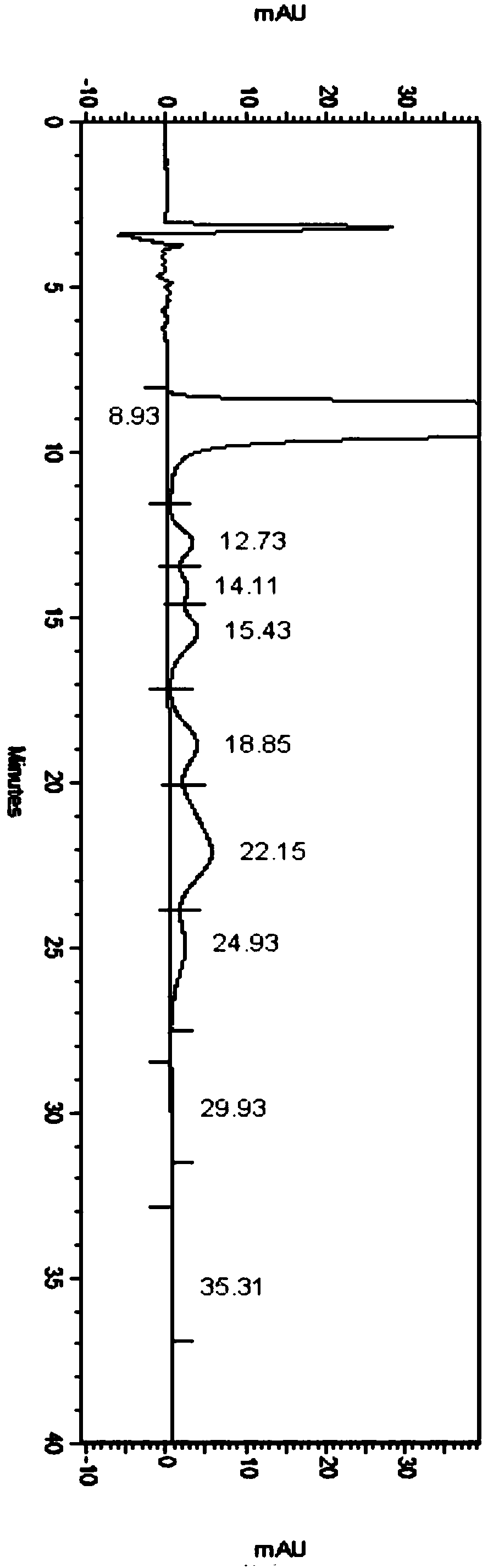
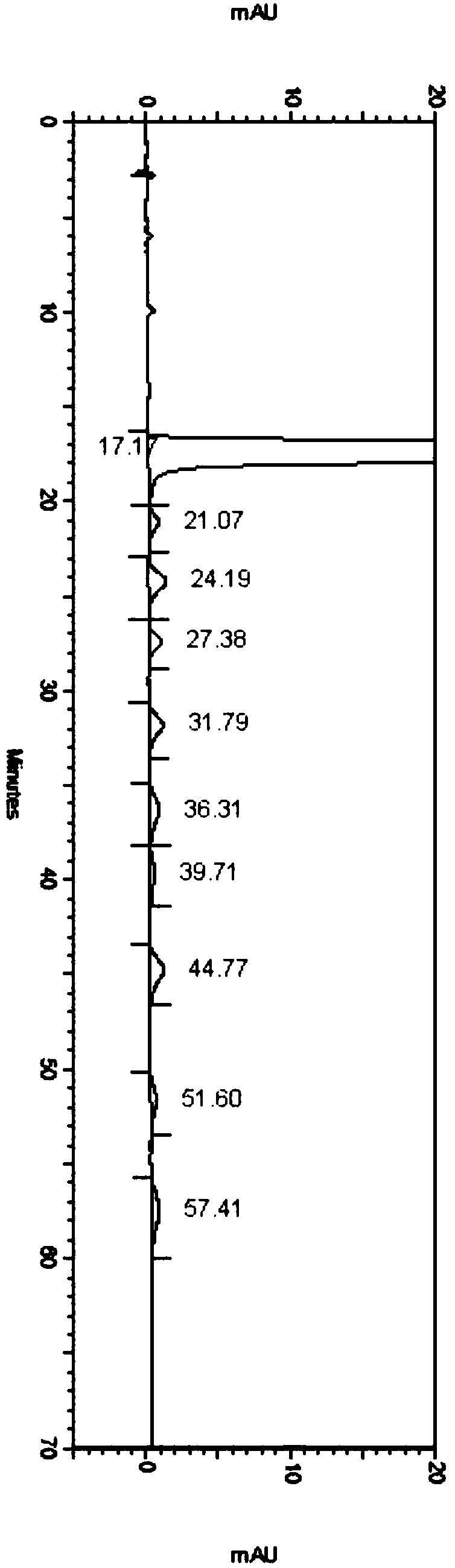
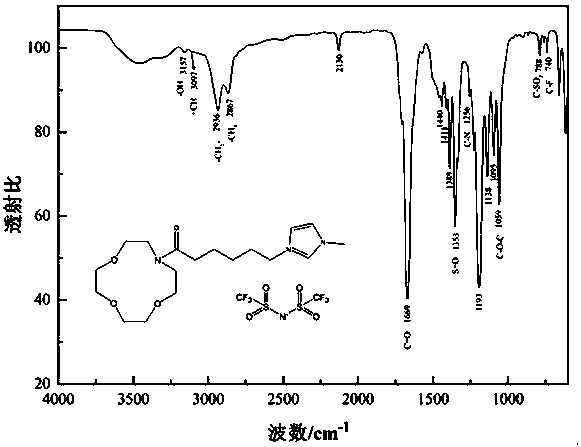
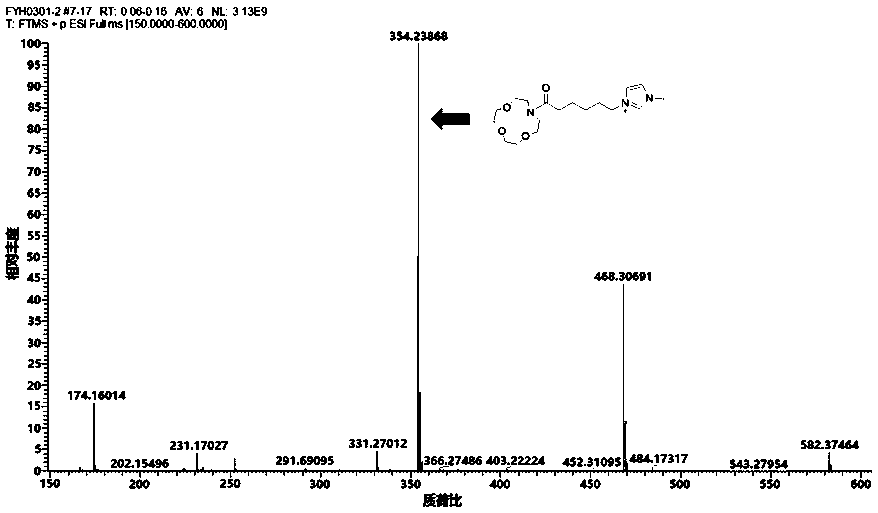
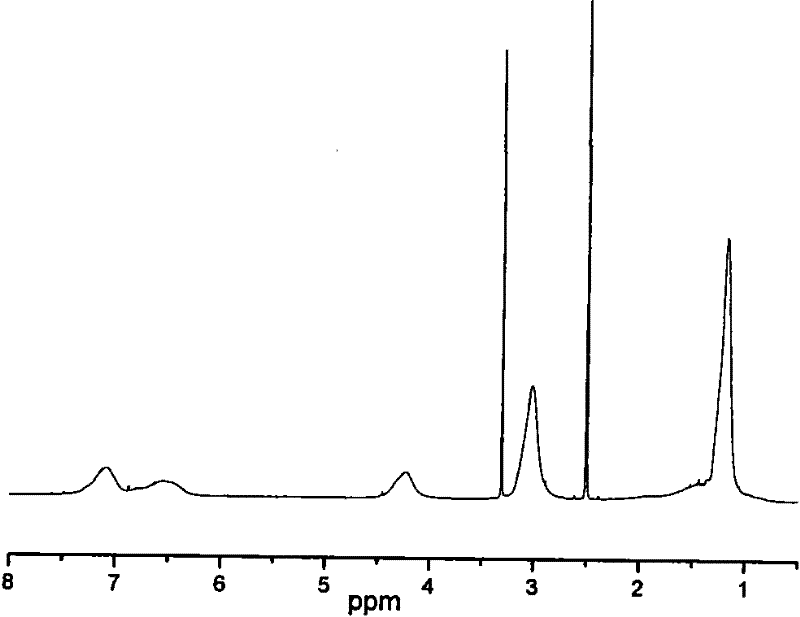
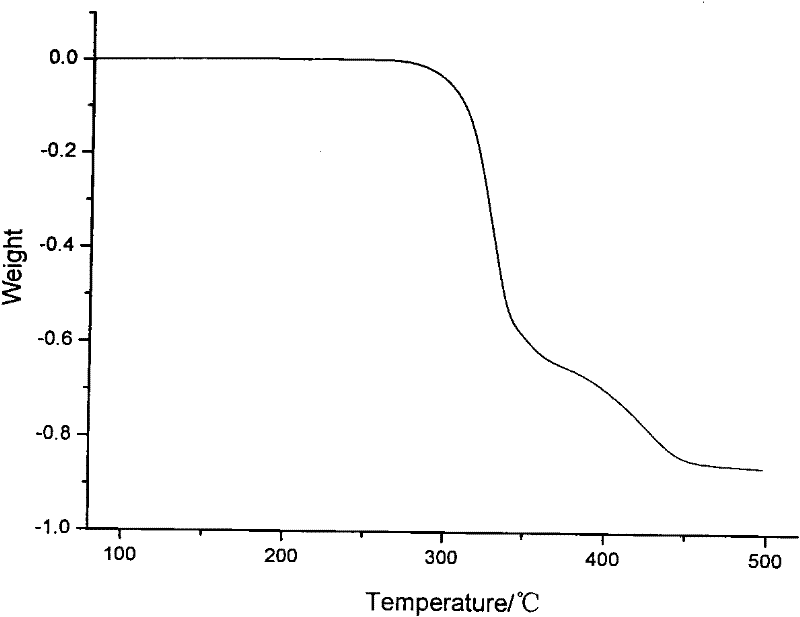
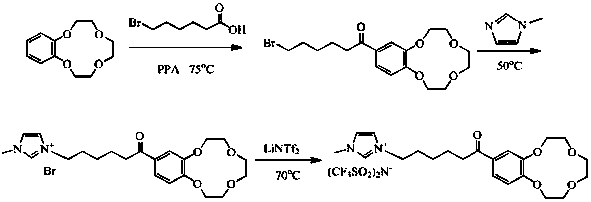
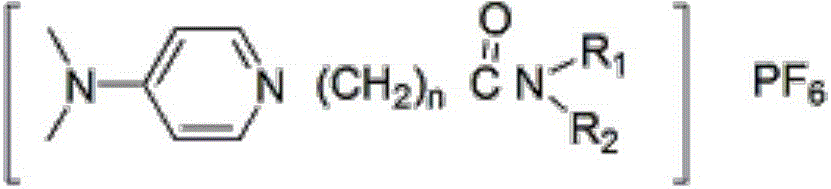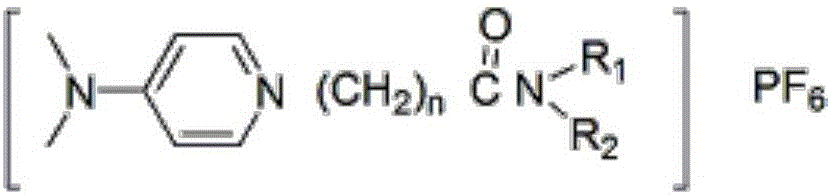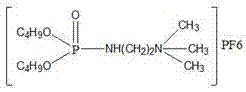Patents
Literature
66 results about "Potassium hexafluorophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium hexafluorophosphate is the chemical compound with the formula KPF₆. This colourless salt consists of potassium cations and hexafluorophosphate anions. This exothermic reaction is conducted in liquid hydrogen fluoride. The salt is stable in hot alkaline aqueous solution, from which it can be recrystallized. The sodium and ammonium salts are more soluble in water whereas the rubidium and caesium salts are less so.
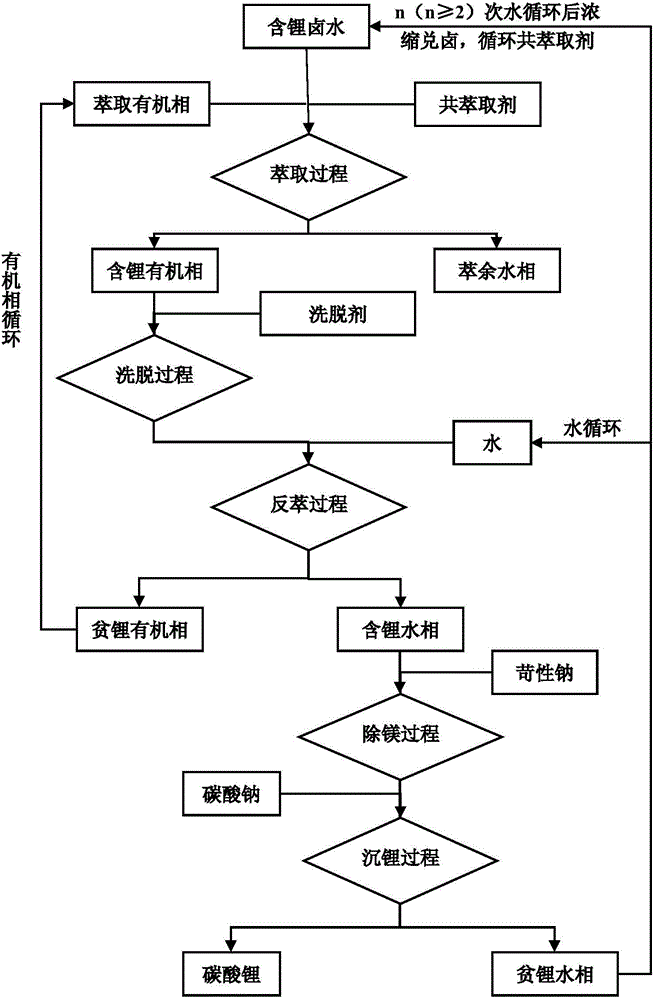
Novel co-extraction system for extraction of lithium from high magnesium-lithium ratio bittern and co-extraction method using the same
ActiveCN104404268AHigh extraction rateHigh stripping rateProcess efficiency improvementHigh magnesiumPotassium hexafluorophosphate
The invention discloses a novel co-extraction system for extraction of lithium from high magnesium-lithium ratio bittern. The novel co-extraction system comprises an extraction agent and a co-extraction agent. The co-extraction agent is a mixture of one or more of potassium hexafluorophosphate, sodium hexafluorophosphate, magnesium hexafluorophosphate, calcium hexafluorophosphate, potassium fluoborate, sodium fluoborate, magnesium fluoborate and calcium fluoborate. The novel co-extraction method for extraction of lithium from high magnesium-lithium ratio bittern comprises extraction, back extraction, deposition for magnesium removal and deposition for lithium acquisition, wherein aiming at bittern with a magnesium-lithium ratio greater than 100, an elution process is used between extraction and back extraction. The novel co-extraction system has simple processes and a high lithium extraction rate, effectively reduces a magnesium-lithium ratio of bittern, has obvious advantages than the traditional ferric trichloride extraction system, can be used for extraction of lithium from salt lake bittern and is especially suitable for solving the problem of high difficulty of extraction of lithium from salt lake bittern with a high magnesium-lithium ratio.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Spectrum-adjustable gathering, inducing and light-emitting type fluorescent materials as well as preparation method and application thereof
ActiveCN107501170AGood aggregation-induced luminescenceImprove solubilityStyryl dyesMethine/polymethine dyesPotassium hexafluorophosphateFluorescent light
The invention discloses spectrum-adjustable gathering, inducing and light-emitting type fluorescent materials as well as a preparation method and an application thereof. The fluorescent materials have the structure formula shown in the description, wherein Ar denotes phenyl, C1-C4 halogenated alkyl substituted phenyl, C1-C4 alkyl substituted phenyl, C1-C4 alkoxy substituted phenyl, thienyl, C1-C4 alkyl substituted thienyl, furyl, C1-C4 alkyl substituted furyl or naphthyl, and the fluorescent materials with the gathering, inducing and light-emitting functions are obtained as follows: aldehyde derivatives and 4-pyridylacetonitrile hydrochloride as raw materials are subjected to a simple condensation reaction, an intermediate is obtained and reacts with methyl iodide to produce an ionic compound, and finally, the ionic compound is subjected to replacement through potassium hexafluorophosphate. The fluorescent materials have the gathering, inducing and light-emitting characteristics, are cation compounds, can specifically label mitochondria in live cells and are excellent mitochondrion imaging dye.
