Extraction and separation method of alkane/olefin
A separation method, olefin technology, applied in extraction purification/separation, organic chemistry, etc., to achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The mixture to be separated is a mixture of 1-hexene / n-hexane in different proportions, 1-hexene and n-hexane can be in any proportion, and the extractant is N-formylmorpholine (NFM);
[0022] First, according to different ratios (0.25, 0.5, 1.0), add a certain volume of extractant NFM to the mixture of 1-hexene and n-hexane, put the mixture containing the three substances into a volumetric flask, and then adjust the capacity The bottle was shaken in a constant temperature water bath shaker at 25°C for 2h, and then stood still for 1h.
[0023] Change the temperature of the oscillator and repeat the above experiment.
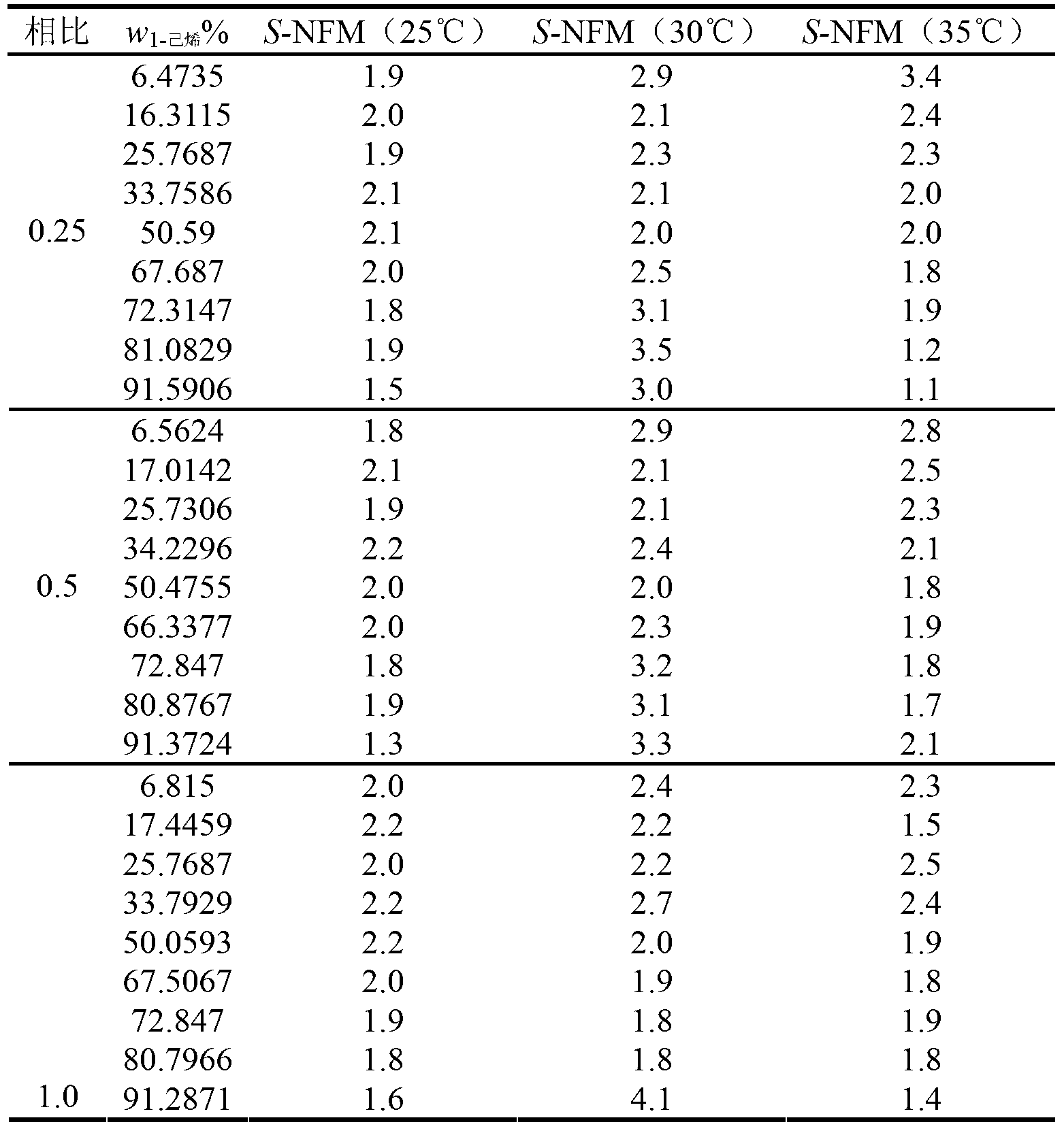
[0024] See Table 1 for the extraction results represented by the extraction selectivity S of the extractant NFM for 1-hexene at different temperatures (25°C, 30°C, 35°C).
[0025] Extraction selectivity of extractant NFM to 1-hexene under table 1 different temperatures
[0026]
[0027] w 1-己烯 % is the molar percentage of 1-hexene in the mixture to b...
