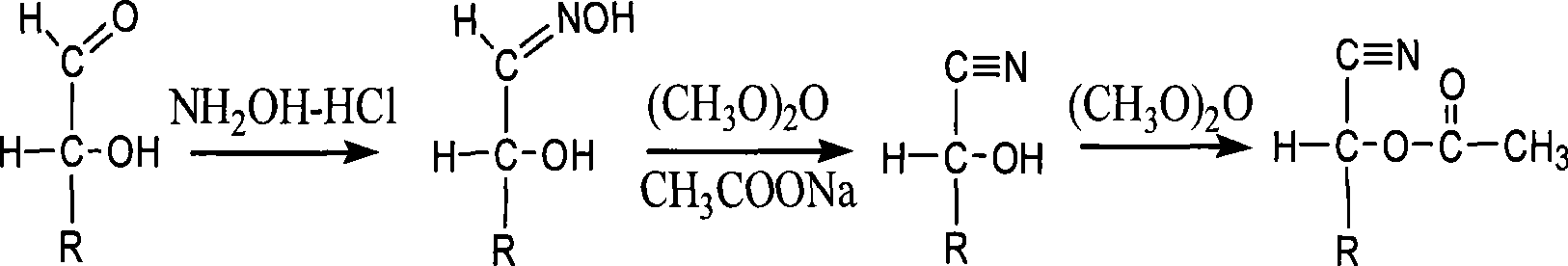Derivatization method of qualitative or quantitative analysis for polyhydroxy compound
A polyol derivatization technology, applied in the field of derivatization of polyols, can solve problems such as long time, research on trace materials or damage to endangered organisms, increased internal pressure of the container, etc., and achieve the effect of structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1. Determination of the hydroxyl derivatization method used for qualitative or quantitative analysis of carbohydrates in the present invention
[0110] 1. Screening of monosaccharide derivatization methods
[0111] In view of various problems existing in the qualitative or quantitative analysis of sugars (such as ketose, aldose, alcohol sugar, etc.) described in the background art, the inventors of the present invention determined the acetylation derivatization method to solve the existing problems. Carry out derivatization experiments with the following 24 monosaccharides and their derivatives, analyze their effects, and screen derivatization technical solutions. The specific method is as follows:
[0112] 1. Experimental instruments and materials
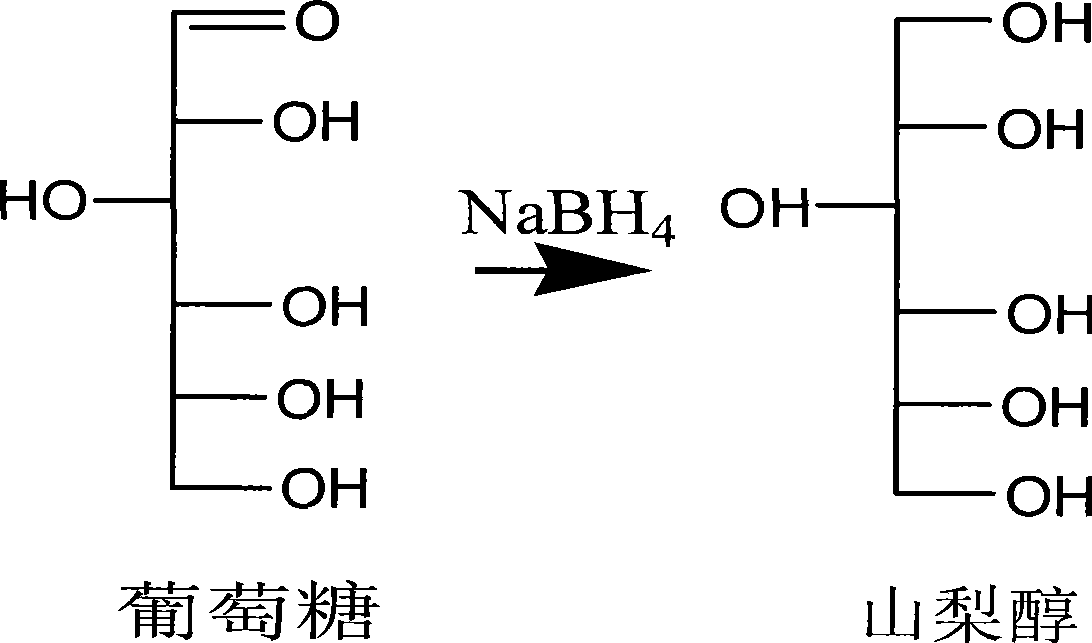
[0113]Vacuum concentrator, vacuum freeze dryer, Finnigan gas chromatography mass spectrometer, micro syringe, etc. The 24 monosaccharide standard samples were all purchased from Sigma (USA), namely: deoxyribose (...