Owner:SHAANXI NORMAL UNIV
Hyperbranched ionic liquid based on HCCP and application of hyperbranched ionic liquid as fire retardant
ActiveCN104558046ASimple processLow equipment requirementsGroup 5/15 element organic compoundsPotassium hexafluorophosphateLithium
The invention provides a method for synthesizing a hyperbranched ionic liquid fire retardant based on HCCP. According to the method, HCCP reacts with trialkylamin, trialkyl phosphorus, and N-alkyl imidazole to realize ionization, salts such as sodium tetrafluoroborate, potassium hexafluorophosphate and bistrifluoromethanesulfonimide lithium, which contain different anions, are used for ion exchange, so that a hyperbranched ionic liquid fire retardant containing different anions is obtained, and synergistic flame retardance of HCCP and the ionic liquid is realized. The synthesized hyperbranched ionic liquid based on HCCP can be used as the fire retardant in various polymers.
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of lithium hexafluorophosphate
ActiveCN101712467AWill not polluteLow costPhosphorus compoundsPotassium hexafluorophosphateLithium chloride
The invention discloses a synthesis method of lithium hexafluorophosphate, aiming to provide a lithium hexafluorophosphate synthesis method with low cost, safety and high efficiency. The method comprises the following steps of: firstly, adding dried lithium chloride and potassium hexafluorophosphate to an organic solvent and stirring; secondly, adding a catalyst to the organic solvent; then, heating while stirring at a controlled temperature of 30-80 DEG C for 2-16 h, controlling the temperature to 20-80 DEG C and the heating time of 2-16 h; and finally, filtering insoluble matter to obtain an organic lithium hexafluorophosphate solution. The invention can be applied to the preparation field of the lithium hexafluorophosphate in a lithium ion battery electrolyte.
Owner:ZHUHAI SMOOTHWAY ELECTRONICS MATERIALS
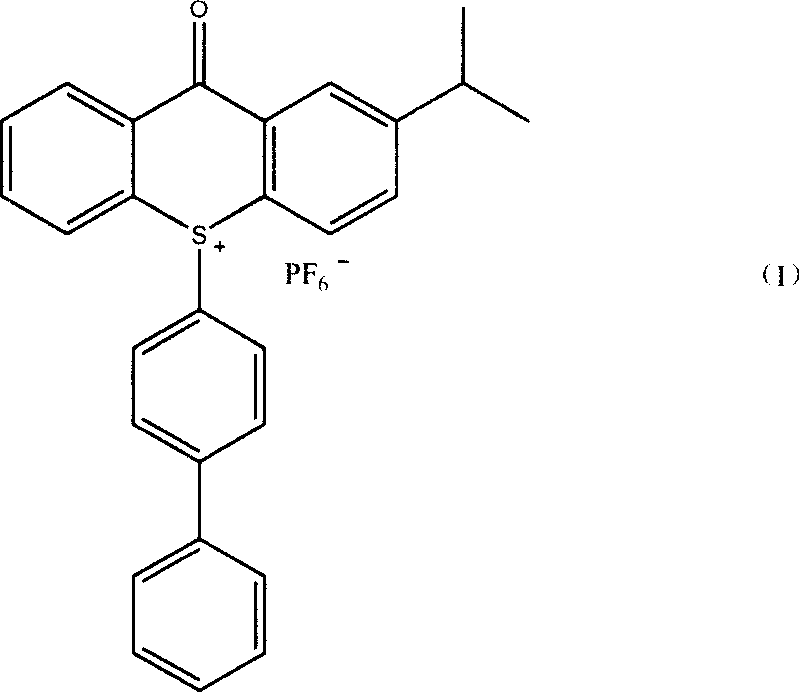
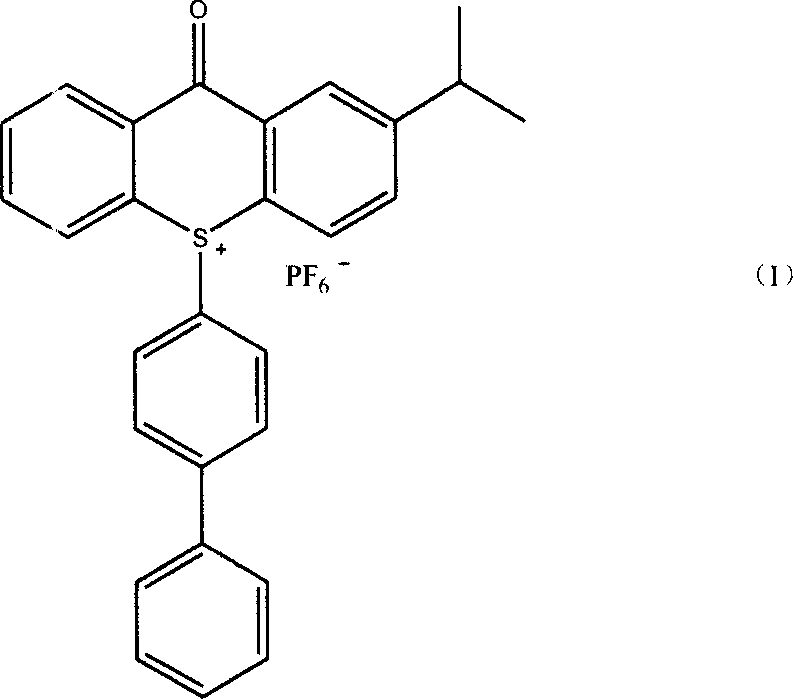
Method of producing 10-(4-xenyl)-2-isopropyl thioxanthone sulfur onium phosphorofluoric acid salt
ActiveCN101153037AImprove solubilityEasy to recycleOrganic chemistryPotassium hexafluorophosphateCerium
The present invention relates to a preparation method of novel cationic photoinitiator10-(4-biphenyl)-2-isopropyl-thiadiazole ketone sulfur hexafluorophosphate. The 2-isopropyl thioxanthone is used as raw material; the mixed oxidant of cerium ammonium nitrate and sodium hypochlorite is used; the hydrohalogenic acid is added for catalysis. After the oxidation reaction, the intermediate sulfoxide can be made; the sulfoxide and biphenyl react to get the intermediate of sulfonium salt through salt reaction. Finally, the mixed solvent of alcohol categories and water is used; and the aqueous solution potassium hexafluorophosphate is dropped into the solution of the intermediate sulfoxide to get the product. The method of the present invention can reduce the dosage of expensive oxidant, and quicken the speed of oxidation reaction. Besides, the ion exchange reaction is complete and the solvent is easy to be recycled and reused; the purity of the target product is high; the optical solidification effects are good; the whole process is easy for realization of industrial production.
Owner:INSIGHT HIGH TECH (BEIJING) CO LTD
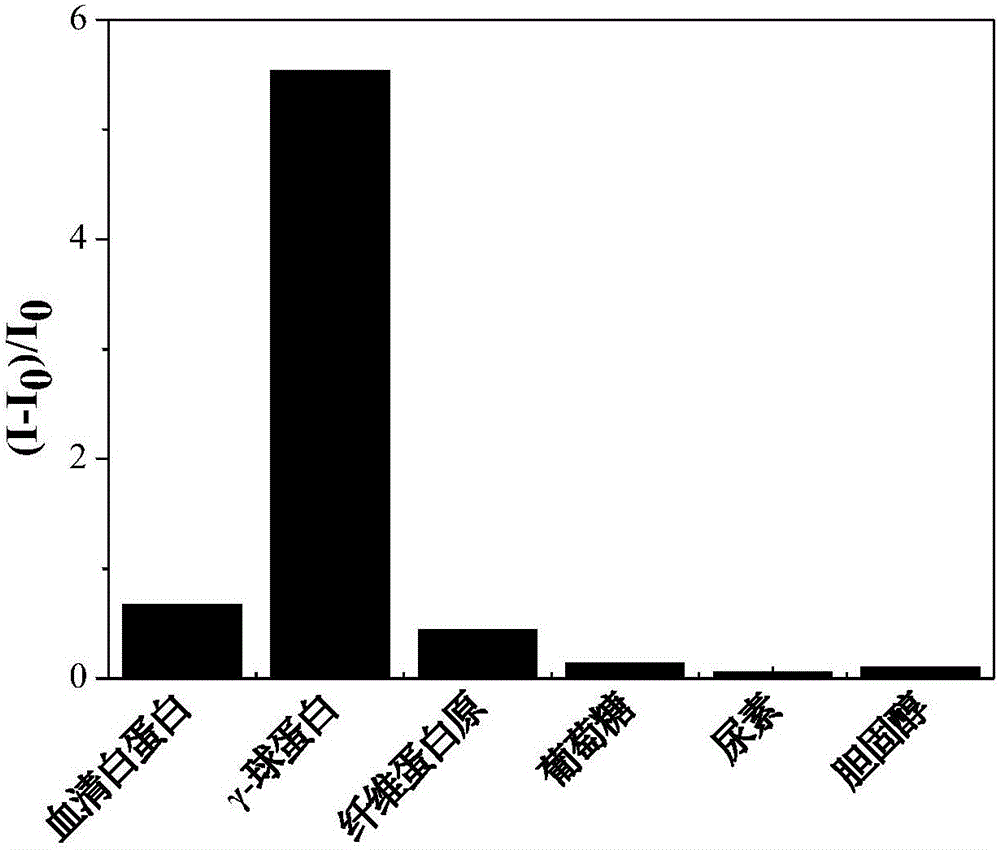
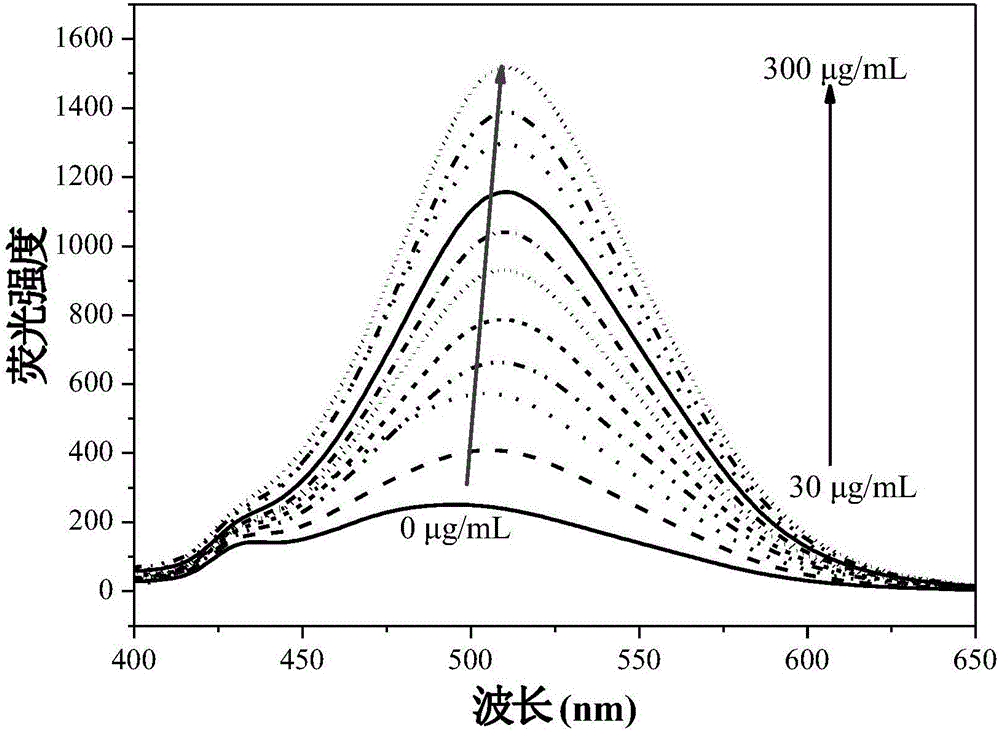
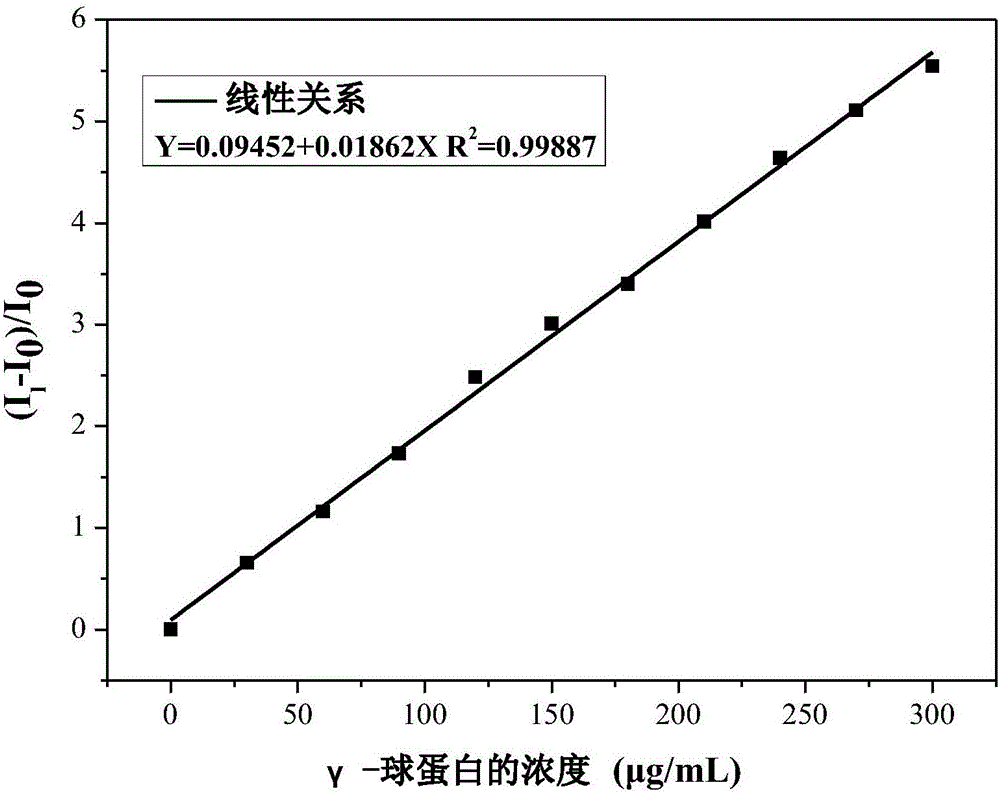
Fluorescent reagent for detecting trace gamma-globulin, as well as preparation method and application thereof
InactiveCN105778894AFluorescent signal enhancementHigh sensitivityOrganic chemistryFluorescence/phosphorescencePotassium hexafluorophosphateSolvent
The invention discloses a fluorescent reagent for detecting trace gamma-globulin, as well as a preparation method and application thereof, and belongs to the field of fluorescent bio-sensors. The fluorescent reagent is prepared from TABD-Py-PF6 and a good solvent. The preparation method comprises the following steps: dissolving (1Z,3Z)-1,4-di(4-methoxycarbonyl) phenyl-1,4-dibromo-1,3-butadiene in the good solvent, adding 4-pyridine phenylboronic acid, a catalyst and a basic salt, and reacting to obtain TABD-Py; dissolving the TABD-Py in a solvent, adding iodomethane to synthesize iodate, adding potassium hexafluorophosphate, and reacting to obtain TABD-Py-PF6; dissolving the TABD-Py-PF6 in the good solvent to obtain the fluorescent reagent. The fluorescent reagent does not need participation of metal ions, has a specific lighting-type fluorescent response on gamma-globulin, has high and quick sensitivity on gamma-globulin detection, and has high selectivity. The fluorescent reagent has an excellent visible detection signal, can be completely separated from high-precision instruments according to actual requirement, and can meet current requirement for detecting the gamma-globulin in serum to a great extent.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
