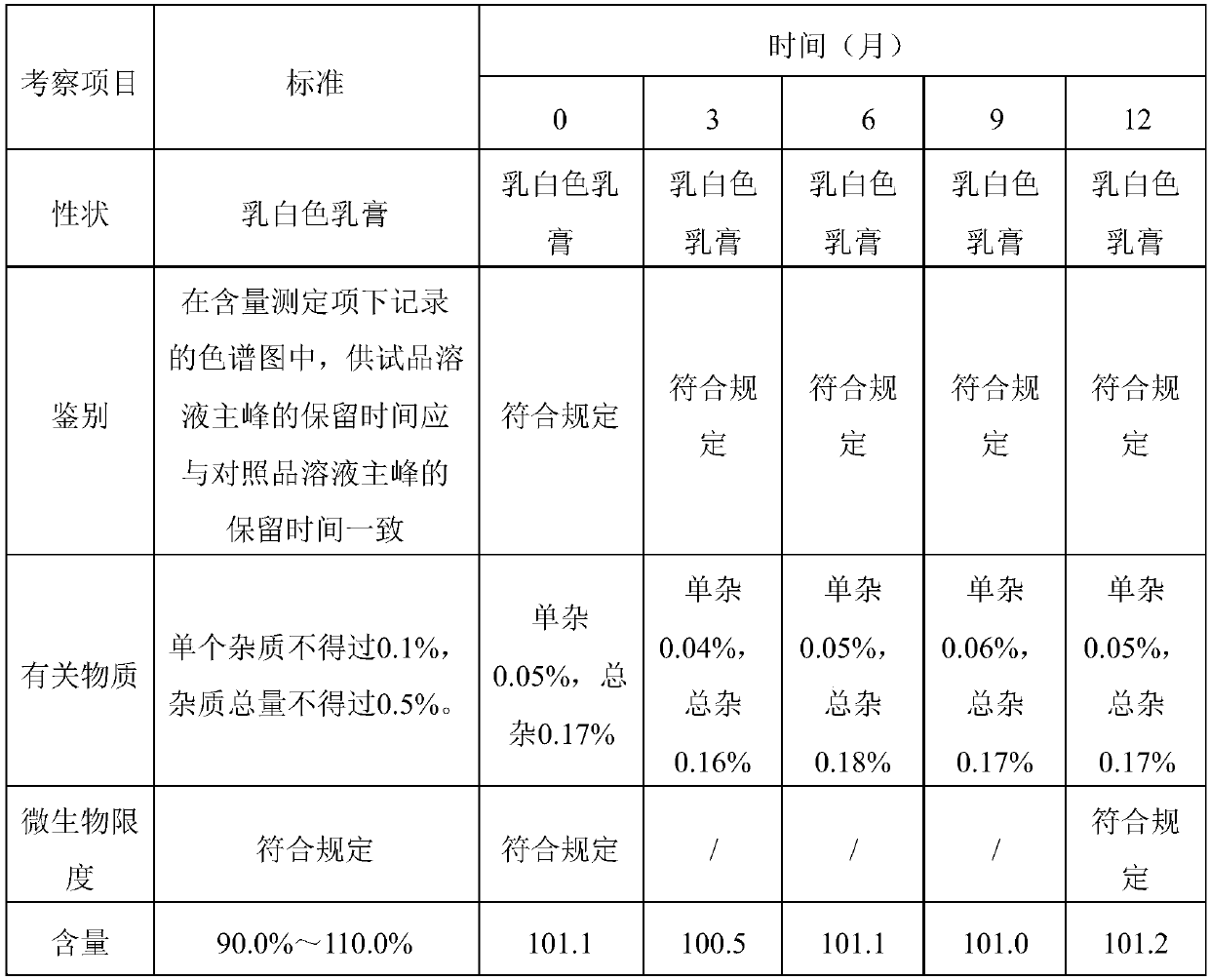
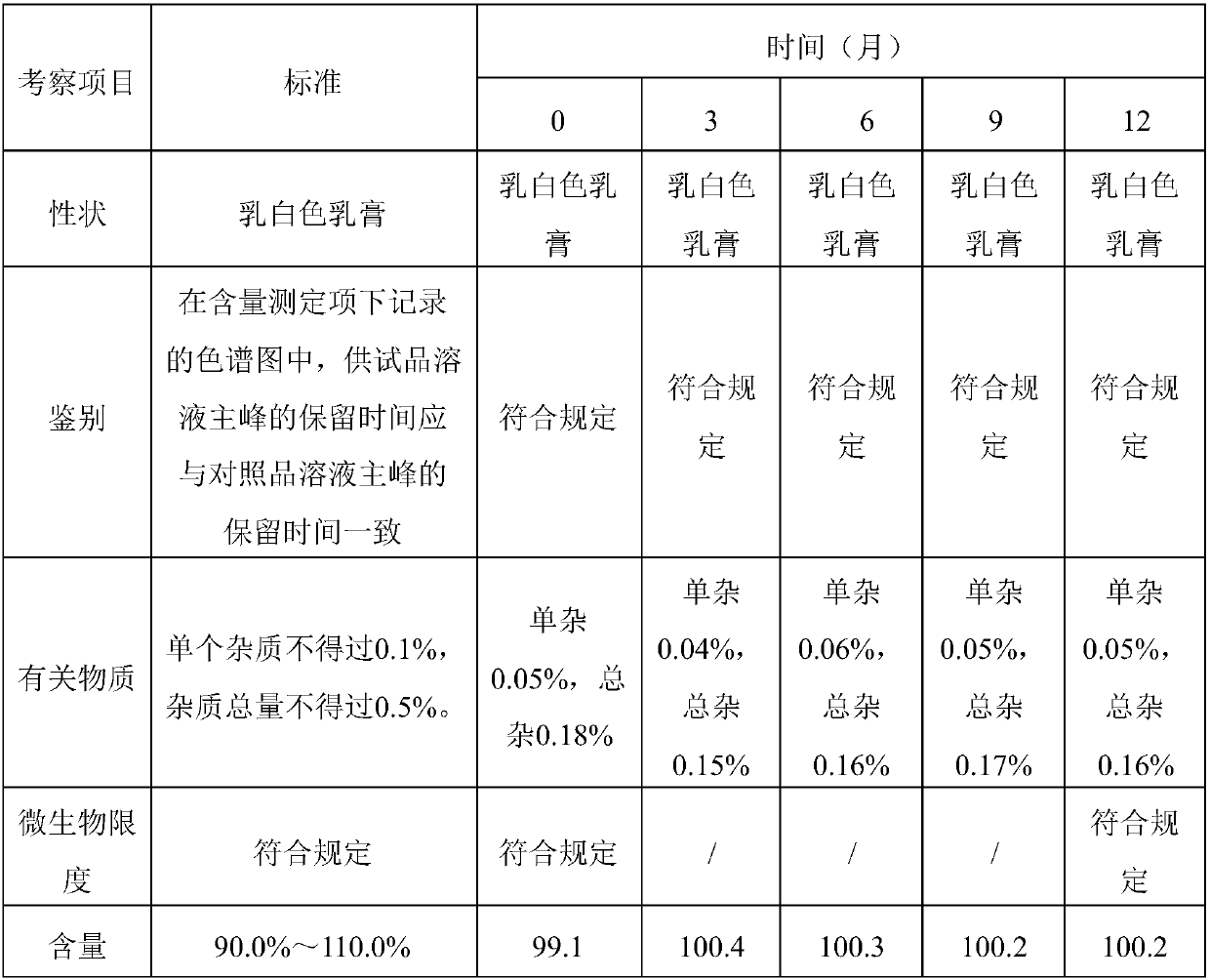
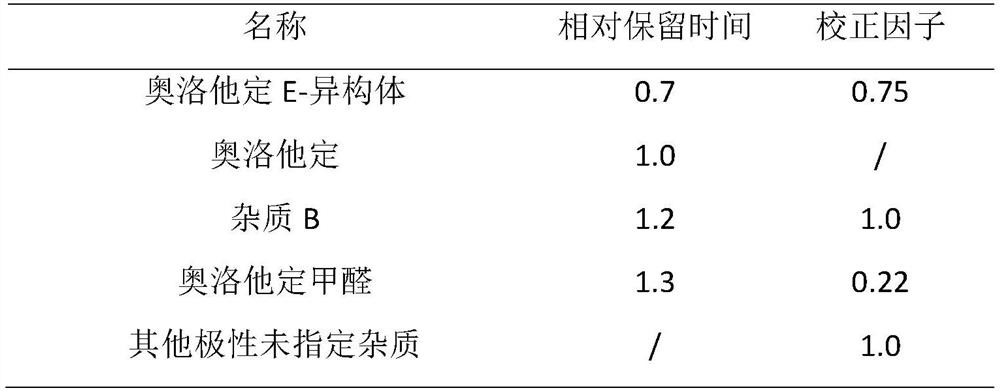
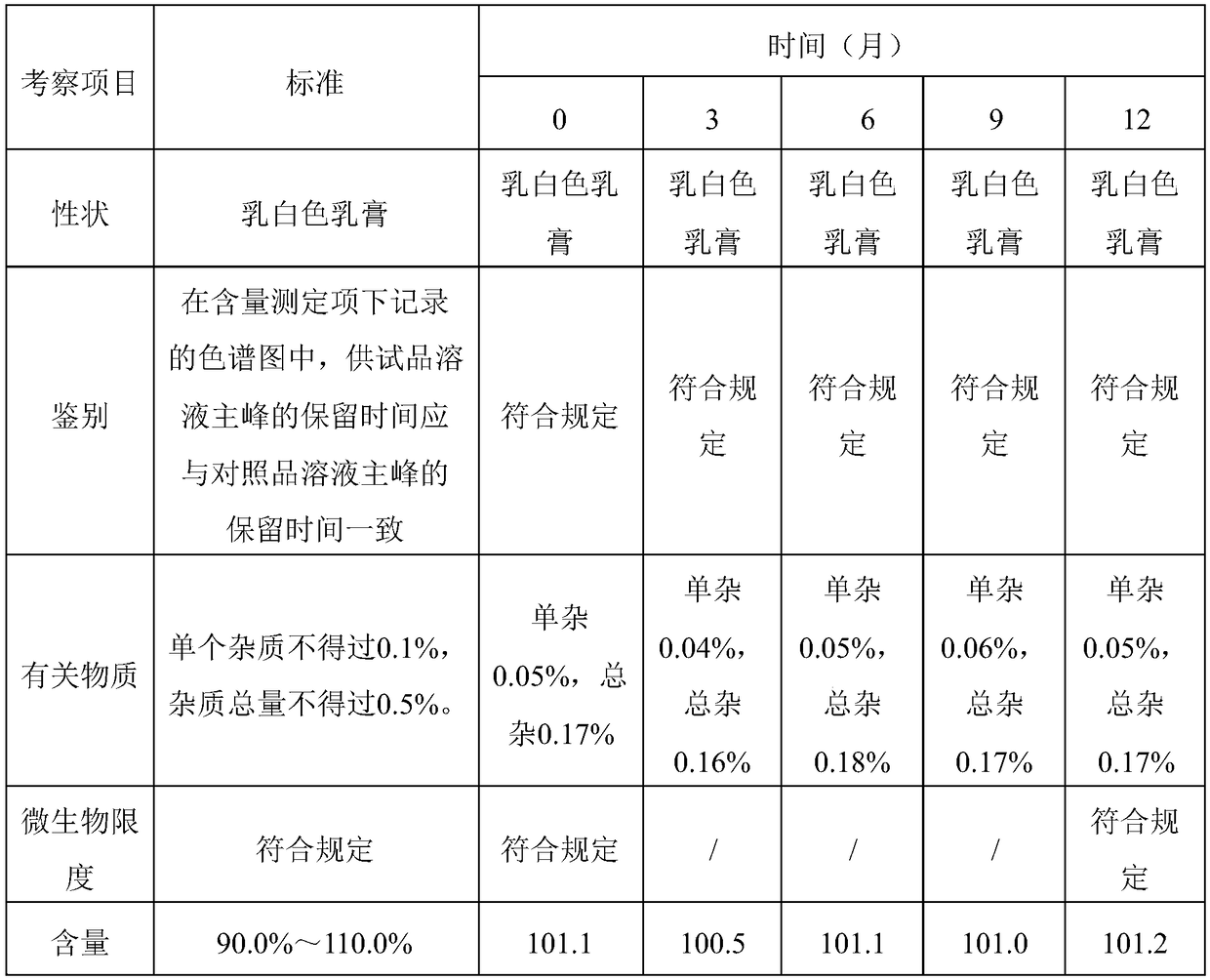
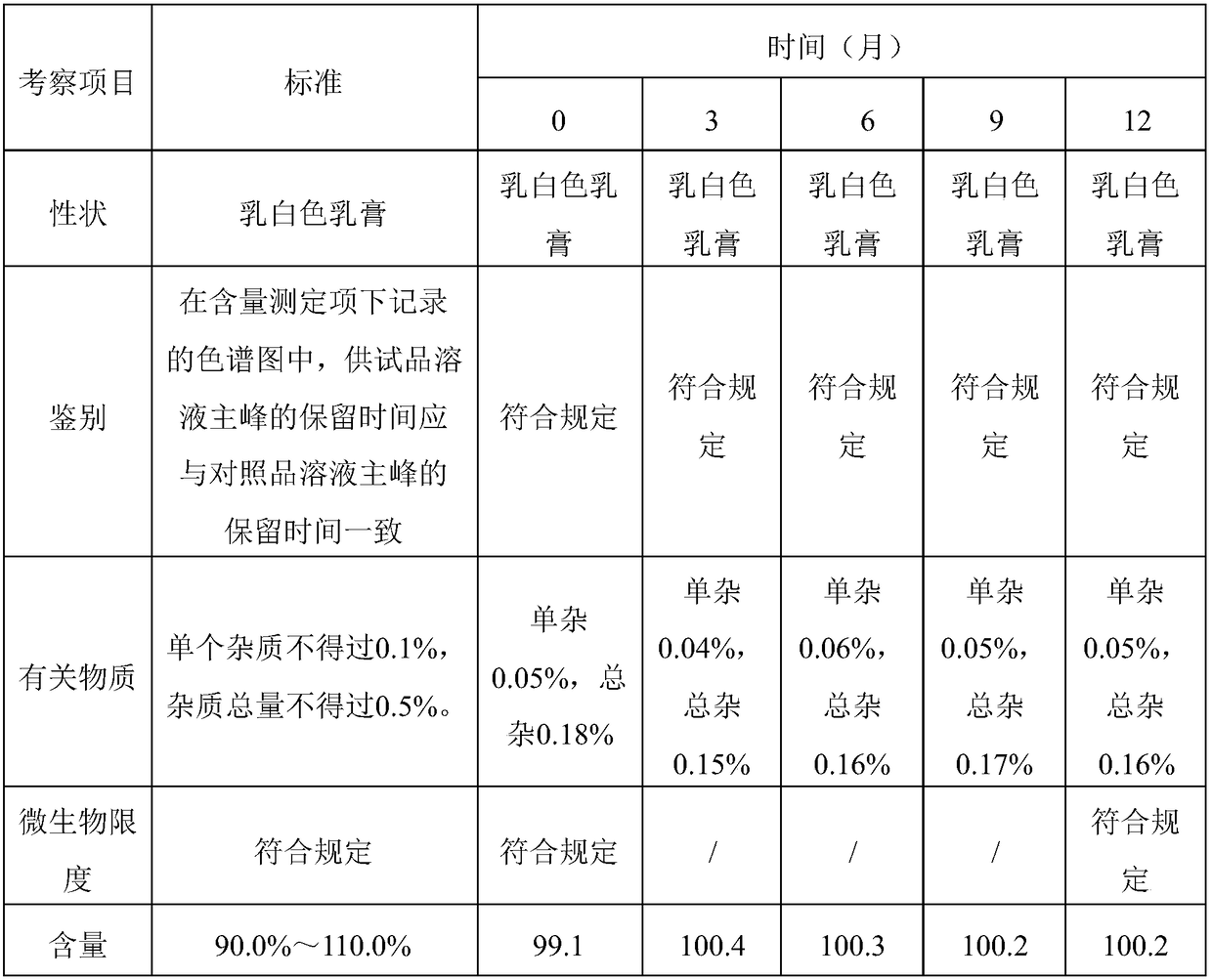
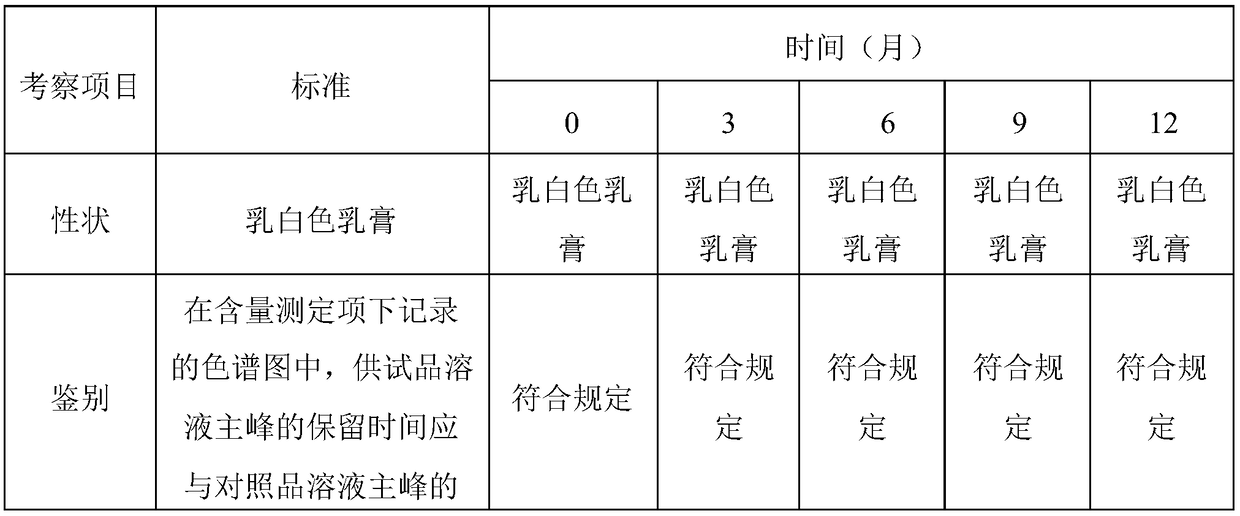
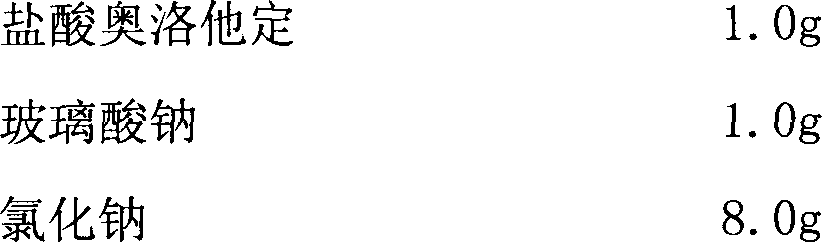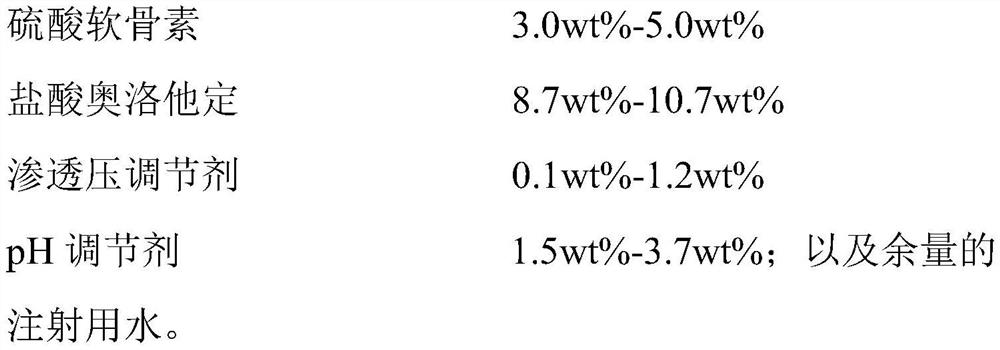Patents
Literature
39 results about "Olopatadine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
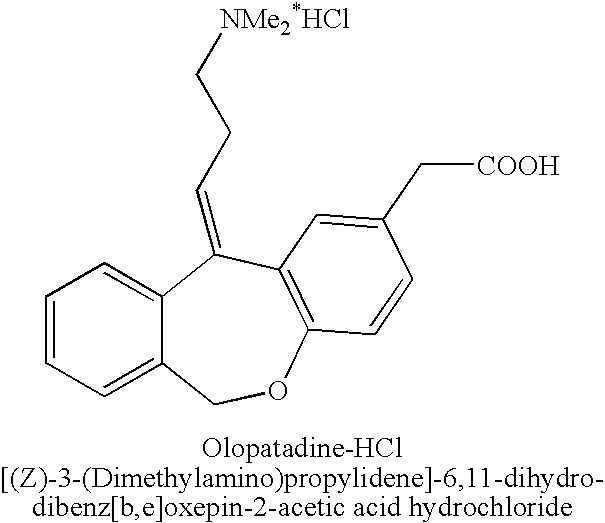
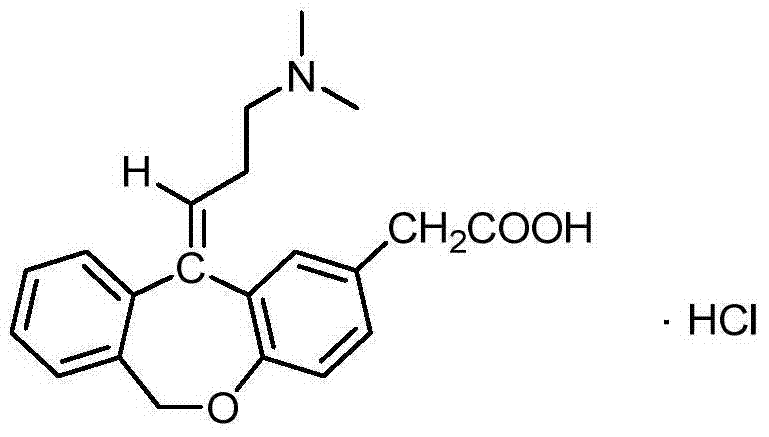
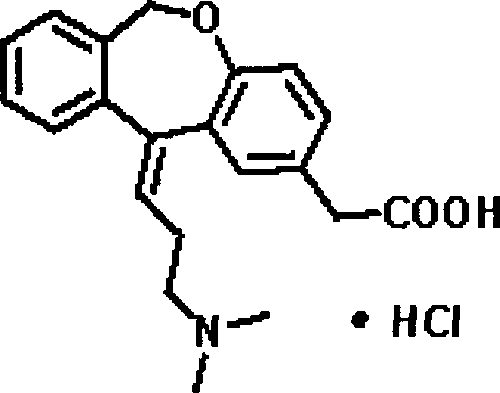
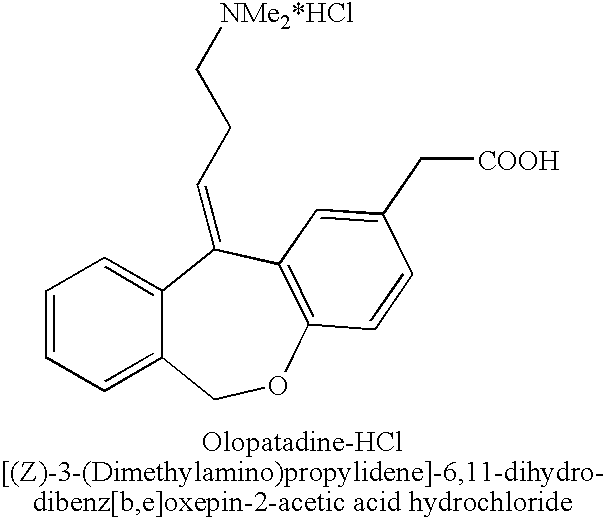
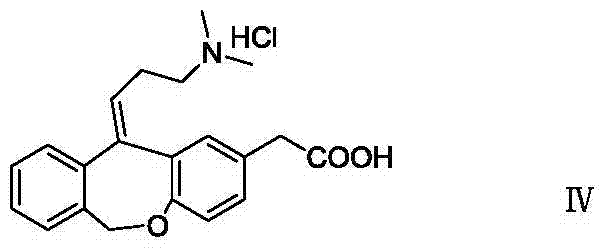
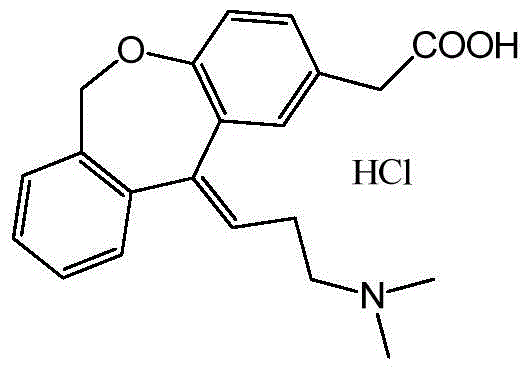
The hydrochloride salt form of olopatadine, a dual action selective histamine H1 receptor antagonist and mast cell stabilizer with anti-allergic activity. Olopatadine stabilizes mast cells and prevents histamine release from mast cells. In addition, this agent also blocks