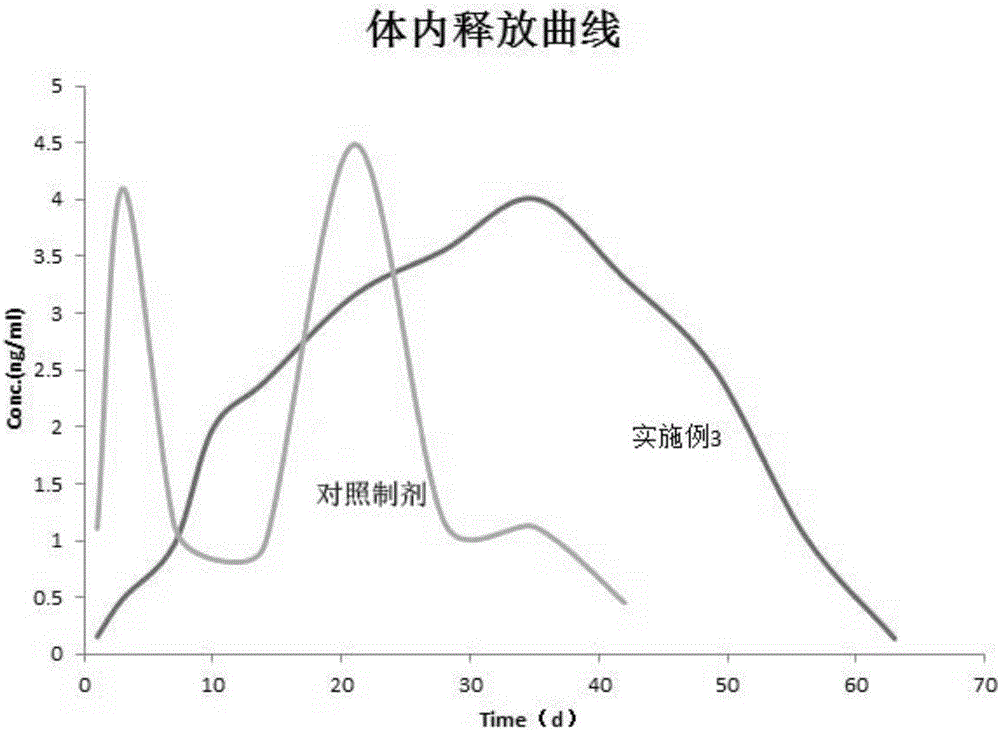
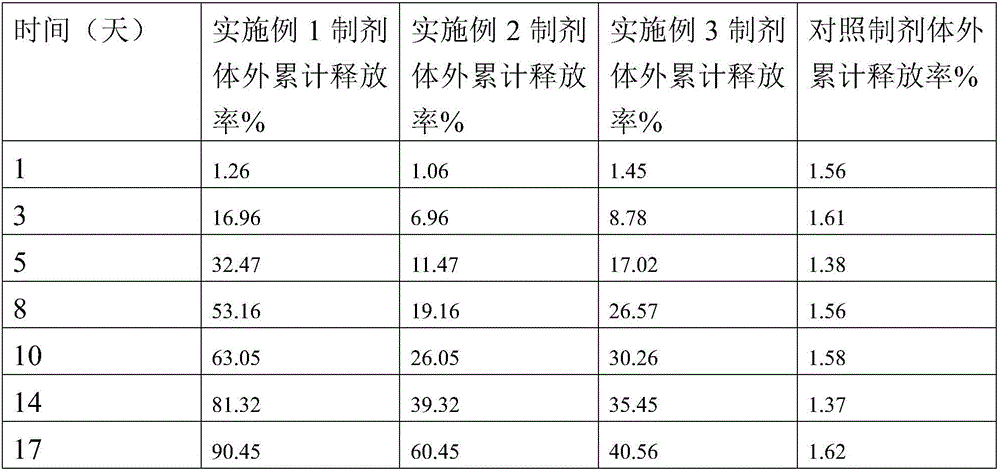
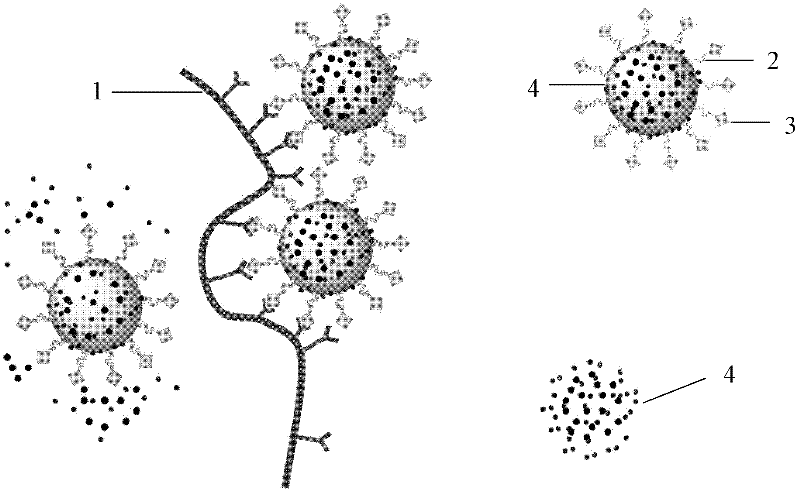
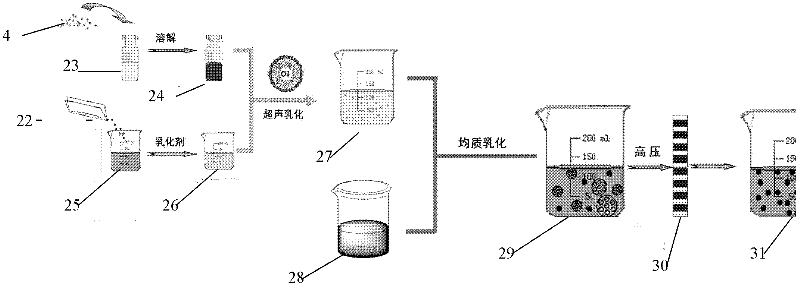
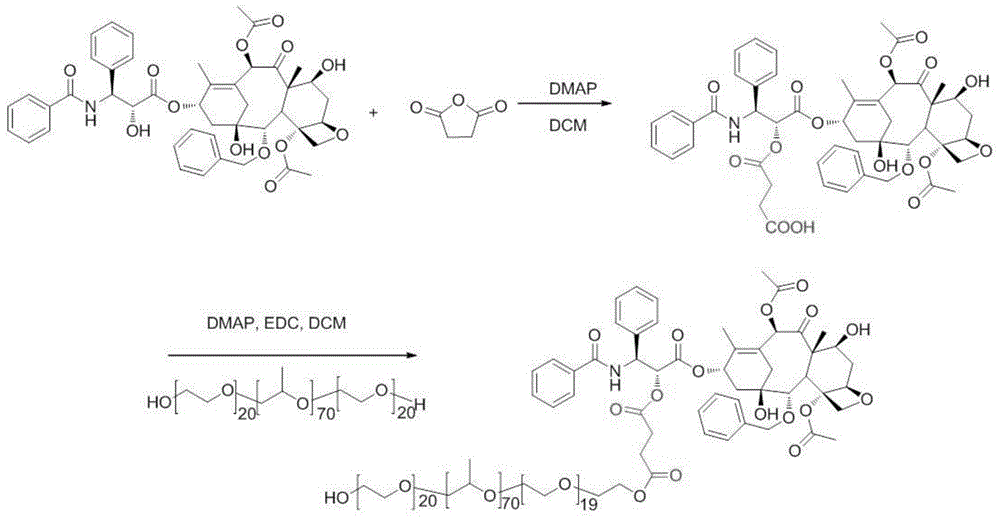
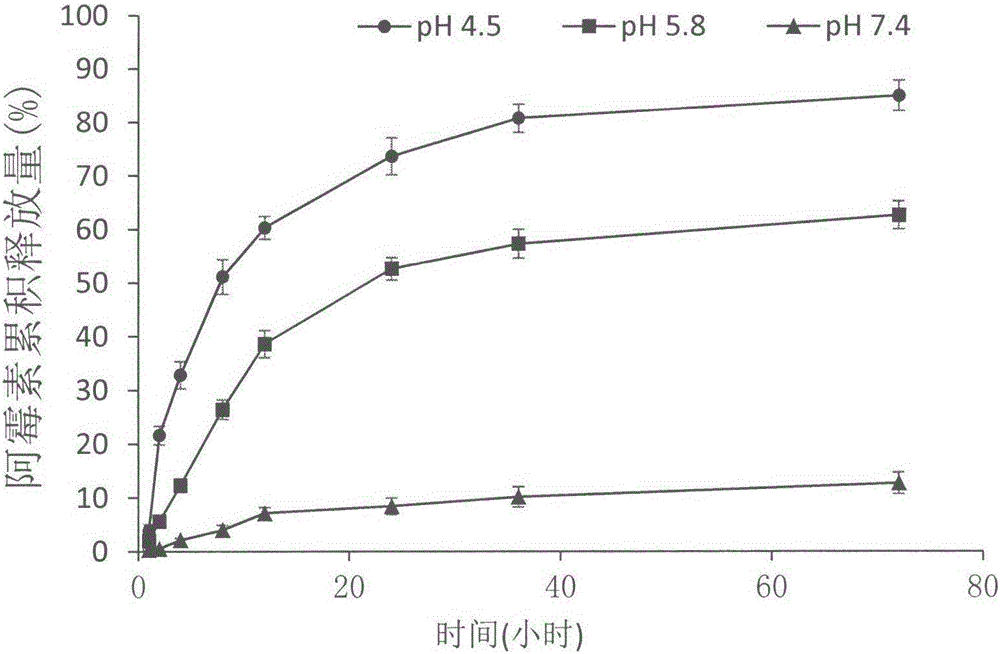
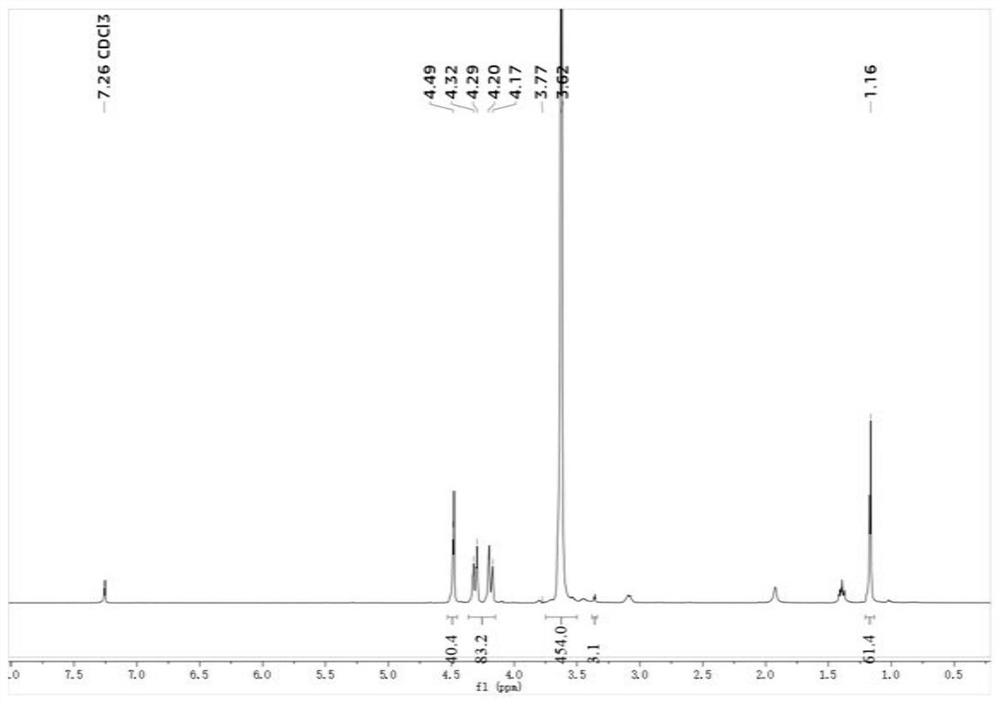
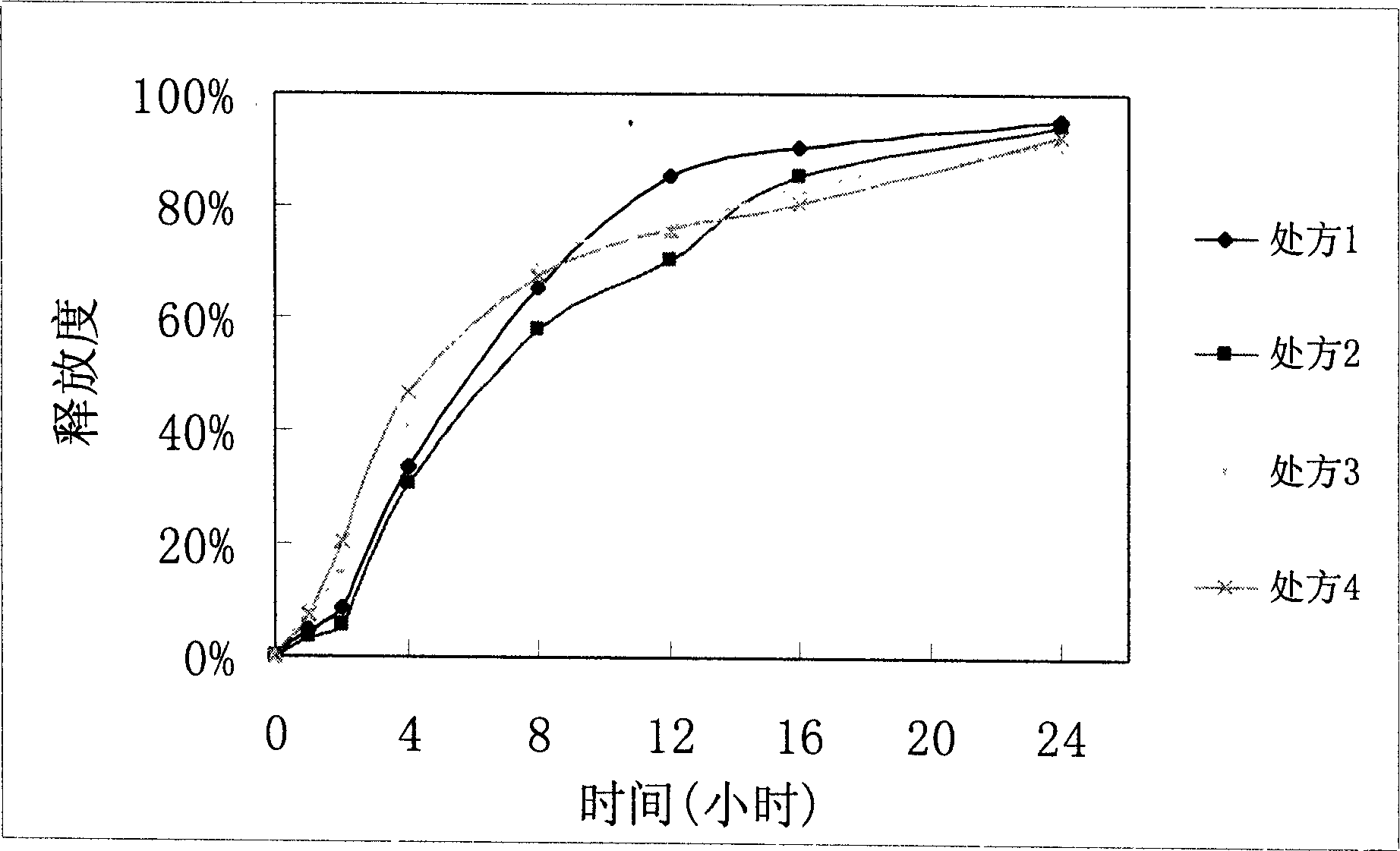
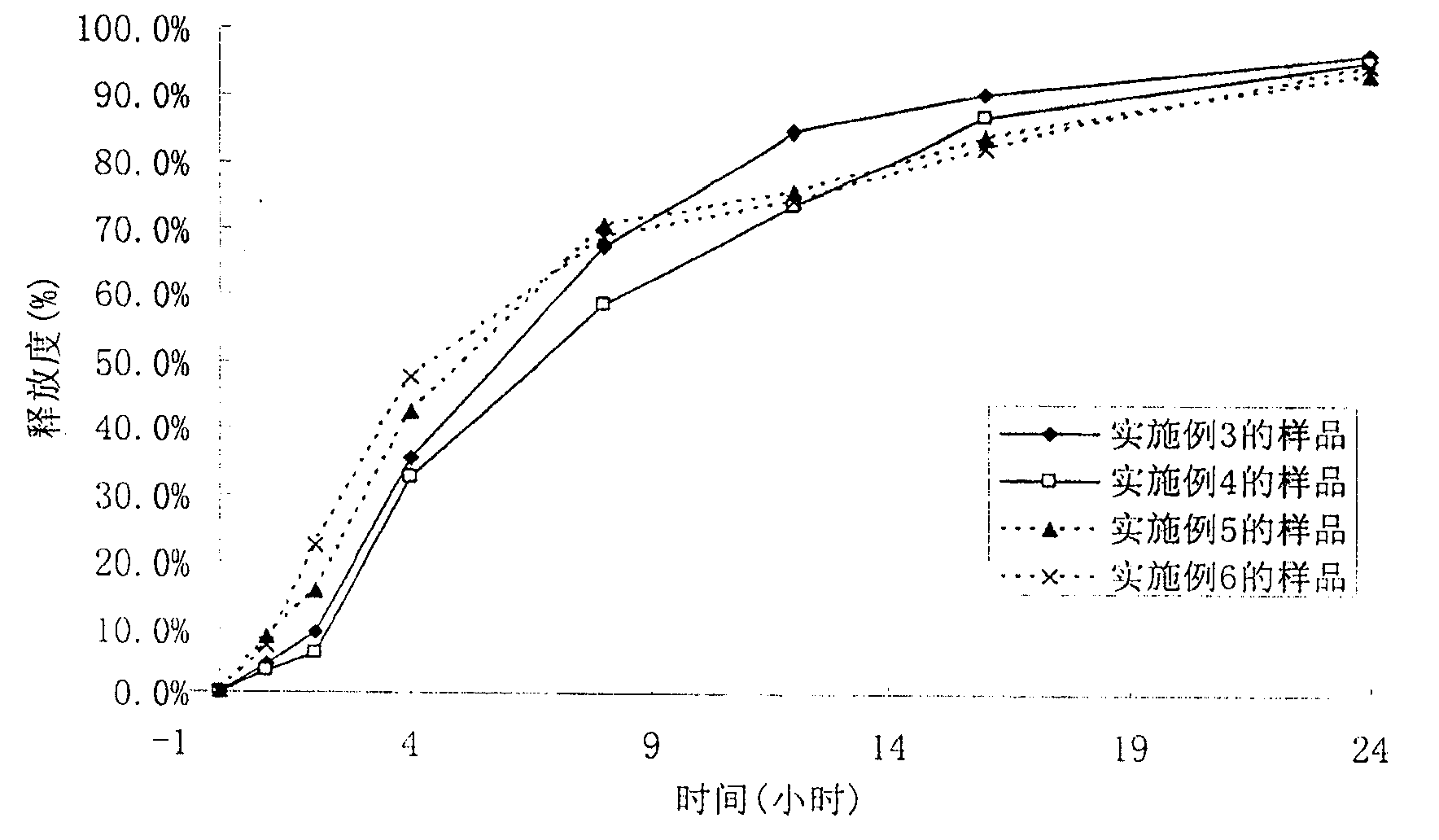
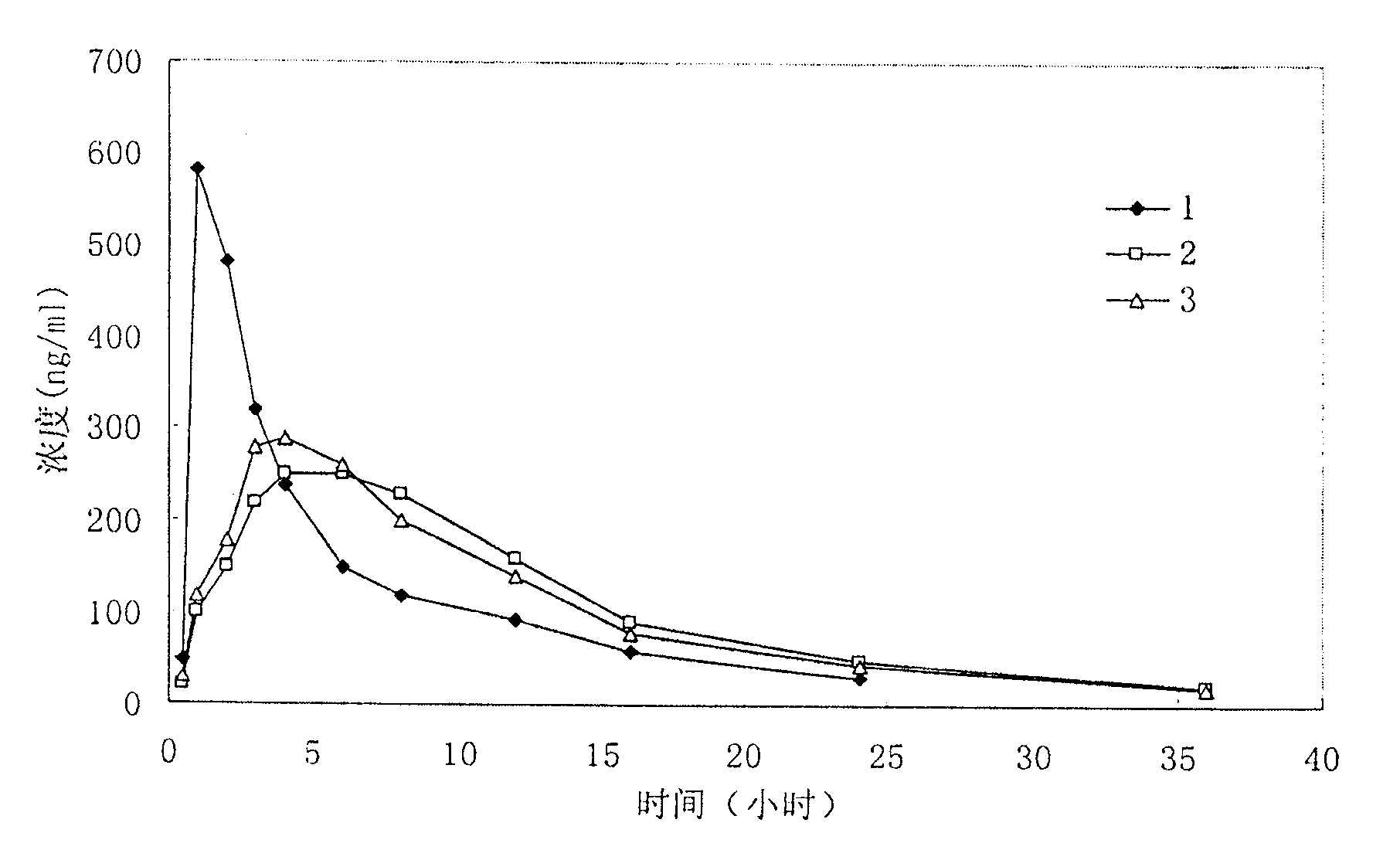
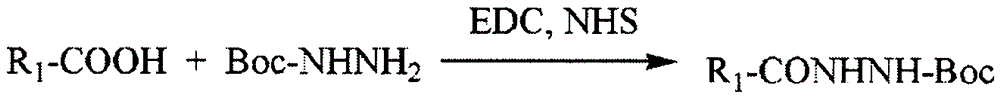Patents
Literature
55results about How to "Avoid burst phenomenon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
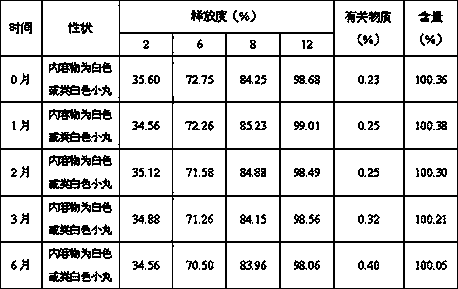
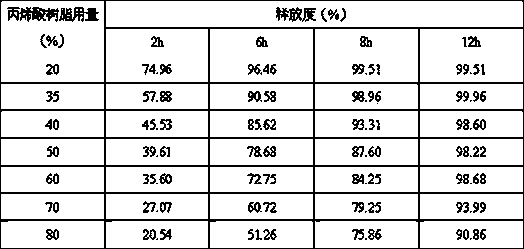
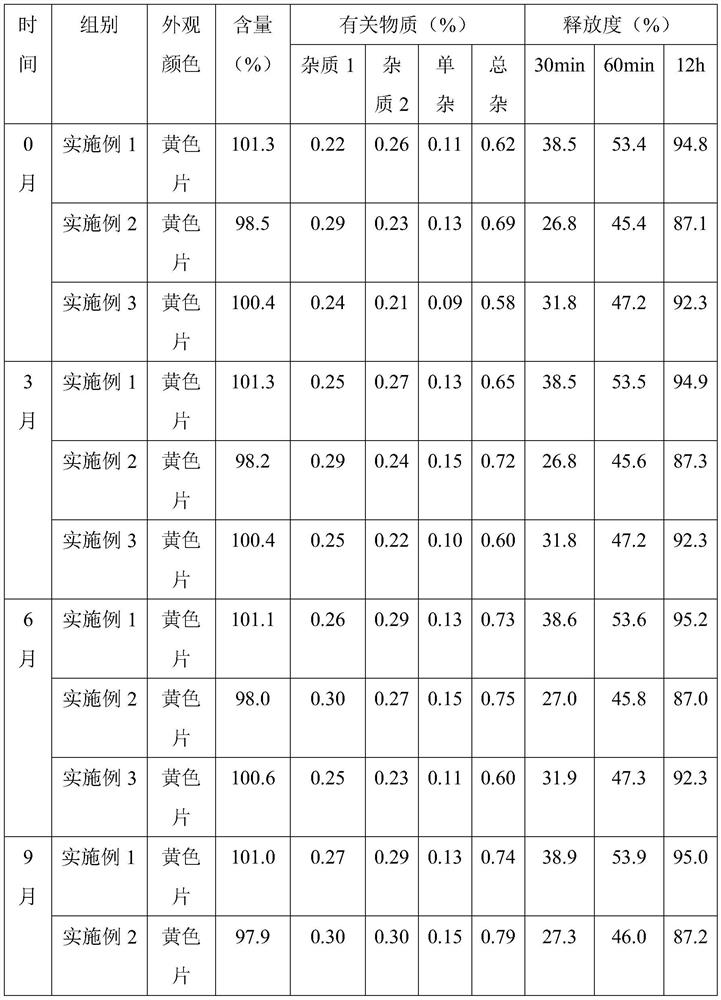
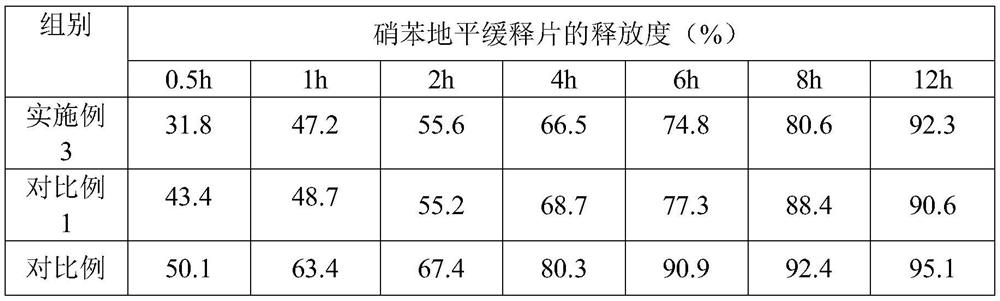
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
InactiveCN103385861AQuality improvementSmooth releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletAdhesive
The invention discloses a trimetazidine hydrochloride sustained release tablet prepared from the following ingredients in percentage by weight: 10-25% of trimetazidine hydrochloride, 20-60% of hydrophilic gel skeleton material, 1-20% of a hydrophobic blocker, 15-55% of filler, 1-10% of a lubricant, 0 or 1-10% of a coating material and a proper dosage of adhesive. A wet granulation method is adopted in the preparation process. After the trimetazidine hydrochloride sustained release tablet disclosed by the invention touches water, the hydrophilic gel skeleton material can expand rapidly to form a gel layer with a plurality of pores, a drug can be slowly released through the pores, and the hydrophobic blocker can partially block the pores to avoid the burst release of the drug. Proper mass and proper proportion of hydrophilic gel skeleton material and hydrophobic blocker are added to realize slow and uniform release of the drug under the cooperation of the two ingredients and well avoid the burst release phenomenon so as to beneficially ensure the safety and effectiveness of drug therapy.
Owner:SHANDONG UNIV
Calcium alginate-graphene oxide nanofiber and preparation method and drug-carrying calcium alginate-graphene oxide nanofiber
ActiveCN109468708AReduced release rateAvoid burst phenomenonAlginate artificial filamentsPharmaceutical non-active ingredientsOxygenDrug release
