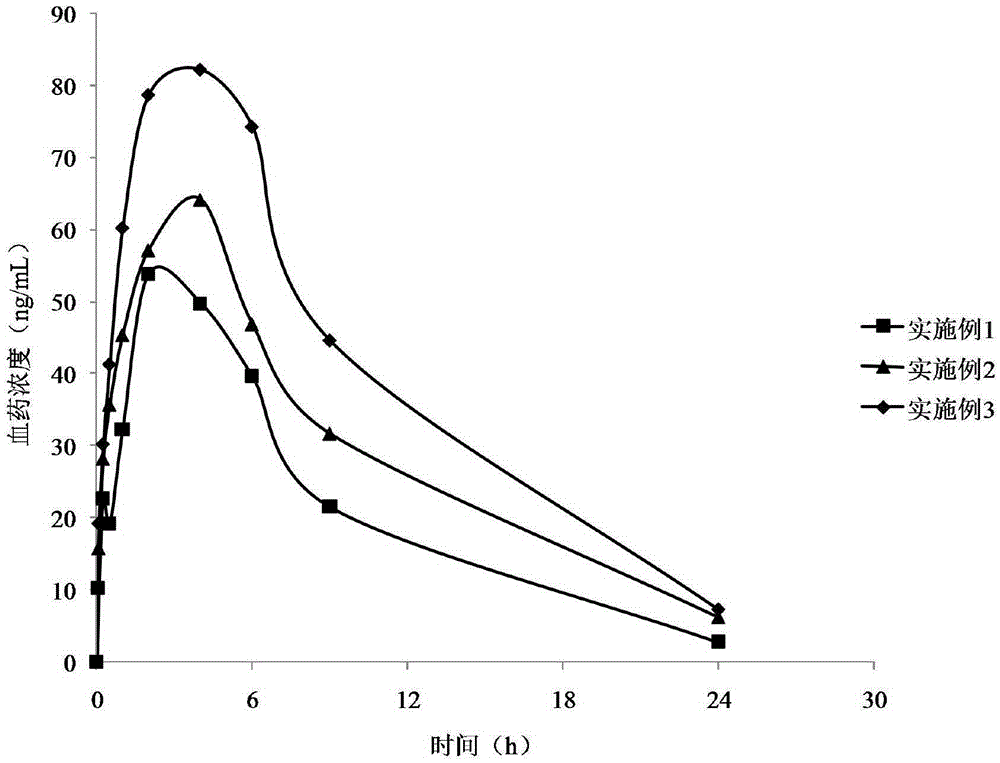
Patents
Literature
48 results about "Medication risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primer combination sequence and kit for detecting child safe medication-related gene mutation sites
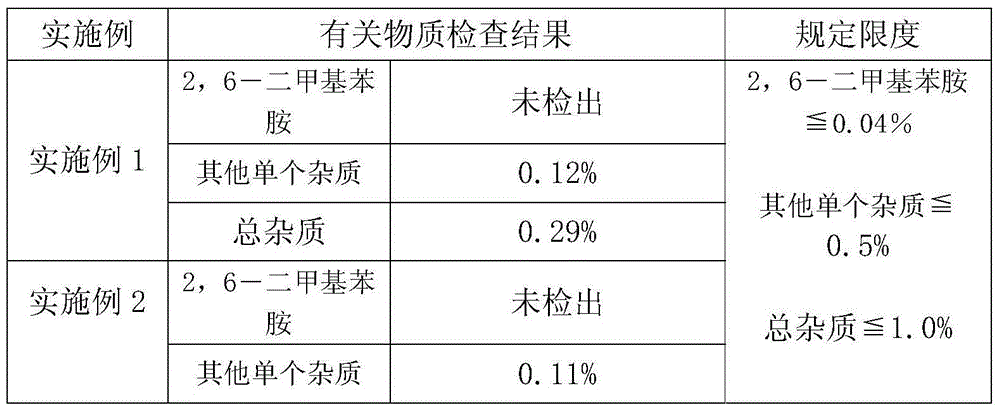
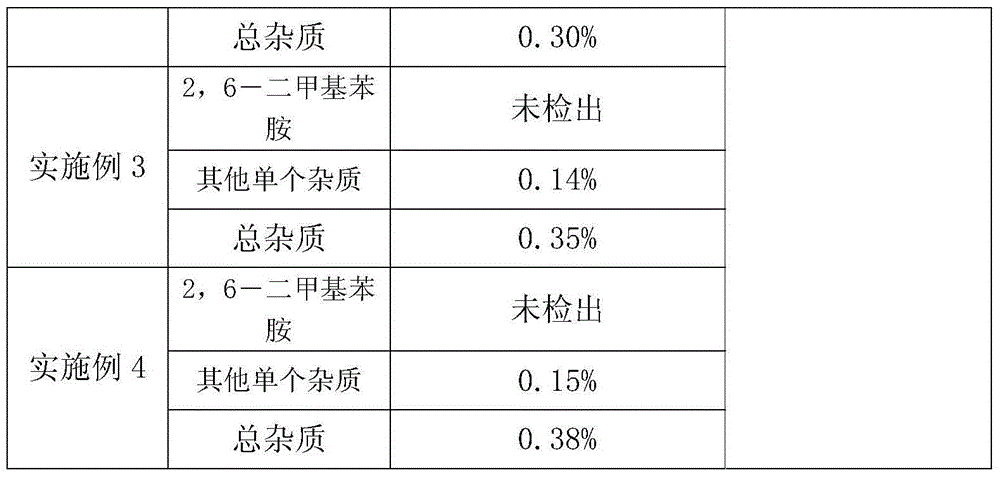
PendingCN111304321AHigh sensitivityGood curative effectMicrobiological testing/measurementDNA/RNA fragmentationMedication riskFull Term Neonate
The invention discloses a primer combination sequence and kit for detecting child safe medication-related gene mutation sites. The kit includes 23 pairs of amplification primers and 23 single-base extension primers; the 23 pairs of amplification primers can specifically amplify the 23 common mutation site regions of child safe medication-related genes, and the 23 single-base extension primers areused to detect the 23 mutation site genotypes of the child safe medication-related genes; and the kit further includes special reagents for pretreatment and detection. The kit provided by the invention can realize one-hole detection of the 23 mutations related to the clinical child safe medication-related genes, has high sensitivity, strong specificity and high accuracy, and is simple to operate,low in cost and high in throughput, the detection is fast, automatic interpretation of results is realized, and the clinical promotion and application are easy to realize; and the kit can be applied to the detection of the genes related to safe medication of newborns or children, guide clinical correct medication, avoid medication risks, reduce medication injury, and truly achieve precise medication.
Owner:ZHEJIANG DIGENA DIAGNOSTIC TECH CO LTD
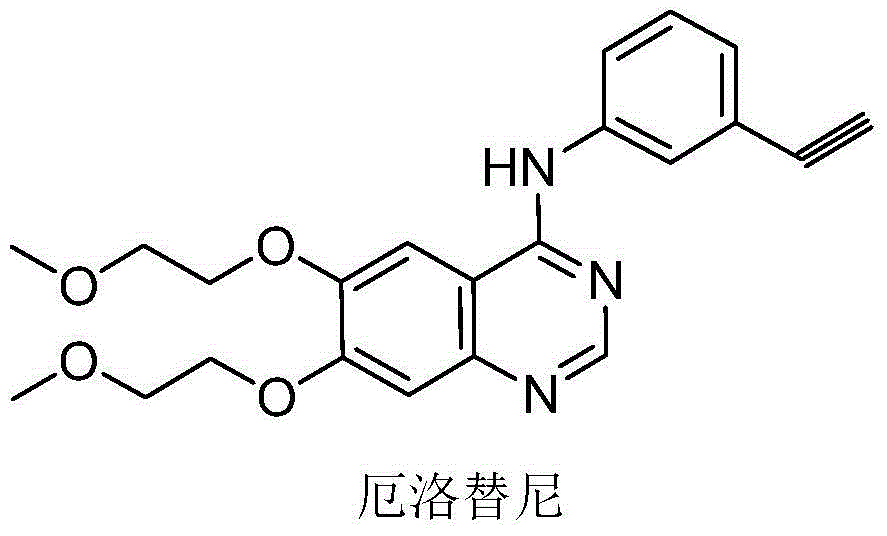
Medicine composition containing erlotinib hydrochloride and preparation method of medicine composition
InactiveCN105362239APromote dissolutionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsTraditional medicineDigestion
The invention provides a medicine composition containing erlotinib hydrochloride. The medicine composition has the advantages of being good in digestion performance, high in stability, low in clinical pharmacy risk and the like. In the production and preparation process, raw materials and auxiliary materials do not need to be specially treated, and the production process is simple.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
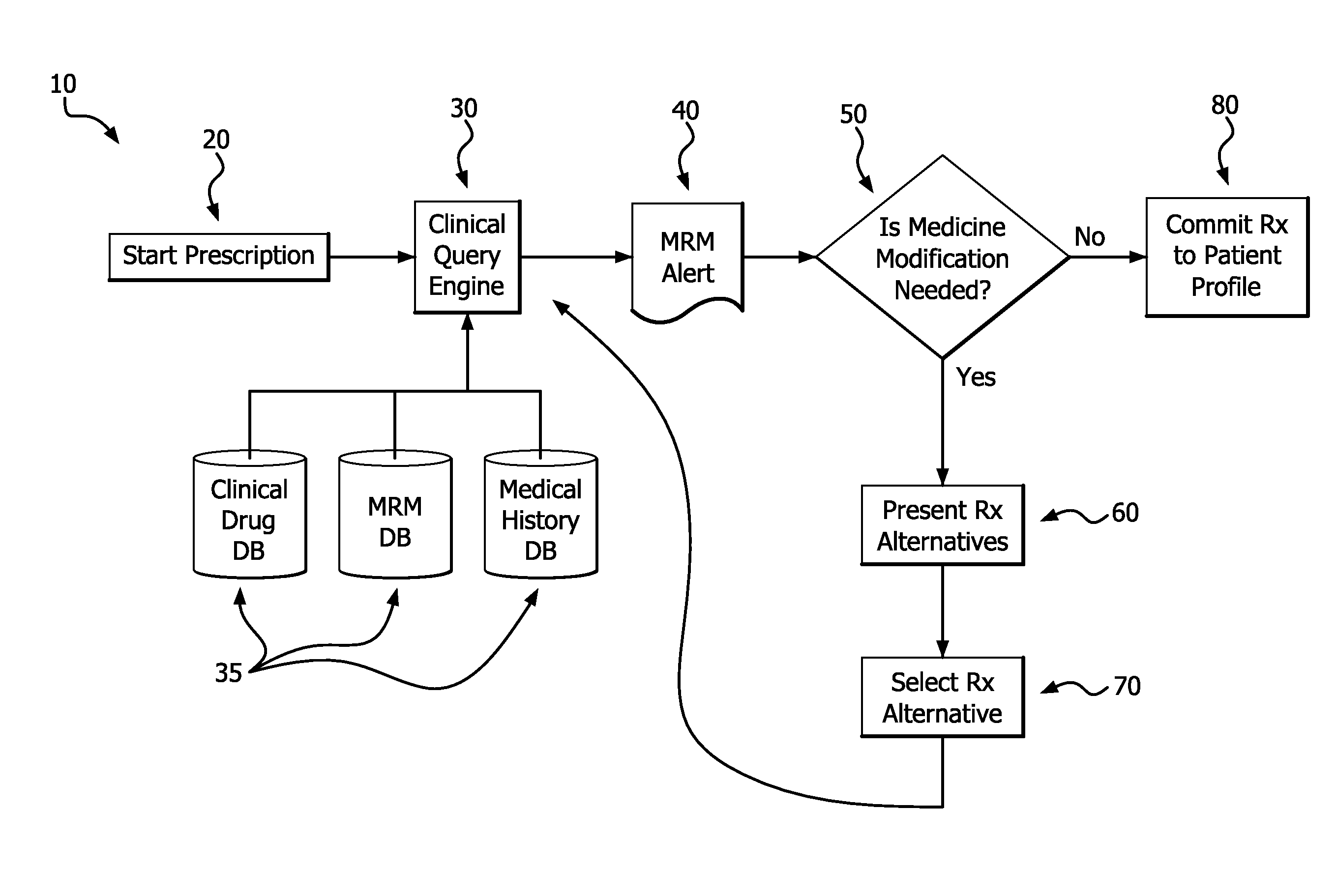