histamine H1 receptors, thereby preventing histamine from binding to these receptors. Both actions prevent the effects of histamine on capillaries, bronchial smooth muscle, and gastrointestinal (GI) smooth muscle, including histamine-induced vasodilation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of GI smooth muscle. This drug also prevents histamine-induced pain and itching of mucous membranes.
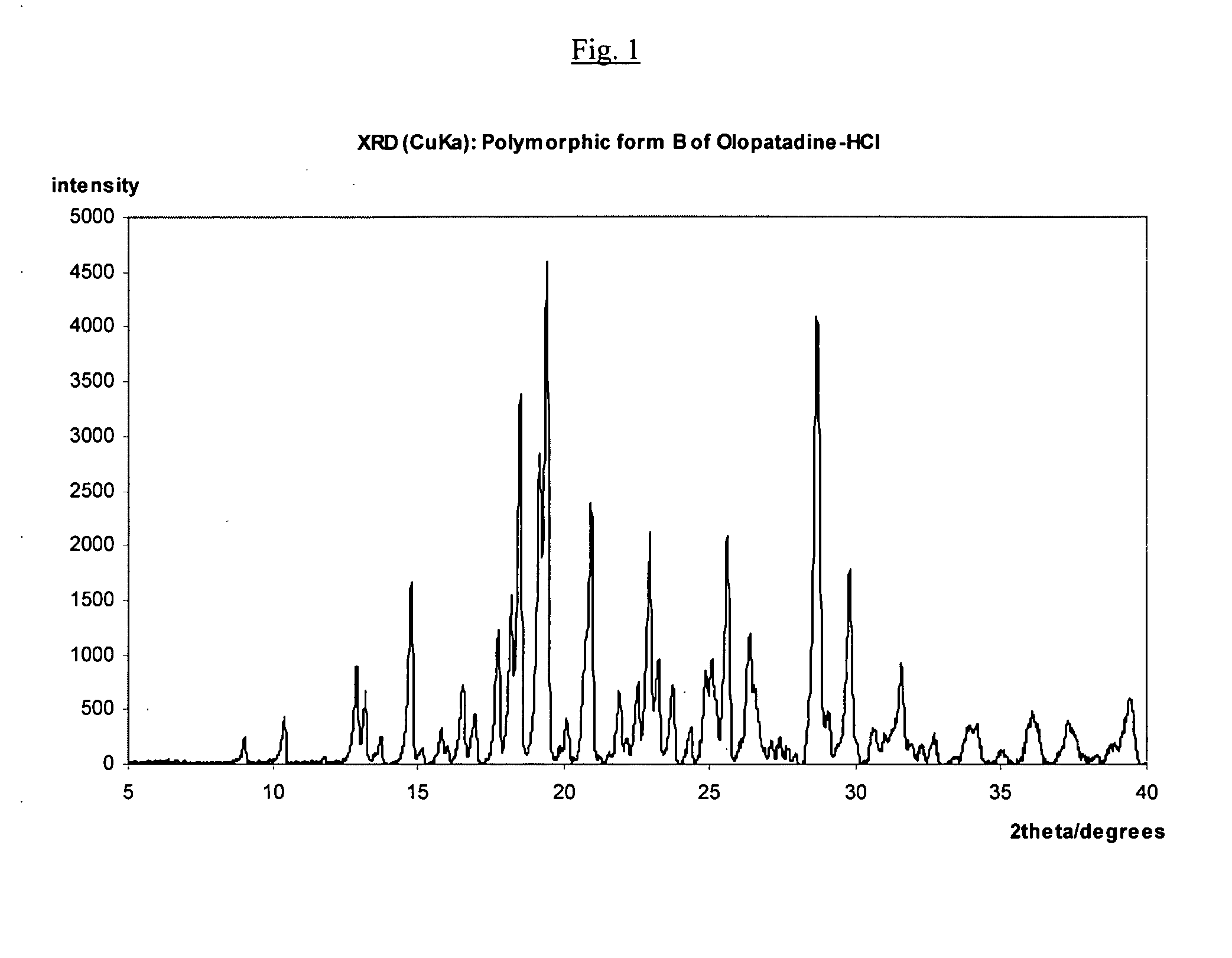
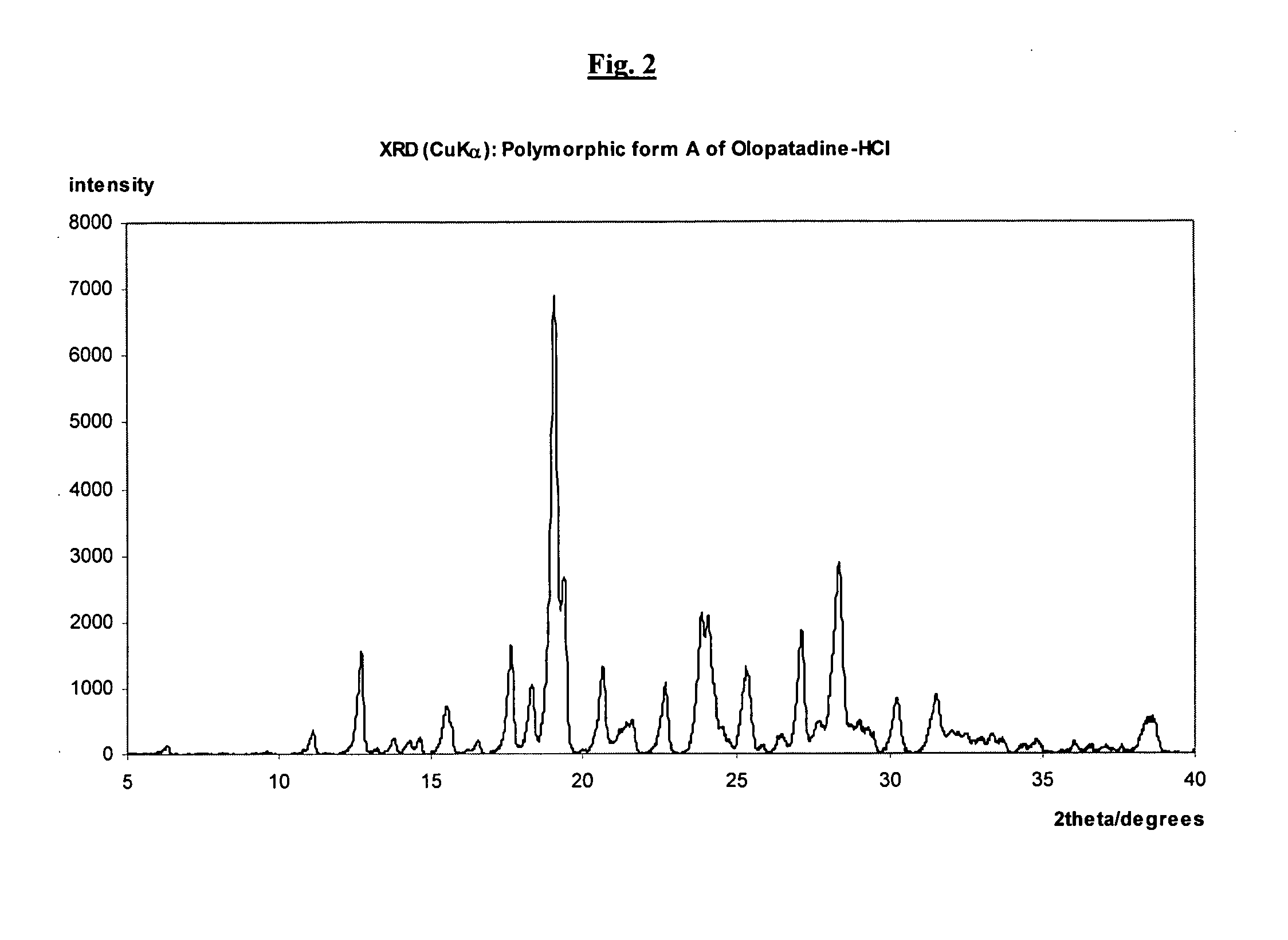
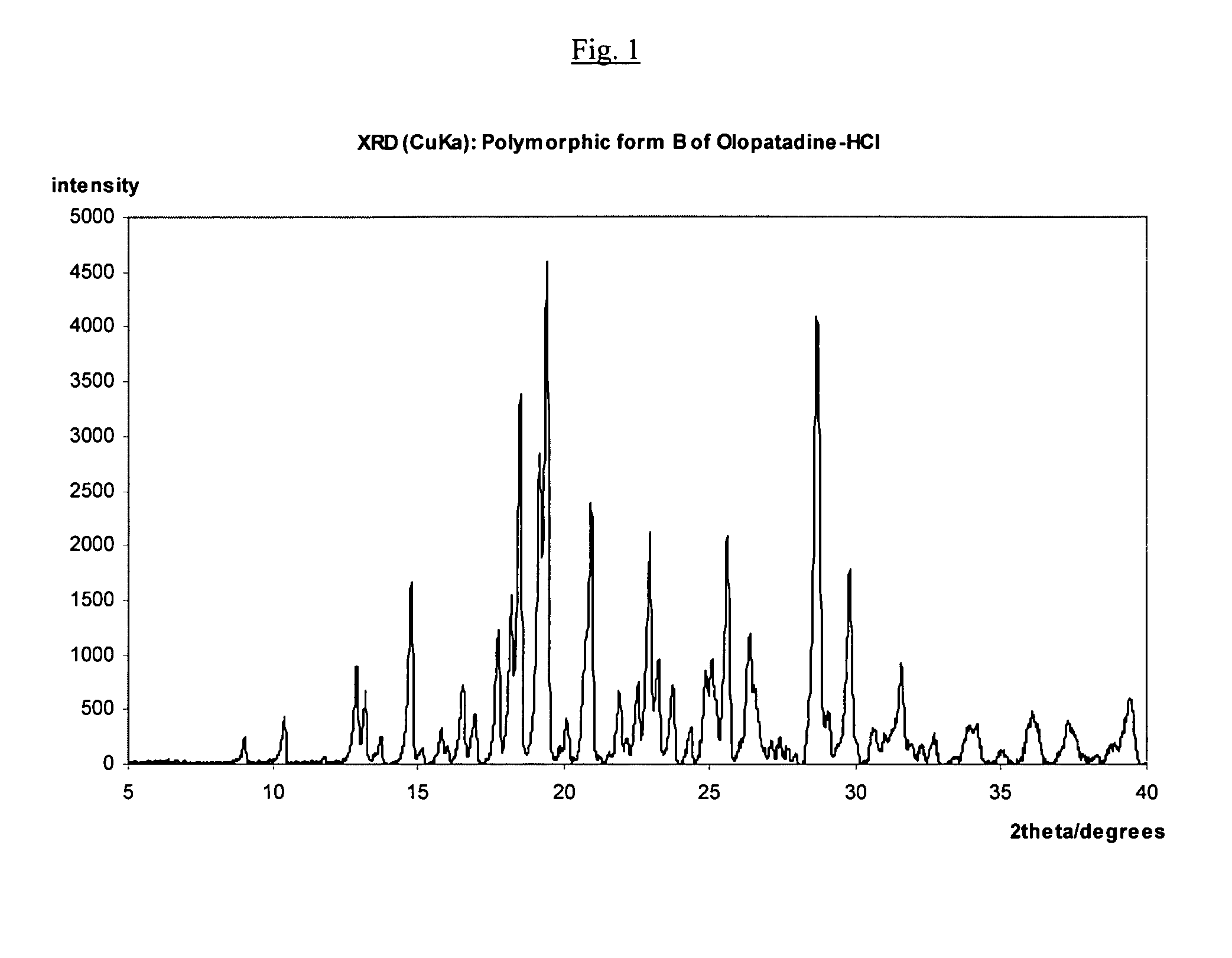
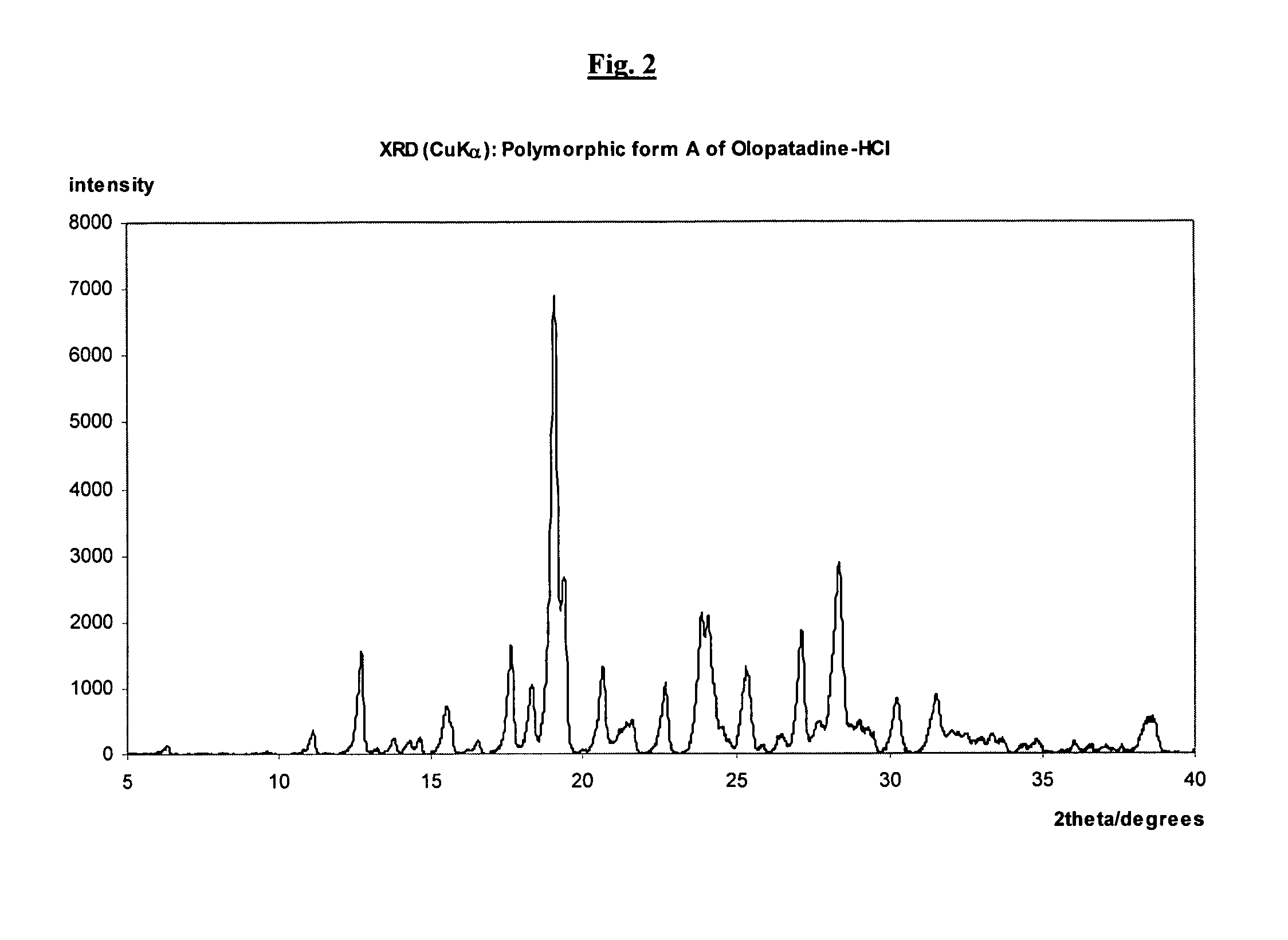
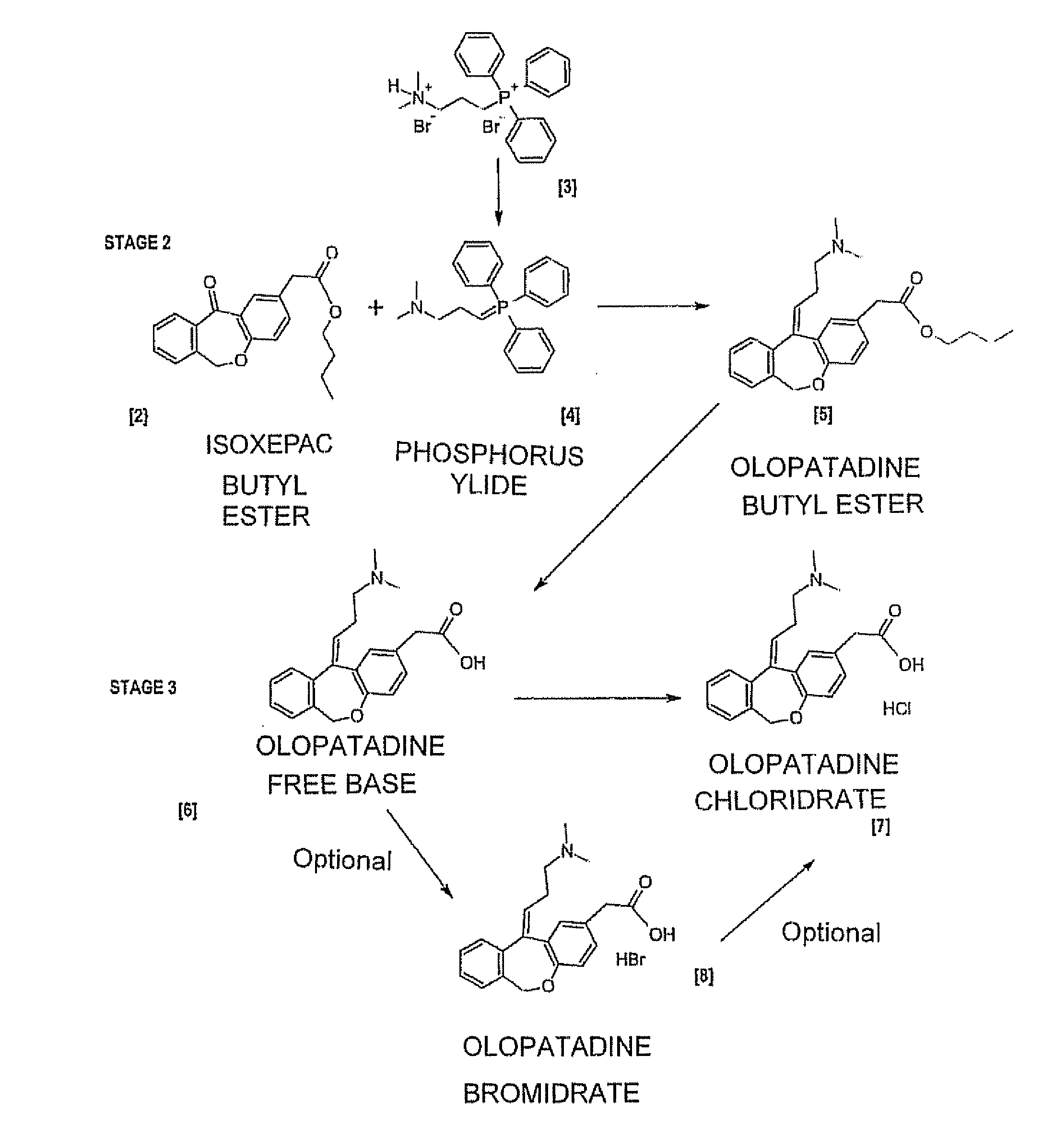
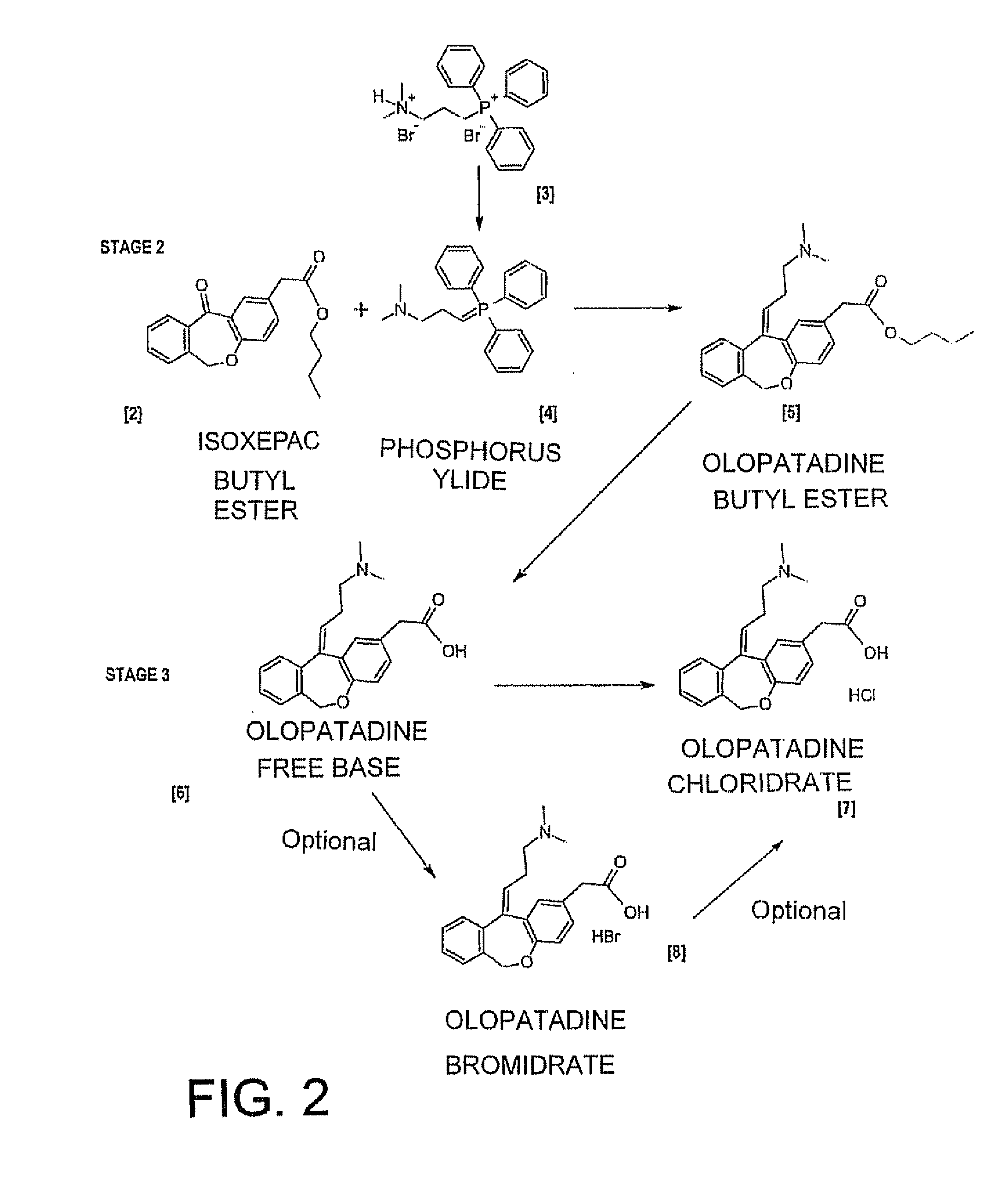
Polymorphic forms of olopatadine hydrochloride and methods for producing olopatadine and salts thereof
ActiveUS20070232814A1Senses disorderGroup 5/15 element organic compoundsHydrobromideSeasonal allergic conjunctivitis
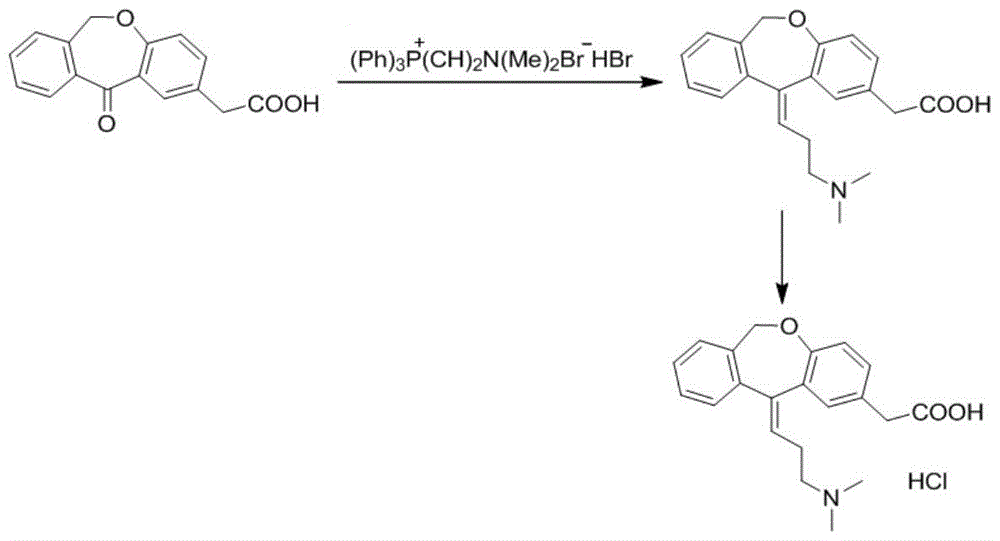
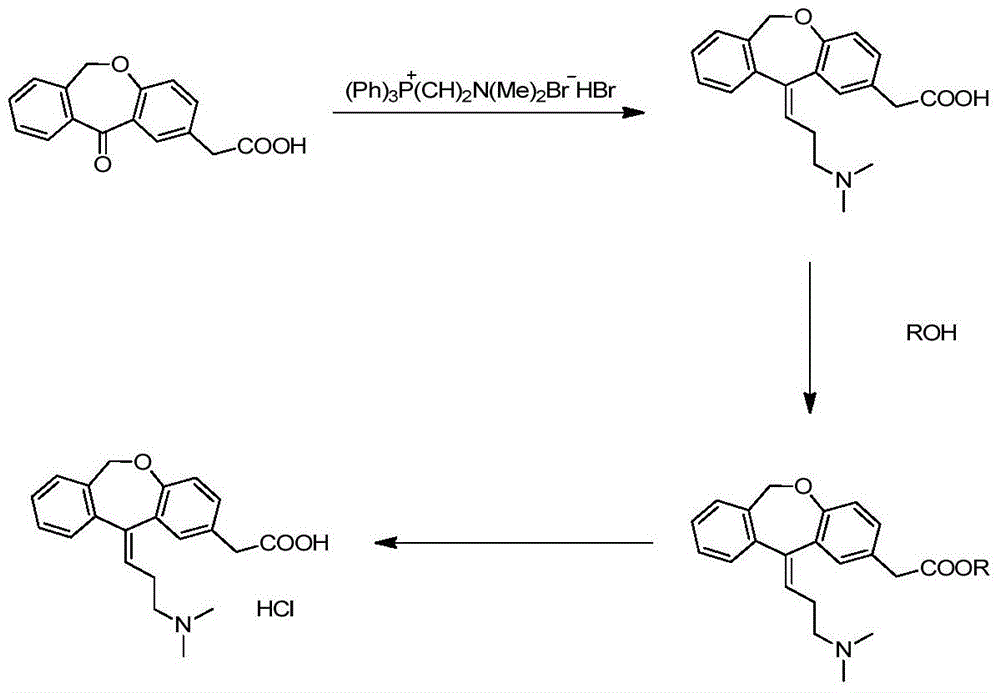
