Method for separating and determining isoxepac and related substances of isoxepac by HPLC (High Performance Liquid Chromatography) method
A technology related to substances, isoxic acid, applied in the field of analytical chemistry, to achieve the effect of strong specificity, accurate sensitivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
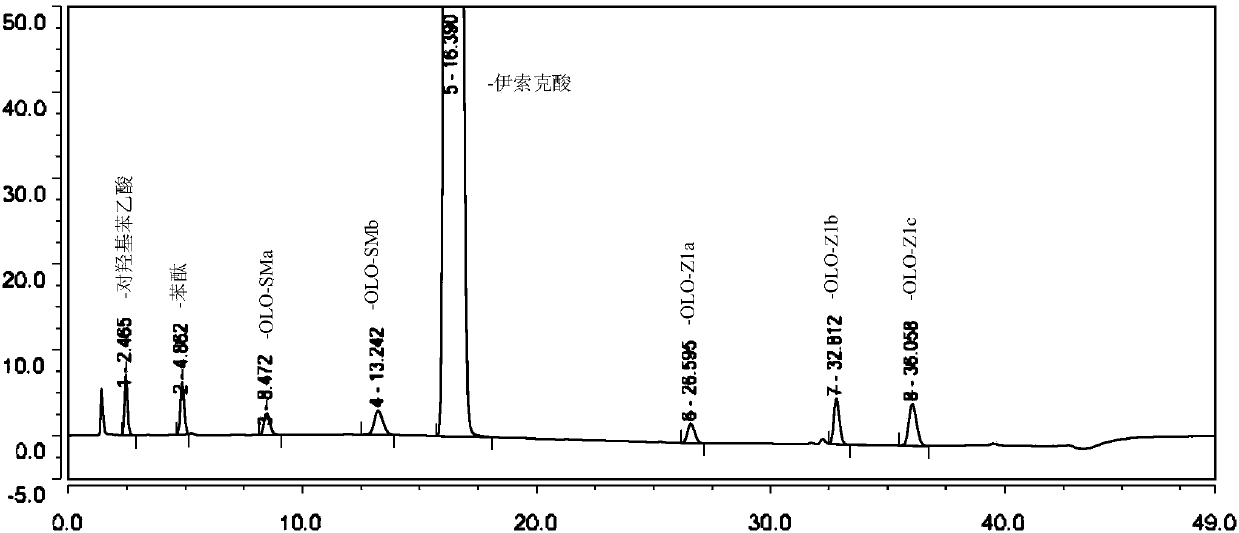
[0057] The mensuration of embodiment 1 isoket acid related substances
[0058] 1. Instruments and conditions
[0059] Instrument: High performance liquid chromatography SHIMADZU LC-20A (LC2019);
[0060] Detector: UV detector;
[0061] Chromatographic column: Octylsilane bonded silica gel filler (Agilent Eclipse XDB-C8, 150mm×4.6mm, 5μm);
[0062] Mobile phase: mobile phase A: 0.02mol / L potassium hexafluorophosphate (adjust the pH value to 3.0 with phosphoric acid); mobile phase B: acetonitrile; use mobile phase A and B for linear gradient elution;
[0063] Detection wavelength: 220nm;
[0064] Column temperature: 25°C;
[0065] Flow rate: 1.0ml / min;
[0066] Injection volume: 20 μl.
