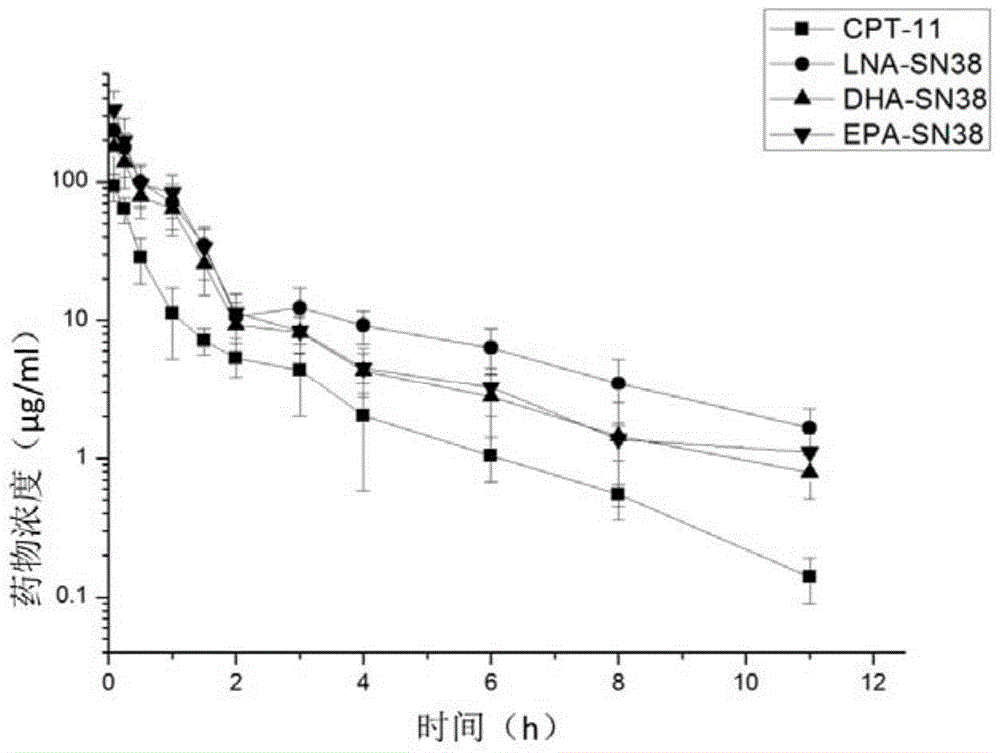
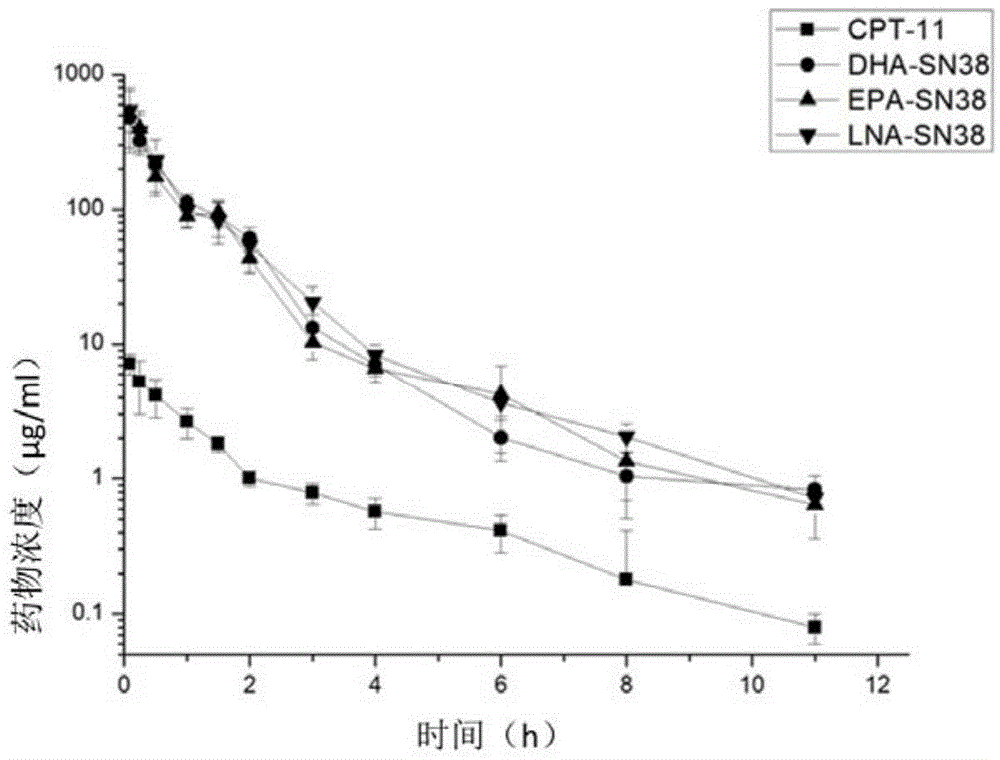
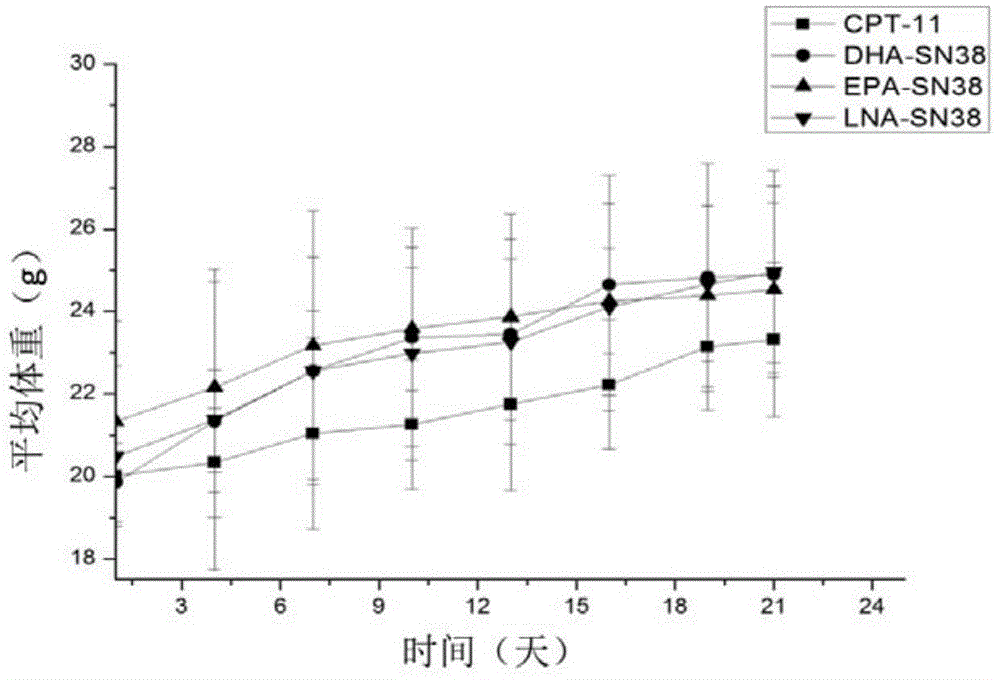
7-ethyl-10-hydroxycamptothecin derivatives, and preparation and application thereof
A technology of hydroxycamptothecin and derivatives, applied in the field of medicine, can solve the problems of carcinogenicity, human safety is not as good as fatty acid, improving and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of 7-ethyl-10-cis-4,7,10,13,16,19-docohexenoyloxycamptothecin
[0050] 1mol 7-ethyl-10-hydroxycamptothecin, 1.2mol cis-4,7,10,13,16,19-docosahexaenoic acid and 1.2mol p-nitrobenzoyl chloride were dissolved in 2L chloroform, After 24 hours of reaction, the solvent was spin-dried, and the obtained drug powder was rinsed twice with ether, dried, dissolved in chloroform, and recrystallized to obtain the pure target product. Yield: 82%.
[0051] 1 HNMR (CDCl 3 , 400MHz, ppm) δ: 8.73 (1H, d, J = 9.14Hz), 8.22 (1H, d, J = 2.46Hz), 8.15 (1H, s), 7.75 (1H, dd, J = 9.14and2.46Hz ), 5.41-5.38 (10H, m), 5.10-5.05 (2H, m), 4.76-4.64 (2H, m), 4.25-4.20 (2H, m), 2.73-2.48 (18H, m), 1.27-1.14 (6H, m), 1.02 (3H, t), 0.87 (3H, t)
[0052] MS (ESI + )m / z:703(M+H + , 100%)
Embodiment 2
[0053] Example 2: Preparation of 7-ethyl-10-cis-4,7,10,13,16,19-docohexenoyloxycamptothecin