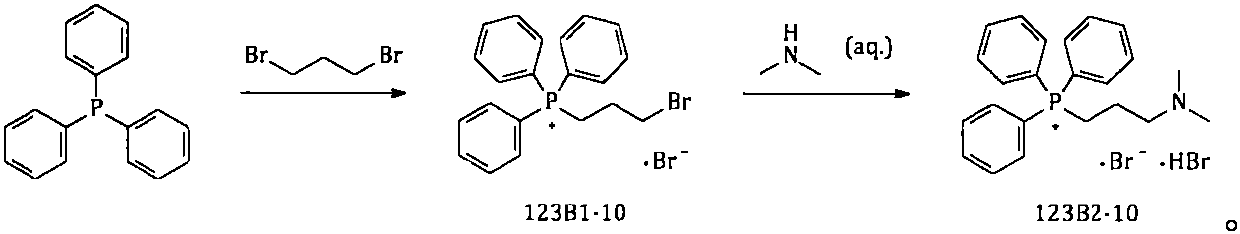
The present invention provides a novel polymorphic form of olopatadine hydrochloride ([(Z)-3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride), a selective histamine H1-receptor antagonist that is used for the treatment of ocular symptoms of seasonal allergic conjunctivitis. The present invention also provides novel methods for producing olopatadine on a large scale, and in a manner that is cost effective, provides a low level of impurities and eliminates the need to use the costly and dangerous base, butyllithium, which is used in prior art reactions for making olopatadine. The present invention further provides novel processes for carrying out a large scale production of 3-dimethylaminopropyltriphenylphosphonium bromide and its corresponding hydrobromide salt, which are employed in the production of olopatadine, and pharmaceutically acceptable salts of olopatadine.