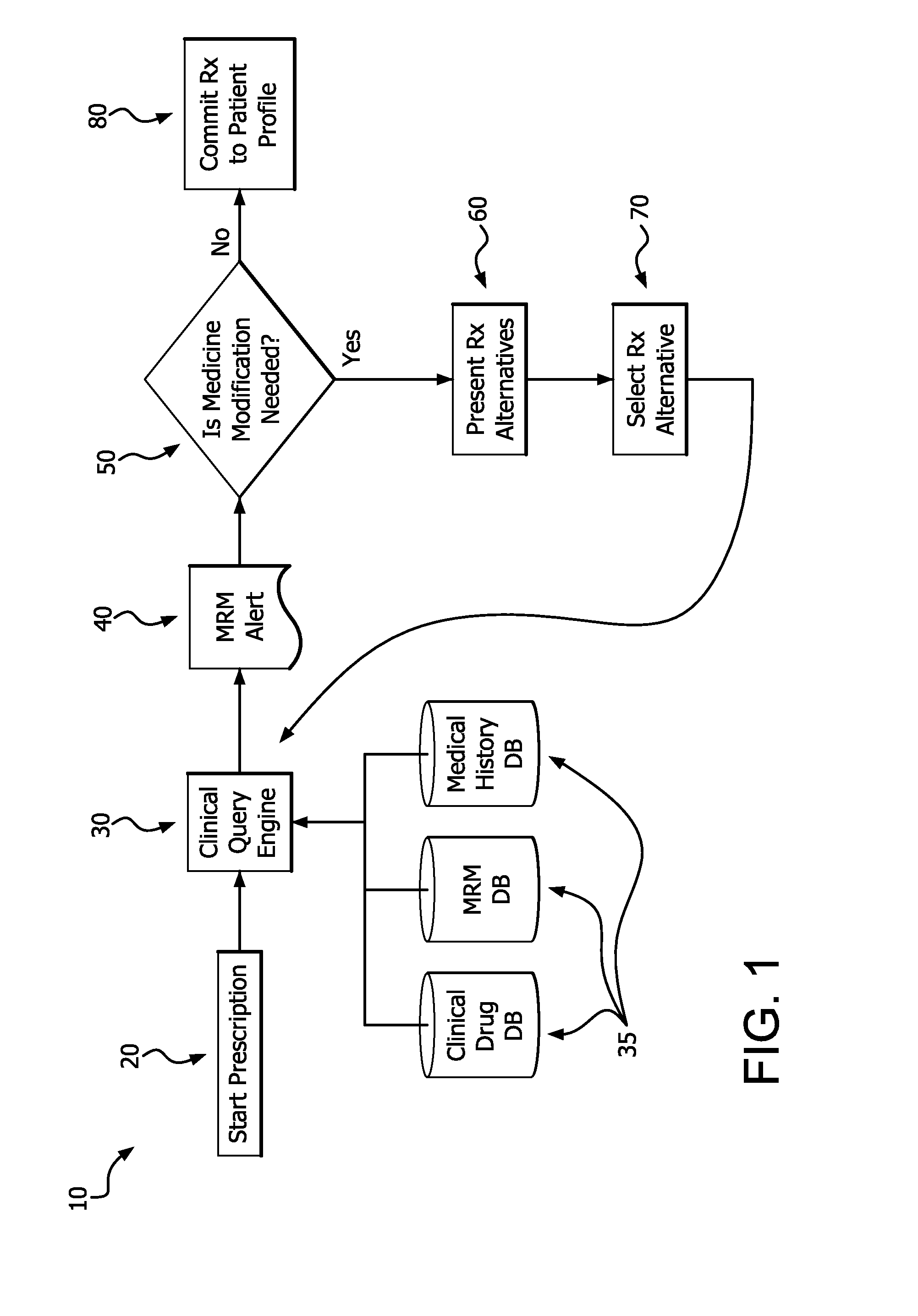
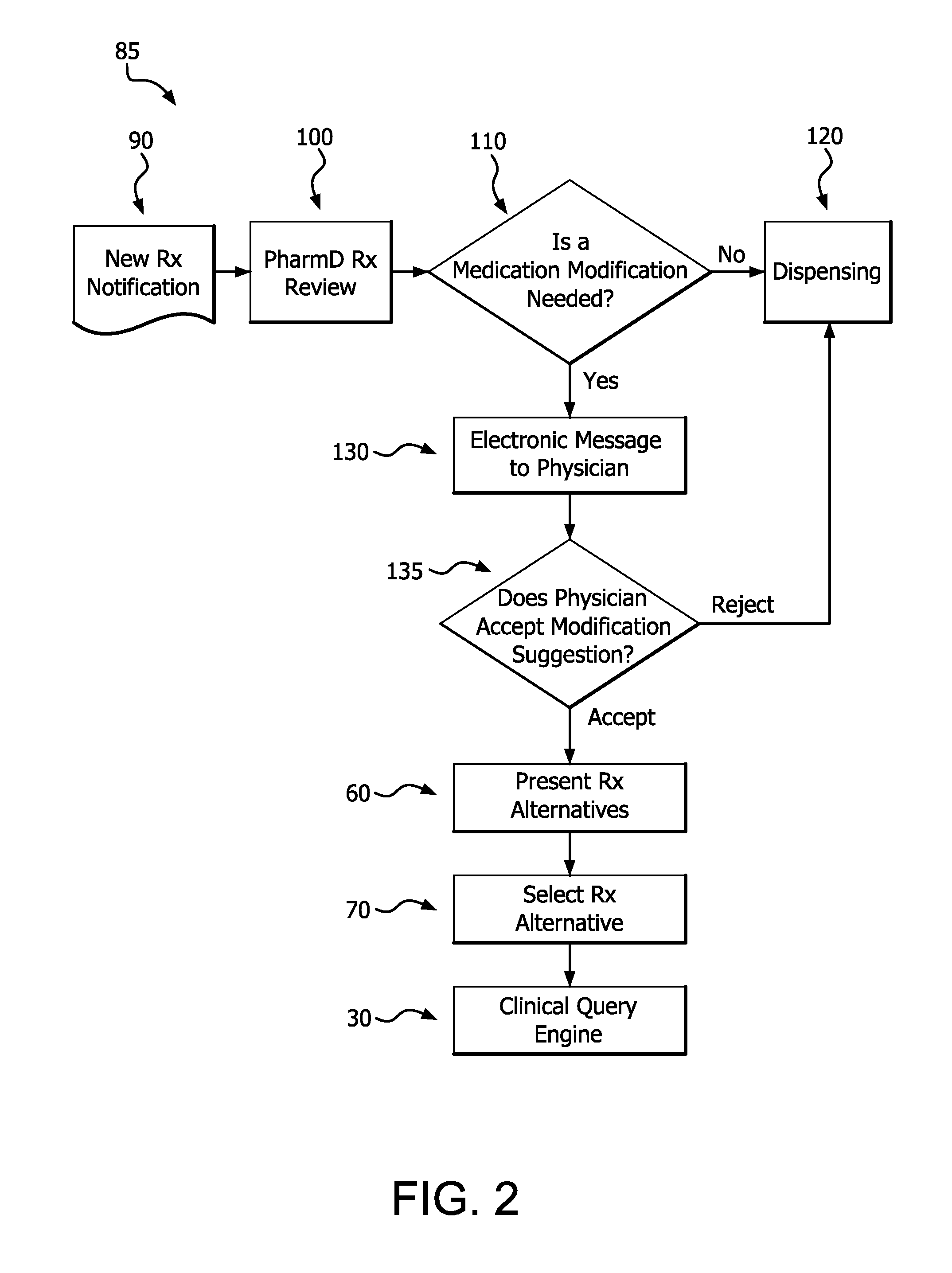
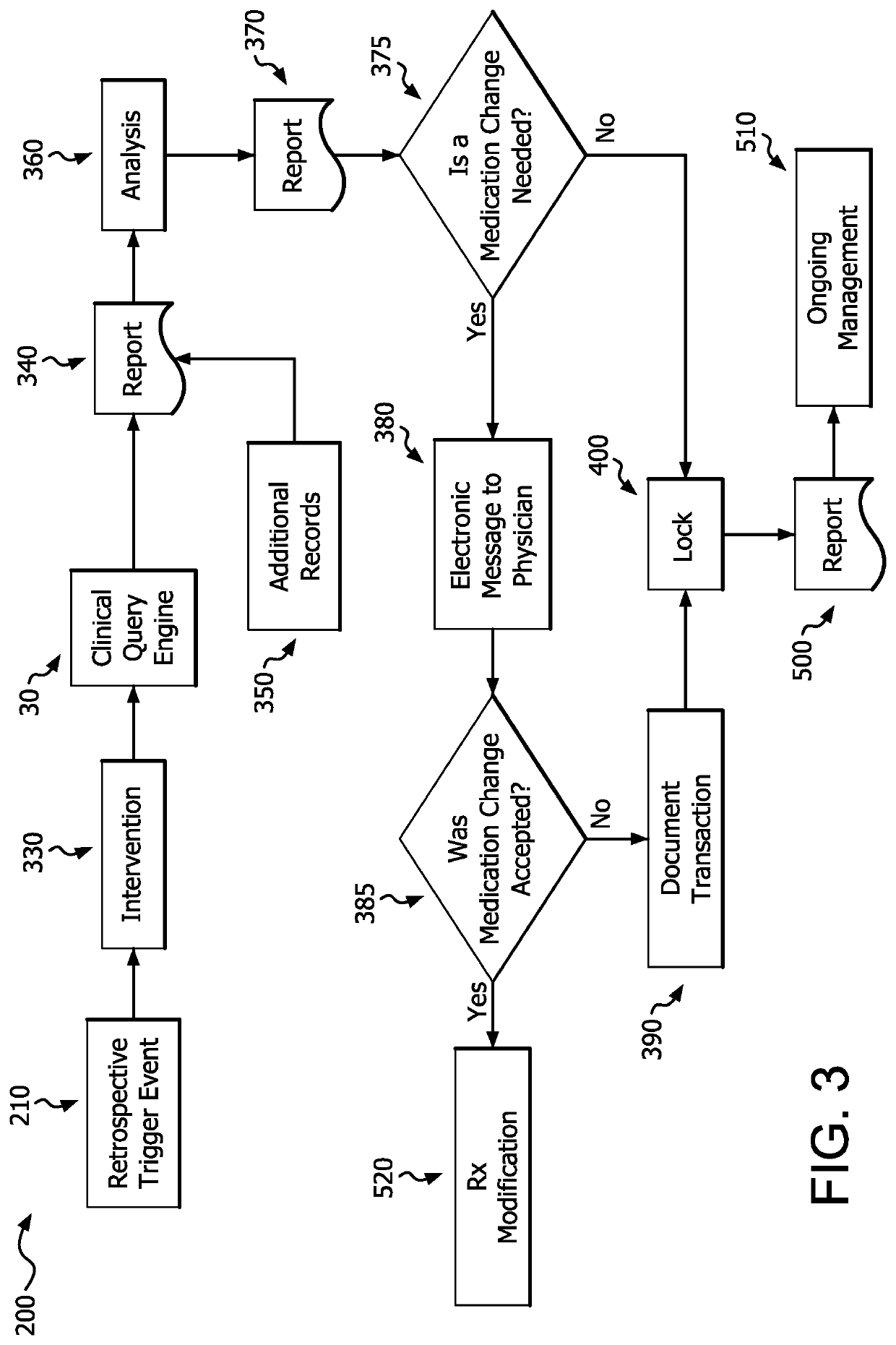
Medication risk mitigation system and method
ActiveUS20150178465A1Reduce drug riskData processing applicationsHealth-index calculationIntervention measuresMedication risk
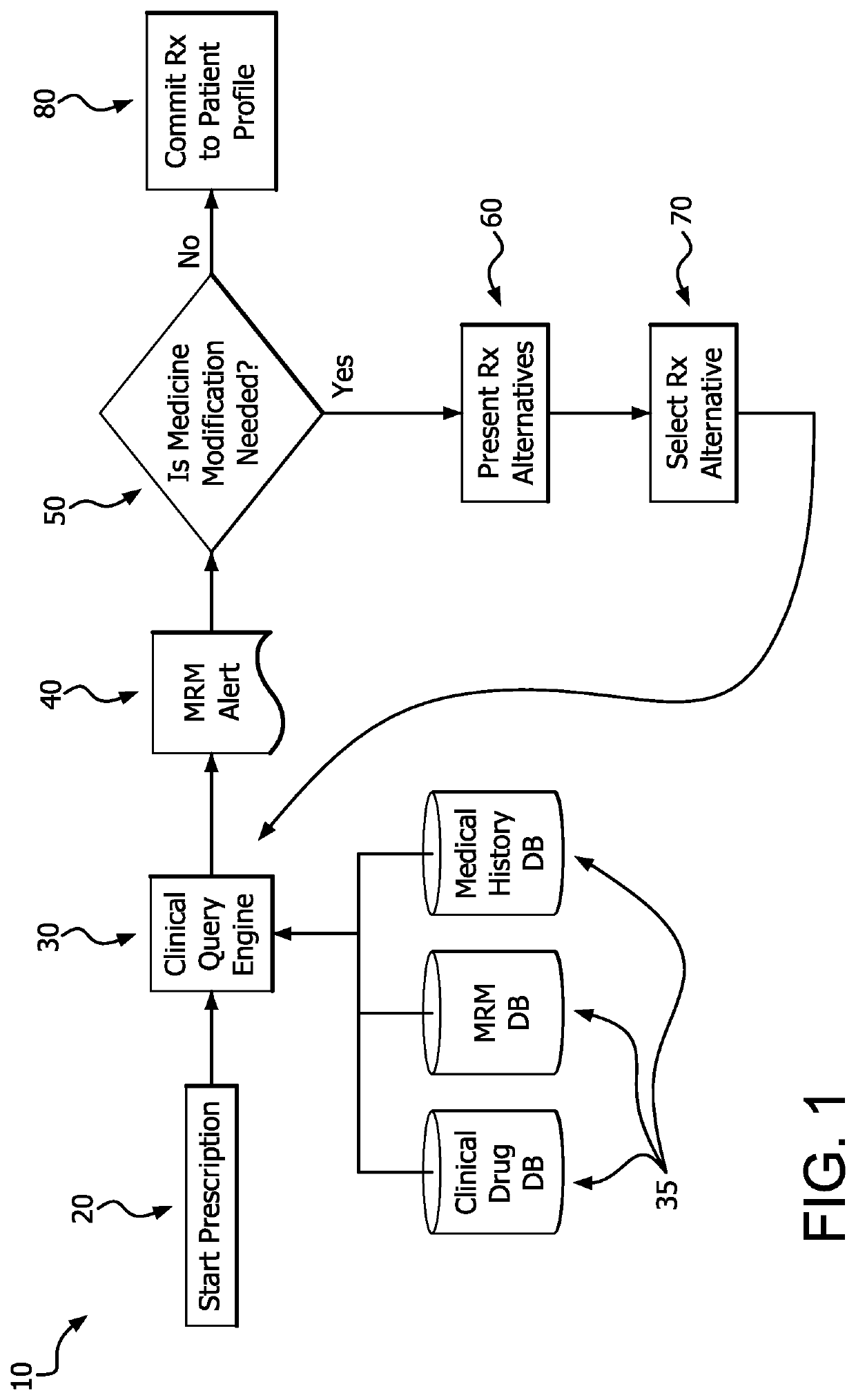
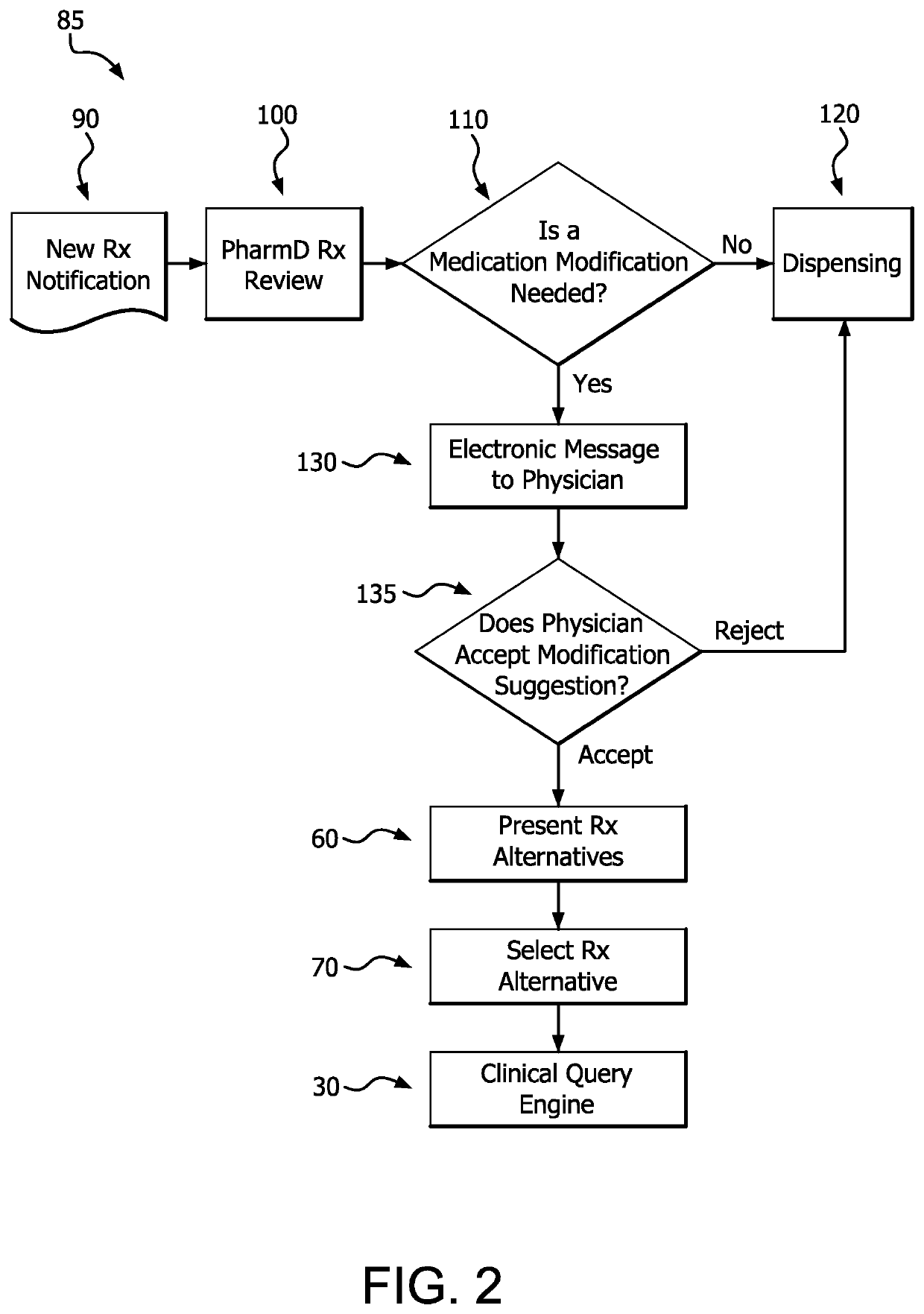
A medication risk mitigation method utilizing three interventions: a prospective intervention performed by a prescriber, a concurrent intervention performed by a pharmacist and a retroactive intervention performed by a pharmacist. At each intervention, the system of the instant invention utilizes a computer program to compare each prescribed medication to a series of intrinsic and extrinsic data sources in order to identify potential contraindications and, if necessary, modify a prescription. The system also permits secure messaging between prescribers and pharmacists, each with access to the computer program, so as to facilitate communication and reduce medication risks. The system of this invention also permits modeling for hypothetical medication modifications based on the same intrinsic and extrinsic data sources.
Owner:CAREKINESIS
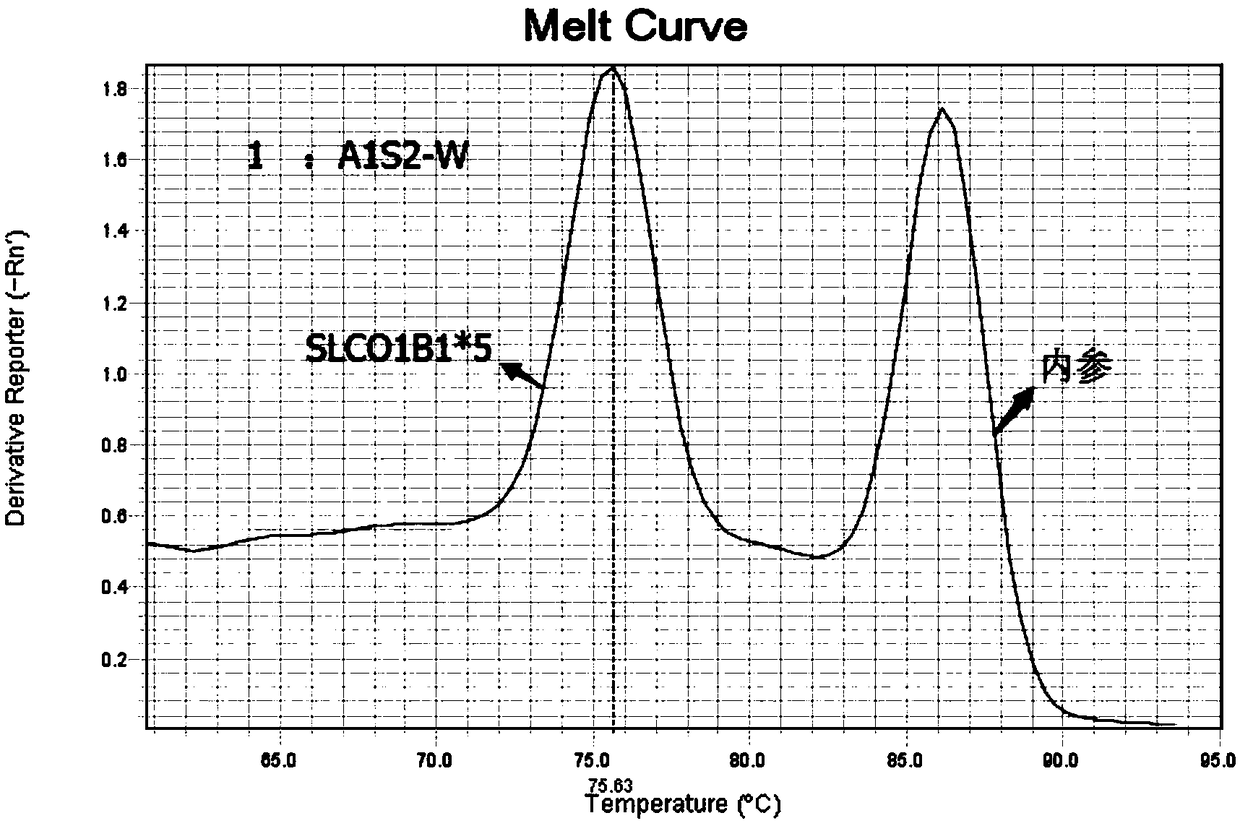
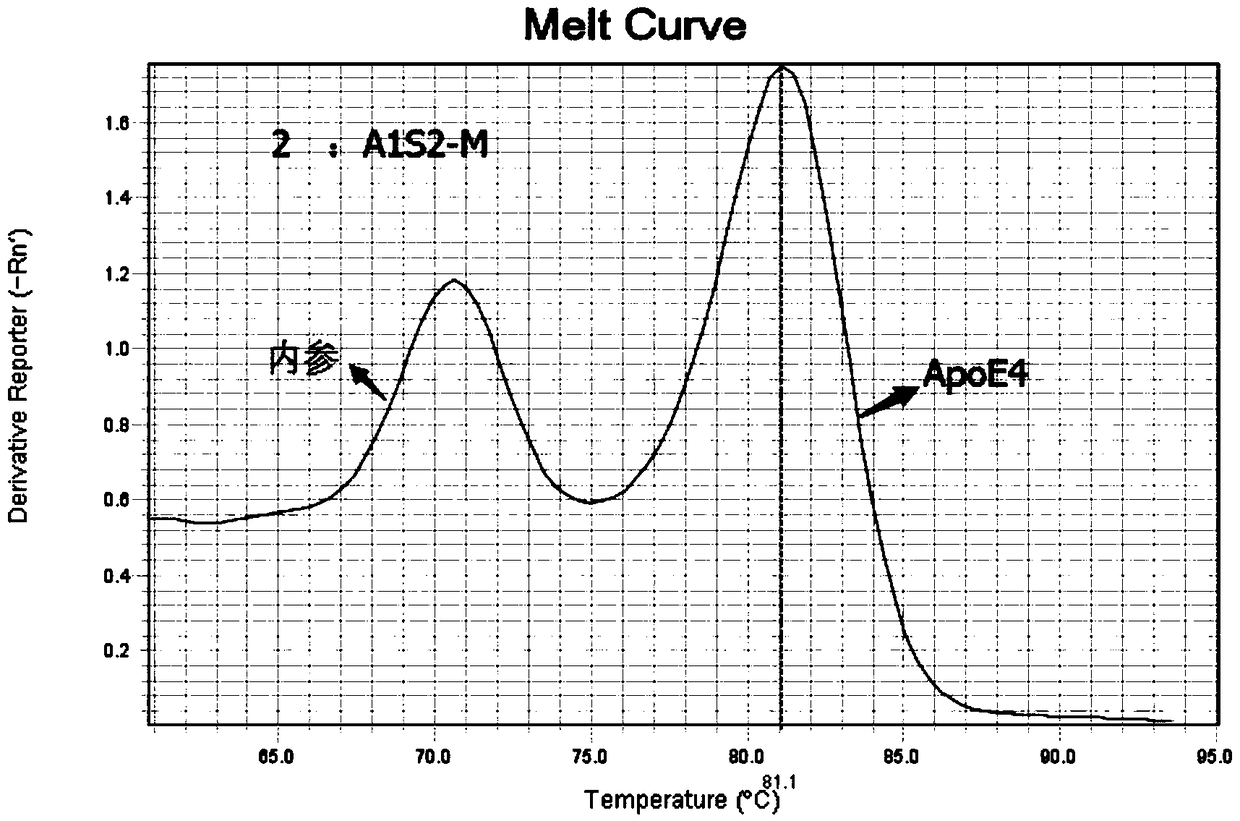
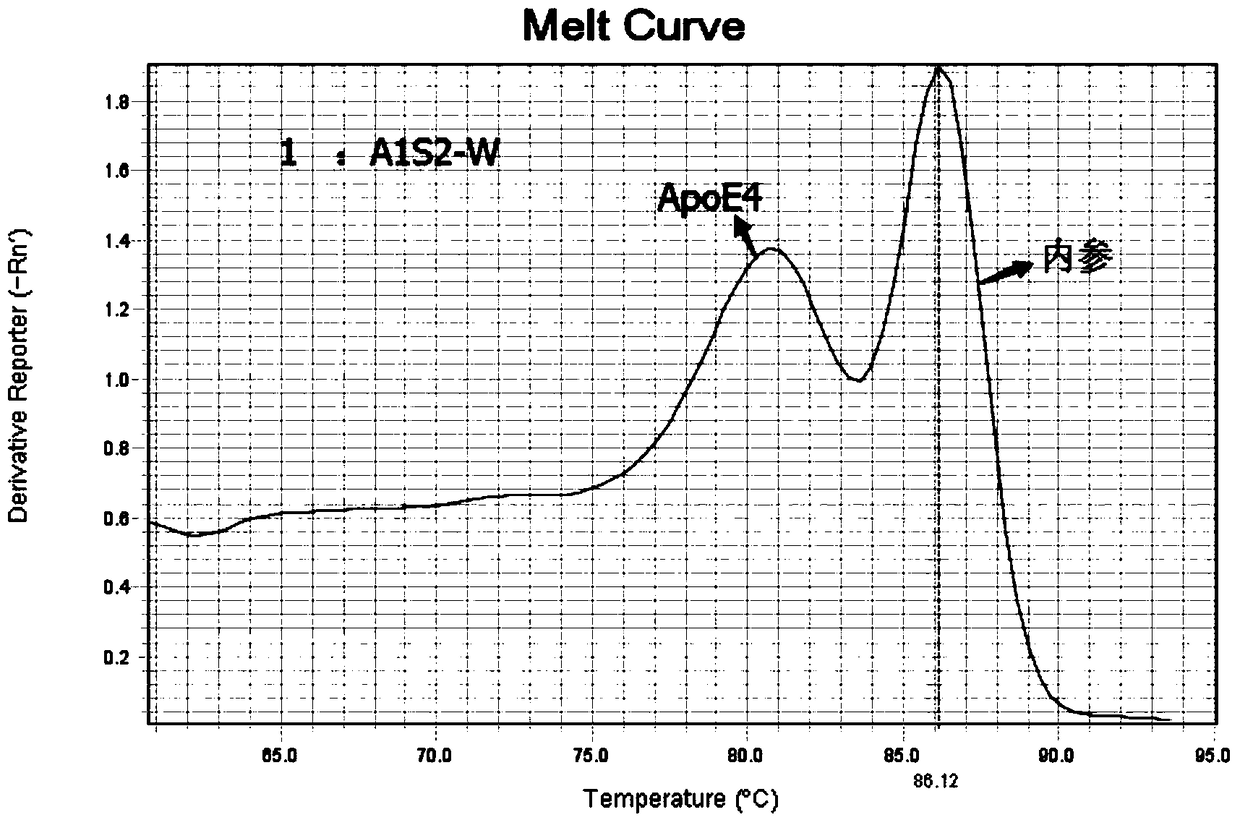
Kit for detecting polymorphism of APOE gene and SLCO1B1 gene
InactiveCN108753951AEasy to operateAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationGeneticsFluorescent pcr
The invention provides a kit for detecting polymorphism of an APOE gene and an SLCO1B1 gene. The kit comprises primers used for detecting polymorphism sites T388C and C526T of the APOE gene and expressed as SEQ ID NO. 1-6, primers used for detecting polymorphism sites A388G and T521C of the SLCO1B1 gene and expressed as SEQ ID NO. 7-12, and internal quality control housekeeping gene primers expressed as SEQ ID NO. 13-20. The kit is used for carrying out fluorescence PCR by using ARMS-PCR and fluorescence fusion curve techniques, is capable of achieving accurate detection of one polymorphism oftwo genes in the same reaction tube, is high in sensitivity and high in specificity, and can meet detection of oral epithelial cells, dried-blood spots and whole-blood samples; the whole detection process is short in time; the medication basis can be provided for the doctor in the first time; the medication risks of the patients can be reduced.