The invention provides a calcium alginate-graphene oxide nanofiber and a preparation method and a drug-carrying calcium alginate-graphene oxide nanofiber and relates to the technical field of nanofibers. The preparation method of the calcium alginate-graphene oxide nanofiber comprises the following step: carrying out microfluidics spinning on a mixed solution of calcium alginate and graphene oxideand a calcium chloride solution, thereby obtaining the calcium alginate-graphene oxide nanofiber. The technical problem that a sudden drug release phenomenon is caused as a conventional calcium alginate nanofiber is good in hygroscopicity and low in mechanical strength is alleviated. By adopting the preparation method provided by the invention, the graphene oxide is put into raw materials, so that the calcium alginate-graphene oxide nanofiber has hydrogen bond interactions with oxygen-containing groups on a graphene oxide sheet and hydroxyl in calcium alginate molecules, the hydrogen bond function of the calcium alginate and water molecules is weakened, the swelling velocity of the calcium alginate is reduced, and the sudden drug release phenomenon can be prevented.
Owner:WUYI UNIV
Method of preparing superfie fiber formulation for carmustine
InactiveCN1687494ASlow release rateSmooth release behaviorFilament/thread formingMonocomponent polyesters artificial filamentSide effectHalf-life
The present invention relates to a method of preparing BCNU which has the biodegradation high polymer ultra-thin fiber form. Dissolve the BCNU into the solution of high polymer which can be biodegraded, electrospinning, so non-woven fabrics or felt of ultra-thin fiber wrapped with BCNU. The preparation is simple and cheap, the diameter of drug loading fiber can be controlled at the dimension of 0.2 - 2 ª–m, drug loading can be controlled at the range of 0.1 - 100%. The release of drug is study. It has high speed of drug releasing and can also release BCNU steadily for a long time. The fiber itself can biodegrades completely so it has little poisonous side effect. On the other hand, because BCNU is wrapped in the fiber and released slowly, so it conquers the disadvantage of short half-life, and also reduces its poisonous side effect. This trademark is suitable for part chemotherapy after the operation of tumour.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Memantine hydrochloride slow-release pellet preparation and preparation method thereof