Owner:UNIV ZURICH
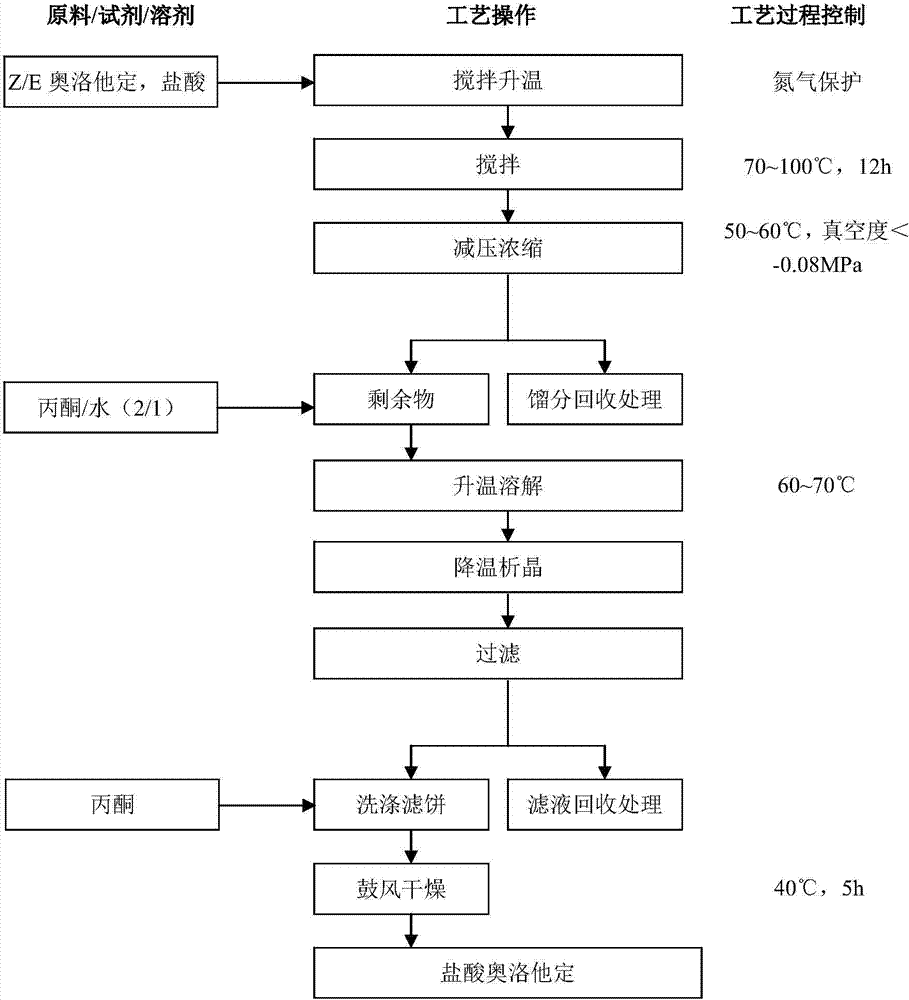
Method for preparing olopatadine hydrochloride
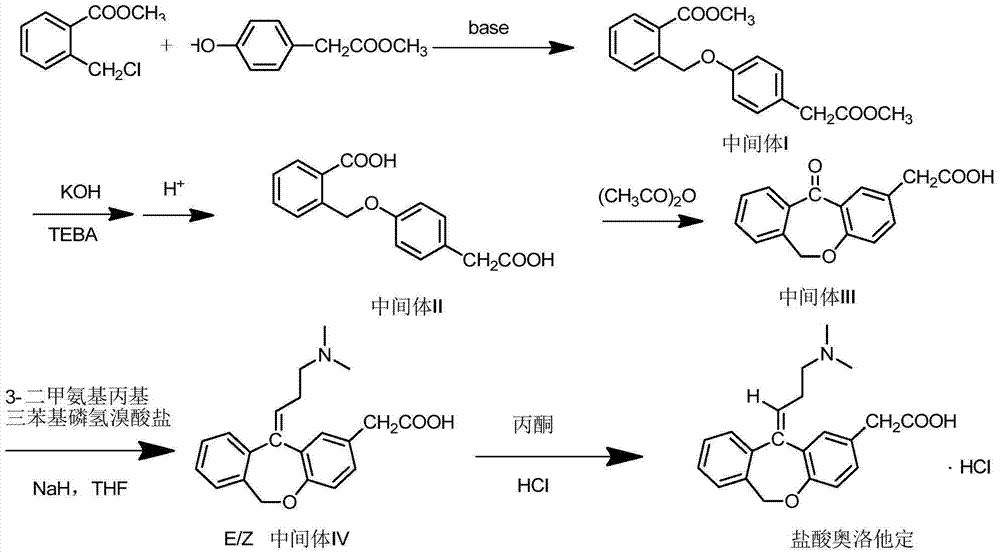
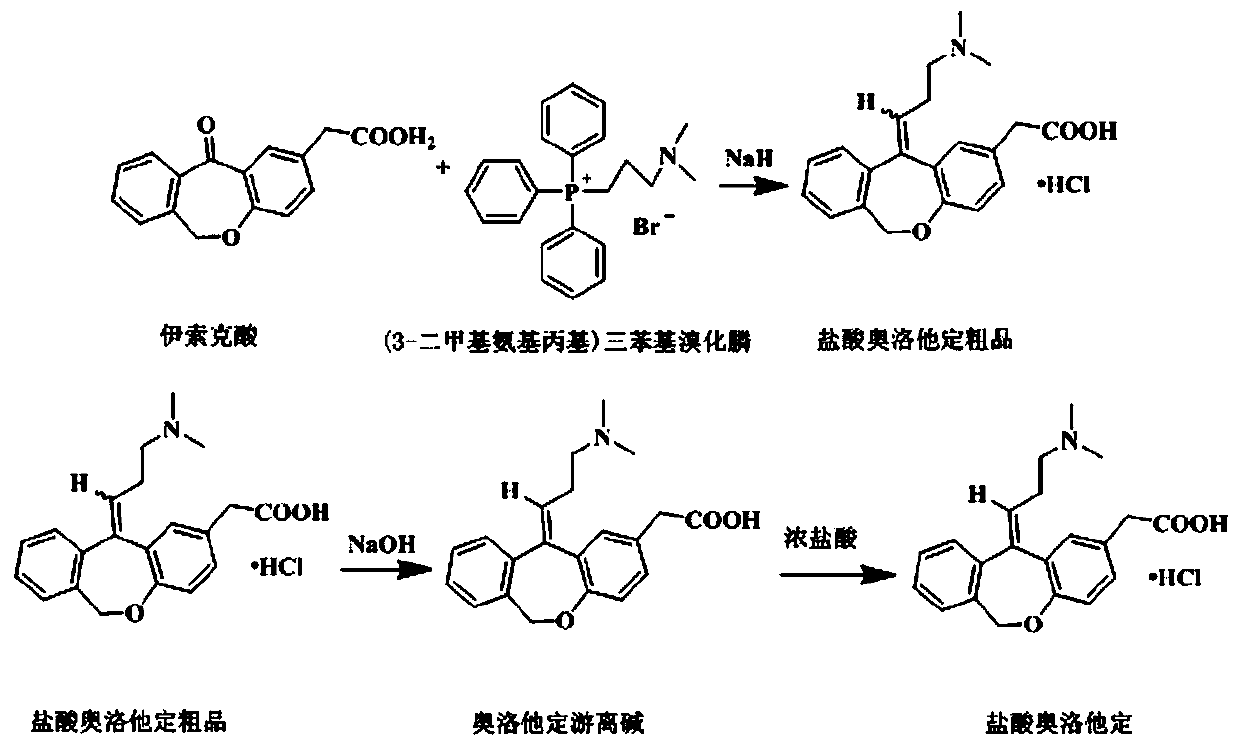
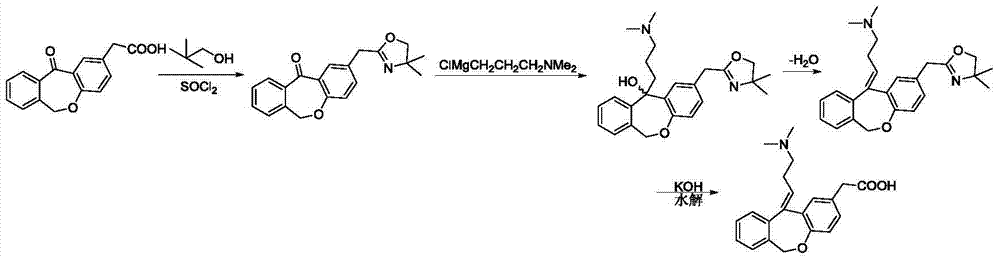
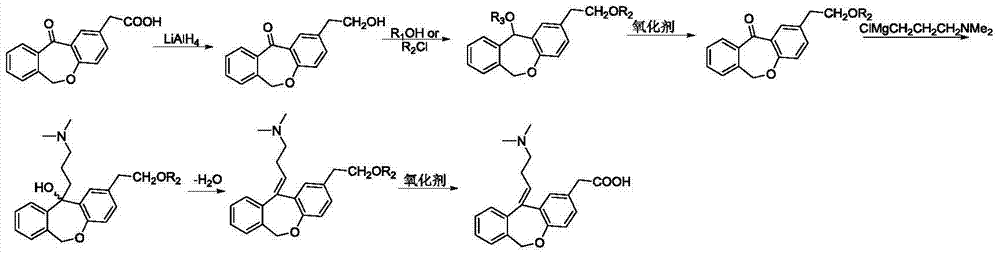
The invention relates to a method for preparing a compound, namely, olopatadine hydrochloride. The method specifically comprises the following steps: by taking 2-chloromethyl methyl benzoate and methyl 4-hydroxyphenylacetate as initial raw materials, performing etherification, hydrolysis and cyclization, further performing wittig reaction, and salifying, thereby synthesizing olopatadine hydrochloride. The process is gentle in process reaction condition, acetic anhydride is adopted to replace polyphosphoric acid, a hydrochloric acid organic solvent is adopted to effectively split Z / E type olopatadine so as to obtain olopatadine hydrochloride, conversion of Z / E configuration is effectively achieved after an E configuration byproduct is treated by using concentrated hydrochloric acid, the yield of olopatadine hydrochloride is increased, the product purity is good, and the feasibility of industrialization production is greatly improved.
Owner:仁合益康集团有限公司
Compound composition of intal and Statins
InactiveCN101766617ASolve the irritatingOrganic active ingredientsSenses disorderDiseaseAdditive ingredient
The invention relates to a compound composition containing intal and statins claritin. The compound composition consists of the following components: a) a certain amount of intal; b) a certain amount of one of olopatadine hydrochloride, ioratadine, desloratadine, degreasing ioratadine, desloratadine, rupatadine and betahistine; c) other medicinal excipients. The compound composition can be made into external preparations such as eye drops, nose drops, aerosol, spray, inhalant, gelata, eye ointments, ointments or patch and the like. The compound composition can be used for curing the diseases such as anaphylactic eye diseases, anaphylactic rhinitis, skin urtication, urticaria, allergic asthma and the like, can significantly improve allergic symptoms, and has the characteristics of high efficiency, stability, safety, low adverse reaction rate, convenient use and the like.
Owner:北京华禧联合科技发展有限公司
Leukotriene antagonist and antihistaminics composition
The invention relates to a pharmaceutical composition for treating asthma, allergic reaction and inflammation. The composition comprises a leukotriene antagonist, antihistaminics and medicinal carriers, wherein the leukotriene antagonist is Montelukast and salts thereof, and the antihistaminics is olopatadine hydrochloride and salts thereof. The composition is suitable for preventing and long-term treating of asthma for children and adults, and can treat asthma patients sensitive to aspirin, prevent bronchoconstriction induced by movement, treat pruritus concomitant with allergic rhinitis, urticaria and skin diseases, and the like. The composition has better curative effect compared with that the two medicines are used independently.
Owner:北京华禧联合科技发展有限公司
Novel olopatadine hydrochloride eye drop and preparation method thereof
InactiveCN102885767AQuality improvementSafe storageOrganic active ingredientsSenses disorderClinical efficacyCurative effect
The invention discloses a novel olopatadine hydrochloride eye drop and a preparation method thereof. The preparation method of the eye drop disclosed by the invention comprises the steps of: dissolving olopatadine hydrochloride and relevant accessories in water; adding a swelled sodium hyaluronate aqueous solution to the liquid medicine and then uniformly stirring; adding a sufficient amount of water; and finally, filtering and dispensing so as to obtain the eye drop. According to the novel olopatadine hydrochloride eye drop and the preparation method thereof, the defects of the current olopatadine hydrochloride eye drop are overcome; the residence time of the medicine on ocular surface is prolonged by the physical thickening and moisturizing lubrication effects of sodium hyaluronate; and furthermore, the medicine seldom flows into mouth and nasal cavity so as to reduce medicine loss and avoid bitterness from the olopatadine hydrochloride eye drop, and therefore, compliance of patients is increased and clinical curative effect is ensured.
Owner:JIANGSU JIBEIER PHARMA
Polymorphic forms of olopatadine hydrochloride and methods for producing olopatadine and salts thereof
The present invention provides a novel polymorphic form of olopatadine hydrochloride ([(Z)-3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride), a selective histamine H1-receptor antagonist that is used for the treatment of ocular symptoms of seasonal allergic conjunctivitis. The present invention also provides novel methods for producing olopatadine on a large scale, and in a manner that is cost effective, provides a low level of impurities and eliminates the need to use the costly and dangerous base, butyllithium, which is used in prior art reactions for making olopatadine. The present invention further provides novel processes for carrying out a large scale production of 3-dimethylaminopropyltriphenylphosphonium bromide and its corresponding hydrobromide salt, which are employed in the production of olopatadine, and pharmaceutically acceptable salts of olopatadine.
Owner:UNIV ZURICH
Eye drop containing olopatadine hydrochloride and preparation method thereof
InactiveCN110013498AImprove stabilityGuaranteed stabilityOrganic active ingredientsSenses disorderTreatment effectSide effect
The present invention belongs to the technical field of medicines and particularly relates to an eye drop containing olopatadine hydrochloride and a preparation method thereof. The provided eye drop containing the olopatadine hydrochloride mainly consists of the olopatadine hydrochloride, a cassia seed extract, pharmaceutically acceptable eye drop accessory materials and water for injection. Every100 mL of the eye drop contains 0.095-0.110 g of the olopatadine hydrochloride, and a weight ratio of the olopatadine hydrochloride to the cassia seed extract is 1:(1-3). The provided eye drop containing the olopatadine hydrochloride has advantages of remarkable therapeutic effects, high in stability, small toxic and side effects, etc., and is a relatively ideal eye drop for treating allergic conjunctivitis.
Owner:合肥华威药业有限公司
Preparation method of olopatadine hydrochloride
InactiveCN110343086AReduce generationFew reaction stepsOrganic chemistry methodsHydrogenReaction temperature
The invention discloses a preparation method of olopatadine hydrochloride. The preparation method comprises the following steps of preparing olopatadine hydrochloride crude products, preparing olopatadine freebase and preparing olopatadine hydrochloride. According to the preparation method of olopatadine hydrochloride, the key steps are upgraded, and the preparation method has the following advantages that 1, reaction steps are reduced, the loss is reduced, and the production efficiency is improved; 2, generation of hydrogen is reduced, and potential safety hazards are reduced; 3, the processing difficulty is reduced while ensuring the quality and yield unchanged; 4, a reaction temperature is optimized, a low-temperature harsh reaction is eliminated, and the dependence on equipment is reduced; 5, an original separate heterogeneous removal step is eliminated; and 6, the yield is high and the quality is good.