Owner:SHENZHEN YOU SHENGKANG BIOSCI CO LTD
Medication Risk Assessment System
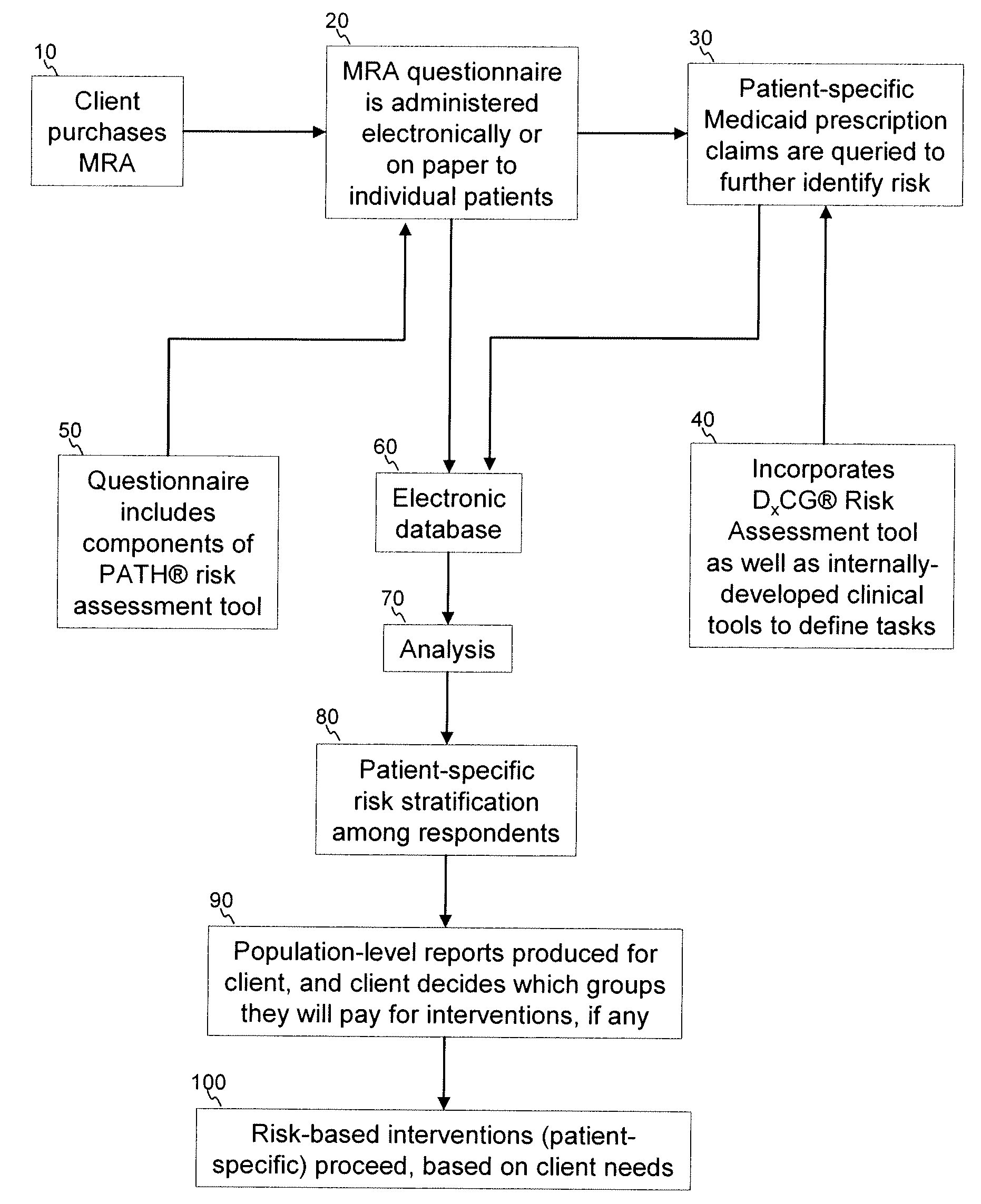
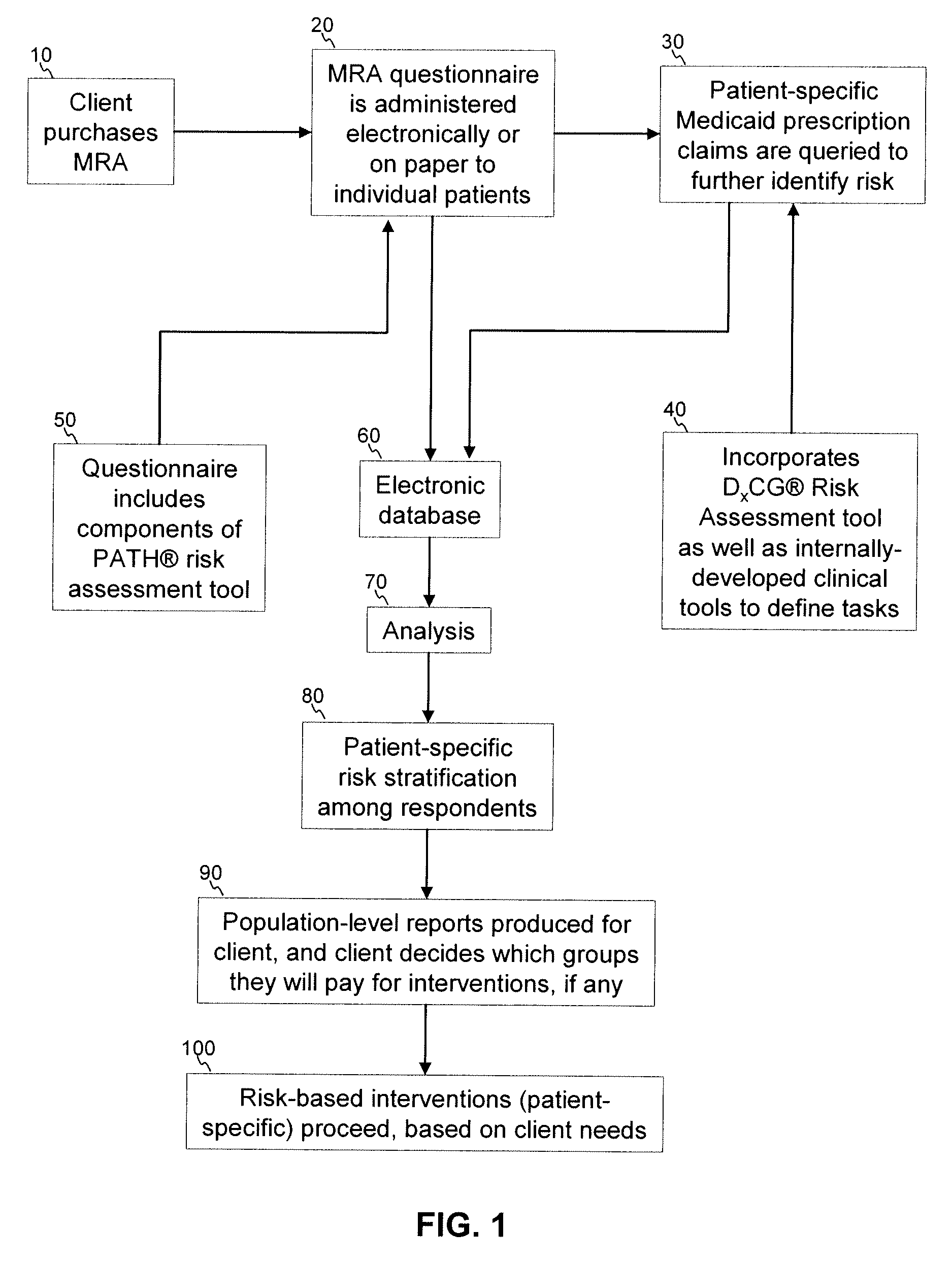
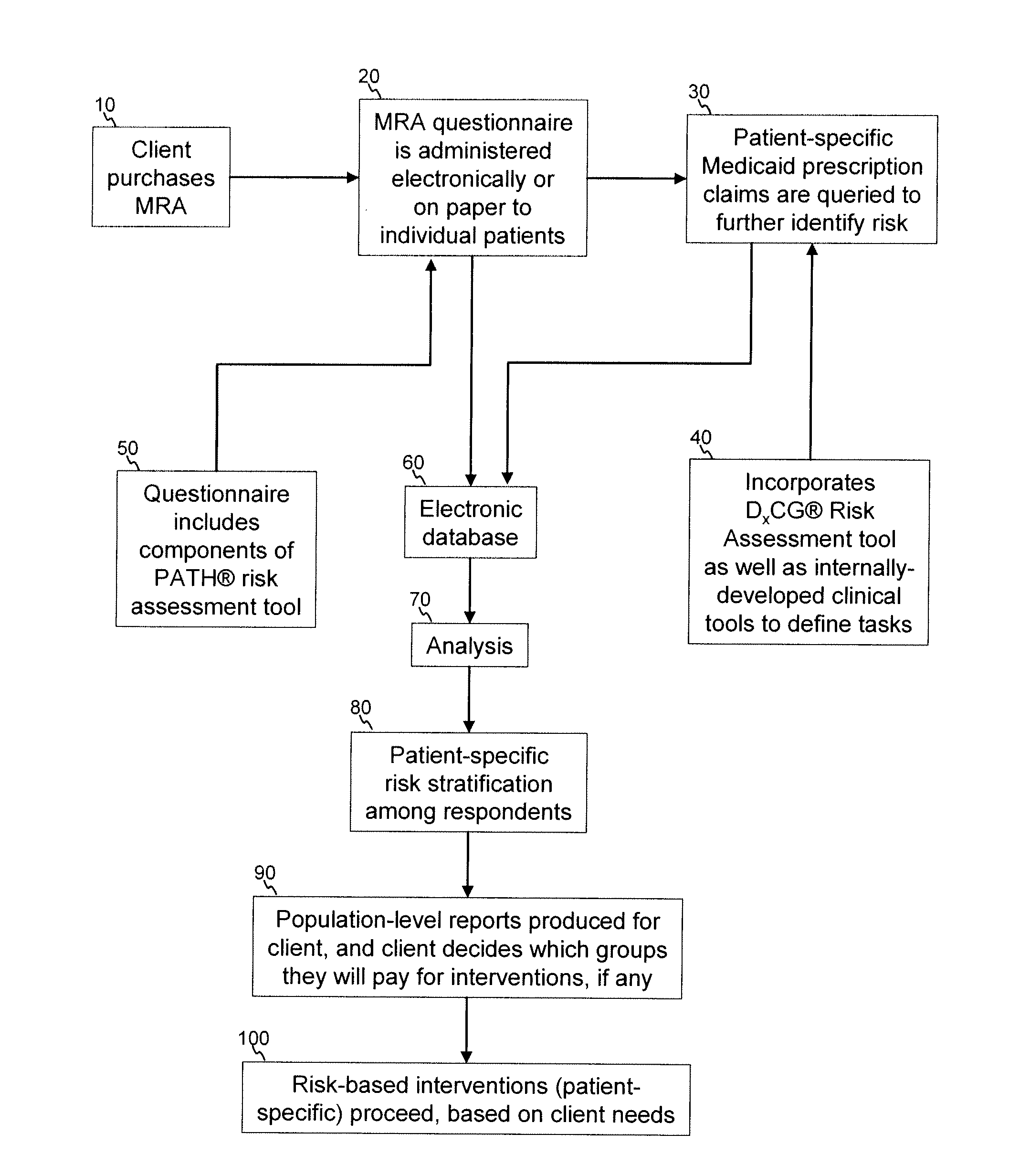
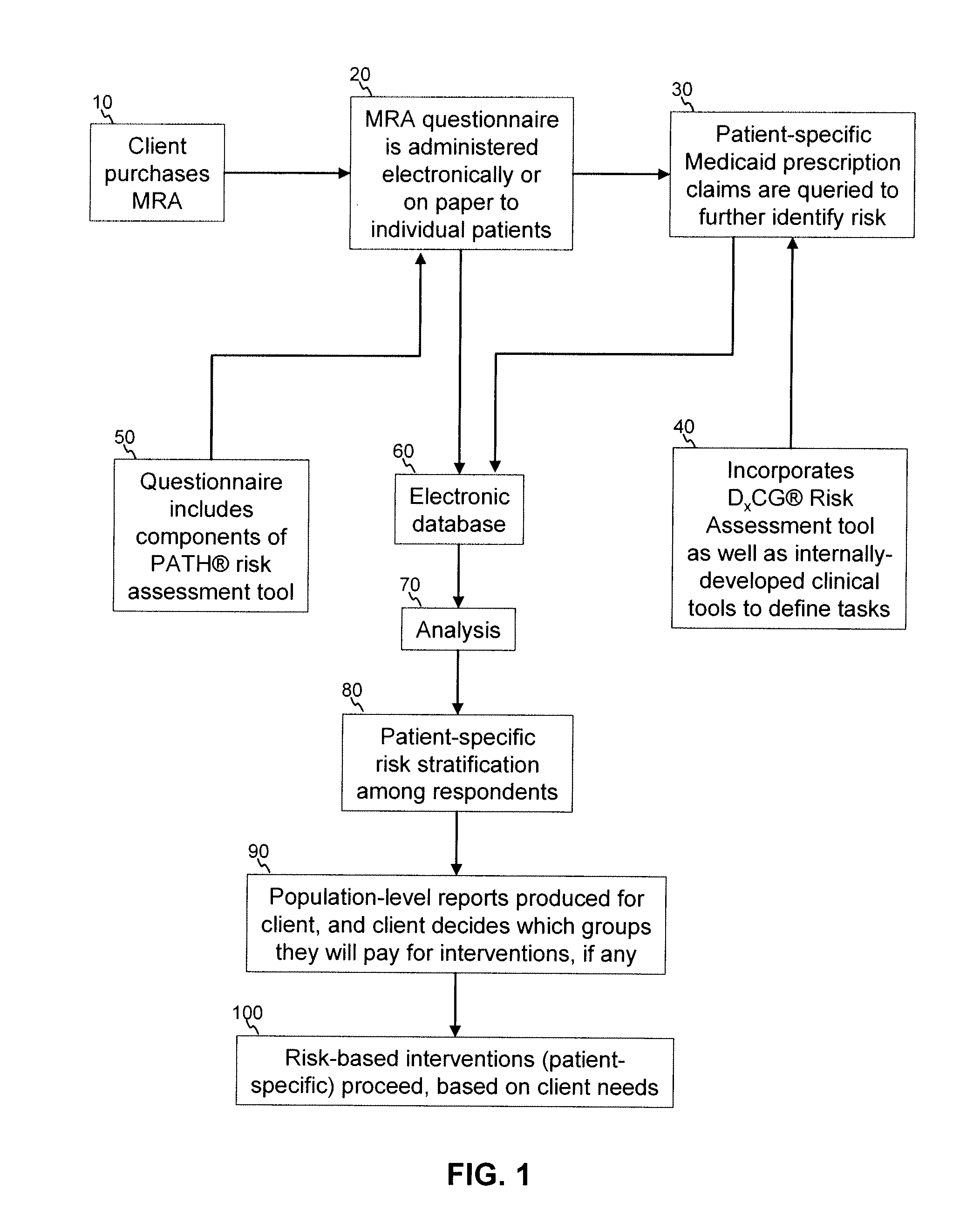
A medication risk assessment method implemented using a computer particularly adapted for a health care management or delivery organization is provided, wherein the method includes at least the steps of: providing a medication risk assessment tool to a client, wherein the medication risk assessment tool includes a questionnaire, and wherein the client maintains a relationship with a population of individuals; administering the questionnaire to the population of individuals, thereby generating questionnaire data; obtaining prescription claims data from the population of individuals; combining the questionnaire data and the prescription claims data in an electronic database, thereby generating population data; analyzing the population data according to a set of rules; ranking the analyzed data according to risk; and generating at least one report based on the ranked data.
Owner:PHARMMD SOLUTIONS
Composition containing paclitaxel and preparation method thereof
ActiveCN103736096AAvoid hemolytic reactionsReduce drug riskOrganic active ingredientsPowder deliveryPatient complianceMedication risk
The invention relates to the technical field of antitumor drugs, and discloses a composition containing paclitaxel and a preparation method thereof. The composition containing paclitaxel comprises 1 part by weight of paclitaxel and 1.2-15 parts by weight of egg yolk lecithin; the pH value range of the composition is 4.0-6.0. According to the composition containing paclitaxel of the invention, egg yolk lecithin is adopted as an emulsifier; the pH value of the composition is adjusted to be within a proper range; no polyoxyethylated castor oil with large toxicity is adopted; no ethanol is contained; the medication risk of the composition containing paclitaxel is reduced; and the patient compliance is improved.
