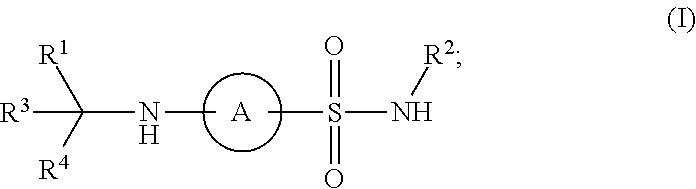
Patents
Literature
151 results about "Phenylsulfonamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
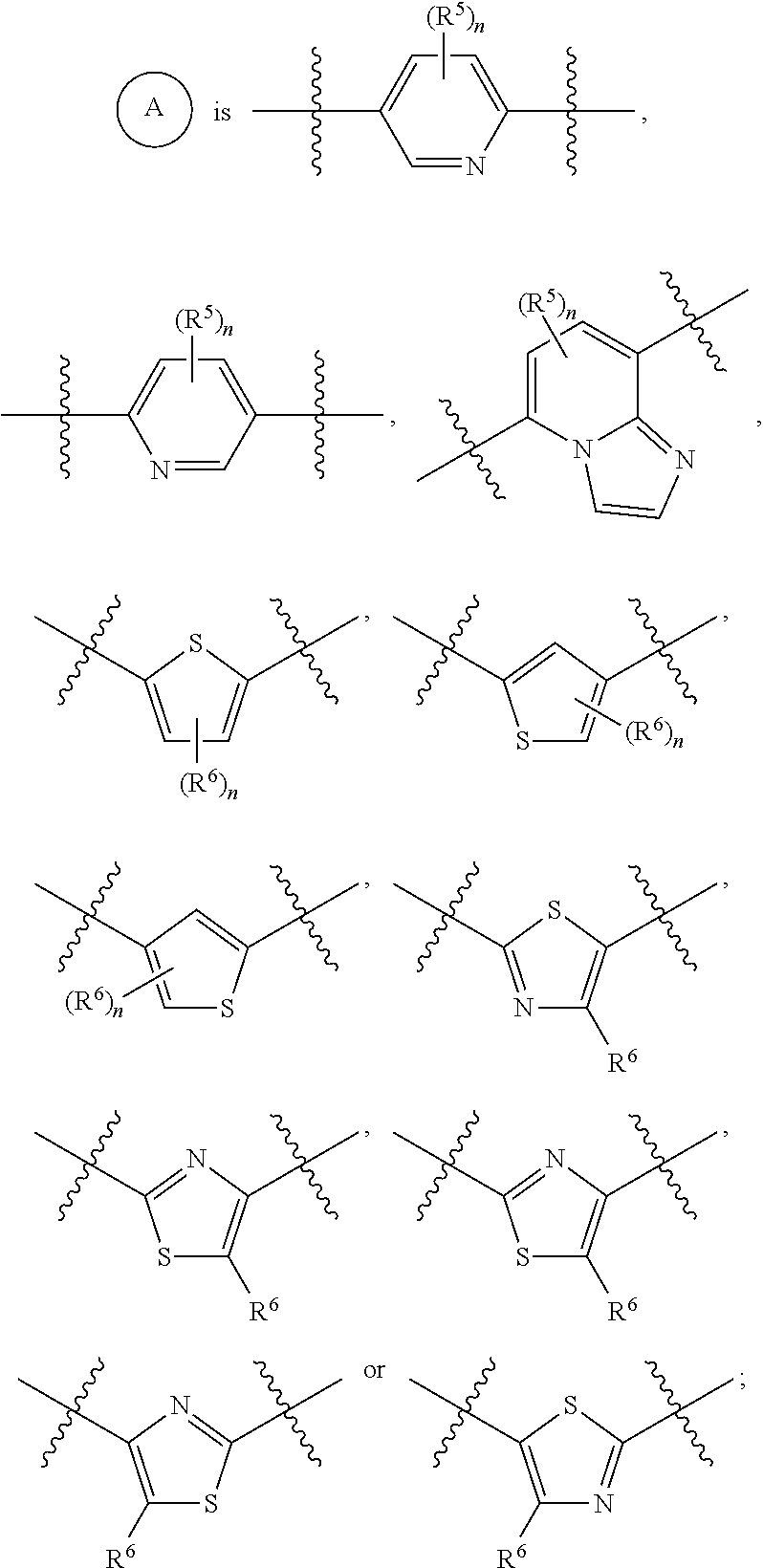
Arylsulfonamide compounds
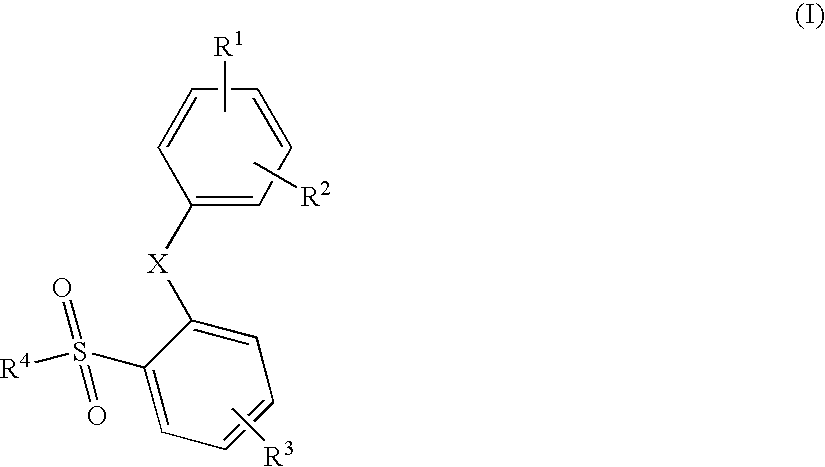
The invention relates generally to small molecules that mimic the biological activity of certain peptides and proteins, to compositions containing them and to their use. In particular, the invention relates to compounds of the general formula (I) that mimic the biological activity of BH3-only proteins and are capable of binding to and neutralizing pro-survival Bcl-2 proteins:wherein A1, A2, B1, B2, B3, X, Z, R1, R2, R3 and t are as described herein. The invention also relates to processes of preparing the benzenesulfonamide compounds that mimic portions of peptides and proteins, and to the use of such compounds in the regulation of cell death and the treatment and / or prophylaxis of diseases or conditions associated with the deregulation of cell death.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES +1
2-phenoxy- and 2-phenylsulfonamide derivatives with CCR3 antagonistic activity for the treatment of asthma and other inflammatory or immunological disorders
The present invention relates to a benzenesulfonamide derivative of formula (I), which is useful as an active ingredient of pharmaceutical preparations. The benzenesulfonamide derivatives of the present invention have CCR3 (CC type chemokine receptor) antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with CCR3 activity, in particular for the treatment of asthma, atopic dermatitis, allergic rhinitis and other inflammatory / immunological disorders. In said formula, X represents O or S; R4 represents formulae (a), (b), (c), (d), (e), (f), (g), (h), (i) or (j), the other substituents are as defined in claim 1.
Owner:AXIKIN PHARMA
2-Phenoxy- and 2-Phenylsulfonamide Derivatives with CCR3 Antagonistic Activity for the Treatment of Inflammatory or Immunological Disorders
Provided herein are 2-phenoxy- and 2-phenylsulfonamide derivatives with CCR3 antagonistic activity. These compounds are useful for the treatment of diseases associated with CCR3 activity, including but not limited to, atopic dermatitis, allergic rhinitis, rheumatoid arthritis, Grave's disease, HIV infection, Alzheimer's disease, atherosclerosis and other inflammatory and / or immunological disorders.
Owner:LI YINGFU +10
Celecoxib preparation process
InactiveCN102746231AMild reaction conditionsEasy to operateOrganic chemistryTrifluoromethylCyclooxygenase
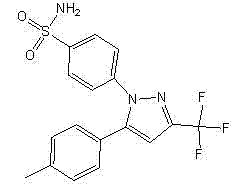
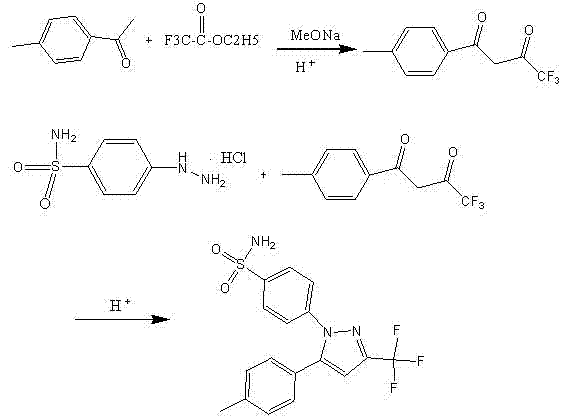
The invention provides a process for simply and efficiently preparing a selective cyclooxygenase-2 (COX-2) inhibitor 4-[3-trifluoromethyl-5-(4-methylphenyl)-1H-pyrazolyl]benzenesulfonamide (celecoxib, I). The process comprises the following steps: reacting an alkyl trifluoroacetate and 4-methylacetophenone which are initial raw materials under the action of an organic alkali solution, directly adding a dilute acid, an organic solvent and 4-aminosulfonylhydrazinobenzene or its acid salt to the resulting system, and heating to prepare the celecoxib. Above reaction adopts one kettle way, so the process has the advantages of mild condition, simple operation, high product yield and high product purity, and is suitable for industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of high-yield and high-purity celecoxib
The invention belongs to the technical field of preparation methods of drugs, and particularly relates to a preparation method of high-yield and high-purity celecoxib. The preparation method of the high-yield and high-purity celecoxib comprises the following steps: preparing a salt solution of 1-p-methylphenyl-4,4,4-trifluoro-1,3-butanedione, preparing an alcohol water solution of hydrazinobenzene-1-sulfonamide hydrochloride, conducting reaction, drying, and refining. The preparation method has the benefits that compared with the traditional method, with the adoption of the preparation method of the celecoxib, the yield and purity are increased; the yield of the celecoxib reaches above 91%; the purity reaches above 99%; and the method is simple in technology, and can be applied to large-scale production easily.
