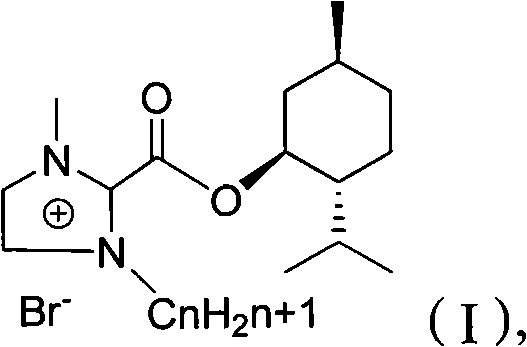
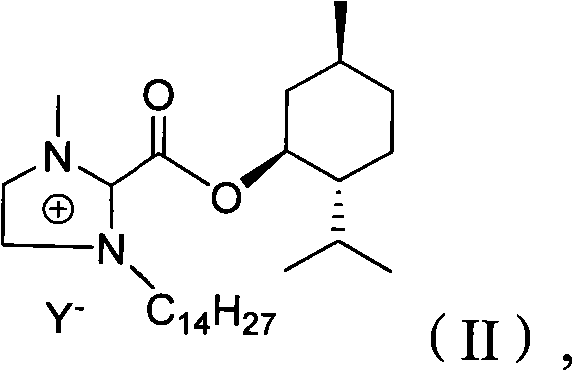
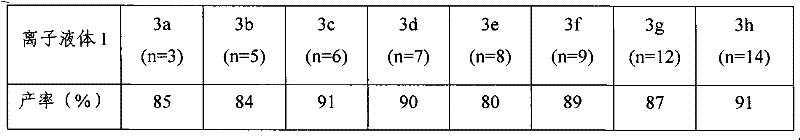
Preparation method of imidazole chiral ionic liquid replaced by C-2
A chiral ionic liquid, imidazole technology, applied in the direction of organic chemistry, etc., can solve the problem of single synthesis method, and achieve the effects of simple preparation method, easy availability of raw materials, and broad development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of trichloroacetyl chloride: add 32.7g (0.2mol) of trichloroacetic acid and 32.1g (0.26mol) of thionyl chloride into a two-necked bottle equipped with a thermometer and a spherical condenser (connected to the drying tube), drop into dimethyl DMF 0.286g, the condenser tube was connected to the calcium chloride drying tube, reacted for 3h, and collected 31g of fractions at 119°C to 120°C by atmospheric distillation, with a yield of 90%.
Embodiment 2
[0025] Synthesis of 2-trichloroacetyl-1-methylimidazole: under nitrogen protection, add 7mL (11.2g, 61mmol) trichloroacetyl chloride and 40mLCH to a 100mL three-necked flask 2 Cl 2 . Charge 5 mL (5.00 g, 63 mmol) of 1-methylimidazole (3) and 30 mL of CH into a constant pressure funnel 2 Cl 2 , Slowly drop into the three-necked flask, about 2h after the dropwise addition, continue to stir at room temperature for 8h. The reaction mixture was cooled to 0° C. with an ice-water bath, 8.8 mL of triethylamine was added dropwise, and stirred for another 1 h. After filtration, the solvent was removed from the filtrate under reduced pressure, and recrystallized (n-hexane was used as solvent) to obtain 9.43 g of white crystals, with a yield of 68%. mp76℃~77℃. MS (EI) m / z 226 (M + , C 6 h 5 N 2 OCl 3 The theoretical value of 226).
Embodiment 3
[0027] Synthesis of menthyl 1-methylimidazole-2-carboxylate (2): Add 7.13g (0.03mol) 2-trichloroacetyl-1-methylimidazole and 4.68g (0.03mol) menthol to a 15mL two-neck bottle . Magnetic stirring was performed at 80° C. for 5 h, the generated chloroform was removed under reduced pressure, and recrystallized (n-hexane or ethyl acetate) to obtain 4 g of a white solid with a yield of 57%. MS (EI) m / z 264 (M + , C 15 h 24 N 2 The theoretical value of O is 264). IR (KBr) 3105, 2951, 2933, 2871, 2846, 1705, 1477, 1419, 1414, 1130, 1259, 982, 957, 924, 785, 667. 1 H NMR (DCCl 3 , 500MH Z )7.26(s, 1H), 4.95(m, 3H, J=26.5), 4.00(s, 3H), 2.00(m, 2H, J=43.5), 0.77(m, 3H, J=7). 13 C NMR (CDCl 3 , 500H Z )δ159.0, 136.9, 129.3, 126.0, 77.0, 46.6, 40.8, 35.9, 34.15, 31.5, 26.0, 23.2, 22.0, 20.7, 16.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com