Method for preparing chitosan immobilized Lewis acidic ionic liquid and DCPP synthesized by using chitosan immobilized Lewis acidic ionic liquid in presence of high-efficient catalyst
A technology of Lewis acidic and ionic liquids, which is applied in the field of preparation of chitosan-immobilized Lewis acidic ionic liquids, can solve problems that have not been reported in the literature, and achieve the effects of low price, easy scale-up production, and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: PmimCl-FeCl 3 -CS Catalytic Synthesis of DCPP
[0015] 1. Preparation of Lewis acid ionic liquid
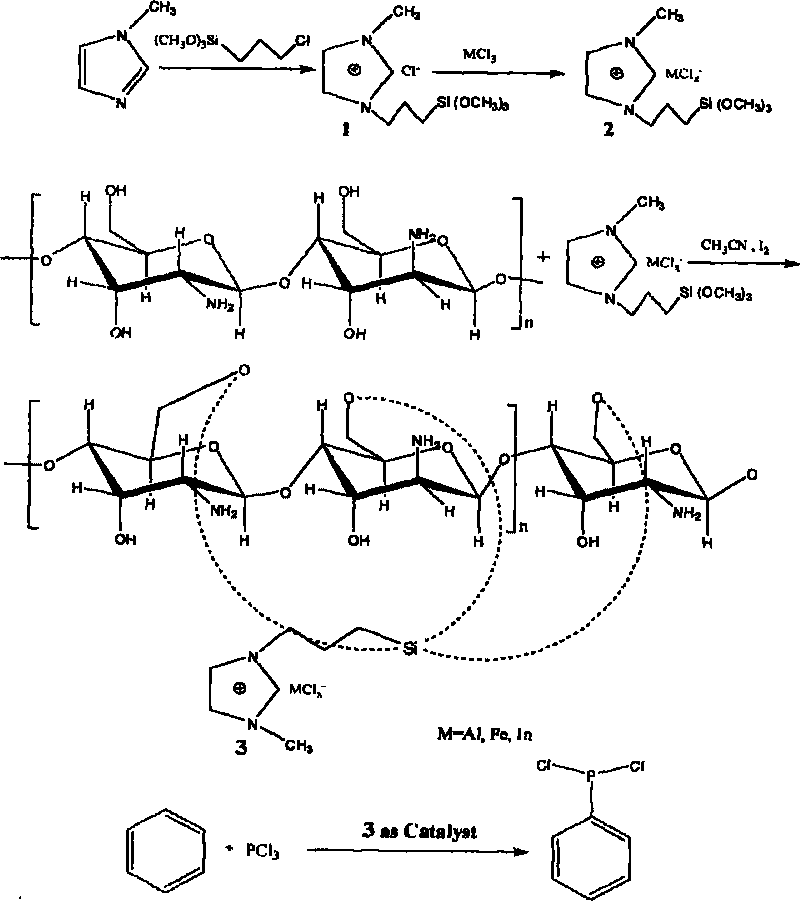
[0016] 1-Methylimidazole and Y-chloropropyltrimethoxysilane were refluxed and reacted at 70°C for 70h, and then reacted at 70°C according to the molar ratio of Lewis acid anhydrous ferric chloride to the prepared product of 2:1 24h.
[0017] 2. Preparation of chitosan immobilized Lewis acid ionic liquid
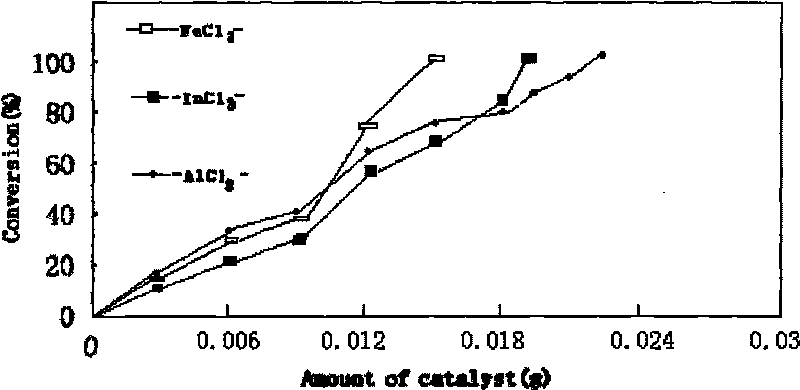
[0018] Such as figure 1 As shown, the obtained ionic liquid containing chloroferric acid and 2 times the mass of chitosan were dispersed in acetonitrile and refluxed for 20 hours, and the solvent was removed by rotary evaporation. The obtained solid was Soxhlet extracted with dichloromethane for 30 hours to obtain a solid chitosan. Supported acidic ionic liquid catalyst PmimCl-FeCl 3 -CS.
[0019] 3. The prepared catalyst can efficiently synthesize DCPP
[0020] Add appropriate amount of catalyst PmimCl-FeCl in a round bottom flask equipped with reflux device 3 -CS, ph...
Embodiment 2
[0021] Example 2: PmimCl-AlCl 3 -CS Catalytic Synthesis of DCPP
[0022] 1. Preparation of Lewis acid ionic liquid
[0023] 1-Methylimidazole and Y-chloropropyltrimethoxysilane were refluxed and reacted at 70°C for 70h, and then reacted at 70°C according to the molar ratio of Lewis acid anhydrous aluminum chloride to the prepared product of 2:1 24h.
[0024] 2. Preparation of chitosan immobilized Lewis acid ionic liquid
[0025] Such as figure 1 As shown, the obtained chloroaluminate-containing ionic liquid and 2 times the mass of chitosan were dispersed in acetonitrile and refluxed for 20 hours, and the solvent was removed by rotary evaporation. The obtained solid was Soxhlet extracted with dichloromethane for 30 hours to obtain a chitosan solid Supported acidic ionic liquid catalyst PmimCl-AlCl 3 -CS.
[0026] 3. The prepared catalyst can efficiently synthesize DCPP
[0027] Add an appropriate amount of catalyst PmimCl-AlCl in a round bottom flask equipped with a reflux device. 3 -C...
Embodiment 3
[0028] Example 3: PmimCl-InCl 3 -CS Catalytic Synthesis of DCPP
[0029] 1. Preparation of Lewis acid ionic liquid
[0030] 1-Methylimidazole and Y-chloropropyltrimethoxysilane were refluxed and reacted at 70°C for 70h, and then reacted at 70°C for 24h according to the molar ratio of Lewis acid indium chloride to the prepared product of 2:1.
[0031] 2. Preparation of chitosan immobilized Lewis acid ionic liquid
[0032] Such as figure 1 As shown, the obtained ionic liquid containing chloroindium acid and 2 times the mass of chitosan were dispersed in acetonitrile and refluxed for 20 hours, and the solvent was removed by rotary evaporation. The obtained solid was Soxhlet extracted with dichloromethane for 30 hours to obtain a solid chitosan. Supported acidic ionic liquid catalyst PmimCl-InCl 3 -CS.
[0033] 3. The prepared catalyst can efficiently synthesize DCPP
[0034] Add appropriate amount of catalyst PmimCl-InCl in a round bottom flask equipped with reflux device 3 -CS, phosphoru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com