Owner:山东诚汇双达药业有限公司
Benzenesulfonamide Compounds and the Use Thereof
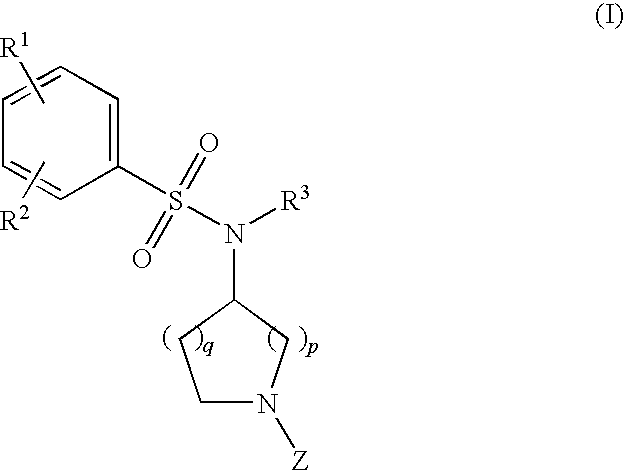
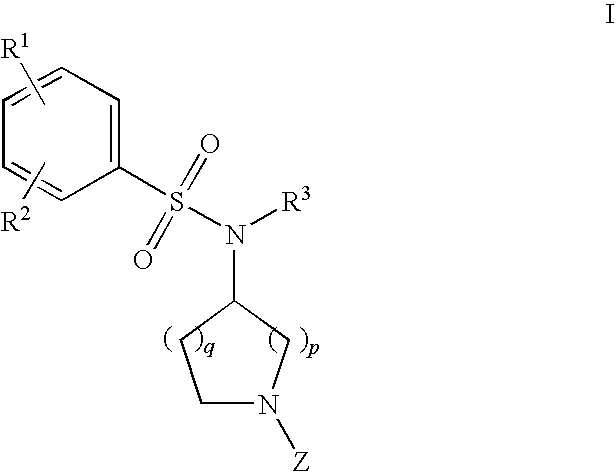
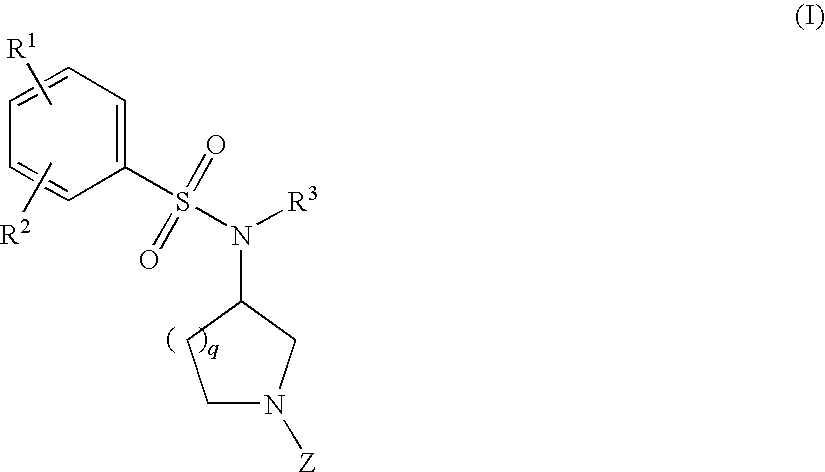
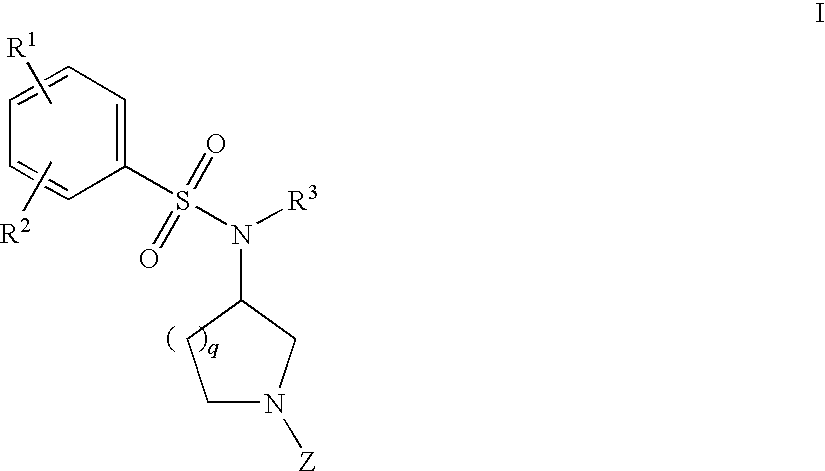
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula (I): and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3, Z, p and q are defined as set forth in the specification. The invention is also directed to the use compounds of Formula (I) to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
Benzenesulfonamide Compounds and Their Use
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula I and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3 and Z are defined as set forth in the specification. The invention is also directed to the use compounds of Formula I to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
N-4-phenylsulfonamido--N'-1-desoxy-(2-desoxy-2-substituted-amino)-beta-D-glucopyransoylthiocarbamide compounds and application thereof
ActiveCN106279303AInhibition of catalytic activityExert anti-tumor effectOrganic active ingredientsSugar derivativesAnhydrase activityThiourea
The invention discloses novel N-4-phenylsulfonamido--N'-1-desoxy-(2-desoxy-2-substituted-amino)-beta-D-glucopyransoylthiocarbamide compounds with the general formula disclosed in the specification, which have the activity of inhibiting carbonic anhydrase and further achieve the effect of resisting tumor metastasis and invasion. The compounds structurally have the three active segments sulfamine, substituted glucosamine and thiocarbamide, and the three active segments and Zn<2+> ions in the carbonic anhydrase form coordinate bonds, thereby inhibiting the catalytic activity of the enzyme and performing the function of resisting tumor metastasis and invasion. Thus, the compounds have application potential in the aspect of antitumor drugs. In the general formula, R is defined in the specification.
Owner:SHENYANG PHARMA UNIVERSITY
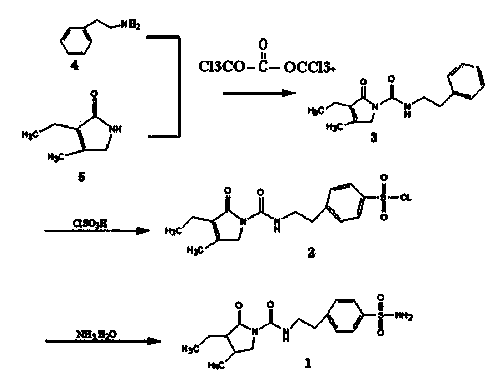
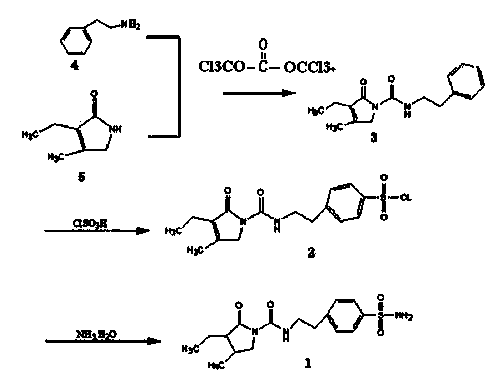
Triphosgene method for synthesizing benzene sulphanilamide, intermediate of glimepiride, drug for Type ii Diabetes Mellitus
The invention discloses a triphosgene method for synthesizing benzene sulphanilamide, the intermediate of glimepiride, a drug for Type ii Diabetes Mellitus. Triphosgene is slowly dropped into a solution which contains phenethylamine and 3-ethyl-4-methyl pyrroline ketone, at a controlled temperature, so that N-[2-(3-ethyl-4-methyl-2-oxidation-3-pyrroline-1-formylamino)ethyl]-benzene (3) is obtained; the product reacts with chlorosulfonic acid to generate sulfonated product; the sulfonated product reacts with ammonia water to generate crude product benzenesulfonamide; the crude product is refined through solvent treatment to generate fine product; the reaction formula is show in the specification. The triphosgene method is simple in technology, simple in operation and high in yield; compared with the conventional method of phosgene, the solid phosgene (triphosgene) method is safer and is convenient to operate.