Owner:重庆西南制药二厂有限责任公司
Olopatadine hydrochloride containing externally applied composition and cream thereof
ActiveCN107823124AIncrease local drug concentrationReduce adverse reactionsOrganic active ingredientsAerosol deliveryMast cellOral medication
The invention discloses an externally applied composition and a preparation thereof, in particular an olopatadine hydrochloride containing externally applied composition and cream thereof. The composition contains olopatadine hydrochloride. The olopatadine hydrochloride containing externally applied composition directly acts on an affected part, the local medicament concentration of skin at an itching part is increased, and the effects of resisting histamine and stabilizing mast cells are achieved; adverse reaction of oral administration is avoided; the administration dosage can be selected according to the area of a diseased part, and the olopatadine hydrochloride containing externally applied composition is convenient to use and carry and high in stability; efficacy tests show that the composition has remarkable treatment effects on pruritic dermatosis; a process for the cream is simple, easy and low in cost.
Owner:深圳博瑞医药科技有限公司
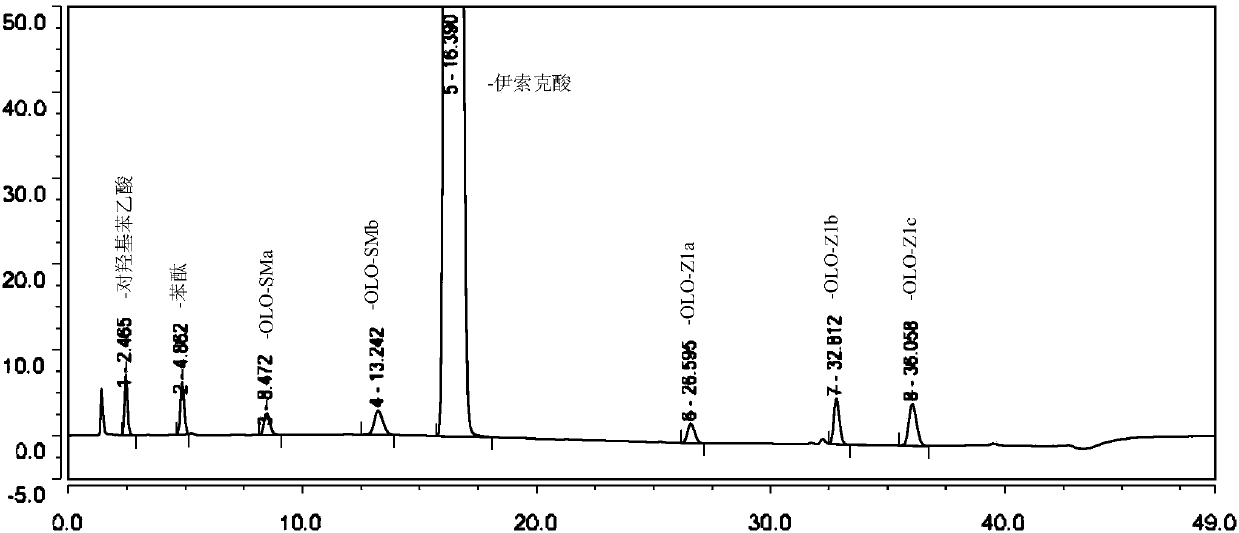
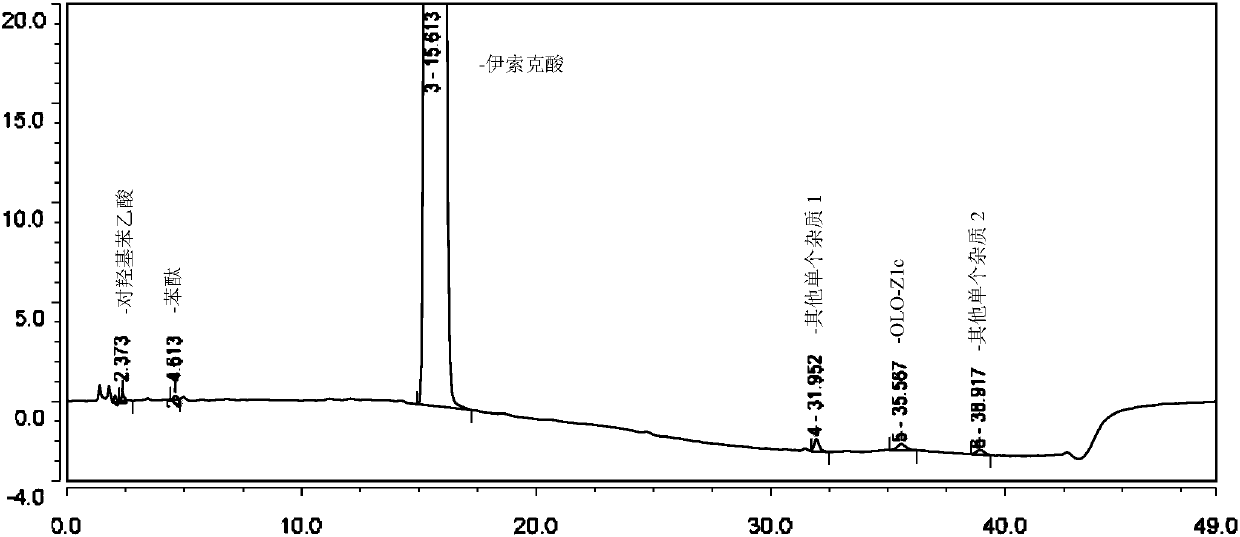
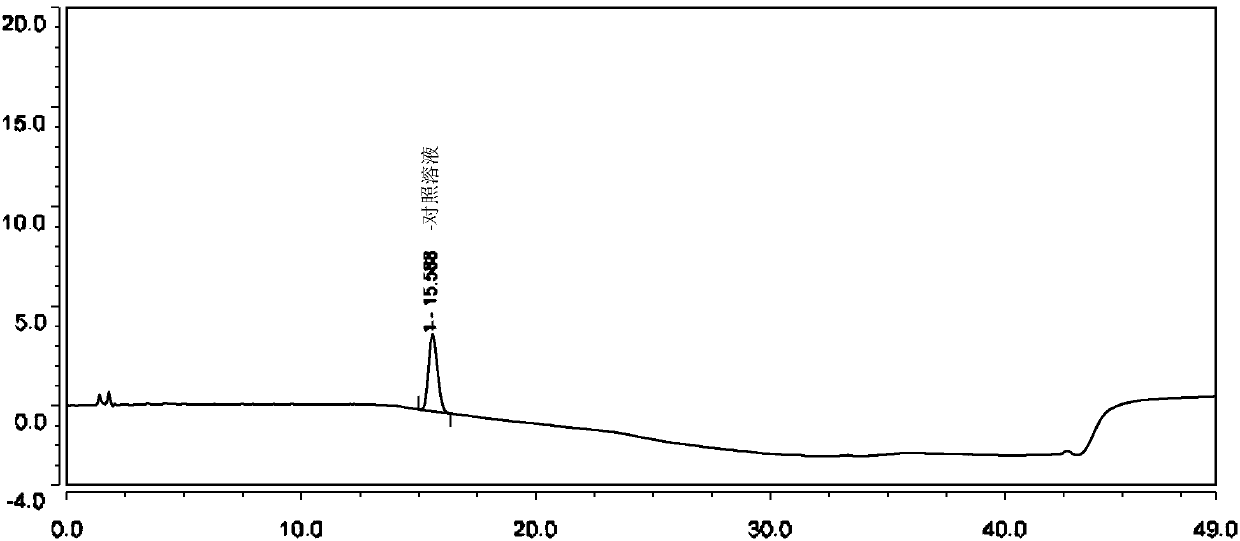
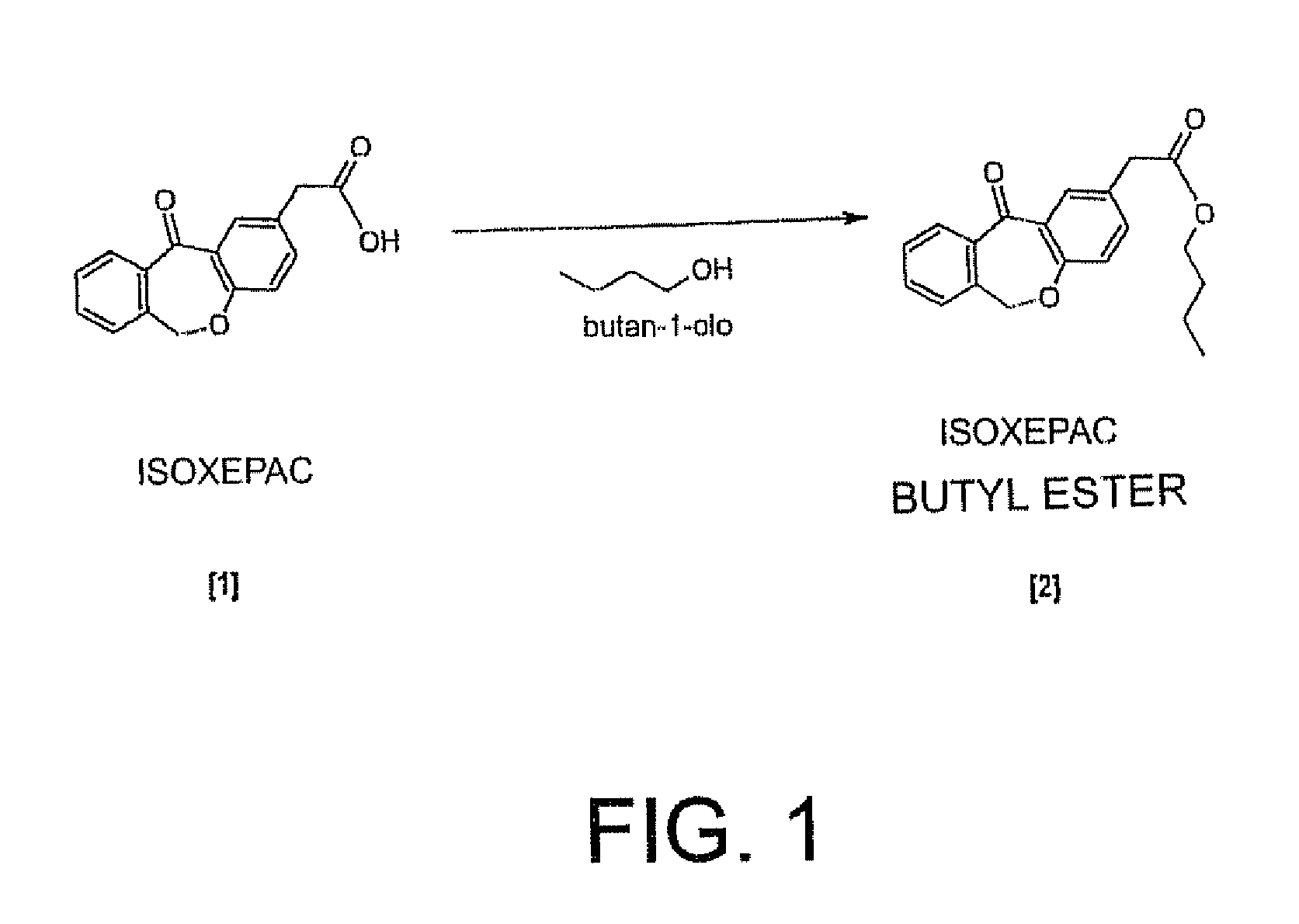
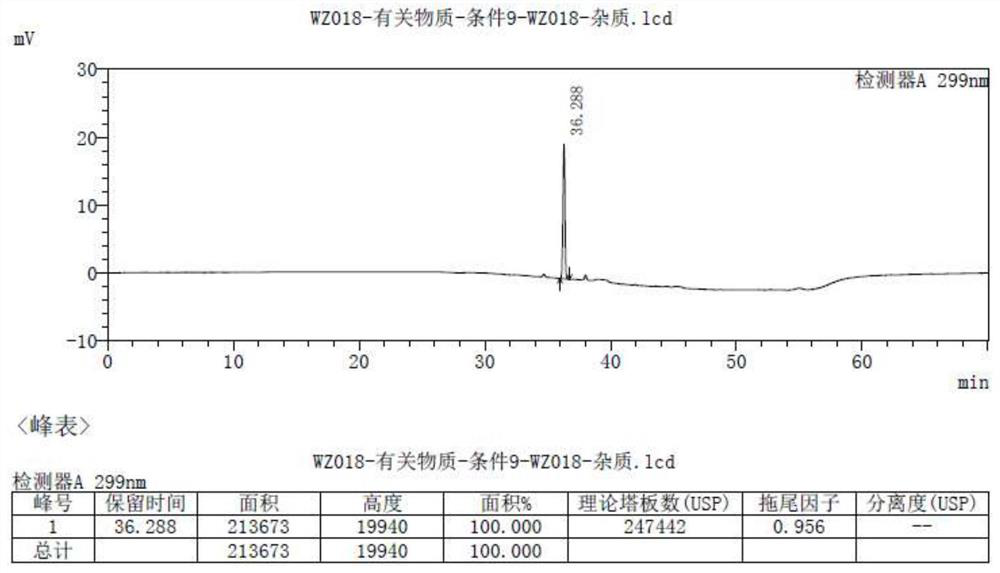
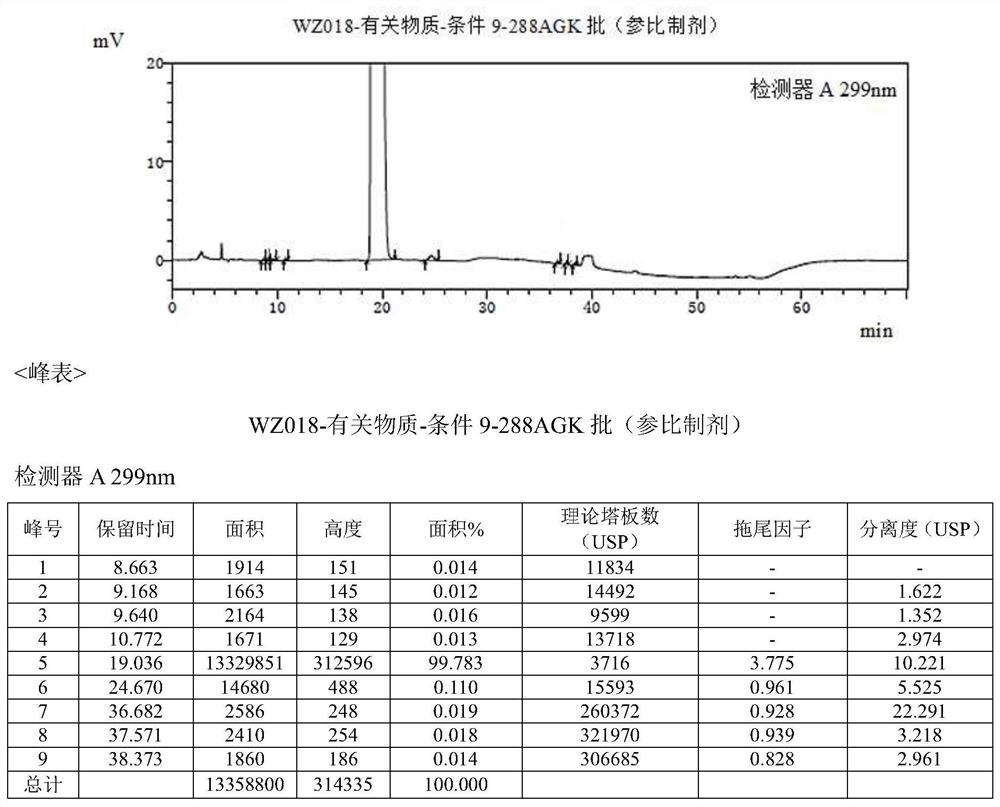
Method for separating and determining isoxepac and related substances of isoxepac by HPLC (High Performance Liquid Chromatography) method
ActiveCN107589197AAccurate detectionQuality is easy to controlComponent separationPotassium hexafluorophosphateImpurity
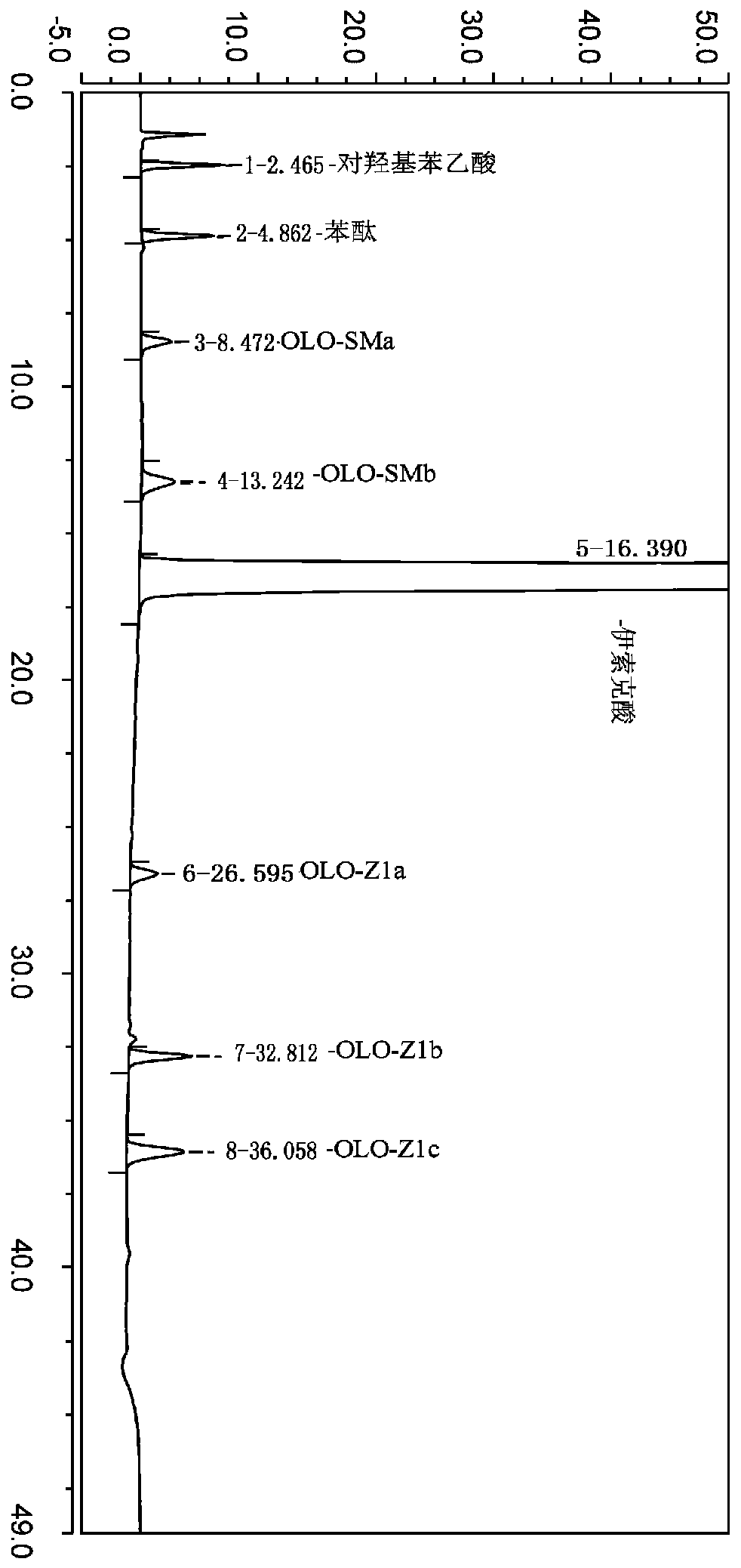
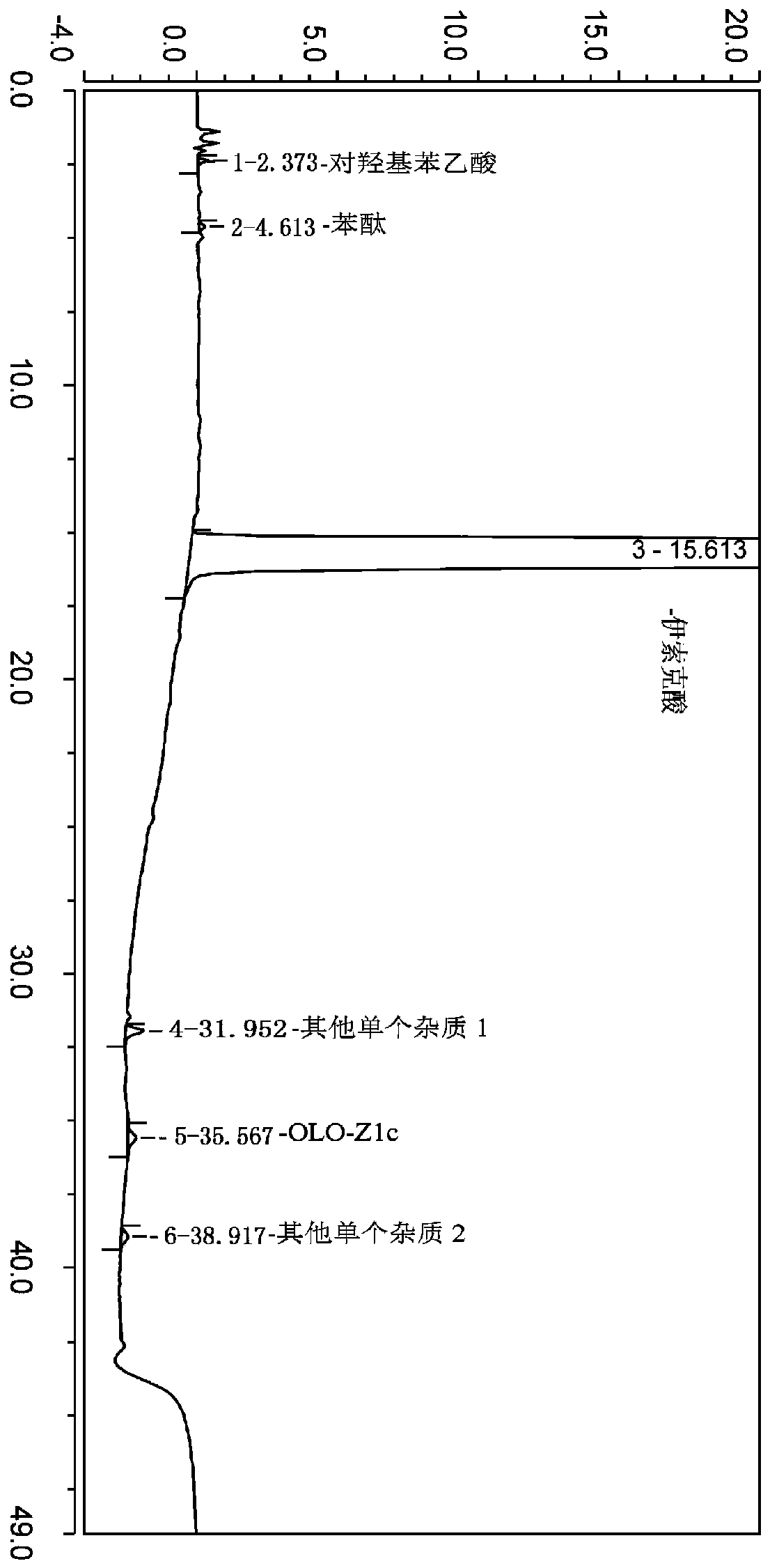
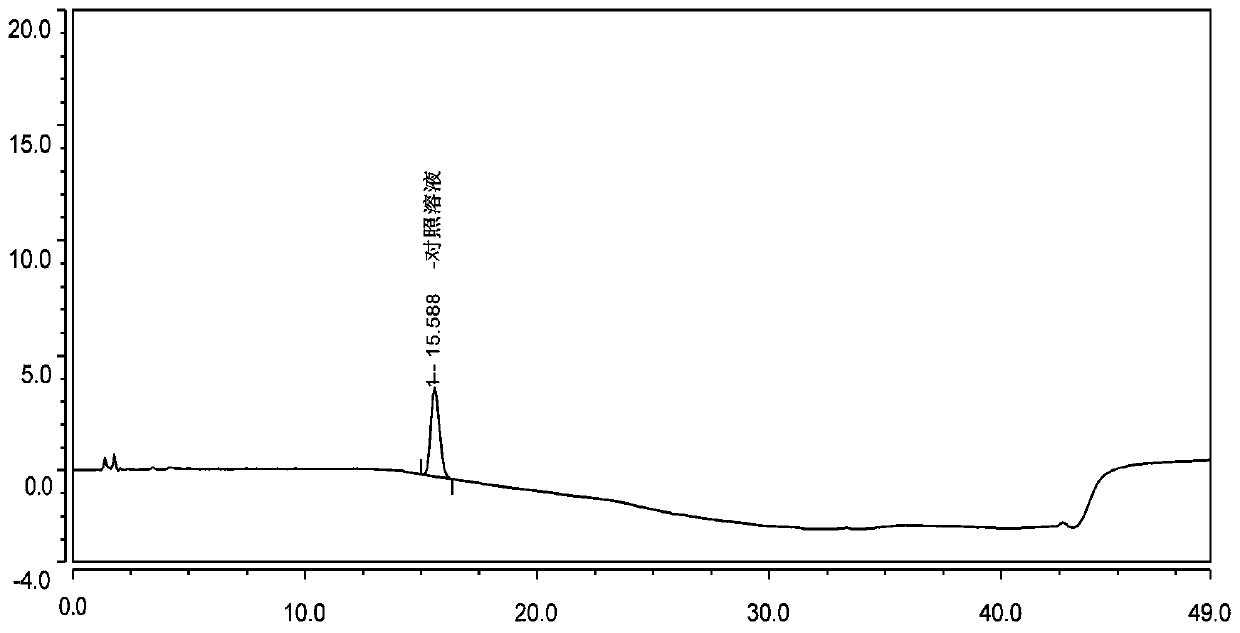
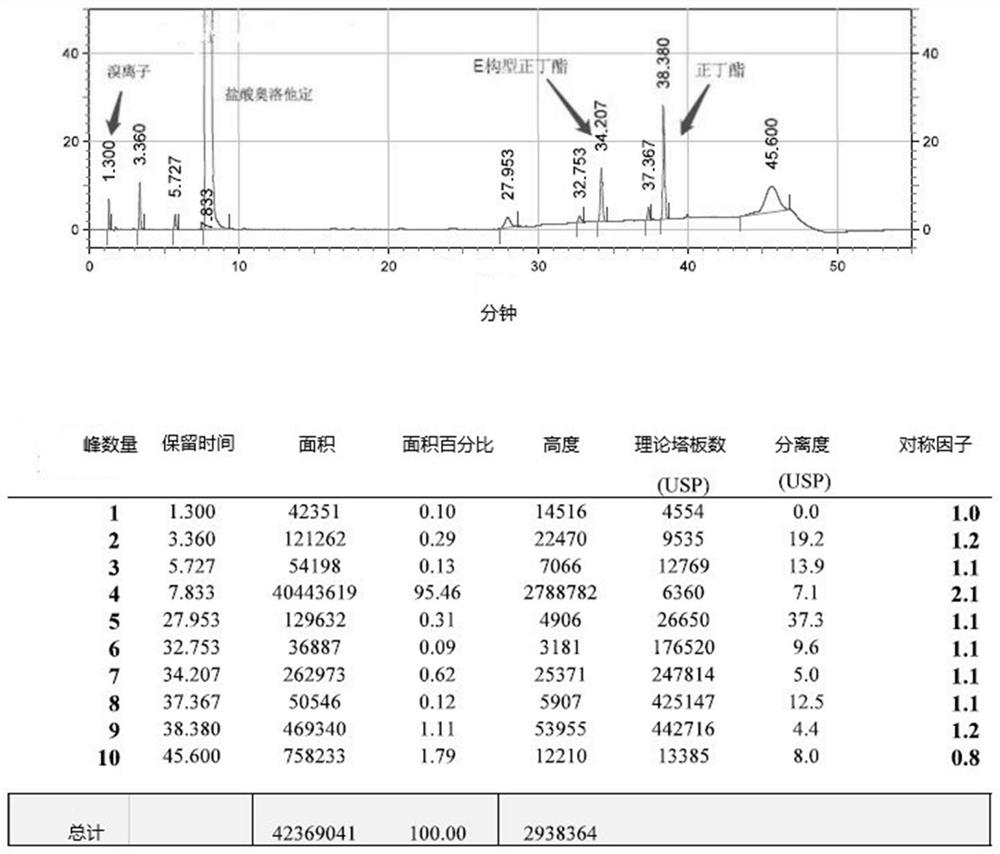
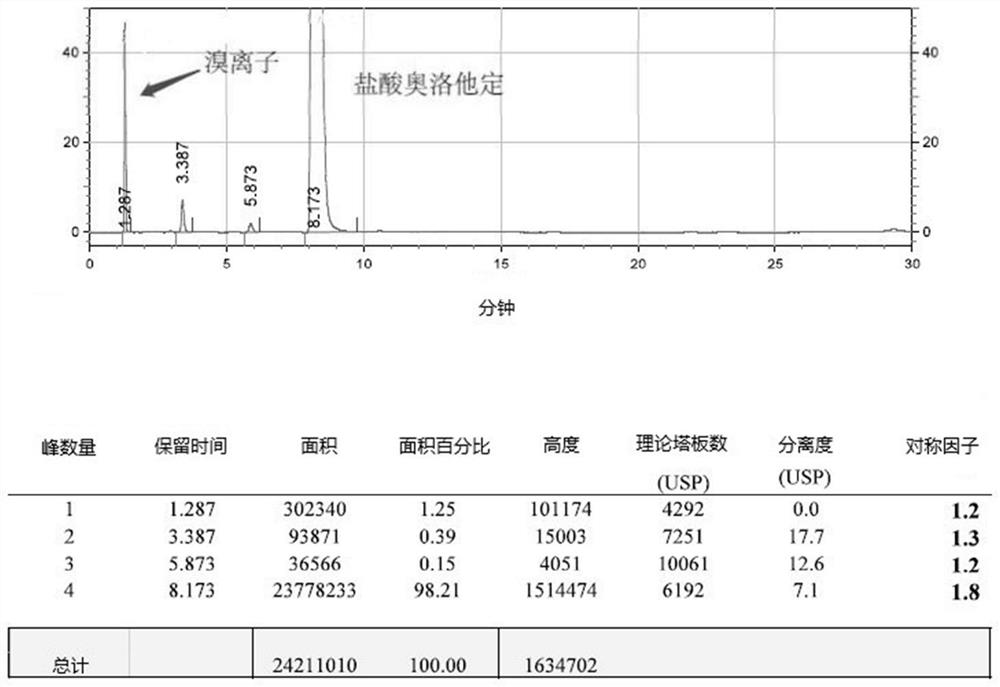
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining isoxepac and related substances of isoxepac by an HPLC (High PerformanceLiquid Chromatography) method. A chromatographic column adopted by the method disclosed by the invention adopts octylsilane-bonded silica gel as filler, a mobile phase A and a mobile phase B are adopted to carry out gradient elution, and detection