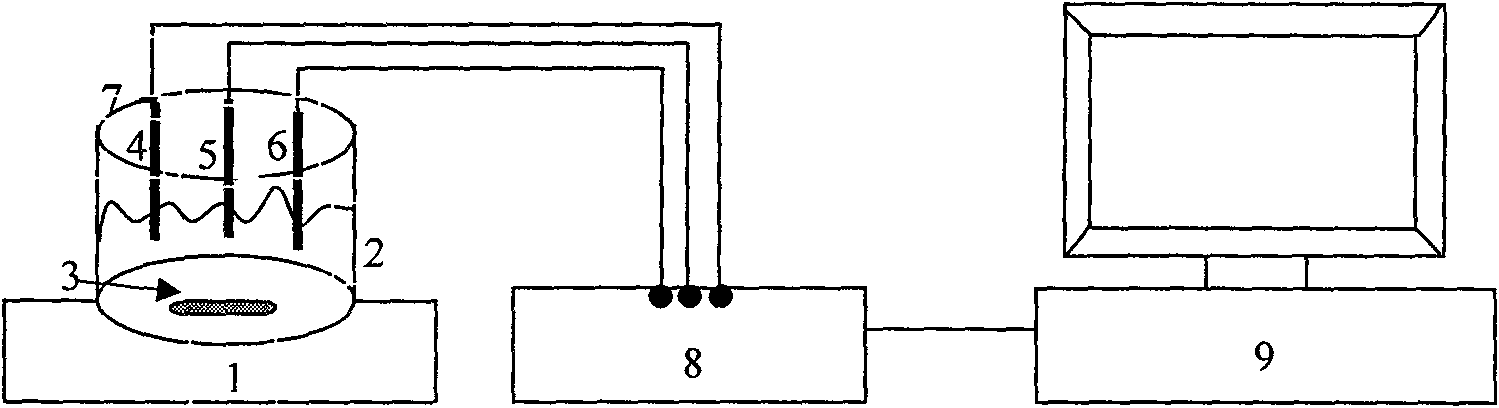
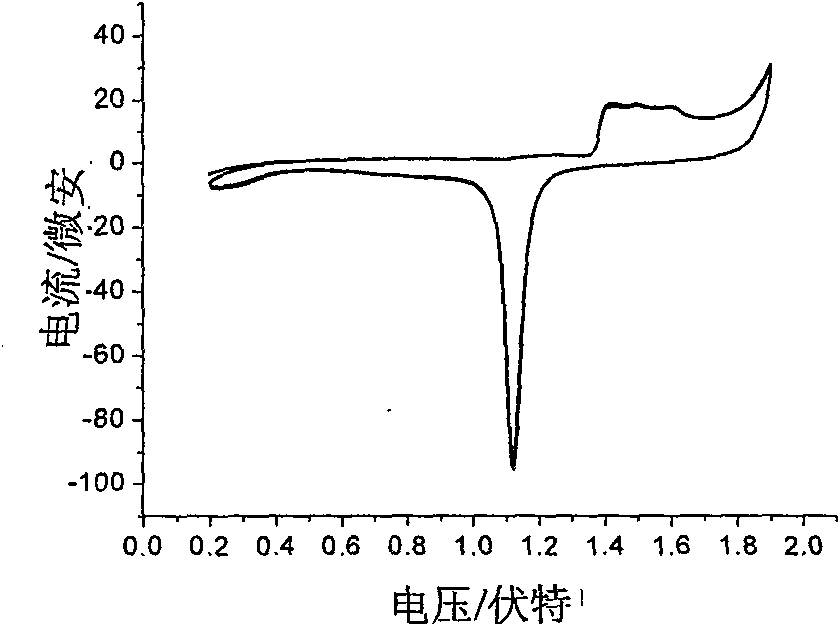
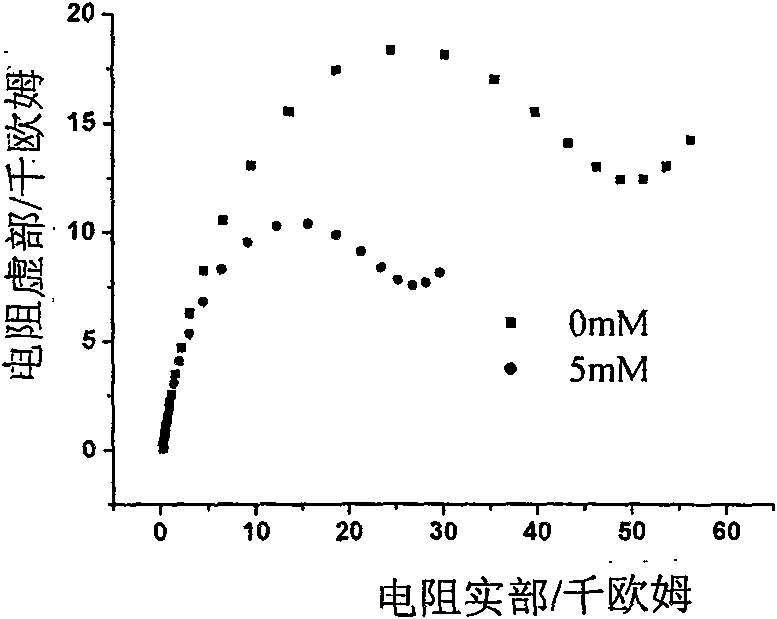
Electrochemical detection method for dihydrogen phosphate ions in water system
InactiveCN101581688ASimple experimental designLow spuriousMaterial electrochemical variablesPotassium hexafluorophosphateThiol
The invention provides an electrochemical detection method for dihydrogen phosphate ions in a water system, which comprises the steps: inserting a gold electrode the surface of which is self-assembled with a porphyrin monolayer, a reference electrode and a counter electrode into an electrochemical detection cell of potassium hexafluorophosphate electrolyte solution containing red potassium prussiate / potassium ferrocyanide probe molecules, performing scanning by an electrochemical workstation, and obtaining a charge transfer resistance value (Rct) of the self-assembled porphyrin monolayer; and then jointly inserting the gold electrode modified by porphyrin, the reference electrode and the counter electrode into the electrochemical detection cell of the electrolyte solution which contains an object to be detected, namely the dihydrogen phosphate ions, performing scanning by the electrochemical workstation, and obtaining a charge transfer resistance value (Rct) of the self-assembled porphyrin monolayer, wherein the charge transfer resistance values (Rct) and the concentrations (C) of the dihydrogen phosphate ions have corresponding relation. The method detects the dihydrogen phosphate ions on the surface of the gold electrode modified by thiol-porphyrin, is convenient and quick, and realizes the selective detection of the dihydrogen phosphate ions in the water system.
Owner:NORTHWEST NORMAL UNIVERSITY
Perovskite photovoltaic cell with potassium hexafluorophosphate film as interface passivation layer
PendingCN110190194AReduce manufacturing costImprove performanceSolid-state devicesSemiconductor/solid-state device manufacturingPotassium hexafluorophosphateOptoelectronics
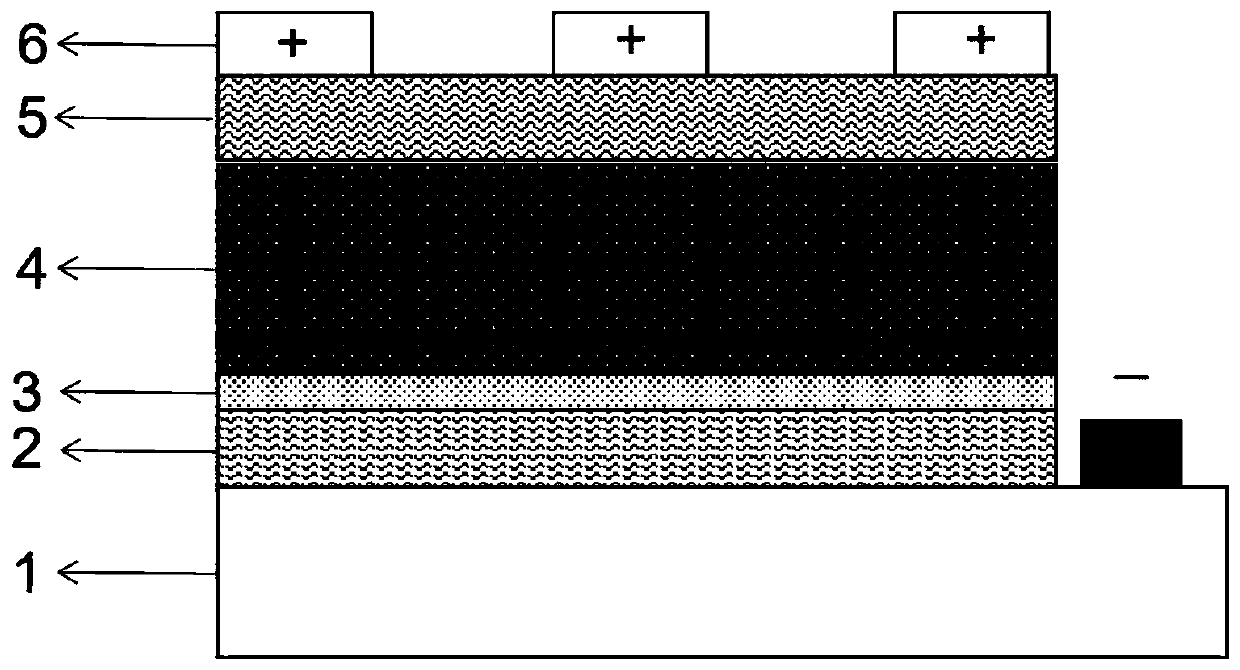
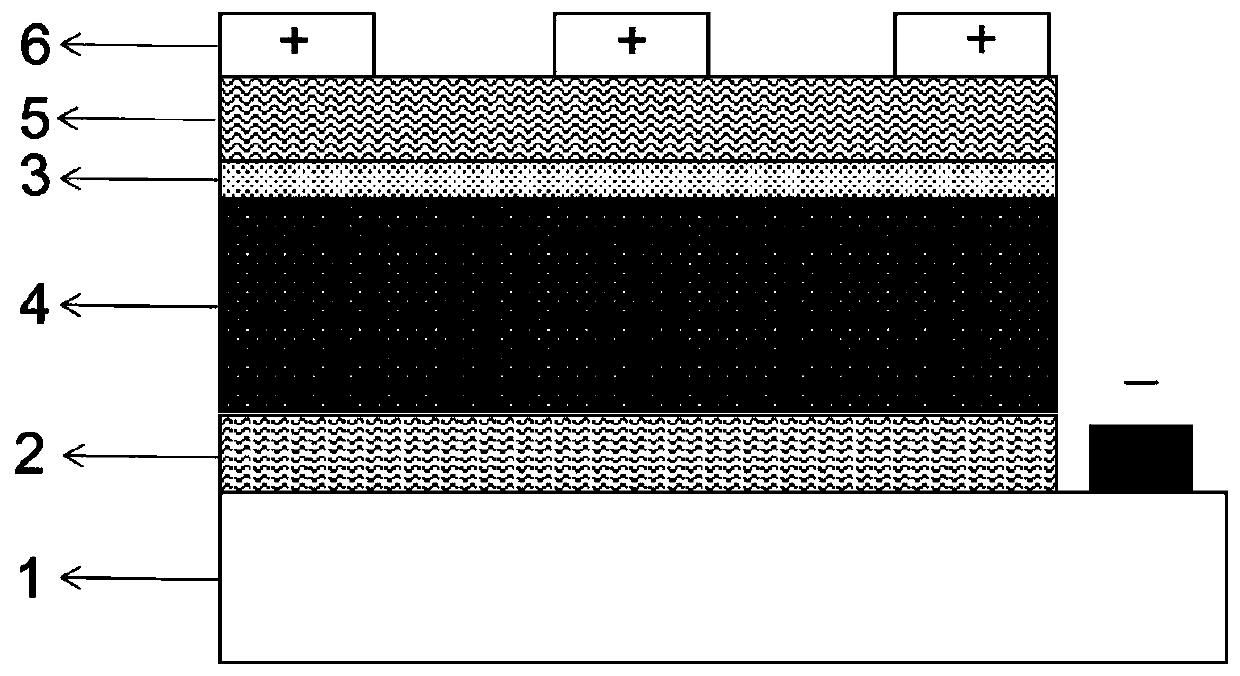
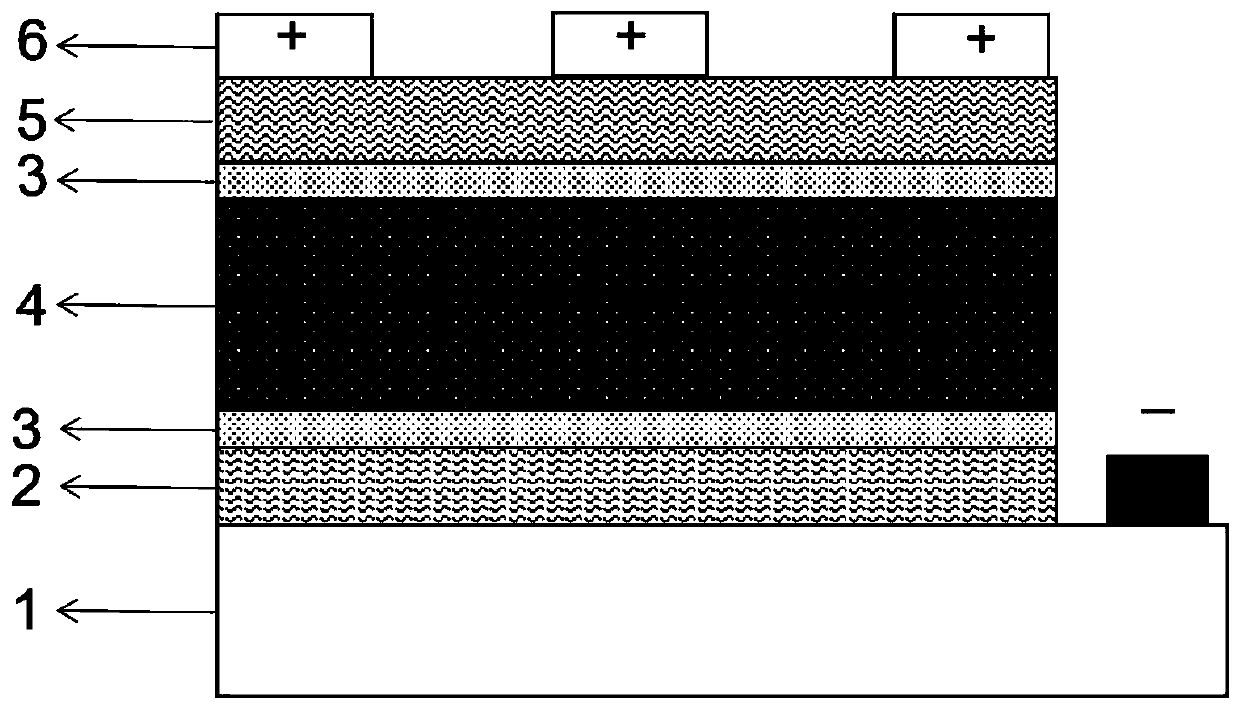
The invention discloses a perovskite photovoltaic cell with a potassium hexafluorophosphate film as an interface passivation layer. The perovskite photovoltaic cell comprises a transparent conductivesubstrate, an electron transport layer, a perovskite photosensitive active layer, a hole transport layer and a metal electrode. An interface passivation layer is arranged between the electron transport layer and the perovskite photosensitive active layer; or an interface passivation layer is arranged between the perovskite photosensitive active layer and the hole transport layer; or interface passivation layers are arranged among the electron transport layer, the perovskite photosensitive active layer and the hole transport layer at the same time. The interface passivation layer is a potassiumhexafluorophosphate film. According to the invention, a KPF6 thin film is used as the interface passivation layer to passivate an adjacent interface of the perovskite photosensitive layer; potassiumion A-site substitution is utilized, so interface defects are reduced, and electron-hole recombination is restrained, charge transfer between interfaces is improved, and filling factors are increased;and he hydrophobicity of the fluorine group is utilized, so the hydrophobicity of a device is improved and the stability of the device is improved.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of gasoline extraction and desulfurization agent
InactiveCN102040553ALow melting pointHighly selective adsorptionOrganic chemistryHydrocarbon oils refiningPotassium hexafluorophosphateSulfur
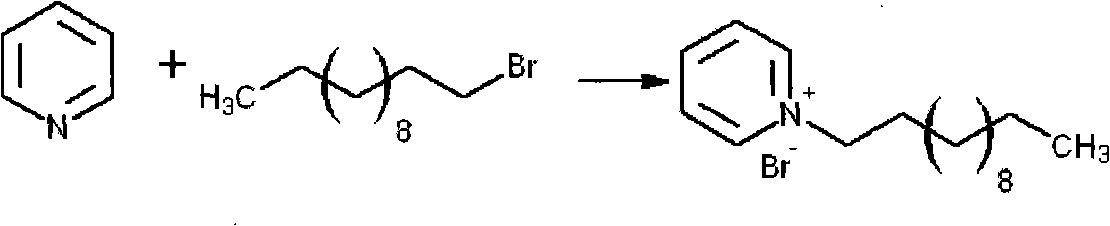
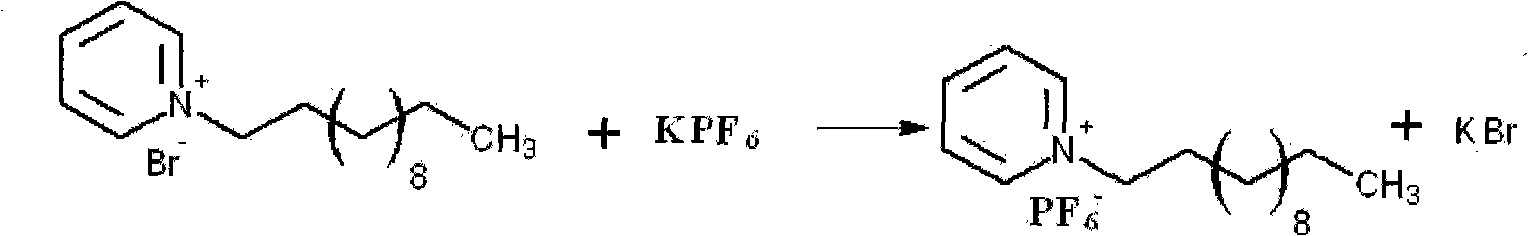
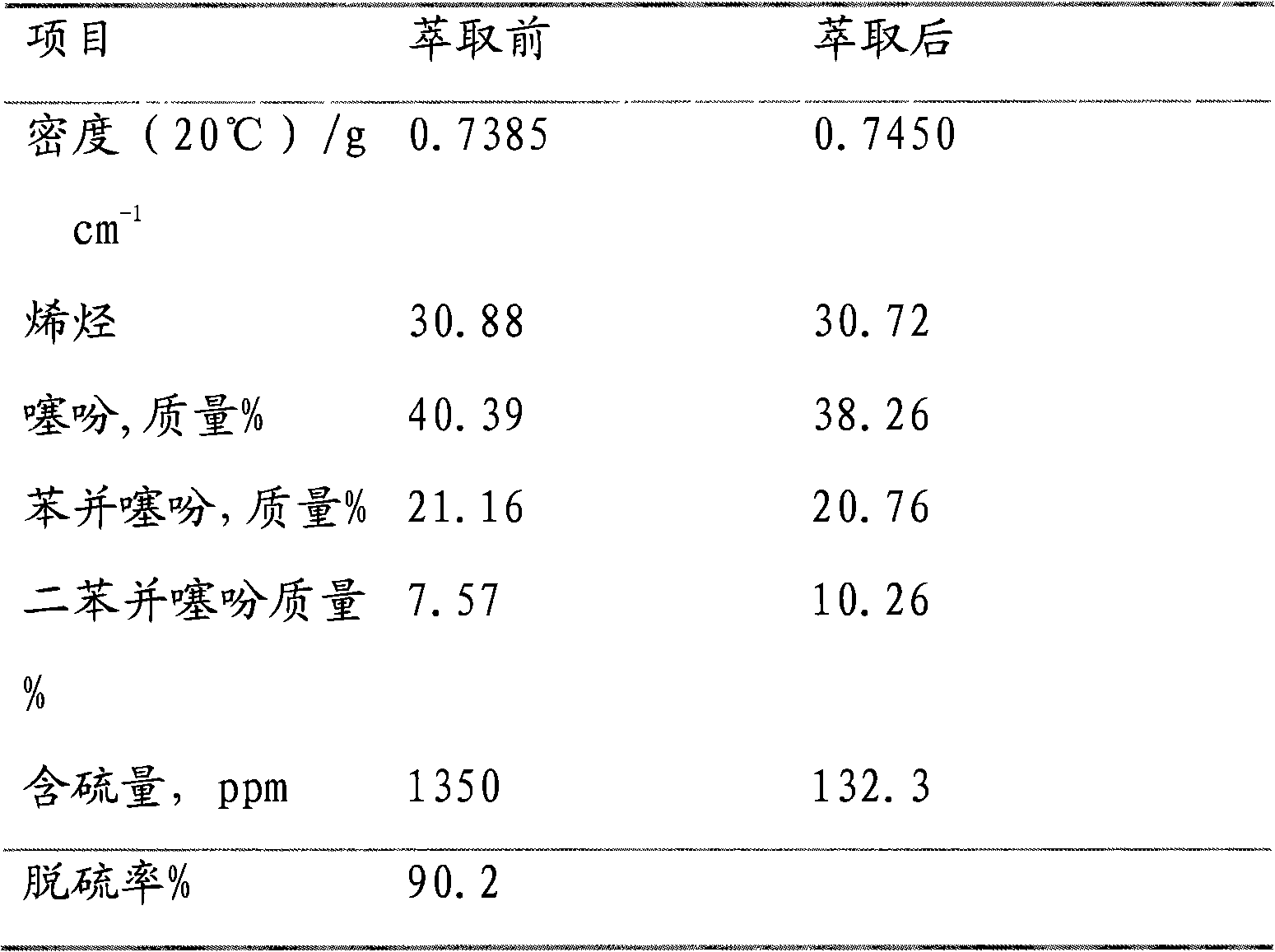
The invention relates to a preparation method of a gasoline extraction and desulfurization agent, belonging to the technical field of gasoline extraction and desulfurization. The preparation method comprises the following steps of: removing sulfur compounds in gasoline by using ionic liquid N-dodecyl pyridine hexafluorophosphate as an extracting agent, firstly preparing N-dodecyl pyridine bromide by using pyridine and bromododecane as raw materials, and then synthesizing a required iconic liquid through ion exchange with potassium hexafluorophosphate under certain conditions. The iconic liquid not only has mild reaction conditions, short reaction time and higher gas desulfurization rate and recovery rate and can properly reduce olefins and aromatic hydrocarbons in oils at the same time, but also is convenient to prepare, higher in yield and more stable in water and air. Thus, the invention provides a basis for industrial production.