Owner:姜树林
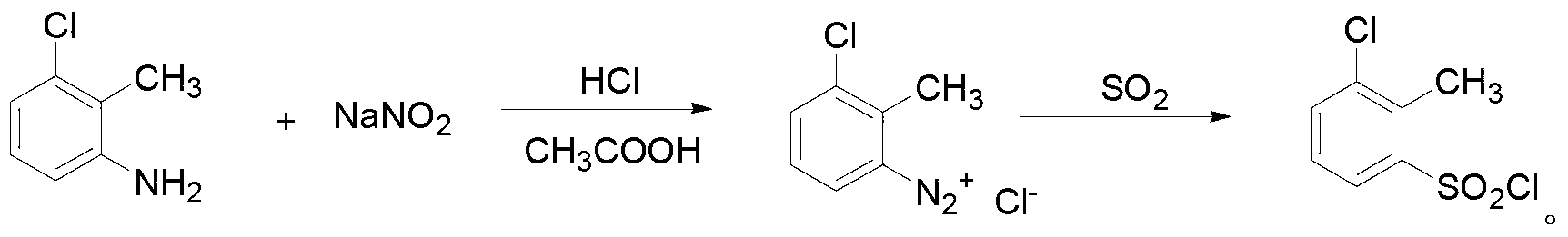
Triazolopyrimidine sulfonamide compound, and synthetic method and application thereof
ActiveCN103319489ASmall molecular weightThe synthesis method is simpleBiocideOrganic chemistrySulfonyl chlorideChemical compound
The invention provides a triazolopyrimidine sulfonamide compound and synthetic methods and application thereof, relating to a penoxsulam analog and a synthetic method and application thereof. The objective of the invention is to overcome the problems of great synthesis difficulty and high cost of conventional penoxsulam. The triazolopyrimidine sulfonamide compound has a structural formula described in the specification. The method 1 comprises the following steps: 1, preparing 2-methyl-3-chlorobenzenesulfonyl chloride; and 2 preparing a finished product so as to obtain 3-chloro-2-methyl-N-(5,8-dimethoxy-[1,2,4] triazolo[1,5-c]pyrimidine-2-yl) benzsulfamide. The method 2 comprises the following steps: 1, preparing 2-mercapto-3-trifluoromethylpyridine; and 2, preparing 3-trifluoromethylpyridine-2-sulfonyl chloride; and 3, preparing a finished product so as to obtain 3-trifluoro-methyl-N-(5,8-dimethoxy-[1,2,4] triazolo[1,5-c]pyrimidine-2-yl) pyridine sulfamide. The triazolopyrimidine sulfonamide compound is used as a herbicide in a rice field.
Owner:HEILONGJIANG UNIV
Synthesis method of tamsulosin hydrochloride
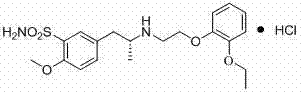
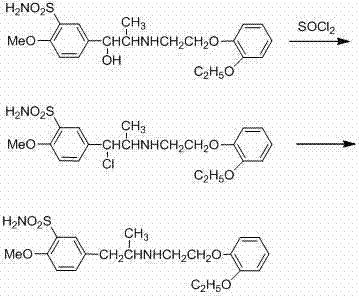
ActiveCN103497126AEasy to synthesizeHigh puritySulfonic acid amide preparationChemical synthesisPhenylsulfonamide
The invention belongs to the technical field of chemical synthesis, and specifically relates to a synthesis method of tamsulosin hydrochloride. According to the synthesis method, benzene sulfonic amide shown as a formula (II) and bromine ether shown as a formula (III) are in a condensation reaction in an aprotic polar solvent in the presence of an acid-binding agent to generate a condensation compound intermediate shown as a formula (IV); the condensation compound intermediate is in organic solvent, in the presence of a catalyst, hydrogen is introduced into the organic solvent under certain pressure so as to hydrogenate the condensation compound intermediate, then, the R-tamsulosin free alkali shown as a formula (V) is obtained, and the R-tamsulosin free alkali further is subjected to a salt formation reaction with hydrochloric acid in an organic solvent C to produce the tamsulosin hydrochloride shown as a formula (I). In the reaction process for preparing the tamsulosin hydrochloride through the synthetic route provided by the invention, the phenomenon that bimolecular bromide and amine react with each other to generate a disubstituted by-product is avoided, and the obtained tamsulosin hydrochloride has high product purity and high yield; according to the synthesis method, the reaction conditions are moderate, and synthesis is convenient to finish.
Owner:天台宜生生化科技有限公司
Method for preparing asymmetric disulfide
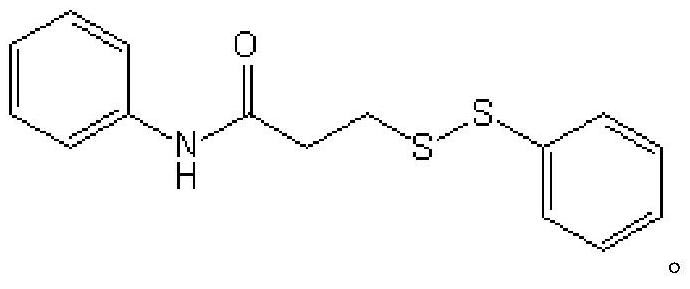
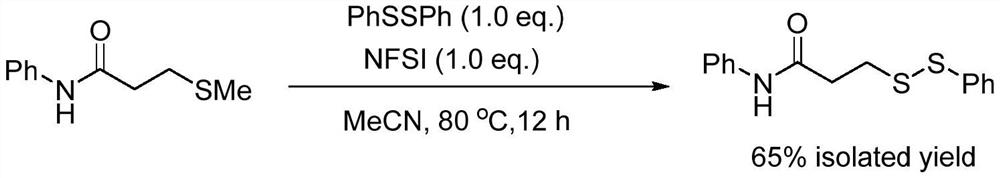
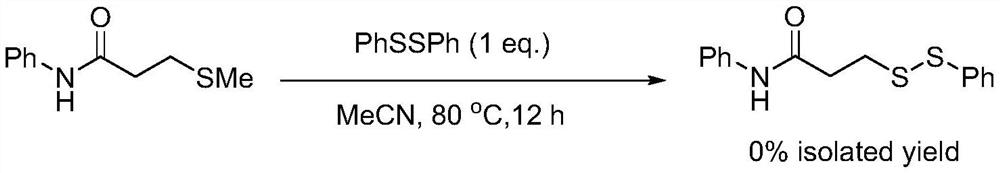
ActiveCN111777536AEfficient synthesisSimple and fast operationHydropoly/poly sulfide preparationPhenylsulfonamidePtru catalyst
The invention relates to the technical field of fine chemical engineering, and discloses a method for preparing asymmetric disulfide. The preparation method comprises the following specific steps: taking 3-methylthio-N-phenylpropionamide and diphenyl disulfide as raw materials, taking N-fluorobisbenzenesulfonamide as an additive, and preferably carrying out a reaction in an acetonitrile solvent under a heating condition so as to obtain a target product, namely the symmetric disulfide N-phenyl-3-(phenyldithioalkyl)propionamide. The method is simple and convenient to operate and mild in reaction, 3-methylthio-N-phenylpropionamide and symmetric diphenyl disulfide are used as reaction raw materials, the asymmetric disulfide product is efficiently synthesized in one step, the use of a thiol rawmaterial with unpleasant smell or a transition metal catalyst is avoided, and the method has a potential application value.
Owner:CHANGZHOU UNIV
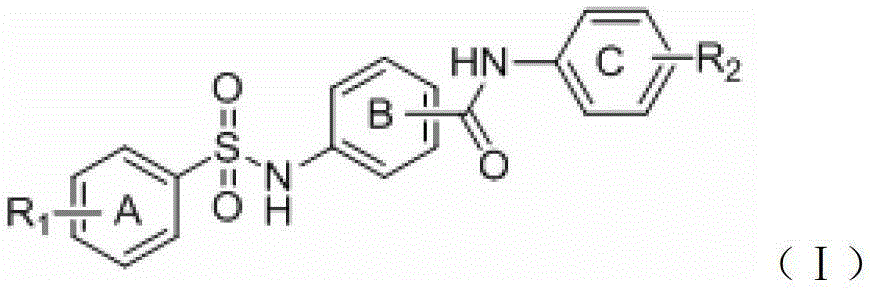
A group of benzene sulfonamido benzamide derivatives, and preparation and application thereof
InactiveCN103183623AEnhanced inhibitory effectAvoid sedimentationMetabolism disorderDigestive systemDiseaseApical sodium-dependent bile acid transporter
The invention relates to a group of benzene sulfonamido benzamide derivatives, and a preparation method and an application thereof. The research results indicate that the compounds obviously inhibit the apical sodium-dependent bile acid transporter (ASBT) at the cellular level, thereby being expected to be researched and developed to become a clinically effective medicament for treating hyperlipemia and cholestatic diseases.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
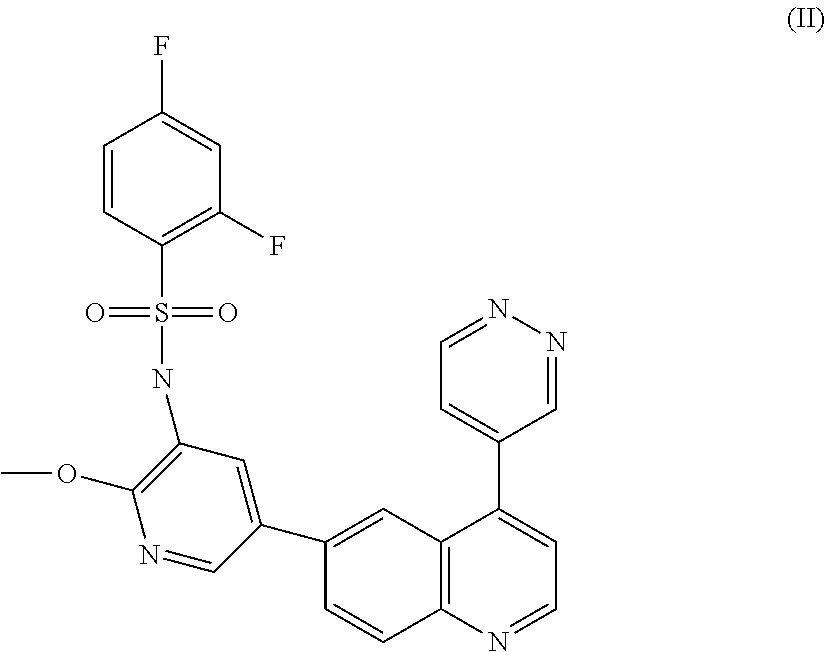
Combination
The present invention relates to a method of treating cancer in a mammal and to pharmaceutical combinations useful in such treatment. In particular, the method relates to a novel combination comprising the MEK inhibitor: N-{3-[3-cyclopropyl-5-(2-fluoro-4-iodo-phenylamino)6,8-dimethy; -2,4,7-trioxo-3,4,6,7-tetrahydro-2H-pyrido[4,3-d]pyrimidin-1-yl]phenyl}acetamide, or a pharmaceutically acceptable salt or solvate thereof, and the PI3 kinase inhibitor: 2,4-difluoro-N-{2-(methyloxy)-5-[4-(4-pyridazinyl)-6-quinolinyl]-3-pyridinyl}benzenesulfonamide, or a pharmaceutically acceptable salt thereof, pharmaceutical compositions comprising the same, and methods of using such combinations in the treatment of cancer.
