methylimidazole and the balance of water. Compared with the traditional three-acid system, the aluminum alloy chemical polishing liquid has the characteristics of environmental friendliness, no yellow fume, good polishing effects, stable polishing rate, long work liquid service life and preparation convenience.
Owner:合肥华清金属表面处理有限责任公司
Preparation method of imidazole chiral ionic liquid replaced by C-2
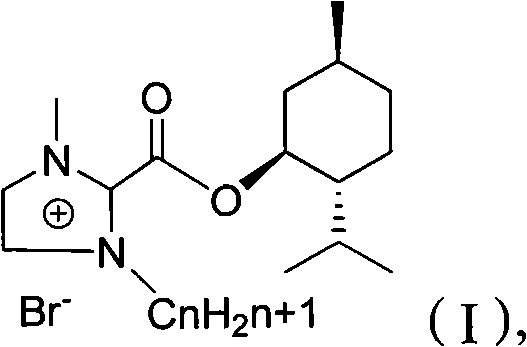
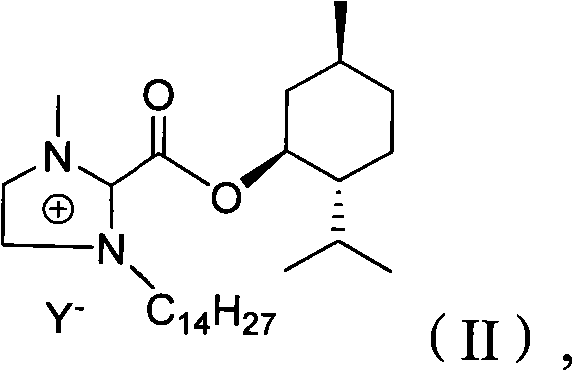
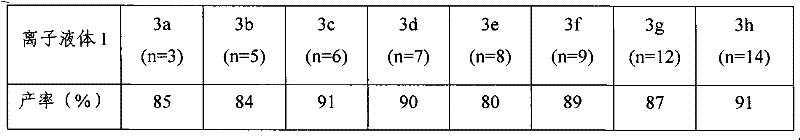
InactiveCN102516180AEasy to prepareRaw materials are easy to getOrganic chemistryMenthol1-Methylimidazole
The invention relates to a preparation method of imidazole chiral ionic liquid replaced by C-2, which comprises the following steps of: acylating 1-methylimidazole and trichloroacetic chloride, esterfying with chiral menthol and carrying out chiral fragment reaction at 1-methylimidazole C-2. According to the preparation method of the imidazole chiral ionic liquid replaced by the C-2, natural menthol is introduced into a chiral center by adopting a green chemical thinking method and utilizing a chiral pool method to prepare the imidazole chiral ionic liquid replaced by the C-2 under the microwave-assisted catalysis. The preparation method has the advantages of simplicity, easiness for raw material obtaining, low cost and short reaction time, thereby having a wide development prospect.
Owner:中国人民解放军63975部队
Gel electrolyte for dye-sensitized nanocrystal solar cell
InactiveCN102360952AImprove stabilityImprove conductivityLight-sensitive devicesCapacitor electrolytes/absorbentsTin dioxideN dimethylformamide
The invention relates to a gel electrolyte for a dye-sensitized nanocrystal solar cell. The gel electrolyte for the dye-sensitized nanocrystal solar cell contains an organic molten salt and a nanometer tin dioxide gel in a volume ratio of 50:(7-15). A preparation method of the gel electrolyte comprises the following steps of: at first, reacting polyethylene oxide with a low molecular weight with thionyl fluoride to generate fluorinated polyethylene oxide, and reacting the fluorinated polyethylene oxide with 1-methylimidazole in N,N-dimethylformamide to obtain the molten salt with fluoride ions as anions; then preparing the nanometer tin dioxide gel from tin dioxide; and finally, mixing the molten salt with the nanometer tin dioxide gel so as to obtain stable gel electrolyte by ageing and purifying. The gel electrolyte prepared by the preparation method has the same properties as a liquid electrolyte, and has the advantages of high stability, high conductivity and long service life. The gel electrolyte provided by the invention plays an important role in the preparation field of the dye-sensitized nanocrystal solar cell and has a wide application prospect.