Owner:李宏 +1
Major disease medical insurance drug auditing and drug treatment management system
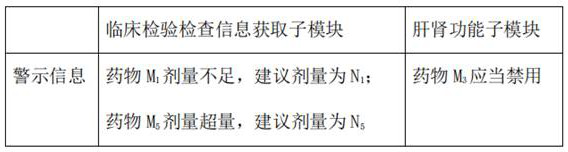
ActiveCN113488134ALiver and kidney function is goodImprove efficiencyMedical data miningHealth-index calculationDrug utilisationCritical illness
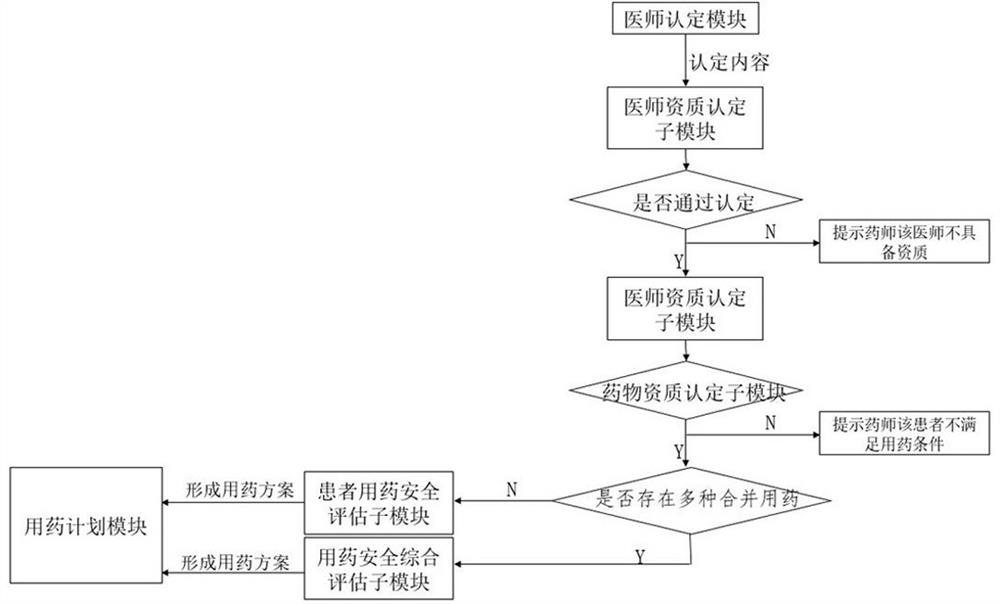
The invention relates to the technical field of medical data processing, in particular to a major disease medical insurance drug auditing and drug treatment management system which comprises a physician affirmation module, a pharmacist affirmation module, a medication planning module, a basic database, a physician end, a pharmacist end and a patient end. Through dual affirmation of physicians and drugs, the medical insurance drug auditing efficiency and precision are improved, and the medication risk of patients is reduced. The risk of medical negligence in drug treatment of the patients is reduced by adopting a patient medication safety evaluation module and a medication safety comprehensive evaluation module, and the reasonability of doctors' prescriptions and medical advice is improved.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
Medication risk assessment system
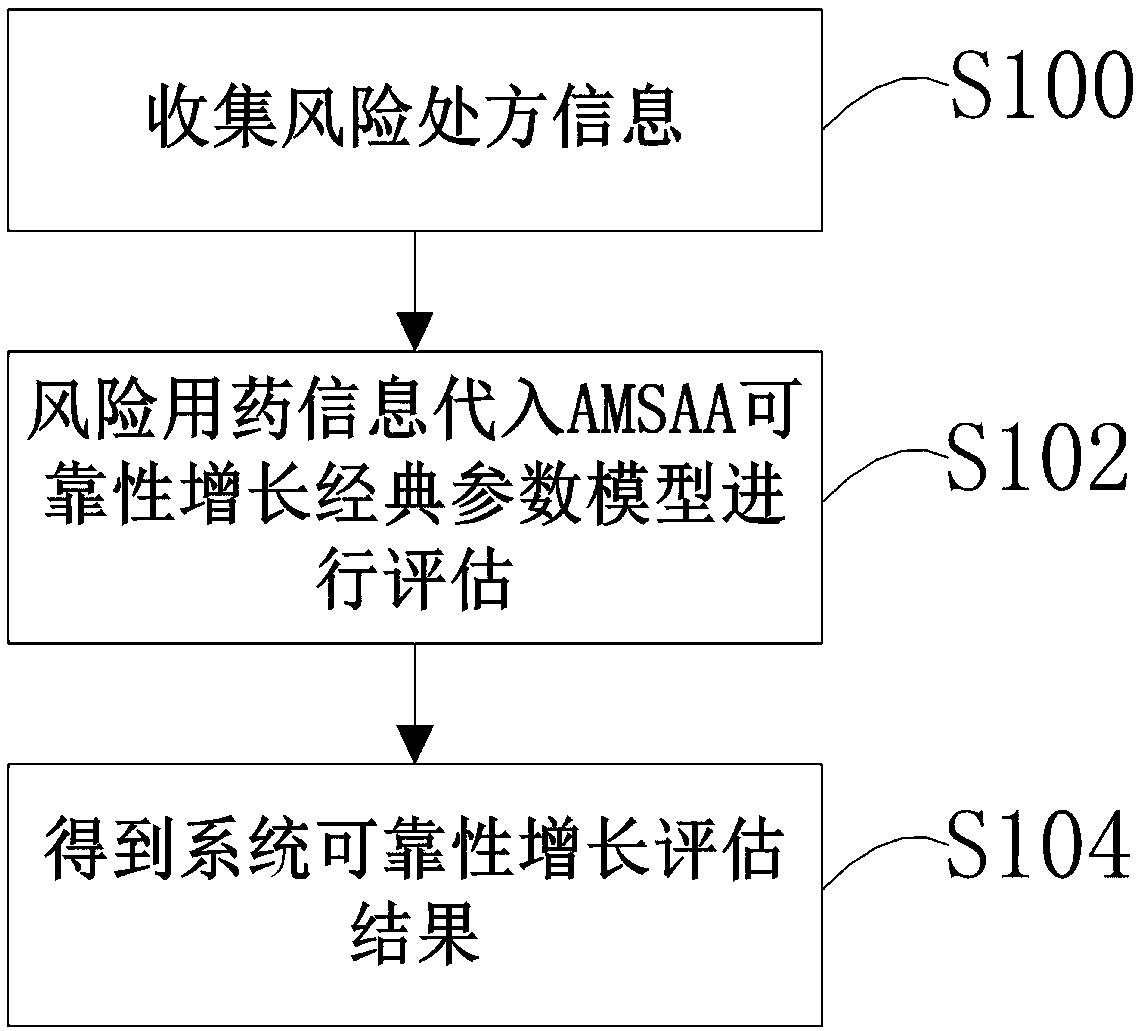
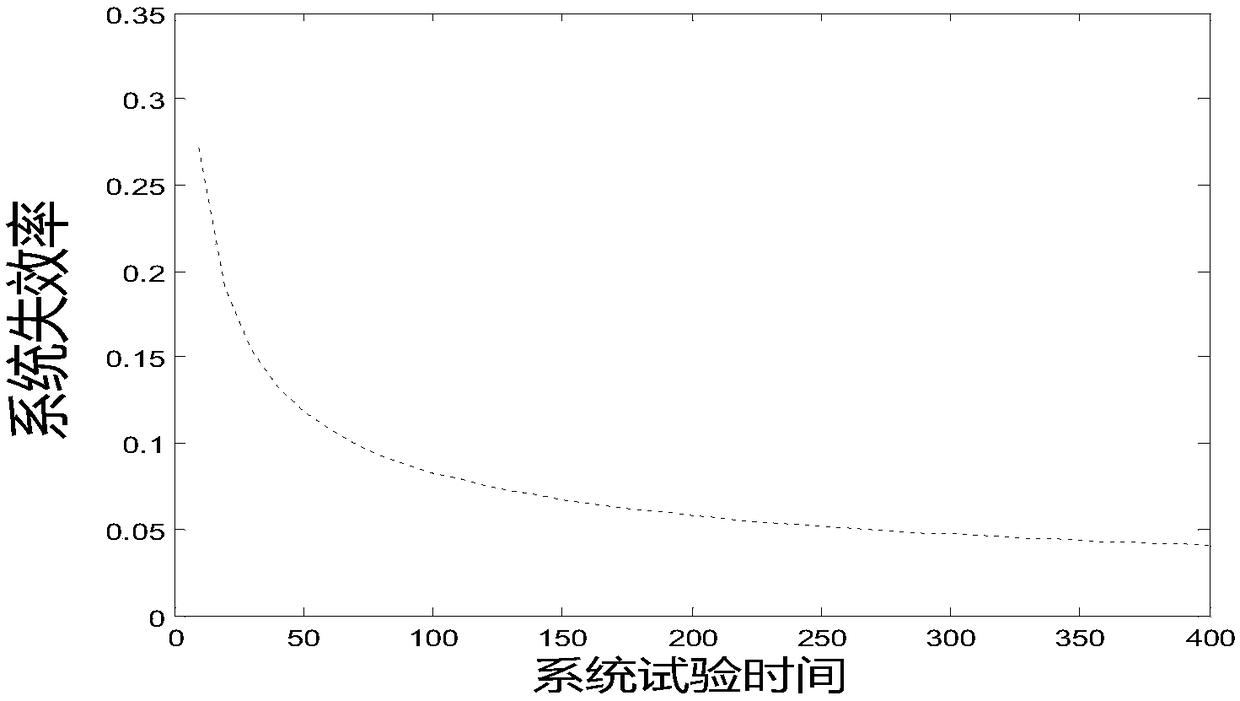
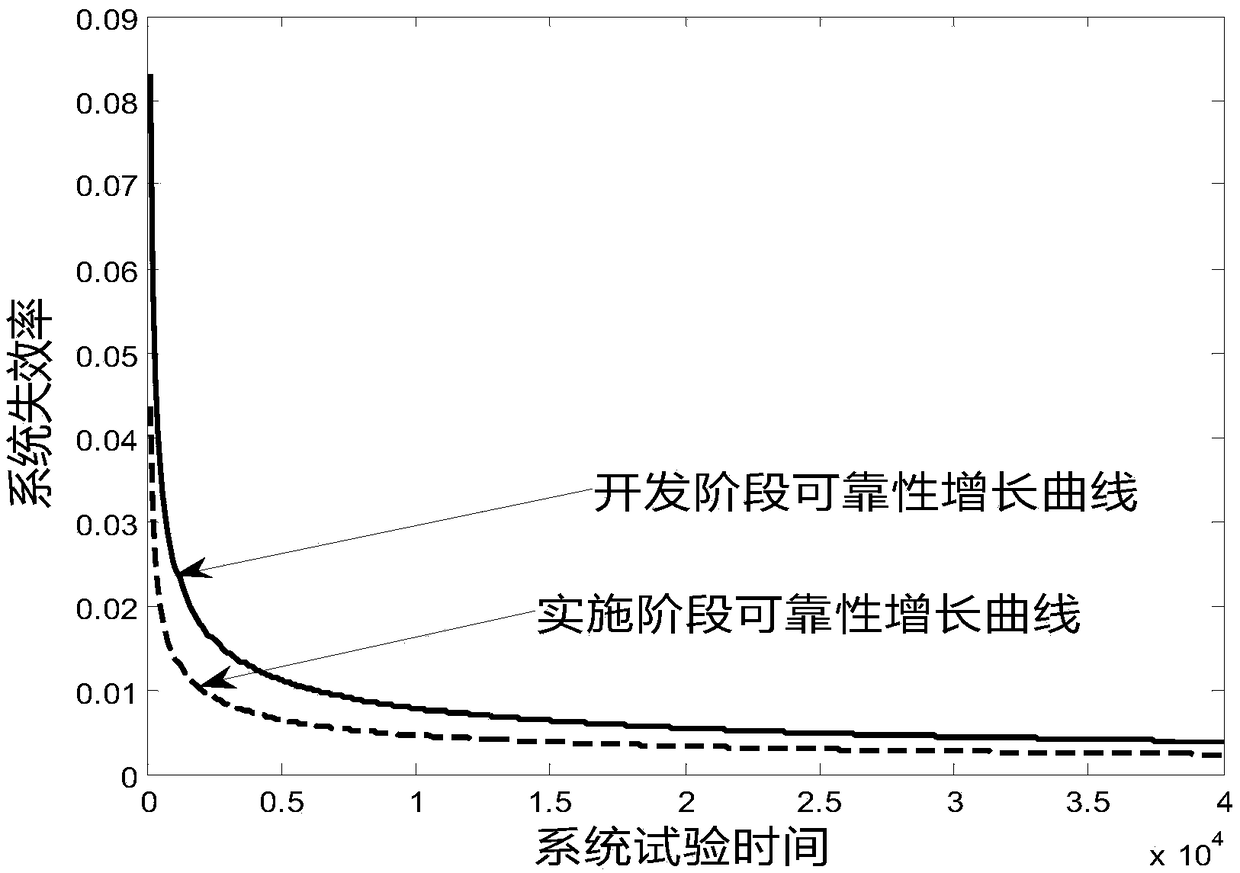
The invention provides a medication risk assessment system comprising an information collection module and a reliability growth assessment module. The information collection module is used for collecting risk medication information, wherein the risk medication information includes the risk prescription information, the risk accumulation time information and the risk medication times. The reliability growth assessment module is used for substituting the risk medication information into the reliability growth classic parameter model for assessment to obtain the mean time between failures so as to further obtain the system reliability growth assessment result. According to the technical scheme, the reliability growth classic parameter model is introduced to perform assessment so as to providetechnical indicators for risk prevention and control in the entire medical treatment system and provide visual suggestions for medical medication management. The reliability of each risk is calculated so as to achieve the effect of visually prompting the systemic risk.
Owner:FUJIAN UNIV OF TRADITIONAL CHINESE MEDICINE
Taxol composition for injection and preparation method thereof
ActiveCN105534903AHigh drug loadingLow in lipidsOrganic active ingredientsEmulsion deliveryMedication riskPhospholipid
The invention provides a taxol composition for injection, which comprises taxol, oil for injection, an emulsifying agent and a stabilizing agent, wherein the oil for injection is selected from at least one of soybean oil, olive oil, coix seed oil, medium chain triglyceride as well as vitamin E and derivatives thereof, and the emulsifying agent is selected from at least one of egg yolk lecithin, soybean lecithin and synthetic lipid as well as amino acid. The invention also provides a preparation method of the taxol composition for injection, wherein an oil phase is prepared by the following steps: mixing and stirring taxol, the emulsifying agent and absolute ethyl alcohol until the solution is clear, then adding the oil for injection and the stabilizing agent, further stirring until the solution is clear, and volatilizing ethyl alcohol in vacuum so as to obtain the oil phase. The taxol composition for injection, provided by the invention, is high in drug loading capacity, low in lipid content and good in stability and is capable of reducing the medication risk of taxol for injection and increasing the compliance of patients.
Owner:慧禹康成(浙江)医药科技有限公司
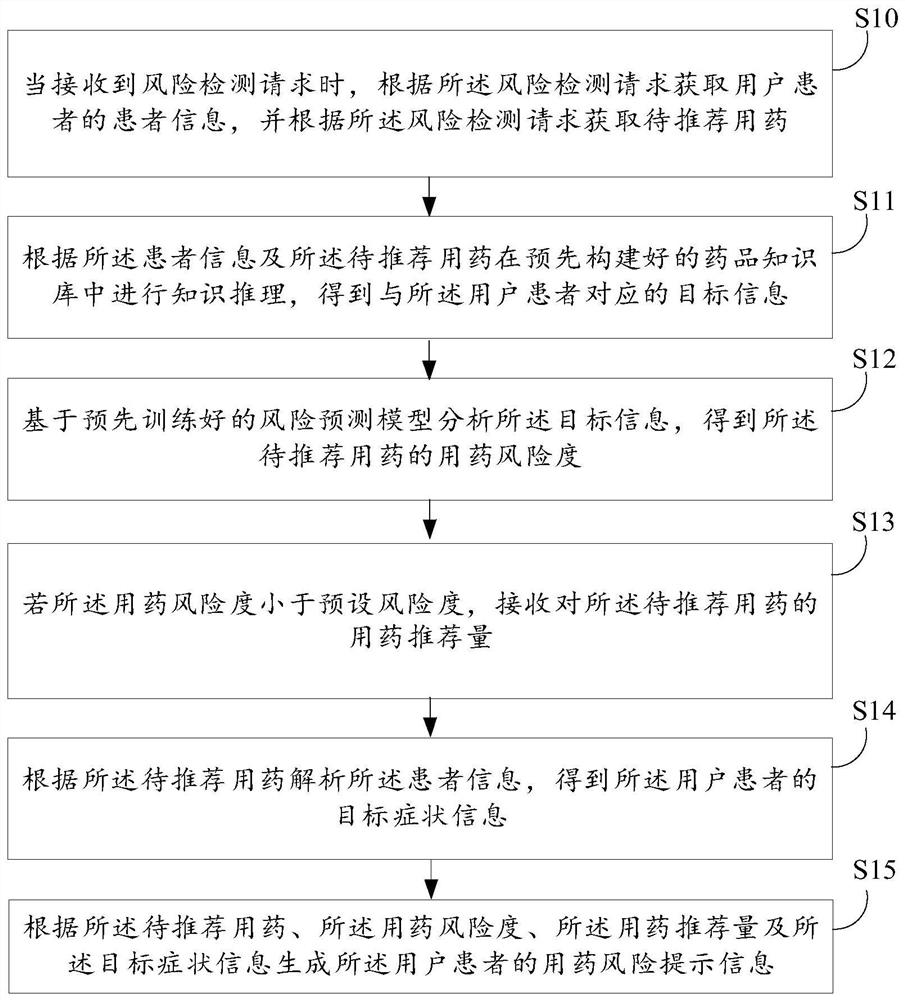
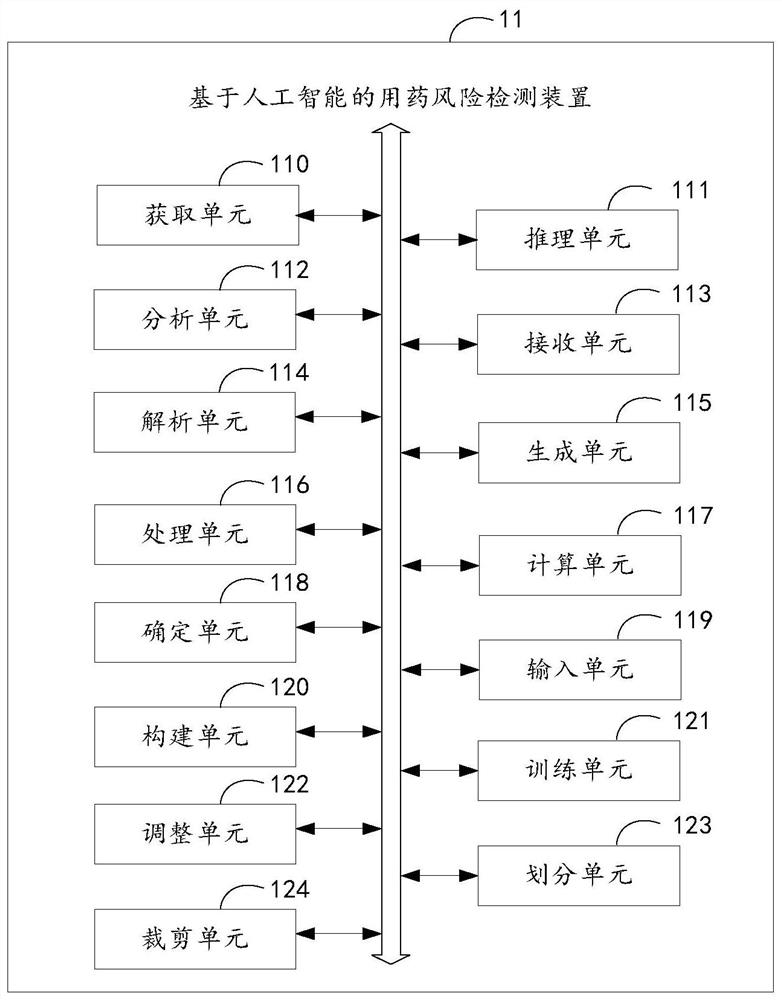
Drug use risk detection method based on artificial intelligence and related equipment
PendingCN113724830AAccurate acquisitionImprove production efficiencyDrug and medicationsNatural language data processingMedicineMedication risk
The invention relates to artificial intelligence, and provides a medication risk detection method based on artificial intelligence and related equipment. The method comprises the steps of obtaining patient information of a user patient according to a risk detection request, and obtaining a to-be-recommended medicine according to the risk detection request; performing knowledge reasoning in a medicine knowledge base according to the patient information and the to-be-recommended medicine to obtain target information corresponding to the user patient; analyzing the target information based on a risk prediction model to obtain the medication risk degree of the to-be-recommended medication; if the medication risk degree is smaller than a preset risk degree, receiving a medication recommendation amount of the to-be-recommended medication; analyzing the patient information according to the to-be-recommended medication to obtain target symptom information of the user patient; and generating prompt information of the user patient according to the to-be-recommended medicine, the medicine risk degree, the medicine recommendation amount and the target symptom information. According to the invention, the accuracy of medication risk detection can be improved. In addition, the invention also relates to a block chain technology, and the prompt information can be stored in a block chain.
