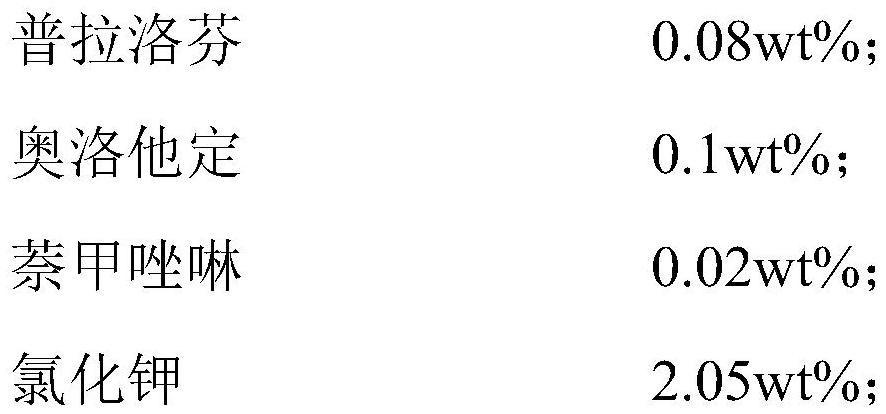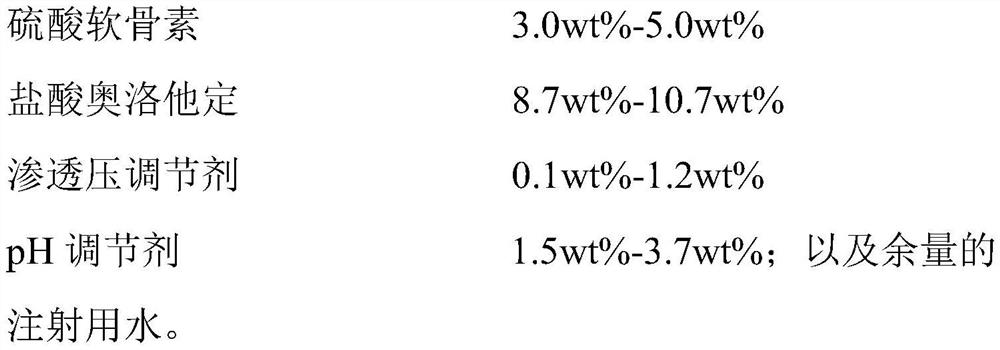Patents
Literature
31 results about "Olopatadin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Olopatadine is an antihistamine (as well as anticholinergic and mast cell stabilizer), sold as a prescription eye drop manufactured by Alcon in one of three strengths: 0.7% solution or Pazeo in the United States, ...
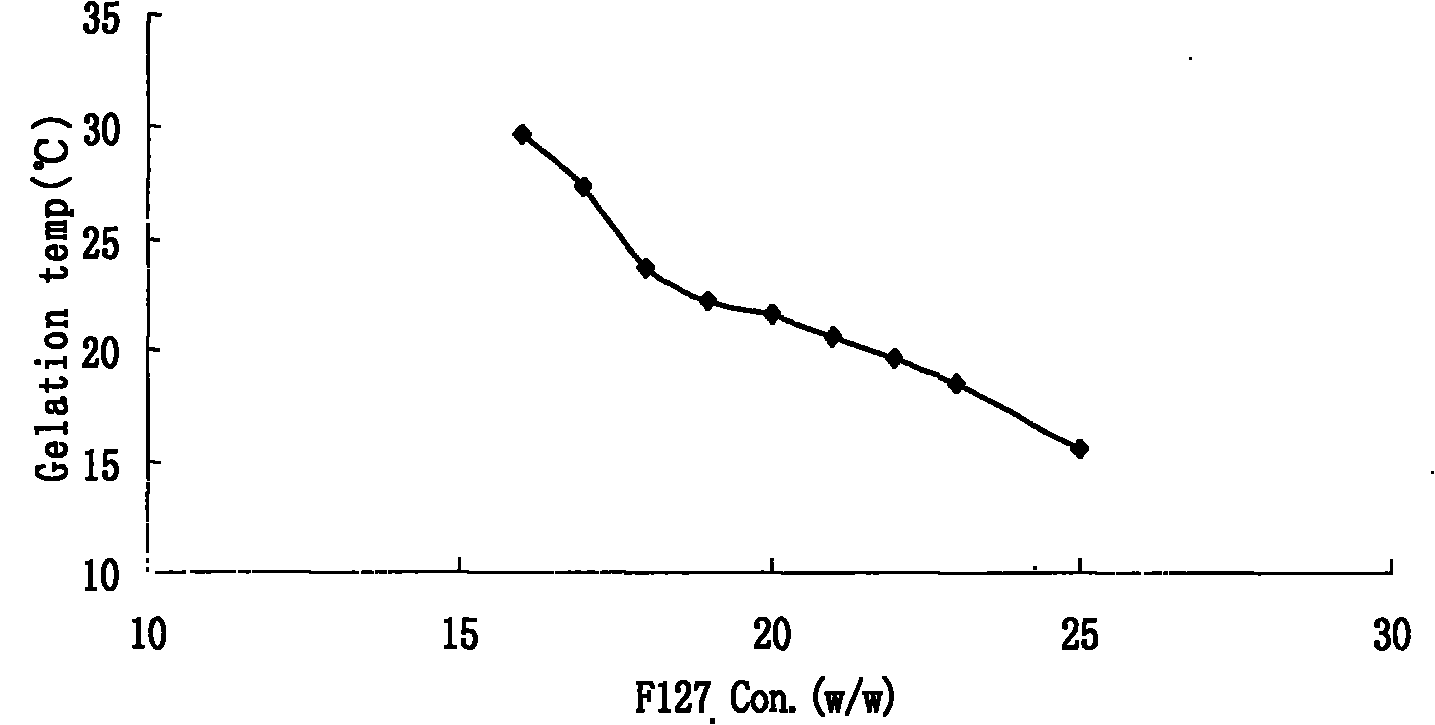
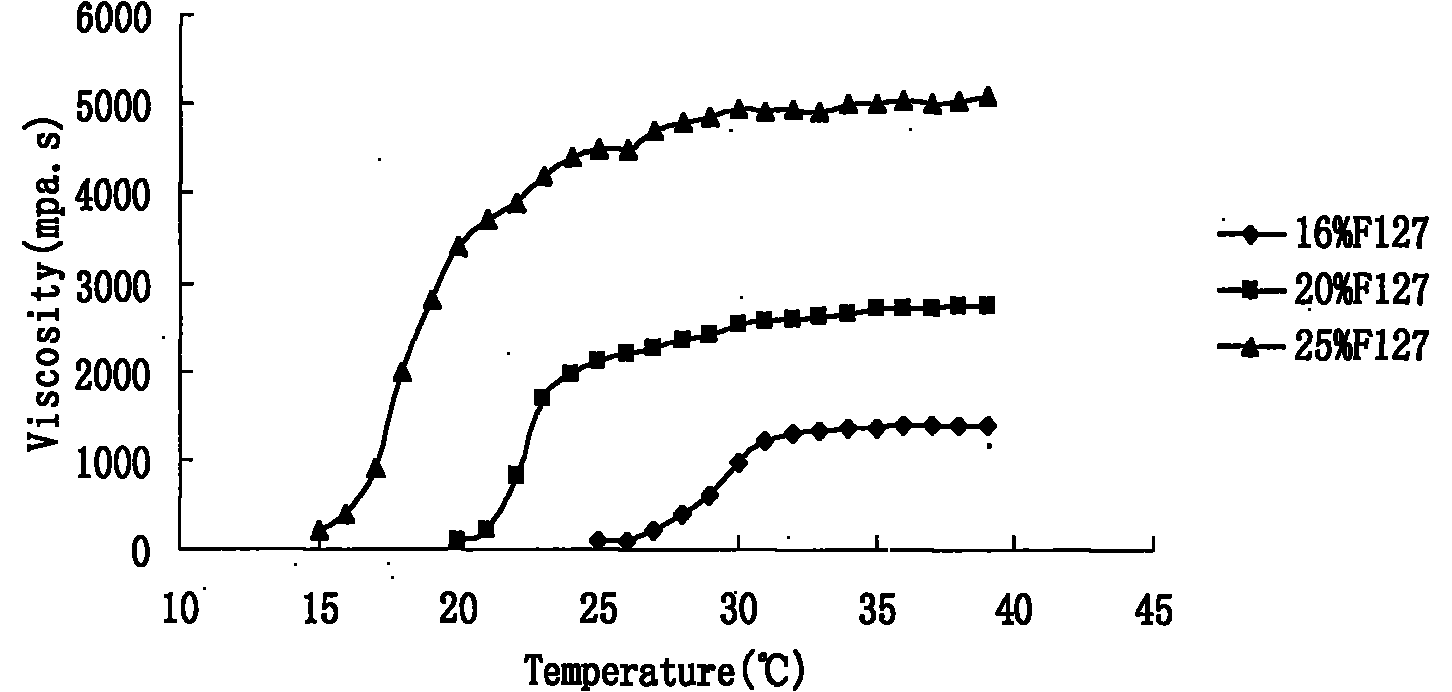
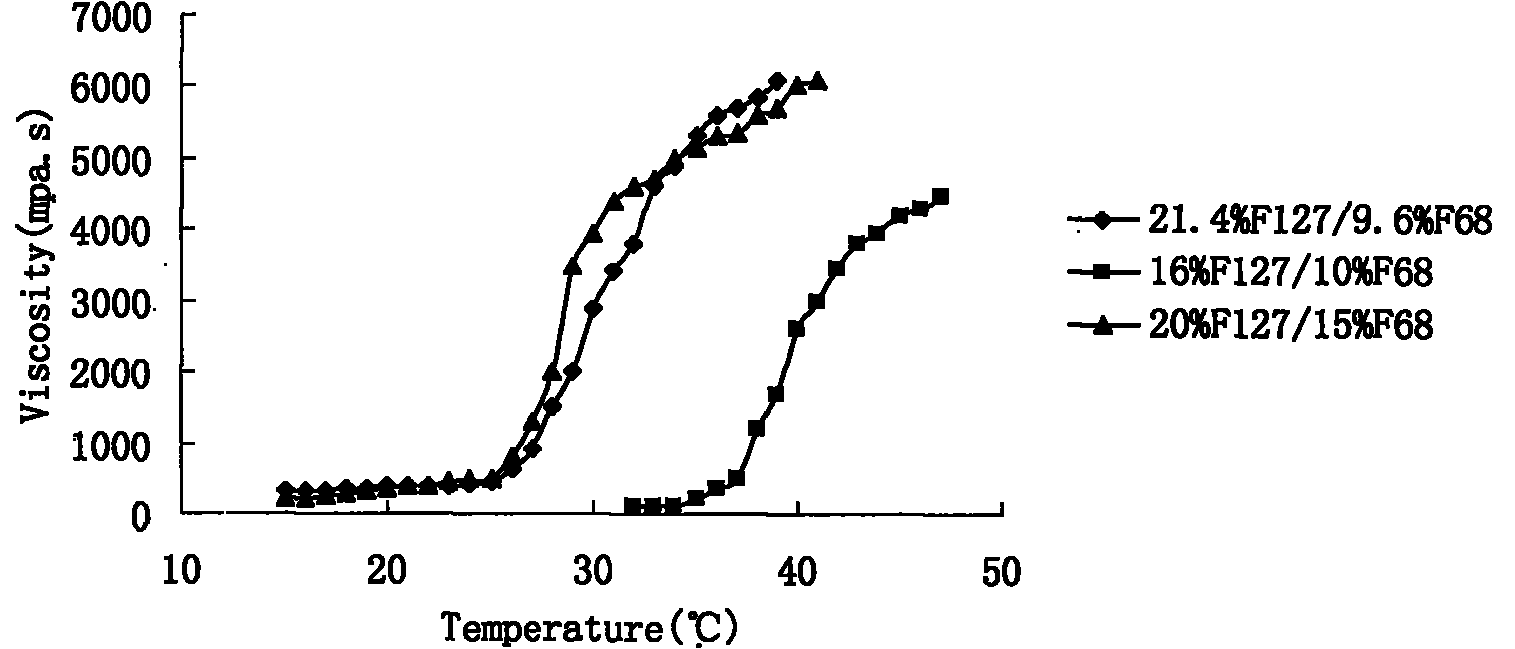
Preparation and application of olopatadine in-situ gel
InactiveCN101966144AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderMethyl cellulosePharmaceutical drug
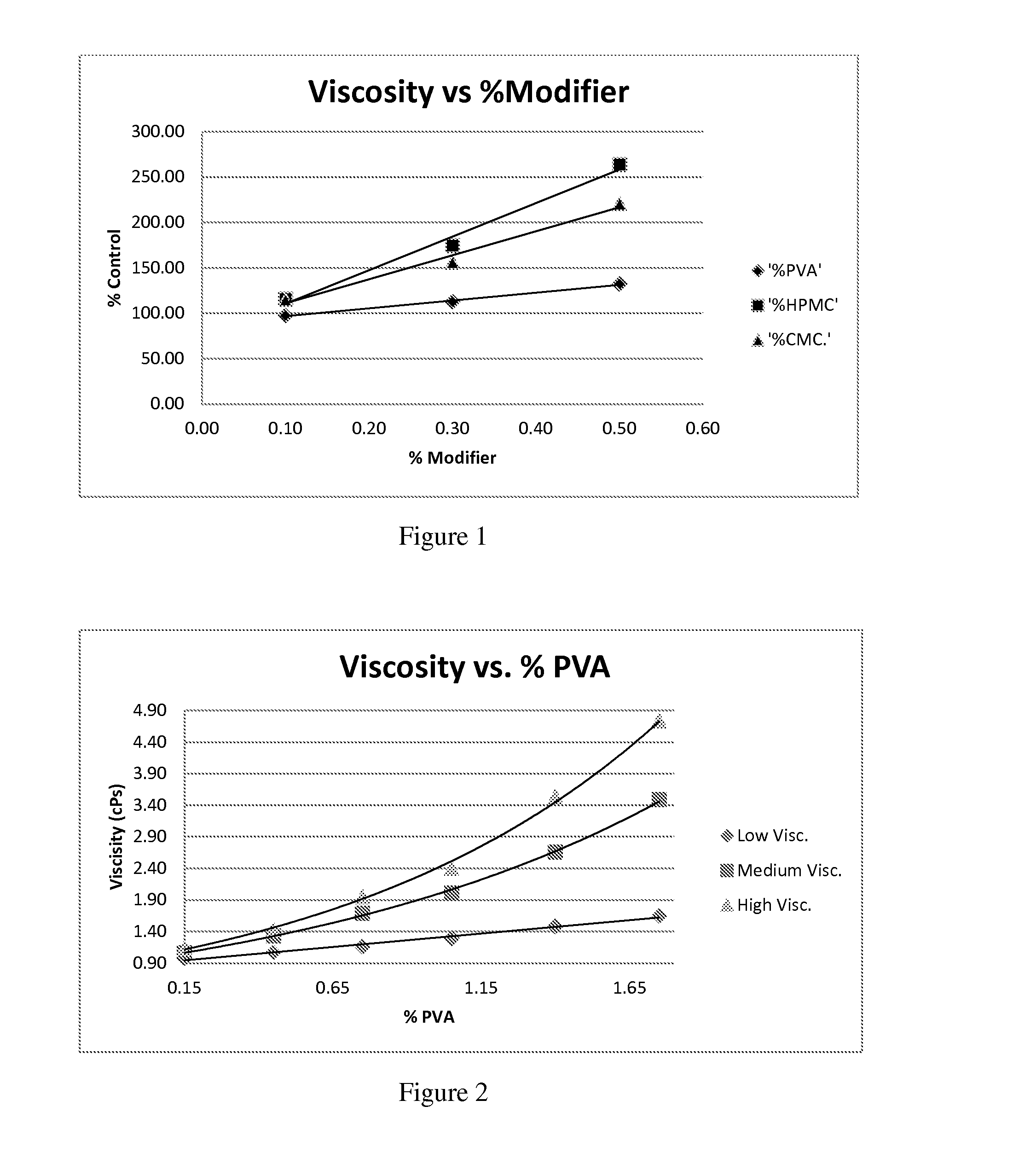
The invention relates to a new dosage form of olopatadine in the technical field of medicines, in particular to the preparation and an application of olopatadine in-situ gel. The invention is characterized in that the preparation is a solution in a non-physiological state and is converted into gel in a physical state. Olopatadine temperature-sensitive gel is prepared by systematically investigating shaft materials and properly proportioning poloxamer 407 (F127 for short) and poloxamer 188 (F68 for short). Olopatadine pH-sensitive gel is prepared by adjusting the consumption of carbomer 980 and hydroxypropyl methyl cellulose (HPMC). The preparations can be applied through the eyes or nasal cavity, the residence time of the drug in the administration parts is greatly prolonged, bioavailability is increased, curative effect is improved, and the preparation has ideal application prospects.