is carried out in a detector; the related substances include one or more of A-G; the mobile phase A is potassium hexafluorophosphate solution, and themobile phase B is organic solvent. The method can effectively separate the seven impurities and other individual impurity in isoxepac and quantitatively determine the existence levels. The method hasthe advantages of high separation degree, high specificity, high sensitivity and accuracy, and is of a great significance in the control of the quality of isoxepac and olopatadine hydrochloride.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Novel method for preparing olopatadine hydrochloride by using high-activity organic zinc reagent
The invention discloses a novel method for preparing olopatadine hydrochloride. The method comprises the steps as follows: (1) high-activity zinc and 3-bromo-N,N-dimethylpropylamine form an organic zinc reagent (I); (2) the organic zinc reagent is eliminated after electrophilic substitution with isoxepac (II) to form olopatadine (III); (3) the olopatadine with higher purity is obtained through recrystalization of the olopatadine, wherein structural formulas of the organic zinc reagent (I), the isoxepac (II) and the olopatadine (III) are as follows.
Owner:北京华禧联合科技发展有限公司
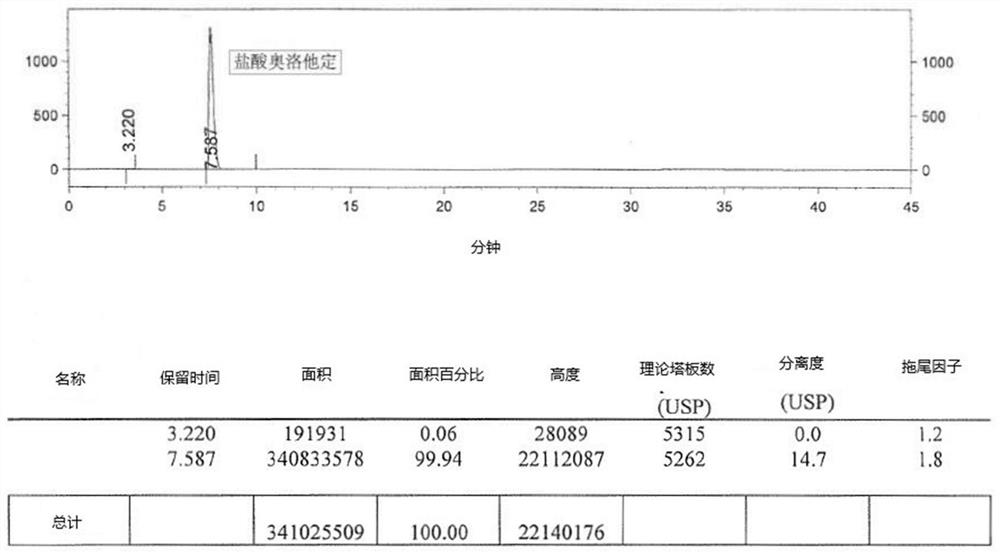
Detection method of olopatadine hydrochloride and related substance thereof
The invention relates to a detection method of olopatadine hydrochloride and related substance thereof. The method comprises a step of using high performance liquid chromatography for detection, wherein liquid chromatographic conditions are as follows: an octyl silane bonded silica gel chromatographic column is used; a mobile phase A is aqueous solution containing 0.01%-1% ion pair reagent, 0.3%-1% monoamine and 0.001-0.1mol / L buffer salt, a mobile phase B is acetonitrile and / or methyl alcohol, and gradient eluting is executed; and a pH value of buffer solution is 2.5-3.5, and detection wavelength is 220nm-280nm. The detection method of the olopatadine hydrochloride and the related substance thereof provided by the invention can effectively separate the olopatadine hydrochloride and impurities thereof, improve accuracy of a detection result and reduce clinical medication risks.