Owner:JINAN DEV ZONE XINGHUO SCI & TECH RES INST
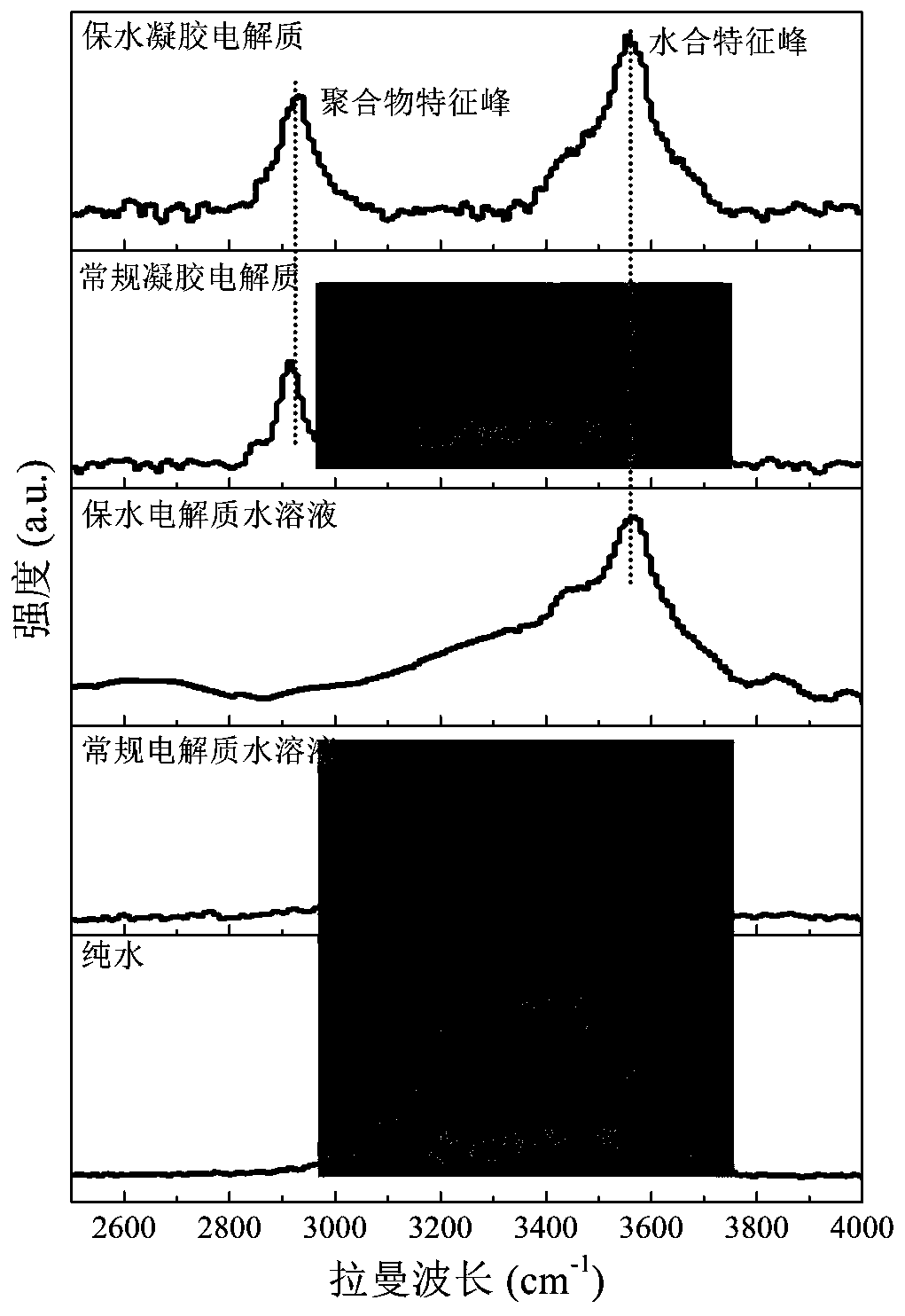
Water-retention gel electrolyte, preparation method thereof, and water-based supercapacitor and preparation method and application thereof
InactiveCN110246705AEasy to manufactureImprove water retentionHybrid capacitor electrolytesDouble layer capacitorsWater basedPotassium hexafluorophosphate
The invention provides a water-retention gel electrolyte , a preparation method thereof, and a water-based supercapacitor and a preparation method and application thereof, and relates to the field of gel electrolytes and supercapacitors. The water-retention gel electrolyte provided by the invention is prepared from an electrolyte aqueous solution and an oxygen-containing functional group polymer. The electrolyte in the electrolyte aqueous solution is one or more of lithium perchlorate, bis(trifluoromethane)sulfonimide lithium salt, lithium hexafluorophosphate, lithium nitrate, sodium chloride, sodium perchlorate, bis(trifluoromethane)sulfonimide sodium salt, sodium hexafluorophosphate, sodium nitrate, potassium chloride, potassium perchlorate, bis(trifluoromethane)sulfonimide potassium salt, potassium hexafluorophosphate and potassium nitrate. The oxygen-containing functional group polymer is one or more of polyvinylpyrrolidone, polyethylene glycol, polyvinyl alcohol, carboxymethyl cellulose and polyacrylamide. The water-based supercapacitor based on the water-retention gel electrolyte has ultra-long cycle life at the normal temperature, and can be normally charged and discharged at the high temperature of 120 DEG C and stably circulated.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
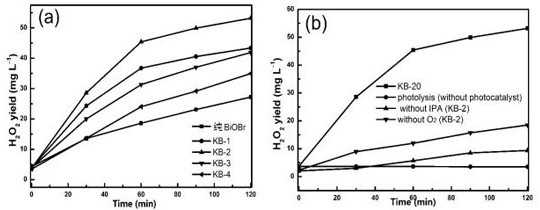
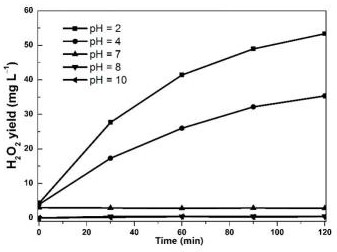
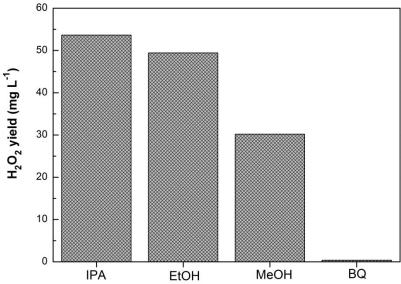
Photocatalytic material as well as preparation method and application thereof
ActiveCN112642451AExcellent photocatalytic hydrogen peroxide production performanceImprove performancePeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesCatalyst activation/preparationPotassium hexafluorophosphateBismuth oxybromide
The invention relates to the technical field of photocatalysis, and particularly discloses a photocatalytic material and a preparation method and application thereof, and the main components of the photocatalytic material are KPF6 and BiOBr. The photocatalytic material provided by the embodiment of the invention has excellent performance of preparing H2O2 by photocatalysis, is a BiOBr composite material improved by KPF6, can be used for efficiently preparing H2O2 by photolysis of water, is prepared by dropwise adding a potassium hexafluorophosphate solution into a bismuth oxybromide precursor solution by adopting a one-step hydrothermal method for hydrothermal reaction, is simple to operate. The problem that an existing BiOBr photocatalyst is not high in hydrogen peroxide synthesis efficiency in the process of preparing hydrogen peroxide through water photolysis is solved. Moreover, the preparation method provided by the invention is simple, and the prepared photocatalytic material is stable in performance and low in cost and has a good market application prospect.
Owner:HENAN NORMAL UNIV
Lithium hexafluorophosphate preparation method
InactiveCN105692574ARelative density is smallEasy to handlePhosphorus halides/oxyhalidesPotassium hexafluorophosphateDistillation
The invention relates to a lithium hexafluorophosphate preparation method. The method comprises the following steps: processing highly pure lithium fluoride by anhydrous hydrogen fluoride to obtain porous lithium fluoride and release a large amount of heat, using the heat to catalyze dissolving of potassium hexafluorophosphate and lithium fluorine in an anhydrous organic solvent, and absorbing too high heat by the anhydrous organic solvent to form a high efficiency reaction system; and reducing the relative density of a fluorine-containing gas generated in the reaction by the volatilized anhydrous organic solvent, easily processing discharged exhaust gas, reacting gaseous phosphorus pentafluoride with lithium fluoride incompletely reacted in the reaction system, and carrying out reduced pressure distillation to obtain lithium hexafluorophosphate. The method avoids the reaction at an ultralow temperature (-30DEG C or below), reduces the requirements and the operating difficulty of a device, and guarantees the safety.
Owner:庄祥荣
Method for separating and detecting daclatasvir hydrochloride and optical isomers thereof
ActiveCN108732280AQuality improvementEfficient separationComponent separationPotassium hexafluorophosphateCellulose
The invention in particular relates to a method for separating and detecting daclatasvir hydrochloride and optical isomers thereof. The method for separating and determining daclatasvir hydrochlorideand optical isomers thereof (impurities) by using liquid chromatography is characterized by comprising the following steps: adopting a chiral chromatographic column taking cellulose tris(3,5-dimethylphenylcarbamate) as a filler, and taking a mixed solution which takes sodium hexafiuorophosphate, potassium hexafluorophosphate, formic acid, acetic acid, phosphoric acid or aqueous phosphate solutionas an aqueous phase and takes acetonitrile or methanol as an organic phase as a mobile phase. According to the separating and detecting method disclosed by the invention, the daclatasvir hydrochlorideand optical isomers thereof (impurities) can be effectively separated, the degree of separation reaches 3.0 or higher, and complete baseline separation is realized, so that the quality of the daclatasvir hydrochloride can be accurately and effectively controlled. With the adoption of the separating method disclosed by the invention, the time of separating and detecting the daclatasvir hydrochloride and optical isomers thereof is within 30-80 minutes. The method disclosed by the invention has the advantages of being simple, rapid, accurate and the like.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of potassium hexafluorophosphate
ActiveCN107758701AHigh yieldReduce usageSodium/potassium compoundsPotassium hexafluorophosphateEvaporation
The invention relates to the field of synthesis of an inorganic compound, and particularly relates to a preparation method of potassium hexafluorophosphate. The preparation method of the potassium hexafluorophosphate comprises the following steps: performing an initial reaction under a certain condition by taking potassium bifluoride (or potassium chloride), anhydrous hydrogen fluoride and phosphorus oxychloride as reaction raw materials to obtain a potassium hexafluorophosphate crude product, adjusting the acidity by using potassium hydroxide, eliminating iron ions, adsorbing and decoloring by activated carbon, performing evaporation and concentration on the crude product after removing impurities, and performing recrystallization to obtain the potassium hexafluorophosphate finished product. Compared with the traditional preparation method, the preparation method of the potassium hexafluorophosphate has the following advantages: the preparation method is simple in operation, mild in reaction and easy to control, and the use of a large amount of unstable, corrosive and toxic raw materials is avoided, so that the operation dangerousness is reduced; by adopting the potassium hexafluorophosphate produced by the method, the yield is up to 88 percent and the purity exceeds 99 percent.
Owner:JIANGXI DONGYAN PHARMA
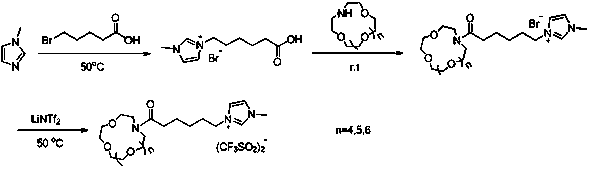
Synthesis method of aza-crown ether functionalized ionic liquid
PendingCN110396082AImprove stabilityHigh yieldOrganic chemistryPotassium hexafluorophosphateSynthesis methods
The invention discloses a synthesis method of aza-crown ether functionalized ionic liquid, and belongs to the technical field of organic synthesis and supermolecules. The invention aims to provide a synthetic method of hydrophobic crown ether functionalized ionic liquid. The method comprises the following steps of: 1) performing a reaction on 1-methylimidazole and bromoalkyl acid serving as raw materials to obtain 1-carboxyl-3-methylimidazole bromide; 2) carrying out a reaction with aza-crown ether to obtain 1-alkanoyl-aza-crown ether-3-methylimidazole bromide; and 3) dissolving the 1-alkanoyl-aza-crown ether-3-methylimidazole bromide and lithium / sodium / potassium bistrifluoromethanesulfonimide (or lithium / sodium / potassium hexafluorophosphate) in water for anion exchange, and finally washing and drying the products to obtain 1-alkanoyl-aza-crown ether-3-methylimidazole bistrifluoromethanesulfonimide / hexafluorophosphate ionic liquid. The synthesis method can effectively avoid the loss ofcrown ether molecules and improve the stability of the chelate. In addition, the anionic bistrifluoromethanesulfonimide salt of the ionic liquid is hydrophobic, so that the ionic liquid can be used for recycling metal ions in a water phase.