Owner:BEIJING JIAOTONG UNIV
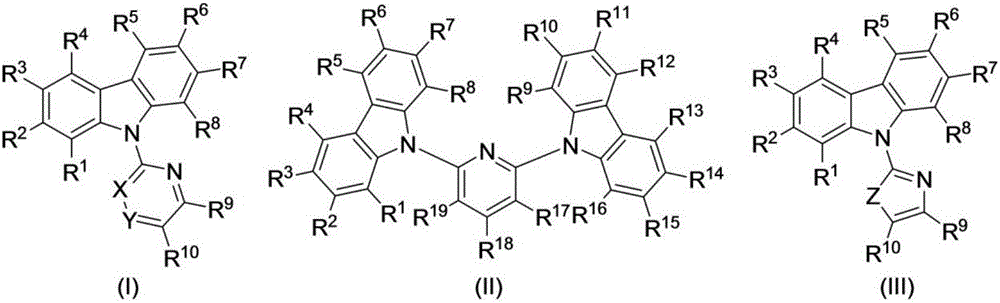
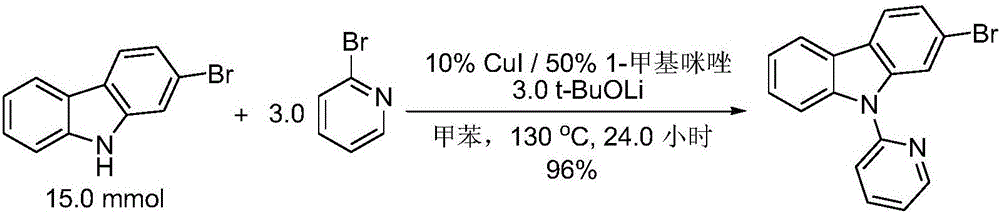
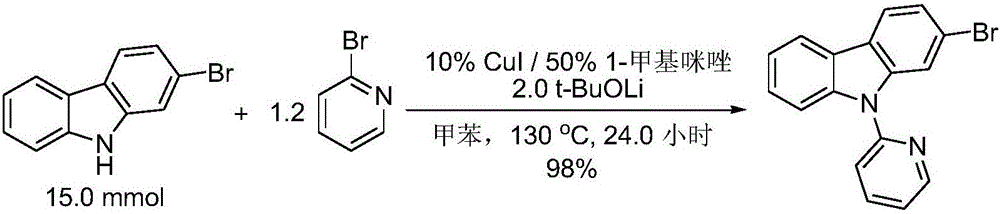
Preparation method of N-heteroaryl carbazole compounds
The invention relates to a preparation method of N-heteroaryl carbazole compounds. The method comprises the following steps: by using carbazole compounds and heteroaryl halides as raw materials, Cu (I) salt as a catalyst and 1-methylimidazole as a ligand, carrying out reaction in an organic solvent at 110-130 DEG C in a nitrogen protective atmosphere in the presence of an alkaline matter tert-butyl alcohol lithium for 1.2 hours-5.0 days; and after the reaction finishes, carrying out separation purification on the reaction mixture to obtain the N-heteroaryl carbazole compounds disclosed as Formula (I), Formula (II) or Formula (III), wherein the heteroaryl halides are heteroaryl bromides or heteroaryl iodides. The method has the advantages of low raw material price, simple reaction technique and operation and high yield (up to 90% or above), is suitable for mass preparation and industrialization, and has wide application prospects.