Owner:北京海晶生物医药科技有限公司
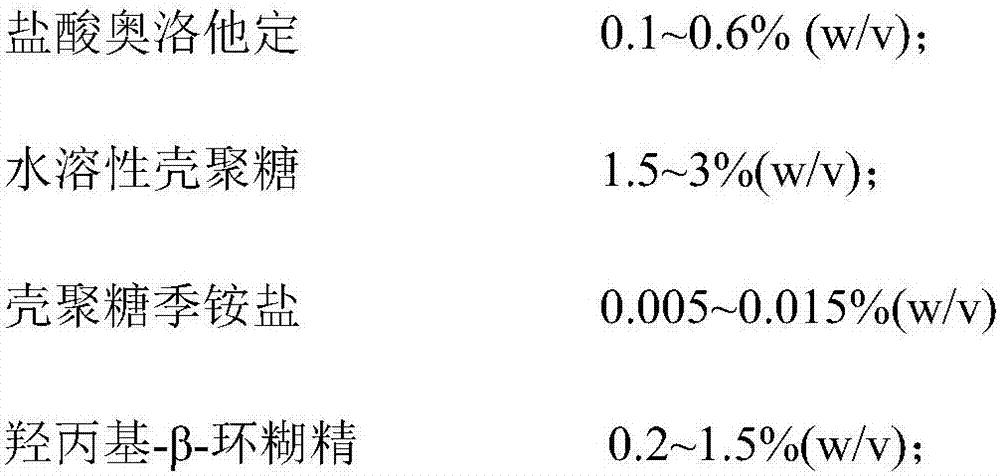
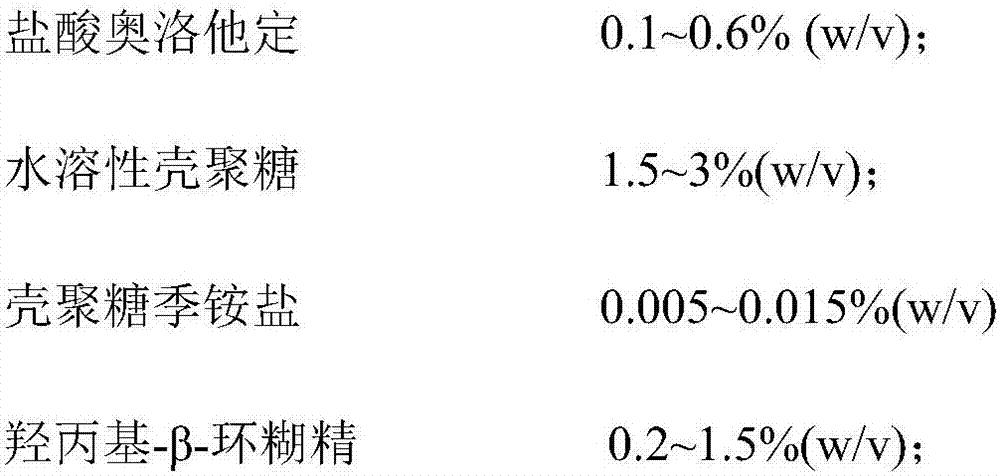
Olopatadine hydrochloride nasal spray and preparation method thereof
ActiveCN107569454AExtended stayImprove bioavailabilityOrganic active ingredientsAerosol deliveryNasal cavitySide effect
The invention belongs to the technical field of medicines, and provides olopatadine hydrochloride nasal spray and a preparation method thereof. The nasal spray is prepared from olopatadine hydrochloride, water-soluble chitosan, chitosan quaternary ammonium salt, hydroxypropyl-beta-cyclodextrin, an osmotic pressure regulator, a buffer agent, a pH regulator and purified water. The olopatadine hydrochloride nasal spray prolongs residence time, in a nasal cavity, of olopatadine hydrochloride and improves bioavailability of the olopatadine hydrochloride. Besides, stability of the product is furtherimproved, bitter taste of the product is reduced, an occurrence rate of untoward effects such as epistaxis and a sore throat is reduced, thrill on nasal mucosa is low, side effect is small, and the safety of nasal cavity drug delivery is improved.
Owner:深圳大佛药业股份有限公司
Preparation technique of olopatadine hydrochloride
ActiveCN105693685AMild reaction conditionsEasy to operateOrganic chemistryBenzoic acidP-hydroxyphenylacetic acid
The invention relates to a preparation technique of olopatadine hydrochloride. The technique comprises the following steps: carrying out reaction on 2-(chloromethyl)benzoic acid and p-hydroxyphenylacetic acid to generate Isoxepac, and carrying out reaction on the Isoxepac and a Wittig-horner agent generated by N,N-dimethylaminochloropropane hydrochloride to generate hydrochloride, thereby obtaining the olopatadine hydrochloride. The method uses the low-cost raw materials for reaction, avoids using toxic agents in the reaction process, has the advantages of short process route, mild reaction conditions, controllable operation, short production cycle, low energy consumption, high yield, high purity and safe technique, and is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Olopatadine hydrochloride gel eye drops and preparation method thereof
InactiveCN106256351ANon-irritatingLess irritatingOrganic active ingredientsSenses disorderGel matrixInjections water
The invention discloses an olopatadine hydrochloride gel eye drop and a preparation method thereof. The main raw material is olopatadine hydrochloride, and a gel matrix, an isotonic regulator, a preservative, water for injection and other auxiliary materials are added. Swell and stir evenly, adjust pH and sterilize, and then prepare 0.1%-0.2% olopatadine hydrochloride gel eye drops. The purpose of the invention is to provide a practical, convenient and reliable ophthalmic preparation for treating allergies for the clinic, and to solve the problems of short residence time and poor drug absorption of eye drops dosage forms in the eyes.
Owner:JIANGSU JIBEIER PHARMA
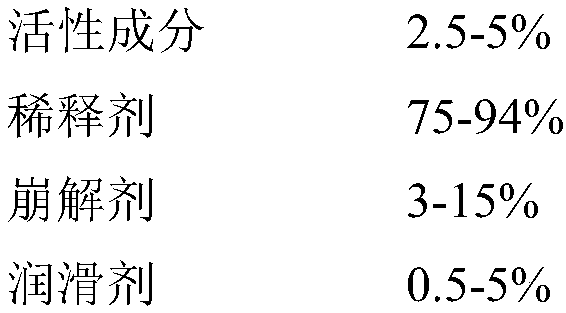
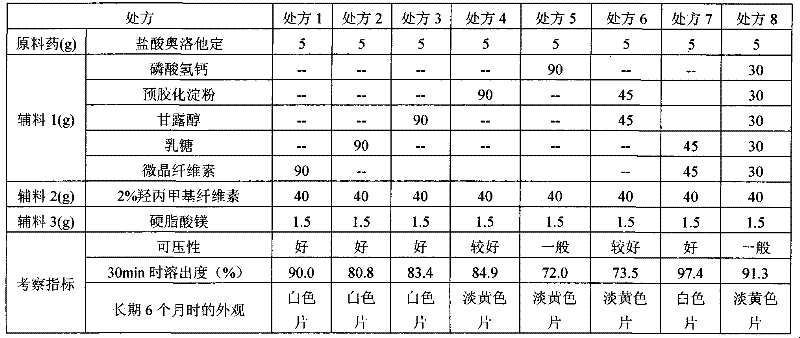
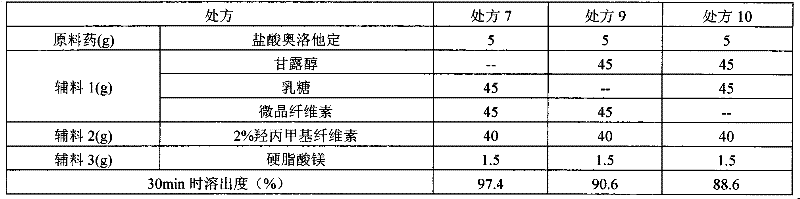
Formula of directly tableted olopatadine hydrochloride tablet
PendingCN110882223ARapid dissolutionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsTabletingMedicinal chemistry
The invention provides a formula of directly tableted olopatadine hydrochloride tablet which has the advantages of rapid dissolution and good stability.
Owner:HAINAN ZHONGJI MEDICAL TECH
Process for the preparation of olopatadine
ActiveUS20110065936A1Organic active ingredientsSenses disorderMedicinal chemistryOlopatadine Hydrochloride
The present invention relates to a novel process for the preparation of olopatadine hydrochloride starting from an advanced intermediate.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Olopatadine alpha methyl compound and its preparation method and use
ActiveCN111808063BImprove medication safetyImprove and ensure medication safetyOrganic active ingredientsOrganic chemistryDiseaseMethyl palmoxirate
Owner:唯智医药科技(北京)有限公司 +1
Method for the separation and determination of isoket acid and its related substances by hplc
ActiveCN107589197BAccurate detectionQuality is easy to controlComponent separationPotassium hexafluorophosphateHplc method
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining isoxepac and related substances of isoxepac by an HPLC (High PerformanceLiquid Chromatography) method. A chromatographic column adopted by the method disclosed by the invention adopts octylsilane-bonded silica gel as filler, a mobile phase A and a mobile phase B are adopted to carry out gradient elution, and detection is carried out in a detector; the related substances include one or more of A-G; the mobile phase A is potassium hexafluorophosphate solution, and themobile phase B is organic solvent. The method can effectively separate the seven impurities and other individual impurity in isoxepac and quantitatively determine the existence levels. The method hasthe advantages of high separation degree, high specificity, high sensitivity and accuracy, and is of a great significance in the control of the quality of isoxepac and olopatadine hydrochloride.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Post-treatment purification method of olopatadine hydrochloride
ActiveCN112375060AReduce residual riskLow Inorganic Salt ContentOrganic chemistryInorganic saltsAcetic acid
The invention provides a post-treatment purification method of olopatadine hydrochloride. The method comprises the steps of 1) washing a reaction liquid crude product with a sodium chloride solution to remove bromide ions so as to obtain an olopatadine hydrochloride salt-containing crude product; (2) dissolving the olopatadine hydrochloride salt-containing crude product prepared in the step (1) into a mixed solvent of dichloromethane, glacial acetic acid, acetic acid and alcohols, and carrying out desalting treatment so as to obtain an olopatadine hydrochloride crude product; and (3) recrystallizing the olopatadine hydrochloride crude product obtained in the step (2), wherein a solvent adopted for recrystallization is a mixed solvent of dimethyl sulfoxide and isopropyl ether. The target product olopatadine hydrochloride obtained through the method is extremely low in inorganic salt content, meanwhile, the preparation yield and the product purity of olopatadine hydrochloride are greatlyimproved, the production cost is reduced, and the method is suitable for industrial production.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
A kind of olopatadine hydrochloride nasal spray and preparation method thereof
ActiveCN107569454BExtended stayImprove bioavailabilityOrganic active ingredientsAerosol deliveryNasal cavityNasal irritation
The invention belongs to the technical field of medicine, and provides an olopatadine hydrochloride nasal spray and a preparation method thereof. The nasal spray is composed of olopatadine hydrochloride, water-soluble chitosan, chitosan quaternary ammonium salt, hydroxyl Propyl-β-cyclodextrin, osmotic pressure regulator, buffer, pH regulator and purified water. The olopatadine hydrochloride nasal spray provided by the present invention prolongs the residence time of olopatadine hydrochloride in the nasal cavity, improves the bioavailability of olopatadine hydrochloride, in addition, improves the stability of the product and reduces the The incidence of adverse reactions such as bitter taste, nosebleeds, and sore throat has low irritation to the nasal mucosa, and the side effects are small, which improves the safety of nasal medication.
Owner:深圳大佛药业股份有限公司
Method for simply and conveniently preparing high-purity olopatadine hydrochloride intermediate
ActiveCN111548369AAvoid harmReduce chemical damageGroup 5/15 element organic compoundsHydrobromideAlcohol
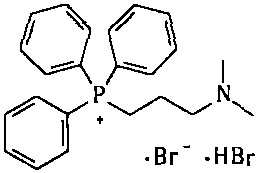
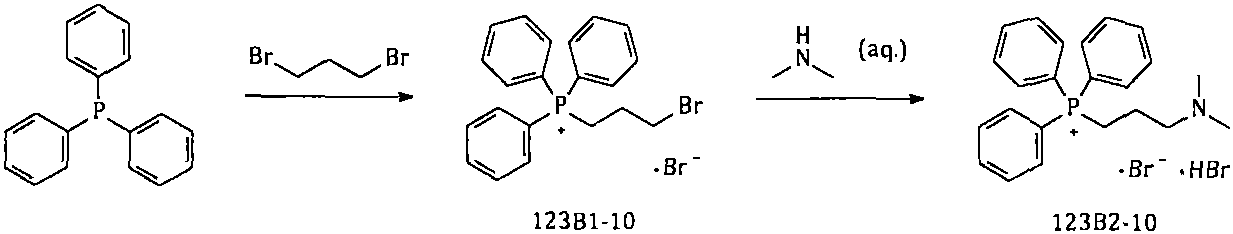
The invention provides a simple and convenient method which is more suitable for industrial large-scale production and preparation of high-purity [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide. According to the preparation method, with triphenylphosphine and 1, 3-dibromopropane adopted as starting materials, reflux reaction is carried out in n-heptane to obtain (3-bromopropyl) triphenylphosphonium bromide; the obtained (3-bromopropyl) triphenylphosphonium bromide does not need to be separated, and directly reacts with a dimethylamine aqueous solution by means of a one-potmethod; after the reaction is finished, the n-heptane is concentrated, water in a system is taken out, so that a [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide crude product canbe obtained; and the crude product is thermally pulped with absolute ethyl alcohol, so that the high-purity [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide can be obtained.