Owner:胡容峰
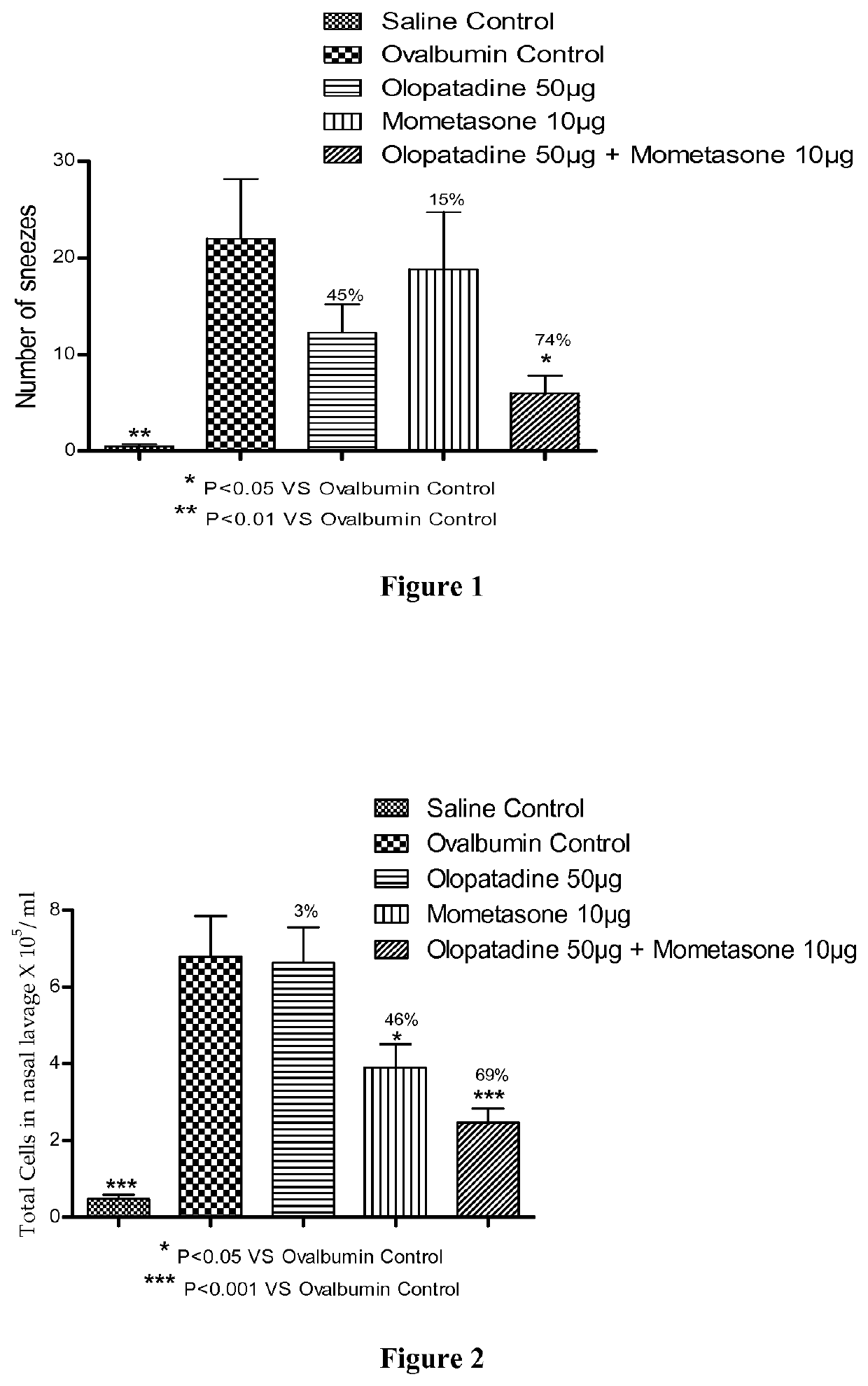
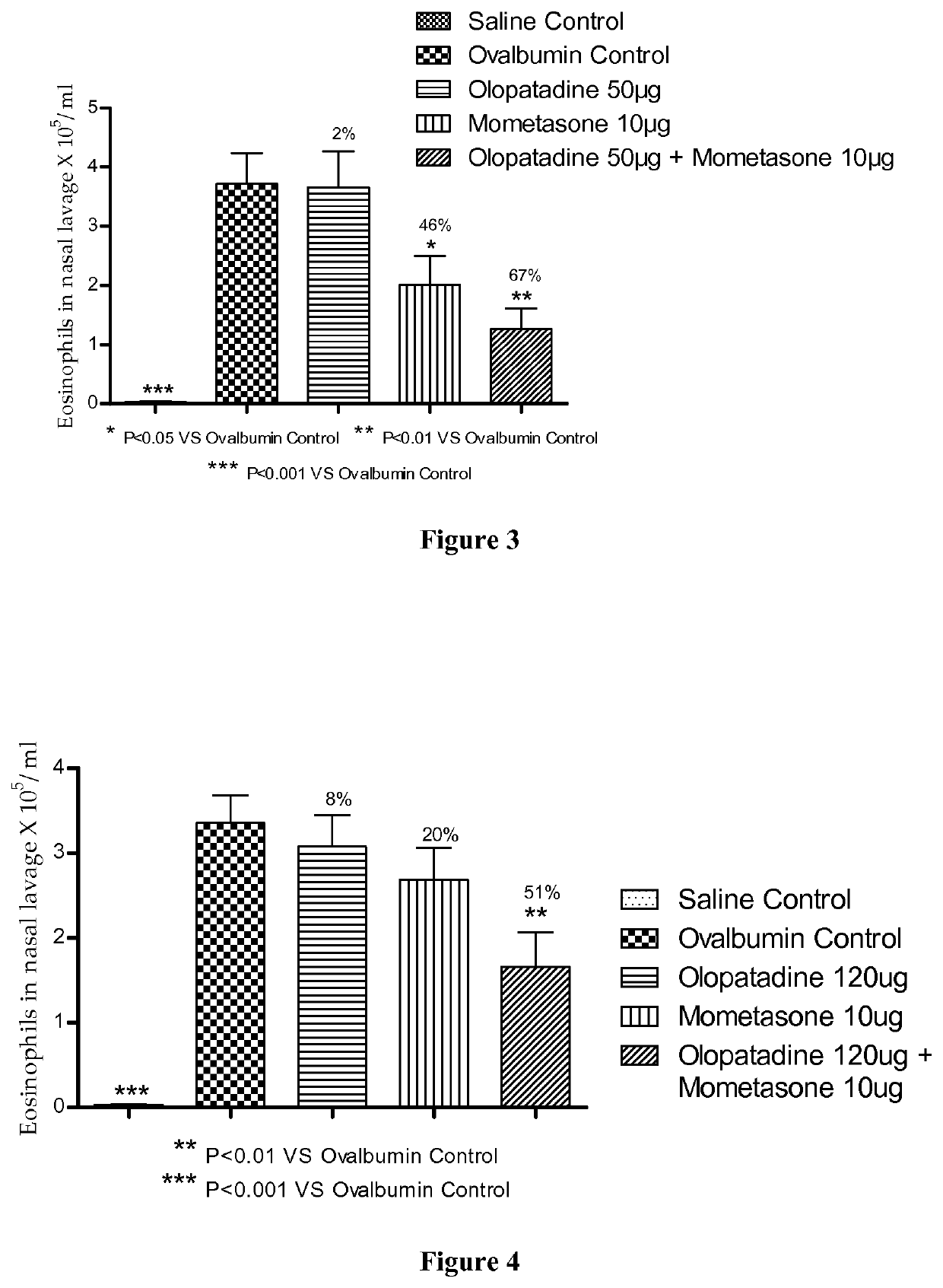
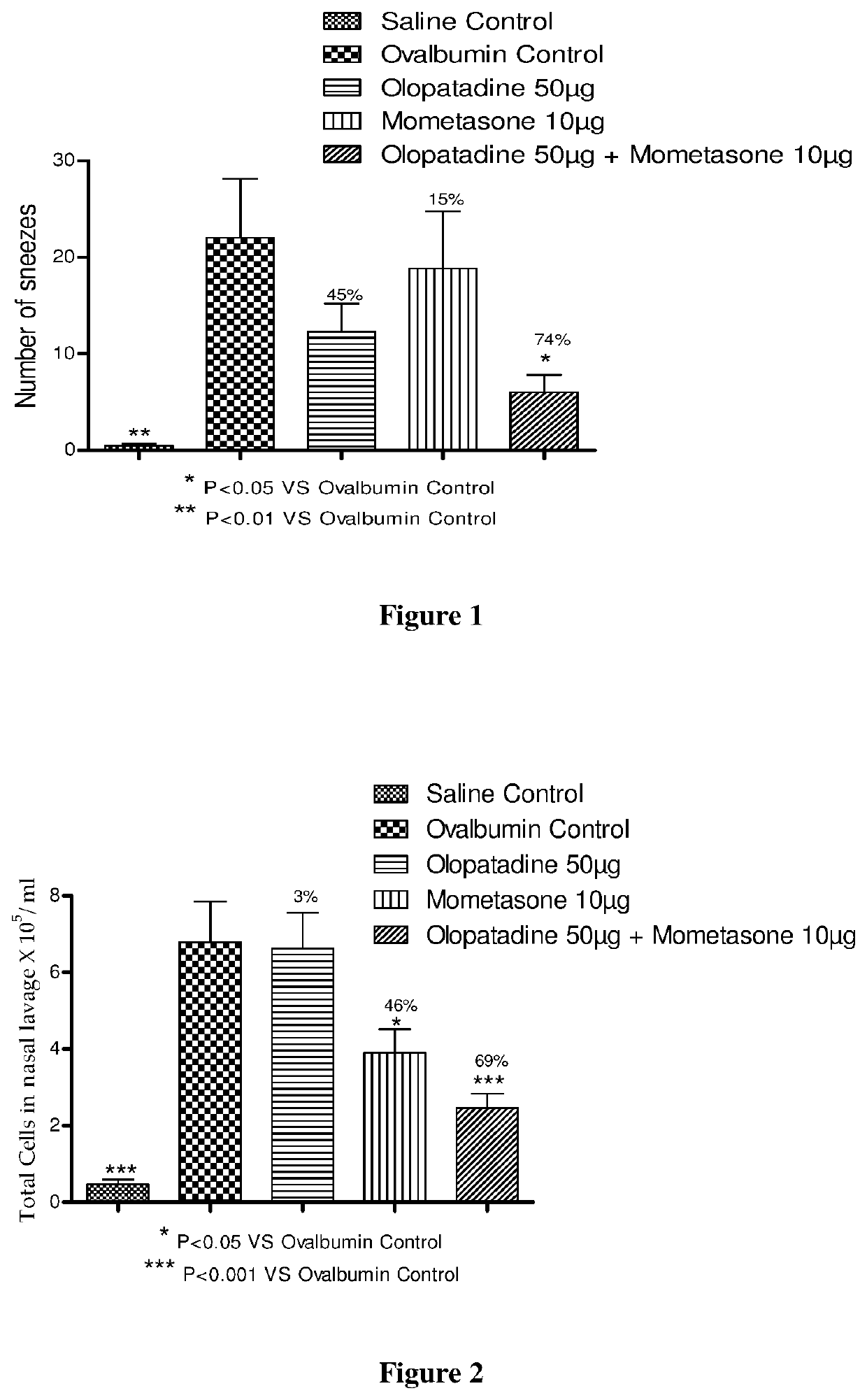
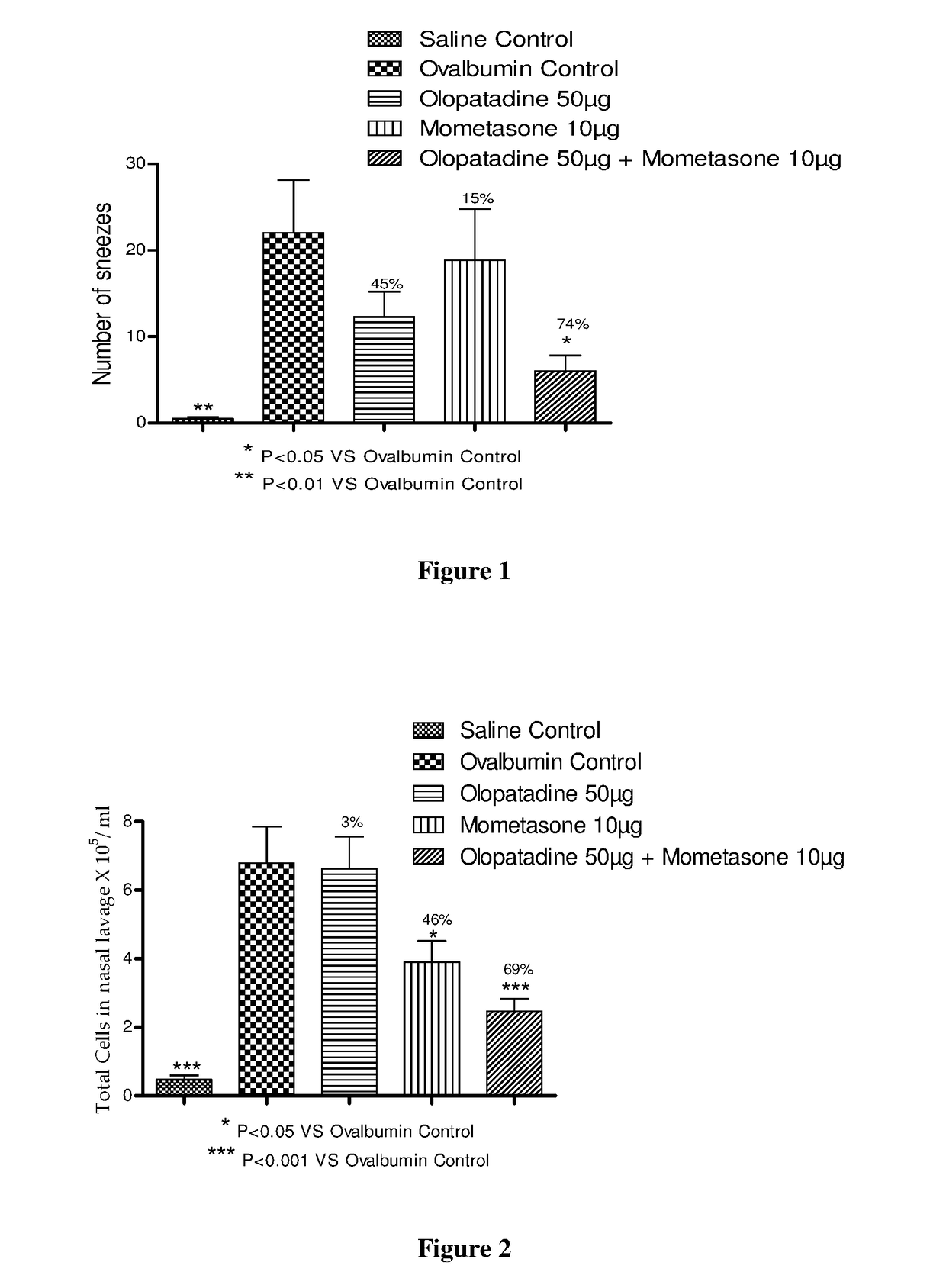
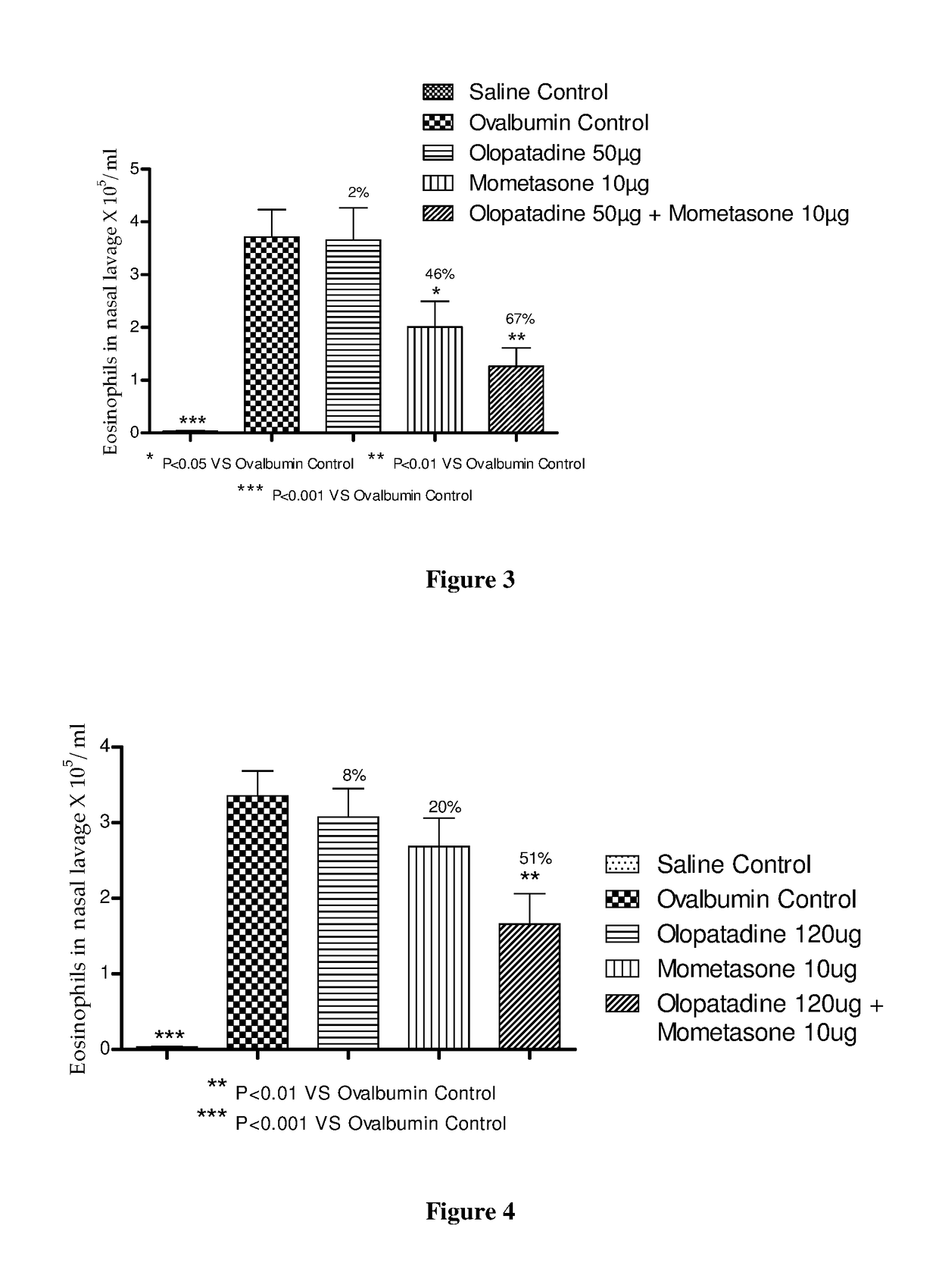
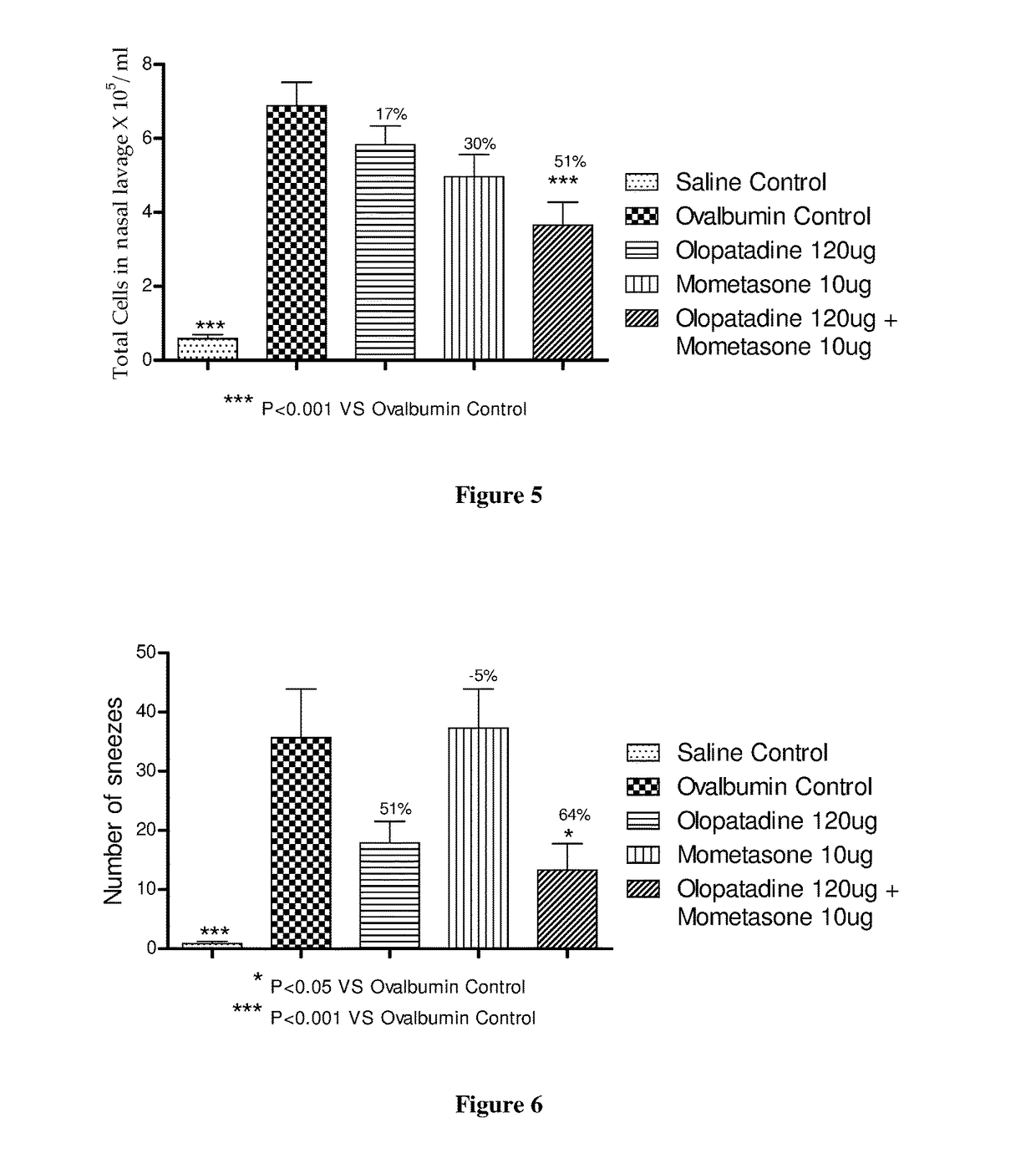
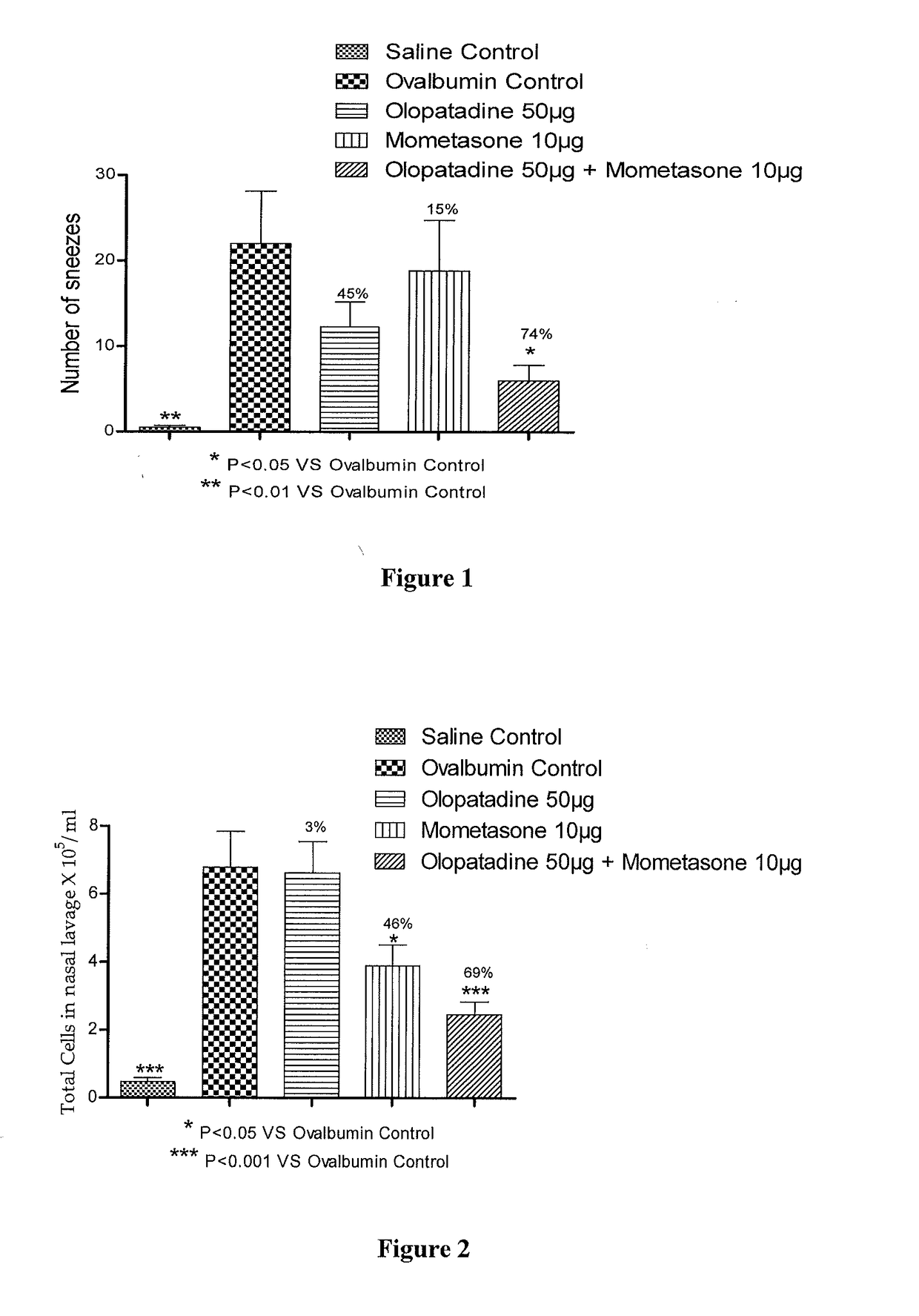
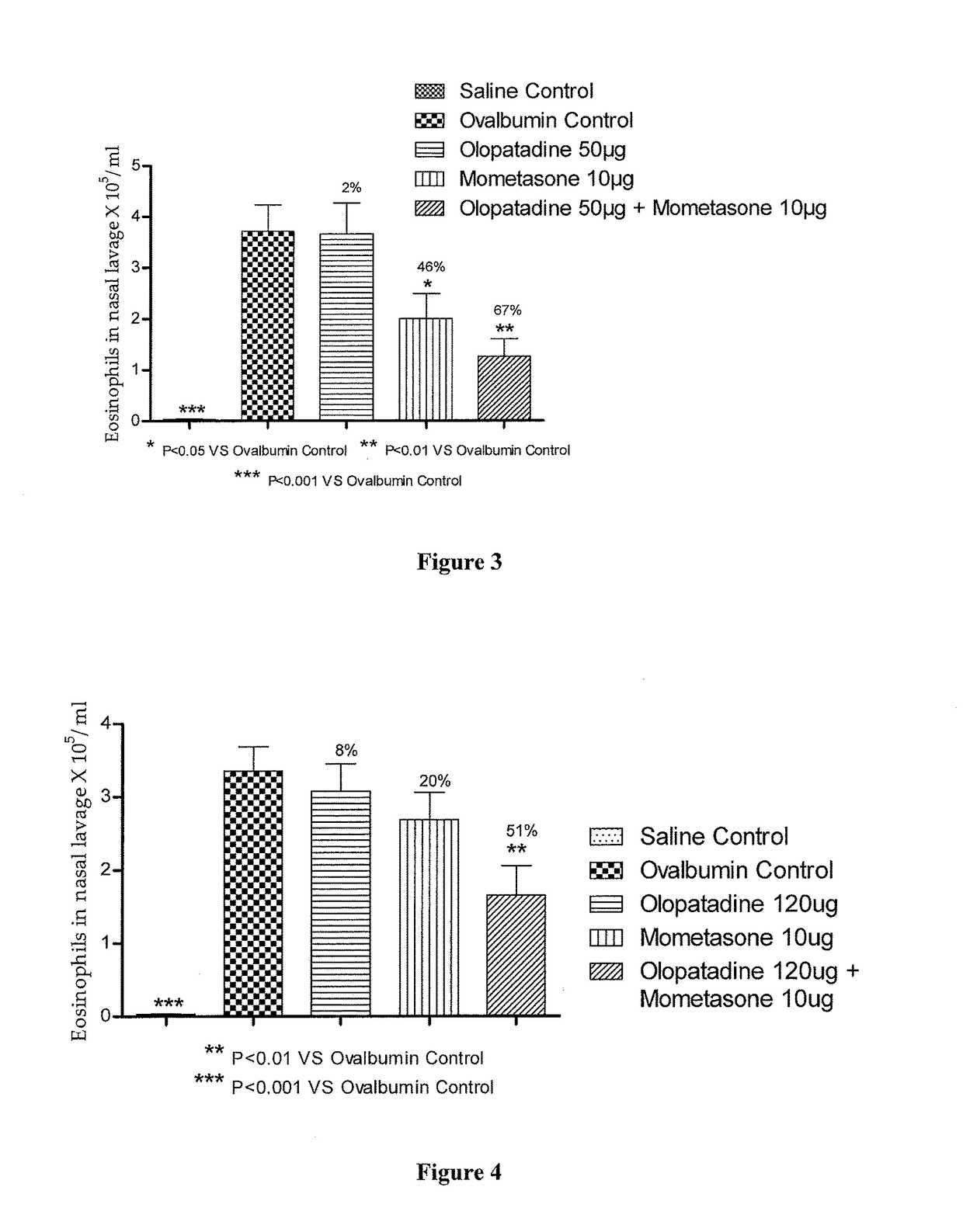
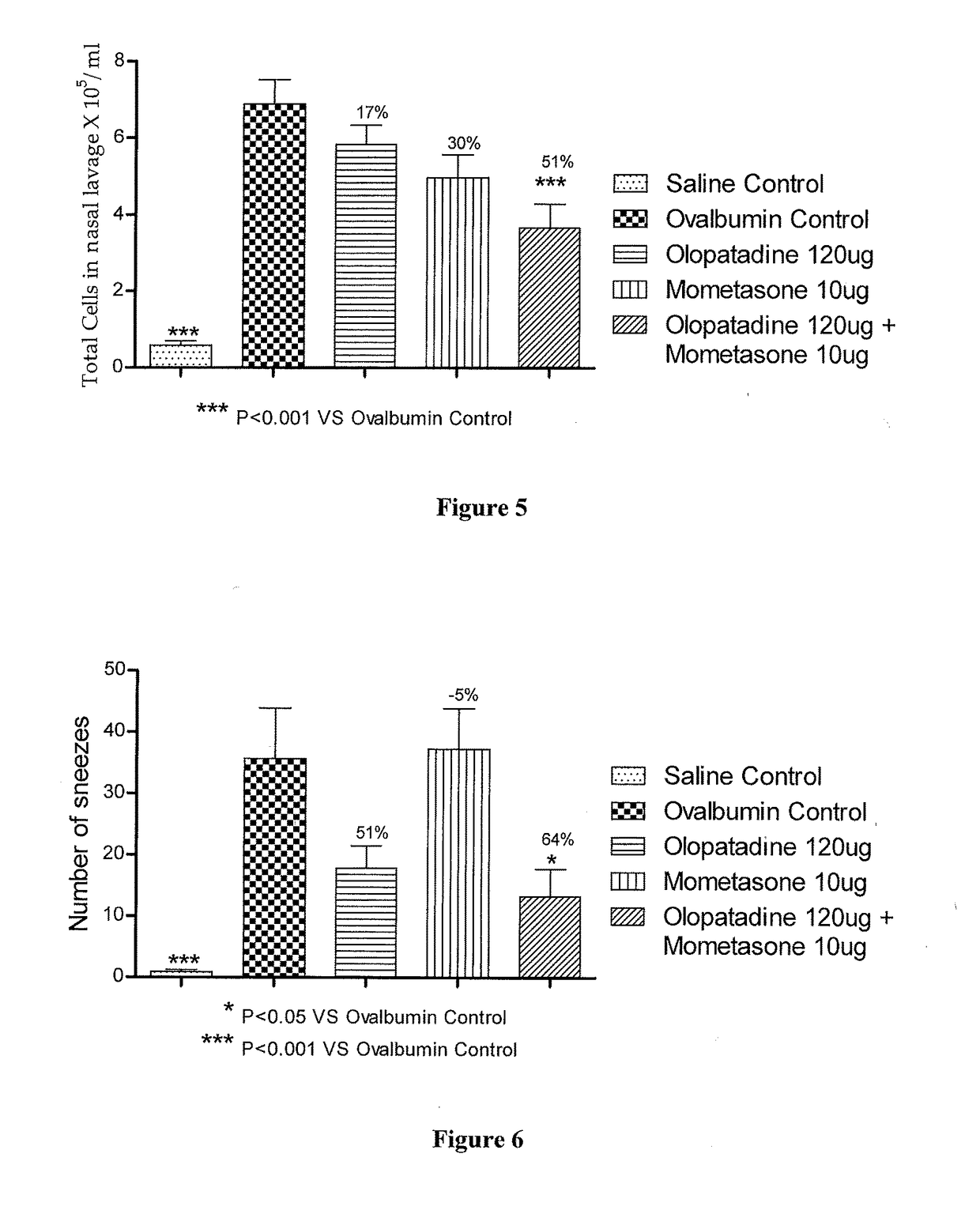
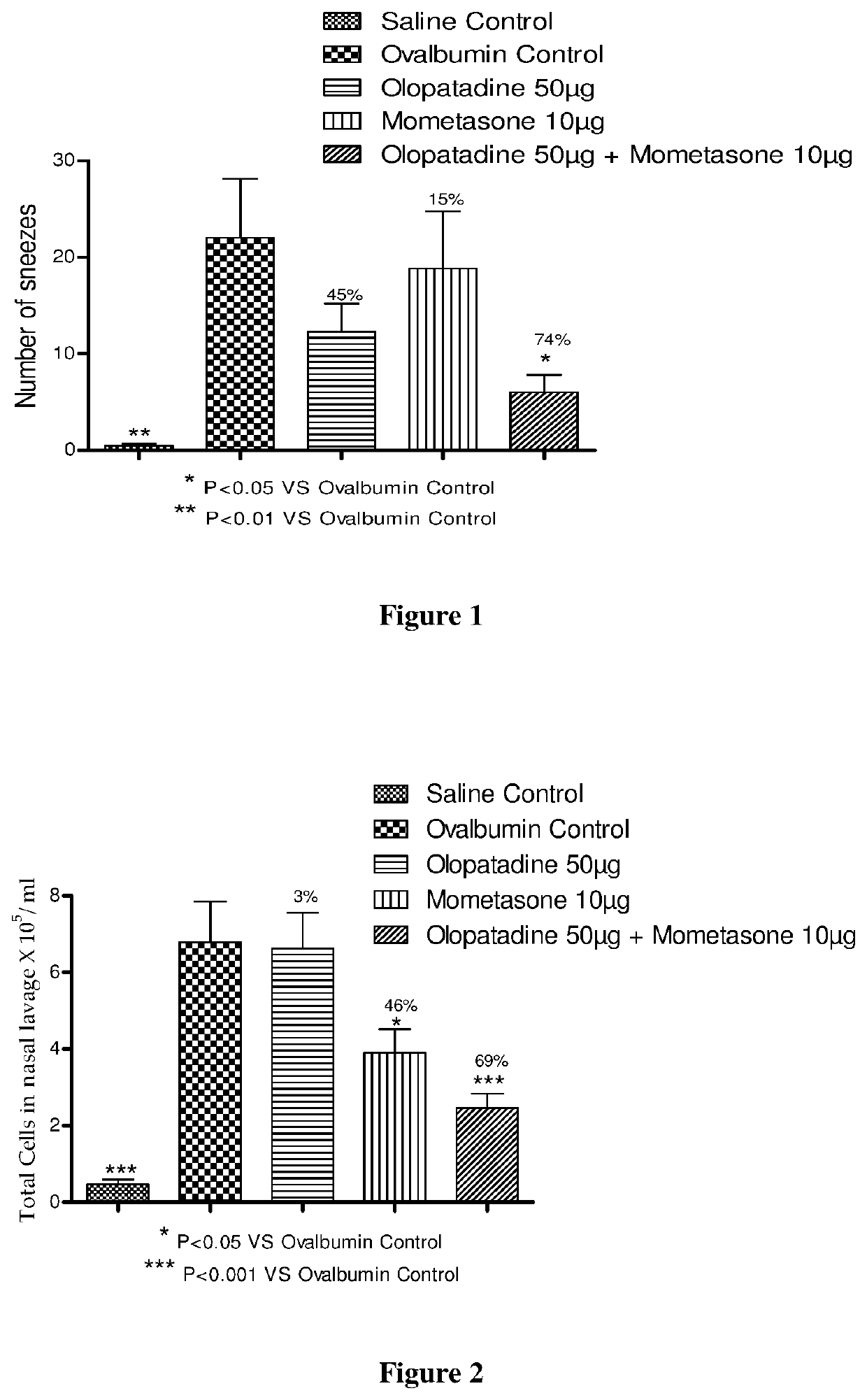
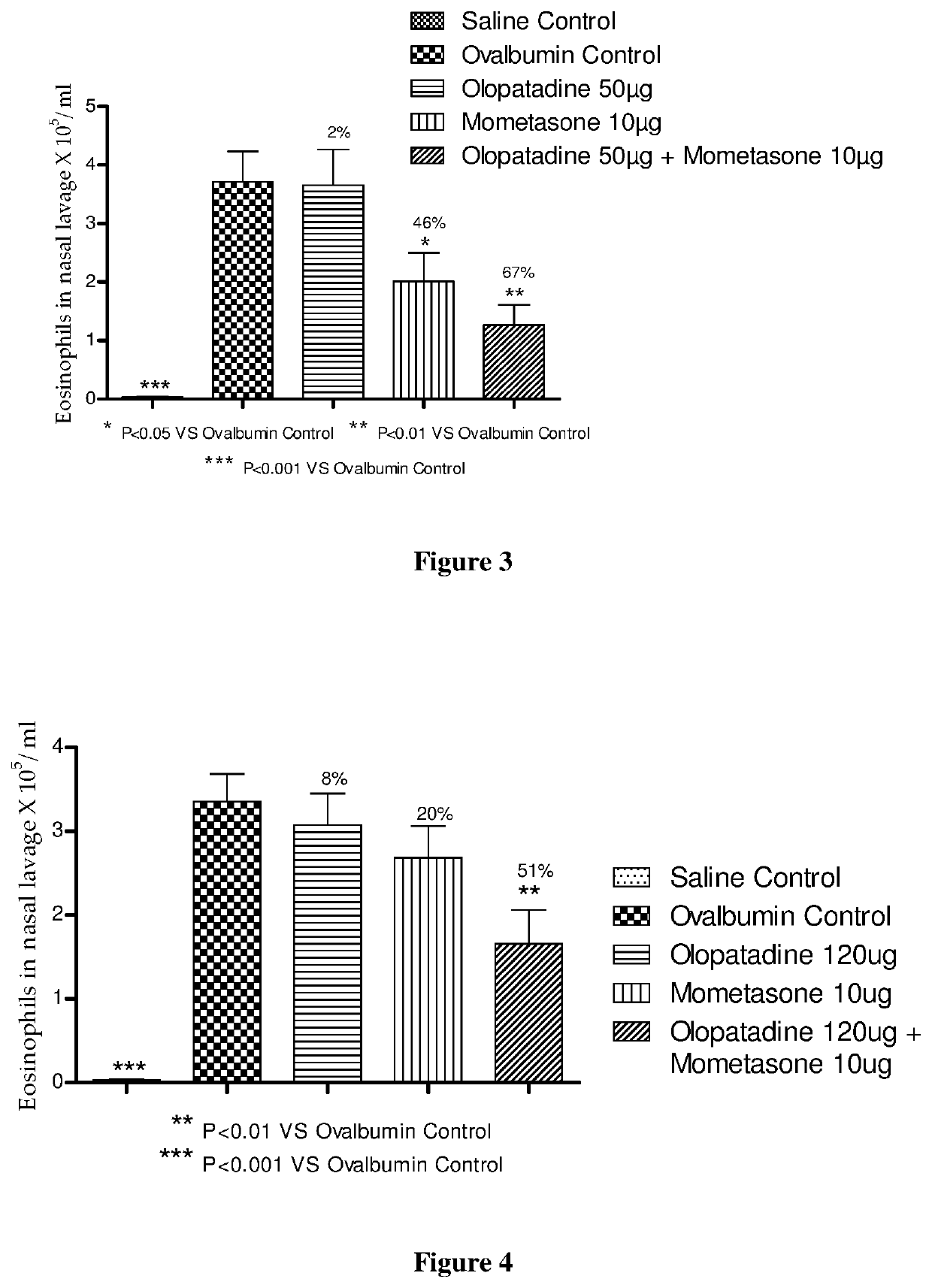
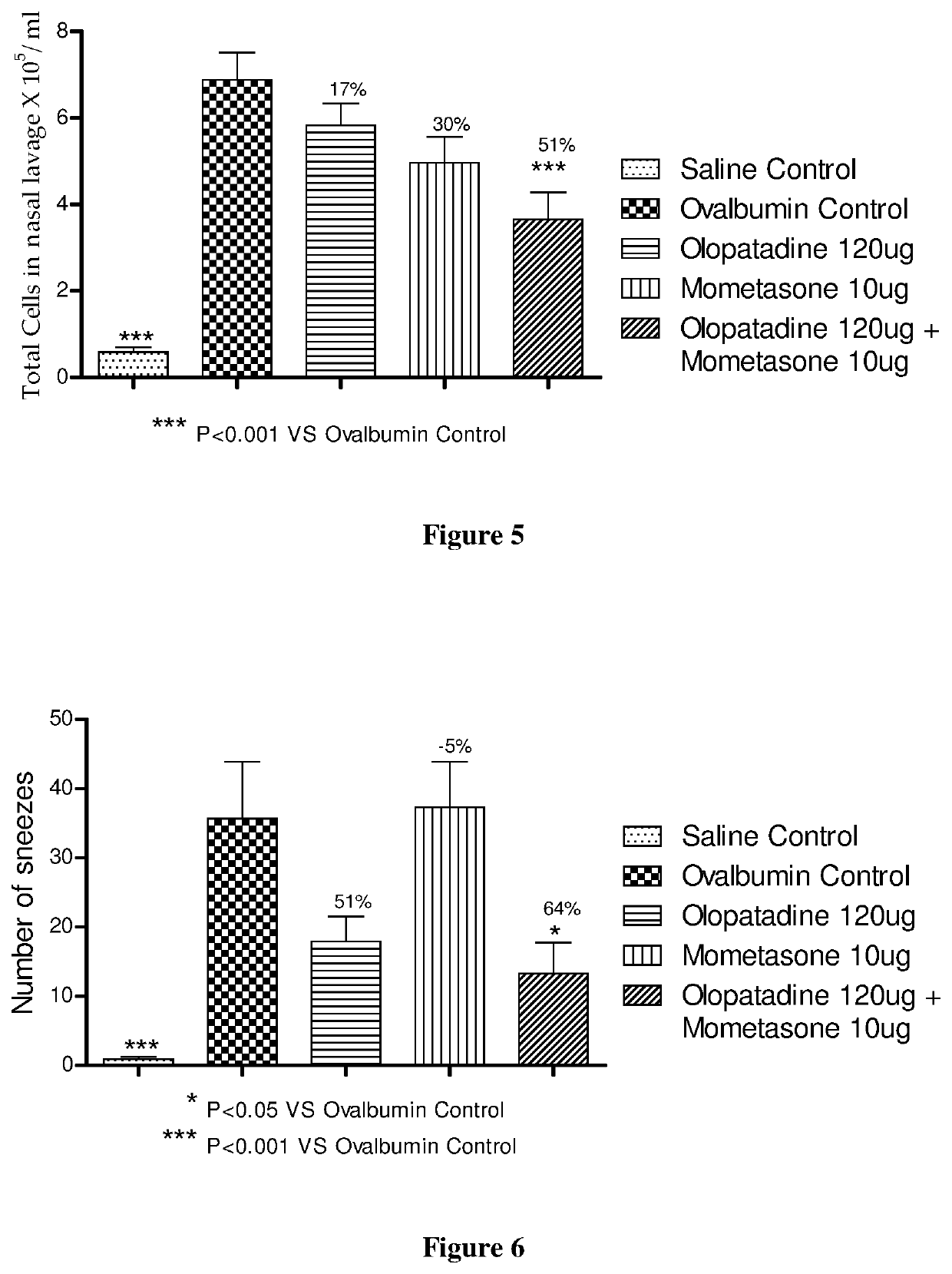
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
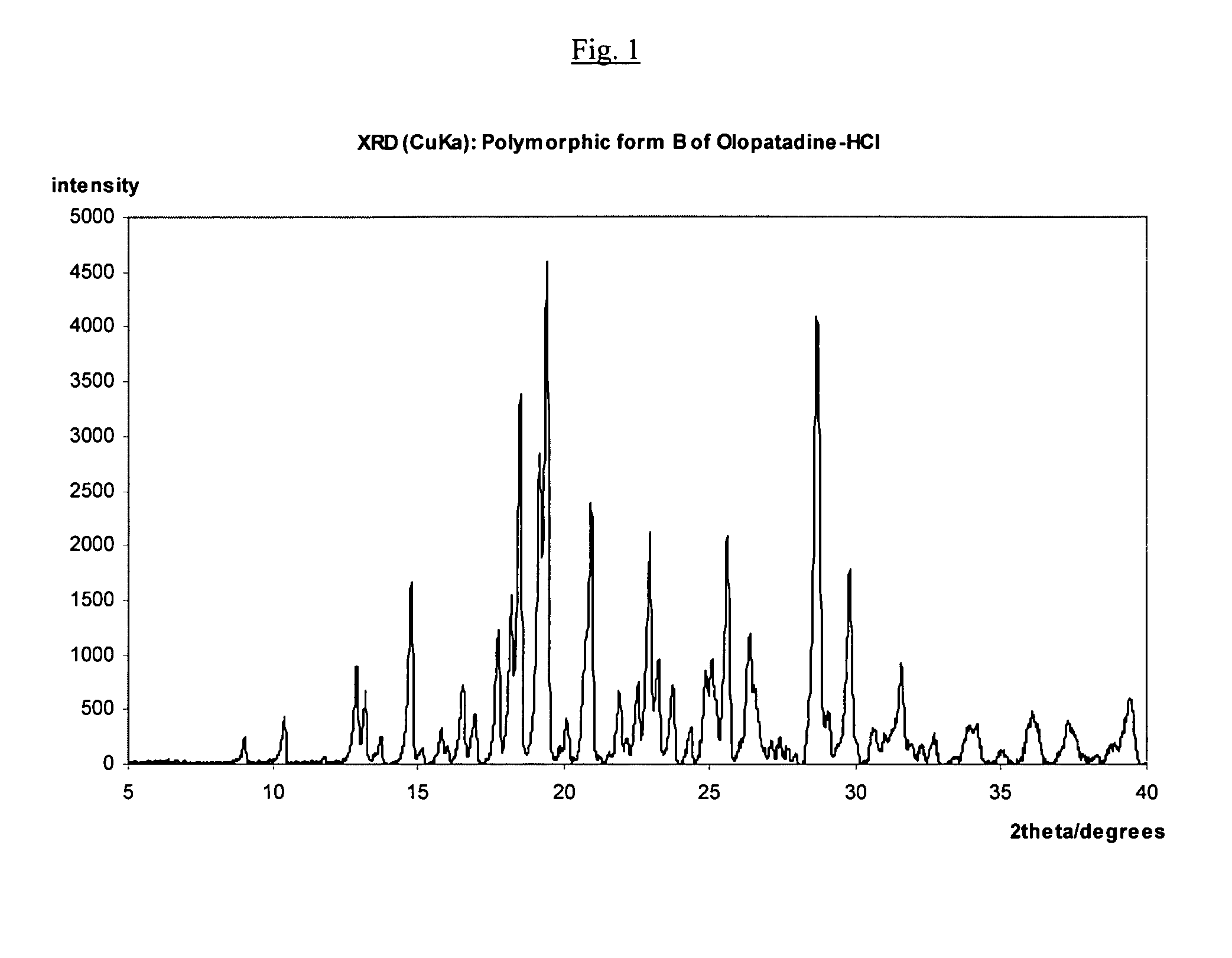
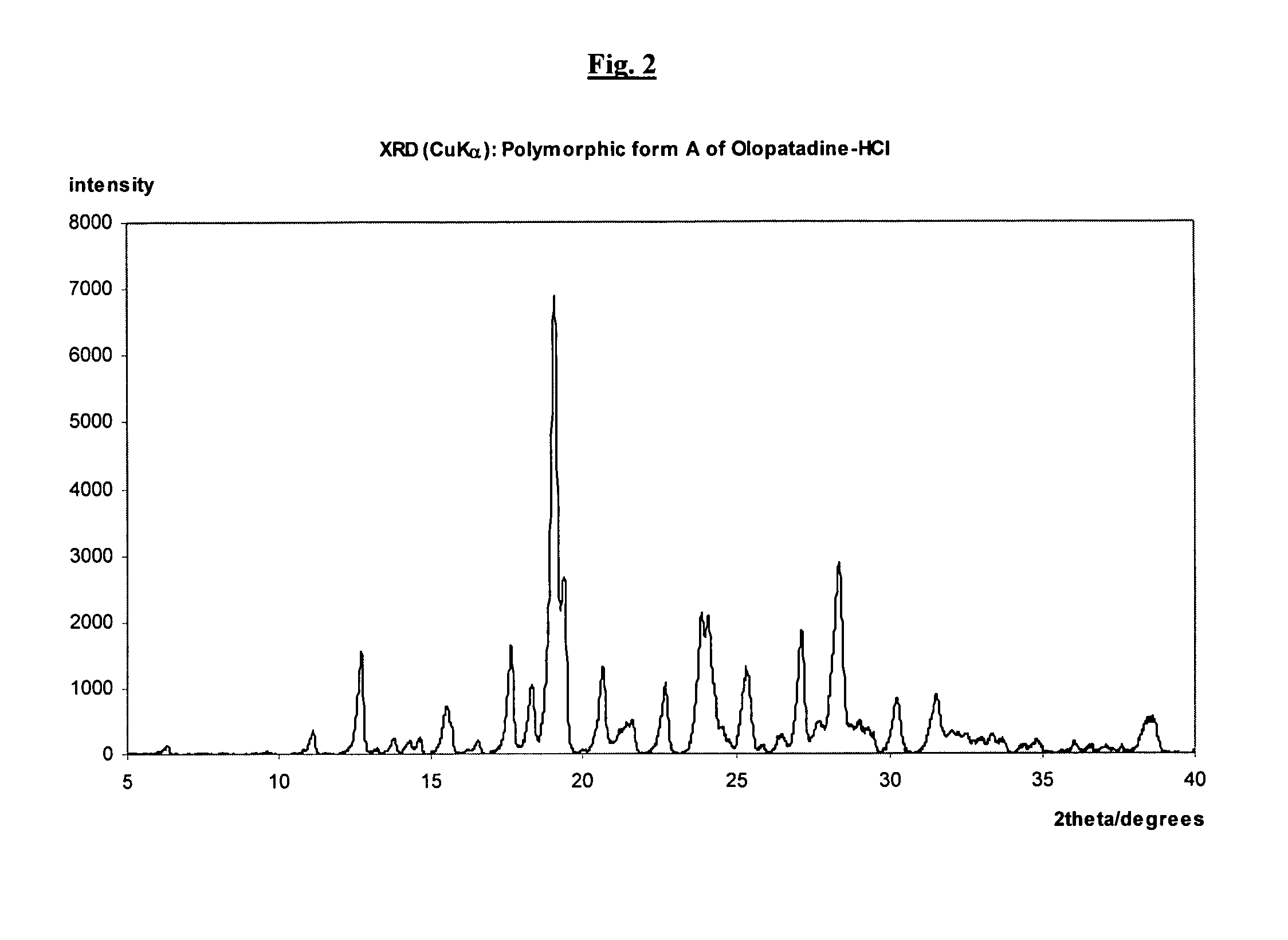
Polymorphic forms of olopatadine hydrochloride and methods for producing olopatadine and salts thereof
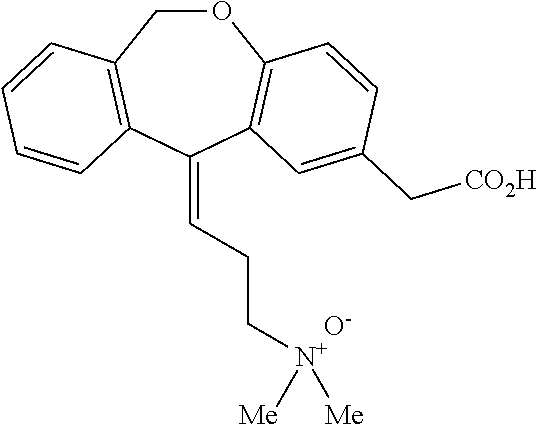
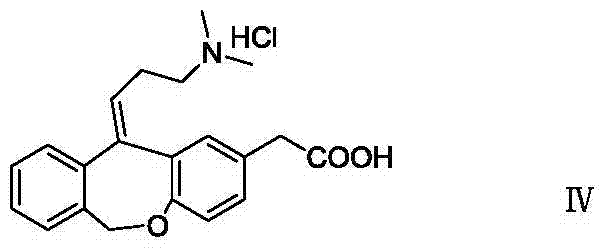
The present invention provides a novel polymorphic form of olopatadine hydrochloride ([(Z)-3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride), a selective histamine H1-receptor antagonist that is used for the treatment of ocular symptoms of seasonal allergic conjunctivitis. The present invention also provides novel methods for producing olopatadine on a large scale, and in a manner that is cost effective, provides