Owner:深圳平安智慧医健科技有限公司
Device for automatically dispensing medicine for elderly
InactiveCN110697228AEasy to useEasy to operateSmall article dispensingSpecial dispensing meansMedication riskDispensing medications
The invention discloses a device for automatically dispensing medicine for the elderly, and relates to the technical field of elderly services. The device for automatically dispensing medicine for theelderly includes a medicine dispensing layer part, a medicine placing layer part, a drawer layer part, a circular rail part, a power part, a medicine taking part, a shell and a lower body part; the medicine dispensing layer part and the medicine placing layer part are connected through the shell; the medicine placing layer part is located at the upper end of the medicine dispensing layer part; the medicine dispensing layer part is fixedly connected to the power part by welding; the drawer part and the circular rail part are fixedly connected by screws; the medicine taking part is located inside the power part; and the shell and the drawer layer part are fixedly connected by welding. The storage safety of medicines is guaranteed, accurately dispensing of the medicines used by the elderly is achieved, the problems that the elderly often forget to use the medicines and the amount of the medicines to be used are effectively solved, and the medicine use risk of the elderly is effectively reduced.
Owner:ANHUI UNIV OF SCI & TECH
Preparation method of lidocaine hydrochloride injection
ActiveCN104688675AImprove stabilityReduce drug riskOrganic active ingredientsNervous disorderMedication riskWater temperature
The invention provides a preparation method of a lidocaine hydrochloride injection. The method provided by the invention is improved as follows: the sterilization condition is improved to 116 DEG C for 30-40 minutes from 100 DEG C for 30 minutes; and the temperature of liquid-dosing injection water is improved to 30-50 DEG C from 70-80 DEG C. The defects of an existing method are overcome; by virtue of test research on the prepared lidocaine hydrochloride injection, the indexes such as sterile detection, related substances and content reach the quality standard of the second part lidocaine hydrochloride injection of the Chinese pharmacopoeia 2010; the quality risk of the product and the medication risk of patients are relatively well reduced. By virtue of an accelerated test and a long-term stability test, the result shows that the lidocaine hydrochloride injection prepared by the method provided by the invention is good in stability. The method provided by the invention is simple; new cost is not increased; and the method is suitable for large-scale industrial production, and has relatively great application value.
Owner:SHANGHAI ZHAOHUI PHARMA
Detection method for triptolide in tripterygium preparation
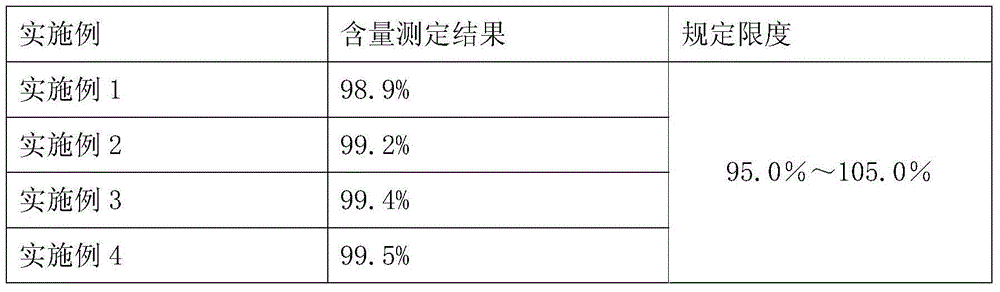
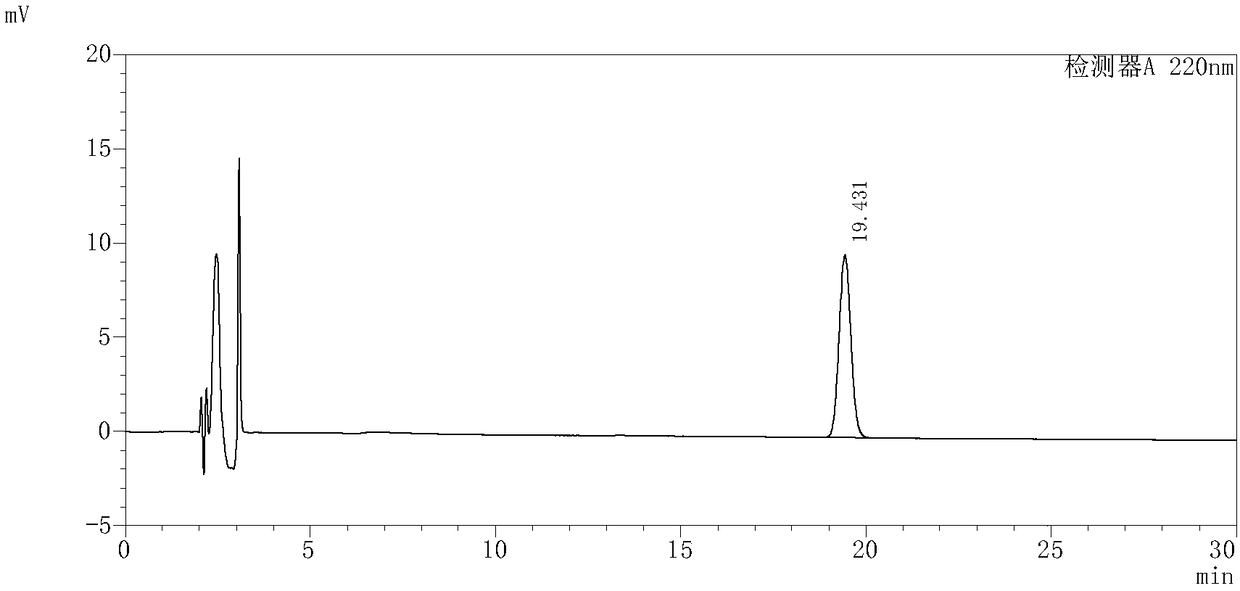
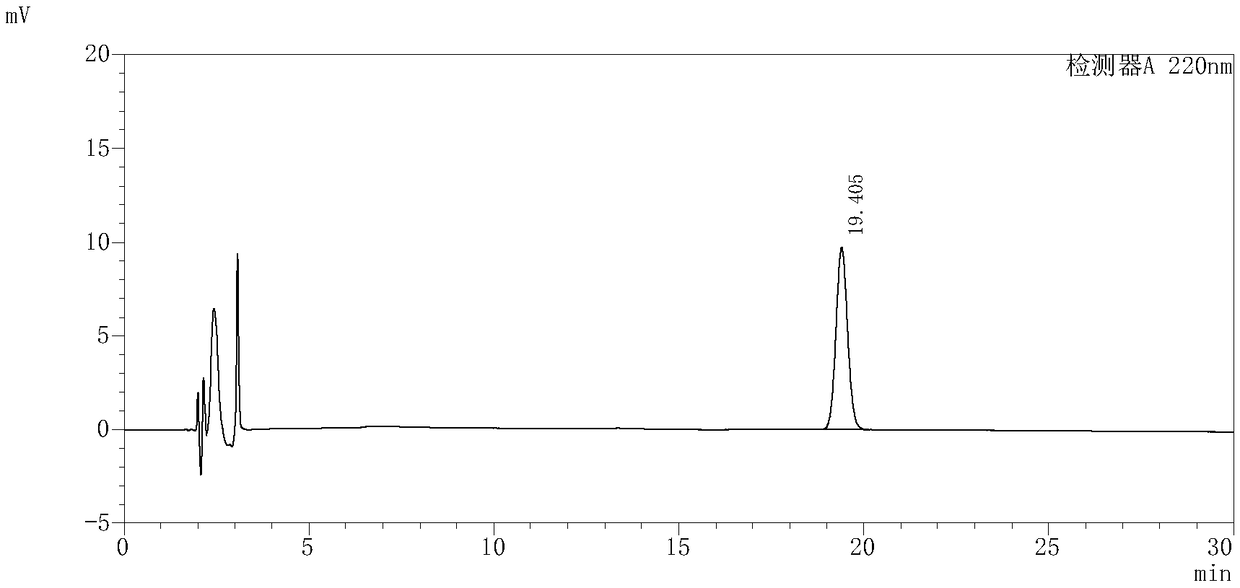
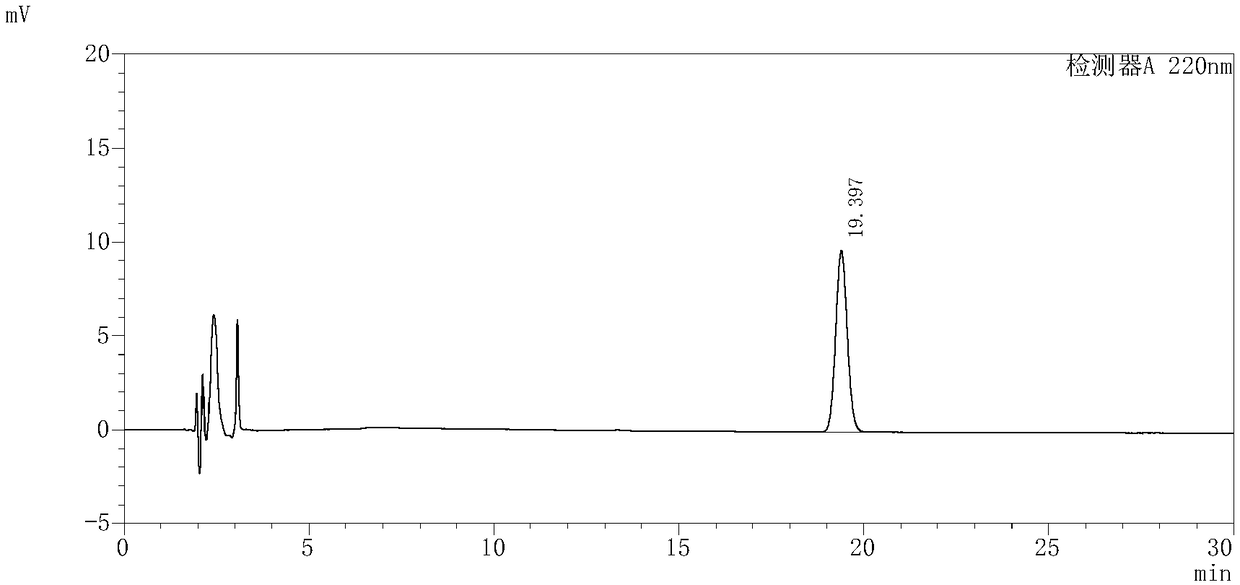
InactiveCN109239213AAccurate detection of triptolide contentEnsure safetyComponent separationMedication riskSilanes
The invention discloses and provides a detection method for triptolide in tripterygium preparation. The detection method comprises the following steps of (1) chromatographic condition, in which tadecyl silane chemically bonded silica is used as a filler, acetonitrile and water are used as a moving phase according to a volume proportion being 25:75, and the detection wavelength is 220 nanometers; 2) system applicability experiment, in which the theoretical plate number is calculated not to be lower than 2,000 according to the triptolide; 3) preparation of a contrast solution, in which a first methanol solution is added into the triptolide to prepare the contrast solution with triptolide having special concentration; 4) preparation of an experiment sample solution; and 5) measurement, in which a certain of the contrast solution obtained in the step 3) and the experiment sample solution obtained in the step 4) are absorbed and injected to a high performance liquid chromatograph for measurement, the column temperature is 30-40 DEG C, the flowing speed is 1.0ml / min, and the calculation is performed by an external standard method. By the detection method, the content of the triptolide inthe tripterygium preparation can be accurately detected, the clinical medication risk is judged in advance, and the clinical medication safety and effectiveness are ensured.
Owner:重庆市药研院制药有限公司
Clinical medication risk intelligent assessment method
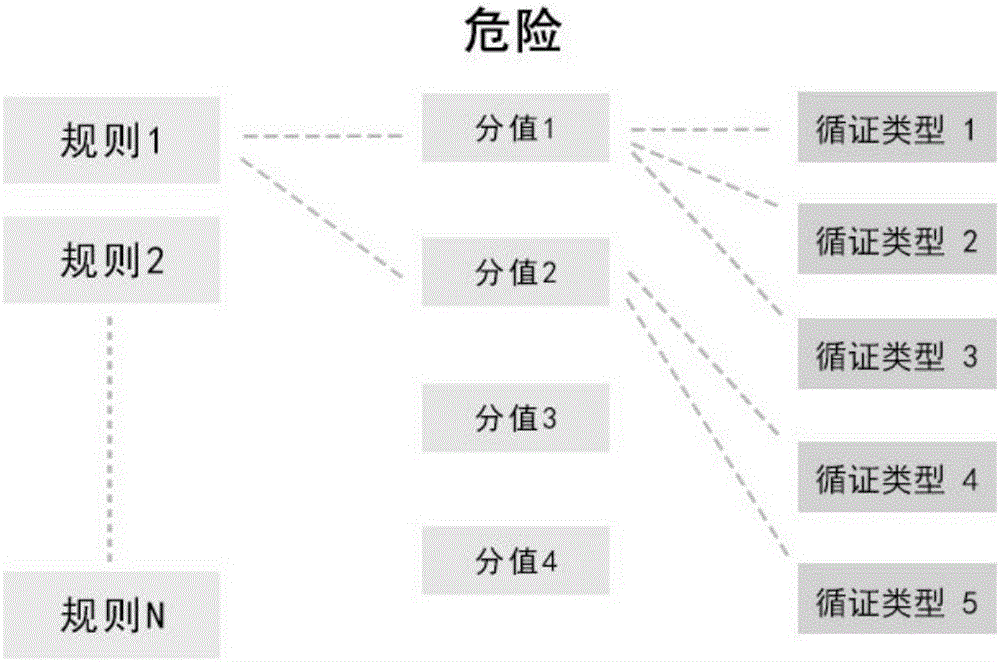
InactiveCN106021851AScientific serviceSpecial data processing applicationsComputer-assisted medical procedureClinical psychologyMedication risk
The embodiment of the invention discloses a clinical medication risk intelligent assessment method. The clinical medication risk intelligent assessment method includes: establishing a medicine association knowledge base, dividing the knowledge into two layers of structures, associating a rule knowledge base of the top layer with an evidence-based knowledge base of the bottom layer, performing type division on evidence-based bases, dividing the evidence-based bases into 5 grades according to the credibility of sources of the evidence-based bases, and giving weight values to the grades; extracting a risk rule from the evidence-based bases, establishing a association relationship between the risk rule and the corresponding evidence-based type; giving a corresponding dangerous score to the risk rule according to the description of the risk rule in the evidence-based bases; and calculating a risk grade index of the risk rule according to a calculation rule.
Owner:北京快马互联科技有限公司
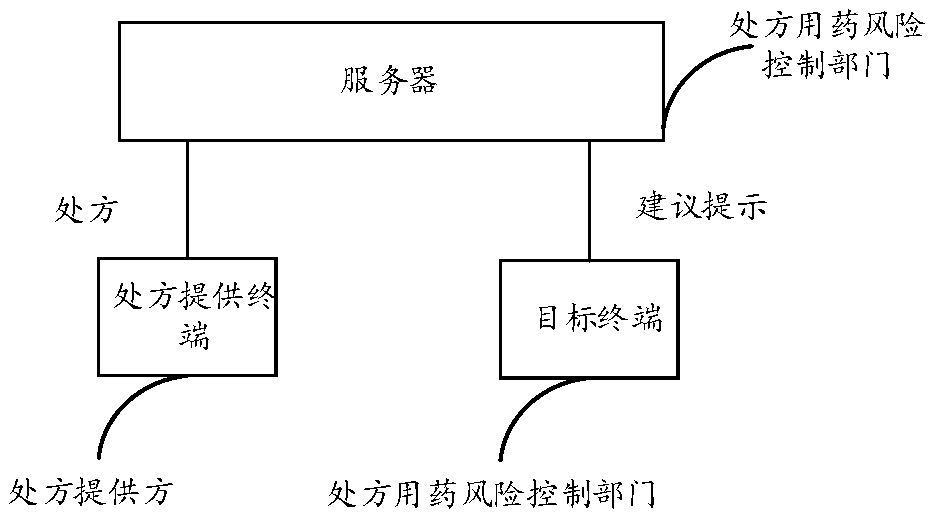
Risk control method for prescription medication, server and system
PendingCN109545314AGuaranteed rationalityReduce doctor-patient conflictsDrug and medicationsRisk ControlMedication risk
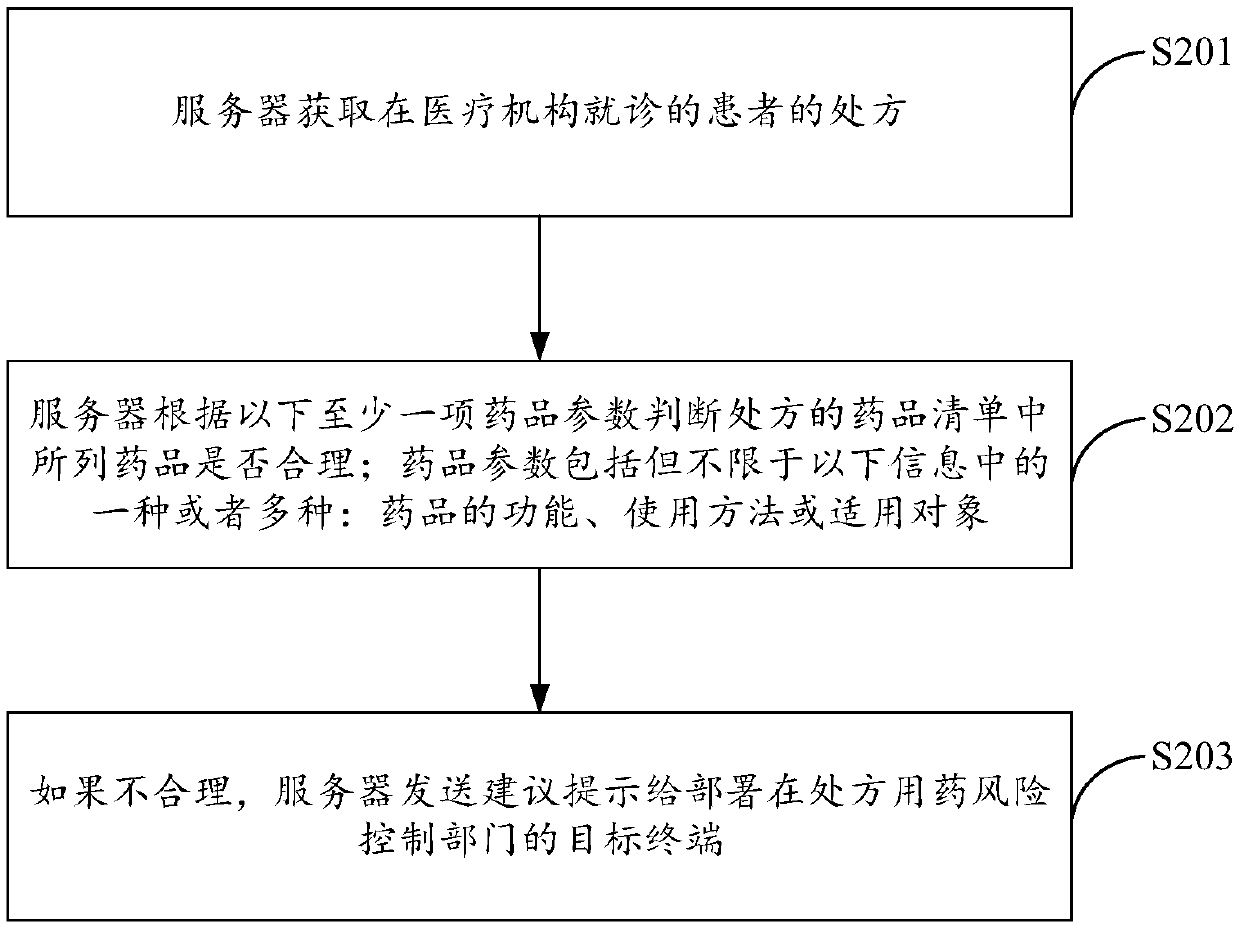
The invention discloses a risk control method for prescription medication, a server and a system and relates to analysis and processing of medical information. The method comprises the following steps: acquiring a prescription of a patient seeing a doctor in a medical institution by a server; confirming whether drugs listed in a drug list of the prescription are reasonable by the server accordingto at least one of the following drug parameters, wherein the drug parameters comprise but not limited to one or more of the following information: functions, application methods or applicable targetsof the drugs; if not reasonable, transmitting a suggestion to a target terminal deployed in a prescription medication risk control department by the server, wherein the suggestion is used for prompting the unreasonable aspect of the prescription; the prescription medication risk control department is a prescription medication risk control department of the medical institution. According to the risk control method disclosed by the invention, real-time intervention can be performed on the unreasonable prescription, conflicts between doctors and patients are decreased, and the prescription normalization and patient medication reasonability are ensured.