[0054] 1mol 7-ethyl-10-hydroxycamptothecin, 1.1mol cis-4,7,10,13,16,19-docosahexaenoic acid and 1.5mol N,N'-dicyclohexylcarbodiimide were dissolved In 2L of chloroform, add a small amount of chloroform to dissolve 2.1mol N, N-diisopropylethylamine, and then add it to the dissolved drug solution using a constant pressure titration funnel. Rinse twice with ether, dry, dissolve in chloroform, and recrystallize to obtain the pure target product. Yield: 74%.
Embodiment 3
[0055] Example 3: Preparation of 7-ethyl-10-cis-4,7,10,13,16,19-docohexenoyloxycamptothecin
[0056] 1mol 7-ethyl-10-hydroxycamptothecin, 1.5mol cis-4,7,10,13,16,19-docosahexaenoic acid and 1.5mol 1-ethyl-(3-dimethylaminopropane Base) carbodiimide hydrochloride was dissolved in 2L dichloromethane, 2.1mol N-hydroxysuccinimide and 1.2mol triethylamine were dissolved in a small amount of dichloromethane and then added to the dissolved drug solution using a constant pressure titration funnel , reacted for 10 hours after sample addition, spin-dried the solvent, rinsed the obtained drug powder twice with ether, dried and dissolved in chloroform, and recrystallized to obtain the pure target product. Yield: 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com