Owner:NOVARTIS AG
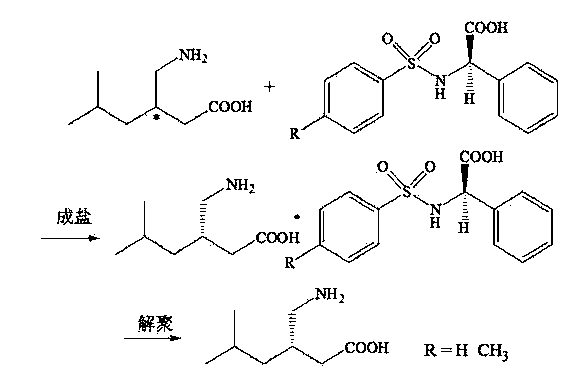
Chemical resolution preparation method of S-configuration pregabalin
InactiveCN103626668AOrganic compound preparationAmino-carboxyl compound preparationGlycineDepolymerization
The invention provides a method for preparing S-configuration pregabalin by resolving a pregabalin racemate. The method comprises the following steps: by taking toluenesulfonamide-D-phenylglycine or benzenesulfonamido-D-phenylglycine as a resolving agent, resolving in a water-borne alcoholic solution; and fully washing by using a resolving solvent, thus obtaining a compound of S-pregabalin and toluenesulfonamide-D-phenylglycine or benzenesulfonamido-D-phenylglycine; performing depolymerization in an aqueous solution or an alcoholic solution, thus obtaining the S-configuration pregabalin. The technical method provided by the invention is simple to operate and high in resolution efficiency, hardly causes environmental pollution and is suitable for industrial production, and the resolving agent and the resolving solvent are easy to recycle.
Owner:河北爱弗特精细化工有限责任公司
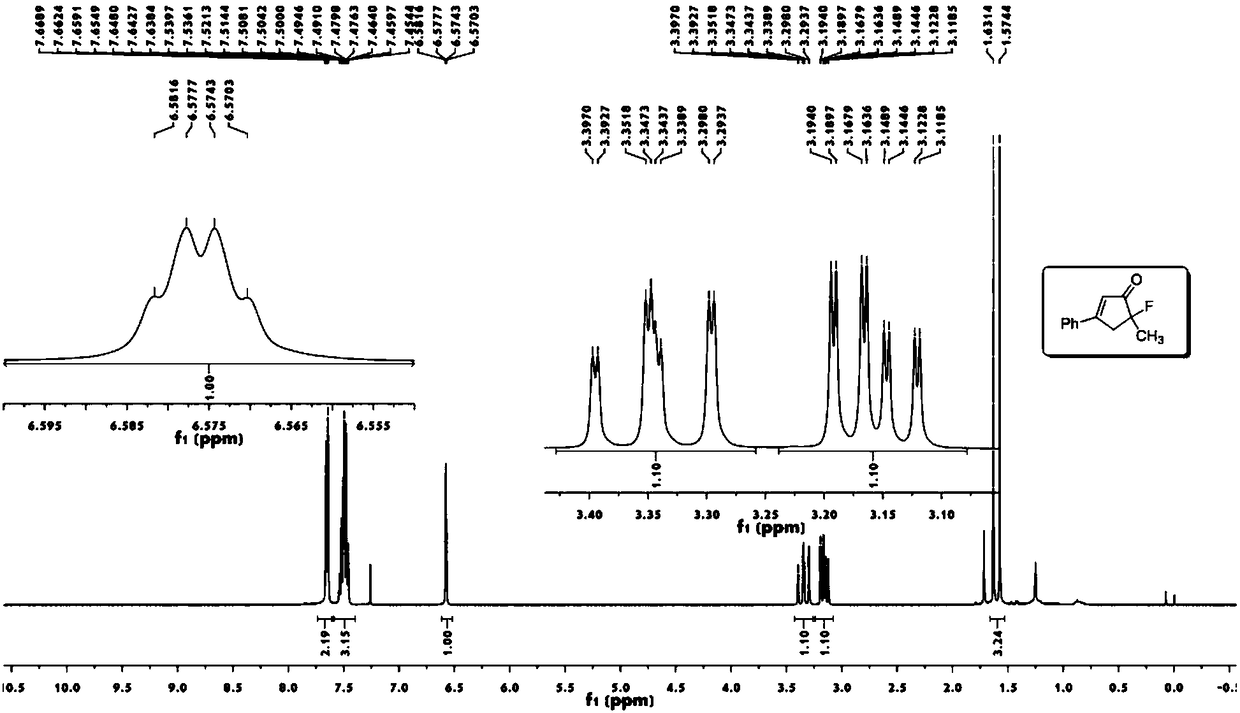
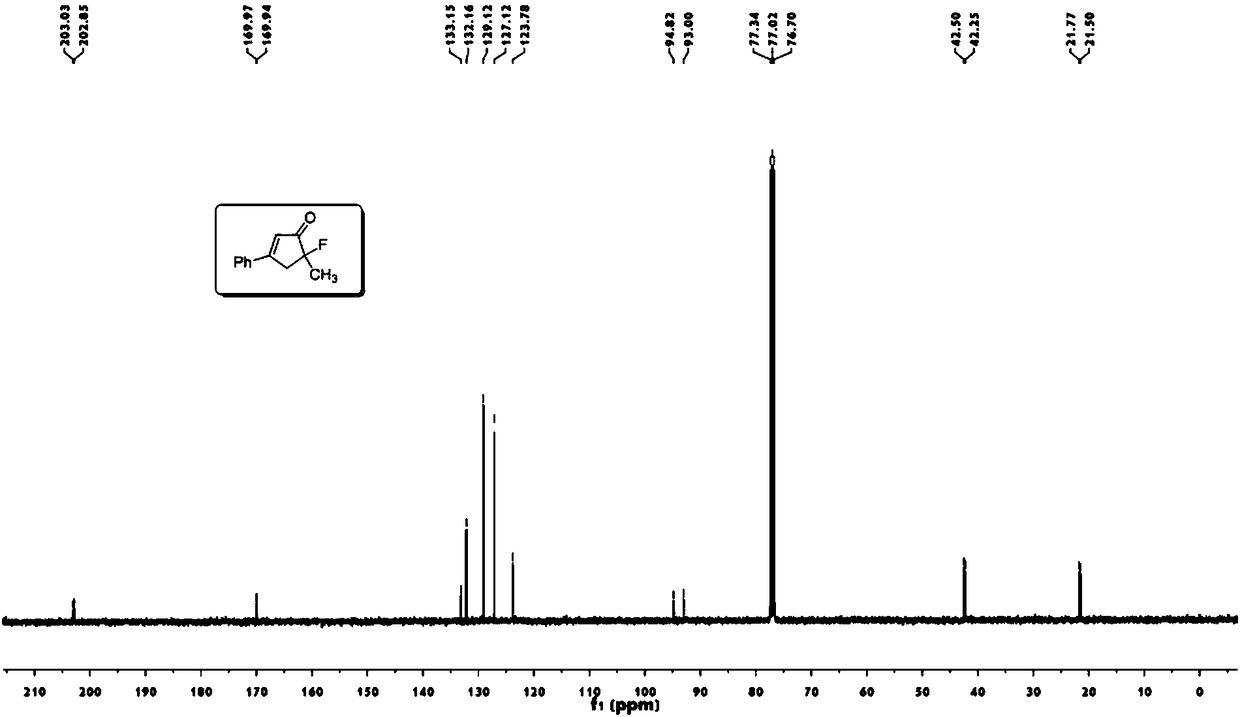
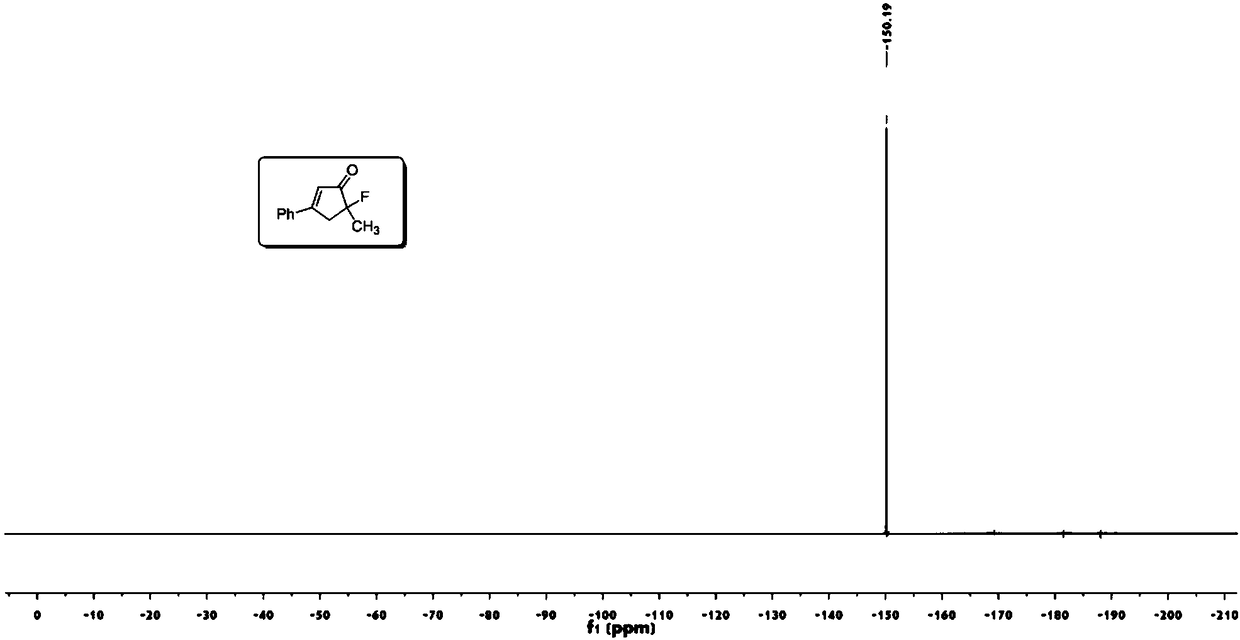
Novel fluorocyclopentenone preparation method and product thereof
InactiveCN108276260AMild conditionsReduce energy consumptionOrganic compound preparationCarboxylic acid esters preparationNatural productAcetonitrile
The invention discloses a novel fluorocyclopentenone preparation method and a product thereof. The novel fluorocyclopentenone preparation method is characterized by comprising the following step: methyl tert-butyl ether, enynic ester, gold (acetonitrile)[(2-biphenyl)di-tert-butylphosphine] hexafluoroantimonate (I) and N-fluorobenzenesulfonimide are added to react, so that fluorocyclopentenone is obtained. The novel method for preparing a fluorocyclopentenone compound which is provided by the invention ensures that an enynic ester compound can be converted into the fluorocyclopentenone compound. The whole reaction is carried out under normal temperature and normal pressure, conditions are mild, and energy consumption is low. The whole reaction is carried out by utilizing a one-pot method, operation is easy, yield is high, and the purity of the product is 98 percent or more. The reaction substrate range is wide, and not only the simple enynic ester compound but also complex compounds containing natural product groups are applicable. The developed fluorocyclopentenone compound has potential bioactivity, and can become a drug by subsequent testing or modification.
Owner:NANJING FORESTRY UNIV
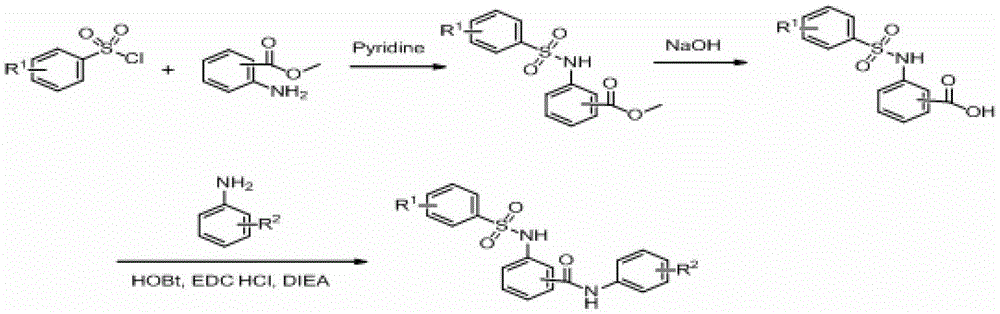
Method for synthesizing benzene sulfonamide compounds