Owner:ZHEJIANG UNIV OF TECH
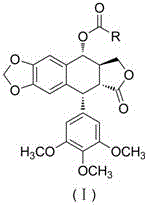
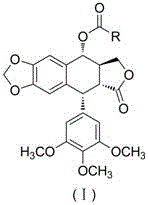
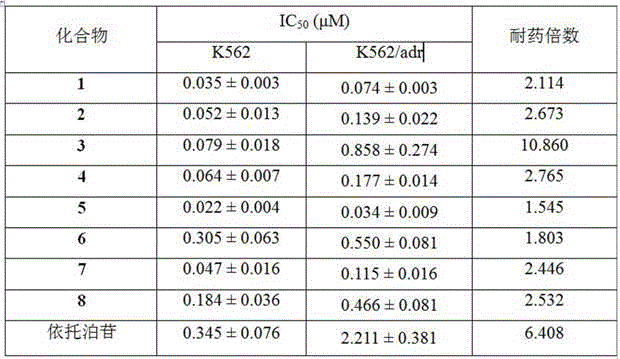
Heterocyclic aromatic acid ester type podophyllotoxin derivatives with anti-tumor activity as well as preparation method and application
InactiveCN106008540AEnhanced inhibitory effectLow drug resistanceOrganic chemistryAntineoplastic agentsQuinoxaline1-Methylimidazole
The invention discloses an aromatic heterocyclic ester podophyllotoxin derivative represented by formula (I), its preparation method and its use in the preparation of antitumor drugs. The present invention combines podophyllotoxin with 4-thiazole formic acid, 1-methylpyrazole-4-formic acid, 1-methylimidazole-4-formic acid, isoquinoline-1-formic acid, 2-quinoxaline formic acid, 1- Esterification with cyclopropyl-1,4-dihydro-4-oxo-6-fluoro-7-chloroquinoline-3-carboxylic acid, 1-methylindazole-3-carboxylic acid or thioindene-2-carboxylic acid , to obtain the aromatic heterocyclic ester podophyllotoxin derivatives represented by the formula (I) with antitumor activity. In vitro cell activity experiments prove that the aromatic heterocyclic ester podophyllotoxin derivatives of the present invention can inhibit the proliferation of tumor cells, have significant antitumor activity, and are expected to be prepared as podophyllotoxin antitumor drugs.
Owner:ZUNYI MEDICAL UNIVERSITY
Composite anion-exchange membrane and preparation method thereof
Disclosed is a composite anion-exchange membrane, which comprises a polytetrafluoroethylene microporous base membrane, an anion-exchange exchange resin and TESO which are uniformly immersed inside the base membrane and are attached to two side surfaces of the base membrane. The carrying capacity of the anion-exchange exchange resin on the polytetrafluoroethylene microporous base membrane is 4-10 mg / cm2, and the carrying capacity of TESO on the polytetrafluoroethylene microporous base membrane is 0.2-2 mg / cm2. The anion-exchange exchange resin is one or more than two of quaternary ammonium salt polyarylether ketone / sulphone, 1-methylimidazole polyarylether ketone / sulphone, 1-methyl-2-alkyl imidazole polyarylether ketone / sulphone, quaternary ammonium salt polystyrene, imidazole polystyrene, 1- methylimidazole polystyrene, and 1-methyl-2-alkyl imidazole polystyrene. Through comparison with the prior art, the composite anion-exchange membrane has advantages of high mechanical strength and good stability in an alkali liquor. The preparation method of the membrane is simple, is wide in applicability, and is suitable for massive production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel activated carbon adsorbing material loaded with imidazole ionic liquid
InactiveCN109289778AImprove stabilityImprove heat resistanceOther chemical processesWater contaminants1-hexyl-3-methylimidazoliumActivated carbon
Provided is a novel activated carbon adsorbing material loaded with an imidazole ionic liquid. The novel activated carbon adsorbing material is prepared by the steps: weighing 1-methylimidazole and bromohexane into a container, carrying out a reaction in a constant-temperature oil bath pot, and thus obtaining a purified 1-hexyl-3-methylimidazolium bromide ionic liquid; adding the purified 1-hexyl-3-methylimidazolium bromide ionic liquid to powdered activated carbon in a flask, mixing evenly by magnetic stirring at constant temperature, drying to constant weight, and thus obtaining the novel activated carbon adsorbing material loaded with the imidazole ionic liquid. The novel activated carbon adsorbing material has the advantages that the novel composite material is good in stability, insoluble in water and most solvents, and high in heat resistance; a stable structure formed between particles is observed under a microscope, and the novel composite material has good adsorption effect oncopper ions and other metal ions, and can be used as a novel activated carbon adsorption material.