a low level of impurities and eliminates the need to use the costly and dangerous base, butyllithium, which is used in prior art reactions for making olopatadine. The present invention further provides novel processes for carrying out a large scale production of 3-dimethylaminopropyltriphenylphosphonium bromide and its corresponding hydrobromide salt, which are employed in the production of olopatadine, and pharmaceutically acceptable salts of olopatadine.
Owner:UNIV ZURICH
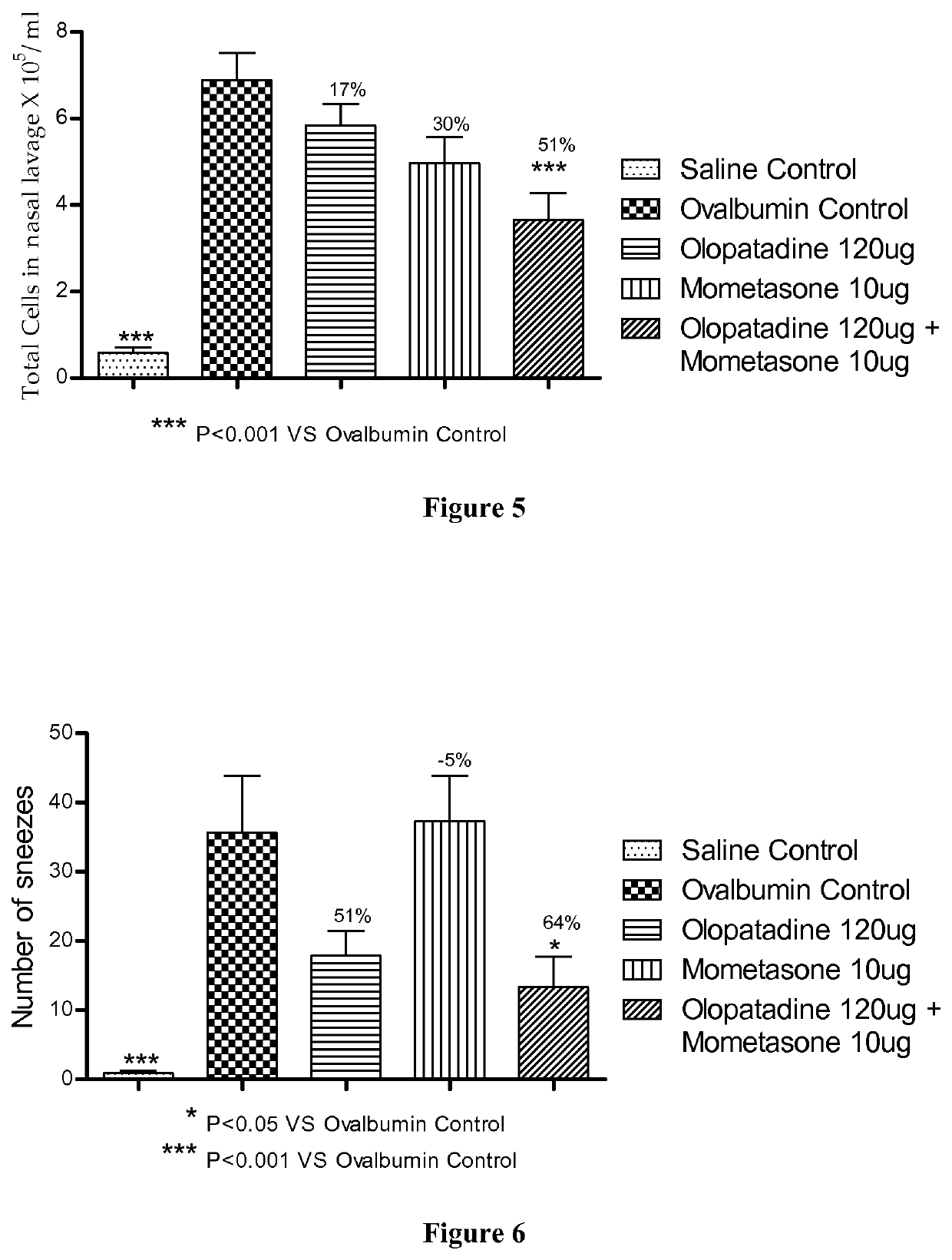
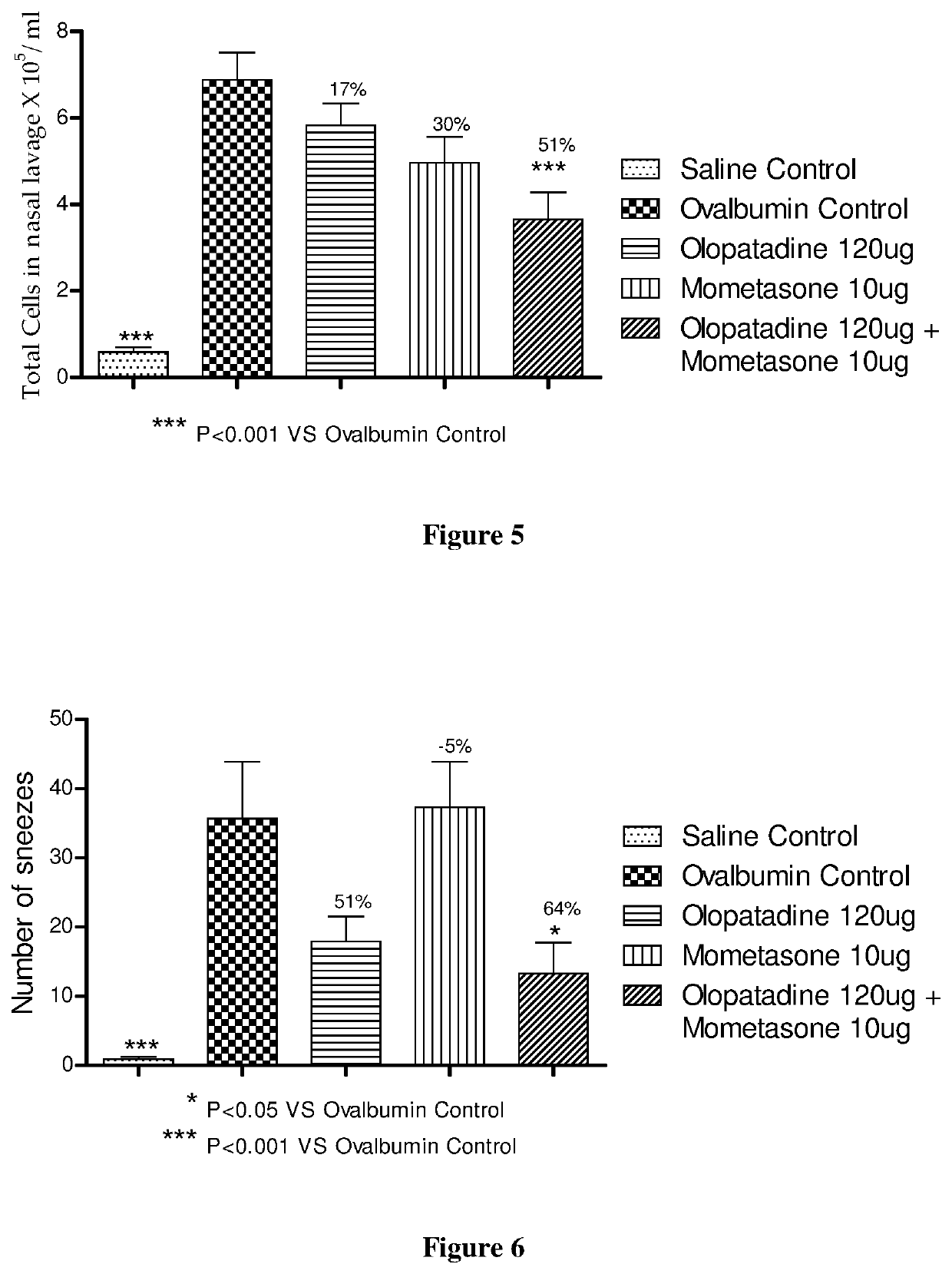
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS20190030047A1Better therapeutic valueIncrease valueOrganic active ingredientsSenses disorderMometasoneOlopatadine
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a pediatric human subject) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
Process for the preparation of olopatadine
ActiveUS20110065936A1Organic active ingredientsSenses disorderMedicinal chemistryOlopatadine Hydrochloride
The present invention relates to a novel process for the preparation of olopatadine hydrochloride starting from an advanced intermediate.
Owner:F I S FAB ILTALIANA SINTETICI SPA
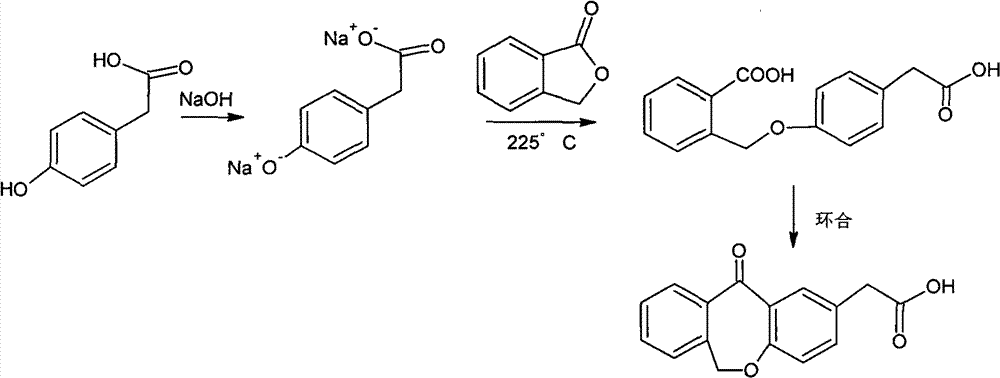