Owner:深圳平安医疗健康科技服务有限公司
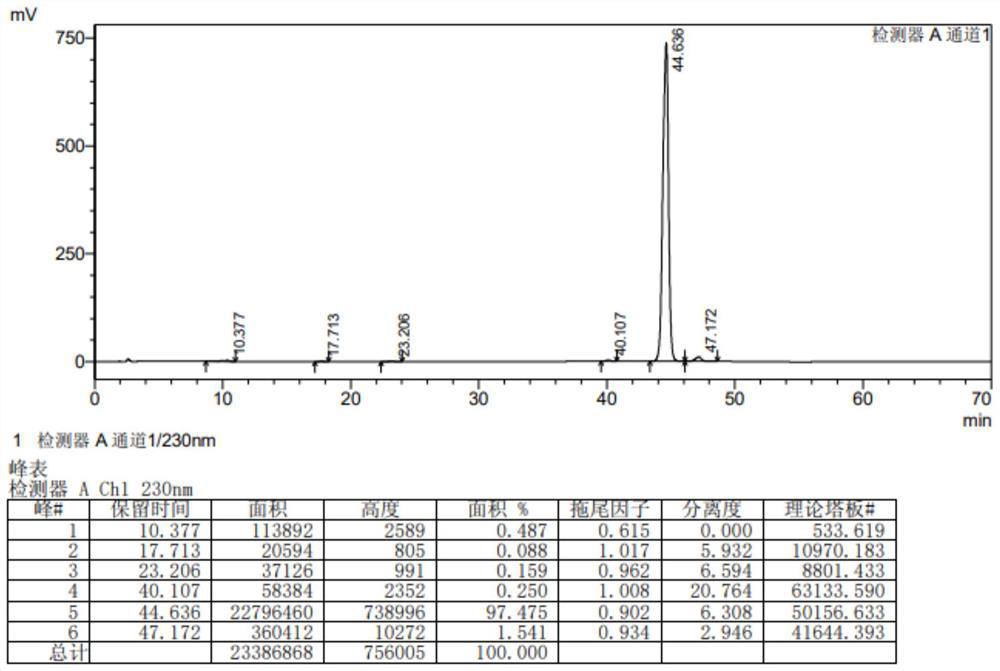
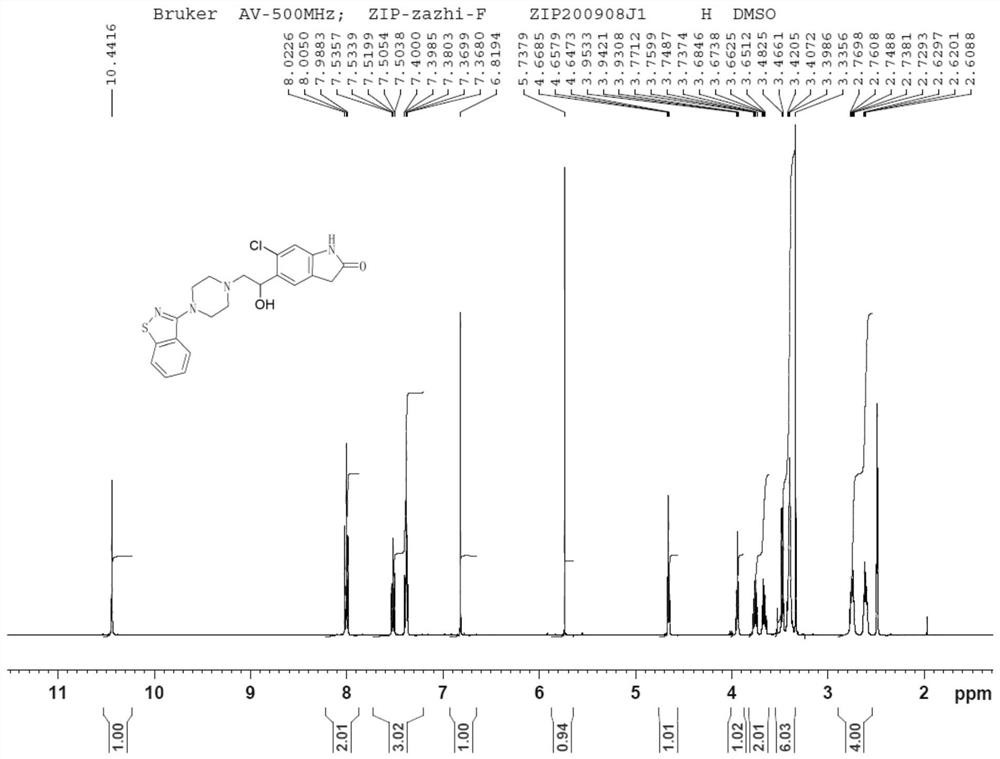
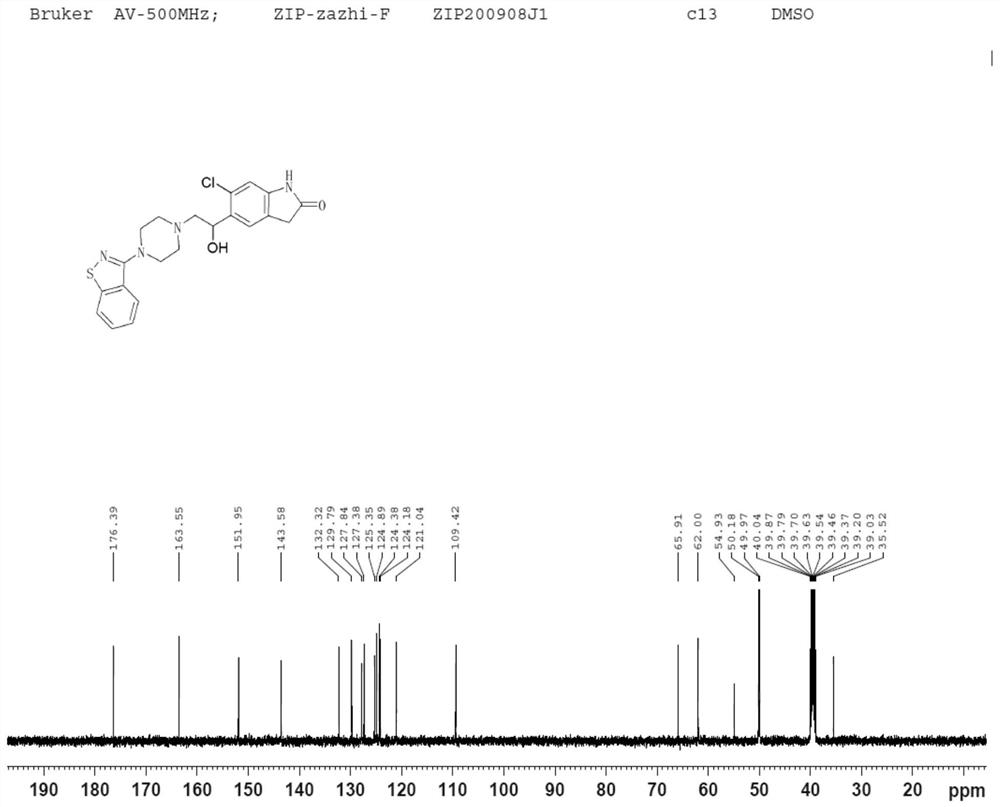
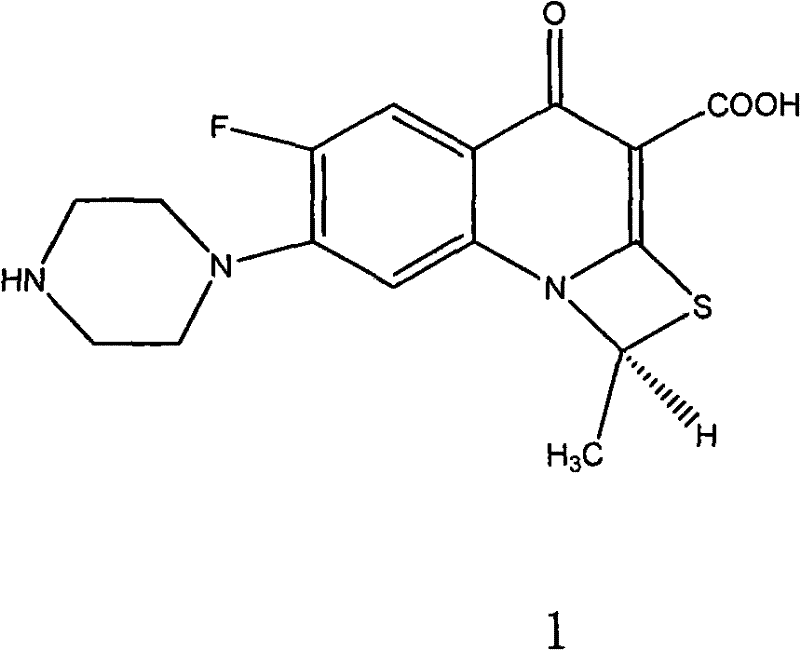
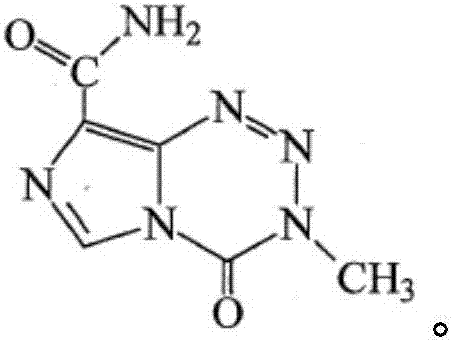
Impurity in ziprasidone hydrochloride and preparation method thereof
The invention provides an impurity in ziprasidone hydrochloride. The impurity has a structure as shown in formula 1. The impurity with a specific structure is obtained on the basis that chlorine impurities are doped in the ziprasidone hydrochloride preparation process, or impurities are brought in and transferred into a final product in the dechlorination step in the acylation reaction process, and the corresponding impurity preparation steps are provided, so that corresponding technical support is provided for preparation of ziprasidone hydrochloride. The synthesis method provided by the invention is simple in process, strong in controllability and mild in condition, can be used for quality standard establishment and quality control links in ziprasidone hydrochloride process research and development, production and the like, and provides technical support for the medication safety of ziprasidone hydrochloride. The method can be used for quality research such as qualitative and quantitative analysis of impurities in ziprasidone hydrochloride synthesis, so that improvement of the quality of ziprasidone hydrochloride is facilitated, and important guiding significance is provided for reducing the medication risk of ziprasidone hydrochloride.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Dexmethylphenidate skeleton pattern transdermal patch containing vitiligo inhibitor and preparing method and application thereof
InactiveCN105395523AAchieve constant releaseAvoid burst phenomenonHeavy metal active ingredientsNervous disorderTransdermal patchMedication risk
The invention discloses a dexmethylphenidate skeleton pattern transdermal patch containing a vitiligo inhibitor and a preparing method thereof. The transdermal patch comprises a skeleton pattern adhesive layer, wherein the skeleton pattern adhesive layer comprises dexmethylphenidate or hydrochloride thereof, the vitiligo inhibitor and an adhesive, the vitiligo inhibitor contains one or more of titanium dioxide, tartaric acid, tretinoin, trichloroacetic acid, zinc oxide, hydroxyl dimethoxy phenylmalonate diethyl hexyl ester and ectoin, and the weight ratio of the vitiligo inhibitor is 5-15%. By the adoption of the transdermal patch, curative effect is guaranteed, and perpetual skin vitiligos are prevented from being generated; the efficacy of the preparation is improved, children clinical medication risks are reduced, and the safety of the preparation is fully guaranteed. The transdermal patch is simple in composition, the technology is simple and easy to apply, cost is low, and production is highly efficient.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
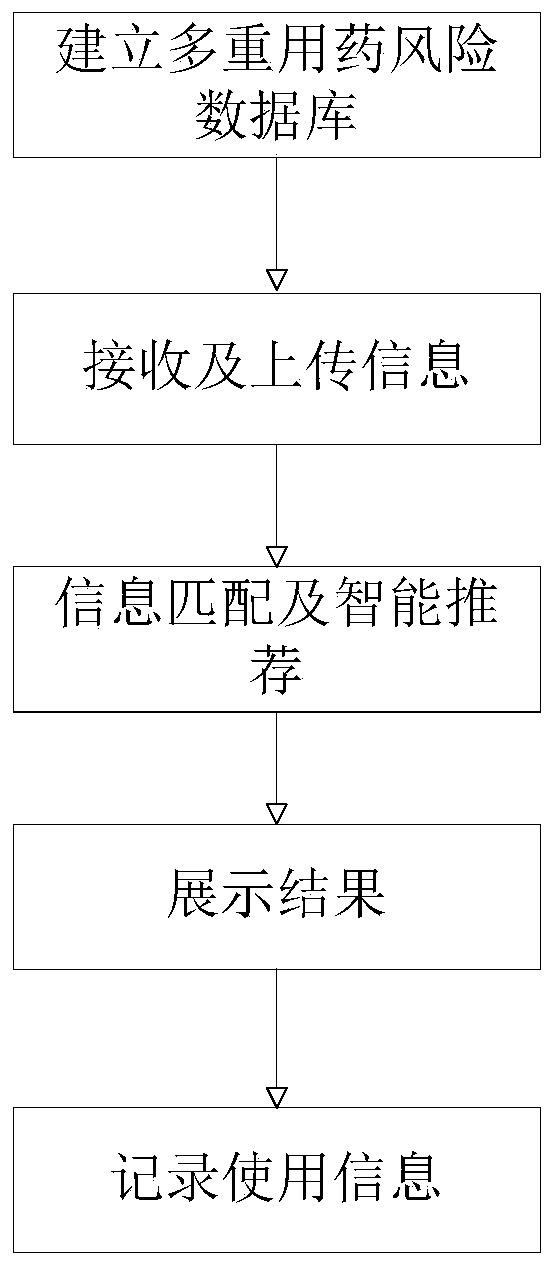
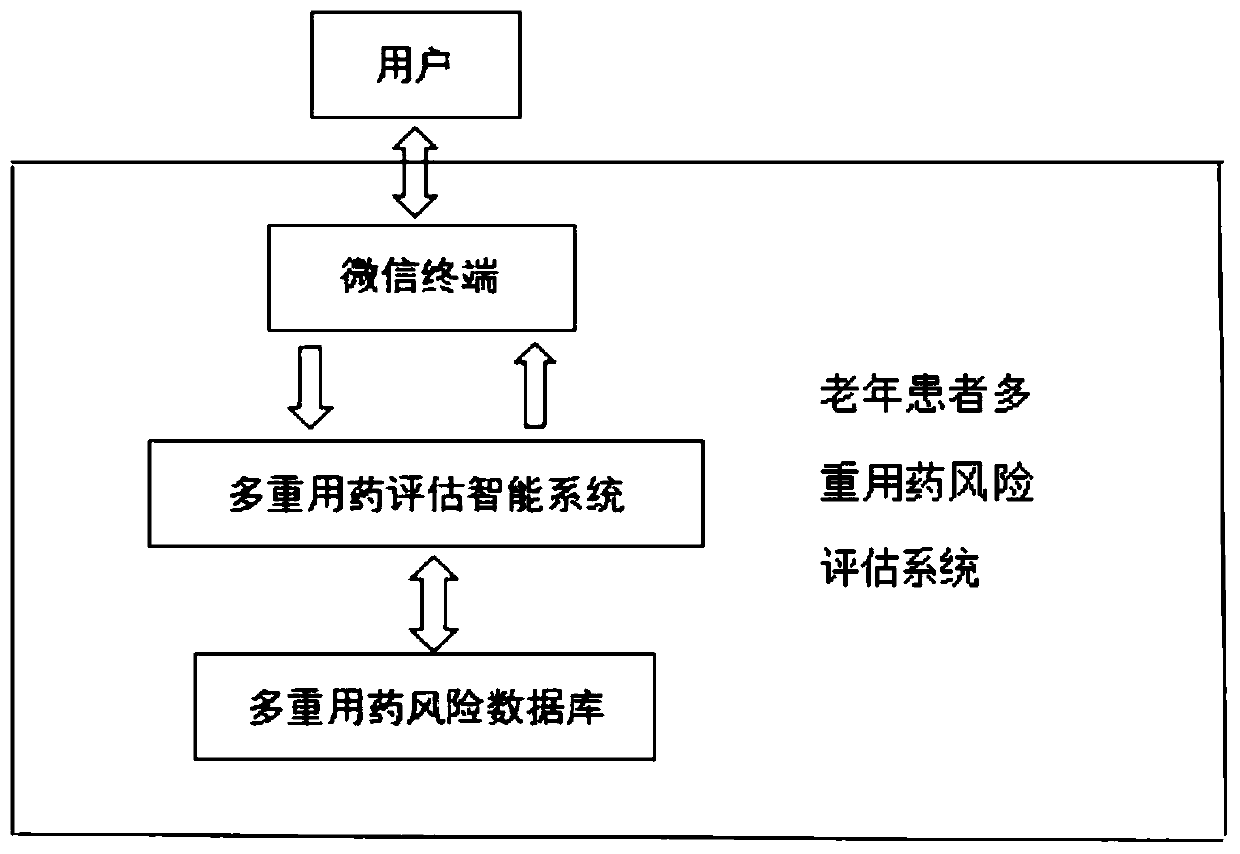
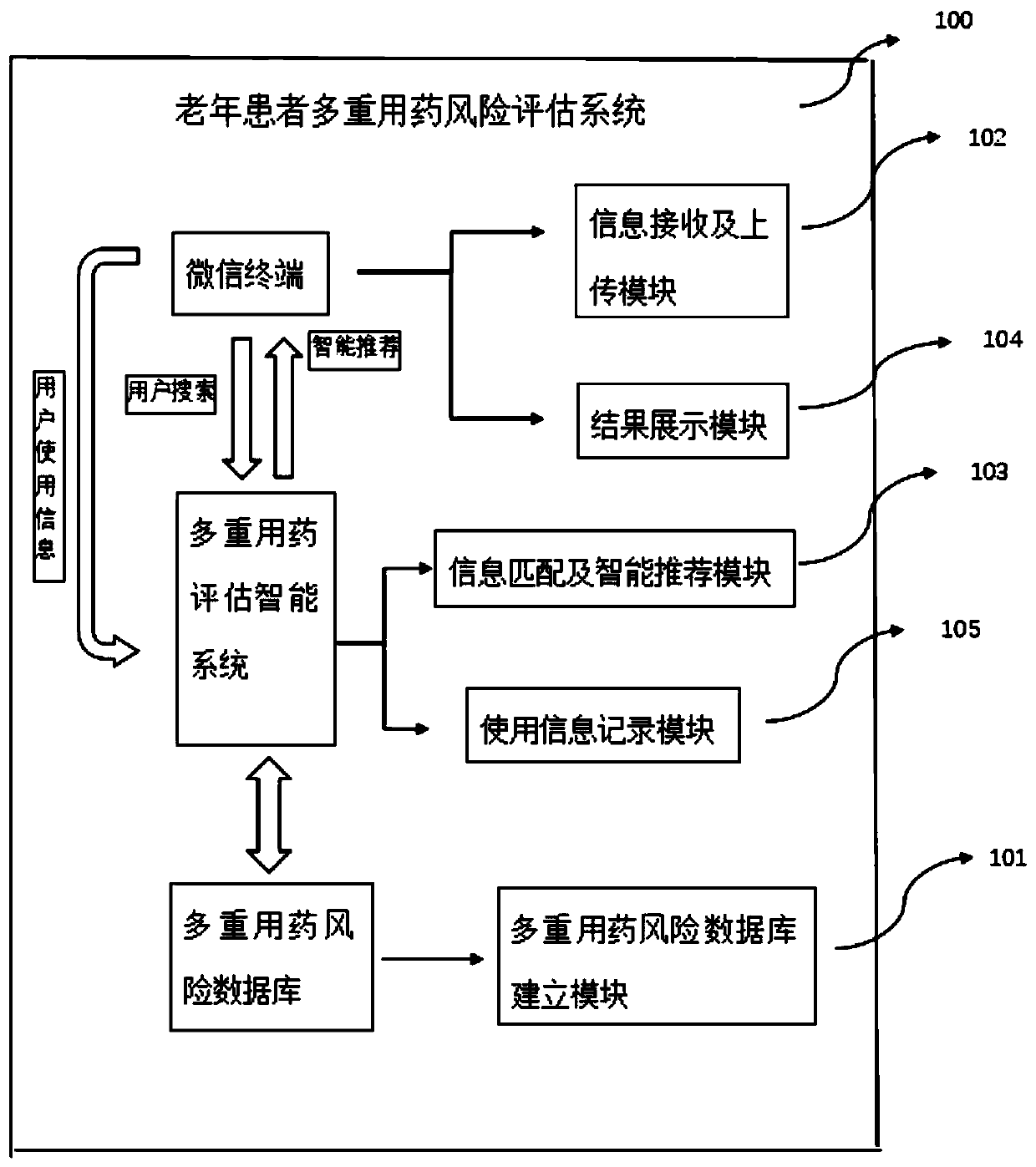
Polypharmacy risk assessment system and method for elderly patients
PendingCN111564195AGuarantee drug safetyQuick searchDrug and medicationsMedication riskInteraction interface
The invention discloses a polypharmacy risk assessment system and method for elderly patients, belonging to the technical field of Internet medical treatment. The system comprises a polypharmacy riskdatabase, an intelligent polypharmacy assessment system, and an interaction terminal. The polypharmacy risk database is used for storing polypharmacy risk data according to medicine and disease information; the intelligent polypharmacy evaluation system is used for carrying out data matching and mining on to-be-evaluated information from the interaction terminal to obtain a data result corresponding to the to-be-evaluated information, and recording the use condition of the user at the interaction terminal for subsequent data analysis; and the interaction terminal is used for providing an interaction interface for the user, receiving and uploading to-be-evaluated information input by the user, receiving a data result corresponding to the to-be-evaluated information, and displaying the dataresult on a corresponding interface. According to the invention, medical staff can be helped to quickly inquire the polypharmacy information of elderly patients, more reasonable medicines are selectedfor the elderly patients, and the medication safety of the elderly is guaranteed.