Owner:LIAONING PETROCCHEM VOCATIONAL & TECH COLLEGE
[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof
The invention discloses a [ethyl (R)-(+)-beta-(1-methylimidazole)-propionate]X chiral ionic liquid and a synthesis method which is used for solving the technical problem that imidazole type chiral ionic liquid prepared by the existing preparation method has high liquid melting point and low yield. The technical scheme is as follows: (S)-(-)-2-chloropropionic acid and (S)-(-)-2-bromopropionic acid are taken as raw materials and undergo esterification reaction with absolute ethyl alcohol to generate stable (S)-(-)-2-chloropropionic acid ethyl ester or (S)-(-)-2-bromopropionic acid ethyl ester; the (S)-(-)-2-chloropropionic acid ethyl ester or (S)-(-)-2-bromopropionic acid ethyl ester undergoes alkylation reaction with N- methylimidazole to generate chlorinated ethyl (R)-(+)-beta-(1-methylimidazole)-propionate or brominated ethyl (R)-(+)-beta-(1- methylimidazole)-propionate chiral ionic liquid; the chlorinated ethyl (R)-(+)-beta-(1- methylimidazole)- ropionate or brominated ethyl (R)-(+)-beta-(1- methylimidazole)-propionat chiral ionic liquid is used as an intermediate to undergo the anion exchange reaction with salts containing anions such