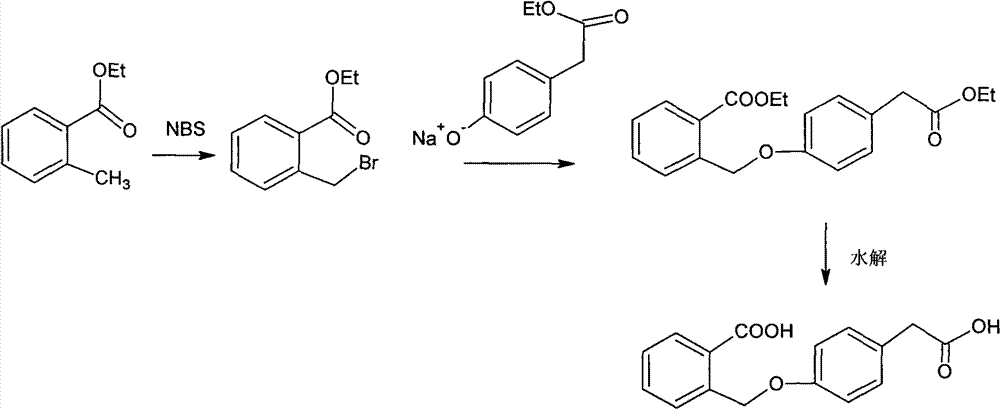
Improved preparation method of 4-(2-carboxybenzyloxy) phenylacetic acid
InactiveCN102757339AReduce process stepsReduce energy consumptionPreparation from carboxylic acid esters/lactonesAcetic acidPhenylacetic acid
The invention provides an improved preparation method of 4-(2-carboxybenzyloxy) phenylacetic acid. 6, 11-dihydro-11-oxo-dibenz (b, e) oxepin-2-acetic acid which is a raw material for producing the anti-allergic drug of olopatatadine can be obtained through a cyclization reaction of the 4-(2-carboxybenzyloxy) phenylacetic acid.
Owner:北京联本医药化学技术有限公司
Novel method for preparing olopatadine hydrochloride by using high-activity organic zinc reagent
The invention discloses a novel method for preparing olopatadine hydrochloride. The method comprises the steps as follows: (1) high-activity zinc and 3-bromo-N,N-dimethylpropylamine form an organic zinc reagent (I); (2) the organic zinc reagent is eliminated after electrophilic substitution with isoxepac (II) to form olopatadine (III); (3) the olopatadine with higher purity is obtained through recrystalization of the olopatadine, wherein structural formulas of the organic zinc reagent (I), the isoxepac (II) and the olopatadine (III) are as follows.