Owner:SHANXI UNIV
High-performance glass micro-bead for reflective film and preparation method thereof
InactiveCN104961344AHigh refractive indexImprove performancePotassium hexafluorophosphateBarium titanate
The invention discloses a high-performance glass micro-bead for a reflective film and a preparation method thereof. The high-performance glass micro-bead for the reflective film is prepared by using the following raw materials in parts by weight: 33 to 45 parts of bryozoatum, 21 to 32 parts of silicon-calcium slag, 24 to 39 parts of topaz, 14 to 23 parts of rubidium carbonate, 18 to 29 parts of kaolin, 25 to 35 parts of barite, 10 to 15 parts of borax, 5 to 10 parts of calcium sulfate, 6 to 12 parts of sodium nitrate, 5 to 10 parts of barium titanate, 4 to 8 parts of potassium hexafluorophosphate and 8 to 12 parts of additive. By using industrial waste silicon-calcium slag as a main raw material and using silicon-calcium slag with components such as bryozoatum, barite, topaz, rubidium carbonate, barium titanate and potassium hexafluorophosphate in a combined manner, not only can the waste be utilized as resources, but also the comprehensive performance of the glass micro-bead is improved, the high-performance glass micro-bead disclosed by the invention has the advantages of high refractive index, high strength, good chemical stability, aging resistance, high temperature resistance, corrosion resistance and the like, and the economic benefit is good.
Owner:HEFEI DINGLIANG OPTICAL TECH
A kind of quaternary ammonium hexafluorophosphate ionic liquid polymer and its synthesis method
The invention provides a kind of synthetic method of novel quaternary ammonium hexafluorophosphate ionic liquid polymer, that is: poly(p-vinylbenzyl-triethyl quaternary ammonium hexafluorophosphate), which first uses triethylamine and p- Chloromethylstyrene is subjected to quaternization reaction, then ion-exchanged with potassium hexafluorophosphate to obtain monomer, and finally poly(p-vinylbenzyl-triethyl quaternary ammonium hexafluorophosphate) is obtained through free radical polymerization. The ionic liquid polymer has good thermal stability and high glass transition temperature, and can be used for the capture of harmful gases such as carbon dioxide, sulfur dioxide, formaldehyde, and dioxin, the solid-phase extraction and separation of sulfur and nitrogen heterocyclic compounds in oil products, and Solid phase electrolyte materials and other fields.
Owner:BEIJING UNIV OF CHEM TECH
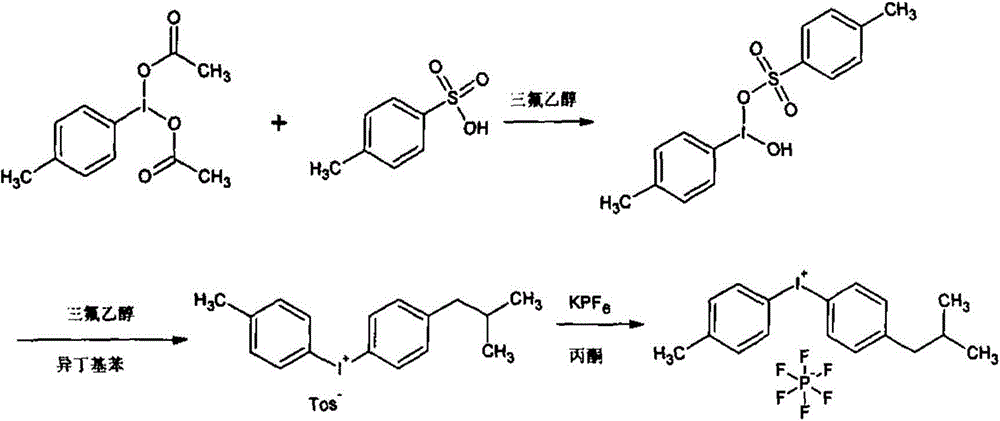
Cation photoinitiator 4-isobutylphenyl-4'-methylphenyl iodonium hexafluorophosphate preparation method
InactiveCN104628516AEasy to separateEasy to purifyHalogenated hydrocarbon preparationPotassium hexafluorophosphateReaction temperature
The present invention relates to a cation photoinitiator 4-isobutylphenyl-4'-methylphenyl iodonium hexafluorophosphate preparation method. According to the preparation method, in a solvent, p-iodotoluene diacetate and isobutyl benzene are subjected to a one-step reaction in the presence of p-toluene sulfonic acid, wherein the reaction temperature is a room temperature, and the reaction time is 4-24 h; concentration is performed after completing the reaction so as to remove the solvent; petroleum ether is added to crystallize to obtain the 4-isopropylphenyl-4'-methylphenyl iodonium p-toluene sulfonate; and finally the 4-isopropylphenyl-4'-methylphenyl iodonium p-toluene sulfonate and potassium hexafluorophosphate are subjected to ion exchange to obtain the product. Compared with the technology in the prior art, the preparation method of the present invention has characteristics of reasonable process, simple operation, and safe production, is suitable for industrial mass production, and provides favorable conditions for industrial production of the 4-isobutylphenyl-4'-methylphenyl iodonium hexafluorophosphate.
Owner:上海予利生物科技股份有限公司
Functionalized ion liquid for lithium extraction, and synthesis method thereof
ActiveCN106478495ASimple methodShort reaction timeOrganic chemistryProcess efficiency improvementChemical synthesisPotassium hexafluorophosphate
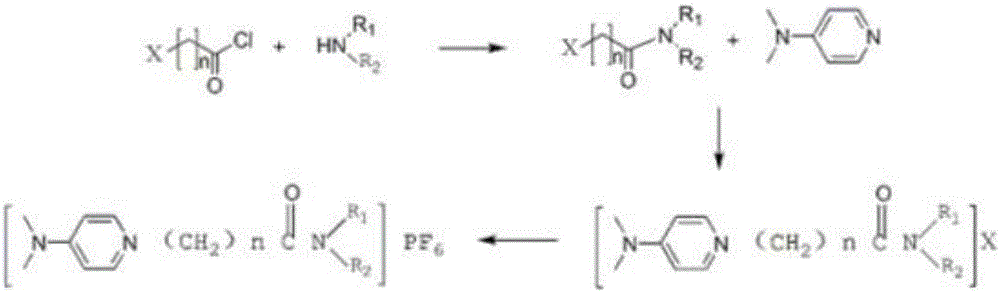
The invention discloses a functionalized ion liquid for lithium extraction, and a synthesis method thereof, and belongs to the field of chemical synthesis. According to the method, halogenated acyl chloride, disubstituted amine, 4-N,N-dimethylpyridine and potassium hexafluorophosphate are adopted as raw materials, and an amidation reaction, a salt formation reaction and an exchange reaction are performed to obtain the functionalized ion liquid having the amide group. Compared to the functionalized ion liquid and the synthesis method thereof in the prior art, the functionalized ion liquid and the synthesis method thereof of the present invention have characteristics of simple operation, industrial application, important application value, and the like.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Phosphate ionic liquids as well as synthetic method and application thereof
InactiveCN107955036ALow viscosityHigh single extraction efficiencyGroup 5/15 element organic compoundsLiquid solutions solvent extractionLithiumPotassium hexafluorophosphate
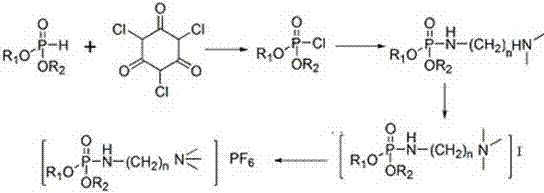