as BF4<->, PF6<->, SCN<->, CH3COO<-> so as to obtain the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid. The melting point of the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid is reduced to minus 40 to minus 36 DEG C from 5 to 16 DEG C of the melting point of the background art; the yield of the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid is increased to 52.8 to 60.5% from 20 to 35% of yield of the background art.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
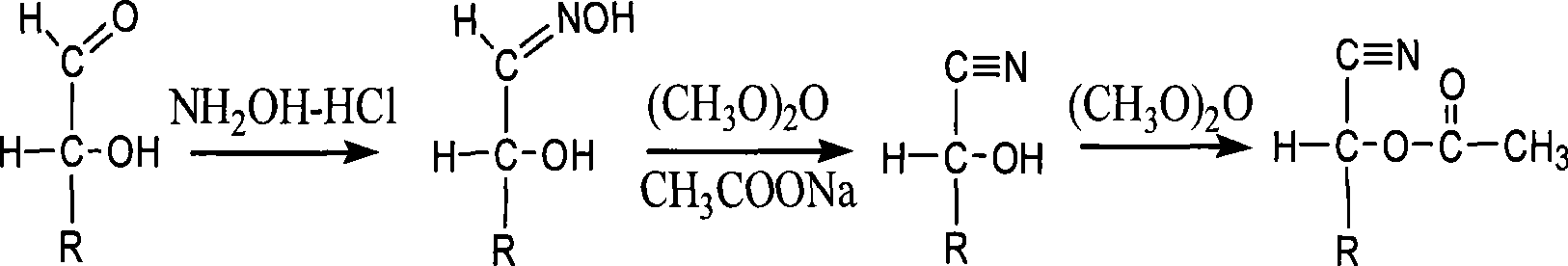
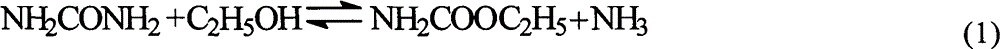
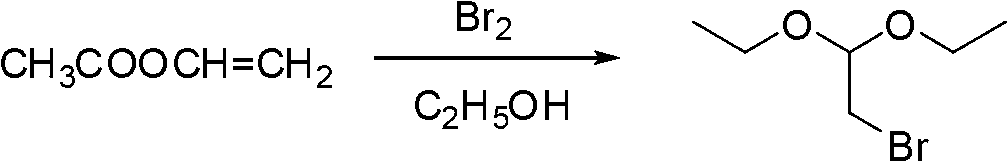