InactiveCN103417491AImprove securityImprove the effect of treatmentNervous disorderGranular deliverySustained release pelletsCombinatorial chemistry
The invention relates to a memantine hydrochloride slow-release pellet preparation and a preparation method thereof. The memantine hydrochloride slow-release pellet preparation comprises a blank pellet core as a nuclear parent and memantine hydrochloride medicine slow-release composite layer wrapped as an outer layer. The preparation is characterized in that the blank pellet core and the memantine hydrochloride medicine slow-release composite layer are in the weight ratio of 2-6:1; the memantine hydrochloride medicine slow-release composite layer contains 10-40% of memantine hydrochloride, 50-70% of a slow-release material and 0.1-20% of other accessories. The slow-release pellet prepared by the invention has high roundness and good fluidity, can obtain high yield during wrapping of medicine and the slow release material, and has good reappearance. The simple preparation technology of the slow-release pellet is not restrained by place or equipment and suitable for industrialized production.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Decitabine sustained release microsphere and preparation method thereof
ActiveCN101966157AExtended half-lifeImprove efficacyOrganic active ingredientsPharmaceutical non-active ingredientsDrug administrationEntrapment
The invention discloses a Decitabine sustained release microsphere, which comprises Decitabine and a carrier material, wherein the weight percentage of the Decitabine and the carrier material is 1-8%, the partical size of the Decitabine sustained release microsphere is below 10 mu m, and the entrapment rate of the Decitabine sustained release microsphere is more than 75%. The Decitabine sustainedrelease microsphere provided by the invention has higher loading rate, no in-vivo burst effect, stable blood concentration, and no drug release delivery deadtime, thus greatly reducing clinical interval of drug administration, reducing dosage, improving compliance of patients, and reducing hazard rating of adverse reaction.