InactiveCN103819369AEfficient responseMild responseOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acid amide preparationBenzeneMethyl tertiary butyl ether
The invention relates to a method for preparing benzene sulfonamide compounds. In the method, a ternary catalyzing system of ethyltriphenylphosphonium bromide-silver compounds-porphyrin is adopted; the method for preparing N-tert-Butylbenzenesulfenamide from the reaction of methyl tertiary butyl ether with a weak reactivity and benzene sulfonamide compounds is realized; remarkably technical effects of preferable reaction temperature, high yield and good universality are achieved; moreover, as appropriate additives are added in the reaction, the collision between molecules is promoted and the reaction time is shortened; the method has favorable industrialization perspective and industrialized production value.
Owner:甘肃皓骏药业有限公司
N-gallic amide-pyrimidine phenyl sulfonamide derivative as well as preparation method and application thereof
ActiveCN104193686AAntibacterial agentsOrganic active ingredientsPhenylsulfonamideCell-Extracellular Matrix
The invention relates to an N-gallic amide-pyrimidine phenyl sulfonamide compound as well as a preparation method and an application of the compound in preparation of medicines for resisting bacteria and promoting the growth of cartilage cells. The N-gallic amide-pyrimidine phenyl sulfonamide compound and a water soluble salt thereof have certain bacteriostatic action on staphylococcus aureus, escherichia coli, pseudomonas aeruginosa, acinetobacter, proteusbacillus vulgaris, pulmonary tuberculosis and the like in vitro, and have an obvious promotion effect on proliferation of bone cells, and synthesis of total protein and glycosaminoglycan in chondrocyte, the gene levels of proteoglycan, II collagen and SOX9 gene can also be regulated upwards, the gene level of I collagen can be inhibited, secretion and synthesis of extracellular matrix of the cartilage can be promoted, and the compound can be applied to preparation of the medicines for treating osteoarthritis.
Owner:湖南沛草堂中医药科技有限公司
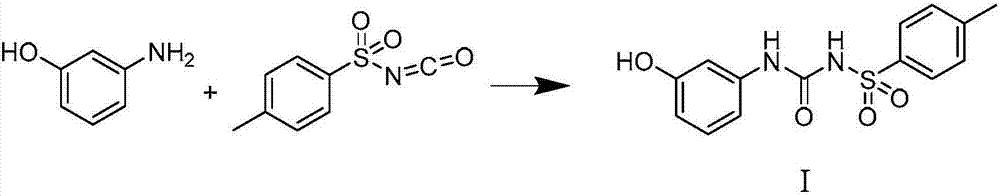
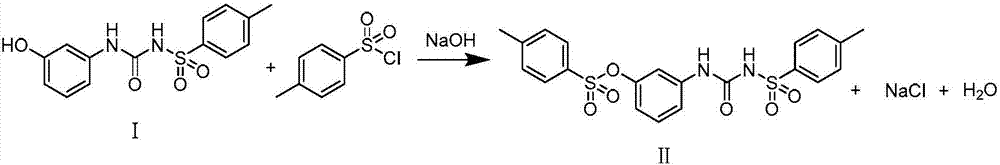
Synthesis method of thermosensitive sensitizer N-p-tolylsulfonyl-N'-(3-p-tolylsulfonyl oxyphenyl) urea
ActiveCN107311893AMild reaction conditionsSimple and efficient operationSulfonic acid amide preparationUreaToluene
The invention provides a synthesis method of a thermosensitive sensitizer N-p-tolylsulfonyl-N'-(3-p-tolylsulfonyl oxyphenyl) urea. The m-aminophenol and the p-toluenesulfonyl isocyanate firstly react to generate N-[(3-hydroxy phenyl) formamyl]-4-toluenesulfonamide, the paratoluensulfonyl chloride is directly reacted with the above mixture without processing the reaction so as to obtain the N-p-tolylsulfonyl-N'-(3-p-tolylsulfonyl oxyphenyl) urea, and the product yield is up to more than 98%.