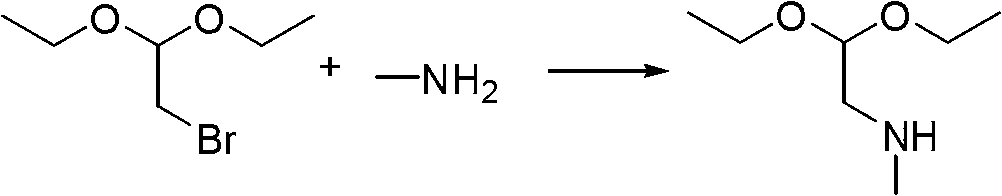
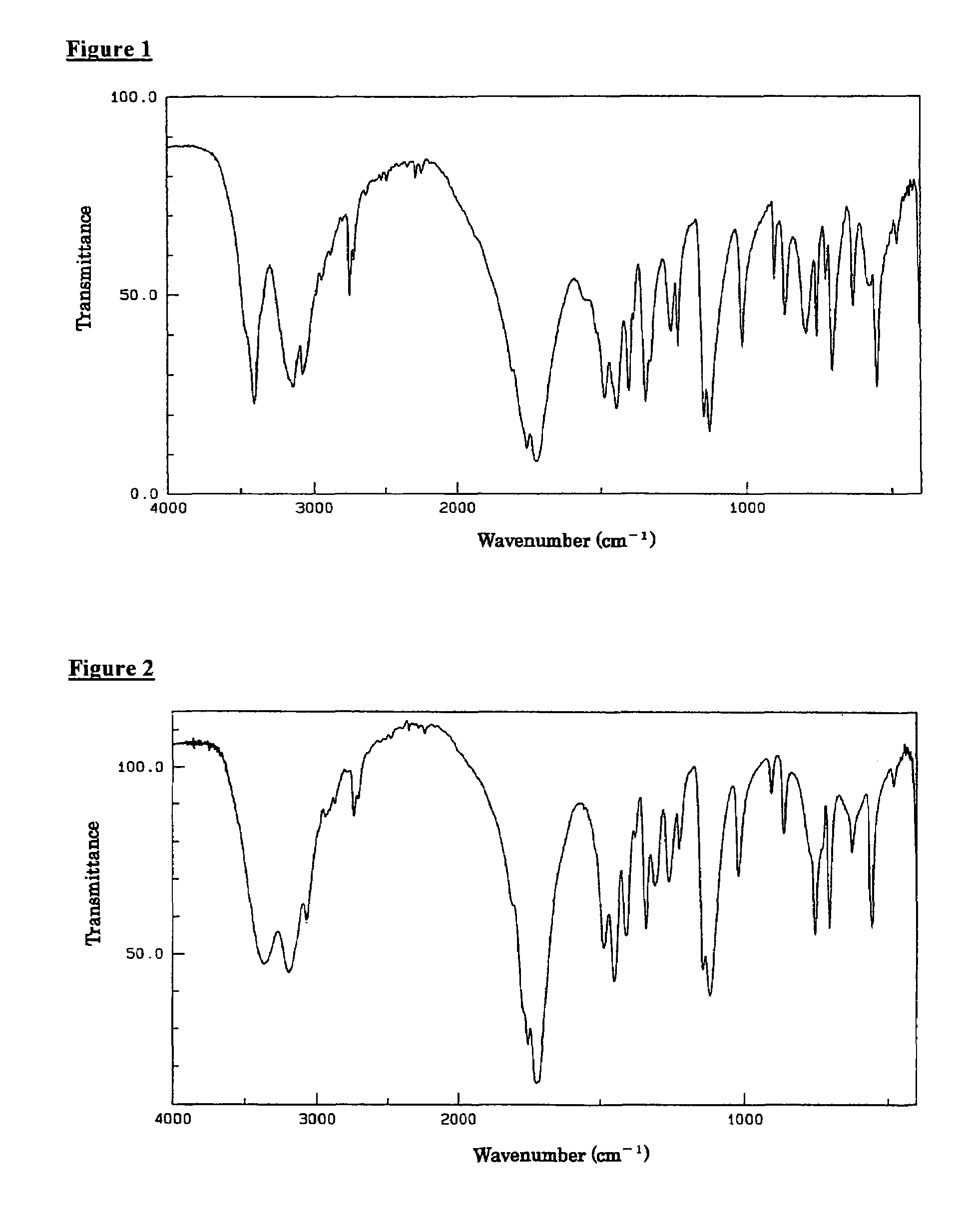
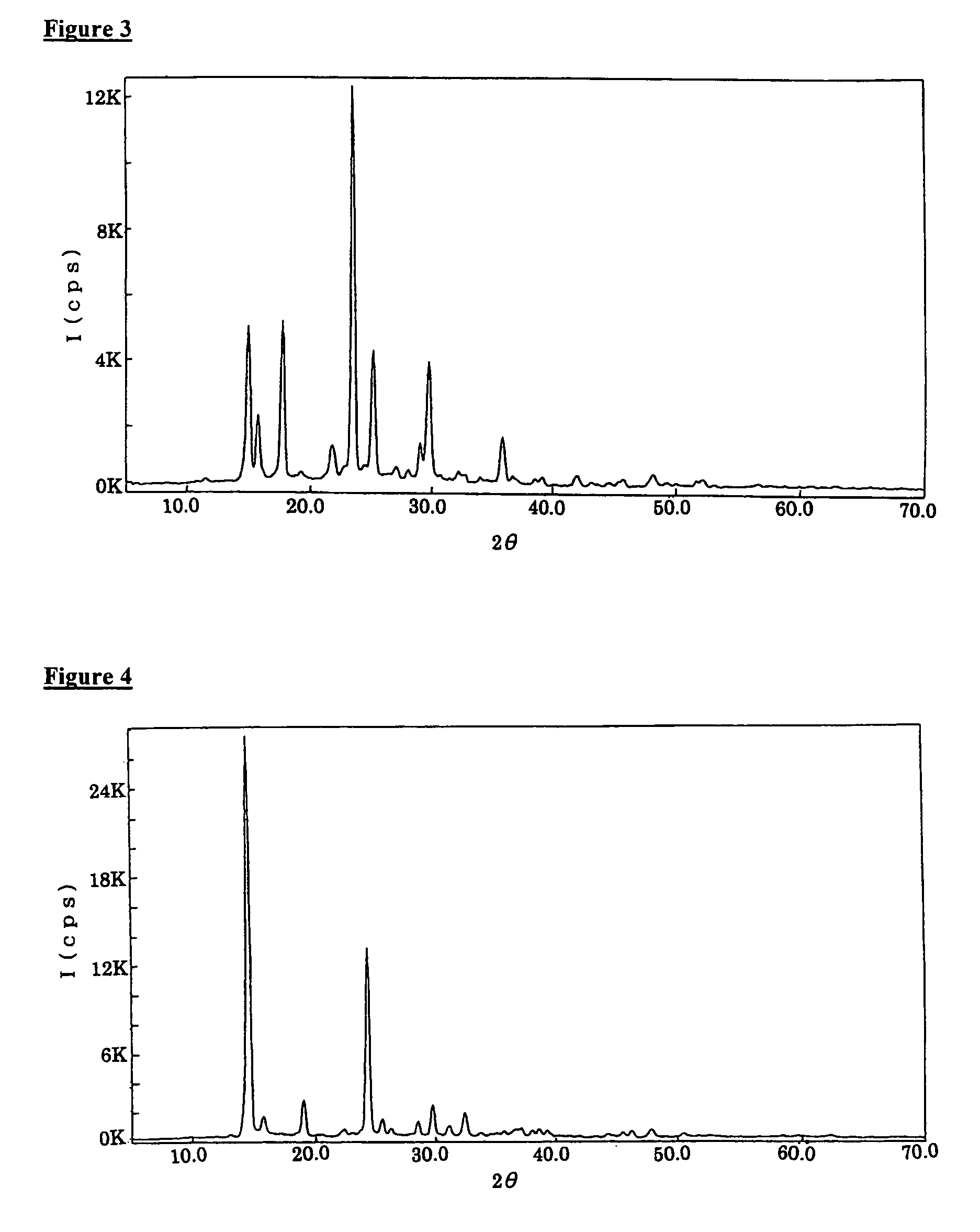
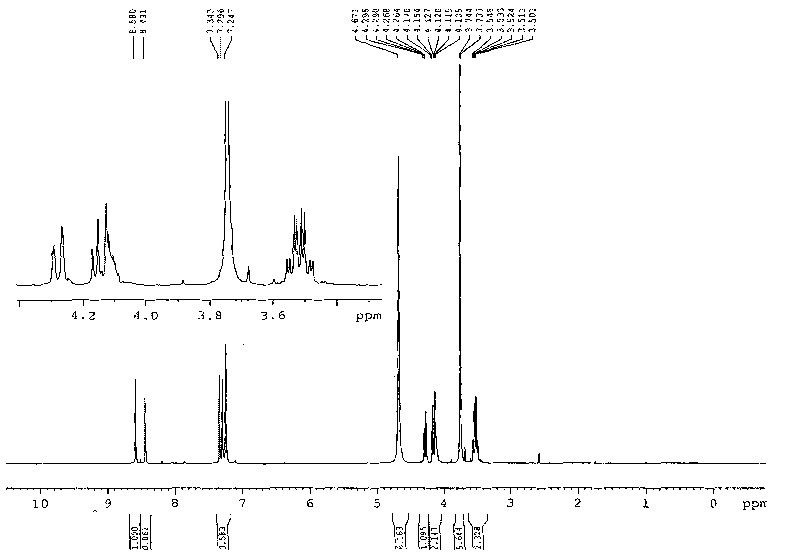
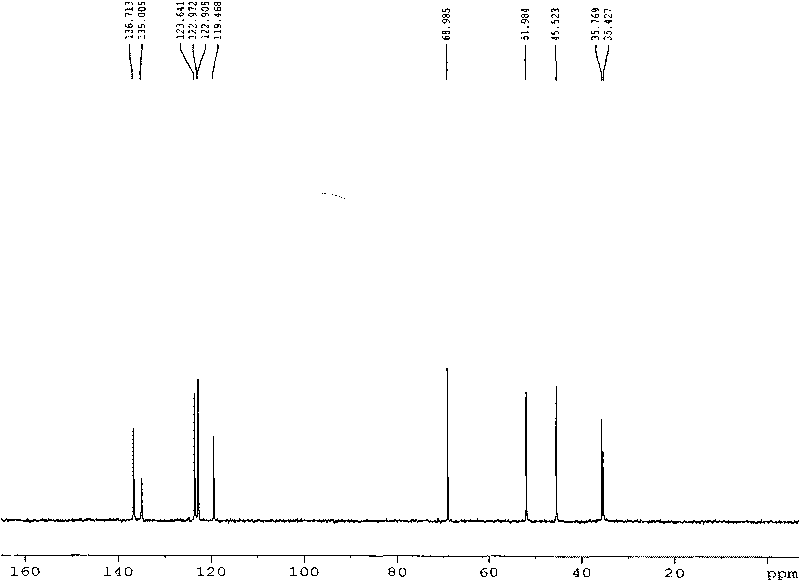
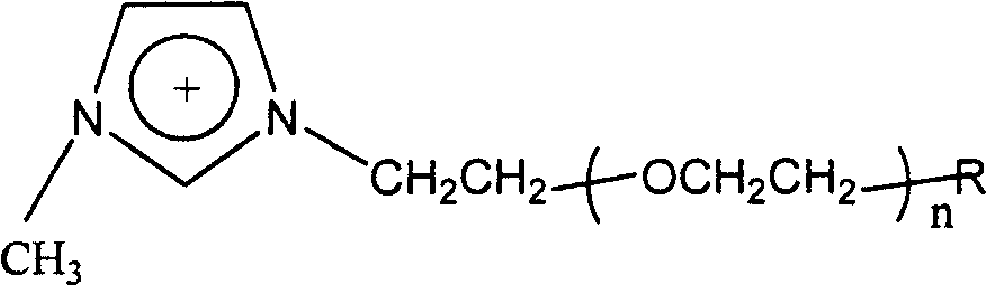
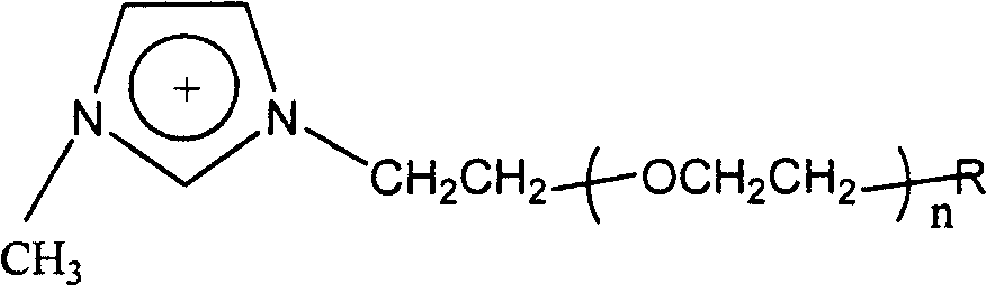



![[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof [ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/24a02186-384f-4593-ba3d-529c35f11ce0/FSA00000436257600011.png)
![[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof [ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/24a02186-384f-4593-ba3d-529c35f11ce0/BSA00000436257700022.png)