Olopatadine hydrochloride alpha methyl compound B crystal form and preparation method and application thereof
A technology for olopatadine hydrochloride and compound, which is applied in the field of olopatadine hydrochloride alpha methyl compound B crystal form and its preparation field, can solve problems such as difference in bioavailability, difficulty in dispersion, unfavorable industrial production and the like, and achieve rapid effect , release the effect of good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of olopatadine hydrochloride α-methyl compound B crystal form
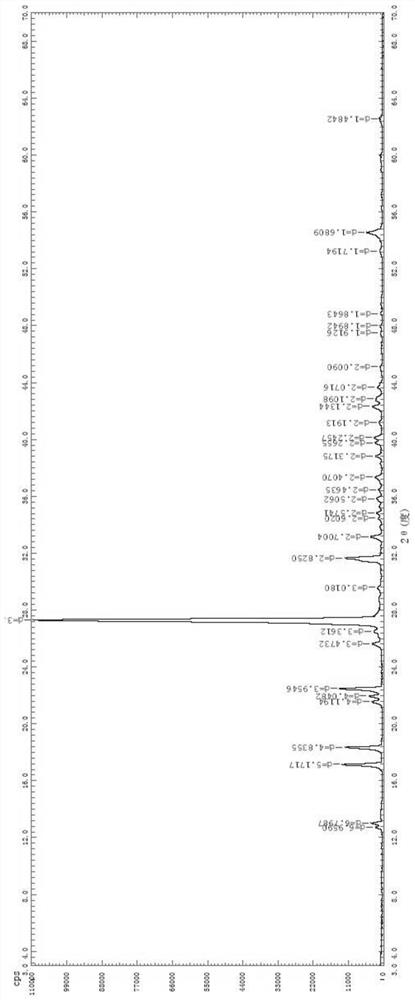
[0033] Add 10.00 g of olopatadine hydrochloride α-methyl compound into the Soxhlet extraction tube, add 80 mL of acetone into the extraction bottle, stir and raise the temperature to reflux for extraction. When there is no obvious solid in the Soxhlet extraction tube, stop heating and remove the acetone. Replace the extraction tube with a distillation device and carry out normal pressure distillation. When solids are obviously separated out in the solution, stop the distillation, keep warm and reflux for 1h, and cool down naturally for crystallization. After filtering and drying, collect the solid precipitation to obtain 5.61g of olopatadine hydrochloride. α-methyl compound B crystal form, see image 3 ; The corresponding filtrate (mother liquor) is collected and preserved for subsequent use.
[0034] The obtained XRPD pattern of Form B has diffraction peaks at 2θ=15.9°, 17.0°, 18.6°, 23.0°, 24....
Embodiment 2
[0036] Preparation of olopatadine hydrochloride α-methyl compound B crystal form
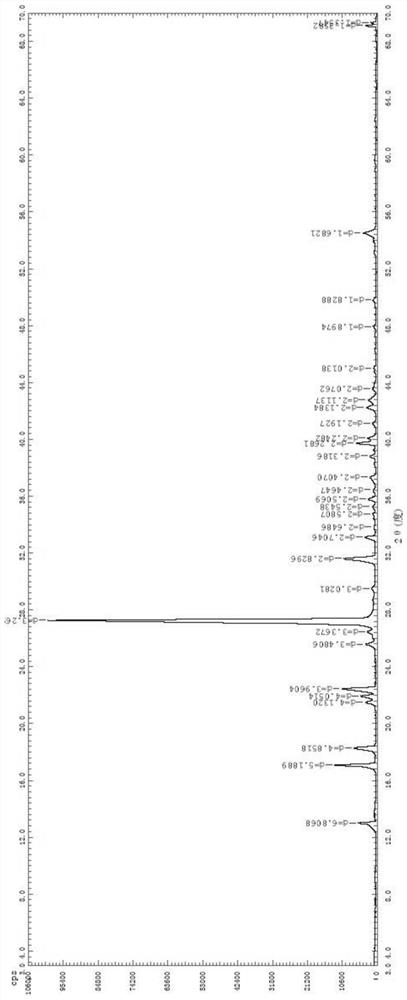
[0037] Add 10.00 g of olopatadine hydrochloride α-methyl compound into the Soxhlet extraction tube, add 20 mL of the mother liquor obtained in Example 1 into the extraction bottle, then add 80 mL of acetone, stir and heat up to reflux for extraction, and wait for Soxhlet extraction When there is no obvious solid in the tube, stop heating, remove the Soxhlet extraction tube, and replace it with a distillation device for atmospheric distillation. When solids are obviously precipitated in the solution, stop the distillation, keep warm and reflux for 1 hour, cool down naturally for crystallization, and filter and dry. 7.83 g of olopatadine hydrochloride α-methyl compound B crystal form was obtained. It shows that this method can be used for repeated recovery and extraction, which is beneficial to save costs.
Embodiment 3
[0039] Preparation of olopatadine hydrochloride α-methyl compound B crystal form
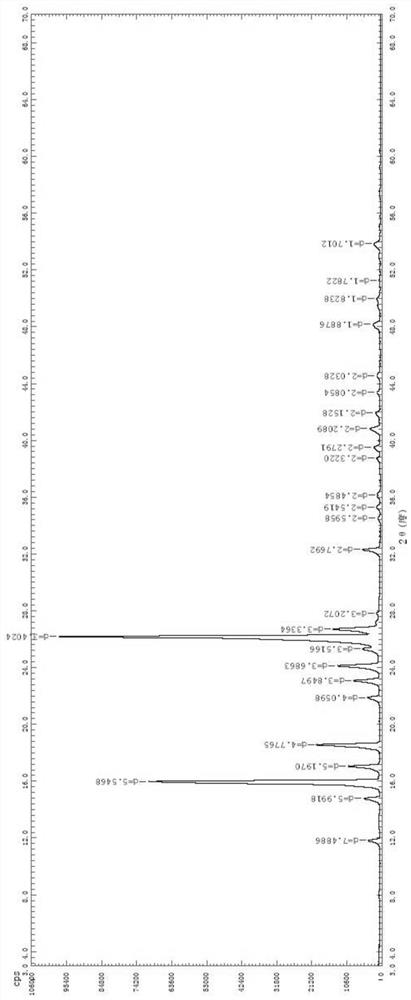
[0040] Add 10.00 g of olopatadine hydrochloride α-methyl compound into the Soxhlet extraction tube, add 100 mL of methanol into the extraction bottle, stir and heat up to reflux for extraction, and maintain a strong reflux state. When there is no obvious solid in the Soxhlet extraction tube, Stop heating, remove the Soxhlet extraction tube, and replace it with a distillation device for atmospheric distillation. When solids are clearly precipitated in the solution, stop the distillation, keep warm and reflux for 1 hour, cool down naturally to crystallize, and filter and dry to obtain 4.77g of olotaxa hydrochloride. Determine the crystal form of α-methyl compound B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com