The invention discloses phosphate ionic liquids, a synthetic method thereof and an application as a lithium extraction agent. According to the method, the functional ionic liquids containing phosphamide groups are prepared from dibutyl phosphite, trichloroisocyanuric acid, N,N'-dimethylethanediamine and potassium hexafluorophosphate taken as raw materials through phosphoryl chlorination reaction,amidation with amine, salt forming reaction and exchange reaction. Compared with the prior art, the ionic liquids and the synthetic method thereof have the characteristics that operation is simple, industrial popularization and application are facilitated and the like, and have important application values.
Owner:青海柴达木兴华锂盐有限公司
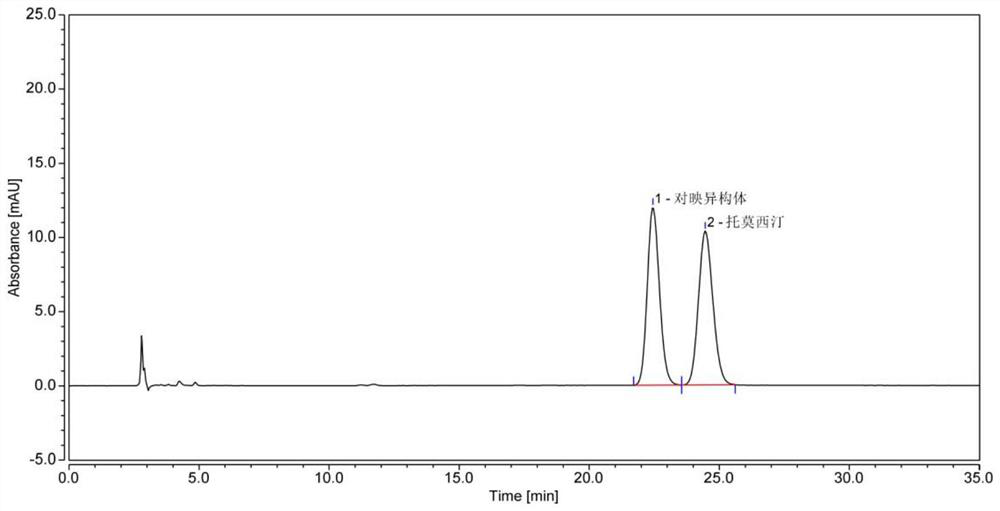
Method for detecting atomoxetine hydrochloride enantiomer by reversed-phase liquid chromatography
ActiveCN113358773AEfficient separationStable performance of chromatographic mediumComponent separationBenzoic acidPotassium hexafluorophosphate
The invention discloses a method for detecting atomoxetine hydrochloride enantiomer by reversed-phase liquid chromatography, which belongs to the field of pharmaceutical analysis, and adopts a polysaccharide derivative reversed-phase coating type chiral chromatographic column of which the surface of silica gel is coated with cellulose-tri (4-methyl benzoate) as a filler; and a mixed solution of a potassium hexafluorophosphate solution and acetonitrile is used as a mobile phase, the column temperature is 25-35 DEG C, the flow velocity is 0.5-0.8 ml / min, an ultraviolet detector is adopted, and the wavelength is 200-250 nm. According to the present invention, the atomoxetine hydrochloride and the enantiomer can be directly and effectively separated, the accuracy of the detection result is good, the sensitivity is high, and compared with the existing normal phase chromatography or precolumn derivatization reverse chromatography, the operation is simple, the cost is low, and the analysis time is short, such that the good guarantee is provided for the quality control of the atomoxetine hydrochloride and the preparation thereof.
Owner:JIANMIN PHARMA GRP CO LTD
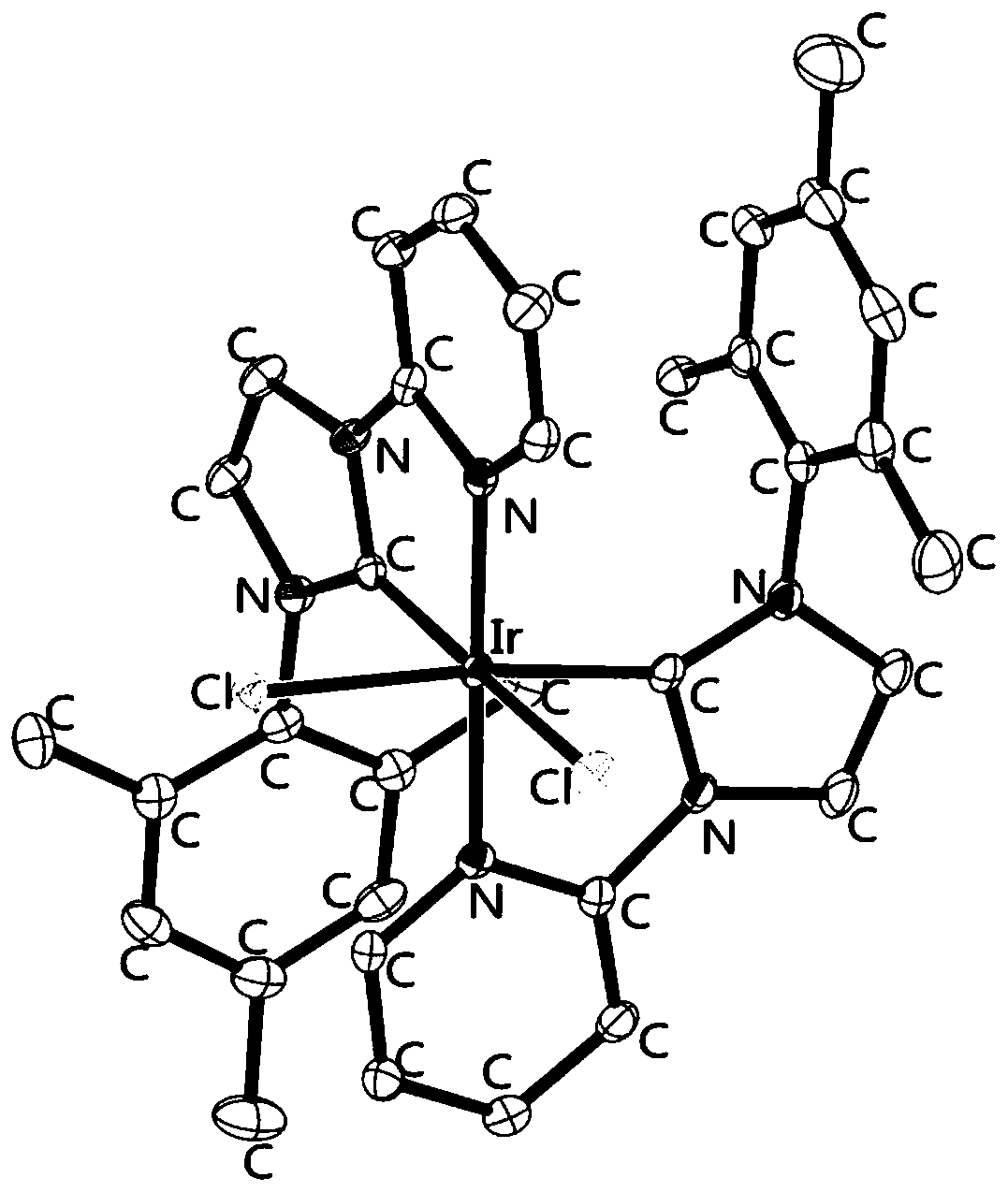
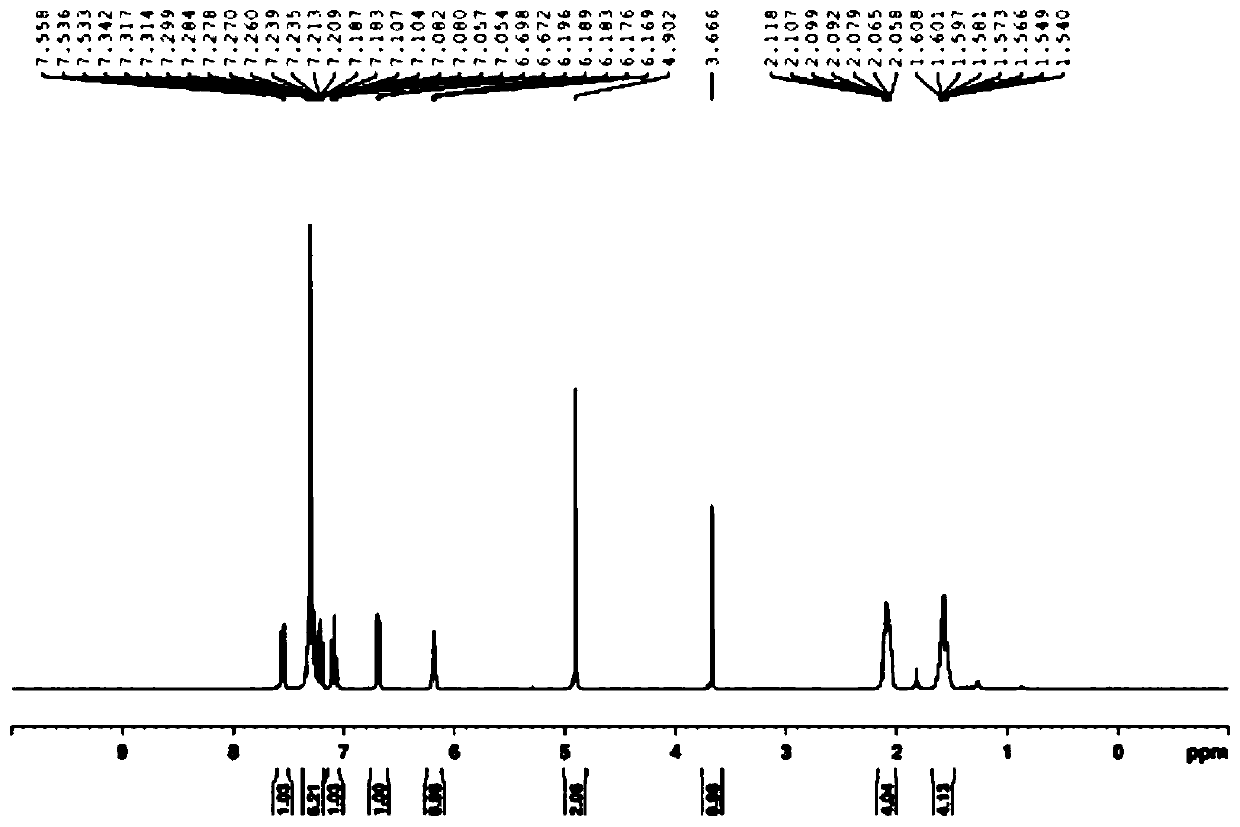
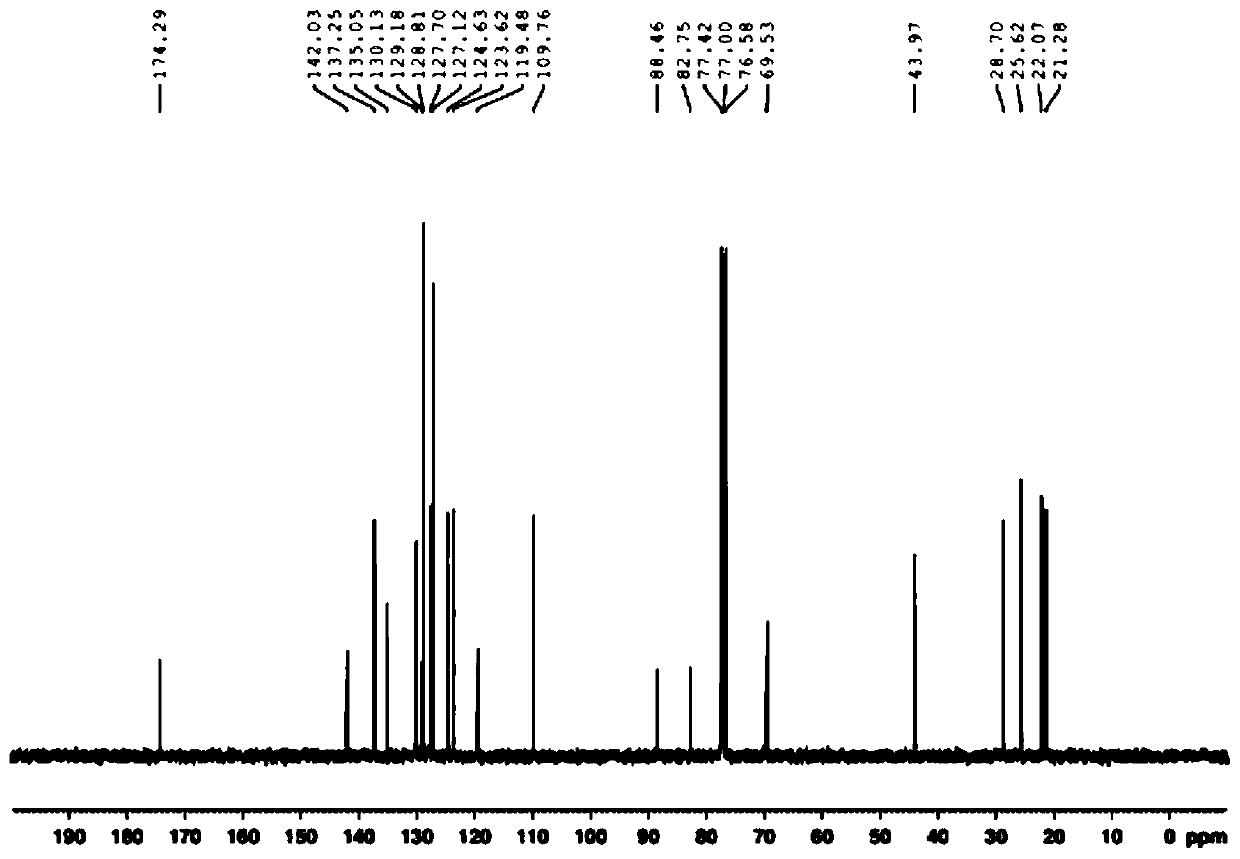
Metal iridium-carbene complex with photocatalytic performance as well as preparation method and application thereof
InactiveCN111548372AImprove photocatalytic efficiencyImprove catalytic performanceIndium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPotassium hexafluorophosphateOrganic base
The invention relates to a metal iridium-carbene complex with photocatalytic performance as well as a preparation method and application thereof, the molecular formula of the metal iridium-carbene complex is C34H36Cl2IrN6PF6, and the metal iridium-carbene complex is a mononuclear-hexadentate coordination compound of a monoclinic system and belongs to a P21 / c space group; the preparation method comprises the following steps: firstly, preparing carbene precursor pyridine-imidazole bromide from N-(2, 4, 6-trimethylphenyl) imidazole and 2-bromopyridine, then reacting with silver oxide to prepare asilver-carbene intermediate complex, and finally reacting with iridium trichloride and potassium hexafluorophosphate to prepare the metal iridium-carbene complex. The complex is applied to an addition reaction of a terminal alkyne compound and an isatin derivative, a visible light source is additionally arranged, and the addition reaction can be well carried out at room temperature by weak organic base; the metal iridium-carbene complex disclosed by the invention is relatively high in efficiency when being used as a photocatalyst for the addition reaction, and the yield of an addition productis 82 wt% or above.
Owner:CHANGZHOU INST OF TECH
A kind of preparation method of potassium hexafluorophosphate
ActiveCN107758701BHigh yieldReduce usageSodium/potassium compoundsPotassium hexafluorophosphateEvaporation
The invention relates to the field of synthesis of an inorganic compound, and particularly relates to a preparation method of potassium hexafluorophosphate. The preparation method of the potassium hexafluorophosphate comprises the following steps: performing an initial reaction under a certain condition by taking potassium bifluoride (or potassium chloride), anhydrous hydrogen fluoride and phosphorus oxychloride as reaction raw materials to obtain a potassium hexafluorophosphate crude product, adjusting the acidity by using potassium hydroxide, eliminating iron ions, adsorbing and decoloring by activated carbon, performing evaporation and concentration on the crude product after removing impurities, and performing recrystallization to obtain the potassium hexafluorophosphate finished product. Compared with the traditional preparation method, the preparation method of the potassium hexafluorophosphate has the following advantages: the preparation method is simple in operation, mild in reaction and easy to control, and the use of a large amount of unstable, corrosive and toxic raw materials is avoided, so that the operation dangerousness is reduced; by adopting the potassium hexafluorophosphate produced by the method, the yield is up to 88 percent and the purity exceeds 99 percent.
Owner:JIANGXI DONGYAN PHARMA
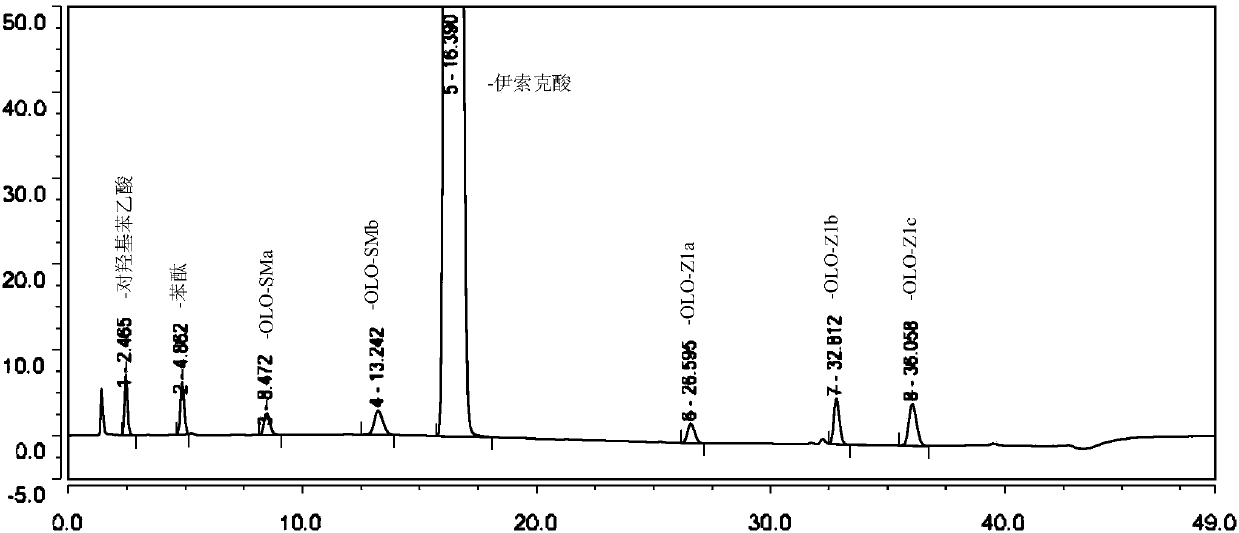
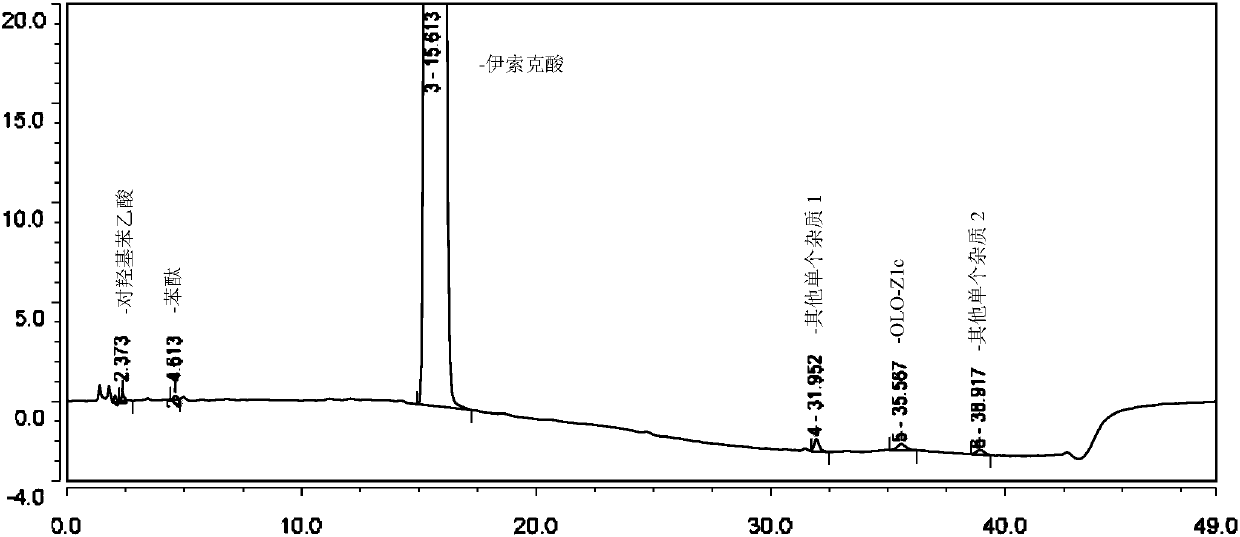