Owner:苏州科耐尔医药科技有限公司
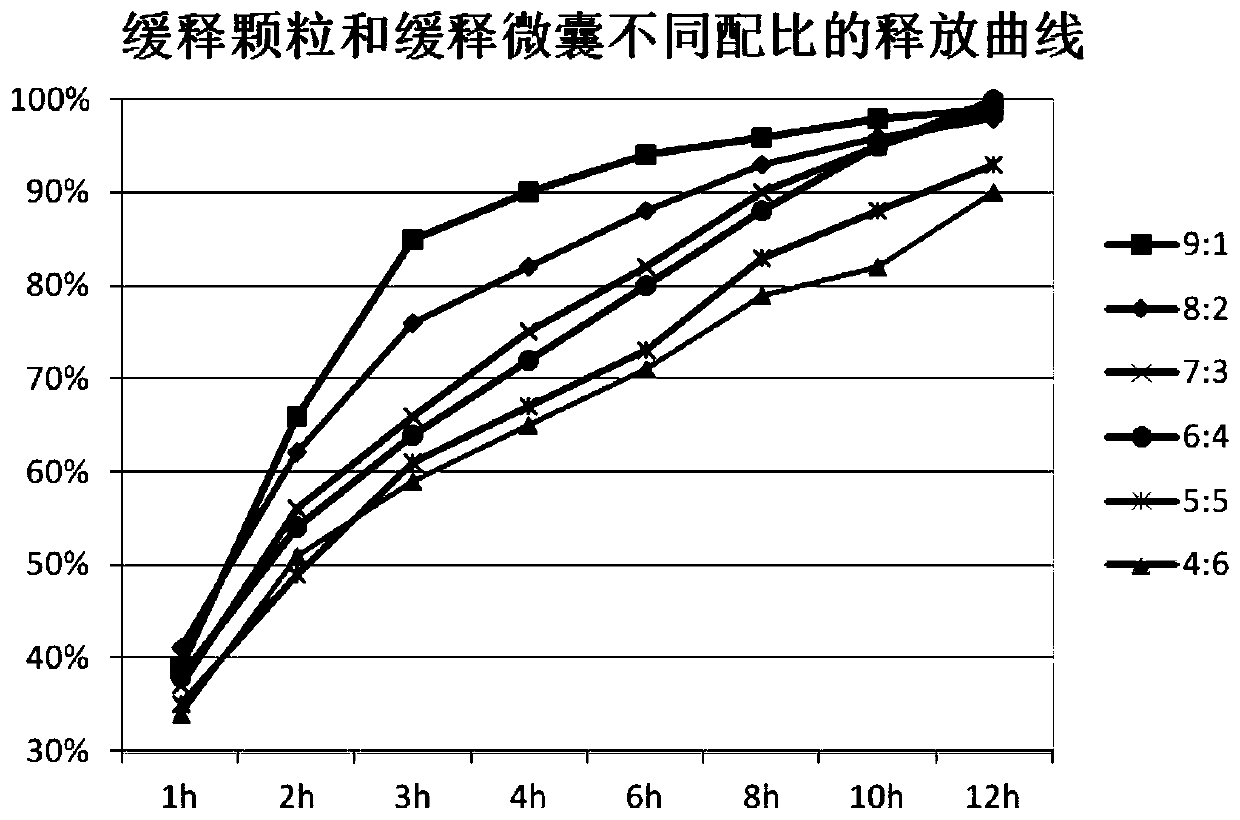
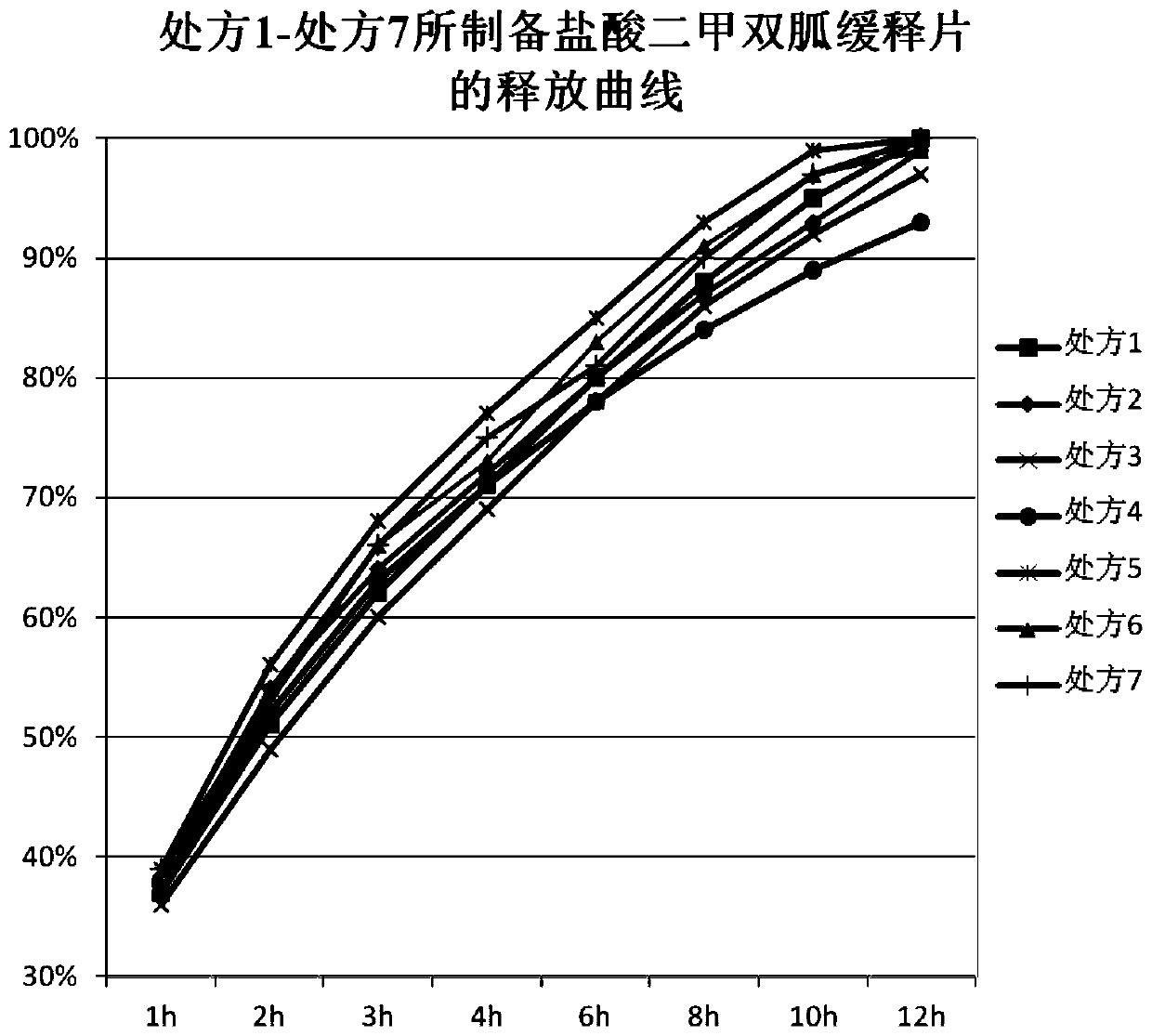
Metformin hydrochloride sustained-release tablet and preparation method thereof
ActiveCN110354090AImprove release behaviorOvercome sudden releaseOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
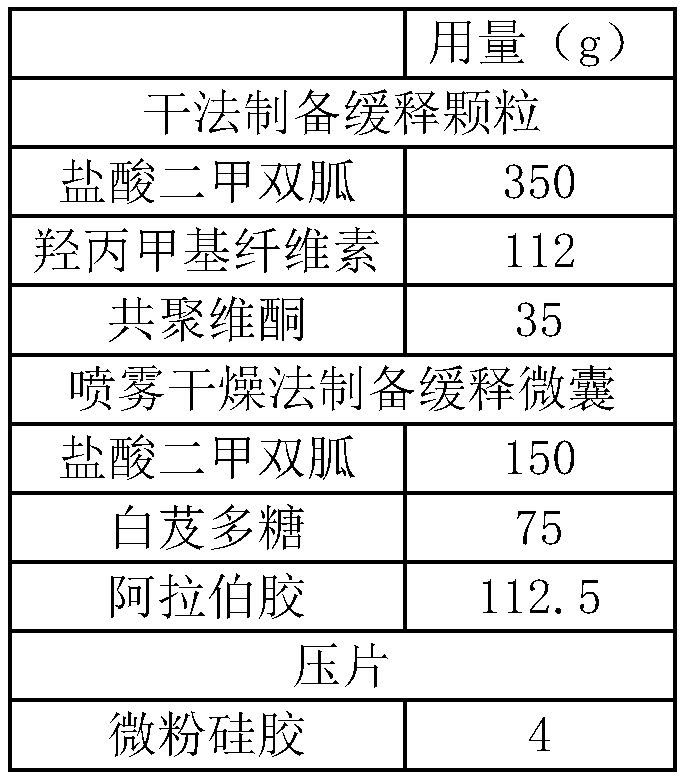
The invention relates to a metformin hydrochloride sustained-release tablet and a preparation method thereof, and belongs to the technical field of medicine. The metformin hydrochloride sustained-release tablet is composed of sustained-release granules, sustained-release microcapsules and a lubricant, and is prepared through tabletting, wherein the weight ratio of the metformin hydrochloride in the sustained-release granules and the metformin hydrochloride in the sustained-release microcapsules is (3:7)-(6:4), and the lubricant is selected from superfine silica powder or magnesium stearate. According to the preparation method, a traditional spray drying method is improved, by combining a dry granulation process, the sustained-release microcapsules and sustained-release granules of different processes are prepared, tabletting is further carried out to prepare the metformin hydrochloride sustained-release tablet, the specific ratio of the sustained-release microcapsules prepared by the spray drying process to the sustained-release granules prepared by the dry granulation process is explored, and the metformin hydrochloride sustained-release tablet of which the inner portion has the structure of the sustained-release microcapsules and the sustained-release granules is finally obtained. The metformin hydrochloride sustained-release tablet and the preparation method thereof achievea good release behavior of the sustained-release tablet, overcome the shortcomings of sudden release and incomplete release, obtain a better release curve, and also greatly reduce the impurity content.
Owner:CSPC OUYI PHARM CO LTD
Process for preparing slow and controlled micro pills
InactiveCN101513392AImprove bioavailabilityAvoid burst phenomenonCapsule deliveryMacromolecular non-active ingredientsHigh energyMetastability
The invention discloses a process for preparing slow and controlled micro dripping pills and belongs to the technical field of medicine production. The process is to prepare micro dripping pills by using a solid dispersion technology, hydrophilic and hydrophobic materials as a carrier and a spray or dripping method. The key points of the technical proposal of the invention include: a mixed carrier is adopted; the medicine exists in molecular, gel micro-crystal metastable state microparticle and other high energy states; a solid dispersion system with highly-dispersed multiple systems is formed; a centrifugal spray, pressure spray or dripping method is used for preparing the micro dripping pills; and the micro dripping pills can be tableted, or filled into capsules, or packaged with bottles, bags, bubble cap and the like. The invention relates to a method for preparing the slow and controlled micro dripping pills, which is simple, easy to implement, short in process, low in production cost and wide in application range, overcomes the drawbacks of the prior art and has wide market prospects.
Owner:孙民富
Segmented copolymer - docetaxel combination, preparation thereof and preparation method thereof
InactiveCN102626521AAvoid burst phenomenonImprove bioavailabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the technical field of pharmaceutical chemicals and discloses segmented copolymer - docetaxel combination, a preparation thereof and a preparation method thereof. The combination is combined by bonding polyoxyethylene - polypropylene oxide - polyoxyethylene (PEO- b - PPO - b - PEO with a product name as Pluronics (BASF company)) triblock copolymer and docetaxel through ester bond, and the Pluronics - docetaxel combination is obtained. By utilizing amphipathy of the combination and adopting physical methods to prepare micelle aqueous solution, a freeze-dried preparation can also be prepared. Nanomicelle is capable of gathering at tumor parts through 'enhanced permeation and retention effects (EPR effects)', and tumor targeting of docetaxel is enhanced. Besides, an active targeting factor is connected on Pluronics polymer so as to have the function of active targeting. The segmented copolymer - docetaxel combination overcomes the defects that existing docetaxel injection is poor in water-solubility and severe in allergic reaction and the like.