Owner:WEIFANG DAYOU BIOLOGICAL CHEM CO LTD
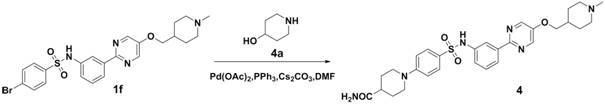
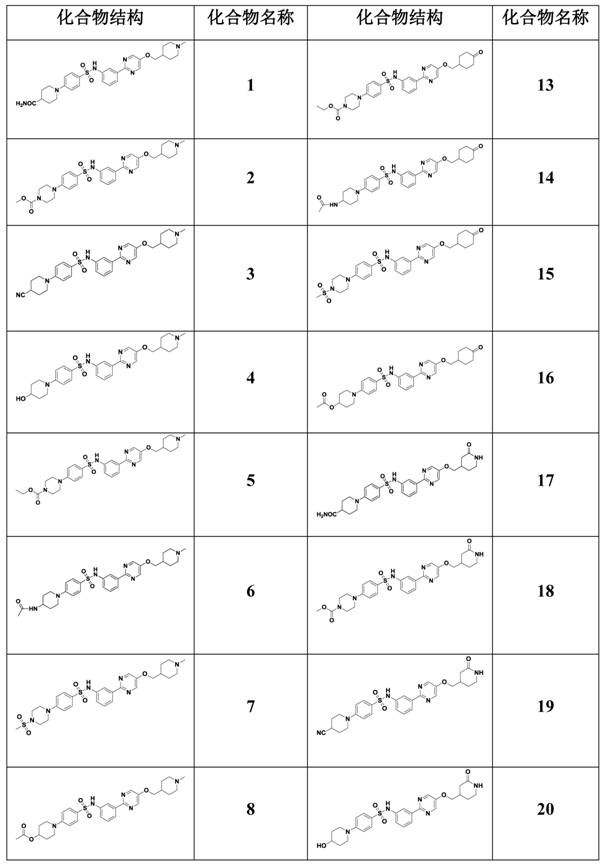
N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative, and pharmaceutical composition, preparation method and application thereof
ActiveCN111732575AGood antitumor pharmacological activityOrganic active ingredientsOrganic chemistryPhenylsulfonamidePharmaceutical medicine
The invention belongs to the technical field of biological medicine, and provides an N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative, and a pharmaceutical composition, a preparation methodand application thereof. The N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative is a compound with a structure of formula I, or a stereoisomer or pharmaceutically acceptable salt and isotope compound thereof. The structure of the formula I is shown in the specification; and in the formula I, R1 and R2 can form a substituted or unsubstituted piperidine or piperazine ring, and R3 representsa (1-methylpiperidine-4-yl)methyl group, a (2-oxopiperidine-4-yl)methyl group or a (4-oxocyclohexyl)methyl group. The N-(3-(pyrimidine-2-yl) phenyl) benzenesulfonamide derivative can be used as an effective c-Met inhibitor, and meanwhile, the pharmaceutical composition of the N-(3-(pyrimidine-2-yl)phenyl)benzenesulfonamide derivative has good multiple anti-tumor pharmacological activities.
Owner:北京鑫开元医药科技有限公司
Indolin phenylsulfonamide derivatives
InactiveCN1678581AOrganic active ingredientsOrganic chemistryCoronary artery diseasePhenylsulfonamide
The invention relates to novel substituted indolin phenylsulfonamide derivatives, to a method for the production thereof and to the use thereof in medicaments, especially as potent PPAR-delta activating compounds for the prophylaxis and / or treatment of cardiovascular diseases, especially dyslipidaemia and coronary heart diseases.
Owner:BAYER HEALTHCARE AG
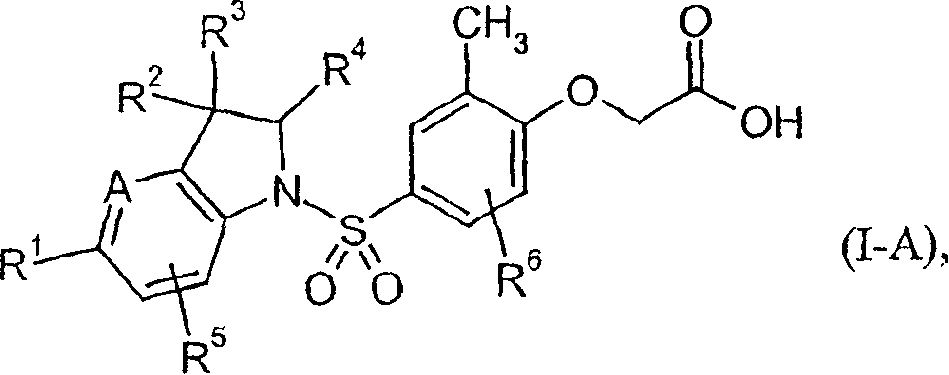
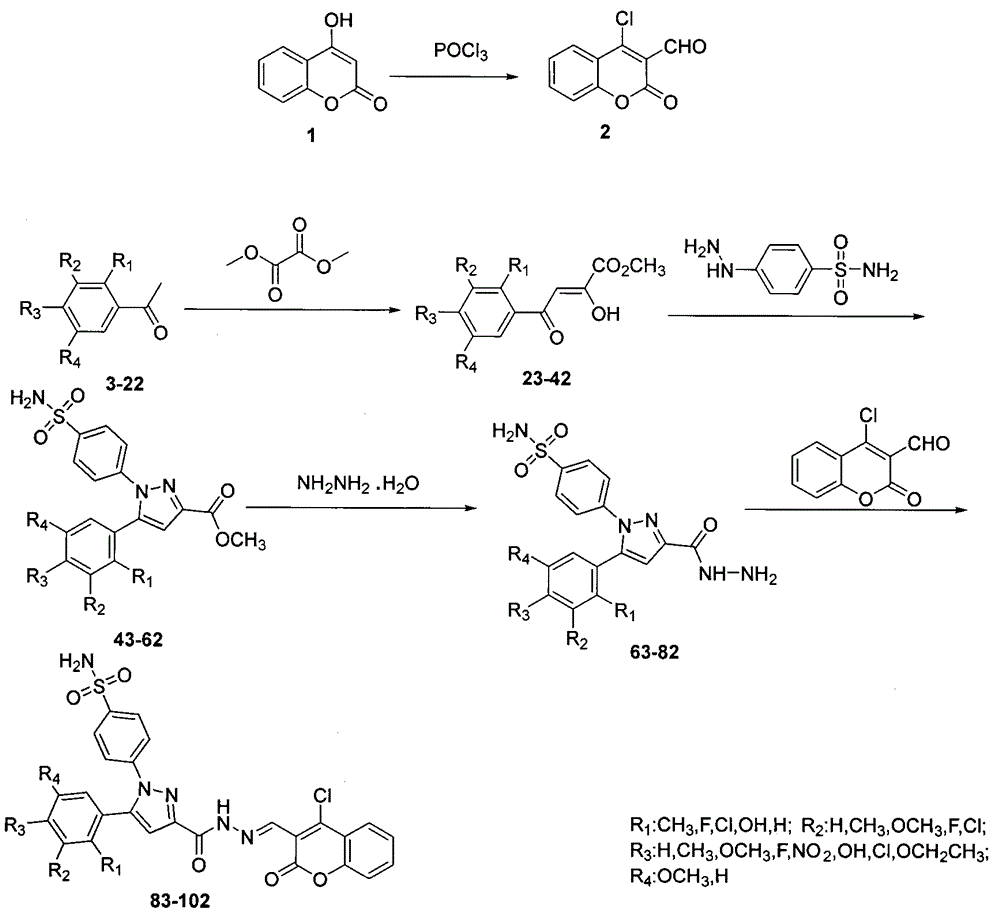
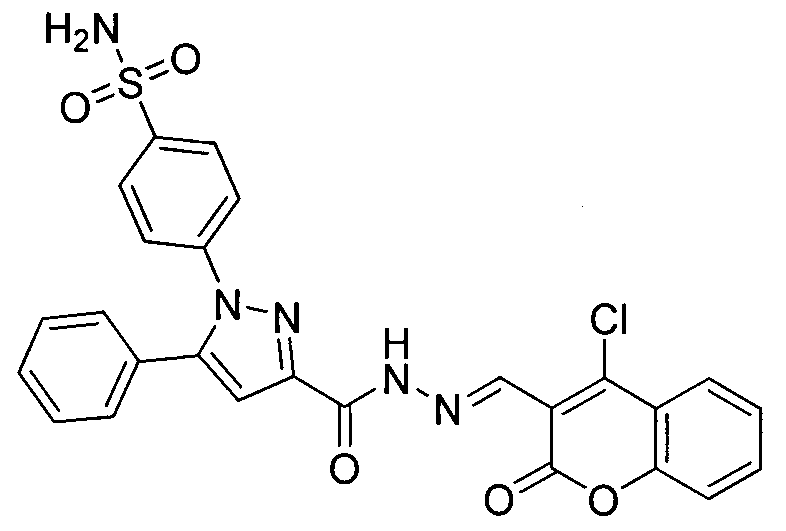
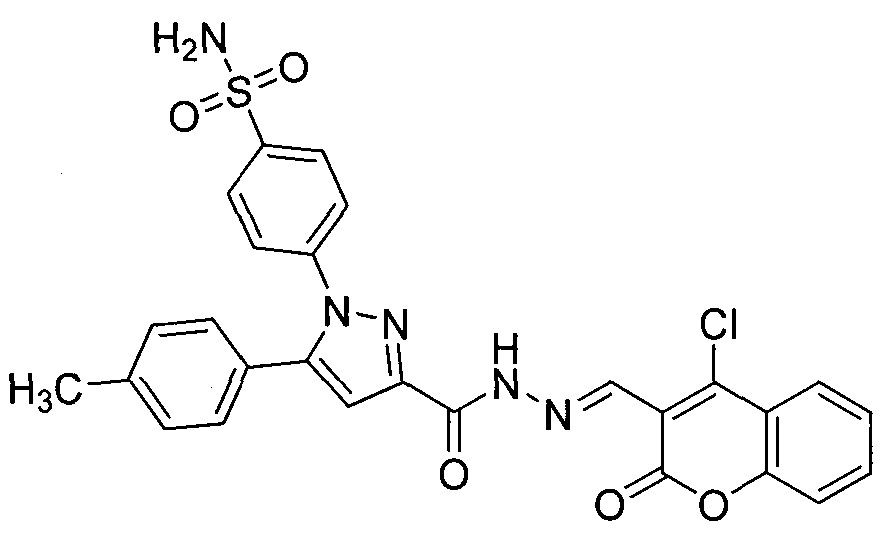
Preparing method for 4-(3-(3-(4-clocoumarol)-acylhydrazone)-5-phenyl-pyrazol) benzene sulfonamide derivate and application to anti-cancer drugs
The invention provides synthesis for a 4-(3-(3-(4-clocoumarol)-acylhydrazone)-5-phenyl-pyrazol) benzene sulfonamide derivate. The synthesis is characterized by being implemented according to the general formula (please see the formula in specification). The 4-(3-(3-(4-clocoumarol)-acylhydrazone)-5-phenyl-pyrazol) benzene sulfonamide derivate has the obvious inhibiting effect on culture of cervical cancer cells (Hela), human melanoma cells (F10) and hepatoma carcinoma cells (HepG2) and has cytotoxicity equivalent to or better than that of positive control drugs for human kidney epithelial cells (293T). Accordingly, the 4-(3-(3-(4-clocoumarol)-acylhydrazone)-5-phenyl-pyrazol) benzene sulfonamide derivate can be used for preparing anti-tumor drugs. The invention discloses a preparing method of the benzene sulfonamide derivate and anti-tumor biological activity.