Owner:北京华禧联合科技发展有限公司
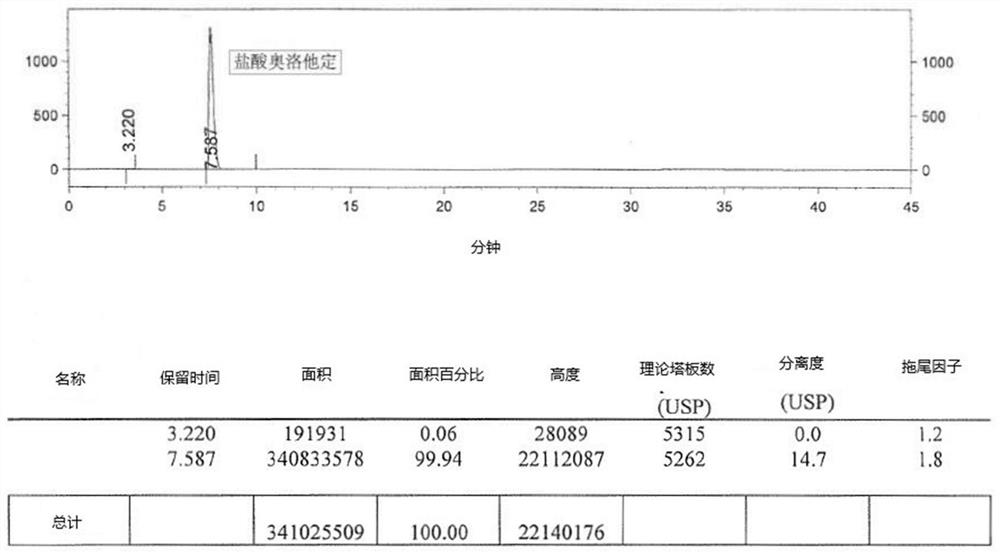
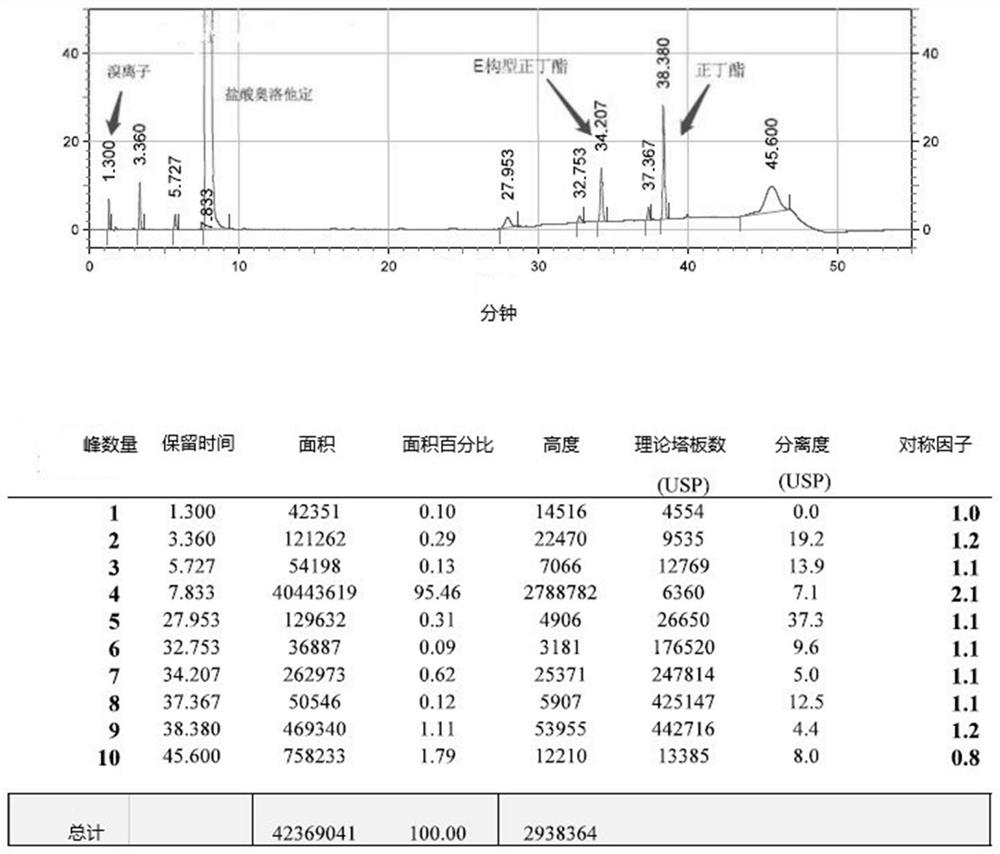
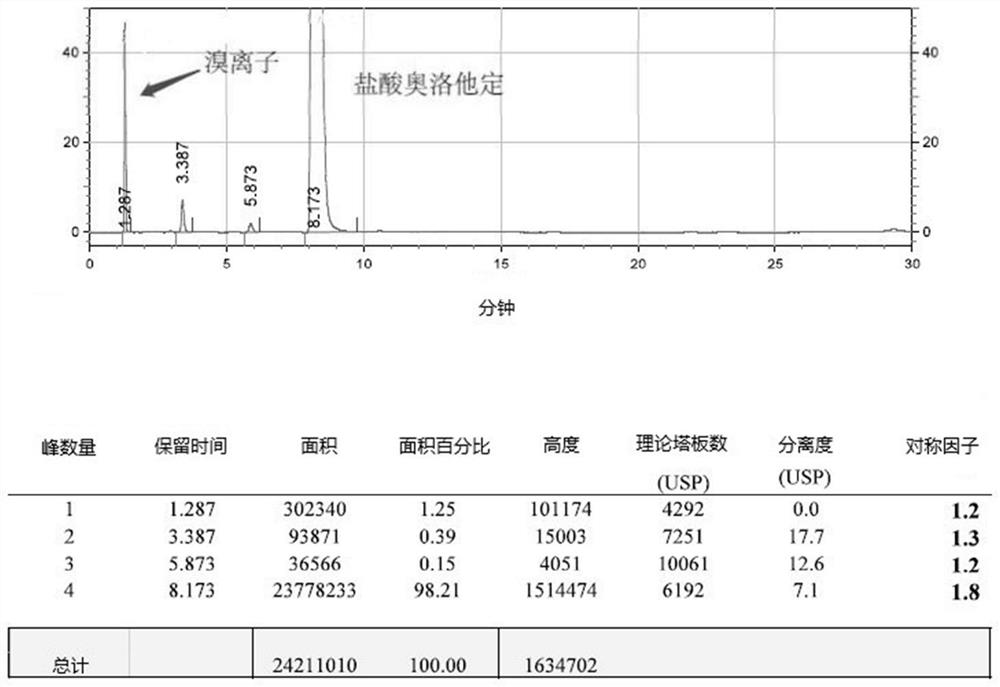
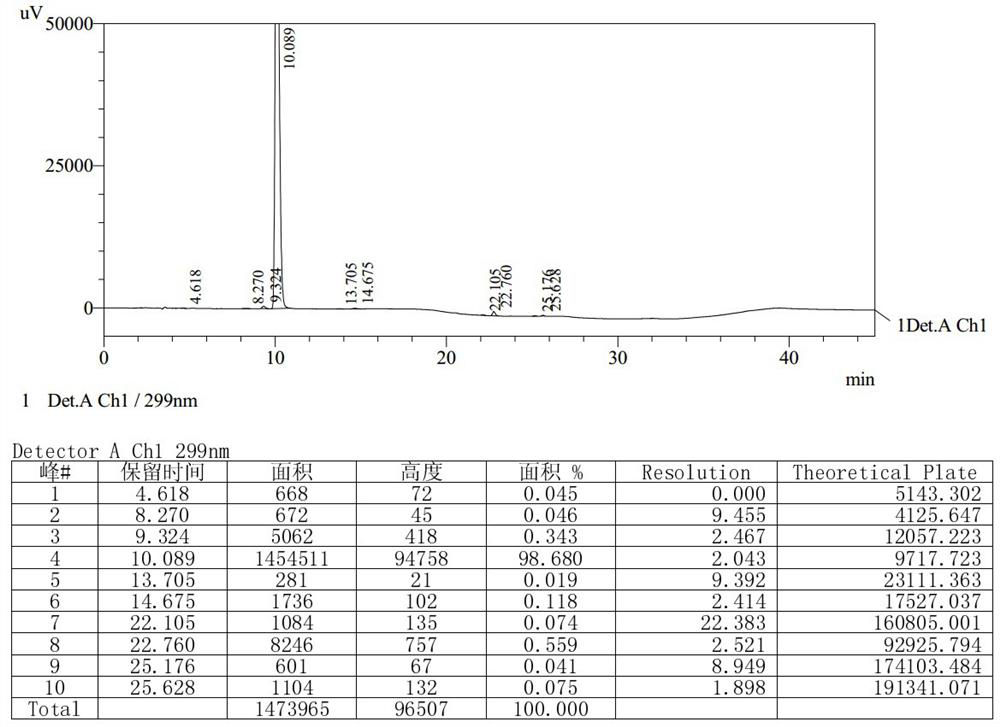
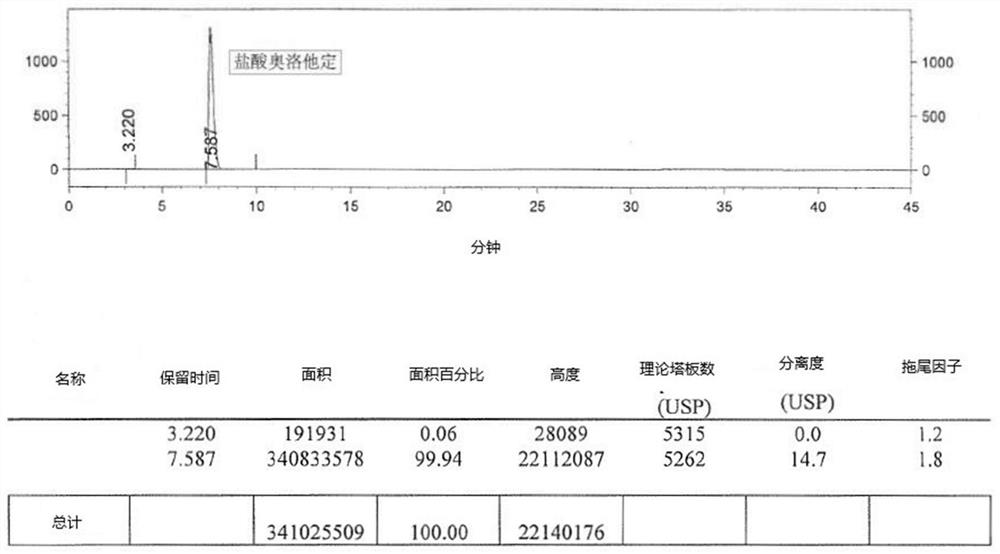
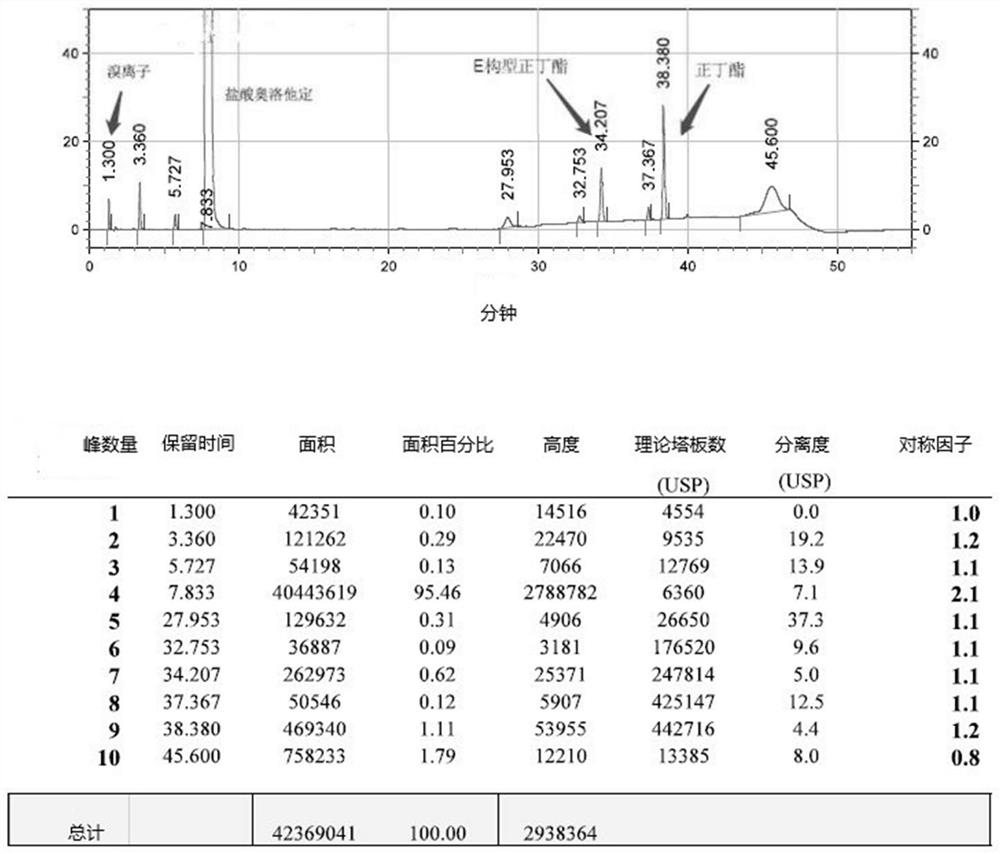
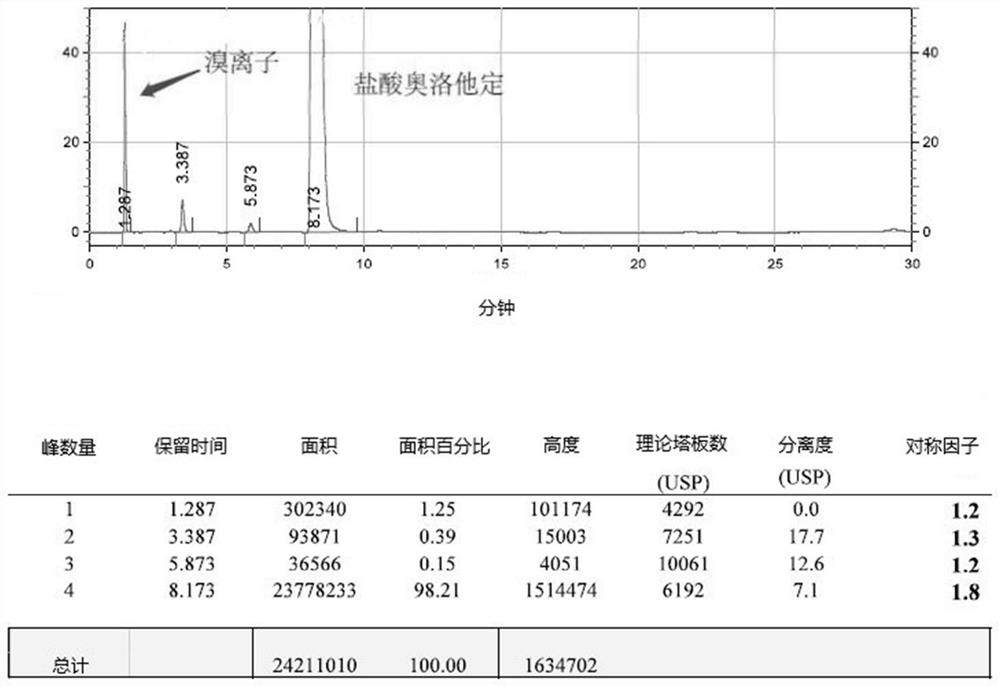
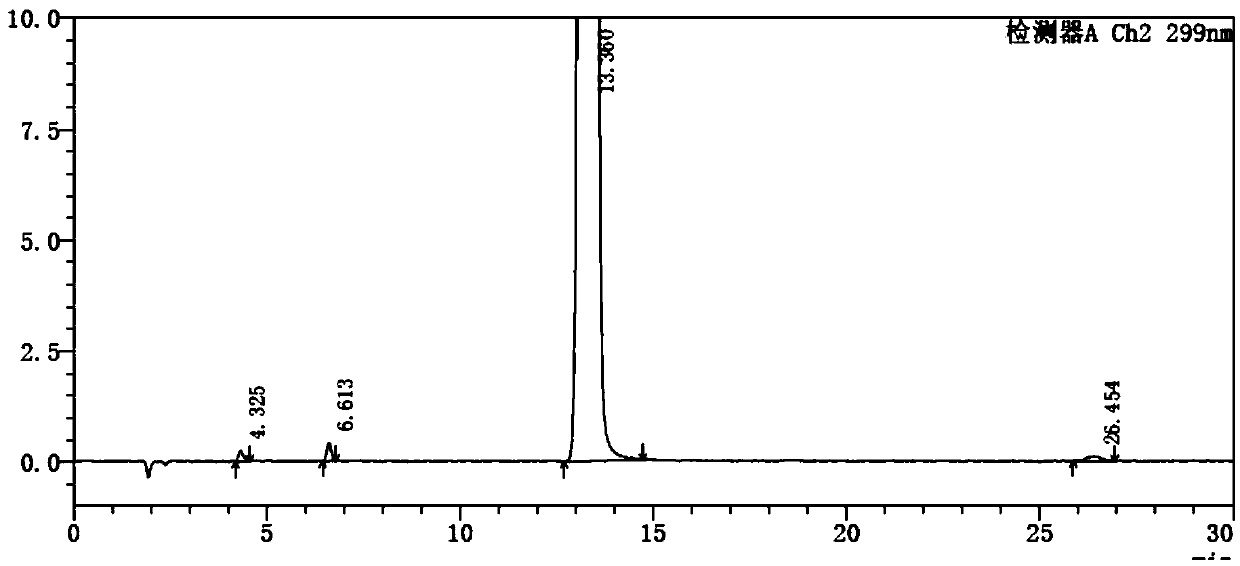
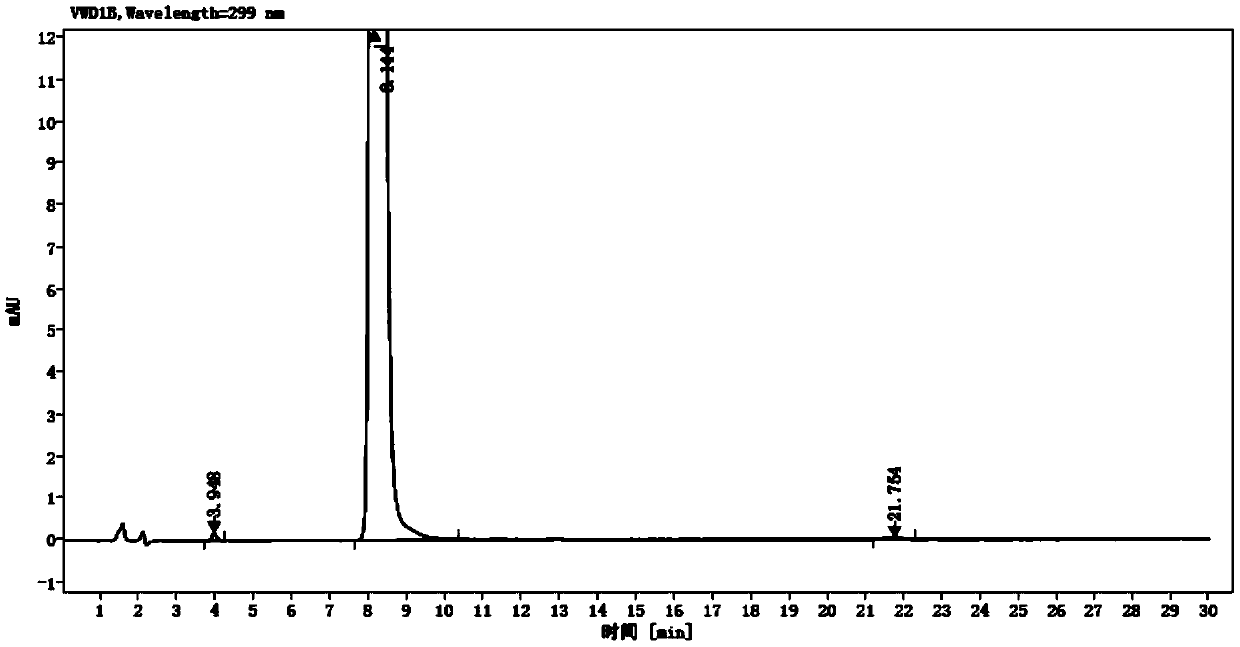
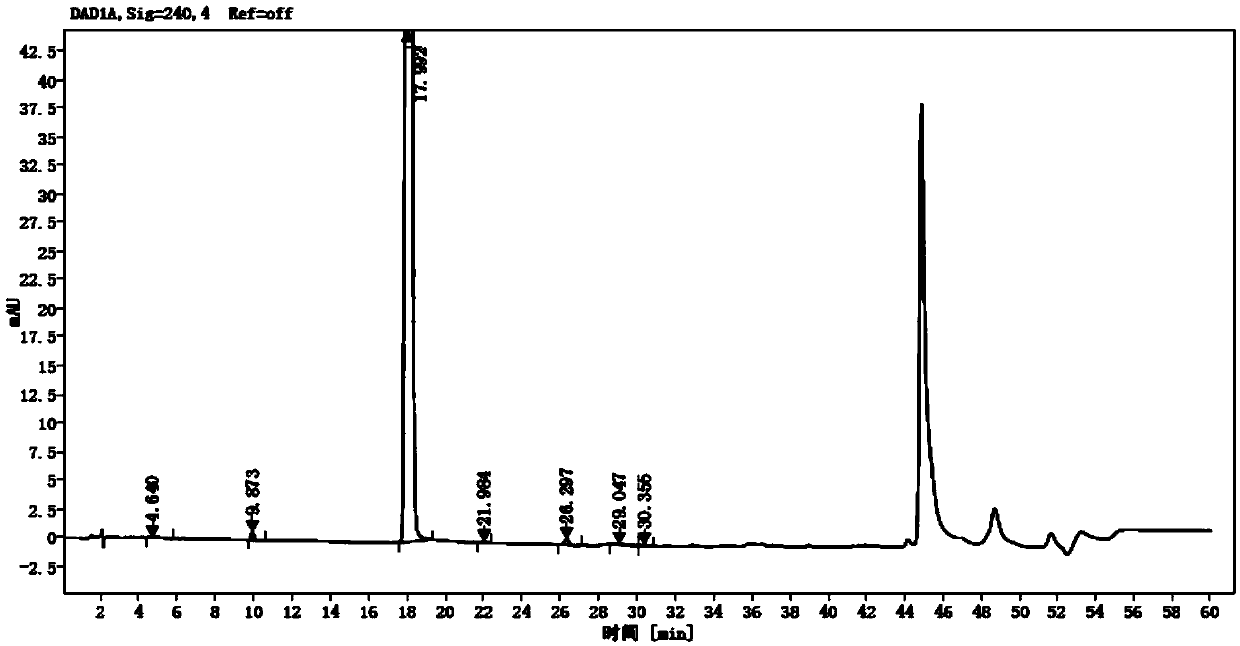
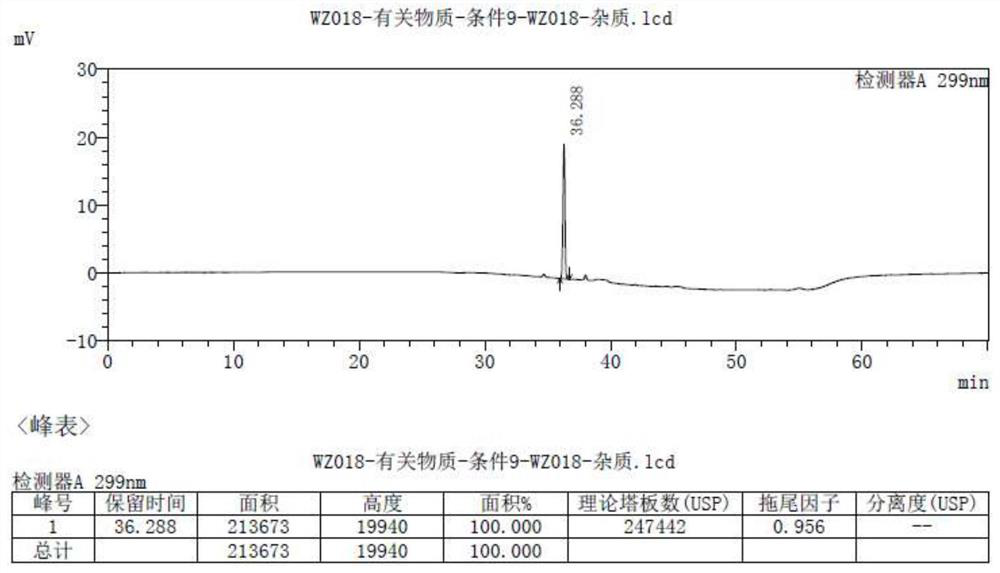
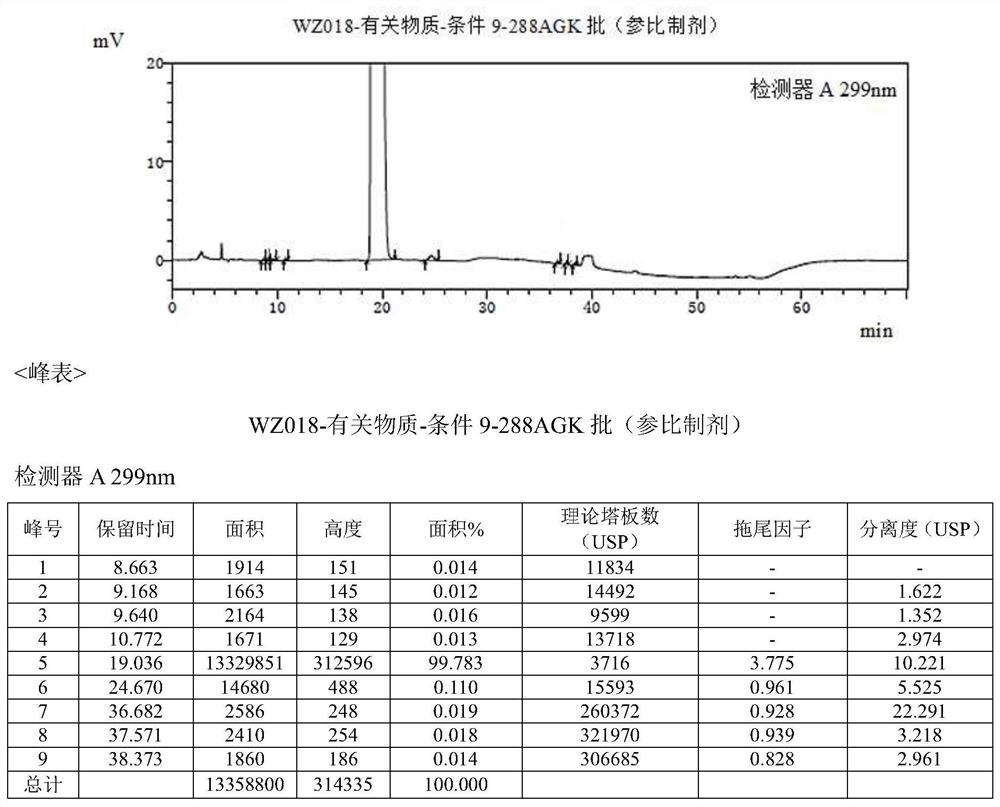
Detection method of olopatadine hydrochloride and related substance thereof
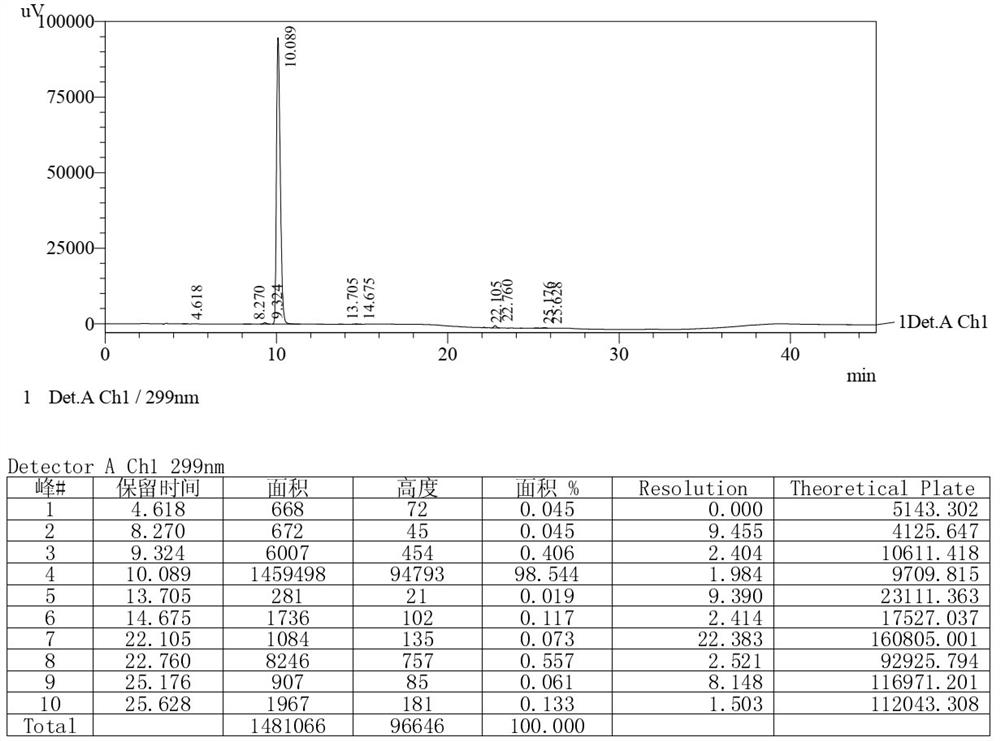
The invention relates to a detection method of olopatadine hydrochloride and related substance thereof. The method comprises a step of using high performance liquid chromatography for detection, wherein liquid chromatographic conditions are as follows: an octyl silane bonded silica gel chromatographic column is used; a mobile phase A is aqueous solution containing 0.01%-1% ion pair reagent, 0.3%-1% monoamine and 0.001-0.1mol / L buffer salt, a mobile phase B is acetonitrile and / or methyl alcohol, and gradient eluting is executed; and a pH value of buffer solution is 2.5-3.5, and detection wavelength is 220nm-280nm. The detection method of the olopatadine hydrochloride and the related substance thereof provided by the invention can effectively separate the olopatadine hydrochloride and impurities thereof, improve accuracy of a detection result and reduce clinical medication risks.
Owner:北京海晶生物医药科技有限公司
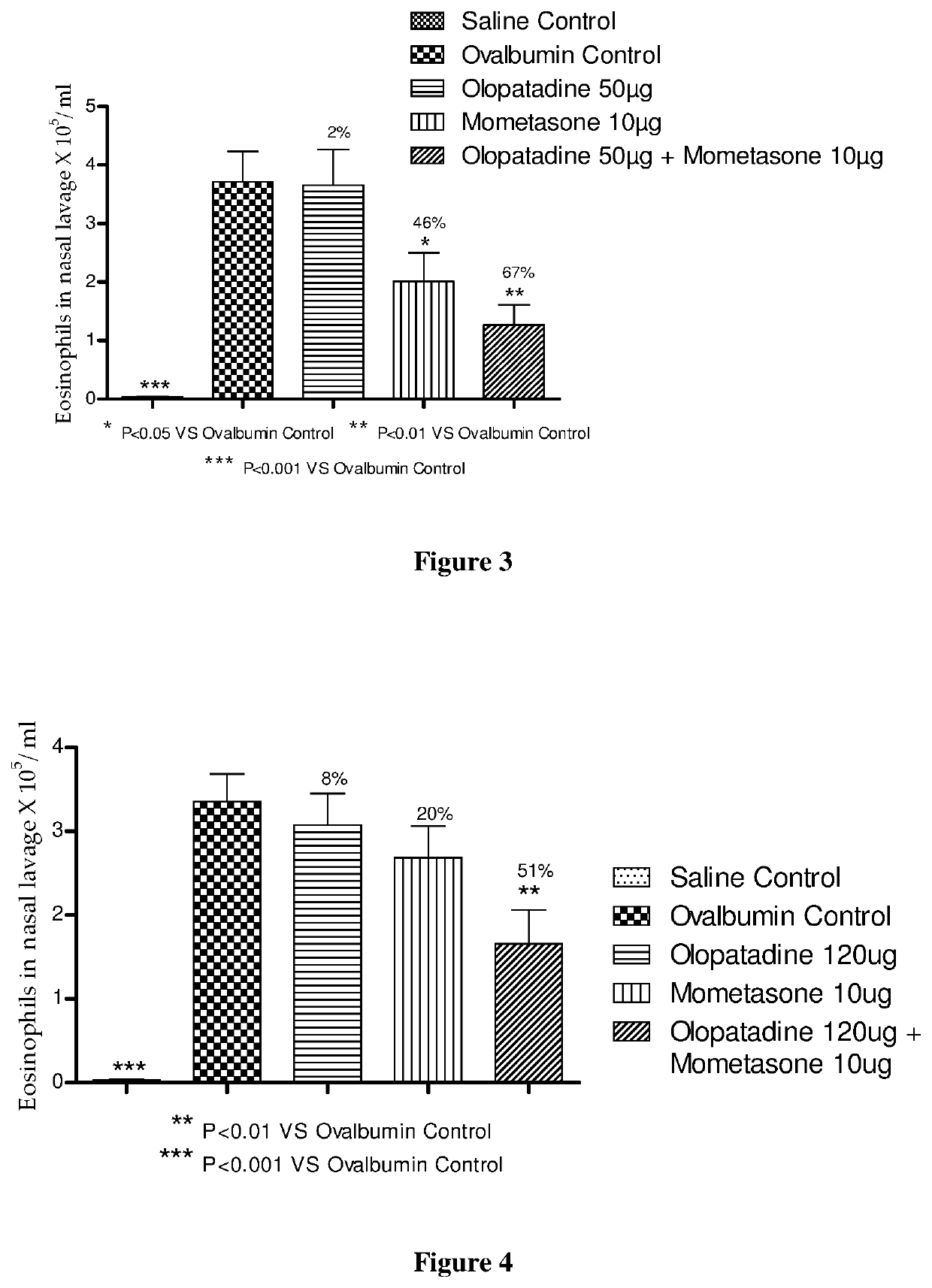
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS20170333449A1Better therapeutic valueIncrease valueOrganic active ingredientsPharmaceutical delivery mechanismMometasonePharmaceutical drug
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a human) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Method for producing dibenzoxepin compound
Disclosed is a method wherein (Z)-11-(3- dimethylaminopropylidene)-6,11-dihydrodibenz[b,e]oxepin-2- acetic acid (general name: olopatadine) or an acid addition salt thereof, which is useful as a pharmaceutical product, is produced by processing a dibenzoxepin derivative represented by the formula [I] below with a dehydrating agent for obtaining a mixture of a dibenzoxepin derivative represented by the formula [II] below and a dibenzoxepin derivative represented by the formula [III] below, and then processing the thus-obtained mixture with an acid. In the formula [I], R1, R2 and R3 independently represent an alkyl group having 1-2 carbon atoms.) (In the formula [II], R1, R2 and R3 are as defined above.) (In the formula [III], R1, R2 and R3 are as defined above.
Owner:SUMITOMO CHEM CO LTD