Owner:SHANDONG UNIV
Antibacterial microcapsule with clove oil embedded in laurinol modified alginic acid derivative and preparation method of antibacterial microcapsule
InactiveCN106433151AImprove hydrophilicityImprove stabilityFlexible coversWrappersMicroorganismAdditive ingredient
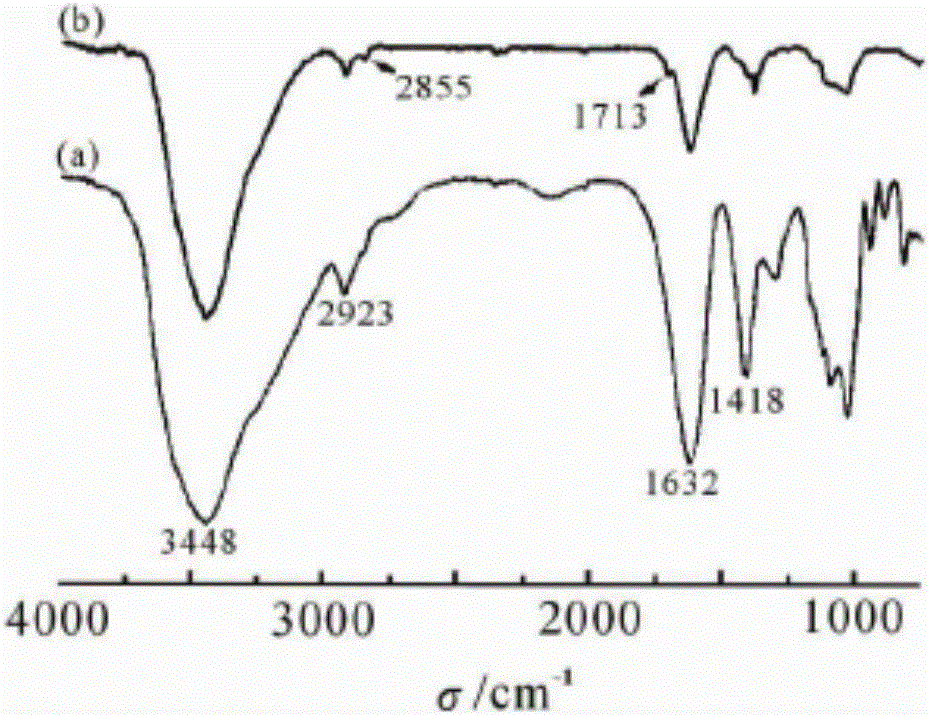
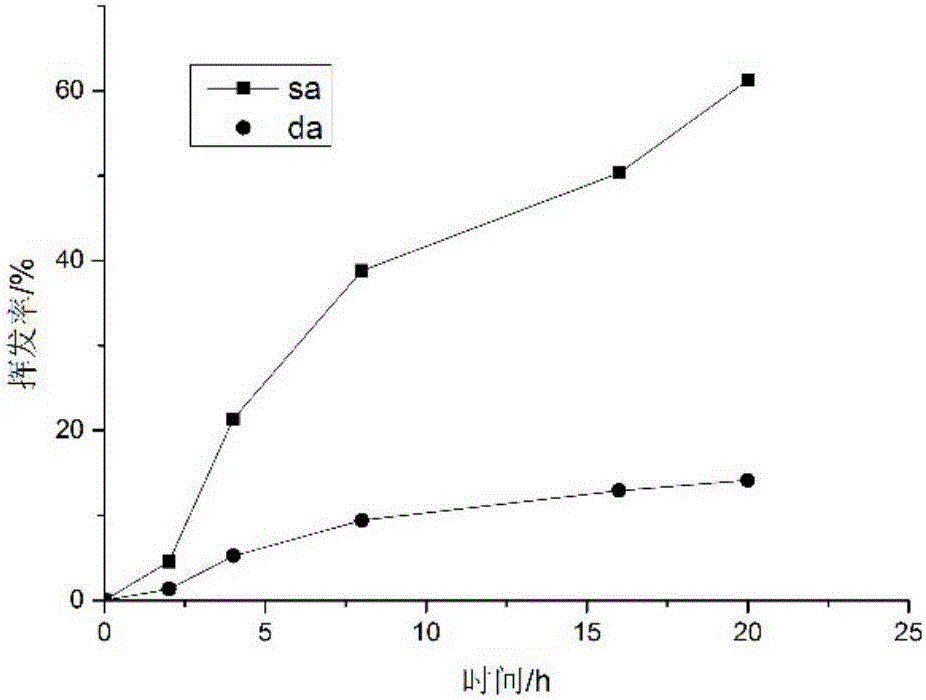
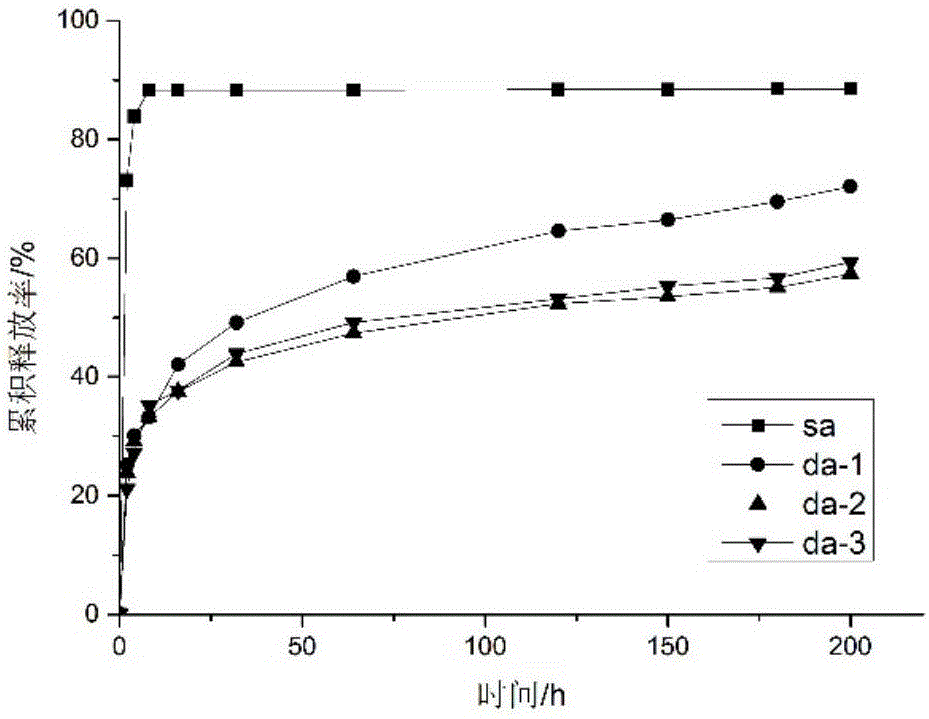
The invention relates to an antibacterial microcapsule with clove oil embedded in a laurinol modified alginic acid derivative and a preparation method of the antibacterial microcapsule, and belongs to the technical field of modification of high polymer materials. The antibacterial microcapsule comprises sodium alginate, laurinol and clove oil.The preparation method comprises the following steps that firstly laurinol is used to modify sodium alginate to obtain sodium alginate graft-copolymerized laurinol SA-Da, and then the clove oil is embedded to obtain the antibacterial microcapsule with the clove oil embedded in the laurinol modified alginic acid derivative. The prepared slow-release antibacterial microcapsule is relatively high in antibacterial property, can effectively inhibit the growth and propagation of microorganisms, has a certain slow release effect, slowly releases the antibacterial ingredient of the clove oil along with the change of time, has an excellent slow-release antibacterial effect, and can be applied to a food packaging film to prolong the food shelf-life and improve the food quality, and therefore, the antibacterial microcapsule has an excellent market prospect.
Owner:JIANGNAN UNIV
Polypeptide sustained-release microsphere preparation and preparation method thereof
ActiveCN106667958ASmall particle sizeLow initial release rate in vitroPeptide/protein ingredientsMetabolism disorderMicrosphereMedicine
The invention discloses a polypeptide sustained-release microsphere preparation. The preparation is composed of 3% to 10% by weight of exenatide or its salt as a raw material and 90% to 97% by weight of a polymer as an auxiliary material. An in-vitro initial release rate of the sustained-release microsphere preparation in 1h is less than 2%. The invention also provides a method for preparing the polypeptide sustained-release microsphere by an O / O phase coacervation method. The polypeptide sustained-release microsphere preparation has low microsphere sizes, low burst release and high entrapment efficiency, further prolongs long drug release time, can continuously release drugs for half a month or more in single-dose administration, can release drugs for 3 months, significantly reduces the frequency of administration, improves the patient's compliance, overcomes the defect of the existing peptide sustained-release microsphere preparation platform period and can get a near-zero order release curve.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Chitosan-carboxylated chitosan nanosphere loading insoluble antitumor drug and preparation method and application thereof
InactiveCN102389572ALong cycleImprove targetingPowder deliveryMacromolecular non-active ingredientsTumor targetCross-link
The invention relates to a chitosan-carboxylated chitosan nanosphere loading an insoluble antitumor drug. The nanosphere has a porous and aperture-controlled structure which controls rapid and stable release of the drug. Polyethylene glycol (PEG) chains and tumor-targeting specific molecules are coupled on the surface of the nanosphere, so as to effectively increase the cycle period of the drug in vivo, improve the affinity of the nanosphere to tumor cells and improve the bioavailability of the drug. Besides, the nanosphere has less toxic side effects and better antitumor effects. The invention also provides a method for preparing the chitosan-carboxylated chitosan nanosphere loading or combining the insoluble antitumor drug. The amino groups of chitosan / carboxylated chitosan are cross-linked to form a sphere, and the carboxyl groups of carboxylated chitosan are coupled with the PEG chains and then grafted with the targeting molecules, so that the drug is uniformly dispersed in the form of in-situ crystallization in chitosan and its derivative nanosphere, as a result, the drug-loading rate is greatly increased and the release rate of the drug is stabilized.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Growth hormone controlled-release coating and preparation method thereof
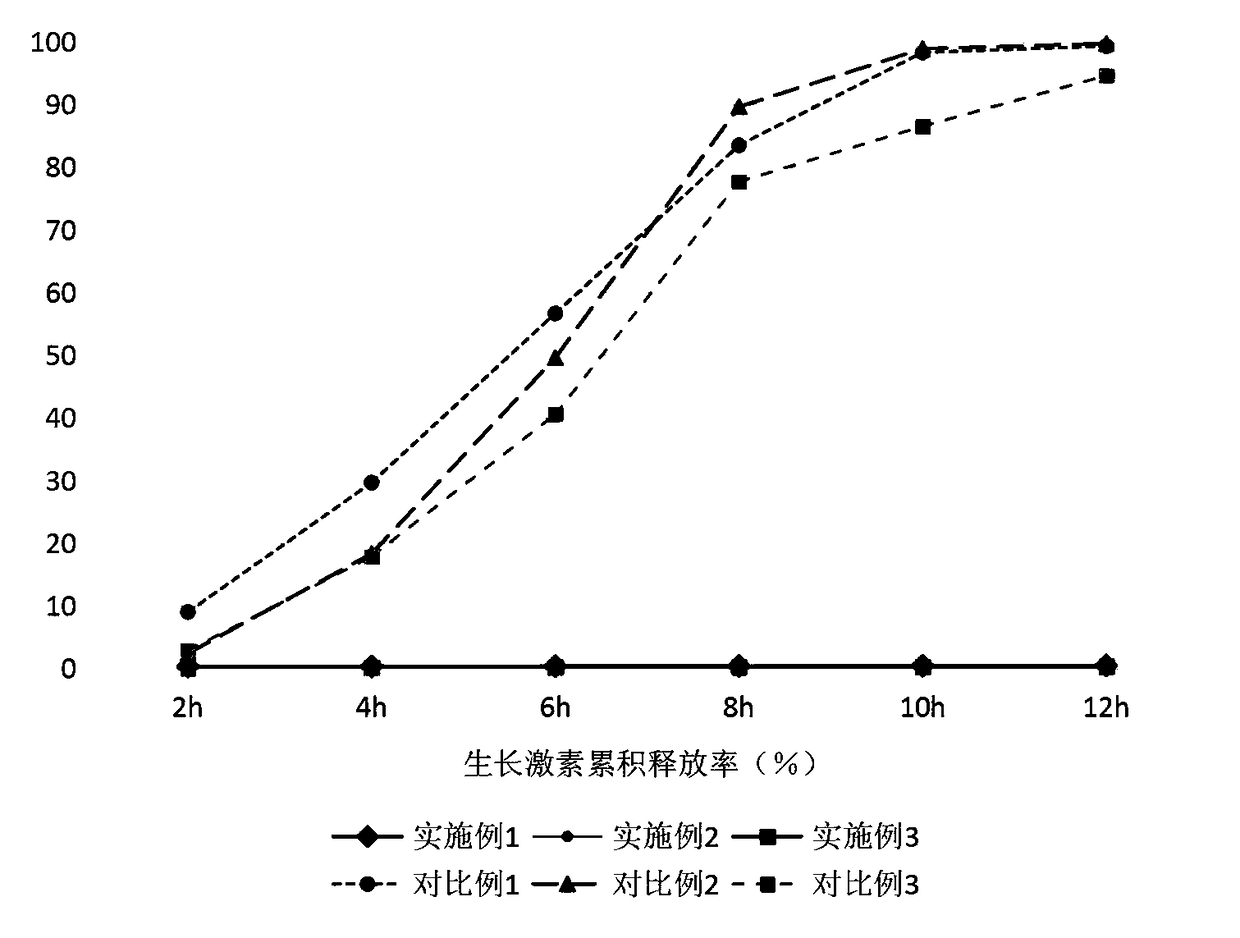
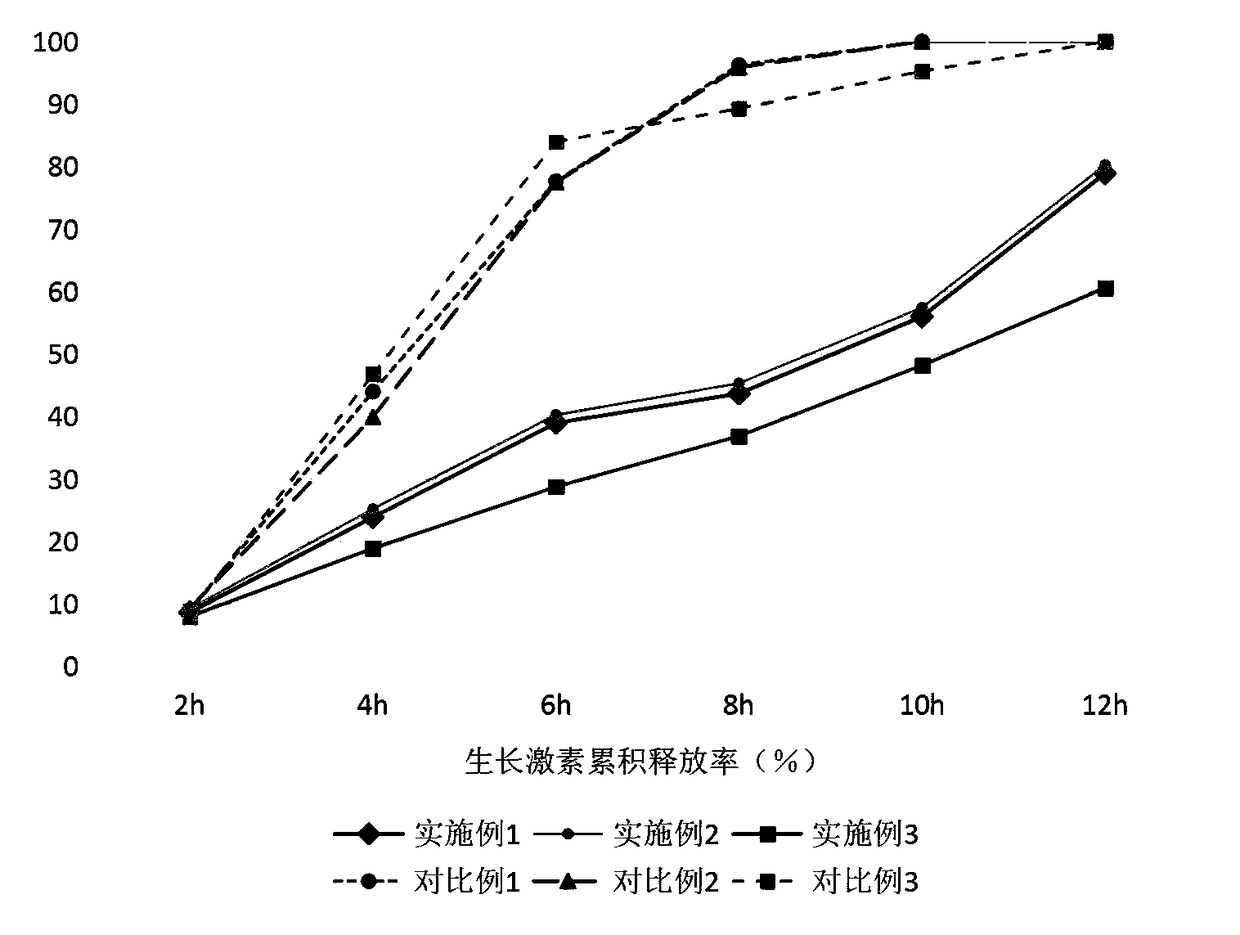
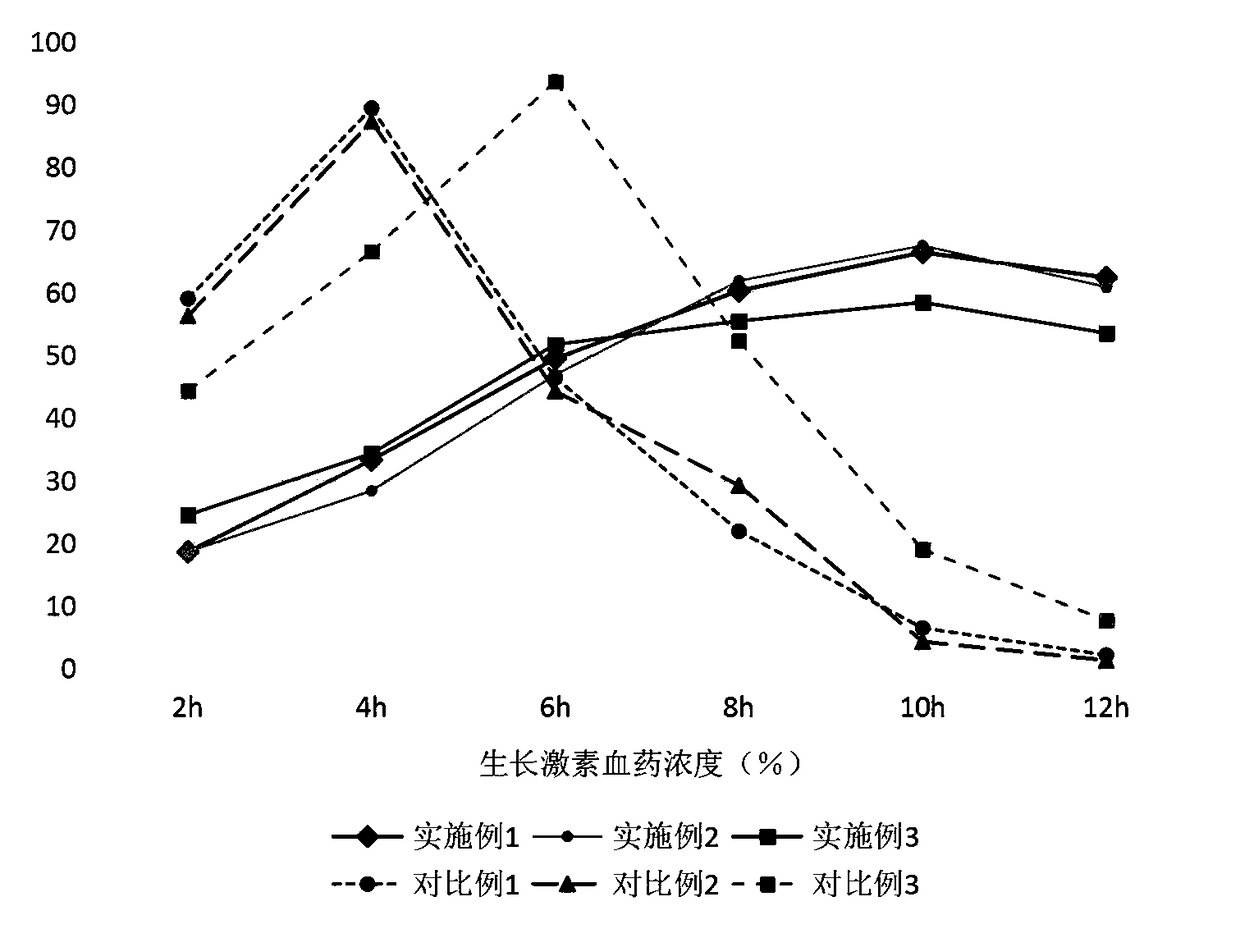