Owner:NANJING UNIV
Preparation method of chiral cyclopropyl amino acid
InactiveCN107445894AHigh boiling pointNot volatileOrganic chemistryN dimethylformamideReaction temperature
The invention discloses a preparation method of chiral cyclopropyl amino acid. The preparation method comprises the following steps: mixing N-(2-formyl-4-methyl phenyl)-4-toluenesulfonamide with ethyl-2-benzoyl-1-chlorocyclopropane carboxylic acid with cesium carbonate in an N,N-dimethylformamide solvent, wherein the molar ratio of N-(2-formyl-4-methyl phenyl)-4-toluenesulfonamide to ethyl-2-benzoyl-1-chlorocyclopropane carboxylic acid to cesium carbonate is 1: 1: 1, the reaction temperature is room temperature and the reaction time is 1-2h; and after reaction, adding water and dichloromethane into a reaction liquid to extract, then spin-drying dichloromethane, purifying a product by means of column chromatography, and then spin-drying the product again to obtain cyclopropyl amino acid. The synthetic method is simple, short in reaction step, easy to operate, small in consumption of an organic solvent, simple in post-treatment, high in product purity, small in environmental protection pressure and convenient for industrial production.
Owner:WUHAN UNIV OF TECH
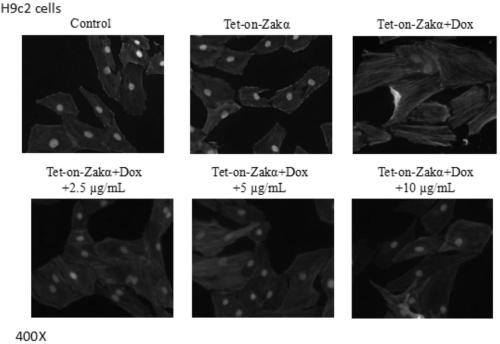
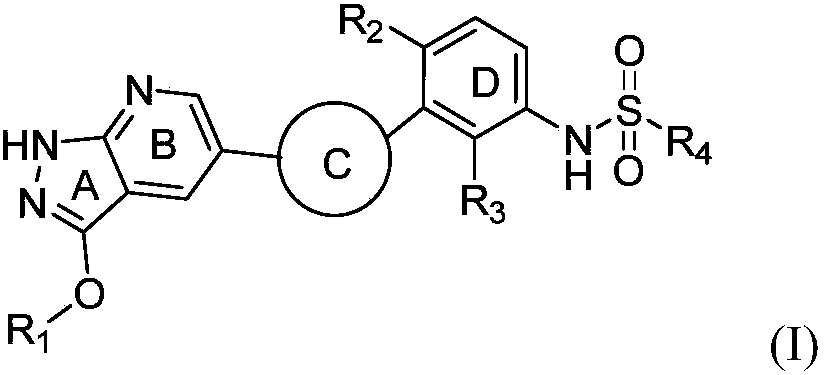
Heterocyclic benzene sulfonamide compound and application thereof
ActiveCN111393433AHigh selectivityImprove druggabilityOrganic active ingredientsOrganic chemistryPhenylsulfonamideAngor pectoris
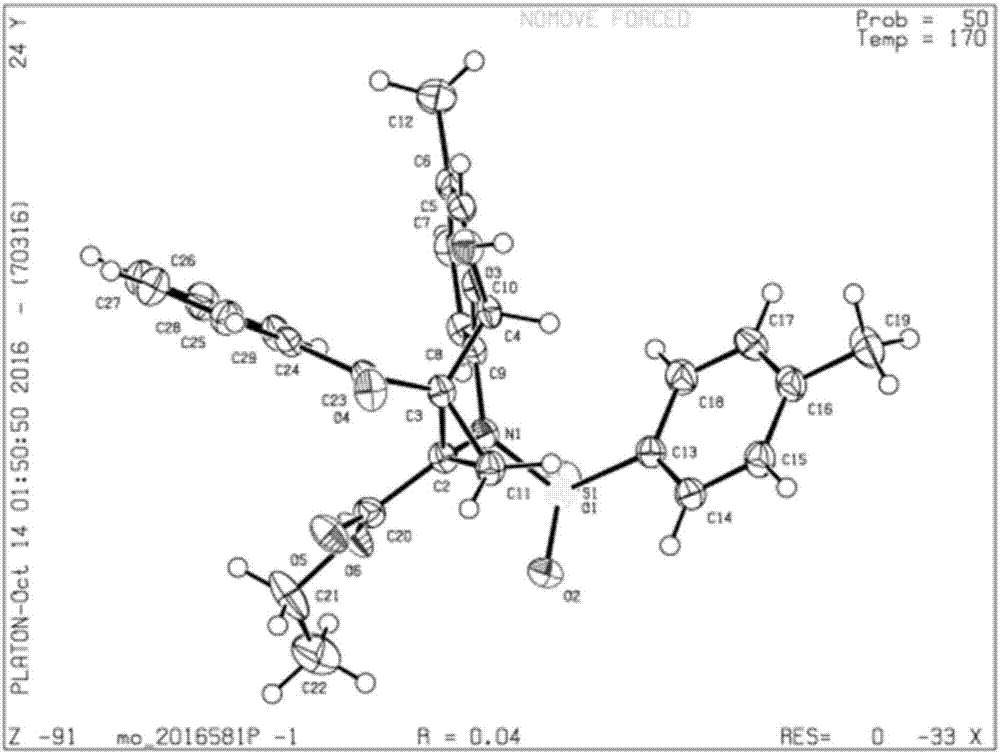
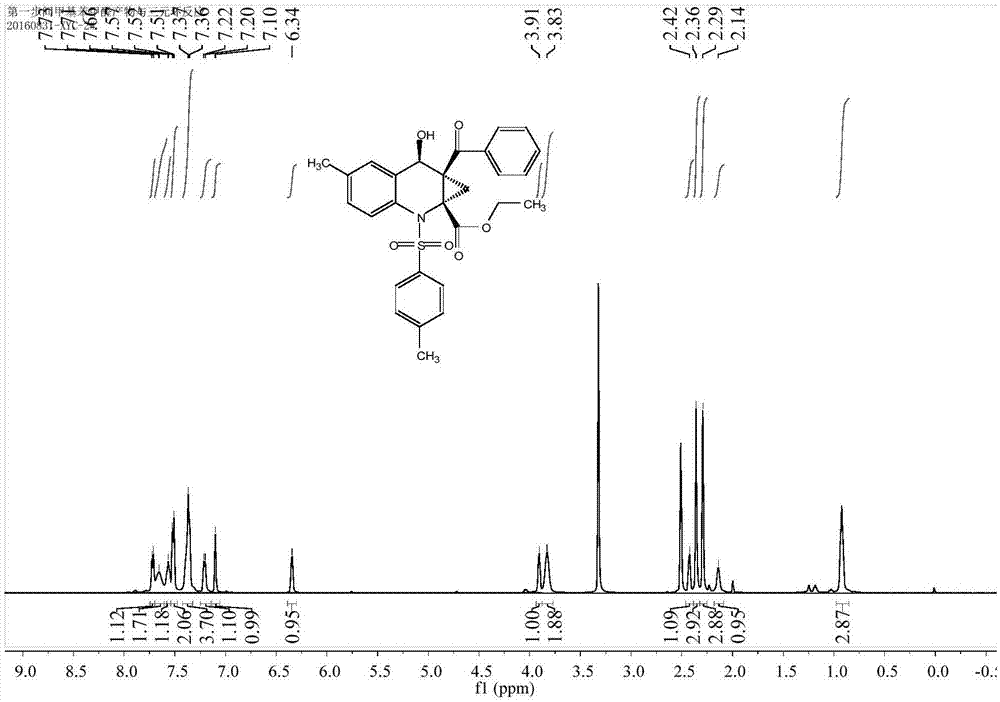
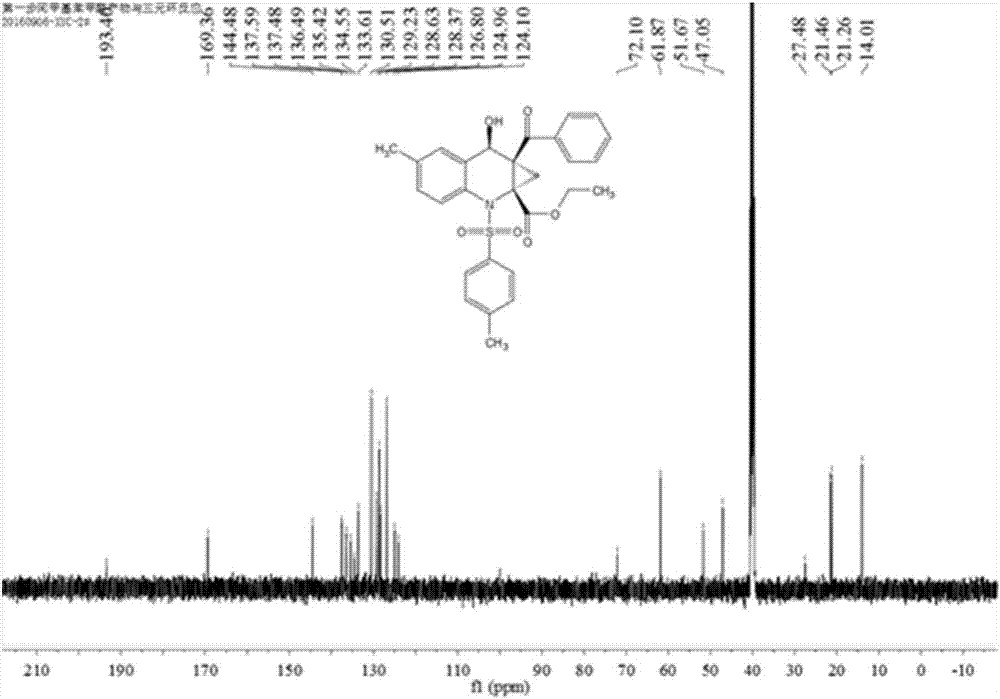
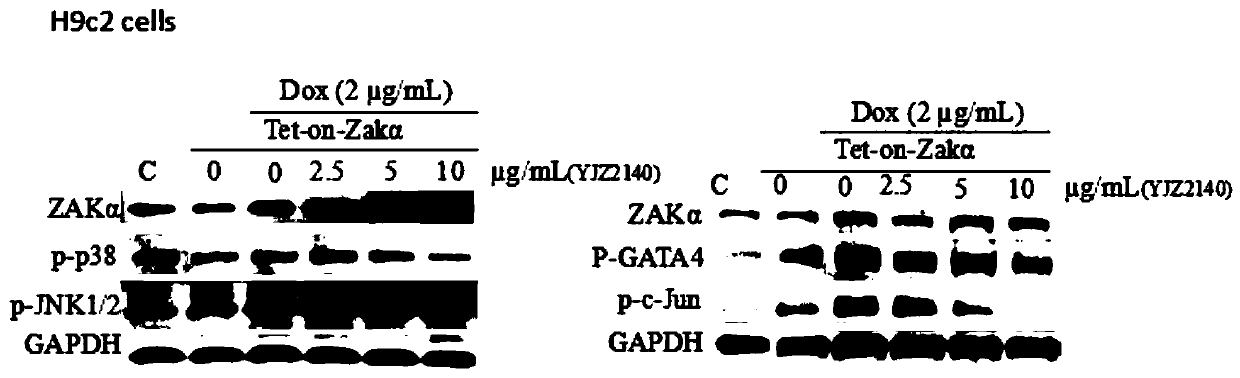
The invention relates to a heterocyclic benzene sulfonamide compound with a structure as shown in a formula (I) which is described in the specification and an application of the heterocyclic benzene sulfonamide compound in preparation of a ZAK inhibitor. The heterocyclic benzene sulfonamide compound can be used for effectively and highly selectively inhibiting ZAK protein kinase and further regulating the activation of downstream JNK / SAPK, p38, ERK and other pathways. The compound can be used for preparing medicines for preventing and treating various diseases related to ZAK kinase, such as myocardial hypertrophy, myocardial fibrosis, angina pectoris, coronary heart disease, heart failure, myocardial infarction and inflammation, and has the characteristics of better pharmacokinetics, low toxicity and higher druggability.
