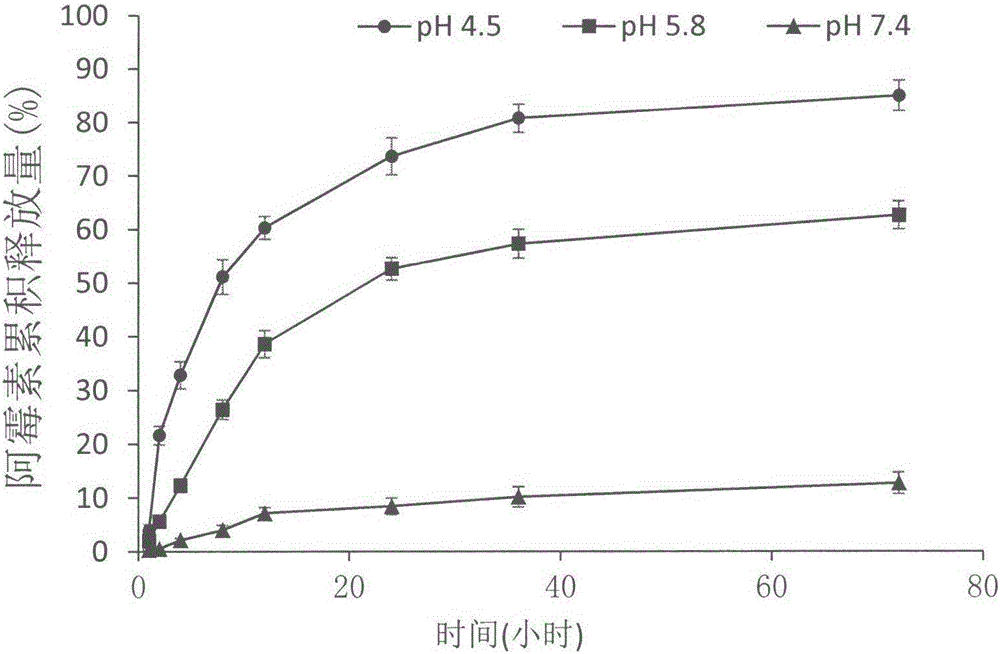
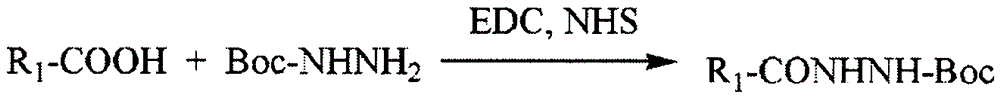Anti-tumor macromolecular prodrug compound, and preparation method and application thereof
A technology of macromolecules and complexes, applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of low bioavailability, skin toxicity, bone marrow toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: Synthesis of low molecular weight heparin-doxorubicin macromolecular prodrug
[0066] Weigh 1 mmol of low molecular weight heparin (LMWH) and add it into 10 mL of formamide, stir magnetically for 1 h until it is completely dissolved, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide ( EDC) and hydroxysuccinimide (NHS), after reacting for 0.5h, slowly add the formamide solution of Boc hydrazine (the molar ratio of LWMH, EDC, NHS, Boc hydrazine is 1:5:5:20 successively), drop The speed is 3 seconds between each drop, after the addition, react at room temperature for 24 hours, precipitate with ice acetone, wash the precipitate repeatedly, and dry it in vacuum for 12 hours to obtain the active intermediate of LMWH modified by Boc hydrazine; the active intermediate of LMWH modified by Boc hydrazine Disperse in 6mL of dichloromethane, add 4mL of trifluoroacetic acid, ultrasonically react for 2h, suction filter to obtain the precipitate, redissolve in water, dialy...
Embodiment 2
[0067] Embodiment 2: Synthesis of heparin-doxorubicin macromolecular prodrug
[0068] Weigh 1 mmol of heparin (heparin) and add it to 10 mL of formamide, stir magnetically for 1 h until it is completely dissolved, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) under the condition of nitrogen protection and ice bath ) and hydroxysuccinimide (NHS), after reacting for 2 hours, slowly drop the formamide solution of Boc hydrazine (the molar ratio of heparin, EDC, NHS, and Boc hydrazine is 1:10:15:60 in turn), and the dropping speed is The interval between each drop is 5 seconds, after the addition is completed, react at room temperature for 24 hours, precipitate with ice acetone, wash the precipitate repeatedly, and dry in vacuum for 12 hours to obtain the active intermediate of heparin modified by Boc hydrazine; disperse 20 mg of active intermediate of heparin modified by Boc hydrazine in Add 4 mL of trifluoroacetic acid to 6 mL of dichloromethane, ultrasonically react for...
Embodiment 3
[0069] Embodiment 3: the synthesis of hyaluronic acid-curcumin macromolecular prodrug
[0070] 1mmol hyaluronic acid (HA) and 3mmol curcumin (CUR) were respectively dissolved in a mixed solvent of formamide and N,N-dimethylformamide (v:v=1:1). Under the protection of nitrogen, add 5 mmol 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.5 mmol 4-dimethylaminopyridine (DMAP) into the HA solution, activate the reaction for 1 h, slowly drop Add CUR solution, the drop rate is 2 seconds per drop, and then react at room temperature for 48 hours after the drop is completed. After the reaction is completed, precipitate with glacial ethyl acetate, wash the precipitate repeatedly, and redissolve it in water, sonicate the probe for 30 minutes, dialyze for 1 day, and freeze-dry , to obtain hyaluronic acid-curcumin macromolecular prodrug (HA-CUR).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com