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS10758550B2Increase valueAct quicklyOrganic active ingredientsSenses disorderMometasonePharmaceutical drug
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a pediatric human subject) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Olopatadine alpha methyl compound and its preparation method and use
ActiveCN111808063BImprove medication safetyImprove and ensure medication safetyOrganic active ingredientsOrganic chemistryDiseaseMethyl palmoxirate
Owner:唯智医药科技(北京)有限公司 +1
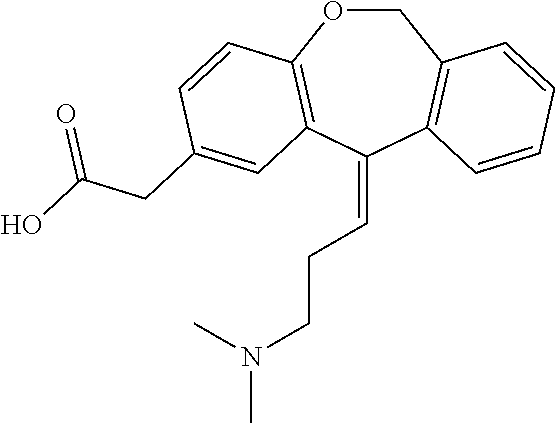
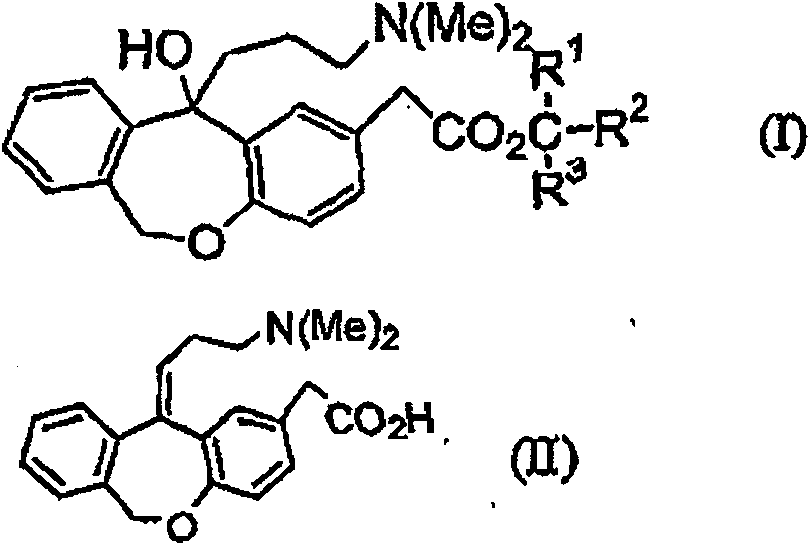
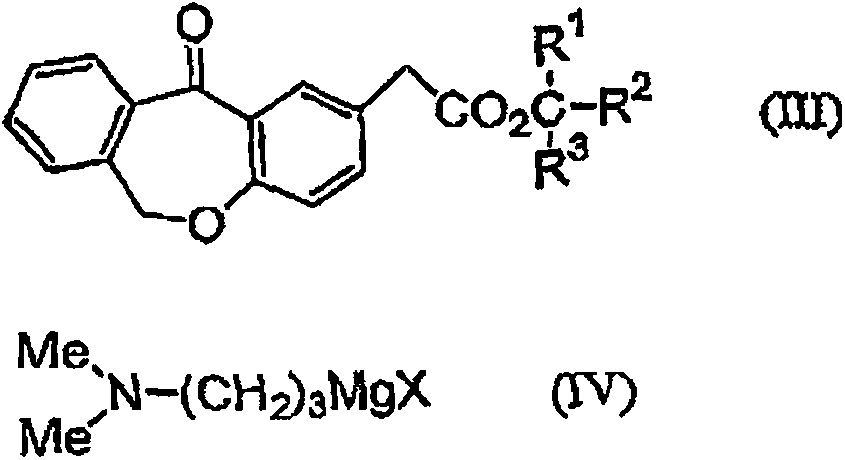
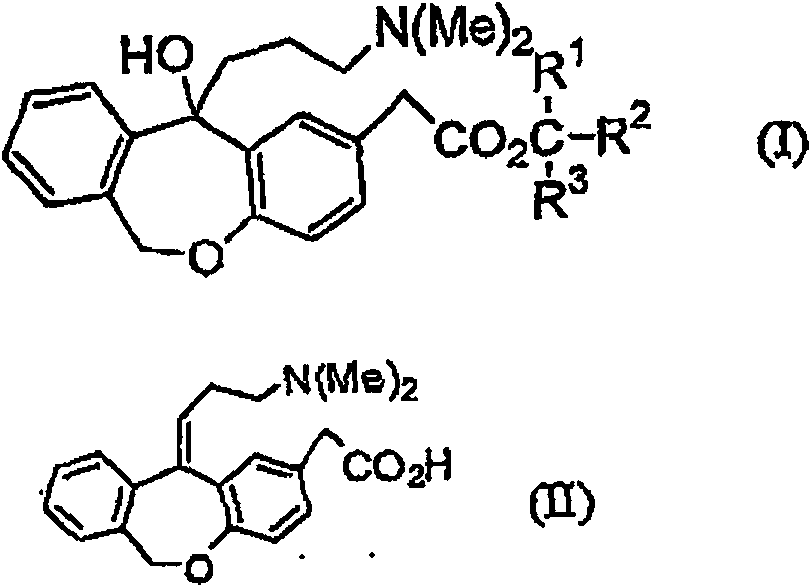
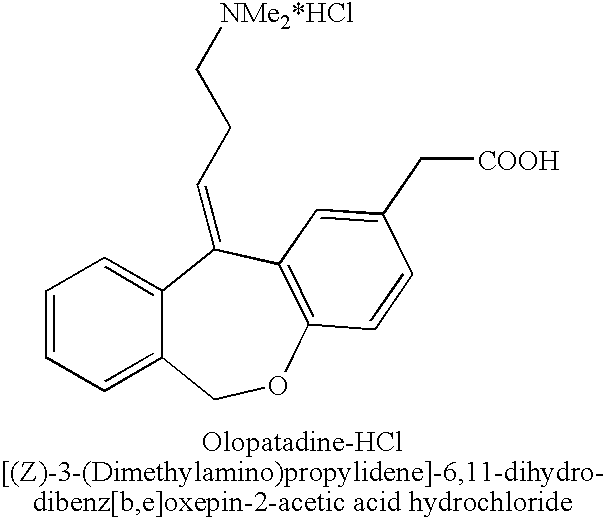
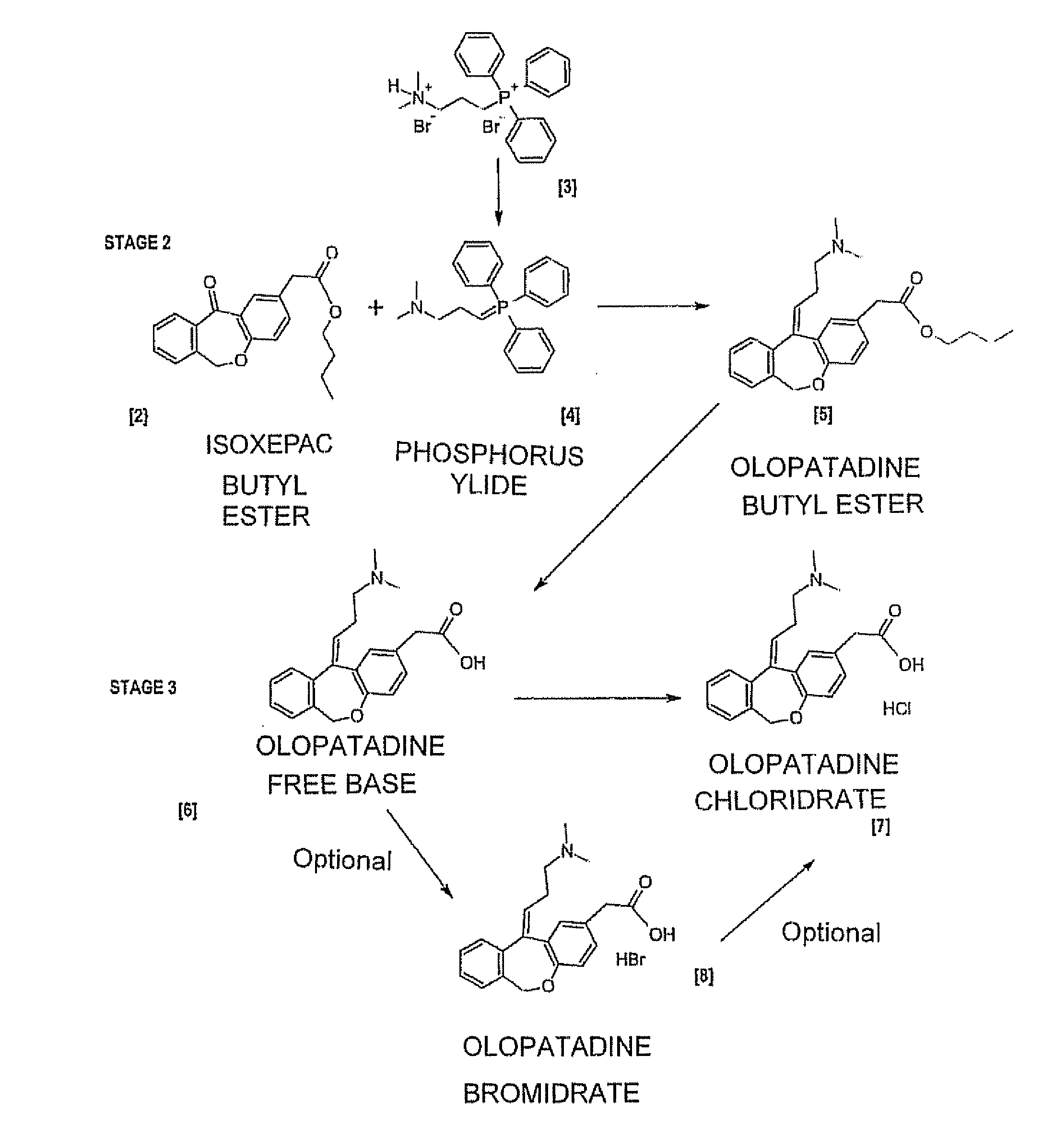
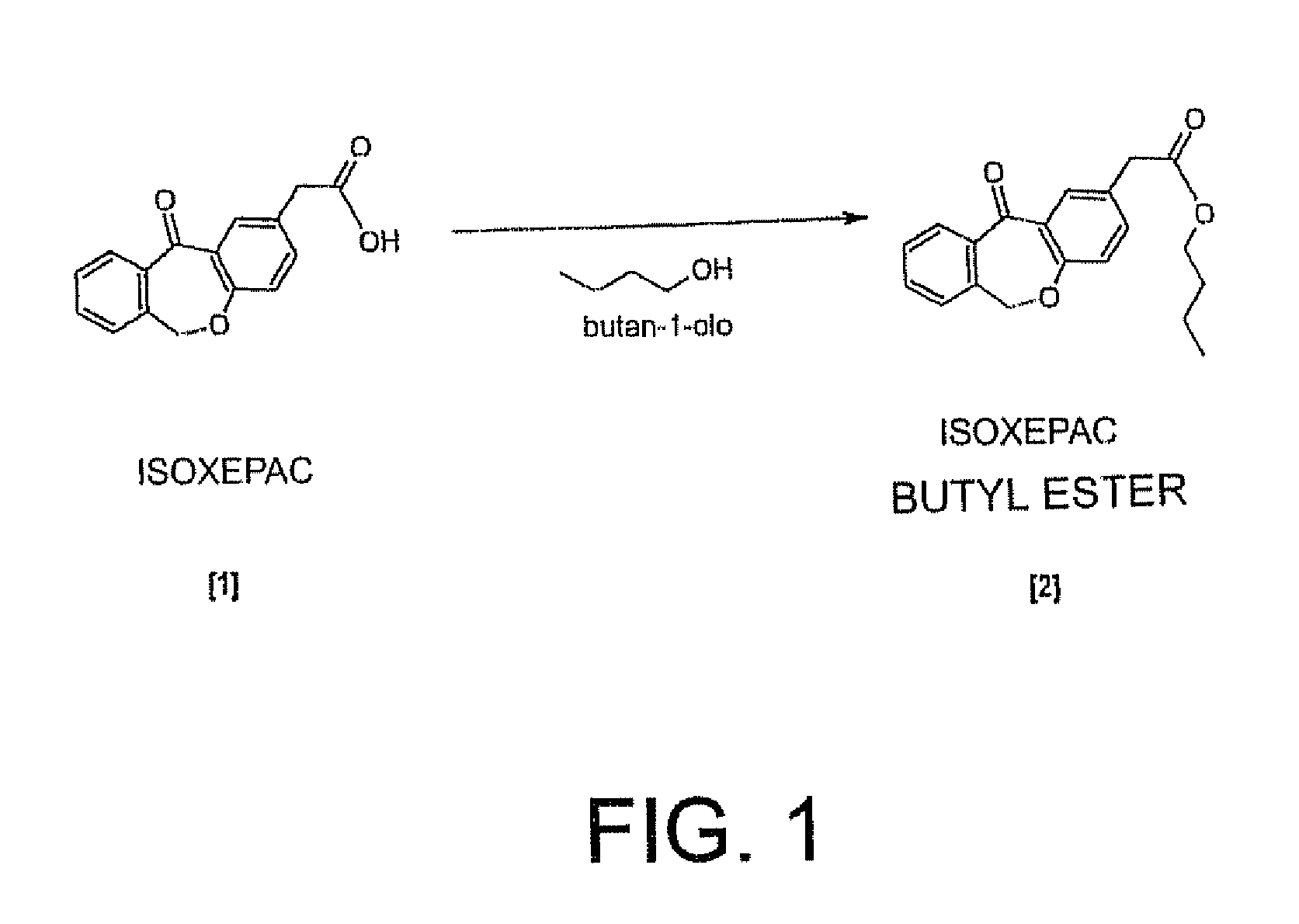
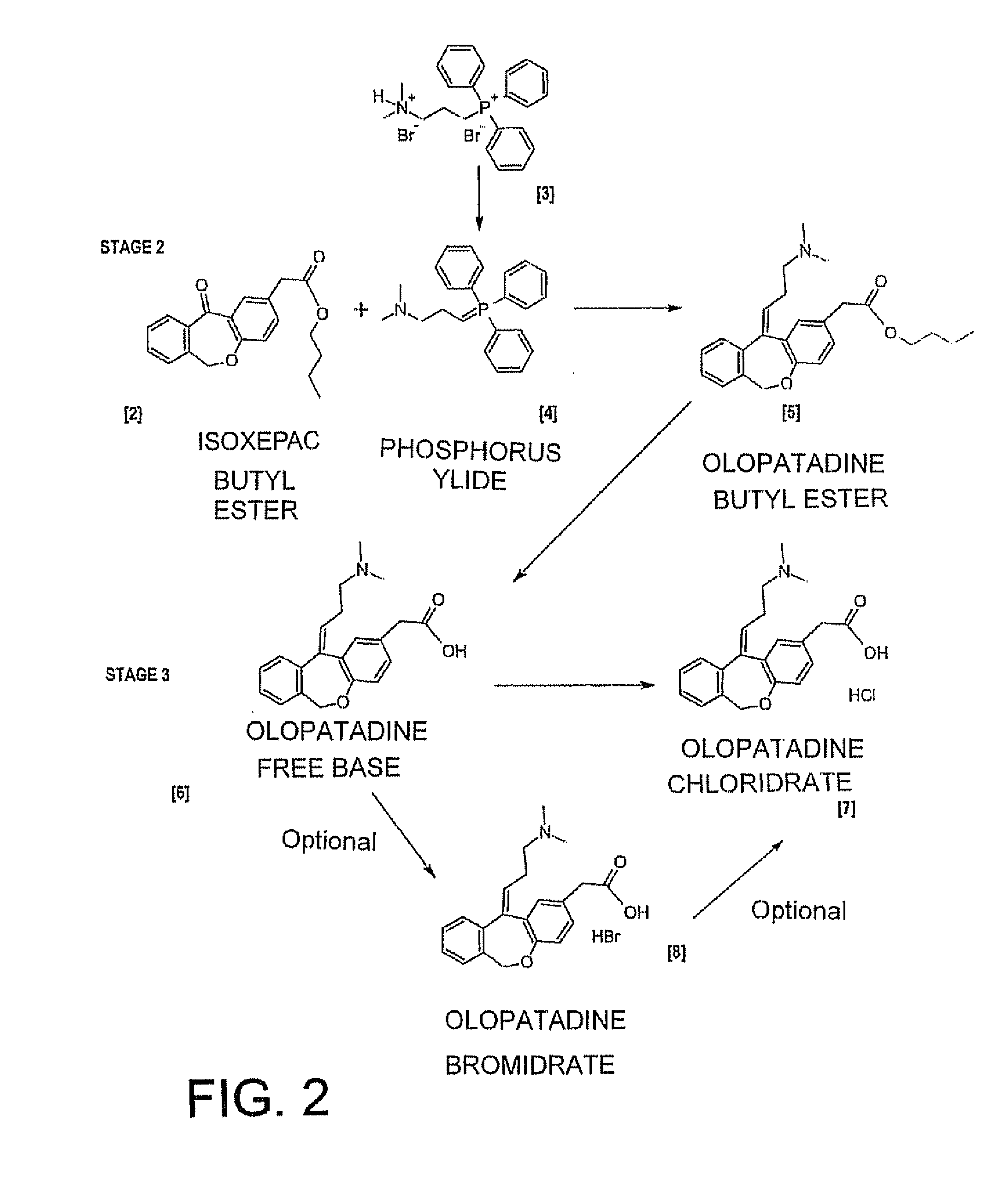
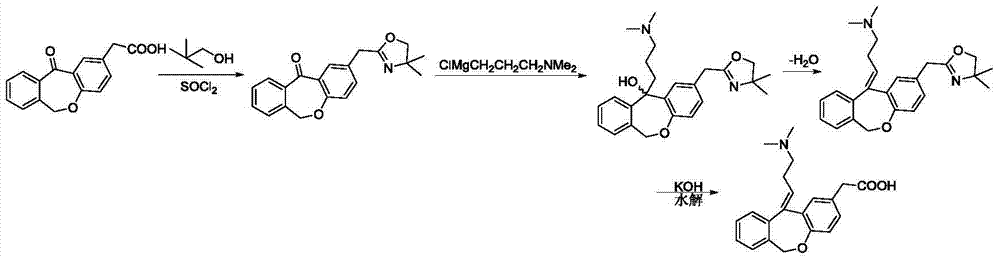
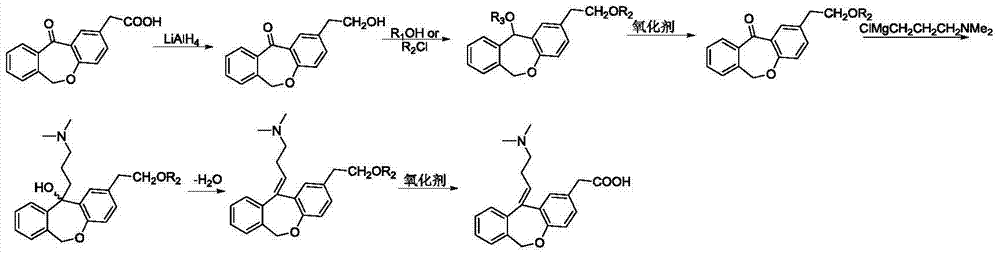
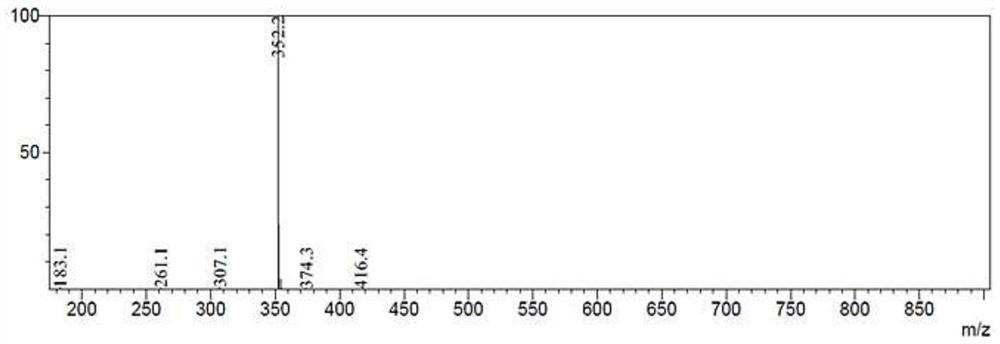
11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid
Process for the preparation of olopatadine (I), which comprises reacting a compound of formula (V) in the presence of a palladium catalyst to, provide a compound of formula (VI), wherein the acid protecting group is removed to provide the compound of formula (I) and if desired, transformation into its salts.
Owner:URQUIMA
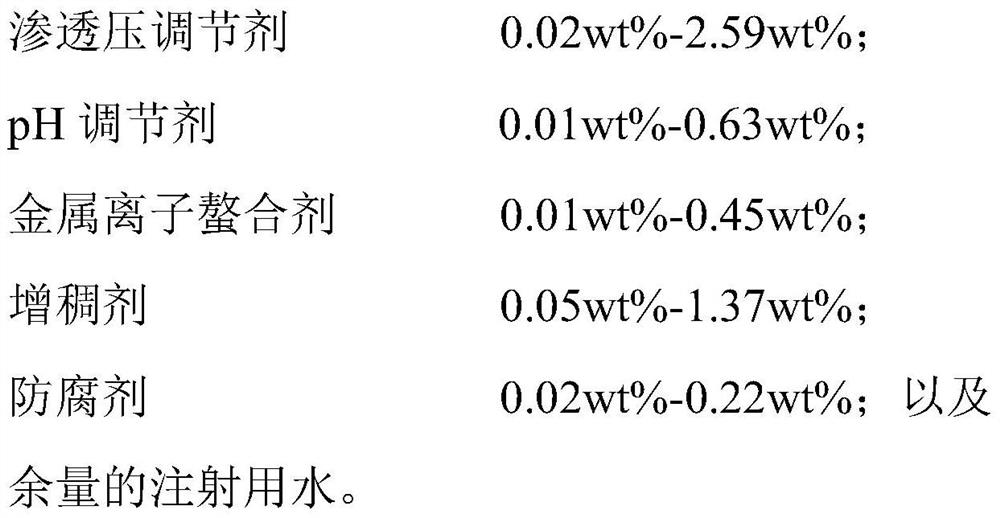
Ophthalmic composition as well as preparation method and application thereof
PendingCN114099505ASuppress generationGood treatment effectAntibacterial agentsOrganic active ingredientsConjunctivaOcular inflammation
The present invention relates to an ophthalmic composition comprising pranoprofen and olopatadine and / or naphazoline. The invention also relates to a preparation method of the ophthalmic composition, which comprises the following steps: mixing the pranoprofen, the olopatadine and / or the naphazoline, the osmotic pressure regulator, the pH regulator, the metal ion chelating agent, the thickening agent, the preservative and the water for injection to obtain the ophthalmic composition. The invention also relates to an application of the ophthalmic composition in preparation of a medicine for treating ocular inflammation, and the ocular inflammation comprises external eye and / or anterior segment inflammation caused by gram-positive bacterium and / or gram-negative bacterium infection. When the ophthalmic composition provided by the invention is used as eye drops, the treatment effect on eye inflammation of a patient can be improved, and adverse effects of eye stimulation, conjunctival congestion, edema and the like after medication can be avoided, so that the medication compliance of the patient is improved, and the recovery time of the patient is shortened.