Owner:ANHUI PROVINCIAL HOSPITAL
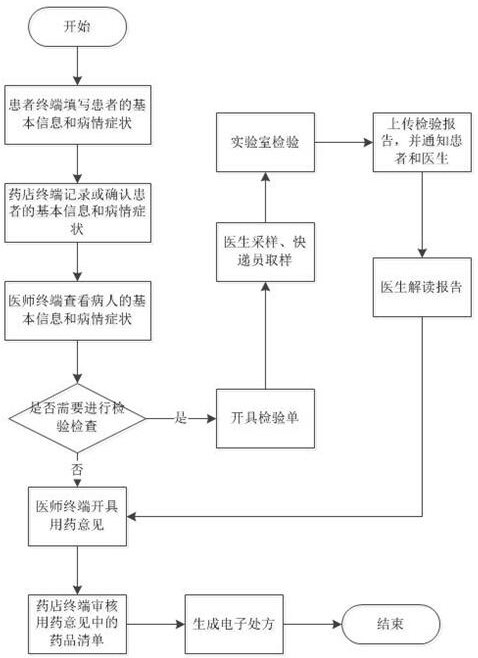
Method and system for issuing an electronic prescription
PendingCN111755088ACause drug riskReduce diagnostic riskDrug and medicationsPatient-specific dataDiseaseDrug utilisation
The invention discloses a method and a system for issuing an electronic prescription. Basic information and disease symptoms of a patient are recorded or confirmed by a pharmacist at a pharmacy terminal and sent to a corresponding doctor terminal; a doctor checks the disease symptoms of the patient through the doctor terminal and diagnoses and gives treatment suggestions to the pharmacy terminal;and after receiving the medication opinions of the doctor, the pharmacy terminal audits a medicine list in the medication opinions and then generates an electronic prescription. In this way, a pharmacist can face the patient and confirm the disease symptoms of the patient, the medication risk caused by the fact that the patient purchases prescription medicine at will is prevented, and meanwhile the diagnosis risk of doctors can be reduced.
Owner:四川好医生云医疗科技有限公司
Impurity in ziprasidone hydrochloride and preparation method of impurity
ActiveCN112778298AQuality improvementReduce drug riskOrganic chemistryBulk chemical productionMedication riskCombinatorial chemistry
The invention provides an impurity in ziprasidone hydrochloride. The impurity has a structure as shown in formula 1. According to the present invention, the impurity with the specific structure is obtained based on incomplete reaction during the ziprasidone hydrochloride preparation process, and the impurity is introduced and transferred to a final product, and the corresponding impurity preparation steps are provided so as to provide the corresponding technical support for the ziprasidone hydrochloride preparation. The synthesis method provided by the invention is simple in process, high in controllability and mild in condition, can be used for quality standard establishment and quality control links such as ziprasidone hydrochloride process research and development, production and the like, and provides technical support for ziprasidone hydrochloride medication safety. The method can be used for quality research such as qualitative and quantitative analysis of impurities in ziprasidone hydrochloride synthesis, so that improvement of the quality of ziprasidone hydrochloride is facilitated, and great guiding significance is provided for reducing the medication risk of ziprasidone hydrochloride.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
Preparation method of bemegride injection
ActiveCN104688674AReduce drug riskGood effectNervous disorderPharmaceutical delivery mechanismMedicineMedication risk
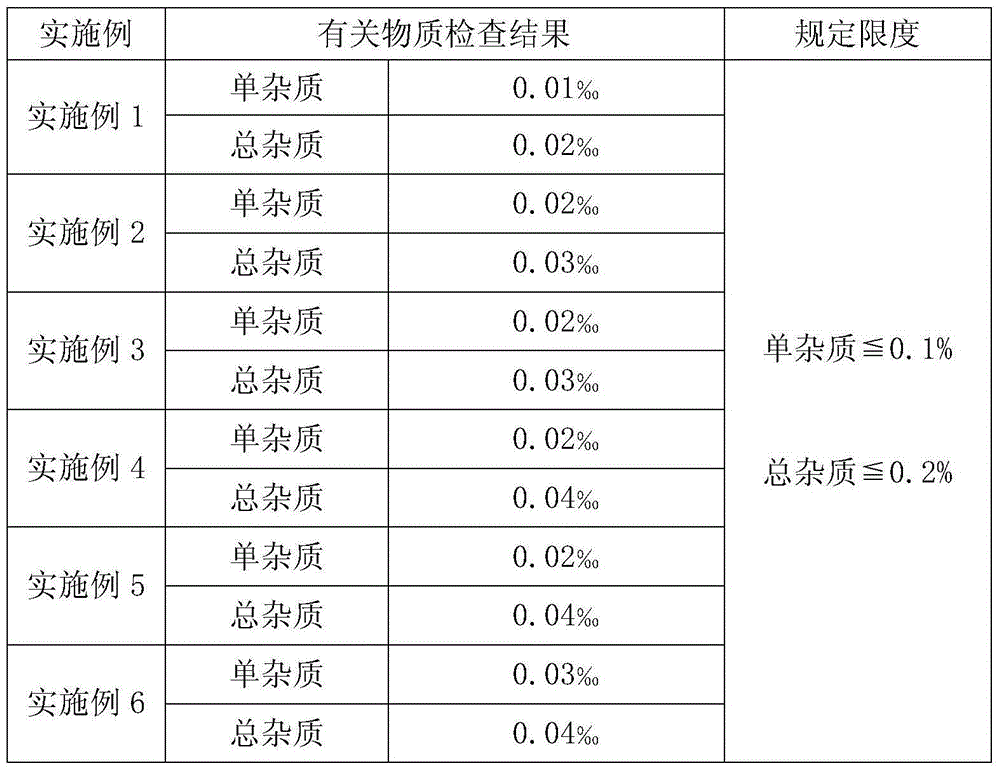
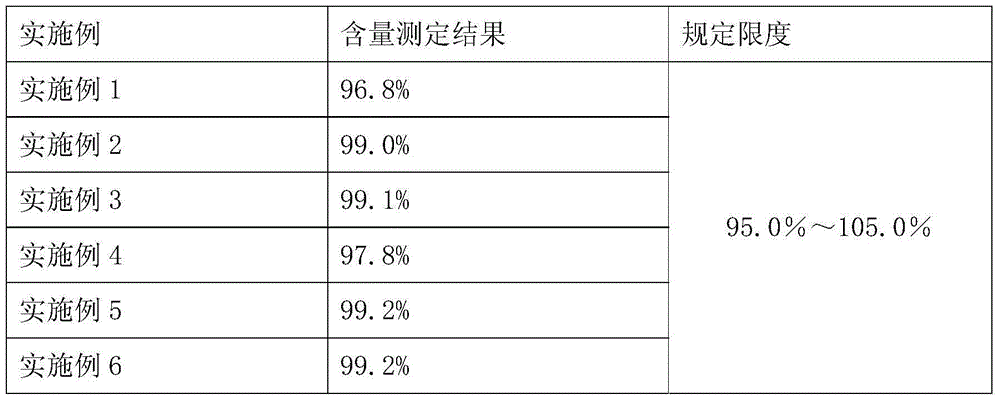
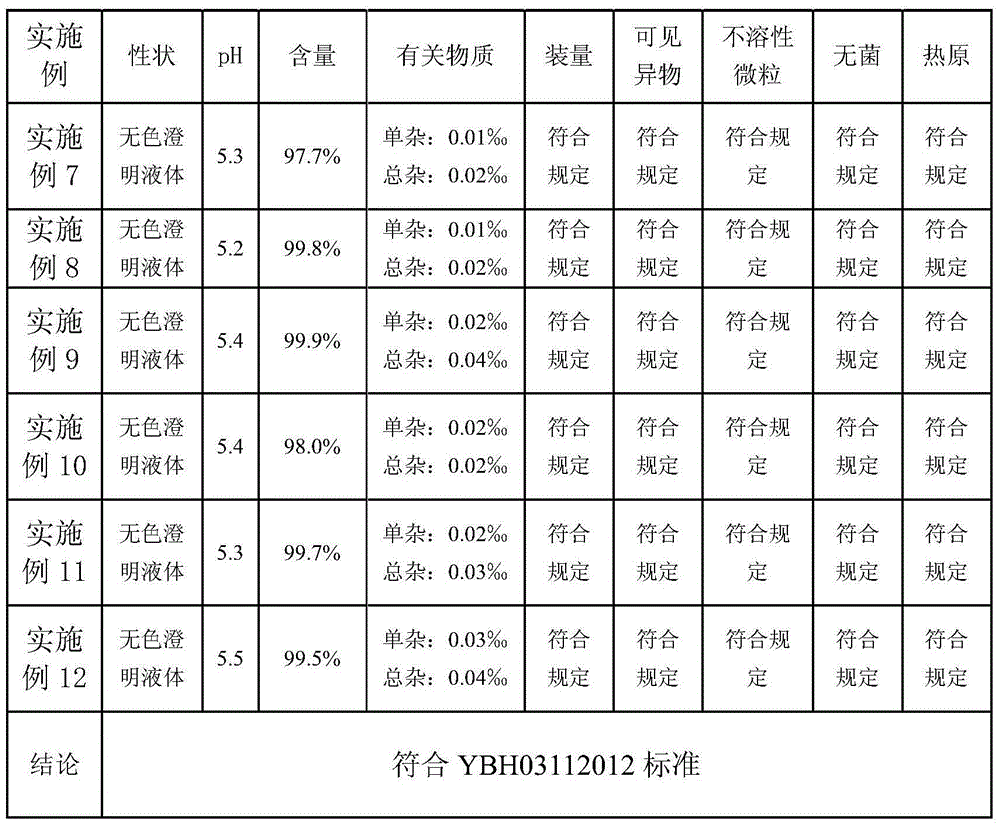
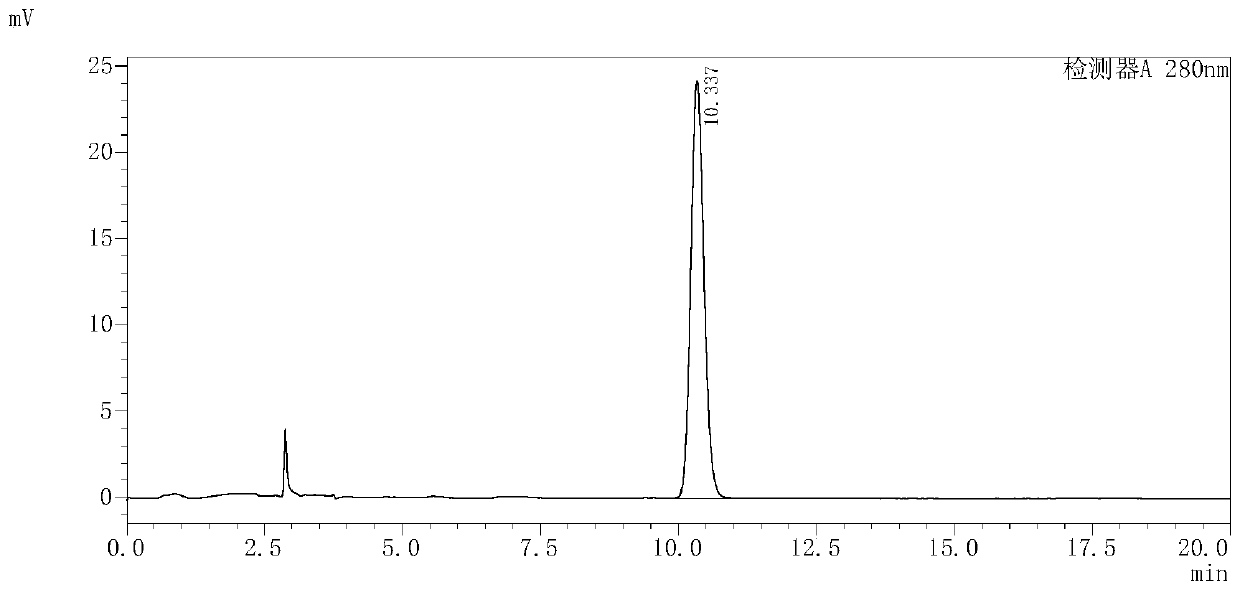
The invention provides a preparation method of a bemegride injection. By virtue of improvement of the preparation method, a terminal sterilization condition is improved to 116 DEG C for 30-45 minutes from 100 DEG C for 45 minutes; and the temperature of liquid-dispensing injection water is improved to 70-85 DEG C from 90 DEG C. By virtue of test detection, the sterile detection of the product reaches the specified requirements; the related substance indexes are reduced. Thus, the quality risk of the product and the medication risk of patients are relatively well reduced. An accelerated test and a long-term stability test prove that the indexes such as sterile detection, related substance and content of the bemegride injection reach the bemegride injection YHB03112012 standard of the Chinese state food and drug administration; and a good effect is achieved. The method disclosed by the invention is simple; new cost is not increased; and the preparation method is suitable for large-scale industrial production, and has relatively great application value.
Owner:SHANGHAI ZHAOHUI PHARMA
Detection method for evaluating quality of colquhounia root medicinal material
InactiveCN110567984AImprove efficacyLow costComponent separationOptically investigating flaws/contaminationTriptolideMedication risk
The invention discloses a detection method for evaluating the quality of a colquhounia root medicinal material. The detection method comprises the following steps: 1) taking peeled dry colquhounia roots for later use; 2) performing powder microscopic identification; 3) determining the nature of epicatechin by using a thin-layer chromatography test method; 4) checking the moisture, the total ash content and the acid-insoluble ash content, and determining a water-soluble extract; 5) determining the epicatechin content and the triptolide content of the colquhounia roots. According to the method,through the following steps: preliminarily screening out raw materials through character observation; performing microscopic identification by powder, and observing the microscopic characteristics ofthe colquhounia roots; then checking the moisture, the total ash content and the acid-insoluble ash content; performing primary determination of impurity content; extracting the medicinal materials, measuring the extract, preliminarily determining the extractability of the effective components of the medicinal materials, and accurately quantifying the conditions of the main components of epicatechin and triptolide by high performance liquid chromatography, thereby comprehensively judging the quality of the colquhounia roots. The method is beneficial to prejudging the clinical medication risk and ensuring the safety and effectiveness of clinical medication.
Owner:重庆市药研院制药有限公司
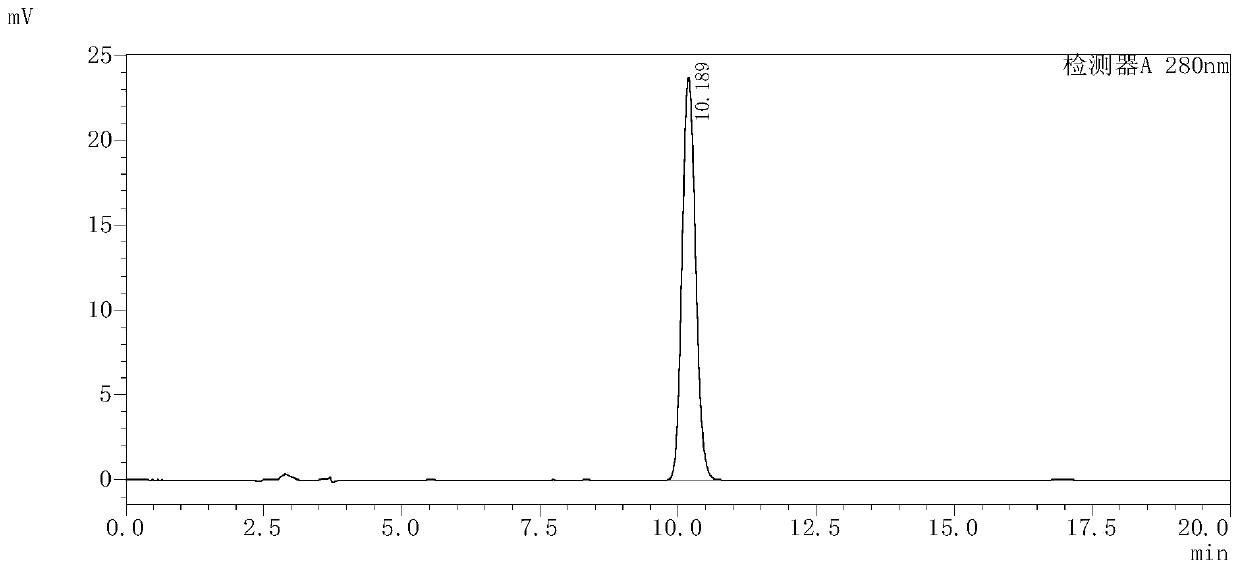
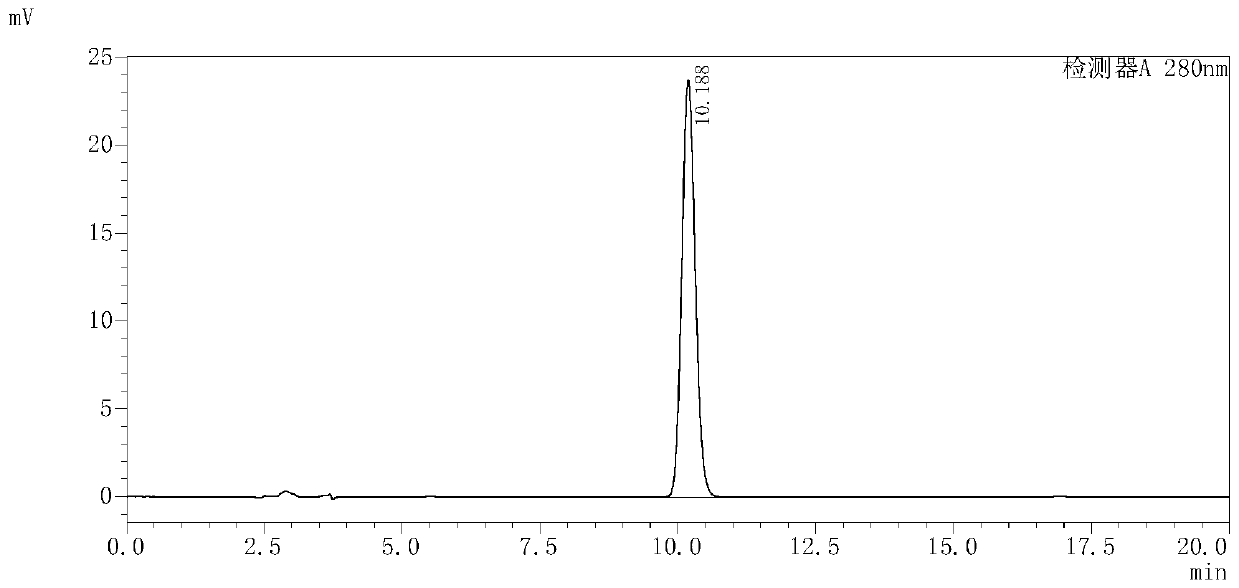
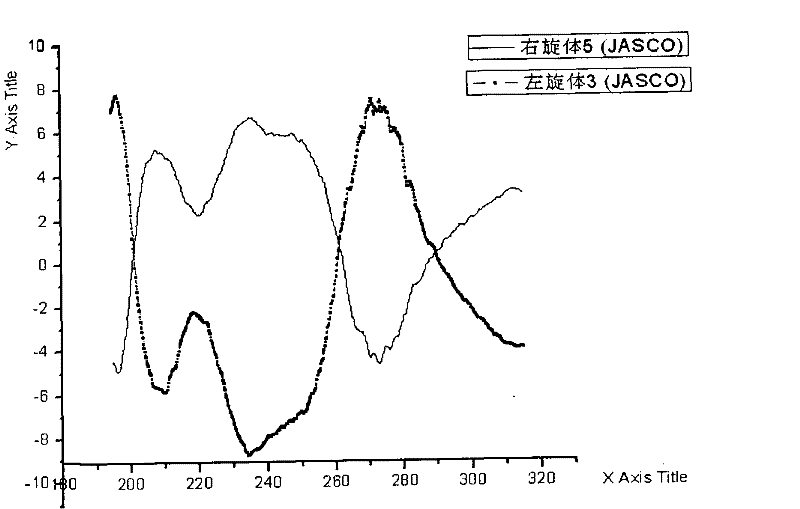
Levo-ulifloxacin injection and preparation method thereof
Levo-ulifloxacin injection and preparation method thereof, relate to pharmaceutical preparations containing fluoroquinolone antibacterial drugs, in particular to injection of prulifloxacin active body Ulifloxacin (S-(-)-Ulifloxacin) and preparation thereof method. The present invention is based on (S)-(-)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H, 4H-[1,3]thiazetidine Alkane[3,2-a]quinoline-3-carboxylic acid (levofloxacin for short) is the active ingredient of the drug, and the rest are pharmaceutically acceptable additives for injections, which are liquid injections or solid injections Injections, wherein each injection or bottle contains 10-100 mg of active pharmaceutical ingredients. The commonly used dose in clinic may be 10-100 mg of levofloxacin, once or twice a day; 1-10 ml for intramuscular injection and 50-1000 ml for intravenous injection. The invention increases the route of administration, expands the range of application groups, and significantly reduces the cost and risk of medication for patients.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Detection method of olopatadine hydrochloride and related substance thereof
The invention relates to a detection method of olopatadine hydrochloride and related substance thereof. The method comprises a step of using high performance liquid chromatography for detection, wherein liquid chromatographic conditions are as follows: an octyl silane bonded silica gel chromatographic column is used; a mobile phase A is aqueous solution containing 0.01%-1% ion pair reagent, 0.3%-1% monoamine and 0.001-0.1mol / L buffer salt, a mobile phase B is acetonitrile and / or methyl alcohol, and gradient eluting is executed; and a pH value of buffer solution is 2.5-3.5, and detection wavelength is 220nm-280nm. The detection method of the olopatadine hydrochloride and the related substance thereof provided by the invention can effectively separate the olopatadine hydrochloride and impurities thereof, improve accuracy of a detection result and reduce clinical medication risks.