InactiveCN108465104AReduce contact dissolution rateMaintain integrityPeptide/protein ingredientsMetabolism disorderReaction ruleSuperhydrophobic coating
The invention belongs to the technical field of biomedical technologies, and discloses a growth hormone controlled-release coating and a preparation method thereof. The coating comprises three layersof structures which are an outermost layer superhydrophobic coating, a middle layer silk fibroin peptide coating and an innermost layer growth hormone controlled-release microballoon sphere. The invention aims at providing a preparation method of an oral administration dosage form. The three layers of structures with different ingredients are targetedly arranged to serve as the coating of growth hormone according to the structure and reaction rules of human digestive tracts, an advanced electrostatic spraying technology is adopted for making the coating, the number of times of administration of a patient is reduced, the growth hormone medicine clinical application range is increased, and the coating has the wide market prospect.
Owner:FOSHAN SHIRUI LEADING MATERIAL RES INST GENERAL PARTNERSHIP
Sustained-release tablet containing trazodone hydrochloride and preparation method of sustained-release tablet
ActiveCN105748421ARelease stabilitySustained releaseOrganic active ingredientsNervous disorderSustained Release TabletTrazodone Hydrochloride
The invention discloses a sustained-release tablet containing trazodone hydrochloride and a preparation method of the sustained-release tablet. The sustained-release tablet is prepared from, in percentage by weight of the whole tablet, 15%-65% of trazodone hydrochloride, 30%-85% of a sustained-release framework and 0.1%-10% of other medical auxiliaries, wherein the sustained-release framework is prepared from high-viscosity hydroxypropyl methylcellulose and water-soluble filler in the weight ratio being 1: (0.3-1.2), and other medical auxiliaries comprise a flow aid and a lubricating agent. Through matched use of high-viscosity hydroxypropyl methylcellulose and the water-soluble filler, the microporous sustained-release framework is formed; the prepared sustained-release tablet containing trazodone hydrochloride can effectively control the release speed of trazodone hydrochloride and can completely release trazodone hydrochloride contained in a sustained release tablet core within certain time, so that water-soluble trazodone hydrochloride is easily stabilized and effectively released, plasma concentration is prevented from fluctuating substantially, the prescription is simple, and the process is simple and convenient.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
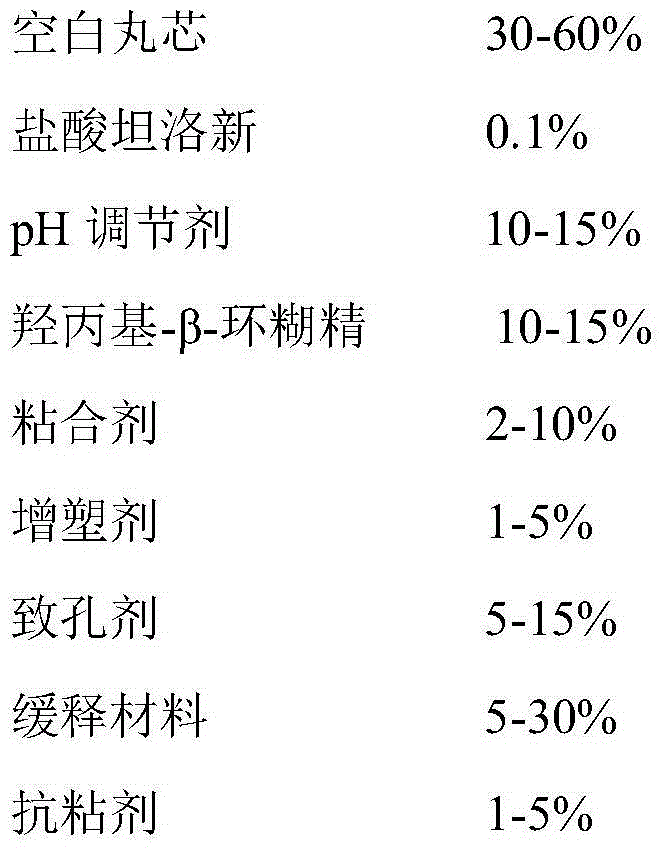
Tamsulosin hydrochloride sustained-release capsule and preparation method thereof
PendingCN104873478AAvoid burst phenomenonImprove securityPharmaceutical delivery mechanismUrinary disorderSustained Release CapsuleEconomic benefits
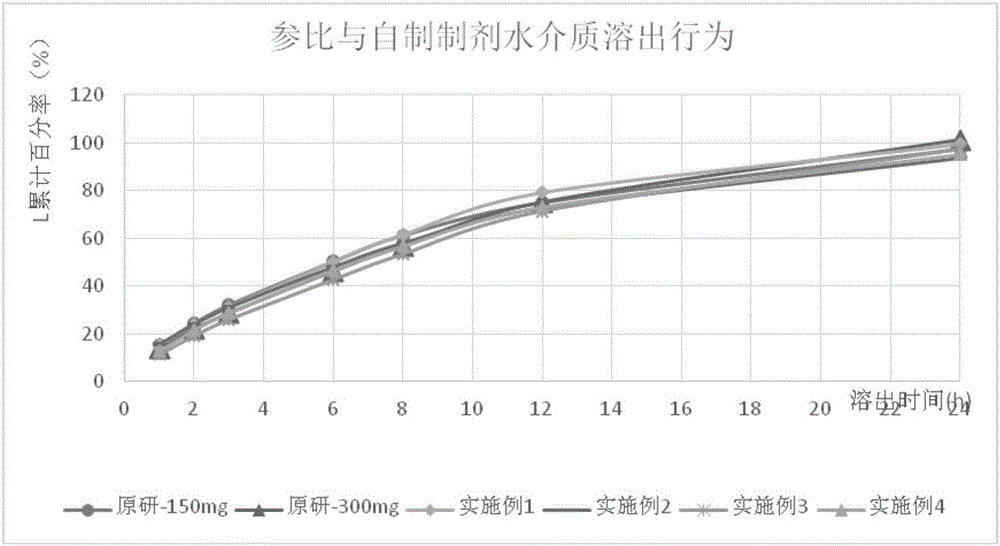
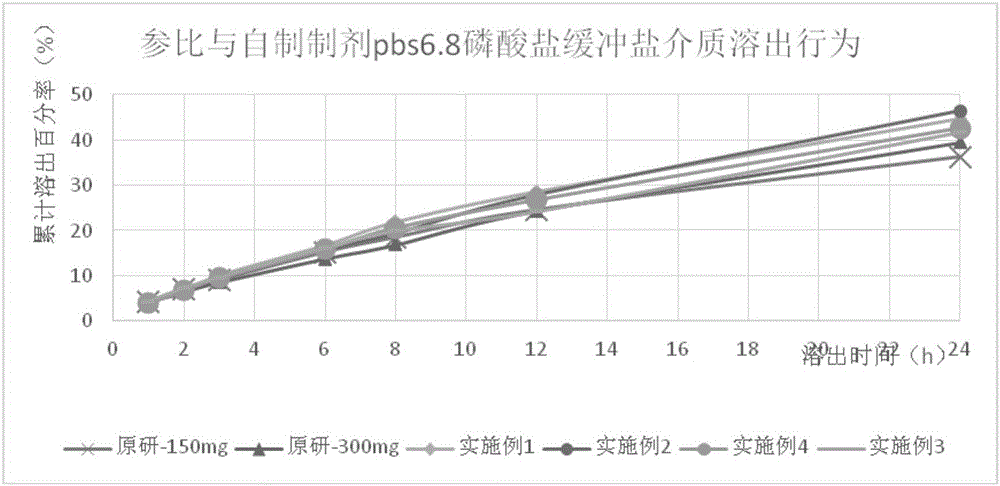
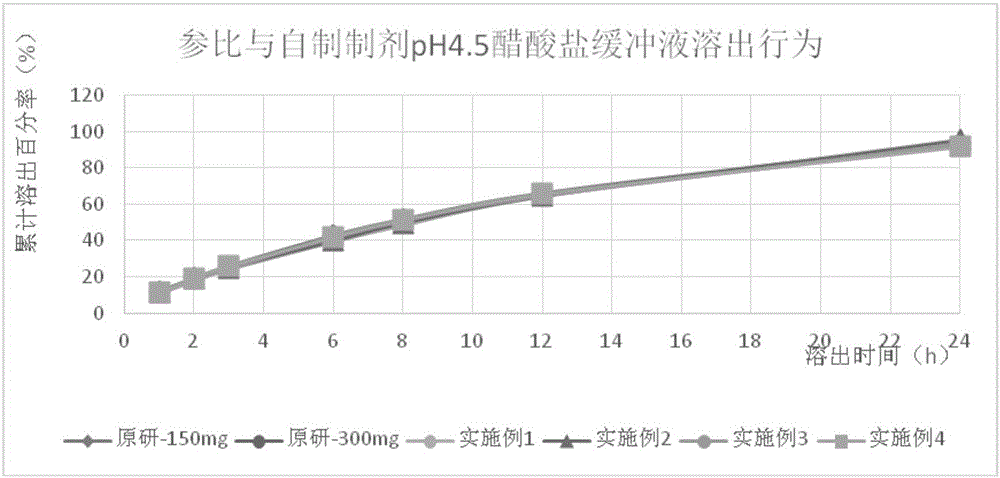
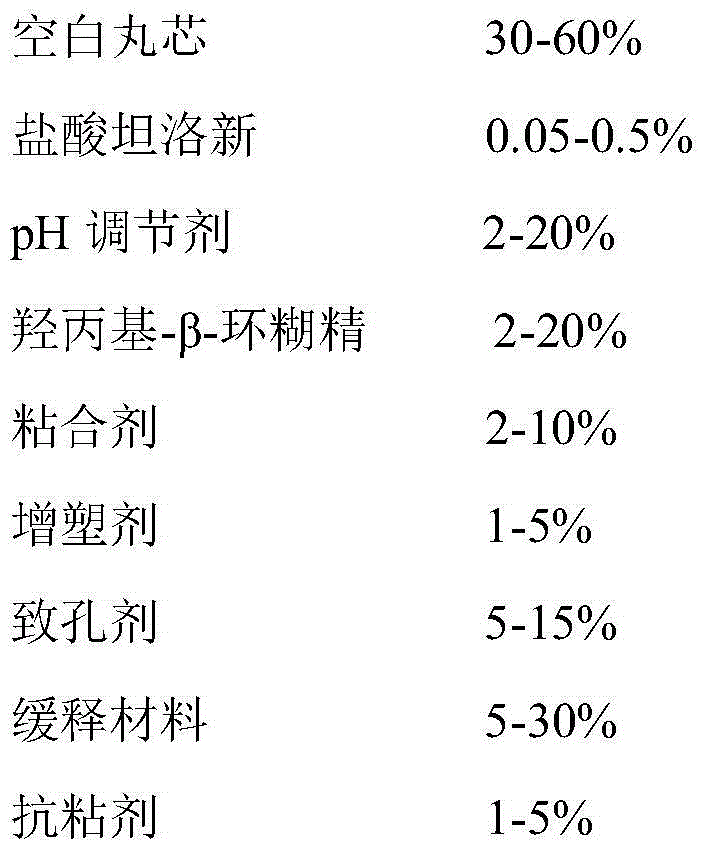
The invention provides a tamsulosin hydrochloride sustained-release capsule and a preparation method thereof. Hydroxypropyl-beta-cyclodextrin is added in a prescription, so as to form clathrate compounds on the outer part of tamsulosin hydrochloride together with the tamsulosin hydrochloride, so that the phenomenon of drug burst release in a drug release process is solved, thereby maintaining stable plasma concentration, reducing the incidence rate of adverse reactions and improving the clinical medication safety. In addition, raw materials are easy to obtain, the preparation process is simple and feasible, the yield is high, the cost is low, industrial mass production can be realized, and obvious economic benefits are achieved.
Owner:LUNAN BETTER PHARMA
Drug-loaded static spinning guided tissue regeneration membrane preparation method and product and application thereof
InactiveCN107875453AAvoid burst phenomenonGood treatment effectFilament/thread formingTissue regenerationFiberTherapeutic effect