Embodiment 2
[0030] The feed liquid is a mixed liquid of 1-hexene / n-hexane in different proportions, and the ratio relationship is that the molar content of 1-hexene in the mixed liquid is between 0-1;
[0031] The extractant is γ-butyrolactone (γ-BL);
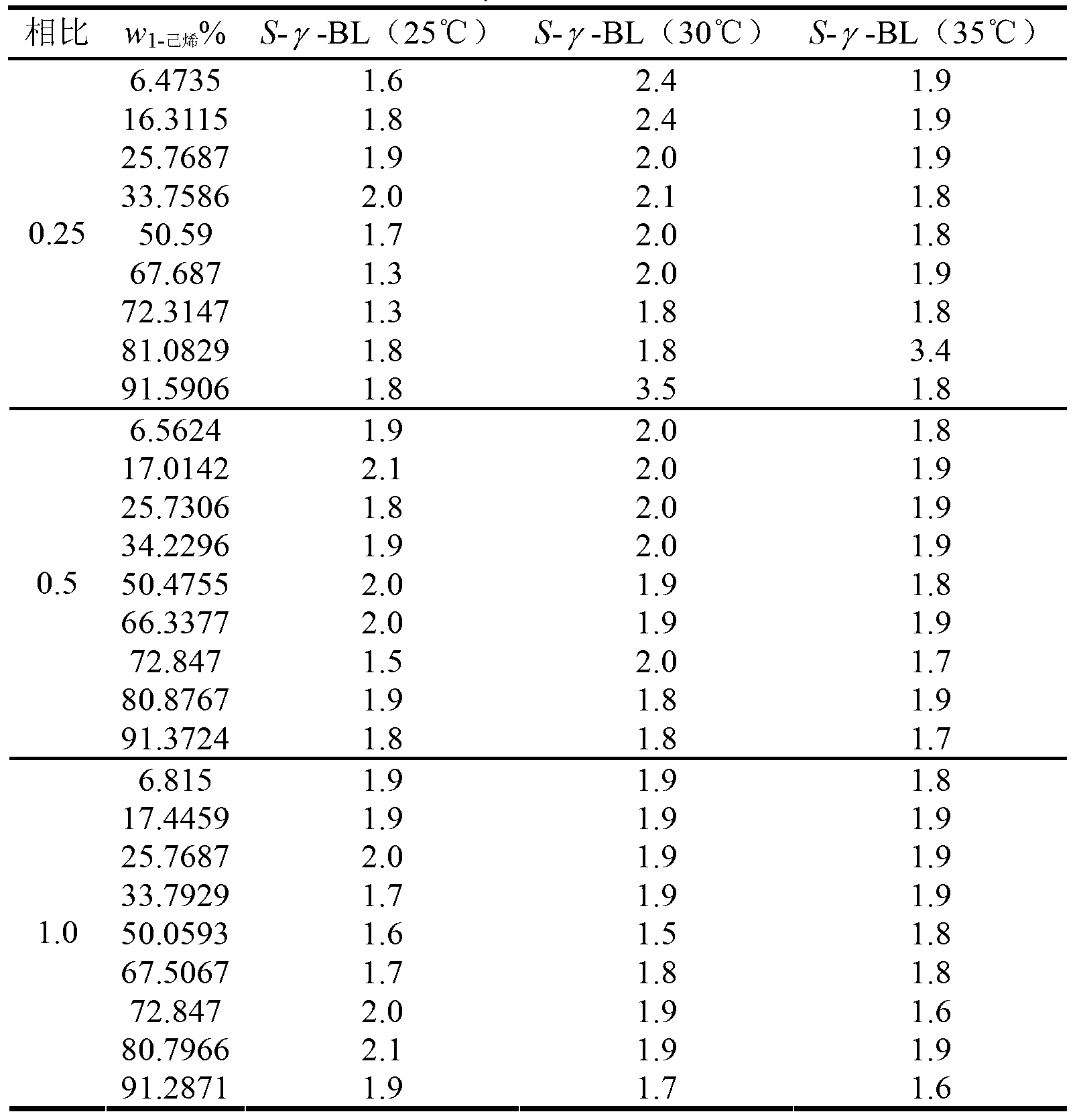
[0032] First, according to different ratios (0.5, 1.0, 2.0), add a certain volume of extractant γ-BL to the mixture of 1-hexene and n-hexane, put the mixture containing the three substances into a small volumetric flask, Then place the volumetric flask in a constant temperature water bath shaker for 2h, and then let it stand for 1h. Change the temperature of the oscillator and repeat the above experiment. The extraction results represented by the extraction selectivity S of the extractant γ-BL to 1-hexene at different temperatures (25°C, 30°C, 35°C) are shown in Table 2.
[0033] Table 2 Extraction selectivity of 1-hexene by extractant γ-BL at different temperatures
[0034]
[0035] It can be seen from Table 2 that at 25°C, 30°C, an...
Embodiment 3
[0037] (1) Carry out fractionation with raw material light oil distillate oil, cut to obtain C 6 + distillate;
[0038] (2) The C obtained in the previous step 6 + The fraction was further fractionated to give C 6 distillate;
[0039] (3) The C obtained in the previous step 6 Carry out extractive distillation, and use a polar solvent as the extractant 1 to remove the organic oxygen-containing compounds contained therein; the extractant 1 used can be N-methylpyrrolidone (NMP), acetonitrile (ACN) or dimethyl Any one of formamide (DMF);
[0040] (4) The C obtained in the previous step 6 The fraction was subjected to liquid-liquid extraction, and the C 6 Alkanes and C 6 Separation of olefins; the extractant used is NMP, N-formylmorpholine (NFM), 1-methylimidazole (1-MI) or γ-butyrolactone (γ-BL); the extractant and the mixture to be separated The total volume ratio (compared to for short) = 0.25, 0.5, 1.0 or 2.0.
[0041] C obtained after two cuts 6 The distillate sect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com