Owner:湖北远大天天明制药有限公司
Stable fixed dose pharmaceutical composition comprising mometasone and olopatadine
The present invention relates to a stable fixed dose aqueous pharmaceutical composition (e.g., contained in a container) for nasal administration to a human, comprising mometasone or its salt, olopatadine or its salt. The composition may further include a hydrocolloid. The invention also relates to a process for preparing the pharmaceutical composition, and the use of the pharmaceutical composition in the treatment of rhinitis in a subject.
Owner:GLENMARK SPECIALTY
Post-treatment purification method of olopatadine hydrochloride
ActiveCN112375060AReduce residual riskLow Inorganic Salt ContentOrganic chemistryInorganic saltsAcetic acid
The invention provides a post-treatment purification method of olopatadine hydrochloride. The method comprises the steps of 1) washing a reaction liquid crude product with a sodium chloride solution to remove bromide ions so as to obtain an olopatadine hydrochloride salt-containing crude product; (2) dissolving the olopatadine hydrochloride salt-containing crude product prepared in the step (1) into a mixed solvent of dichloromethane, glacial acetic acid, acetic acid and alcohols, and carrying out desalting treatment so as to obtain an olopatadine hydrochloride crude product; and (3) recrystallizing the olopatadine hydrochloride crude product obtained in the step (2), wherein a solvent adopted for recrystallization is a mixed solvent of dimethyl sulfoxide and isopropyl ether. The target product olopatadine hydrochloride obtained through the method is extremely low in inorganic salt content, meanwhile, the preparation yield and the product purity of olopatadine hydrochloride are greatlyimproved, the production cost is reduced, and the method is suitable for industrial production.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
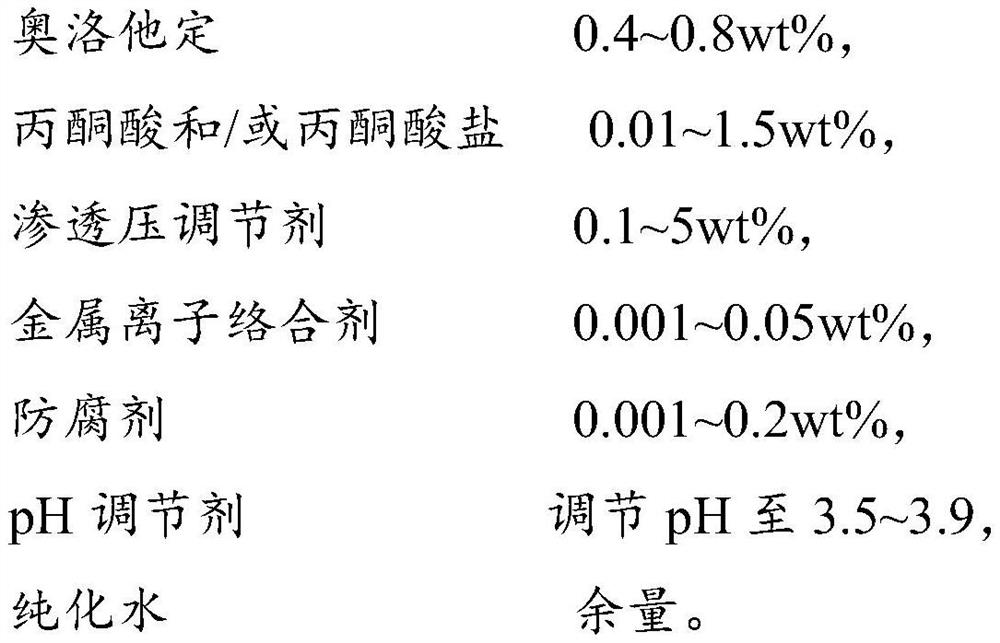
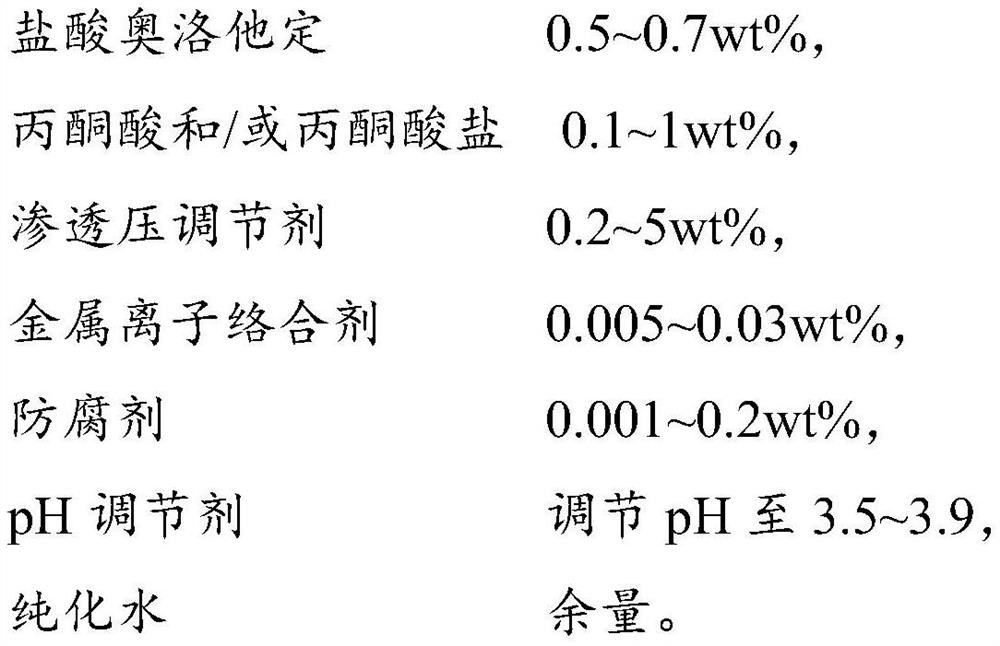
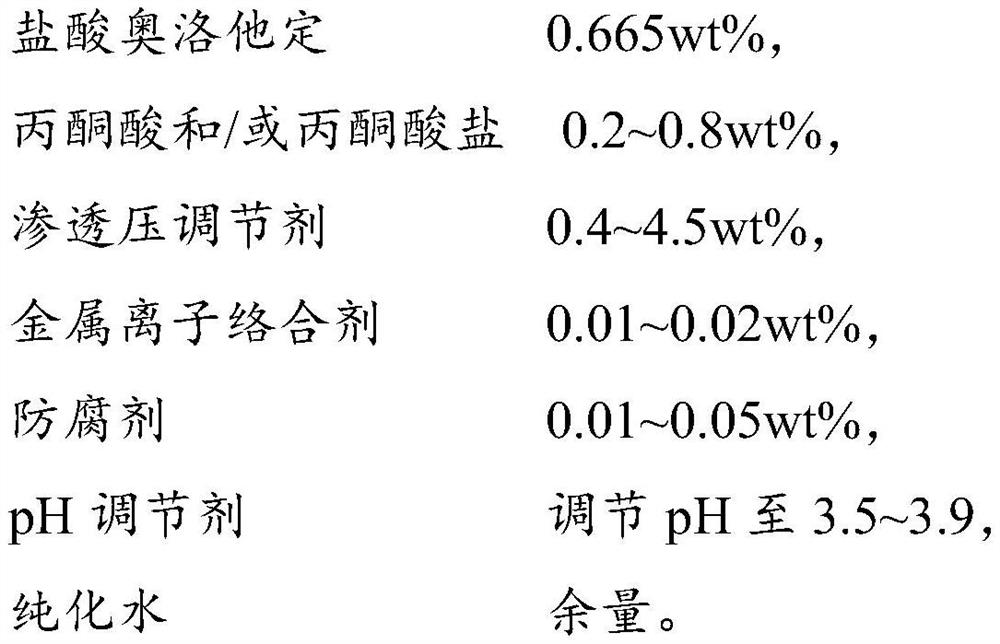
A kind of olopatadine composition and preparation method thereof
ActiveCN109806224BImprove stabilityImprove medication safetyOrganic active ingredientsPharmaceutical delivery mechanismUse medicationIrritation
The present invention provides an olopatadine composition, which comprises olopatadine, pyruvate and / or pyruvate, osmotic pressure regulator, metal ion complexing agent, preservative, pH regulator and purified water. The composition of the invention can improve the stability of the olopatadine, reduce the irritation to the nasal mucosa, accelerate the repair of the nasal mucosa, relieve nasal symptoms and improve the drug safety of the olopatadine.