The invention relates to a drug-loaded static spinning guided tissue regeneration membrane preparation method and a product and an application thereof, and comprises preparation of drug-loaded HNTs and preparation of a static spinning drug-loaded composite nano fiber film. The method employs HNTs having a hollow structure as a drug carrier-loaded anticancer medicine, can avoid burst release phenomenon due to mixed static spinning of the simple medicine and a polymer, and the prepared static spinning guided tissue regeneration membrane has good treatment effect. The doped inorganic HNTs in thestatic spinning polymer nano fiber can increase the mechanical properties of the composite fiber membrane, and has good biological compatibility, degradability and bone growth promotion performance, and is in favor of taking the drug-loaded nano fiber as a functional tissue engineering frame material for complex internal environment; the raw material HNTs is the nano nanotube having good crystallization and low cost, which is environmentally friendly, the preparation method of the guided tissue regeneration membrane is simple, operationality is strong, and production and application can be satisfied.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Preparation method of controlled-release drug
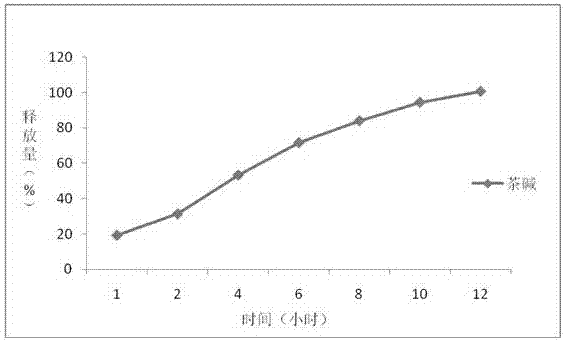
InactiveCN107441055AEvenly dispersedTightly dispersedPill deliveryMicrocapsulesNifedipineOrganic solvent
The invention belongs to the field of pharmaceutical preparations, specially relates to a preparation method of a controlled-release drug, and particularly relates to theophylline and nifedipine controlled-release tablets prepared by using the method. The controlled-release drug is prepared by adopting combination of two techniques of hot melt extrusion and membrane controlling. Compared with the prior art, the method disclosed by the invention has the advantages that the drug is subjected to melting and solidification based on that the drug is preferably controlled to release at a zero level, the quality is uniform, and the phenomenon that burst release of other drugs in controlled-release dosage forms possibly occurs is avoided; for drugs with narrow therapeutic windows, clinical application is safer; organic solvents are not used, so that the security of mass production is good.
Owner:山东则正医药技术有限公司
Pluronics-PTX (Paclitaxel) amphiphilic macromolecular prodrug and micelle preparation thereof
InactiveCN104096237AImprove securityAvoid burst phenomenonOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySolvent
The invention discloses a Pluronics-PTX (Paclitaxel) amphiphilic macromolecular prodrug which is prepared by bonding Pluronics and PTX through an ester bond. The prodrug can be self-assembled in water to form micelle which takes PTX as a hydrophobic core and takes Pluronics as a hydrophilic shell, the content of a PTX drug in a preparation is increased, the problem of low solubility of the PTX drugs in water is solved, and further, the final preparation is free of a solubilizer and an organic solvent, so that the safety of drug use is improved. An active targeting factor can be connected to a Pluronics-terminated hydroxyl to provide the drug with an active targeting function. Further, the defects of poor water solubility, serious allergic reaction and the like of the conventional PTX injection are overcome.
Owner:SHANDONG UNIV
Aripiprazole sustained-release microsphere and preparation method thereof
ActiveCN108498456ASmooth releaseGood compatibilityOrganic active ingredientsNervous disorderOral medicationMicrosphere
Owner:LIVZON PHARM GRP INC
PLGA (Poly Lactic-co-Glycolic Acid)-gelatin composite microspheres carrying genistein and preparation method thereof
InactiveCN106727361ACombined orderlyGood emulsificationOrganic active ingredientsSkeletal disorderControlled releaseMicrosphere
The invention discloses PLGA (Poly Lactic-co-Glycolic Acid)-gelatin composite microspheres carrying genistein and having a controlled-release effect and a preparation method thereof, and belongs to the technical field of biomedical materials. The method comprises the following steps: preparing gelatin nanoparticles by a two-step desolvation method; adsorbing the genistein in the gelatin nanoparticles; entrapping the gelatin nanoparticles adsorbing drugs in PLGA microspheres by an improved S / O / W method to prepare the PLGA-gelatin composite microspheres carrying the genistein, which have the advantages of high drug loading capacity, capability of overcoming burst release of drugs and uniform particle sizes and are applied in the field of drug delivery. The obtained PLGA-gelatin composite microspheres carrying the genistein are white or faint yellow in appearance, and are 3 to 8mu m in particle sizes, the particles are dispersed, and adhesion is prevented; the highest drug loading amount of the genistein is about 12.1 percent by weight. The gelatin nanoparticles prepared in a preparing process are 60 to 300 nanometers in particle sizes, drugs are released hardly within 24 hours, and the release rate of the genistein is about 80 percent within 20 days along with the degradation of the composite microspheres.