Owner:北京海晶生物医药科技有限公司
Active monitoring and early warning system for drug-induced diseases
ActiveCN114005503AStrengthen the risk management and control of clinical drug useQuick searchDrug referencesAlarmsMedication riskPatient treatment
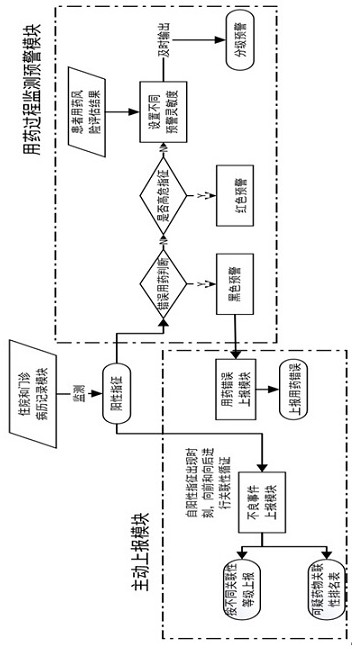
The invention relates to an active monitoring and early warning system for drug-induced diseases, which is used for monitoring clinical treatment process information of a patient, carrying out graded early warning on adverse drug events and drug use errors and actively reporting. After the medication risk information is monitored, graded early warning is output, and the early warning grade is comprehensively obtained by the medication risk assessment result of the patient and the danger degree of the monitored medication risk information; meanwhile, based on the treatment process information of the patient, after the relevance between the adverse reaction and the suspicious medicine is determined, the adverse event of the medicine is actively reported, the medicine use risk is effectively prevented, and the reporting rate and reporting accuracy of the adverse event are improved.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
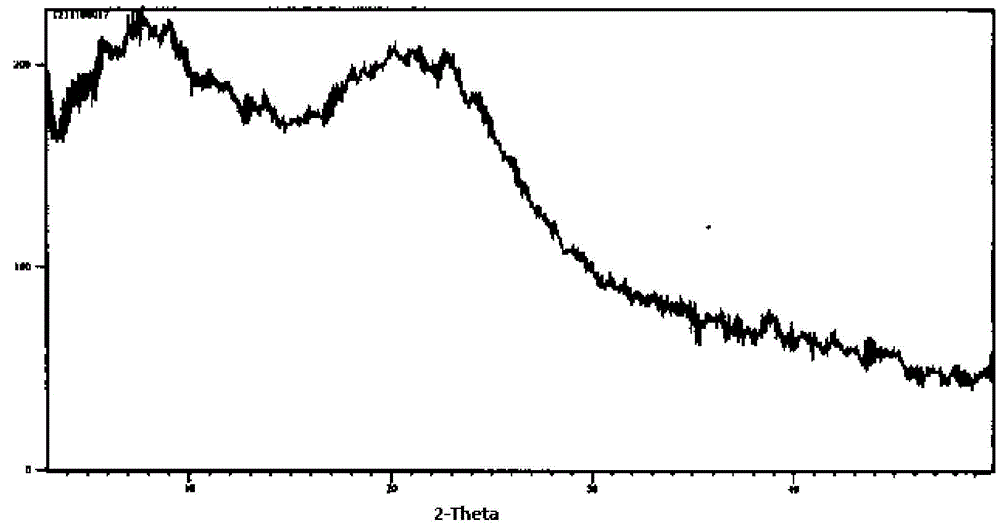
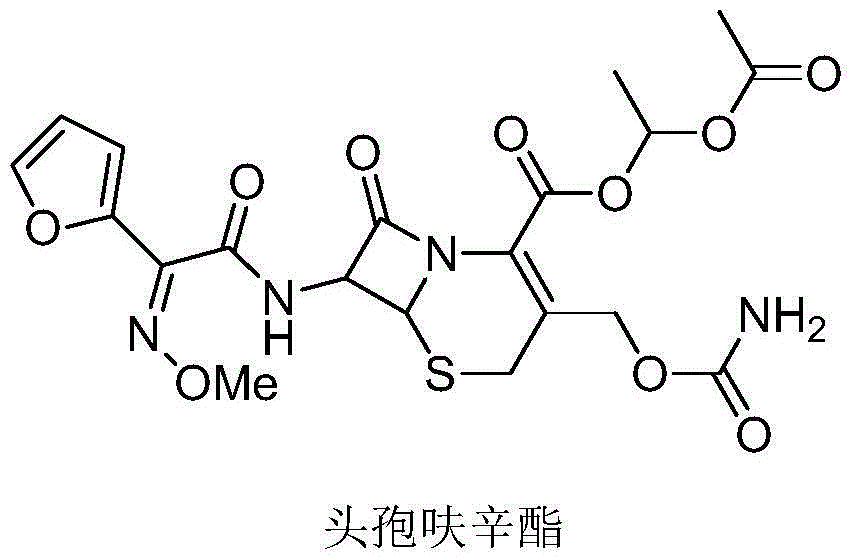
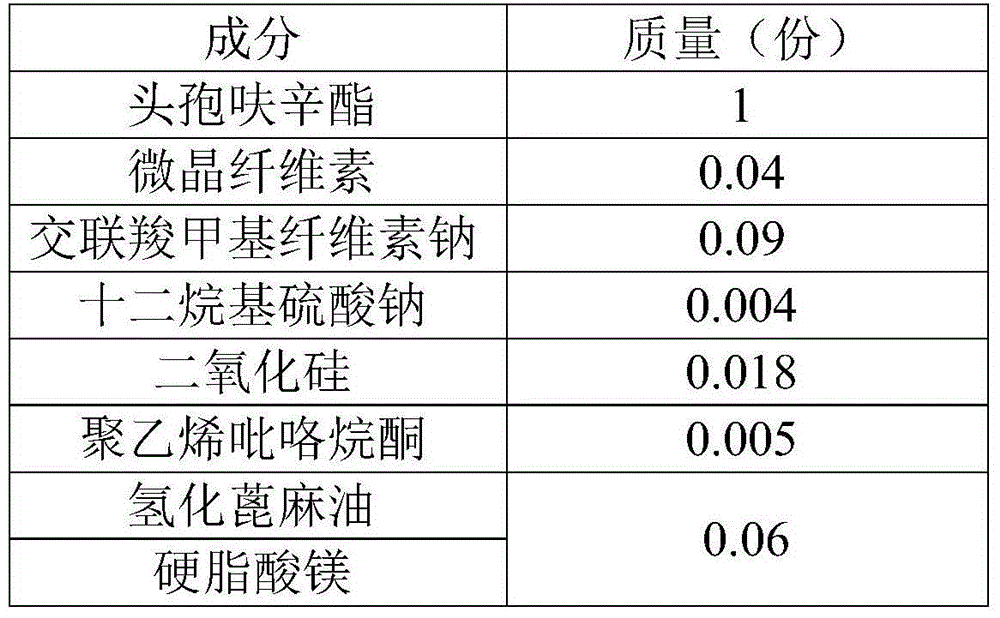
Cefuroxime axetil pharmaceutical composition and preparing method thereof
InactiveCN105456208APromote dissolutionEasy to prepareAntibacterial agentsOrganic active ingredientsMedication riskBiomedical engineering
The invention provides a cefuroxime axetil pharmaceutical composition. The cefuroxime axetil pharmaceutical composition has the advantages that digesting performance is high, the preparing method is simple, product quality is controllable, and the clinical medication risk is small.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Medication Risk Assessment System
InactiveUS20110173025A1Data processing applicationsHealth-index calculationAnalysis dataMedication risk
A medication risk assessment method implemented using a computer particularly adapted for a health care management or delivery organization is provided, wherein the method includes at least the steps of: providing a medication risk assessment tool to a client, wherein the medication risk assessment tool includes a questionnaire, and wherein the client maintains a relationship with a population of individuals; administering the questionnaire to the population of individuals, thereby generating questionnaire data; obtaining prescription claims data from the population of individuals; combining the questionnaire data and the prescription claims data in an electronic database, thereby generating population data; analyzing the population data according to a set of rules; ranking the analyzed data according to risk; and generating at least one report based on the ranked data.
Owner:PHARMMD SOLUTIONS
Medication risk mitigation system and method
A medication risk mitigation method utilizing three interventions: a prospective intervention performed by a prescriber, a concurrent intervention performed by a pharmacist and a retroactive intervention performed by a pharmacist. At each intervention, the system of the instant invention utilizes a computer program to compare each prescribed medication to a series of intrinsic and extrinsic data sources in order to identify potential contraindications and, if necessary, modify a prescription. The system also permits secure messaging between prescribers and pharmacists, each with access to the computer program, so as to facilitate communication and reduce medication risks. The system of this invention also permits modeling for hypothetical medication modifications based on the same intrinsic and extrinsic data sources.
Owner:TABULA RASA HEALTHCARE GRP INC
Temozolomide pharmaceutical composition as well as preparation method and application thereof
ActiveCN107397735AImprove stabilityGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMedication risk
The invention provides a temozolomide pharmaceutical composition as well as a preparation method and application thereof. The temozolomide pharmaceutical composition is prepared from the following components in percentage by weight: 3 to 60 percent of temozolomide, 40 to 95 percent of lactose anhydrous, 1 to 10 percent of croscarmellose sodium, 0.5 to 5 percent of tartaric acid and 0.1 to 2 percent of silicon dioxide. In the preparation method, the components in the pharmaceutical composition are selected specifically, stable capsules which do not exceed + / -6 percent in capsule weight difference can be obtained by adopting the conventional powder capsule filling technology, and the pharmaceutical composition has high stability; under the condition of not adding any extra stabilizer, the prepared temozolomide capsule has higher stability, can be stably stored for three years or longer at normal temperature, and can be dissolved rapidly under human physiological pH; the medication risk caused by undesirable dissolving in clinical is lowered. Moreover, the pharmaceutical composition disclosed by the invention has the advantages of simple preparation process, convenience, feasibility and easiness in amplified production.
Owner:TOT BIOPHARM CO LTD
Risk prediction method and device based on artificial intelligence, electronic equipment and medium
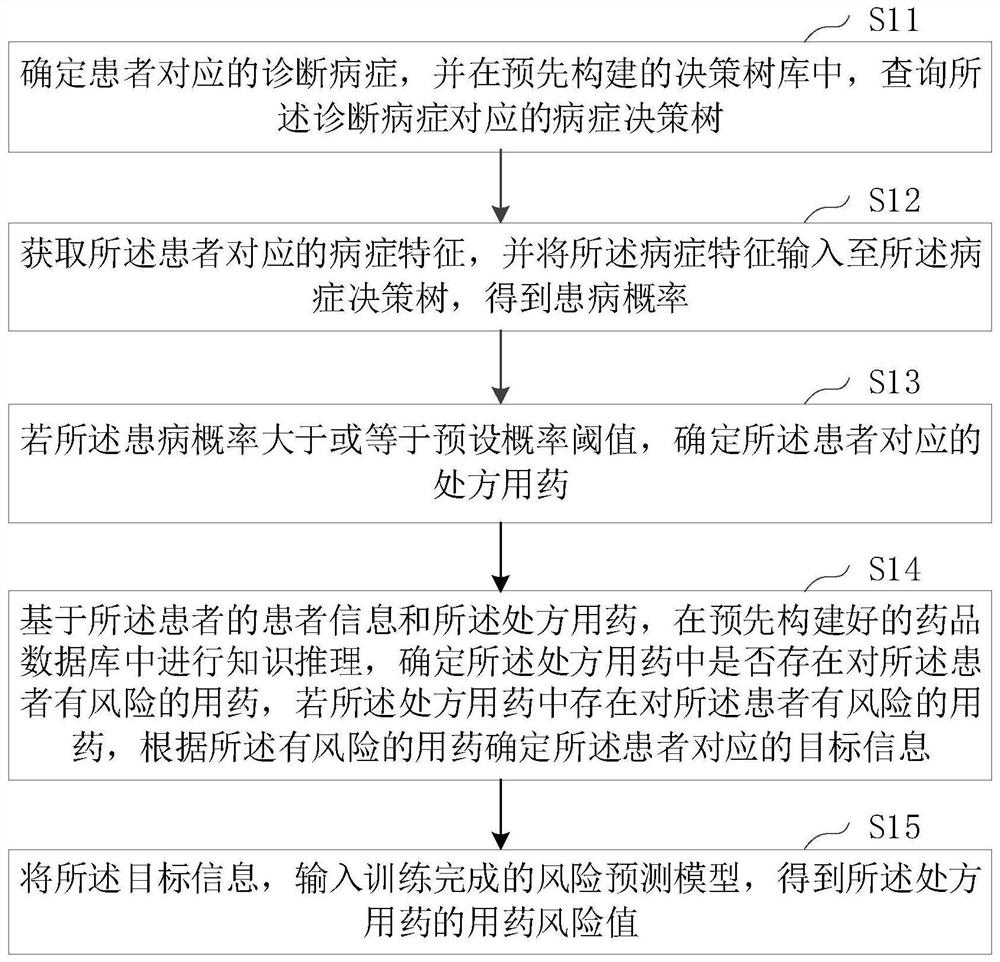
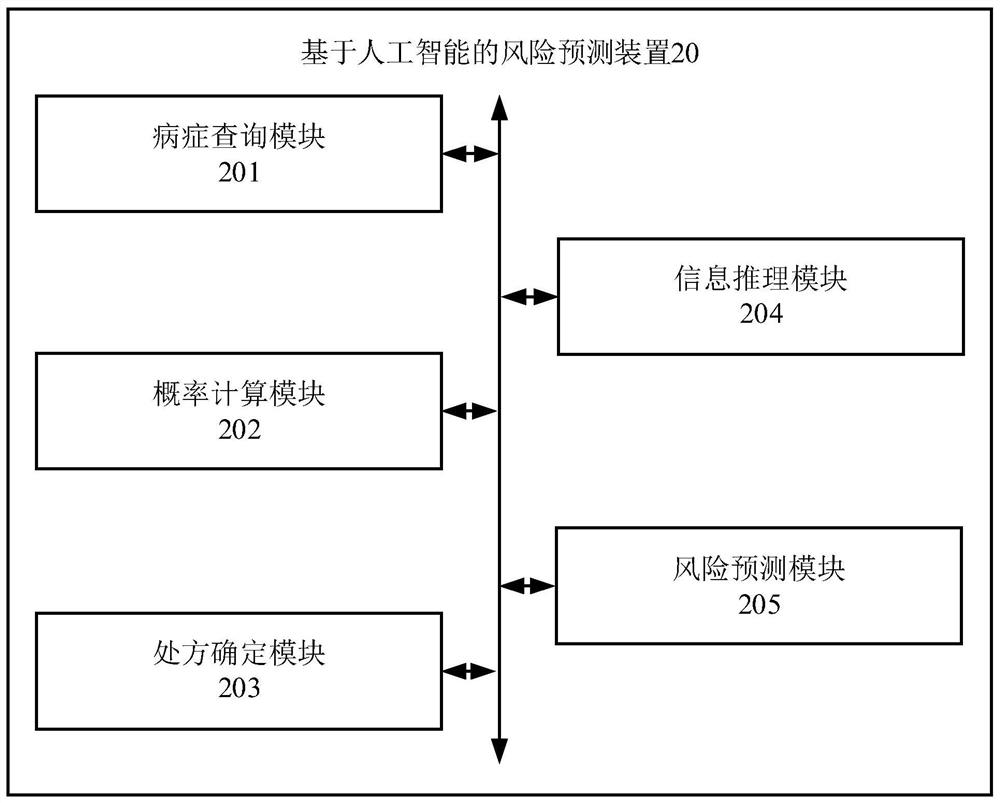
PendingCN113870974AEfficient determinationImprove accuracyDrug and medicationsMedical automated diagnosisDrug utilisationDrug Databases
The invention relates to the technical field of artificial intelligence, and provides a risk prediction method and device based on artificial intelligence, electronic equipment and a medium, and the method comprises the steps: querying a disease decision tree corresponding to a diagnosis disease of a patient in a pre-constructed decision tree library; inputting the disease feature corresponding to the patient into the disease decision tree to obtain a disease probability; if the illness probability is greater than or equal to a preset probability threshold, determining a prescription drug corresponding to the patient; based on patient information of a patient and the prescription drug, performing knowledge reasoning in a pre-constructed drug database, determining whether drugs having risks for the patient exist in the prescription drugs, and if the drugs having risks for the patient exist in the prescription drugs, determining target information corresponding to the patient according to the drugs having risks; and inputting the target information into a trained risk prediction model to obtain a medication risk value of the prescription drug. According to the invention, the accuracy of medication risk prediction is improved.
Owner:深圳平安智慧医健科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com