[0067] The linear gradient elution conditions of mobile phase A and B are:
[0068]
[0069] 2. Experimental steps
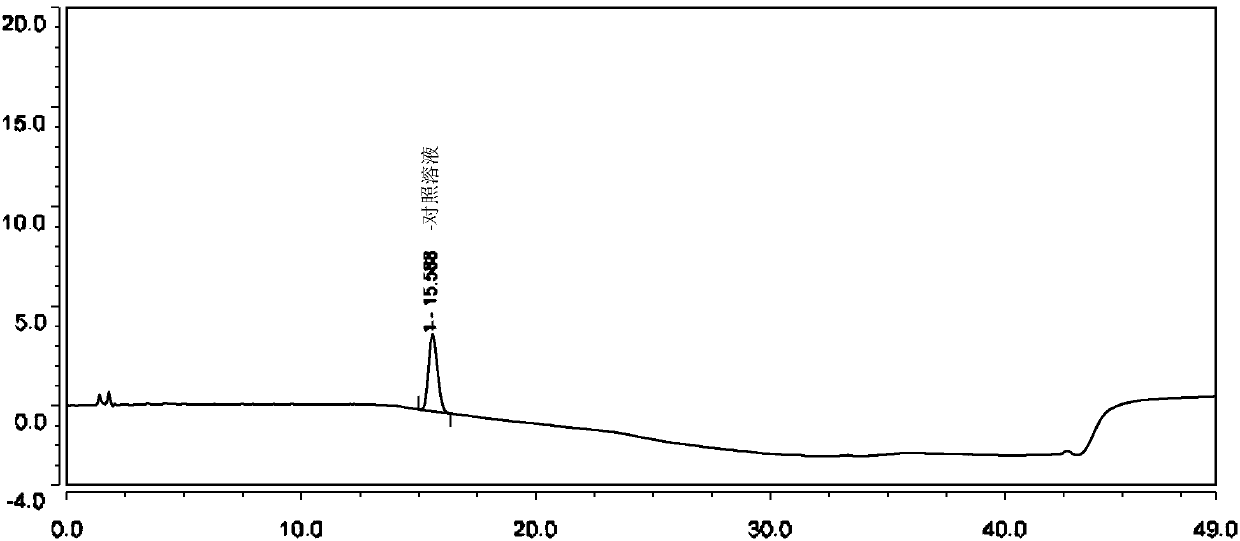
[0070] (1) Impurity mixed stock solution: Accurately weigh 12.46mg of p-hydroxyphenylacetic acid, 12.53mg of phthalide, 12.44mg of OLO-SMb, 12.83mg of OLO-Z1a, 12.35mg of...
Embodiment 2
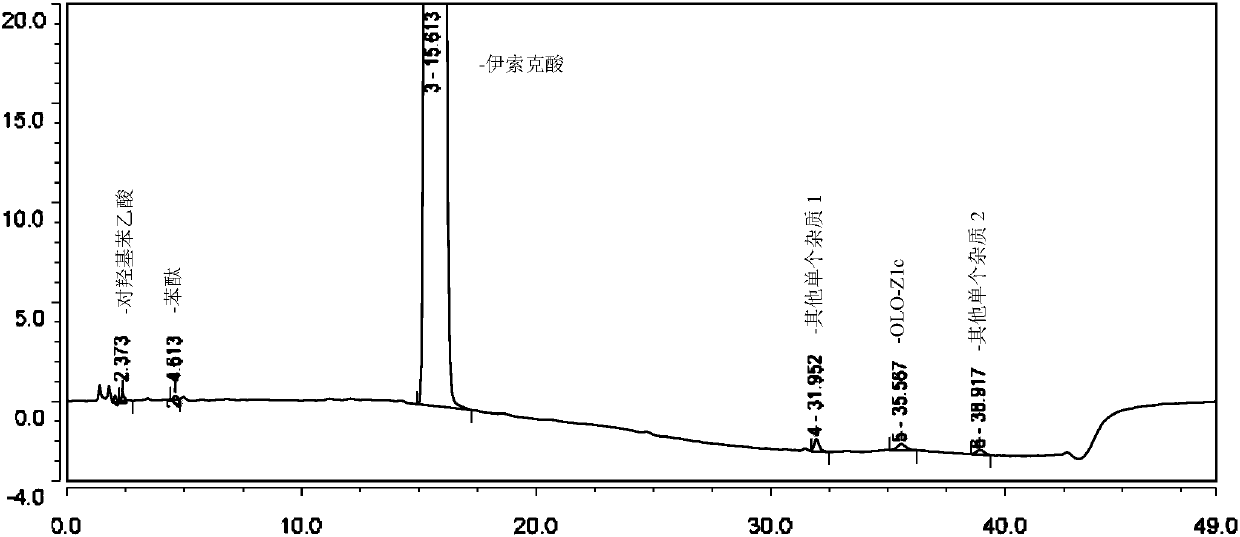
[0086] Example 2 Multiple batches of isoket acid related substances determination results
[0087] According to the method of Example 1, several batches of isoket acid samples of our company were tested for related substances. The calculation results are shown in the table below.
[0088]
[0089]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com