Owner:JILIN UNIV
Anti-tumor macromolecular prodrug compound, and preparation method and application thereof
ActiveCN105797169AGood tumor targetingImprove intake efficiencyHydroxy compound active ingredientsKetone active ingredientsActive componentTherapeutic effect
The invention discloses an anti-tumor macromolecular prodrug compound, and a preparation method and application of the anti-tumor macromolecular prodrug compound. The anti-tumor macromolecular prodrug compound is prepared from heparin or a derivative-a doxorubicin type macromolecular prodrug of the heparin and a hyaluronic acid-natural active component type macromolecular prodrug in a compounding way. The invention also provides the preparation method of the anti-tumor macromolecular prodrug compound and the application of the anti-tumor macromolecular prodrug compound in preparing anti-tumor drugs. Compared with the prior art, by compounding two kinds of macromolecular prodrugs, not only are multiple tumor targeting properties obtained, but also multi-drug resistance of tumor can be effectively reversed; the two kinds of macromolecular prodrugs work together and are in mutual synergy, so that the treating effect is obviously enhanced.
Owner:CHINA PHARM UNIV
Biologically responsive nitric oxide donor type polymer prodrug and preparation method thereof
PendingCN112843241AGood water solubilitySmall side effectsOrganic active ingredientsInorganic active ingredientsChemotherapeutic drugsPolymer
The invention discloses a biologically responsive nitric oxide donor type polymer prodrug and a preparation method thereof, wherein the polymer prodrug is obtained by connecting a hydroxyl-containing chemotherapeutic drug and a nitric oxide donor type polycarbonate macromolecule through a tumor microenvironment acid responsive acetal bond, and then the polymer prodrug nano-drug is obtained through self-assembly. According to the invention, chemotherapeutic drugs are modified, and a nitric oxide donor is introduced to a polymer carrier material, so that the nitric oxide donor type polymer prodrug with high drug loading capacity, high stability and biological response is prepared, drug sensitization and synergistic treatment effects are realized, and the prodrug has a wide application prospect in the aspect of efficient anti-tumor.
Owner:CHINA PHARM UNIV
Decitabine sustained release microsphere and preparation method thereof
ActiveCN101966157BGood physiological compatibilityPhysiological compatibility NoneOrganic active ingredientsPharmaceutical non-active ingredientsBlood concentrationBurst effect
Owner:苏州科耐尔医药科技有限公司
Slow releasing micropill containing active component of indapamide and method for preparing the same
InactiveCN1228049CAvoid burst phenomenonImprove securityOrganic active ingredientsGranular deliveryAdhesiveActive component
A slowly-releasing micropill containing indapamide as its active component is composed of pill core, quick-releasing layer and slow-releasing layer. It is prepared through proportionally mixing filler, adhesive and lubricant, granulating to become (10-30)-mesh pill cores, dissolving indapamide and high-molecular adhesive in solvent, spraying it on the surface of pill cores, dissolving the retarder, pore-forming agent and plasticizer of antisticking agent in solvent, and spraying it on the surface micropill. Its advantages are high slow releasing effect within 24 hr and no sudden release.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Slow release drop pills comprising toraesmide active ingredient and method for preparing same
InactiveCN100548297CAvoid burst phenomenonImprove securityUrinary disorderGranular deliverySustained release pelletsPlasticizer
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Solid lipid magnetic resonance nanoparticle as well as preparation method and application thereof
The invention discloses a solid lipid magnetic resonance nanoparticle as well as a preparation method and application thereof. The preparation method comprises the following steps: using glyceryl monostearate and lecithin as lipid materials; compounding octadecylamine with gadopentetate meglumine serving as a magnetic resonance contrast agent; using trehalose as a freeze-drying protective agent, thus preparing a lipid nanoparticle which is easily absorbed by an intestinal canal. The preparation method disclosed by the invention is optimized on the basis of an existing preparation method of the solid lipid nanoparticle; after a nano contrast agent is ingested by a digestive tract, the release time of the gadopentetate meglumine in the nanoparticle, which is not absorbed by in-vivo tissues in the digestive tract, is prolonged, the imaging time of an MR living body is prolonged, an imaging time window of tumors such as breast cancer is increased, and the cytotoxicity of a material is reduced.
Owner:ZHEJIANG UNIV
Preparation method of nano-particle loaded with two anti-hepatoma medicines and provided with two-layer controlled release-light heat-target function
ActiveCN109125726AEfficient controlled releaseEfficient sustained releaseEnergy modified materialsPharmaceutical non-active ingredientsMicroparticleOil phase
The invention relates to a preparation method of a nano-particle loaded with two anti-hepatoma medicines and provided with a two-layer controlled release-light heat-target function. The method includes the steps: modifying carboxymethyl Pulullan on carboxymethyl chitosan by taking hydrophilic polyacrylamide as a bridge, and enabling the carboxymethyl chitosan to have a target function when the hydrophilcity of the carboxymethyl chitosan is improved; loading doxorubicin serving as an anti-cancer drug on polydopamine, wrapping the doxorubicin with hydrophilic polyvinylpyrrolidone and Arabic gum,loading Sorafenib serving as an anti-cancer drug on the outer surface of an oil phase of the hydrophilic polyvinylpyrrolidone and the Arabic gum, and wrapping a system with carboxymethyl chitosan-polyacrylamide-carboxymethyl Pulullan nano-particle water solution to obtain the nano-particle loaded the two anti-hepatoma medicines and provided with the two-layer controlled release-light heat-targetfunction. The polymer nano-particle can control release of the two-layer anti-hepatoma medicines and is combined with a light heat performance of the polydopamine to treat a liver cancer.
Owner:ZHEJIANG SCI-TECH UNIV
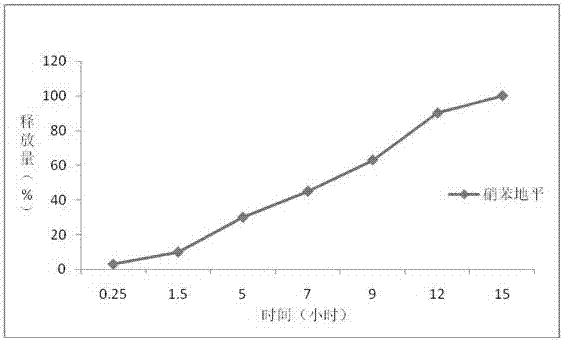
Nifedipine sustained-release tablet and production process thereof
ActiveCN111643467AStable drug releaseAvoid burst phenomenonOrganic active ingredientsPill deliveryNifedipineProlonged-release tablet
The invention belongs to the technical field of medicines and particularly relates to a nifedipine sustained-release tablet and a production process thereof. The provided nifedipine sustained-releasetablet is prepared from components in parts by mass as follows: 20 parts of nifedipine, 13-20 parts of a sustained-release agent, 3-5 parts of a retardant, 72-76 parts of a filling agent, 1-2 parts ofa disintegrating agent, 0.3-0.7 part of a fluidizer and 0.5-1 part of a lubricant. The provided nifedipine sustained-release tablet is stable in drug release, can guarantee full release within 24 hours and maintain necessary blood-drug concentration and avoids the sudden release phenomenon. Besides, the prepared nifedipine sustained-release tablet is uniform in drug dispersion.
Owner:JINAN LIMIN PHARMA
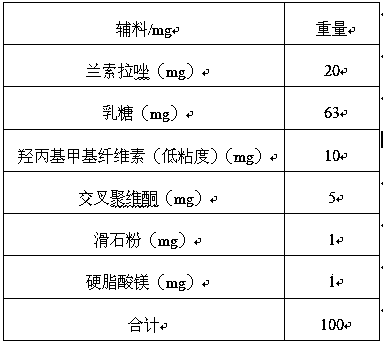
Lansoprazole sustained-release preparation
PendingCN109692161AImprove bioavailabilityAvoid burst phenomenonOrganic active ingredientsDigestive systemPatient complianceMagnesium stearate
The present invention provides a lansoprazole sustained-release preparation, which is characterized in that a sustained-release preparation prescription consists of a core prescription and a coating layer prescription. The core prescription consists of lansoprazole, lactose, hydroxypropyl methylcellulose, cross-povidone, powdered steatile, and magnesium stearate. The coating layer prescription isa mixture of two or more of ethyl cellulose, hydroxypropyl methylcellulose, polyacrylic resin, microcrystalline cellulose, and magnesium stearate. The preparation of lansoprazole sustained-release tablets by a compression coating method can better avoid the sudden release of the drug, and the prescription is simple, and separated addition of a pore forming agent and other sustained-release materials is not required. The invention provides the preparation method of lansoprazole sustained-release tablets with slow release in the intestine and high stability, is suitable for industrial production, and improves patient compliance, medication safety and bioavailability.
Owner:汉寿康运医药科技有限公司
Extrusion molding-photocuring integrated three-dimensional printer and printing method thereof
PendingCN113334761AIncrease printing speedImprove mechanical propertiesManufacturing platforms/substratesManufacturing driving meansDrive motorEngineering
The invention discloses an extrusion molding-photocuring integrated three-dimensional printer and a printing method thereof. The extrusion molding-photocuring integrated three-dimensional printer comprises a rack, a photocuring molding trough module, a composite molding platform module, a non-interference switching device, a light processing device and an X-axis system driving motor, wherein the photocuring molding trough module is fixedly arranged on an axis movement mechanism; the composite molding platform module is fixedly arranged on a Z-axis movement mechanism; the non-interference switching device controls reverse movement of an extrusion molding platform and a photocuring molding platform by utilizing a gear engagement principle; the light processing device is positioned at the bottom end of the trough module, is fixedly arranged on the axis movement mechanism and is used for projecting a photocuring molded graph onto a composite deposition platform; and the X-axis system driving motor is fixed on an X-axis movement mechanism and drives an X-axis system motor lead screw to drive the composite deposition platform to turn over. According to the extrusion molding-photocuring integrated three-dimensional printer disclosed by the invention, a personalized customized high-precision three-dimensional structure is printed by a photocuring technology, and then a biological material is printed by an extrusion molding technology, so that high-precision personalized rapid printing of various materials is realized.
Owner:XINJIANG UNIVERSITY
Injectable artificial dermis for promoting wound healing as well as preparation method and application of injectable artificial dermis
PendingCN114225118APromote repairGood compatibilityPharmaceutical delivery mechanismProsthesisMetaboliteTissue repair
The invention relates to injectable artificial dermis for promoting wound healing as well as a preparation method and application of the injectable artificial dermis. The injectable artificial dermis for promoting wound healing comprises collagen-polysaccharide composite hydrogel microspheres loaded with polyphosphate. The microstructure of the hydrogel microsphere is composed of a cross-linked polymer network, the hydrogel microsphere has high permeability, internal and external circulation of nutrient substances and substance exchange of metabolites are facilitated, and meanwhile, the hydrogel microsphere serves as an injectable stent to support cell ingrowth and induce cell proliferation and differentiation; due to the small size, the materials can be injected to a specific part, and the high viscosity after injection can be guaranteed; and aiming at a wound surface with irregular depth and a cavity, the dressing has good effects of filling and covering and promoting repair of tissues in a lacuna. Meanwhile, the loaded amorphous polyphosphate is hydrolyzed under the action of alkaline phosphatase, chemical energy is released, energy needed for wound healing is provided, cell proliferation, growth and migration are promoted, and formation of a vascular network is accelerated.
Owner:SHENZHEN QIKANG MEDICAL DEVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com