Embodiment 2
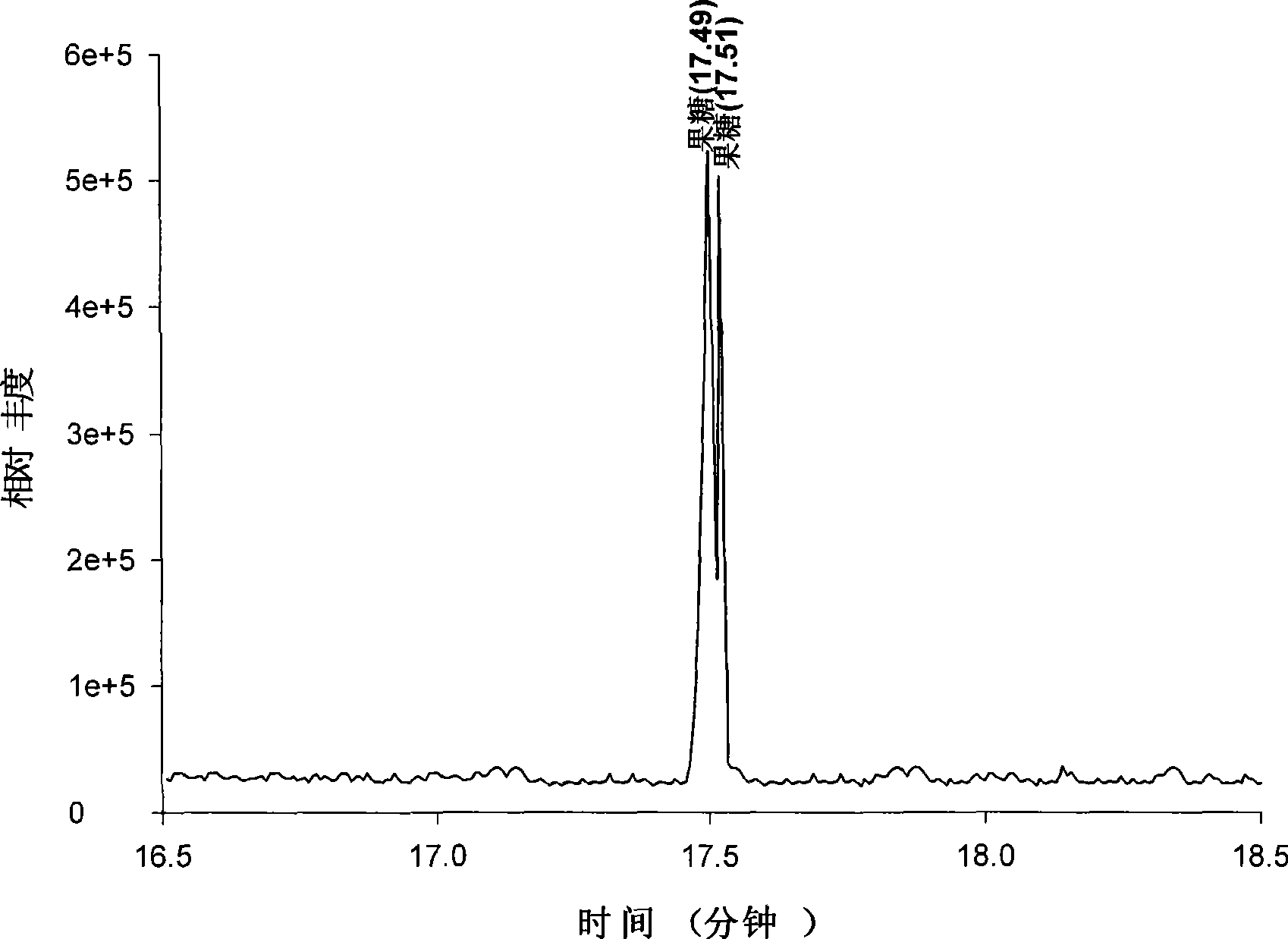
[0153] Example 2. The method of the present invention derivatizes and quantitatively detects soluble sugars in fresh Populus tomentosa leaves
[0154] Utilize the derivatization method of embodiment 1 (DMSO, acetic anhydride and 1-methylimidazole are mixed with 25:3:1) to the detection of soluble monosaccharides and derivatives thereof in the fresh leaves of Populus tomentosa, the specific methods are as follows:
[0155] 1. Experimental instruments and materials
[0156] The 24 sugar standard samples were purchased from Sigma (USA), namely: deoxyribose (purity>99.0%), rhamnose (purity>98.0%), fucose (purity>99.0%), xylose (purity >99.0%), arabinose (purity >99.0%), galactose (purity >99.0%), glucose (purity >99.0%), erythritol (purity >99.0%), deoxyribitol (purity >99.0%) ), rhamnitol (purity>99.0%), fucose (purity>99.0%), xylitol (purity>99.0%), arabitol (purity>99.0%), galactitol (purity> 99.0%), Glucitol (purity>99.0%), Mannitol (purity>99.0%), Ribitol (purity>99.0%), In...
Embodiment 3
[0174] Embodiment 3, fresh poplar tomentosa leaf cell wall sugar component analysis
[0175] Utilize the derivatization method of embodiment 1 screening (DMSO, acetic anhydride and 1-methylimidazole are mixed with 25:3:1) fresh Populus tomentosa leaf cell wall sugar component analysis, concrete method is as follows:
[0176] 1. Experimental materials
[0177] The reagents used are dimethyl sulfoxide (DMSO), acetic anhydride, 1-methylimidazole, ethyl acetate and the like. Monosaccharide and sugar alcohol standard samples are erythritol, deoxyribose, fucose, rhamnose, xylose, arabinose, galactose, glucose, fructose, sorbose, ribitol, xylitol, inositol, Mannitol, Sorbitol, Glucuronic Acid, Galacturonic Acid, N-Acetyl Glucosamine, Trehalose, Sucrose. The above reagents and standard monosaccharides were all analytically pure. Dimethyl sulfoxide was dehydrated with potassium hydroxide before use.
[0178] Plant materials were taken from the fresh leaves of Populus tomentosa grow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com