Owner:JIANG SU PHARMAMAXCORP
Method for simply and conveniently preparing high-purity olopatadine hydrochloride intermediate
ActiveCN111548369AAvoid harmReduce chemical damageGroup 5/15 element organic compoundsHydrobromideAlcohol
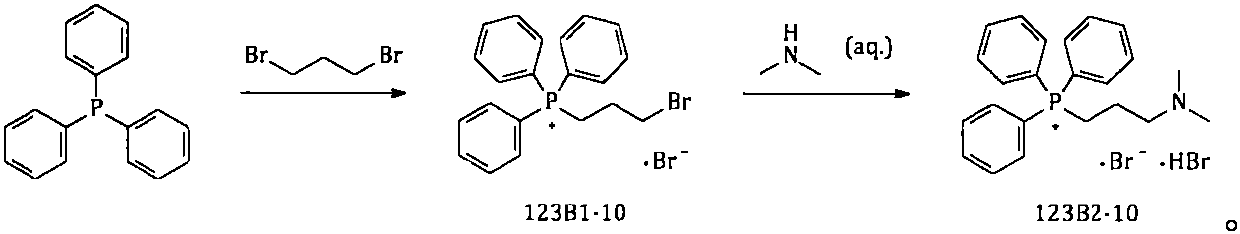
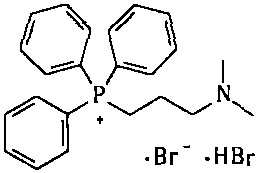
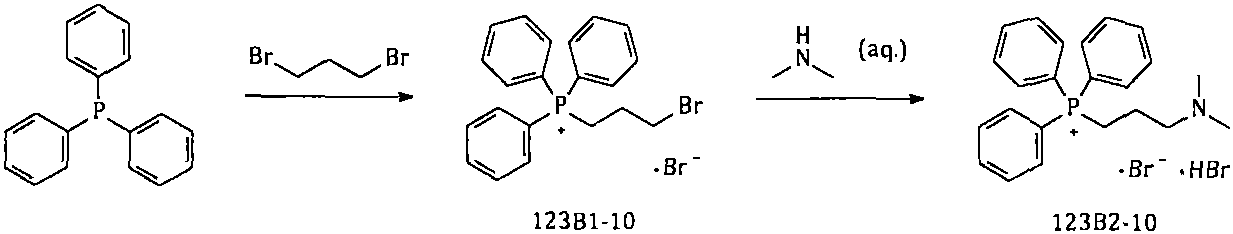
The invention provides a simple and convenient method which is more suitable for industrial large-scale production and preparation of high-purity [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide. According to the preparation method, with triphenylphosphine and 1, 3-dibromopropane adopted as starting materials, reflux reaction is carried out in n-heptane to obtain (3-bromopropyl) triphenylphosphonium bromide; the obtained (3-bromopropyl) triphenylphosphonium bromide does not need to be separated, and directly reacts with a dimethylamine aqueous solution by means of a one-potmethod; after the reaction is finished, the n-heptane is concentrated, water in a system is taken out, so that a [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide crude product canbe obtained; and the crude product is thermally pulped with absolute ethyl alcohol, so that the high-purity [3-(dimethylamine) propyl] triphenylphosphonium bromide hydrobromide can be obtained.
Owner:内蒙古京东药业有限公司
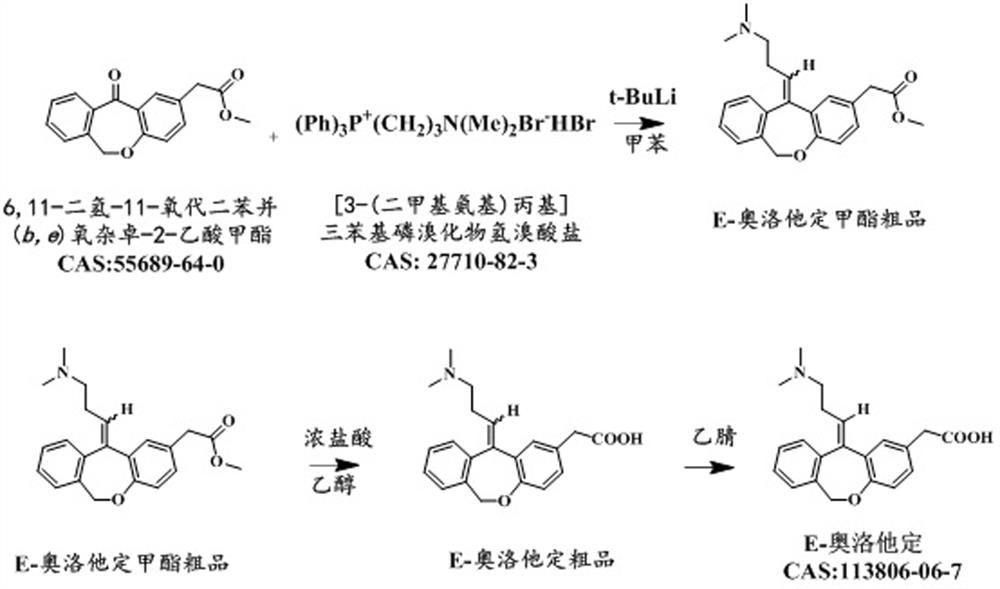
Preparation method of E-olopatadine
PendingCN112457287AFew reaction stepsReduce lossOrganic chemistry methodsBulk chemical productionHydrobromideDistillation
The invention discloses a preparation method of E-olopatadine, which comprises the following steps: adding [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide into a solvent, adding an n-pentane solution of tert-butyl lithium, carrying out heat preservation reaction for 0.5-2 hour, heating to 105-110 DEG C, dropwise adding a toluene solution of methyl 6, 11-dihydro-11-oxo-dibenzo(b, e)oxepine-2-acetate, heating to 105-110 DEG C, stirring for 2-5 hours at the temperature of 105-110 DEG C, then cooling to 0-15 DEG C, carrying out quenching reaction, adding concentrated hydrochloricacid to adjust the pH value to 6+ / -0.2, carrying out reduced pressure distillation to dryness to obtain a solid, enabling the solid to pass through a column to obtain an E-olopatadine methyl ester crude product, and carrying out purification. According to the preparation method of Eolopatadine provided by the invention, on the basis of the prior art, key steps are upgraded and transformed, so thatthe reaction steps can be reduced, and the loss is reduced.
Owner:重庆西南制药二厂有限责任公司
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS10548907B2Increase valueReduce morbidityOrganic active ingredientsPharmaceutical delivery mechanismMometasonePharmaceutical drug
Owner:GLENMARK SPECIALTY
Treatment of allergic rhinitis using a combination of mometasone and olopatadine
ActiveUS10653661B2Increase valueAct quicklyOrganic active ingredientsSenses disorderMometasonePharmaceutical drug
The present invention relates to a method of treating allergic rhinitis in a subject (e.g., a human) in need thereof comprising nasally administering to the subject an effective amount of a fixed-dose pharmaceutical composition comprising mometasone or its salt and olopatadine or its salt.
Owner:GLENMARK SPECIALTY
Formulation of olopatadine
InactiveUS20160271097A1Easy to manufactureAvoid the needOrganic active ingredientsSenses disorderMedicineOlopatadine
Stable formulations of Olopatadine, methods of making such formulations and methods of treatment using such formulations are provided.
Owner:NEPHRON PHARM CORP
A kind of ophthalmic composition and its preparation method and application
ActiveCN112807275BGood anti-inflammatory and anti-viral effectReduce congestion rateSenses disorderAntipyreticConjunctivaPalpebral edema
Owner:湖北远大天天明制药有限公司
Ophthalmic composition as well as preparation method and application thereof
ActiveCN112263545ALittle side effectsAlleviate irritation symptomsOrganic active ingredientsSenses disorderCorneal diseaseEye irritation
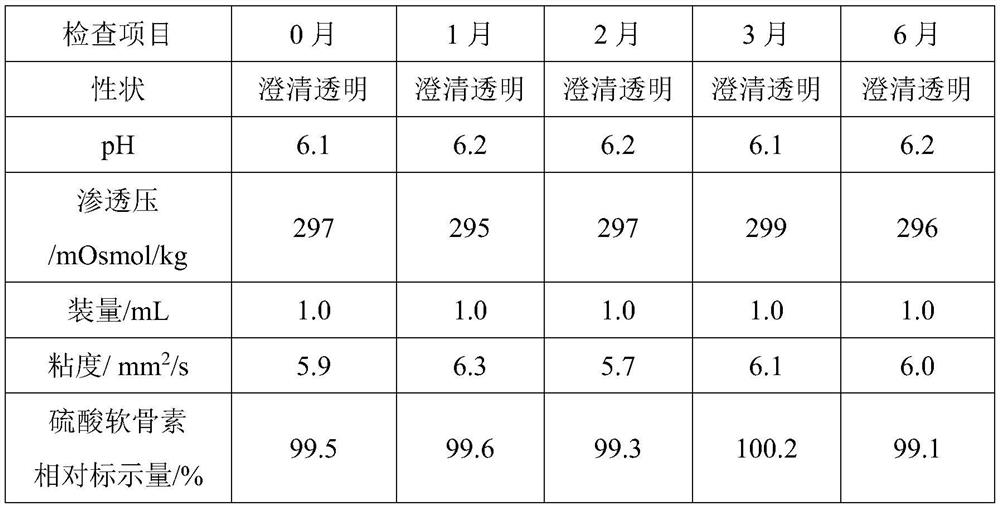
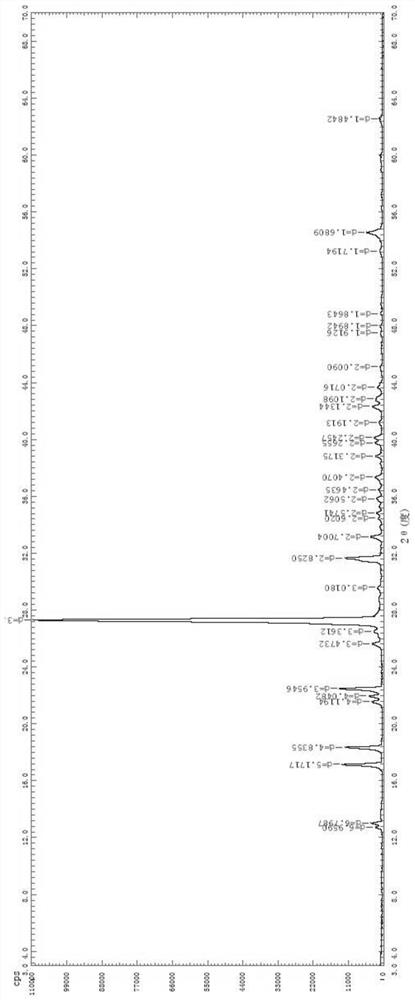
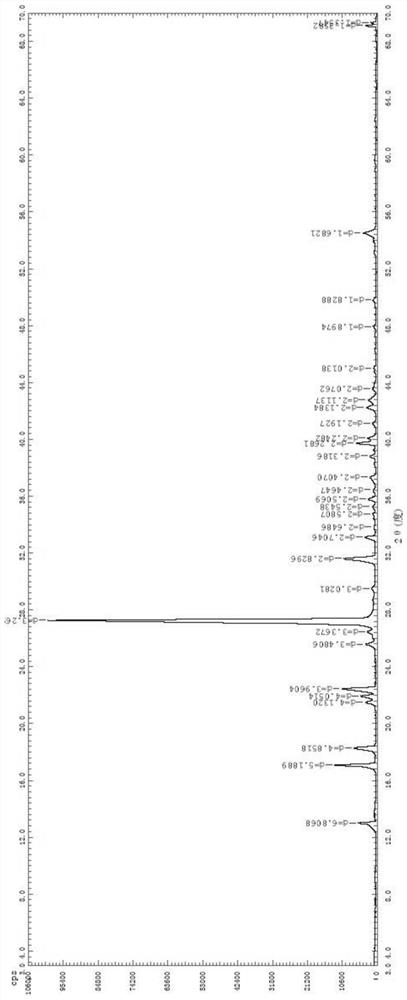
The invention relates to an ophthalmic composition which comprises chondroitin sulfate and olopatadine hydrochloride. The invention further relates to a preparation method of the ophthalmic composition. The preparation method comprises the following steps: S1, mixing a pH regulator, an osmotic pressure regulator and injection water to obtain a mixed solution I; S2, homogeneously stirring and mixing chondroitin sulfate, olopatadine hydrochloride and injection water for 10-15 minutes at 3000-4500 r / min to obtain a mixed solution II; and S3, mixing the mixed solution I and the mixed solution II with the injection water, and filtering the mixture to obtain the ophthalmic composition. Furthermore, the invention relates to application of the ophthalmic composition in preparation of medicines forimproving eye stimulation symptoms caused by treatment of corneal diseases. The ophthalmic composition serving as eye drops can be used for effectively relieving eye irritation caused by chondroitinsulfate at conventional dosage for corneal disease treatment, so that the medication compliance of a patient can be improved, and the treatment time of the patient can be effectively shortened.
Owner:湖北远大天天明制药有限公司
Olopatadine hydrochloride alpha methyl compound B crystal form and preparation method and application thereof
PendingCN114478461AImprove solubilityImprove bioavailabilityOrganic active ingredientsAerosol deliveryChemical compoundPharmaceutical drug
The invention discloses an olopatadine hydrochloride alpha methyl compound B crystal form as well as a preparation method and application thereof, and belongs to the technical field of medicines. The invention develops and prepares an olopatadine hydrochloride alpha methyl compound B crystal form, the XRPD pattern of the olopatadine hydrochloride alpha methyl compound B crystal form contains the following diffraction characteristic peaks: the diffraction angles 2theta are 15.9, 17.0, 18.6, 23.0, 24.1, 26.1 and 26.7, and the error range of each characteristic peak 2theta is + / -0.2. The olopatadine hydrochloride alpha methyl compound B crystal form is obviously improved in the aspects of drug release performance, solubility and the like, the bioavailability is improved, and the pharmaceutical prospect is wide.
Owner:唯智医药科技(北京)有限公司
Process for production of dibenzoxepin compound
The present invention provides a method for producing a dibenzoxe compound. In a solvent, in the presence of an acid, the dibenzoyl compound represented by the formula (I) (in the formula, Me represents a methyl group, and R1, R2 and R3 each independently represent an alkyl group having 1 to 4 carbon atoms) is Olopatadine useful as a pharmaceutical can be produced efficiently and industrially advantageously by heating the oxa derivative or its salt.
Owner:SUMITOMO CHEM CO LTD
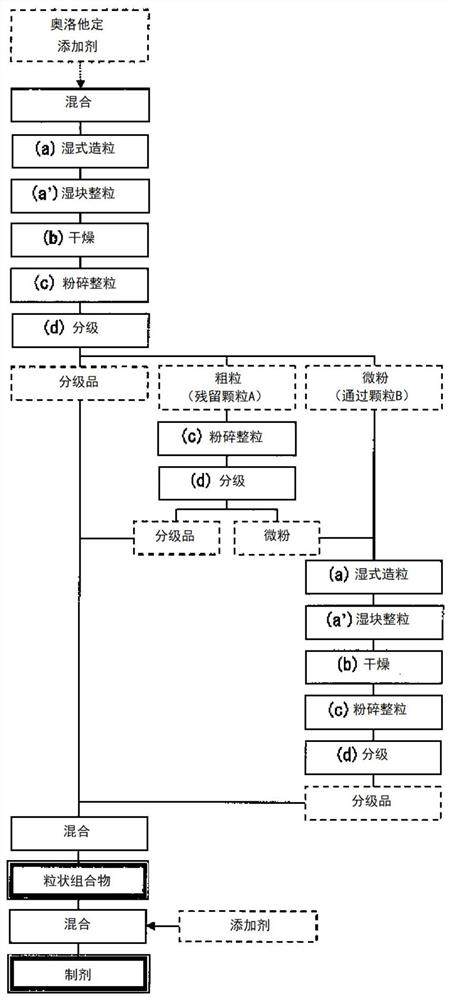
Particulate composition and production method therefor
PendingCN112566635AEasy to takeImprove medication compliancePowder deliveryOrganic active ingredientsPharmaceutical medicineBitter taste
The present invention provides: a particulate composition containing olopatadine or a pharmaceutically acceptable salt thereof and having reduced bitterness and therefore is easy to take; a solid medicinal preparation containing the particulate composition; and a method for uniformly and efficiently producing the particulate composition.
Owner:NIPPON ZOKI PHARM CO LTD
A post-treatment purification method for olopatadine hydrochloride
ActiveCN112375060BReduce residual riskLow Inorganic Salt ContentOrganic chemistryOlopatadinBromide ions
The invention provides a post-treatment purification method of olopatadine hydrochloride. The method of the present invention comprises the following steps: step 1): washing the crude product of the reaction solution through sodium chloride solution to remove bromide ions, and obtaining the crude product of olopatadine hydrochloride containing salt; step 2): washing the crude product of olopatadine hydrochloride obtained in step 1) The salt-containing crude product is dissolved in a mixed solvent of dichloromethane, glacial acetic acid and alcohols for desalination treatment to obtain the crude product of olopatadine hydrochloride; step 3): the crude product of olopatadine hydrochloride obtained in step 2) The product is recrystallized, wherein the solvent used for recrystallization is a mixed solvent of dimethyl sulfoxide and isopropyl ether. The target product olopatadine hydrochloride obtained by the method of the present invention has extremely low inorganic salt content, and at the same time greatly improves the preparation yield and product purity of the olopatadine hydrochloride, reduces production costs, and is suitable for industrial production.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
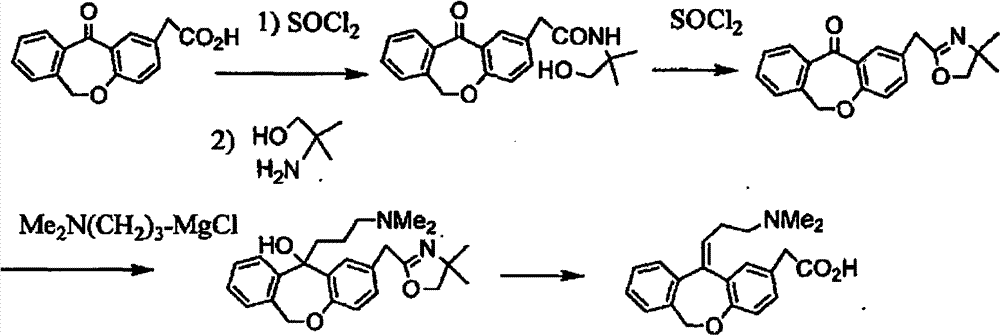
![11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid 11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid](https://images-eureka.patsnap.com/patent_img/2e579ac8-9752-4570-8bb9-a0e171166859/US20090005579A1-20090101-C00001.png)
![11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid 11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid](https://images-eureka.patsnap.com/patent_img/2e579ac8-9752-4570-8bb9-a0e171166859/US20090005579A1-20090101-C00002.png)
![11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid 11 - [ (Z) -3- (Dimethylamino) Propylidene] - 6, 11-Dihydro-Dibenz [B,E] Oxepin-2-Yl] - Acetic Acid](https://images-eureka.patsnap.com/patent_img/2e579ac8-9752-4570-8bb9-a0e171166859/US20090005579A1-20090101-C00003.png)