Owner:内蒙古京东药业有限公司
Olopatadine hydrochloride tablet as well as preparation method and detecting method thereof
The invention discloses an olopatadine hydrochloride tablet as well as a preparation method and a detecting method thereof. The olopatadine hydrochloride tablet comprises the raw materials based on parts by weight: 1-10 parts of olopatadine hydrochloride, 50-150 parts of lactose, 25-75 parts of microcrystalline cellulose, 30-50 parts of 2 percent of hydroxypropyl methyl cellulose and 0.15-2.2 parts of magnesium stearate. The olopatadine hydrochloride tablet has good dissolution performance and few selected auxiliary materials and can achieve good anti-allergic effect.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
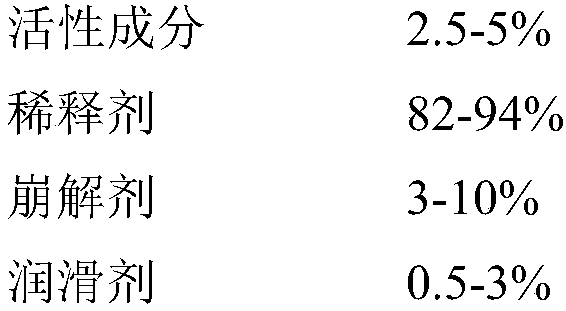
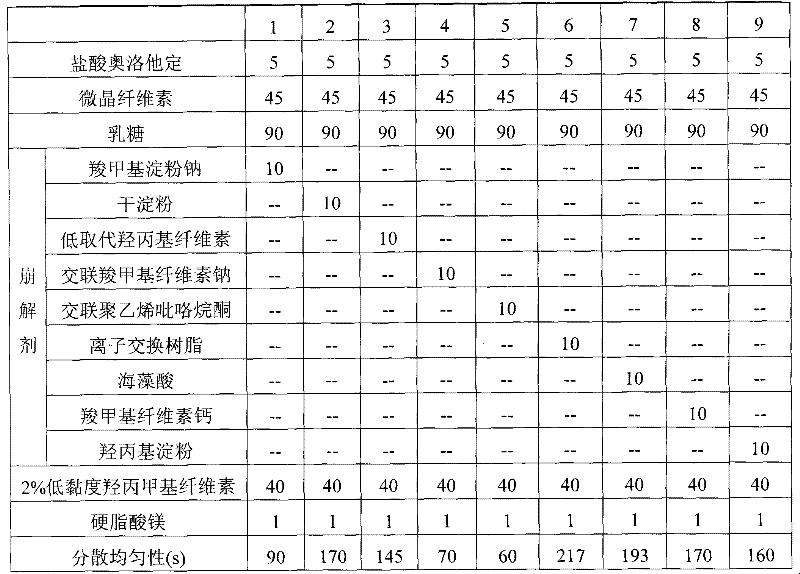
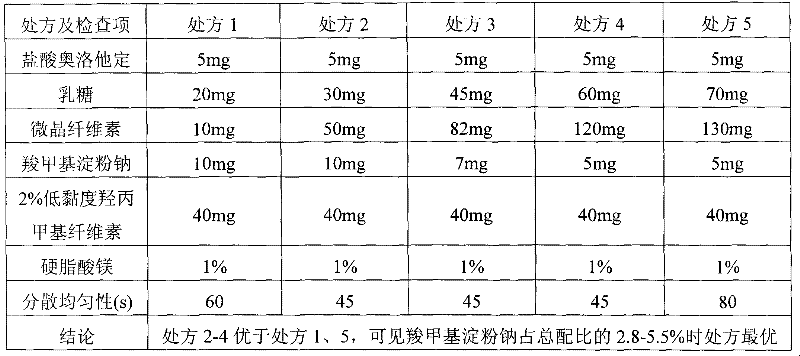
Olopatadine hydrochloride dispersible tablets, preparation method thereof and quality control method thereof
InactiveCN101695480BImprove uniformityImprove comparabilityOrganic active ingredientsComponent separationQuality controlDigestion
The invention discloses olopatadine hydrochloride dispersible tablets, a preparation method thereof and a quality control method thereof. The olopatadine hydrochloride dispersible tablets contain olopatadine hydrochloride serving as a main medicament and a disintegrating agent, a filler, a binding agent and a lubricating agent which serve as auxiliary materials. A wet-method is adopted for granulation and tabletting, and an internal disintegrating agent addition method is adopted. The olopatadine hydrochloride dispersible tablets have the advantages of excellent dispersion state, short disintegrating time, quick medicament digestion, convenient administration, low production cost, no special equipment, convenient and stable carrying and transport and the like.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
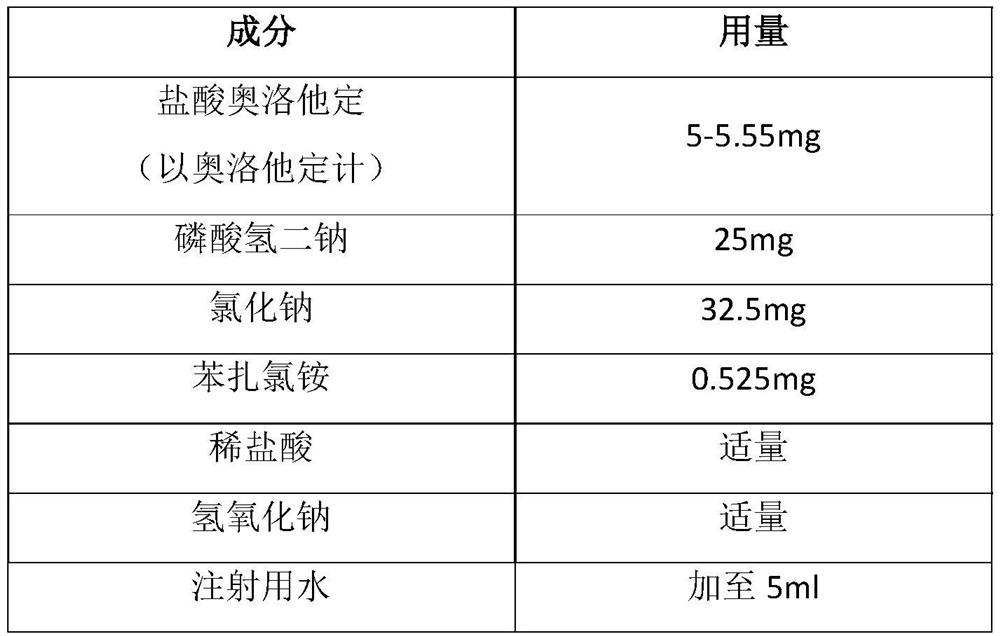
Olopatadine hydrochloride eye drop and preparation method thereof
InactiveCN111700860AOrganic active ingredientsSenses disorderPharmaceutical formulationBuffering agent
The invention relates to the field of pharmaceutical preparations, and particularly provides a novel olopatadine hydrochloride eye drop. The olopatadine hydrochloride eye drop comprises olopatadine hydrochloride, a buffer agent, an osmotic pressure regulator, a preservative, a pH regulator and a solvent.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Traditional Chinese and western medicine compound eye drops for treating bacterial coronitis and preparation method thereof
InactiveCN106138502AGood treatment effectImprove medicinal effectAntibacterial agentsSenses disorderAdditive ingredientBetel leaf
The invention discloses traditional Chinese and western medicine compound eye drops for treating bacterial coronitis and a preparation method thereof, belonging to the technical field of medicines. The eye drops comprise the following raw materials: japanese calystegia, mud snail, marsh horsetail herb, betel leaf, angelica sinensis, zornia gibbosa, semen thlaspi, leaf of Chinese loropetalum, incised spleenwort, three-coloured amaranth seed, smallfruit fig resin, cannalike dona rhizome, common vetch, olopatadine hydrochloride, carbachol and norfloxacin. The eye drops disclosed by the invention adopt a therapeutic method of combining traditional Chinese medicine and western medicine, have excellent treatment effects compared with those of generally pure traditional Chinese and western medicine, take the effects of clearing away heat and toxic materials, diminishing inflammation and sterilizing, improving eyesight and relieving opacity, relieving swelling and pain and tonifying liver and nourishing kidney as treatment principles, can improve the effects of the traditional Chinese medicinal components and western medicine at the highest efficiency and improve medicinal effects, are externally and directly dripped into eyes so as to kill bacteria and realize high antibacterial property, can directly reach lesions, are convenient to use and good in absorption effect, do not have any bad and toxic or side effect, and have good effects of treating decreased eyesight, chemosis and the like caused by bacterial coronitis.
Owner:周艳
A kind of olopatadine hydrochloride external composition and its cream
ActiveCN107823124BIncrease local drug concentrationReduce adverse reactionsOrganic active ingredientsAerosol deliveryMast cellChemical composition
The invention discloses an externally applied composition and a preparation thereof, in particular an olopatadine hydrochloride containing externally applied composition and cream thereof. The composition contains olopatadine hydrochloride. The olopatadine hydrochloride containing externally applied composition directly acts on an affected part, the local medicament concentration of skin at an itching part is increased, and the effects of resisting histamine and stabilizing mast cells are achieved; adverse reaction of oral administration is avoided; the administration dosage can be selected according to the area of a diseased part, and the olopatadine hydrochloride containing externally applied composition is convenient to use and carry and high in stability; efficacy tests show that the composition has remarkable treatment effects on pruritic dermatosis; a process for the cream is simple, easy and low in cost.
Owner:深圳博瑞医药科技有限公司
Multistage filtering device for preparation of olopatadine hydrochloride eye drops
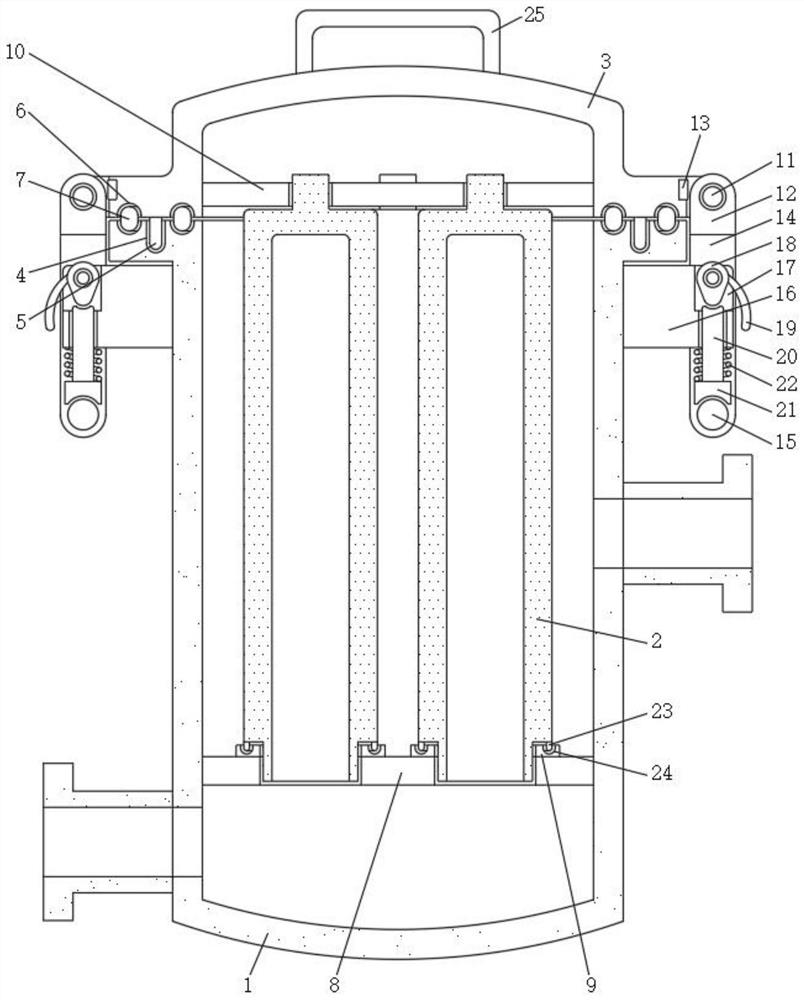
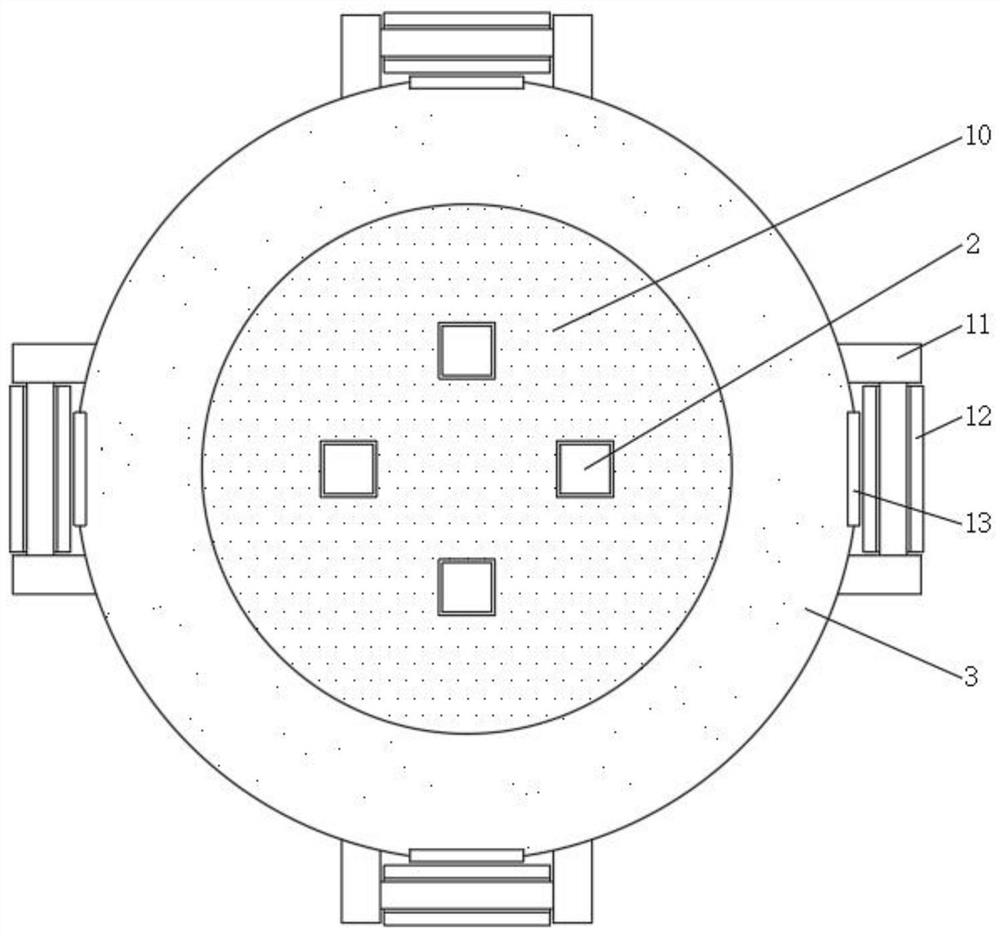
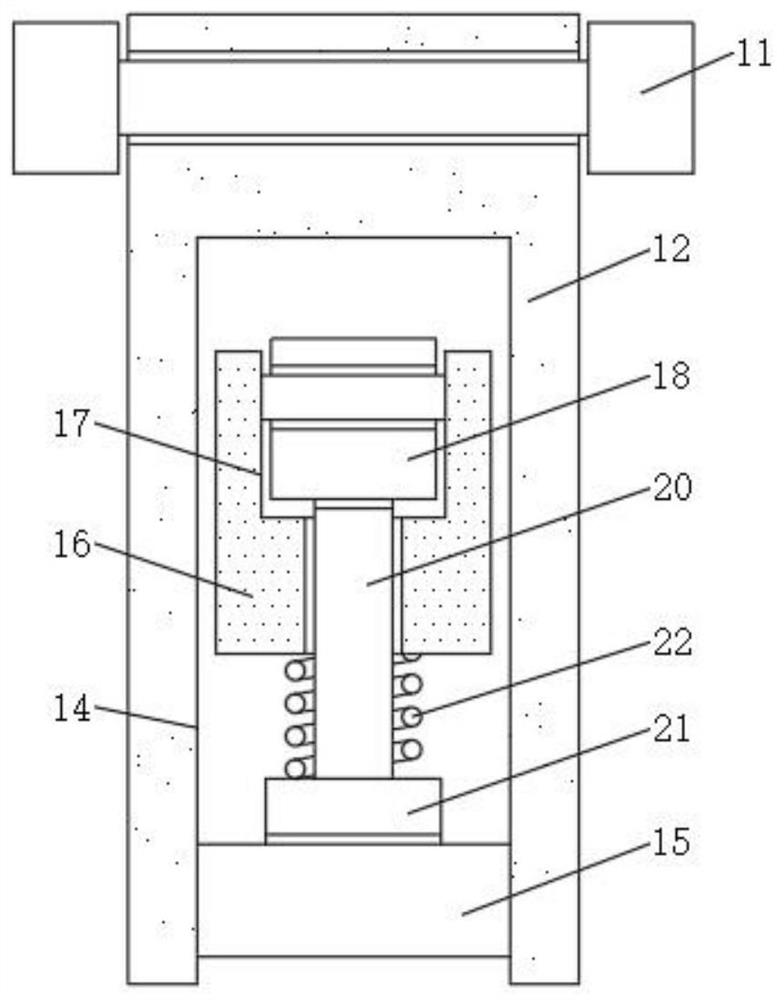
InactiveCN112691429ASolve the problem of inconvenient replacementEasy to operateStationary filtering element filtersCamMechanical engineering
The invention discloses a multistage filtering device for preparation of olopatadine hydrochloride eye drops, and relates to the technical field of eye drop preparation equipment. The multistage filtering device for preparation of olopatadine hydrochloride eye drops comprises a cylinder body and a filter element, the top of the cylinder body is movably connected with a cylinder cover, the bottom of the cylinder cover is fixedly connected with positioning pins, positioning grooves are formed in the top of the cylinder body, the positioning pins are inserted into the positioning grooves, sealing grooves are formed in the top of the cylinder body and the bottom of the cylinder cover, and the inner walls of the sealing grooves are movably connected with the surfaces of sealing strips. By arranging movable plates, round rods, fixed blocks and cams, the problems that in the prior art, when a common multistage filtering device for preparing olopatadine hydrochloride eye drops is used and a polyethersulfone membrane filter element is replaced, due to the fact that the number of bolts for fixing the cylinder cover is large, the cylinder cover is inconvenient to disassemble and assemble, and accordingly the filter element is inconvenient to replace are solved.
Owner:苏州工业园区天龙制药有限公司
Ophthalmic composition as well as preparation method and application thereof
ActiveCN112263545ALittle side effectsAlleviate irritation symptomsOrganic active ingredientsSenses disorderCorneal diseaseEye irritation
The invention relates to an ophthalmic composition which comprises chondroitin sulfate and olopatadine hydrochloride. The invention further relates to a preparation method of the ophthalmic composition. The preparation method comprises the following steps: S1, mixing a pH regulator, an osmotic pressure regulator and injection water to obtain a mixed solution I; S2, homogeneously stirring and mixing chondroitin sulfate, olopatadine hydrochloride and injection water for 10-15 minutes at 3000-4500 r / min to obtain a mixed solution II; and S3, mixing the mixed solution I and the mixed solution II with the injection water, and filtering the mixture to obtain the ophthalmic composition. Furthermore, the invention relates to application of the ophthalmic composition in preparation of medicines forimproving eye stimulation symptoms caused by treatment of corneal diseases. The ophthalmic composition serving as eye drops can be used for effectively relieving eye irritation caused by chondroitinsulfate at conventional dosage for corneal disease treatment, so that the medication compliance of a patient can be improved, and the treatment time of the patient can be effectively shortened.
Owner:湖北远大天天明制药有限公司
Olopatadine hydrochloride alpha methyl compound B crystal form and preparation method and application thereof
PendingCN114478461AImprove solubilityImprove bioavailabilityOrganic active ingredientsAerosol deliveryChemical compoundPharmaceutical drug
The invention discloses an olopatadine hydrochloride alpha methyl compound B crystal form as well as a preparation method and application thereof, and belongs to the technical field of medicines. The invention develops and prepares an olopatadine hydrochloride alpha methyl compound B crystal form, the XRPD pattern of the olopatadine hydrochloride alpha methyl compound B crystal form contains the following diffraction characteristic peaks: the diffraction angles 2theta are 15.9, 17.0, 18.6, 23.0, 24.1, 26.1 and 26.7, and the error range of each characteristic peak 2theta is + / -0.2. The olopatadine hydrochloride alpha methyl compound B crystal form is obviously improved in the aspects of drug release performance, solubility and the like, the bioavailability is improved, and the pharmaceutical prospect is wide.
Owner:唯智医药科技(北京)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com