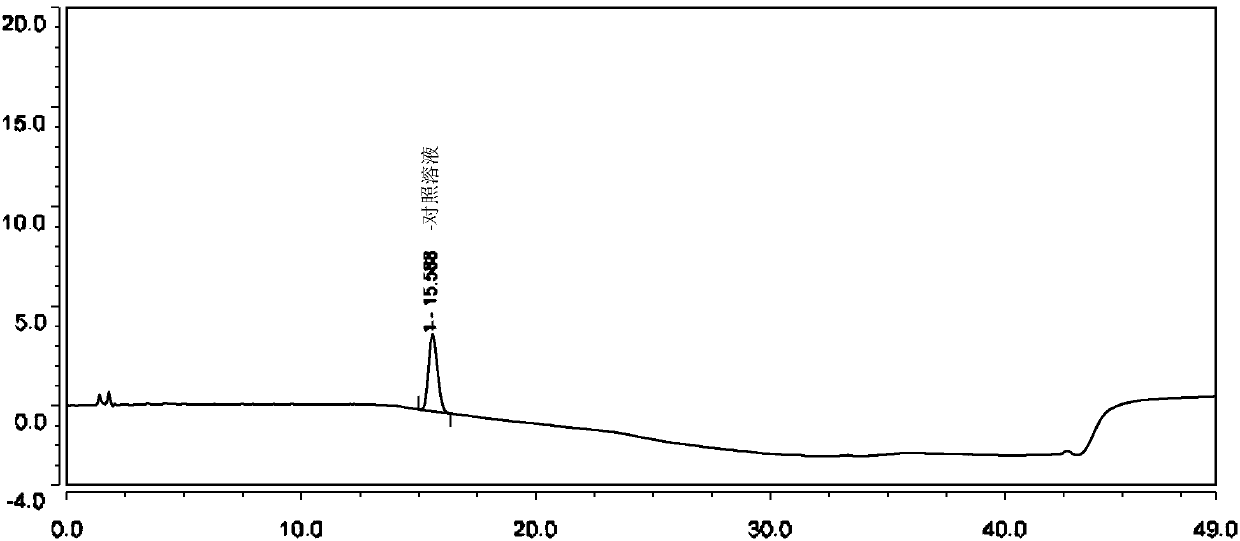
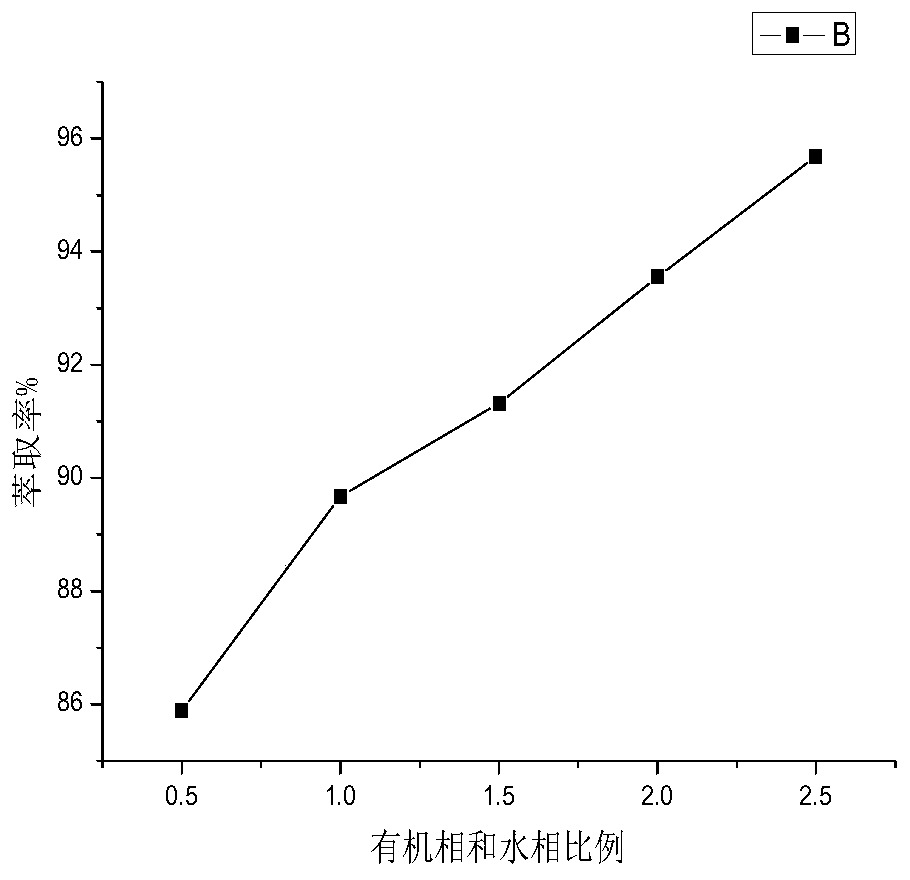
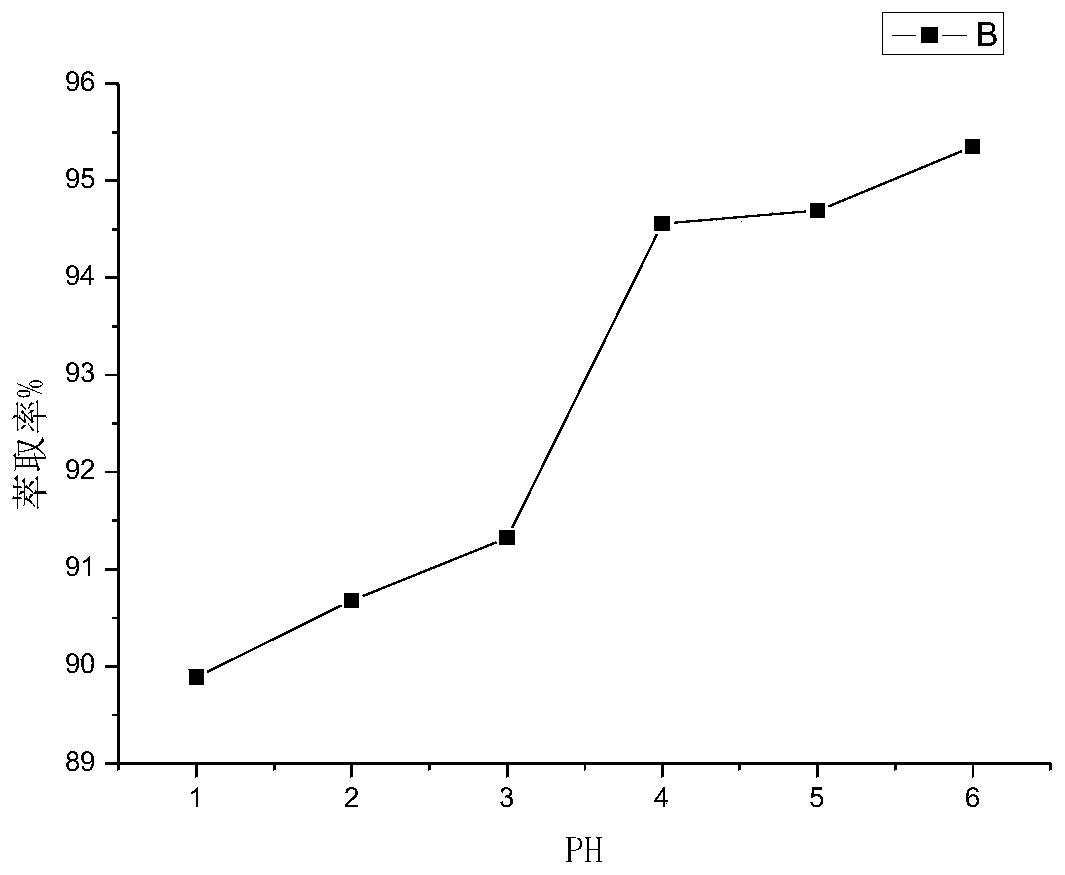
Method for separating and determining isoxepac and related substances of isoxepac by HPLC (High Performance Liquid Chromatography) method
ActiveCN107589197AAccurate detectionQuality is easy to controlComponent separationPotassium hexafluorophosphateImpurity
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining isoxepac and related substances of isoxepac by an HPLC (High PerformanceLiquid Chromatography) method. A chromatographic column adopted by the method disclosed by the invention adopts octylsilane-bonded silica gel as filler, a mobile phase A and a mobile phase B are adopted to carry out gradient elution, and detection is carried out in a detector; the related substances include one or more of A-G; the mobile phase A is potassium hexafluorophosphate solution, and themobile phase B is organic solvent. The method can effectively separate the seven impurities and other individual impurity in isoxepac and quantitatively determine the existence levels. The method hasthe advantages of high separation degree, high specificity, high sensitivity and accuracy, and is of a great significance in the control of the quality of isoxepac and olopatadine hydrochloride.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Lithium hexafluorophosphate waste liquid recovery method
InactiveCN112456519ANitrosyl chlorideLithium hexafluorophosphatePotassium hexafluorophosphateOrganic solvent
The invention discloses a lithium hexafluorophosphate waste liquid recovery method which comprises the following steps: reacting potassium salt with lithium hexafluorophosphate in a solvent, precipitating the generated potassium hexafluorophosphate in a precipitation manner while still dissolving lithium salt in an organic solvent, carrying out solid-liquid separation and drying to obtain pure potassium hexafluorophosphate, carrying out rotary drying on the liquid to obtain lithium salt. and in the rotary drying process, collecting and recycling the organic solvent through condensation. By means of the lithium hexafluorophosphate waste liquid recycling method, potassium hexafluorophosphate with the purity of 99.5% or above and pure lithium salt with the purity of 99% or above can be obtained.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
Compound ionic liquid extraction agent used for extracting copper ions in wastewater and application
InactiveCN110013686AImprove stabilitySimple processWater contaminantsLiquid solutions solvent extractionPotassium hexafluorophosphateButyl bromide
The invention discloses a compound ionic liquid extraction agent used for extracting copper ions in wastewater and application. A production method comprises the steps of placing n-butyl bromide and 1-methylimidazole in a reactor in a round-bottom three-necked flask, carrying out reaction in a constant-temperature oil bath pot for 10 hours, and conducting reduced pressure distillation to remove alight component; and according to a molar ratio of the n-butyl bromide to potassium hexafluorophosphate of 1 to 1, adding the potassium hexafluorophosphate into the reactor, continuously carrying outreaction in the constant-temperature oil bath pot for 10 hours, conducting layering after standing, conducting separation to remove supernatant, keeping lower-layer oily liquid, and placing the lower-layer oily liquid in a vacuum drying box for constant-temperature drying until constant weight to obtain the target product. The compound ionic liquid extraction agent used for extracting the copper ions in the wastewater has the advantages that the technology is simple, and design is reasonable. According to the compound ionic liquid extraction agent, ionic liquid production and extraction agentcompounding are completed at the same time, the stability is good, the extraction agent has the characters of being environmentally friendly and efficient, and meanwhile, a process of adding a chelating agent for compounding in addition is omitted.
Owner:LIAONING PETROCCHEM VOCATIONAL & TECH COLLEGE
Phosphorescent iridium complex, and preparation method and application thereof
ActiveCN102584899AHigh selectivityStrong dyeing functionGroup 8/9/10/18 element organic compoundsBiological testingIridiumPotassium hexafluorophosphate
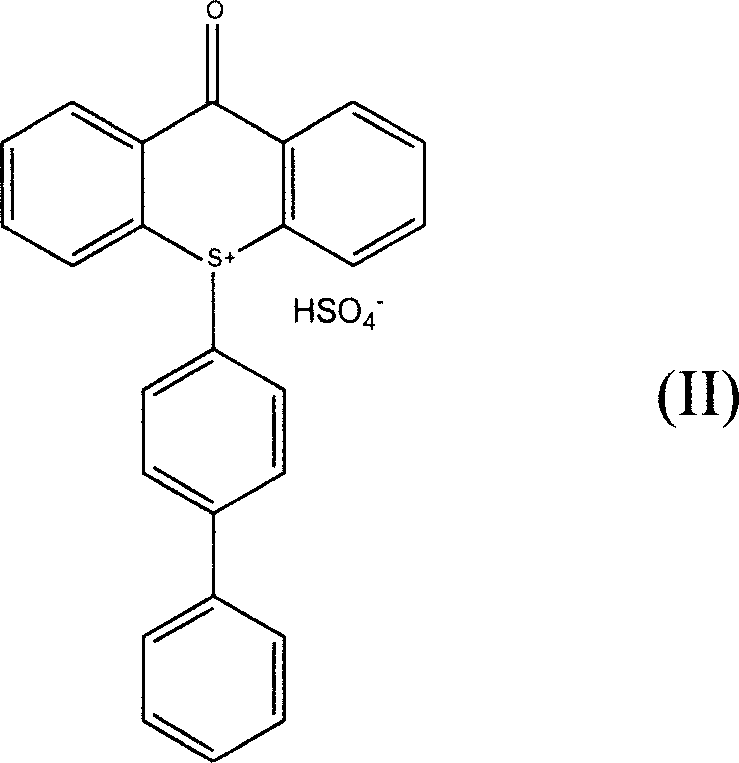
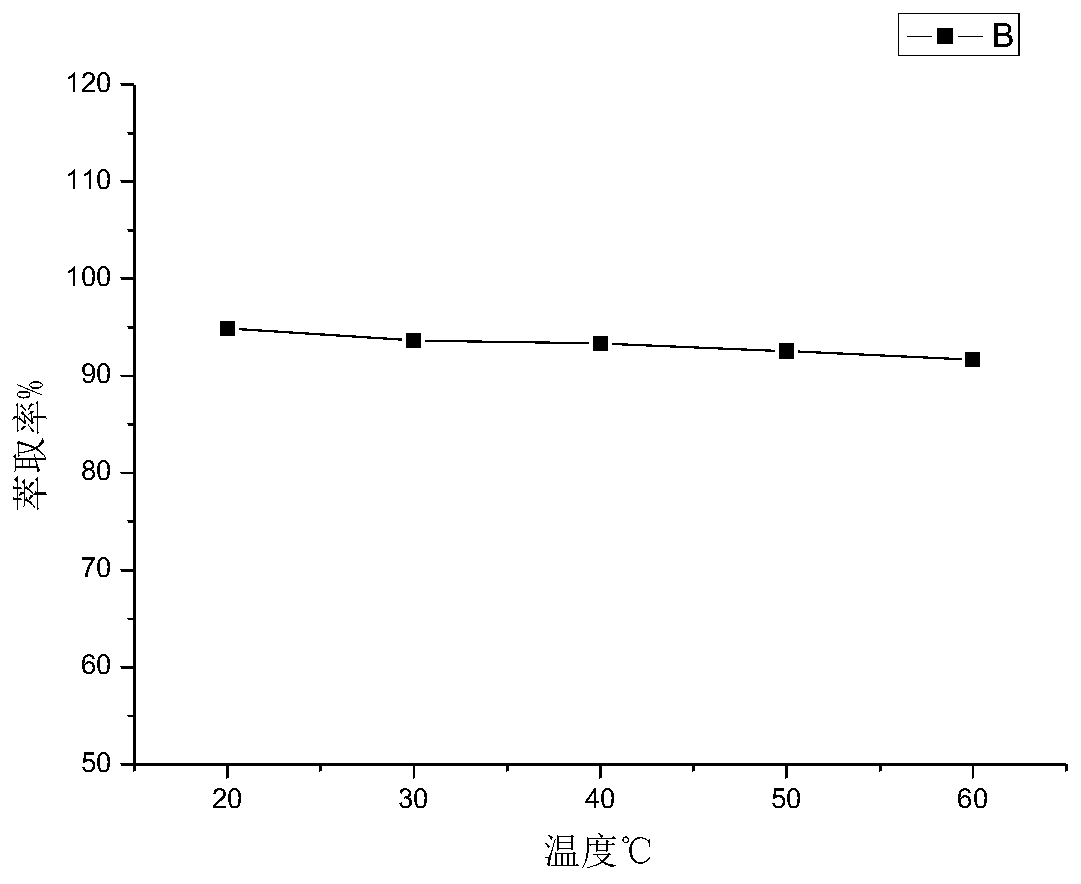
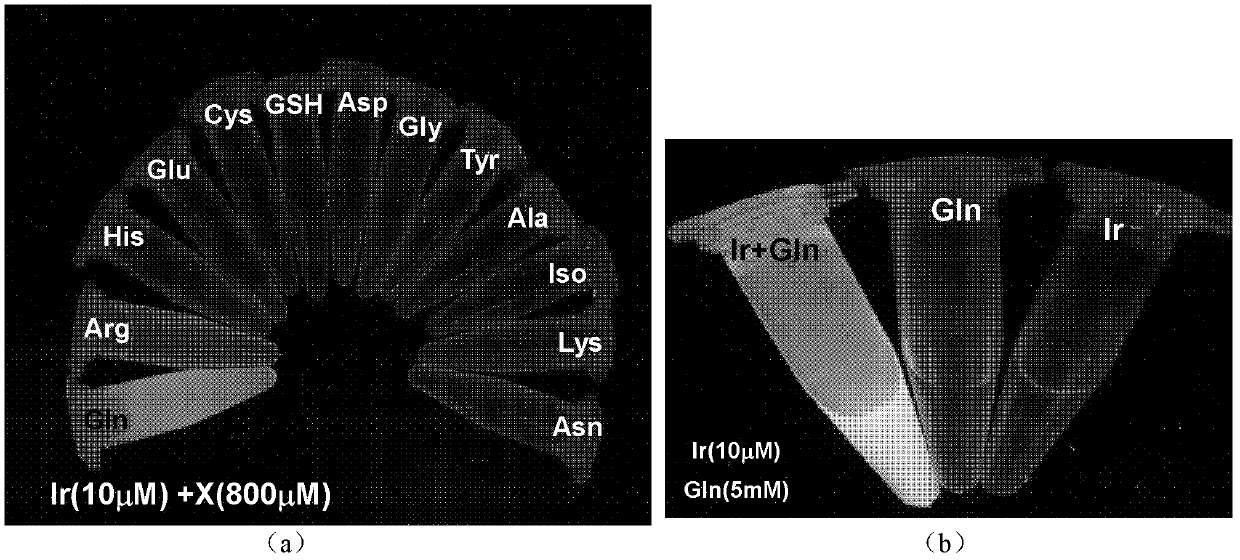
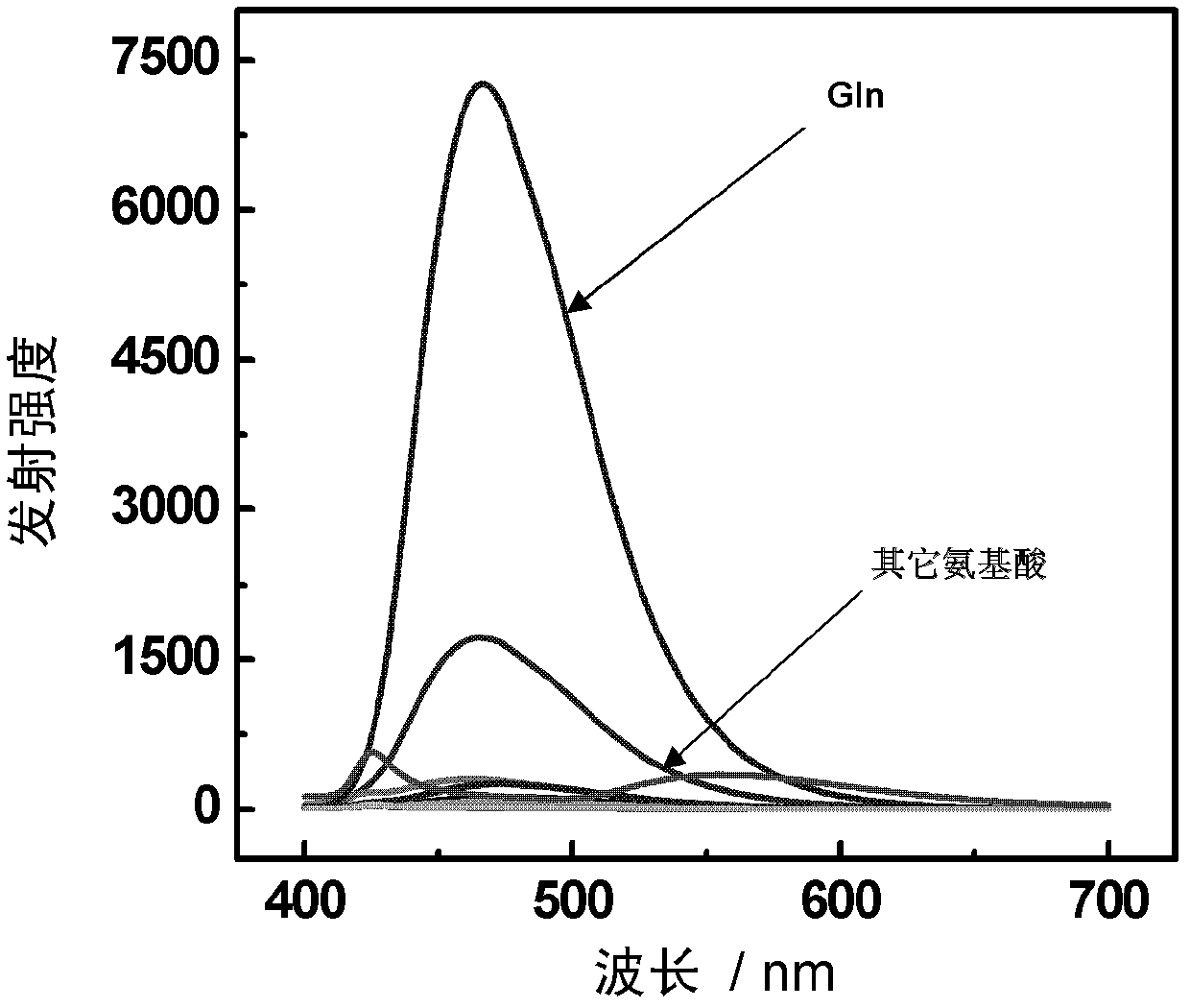
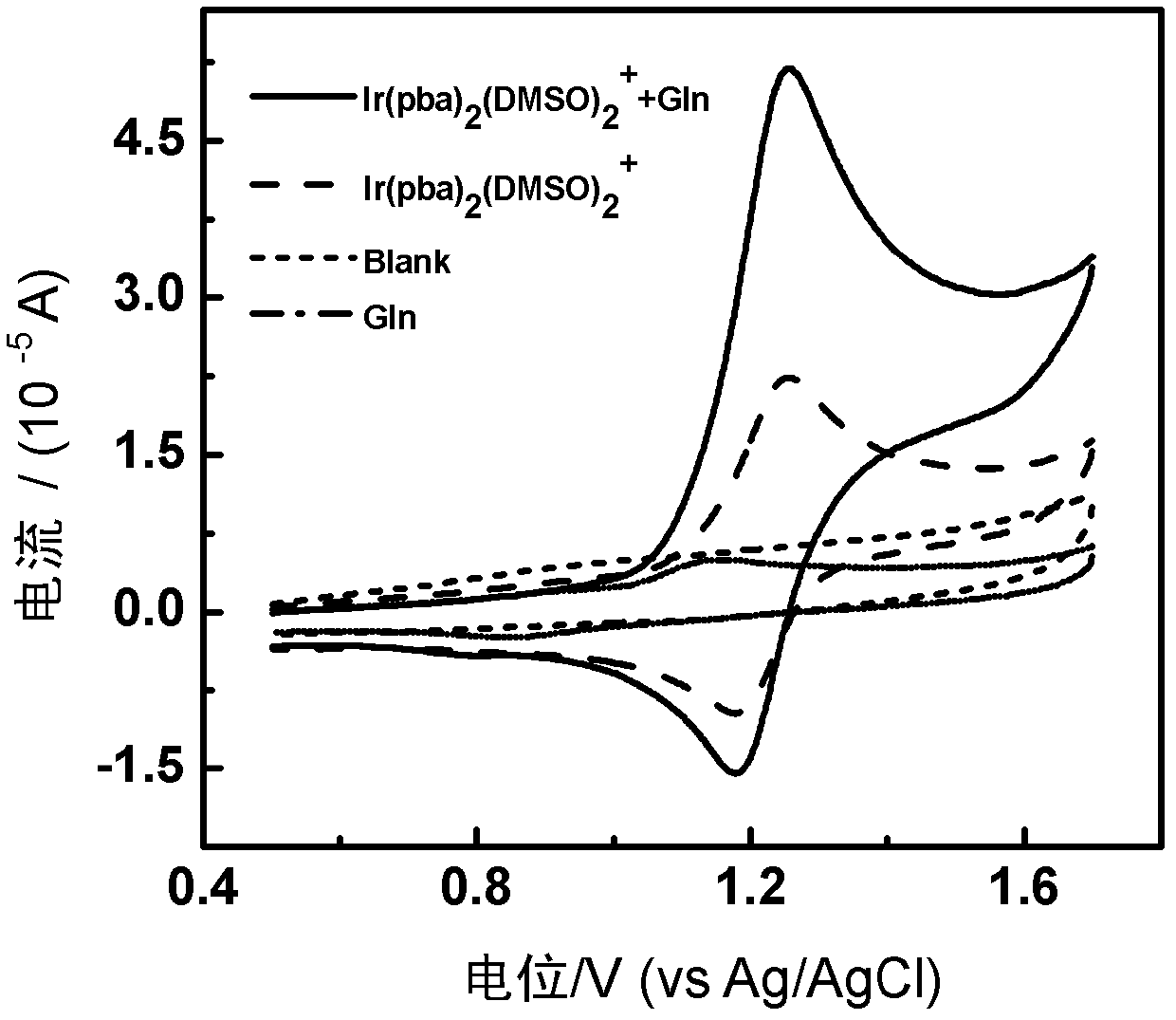
The invention discloses a phosphorescent iridium complex, and a preparation method and application thereof. A chemical formula of the phosphorescent iridium complex is [Ir(pba)2(DMSO)2]PF6 and a structural formula of the phosphorescent iridium complex is shown as a formula (I), wherein pba is expressed as 4-(2-pyridyl)benzaldehyde, DMSO is expressed as dimethylsulfoxide. A method for preparing the phosphorescent iridium complex comprises the following steps of: reacting a complex shown as a formula (II) with the dimethylsulfoxide; and adding potassium hexafluorophosphate into a reaction system and stirring to obtain the phosphorescent iridium complex. The invention also provides application of the phosphorescent iridium complex to the detection of glutamine. By the phosphorescent iridium complex, the high-selectivity and high-sensitivity detection of the glutamine can be realized, and a possibility for constructing a fluorescent chemical sensor for detecting the glutamine in high selectivity and high sensitivity is provided; and the phosphorescent iridium complex has a high dyeing effect on the glutamine in cells, and is expected to be used for detecting the Gln in the physiological and pathological processes.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method of potassium hexafluorophosphate
PendingCN114291805AImprove product qualitySolve the messSodium/potassium compoundsPhosphorus compoundsPotassium hexafluorophosphateMicroreactor
The invention provides a potassium hexafluorophosphate preparation method, which comprises: carrying out a first reaction on hydrofluoric acid and phosphorus pentoxide to obtain a reaction solution; and carrying out a second reaction on the reaction solution and a potassium salt solution to obtain potassium hexafluorophosphate. Compared with the prior art, the preparation method of the potassium hexafluorophosphate provided by the invention has the advantages that water generated by reaction is used as a solvent, the potassium hexafluorophosphate is prepared by liquid-liquid reaction, and the problems of product impurity wrapping and low reaction conversion rate in the traditional process are solved; meanwhile, the microreactor device is adopted, so that the automation degree is high, the reaction is more sufficient, the time is shorter, the control is more accurate, and continuous production can be realized. The preparation method of potassium hexafluorophosphate provided by the invention has the advantages of simple process, controllable reaction, good product quality, no emission of three wastes and the like, and is easy to industrially implement and popularize.
Owner:DO FLUORIDE CHEM CO LTD
Synthesis method of high-purity crown ether functional ionic liquid
PendingCN110372678AImprove stabilityHigh purityOrganic chemistryPotassium hexafluorophosphateSynthesis methods
The invention discloses a synthesis method of high-purity crown ether functional ionic liquid, and belongs to the technical field of organic synthesis. The problems that in synthesis of exiting crownether functional ionic liquid, the synthesis process is complex, the product purity is low, and the exiting crown ether functional ionic liquid cannot be used for recovery of metal ions and organic small molecules in a water phase can be solved. The synthesis method comprises the steps that firstly, benzo-crown ether and bromoalkyl acid are subjected to benzene ring carbon acylation reaction underthe catalysis effect of polyphosphoric acid, and an intermediate product I is produced; the intermediate product I and 1-methylimidazole are subjected to quaternization reaction, and an intermediateproduct II is produced; the intermediate product II is purified; and the intermediate product II and lithium / sodium / potassium bis(trifluoromethanesulphonyl)imide (lithium / sodium / potassium hexafluorophosphate) are dissolved in water for anion exchange, and through washing and vacuum drying, a product is obtained. The synthesis method is adopted to prepare the crown ether functional ionic liquid, operation is easy, and the synthesized ionic liquid is high in purity and good in hydrophobicity, and can be used for selective extraction and separation of the metal ions and the organic matter molecules from the water phase.
Owner:SHANXI UNIV
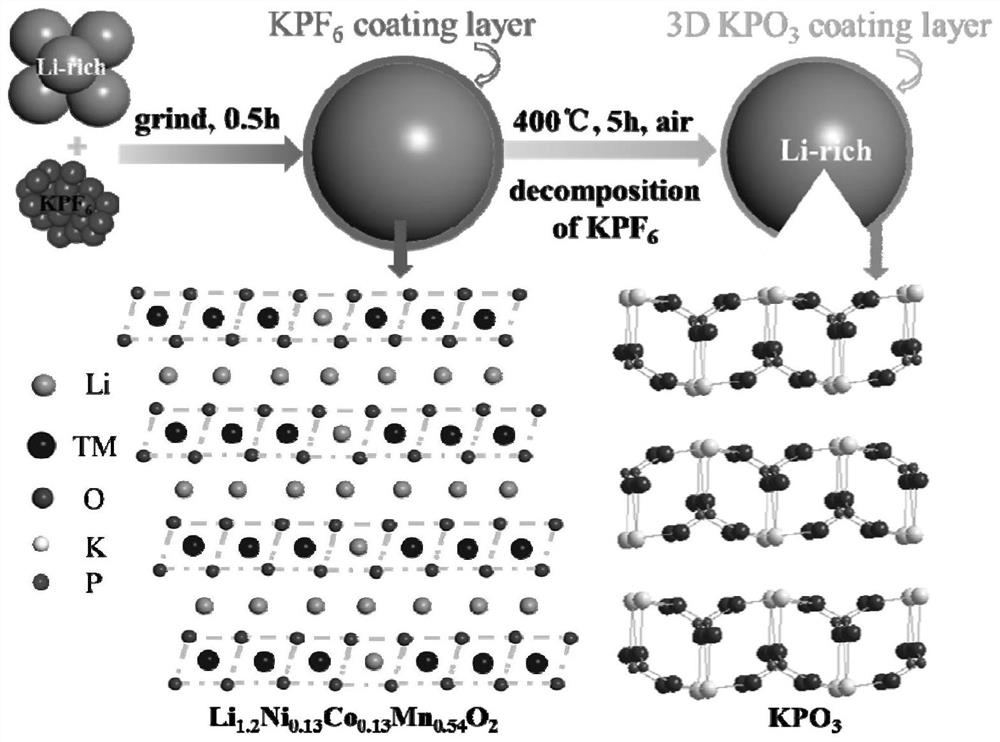
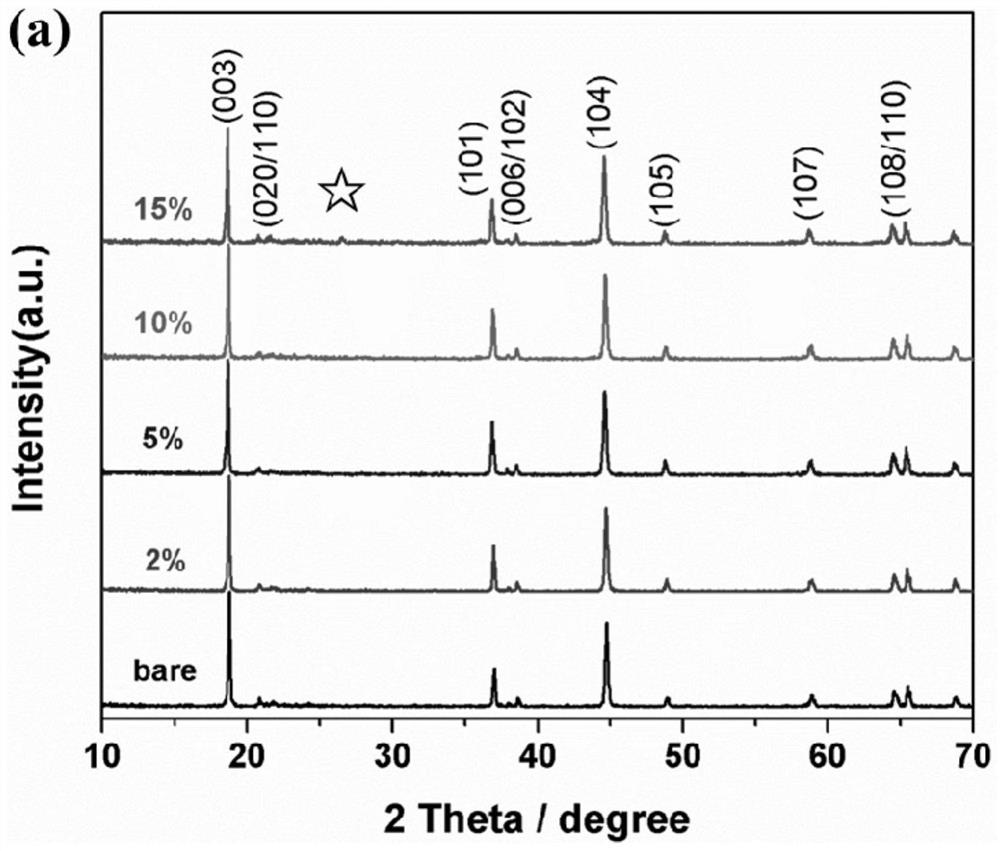
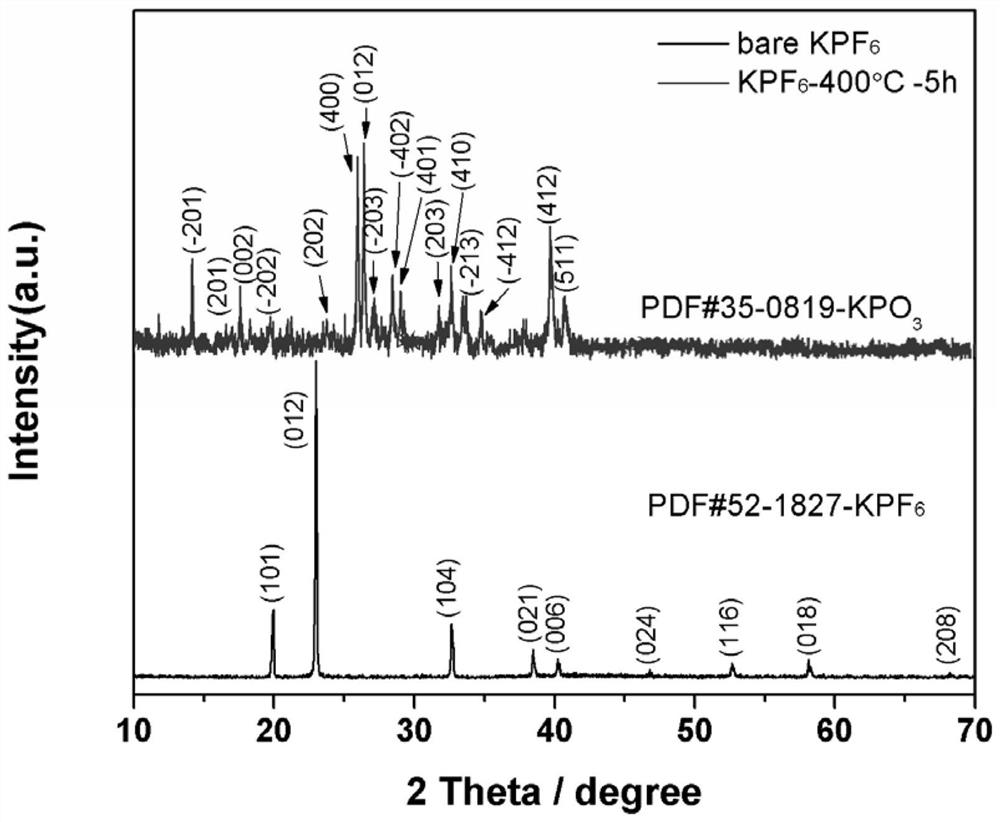
Positive electrode material, modification method thereof and battery
InactiveCN112242502AImprove the first Coulombic efficiencyImprove cycle stabilityFinal product manufactureCell electrodesPotassium hexafluorophosphateElectrical battery
The invention provides a modification method of a positive electrode material. The modification method comprises the following steps: preparing lithium-rich phase powder containing lithium, nickel, cobalt, manganese and oxygen; mixing a coating material with a dispersing agent and a solvent, ultrasonically dispersing, dropwise adding into the lithium-rich phase powder, grinding to be dry, and calcining to obtain a coating product, wherein the coating material is selected from one or more of potassium hexafluorophosphate and potassium metaphosphate. The modification method comprises the following steps: performing surface modification treatment on xLi2MnO3. (1-x) LiNiyCozMn1-y-zO2 lithium-rich phase powder through potassium hexafluorophosphate and / or potassium metaphosphate, and performingin-situ decomposition on the potassium hexafluorophosphate at high temperature to form a potassium metaphosphate coating layer with a three-dimensional structure. The first coulombic efficiency, the cycling stability and the rate capability of the lithium-rich phase material subjected to surface coating treatment are all improved, so that relatively good electrochemical comprehensive performance is obtained, and the development requirements of high-power electronic equipment can be met. The preparation method of the positive electrode material is simple, wide in application range, low in costand suitable for industrial production.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com