Owner:JINAN UNIVERSITY
Process for Preparing Stable Solids Formulations of Sulfonamides
InactiveUS20110190128A1Improve toleranceHigh effectivenessBiocideAnimal repellantsPhenylsulfonamideOrganic chemistry
The present invention relates to a process for preparing storage-stable solid herbicide formulations which comprise herbicidally active compounds from the group of the sulfonamides and their salts, in particular phenylsulfonamides, such as phenylsulfonylaminocarbonyltriazolinones or phenylsulfonylureas, heteroarylsulfonamides and other sulfonamides and their salts.
Owner:BAYER CROPSCIENCE AG
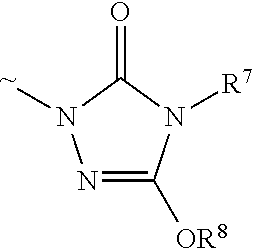
Method for preparing N-[6-chloro-5-(2-methoxyphenoxy)[2,2'-dipyridine]-4-yl]-4-tertiary butyl-benzsulfamide
InactiveCN102250020AInhibit side effectsHigh efficiency purificationOrganic chemistrySodium methoxidePhenylsulfonamide
The invention relates to a method for preparing a medical bosentan intermediate N-[6-chloro-5-(2-methoxyphenoxy)[2,2'-dipyridine]-4-yl]-4-tertiary butyl-benzsulfamide. The method provided by the invention comprises the following steps of: reacting methanol with sodium to obtain a sodium methoxide solution; reacting the sodium methoxide with p-tertiary butyl benzsulfamide to obtain p-tertiary butyl sodium benzsulfamide; dissolving the p-tertiary butyl sodium benzsulfamide and 4,6-dichloro-5-(2-methoxyphenoxy)-2,2'-dipyridine through utilizing dimethyl sulfoxide; raising the temperature for reaction to obtain a rough product; dissolving the rough product by using the methanol and crystallizing by using de-ionized water; after dissolving the rough product by using the methanol, crystallizing by using hydrochloric acid and then filtering. The method provided by the invention can be used for effectively stopping side reactions from happening and can also be used for more efficiently purifying and crystallizing the product; the method has the advantages of simplicity for operation, low manufacturing cost and small pollution and is suitable for the industrial production in a large scale; an advanced post-treatment method is adopted so that the purity reaches more than 99% and the yield reaches more than 93%; solvents utilized for the post-treatment include water and methanol, which are cheap, are easy to process and has little pollution.
Owner:山东致泰医药技术有限公司
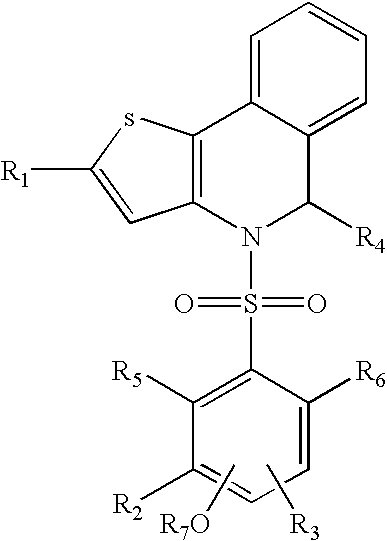
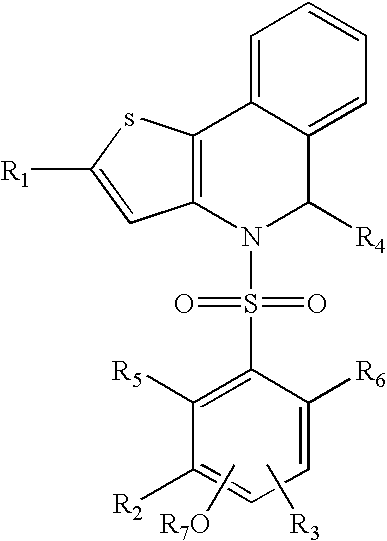
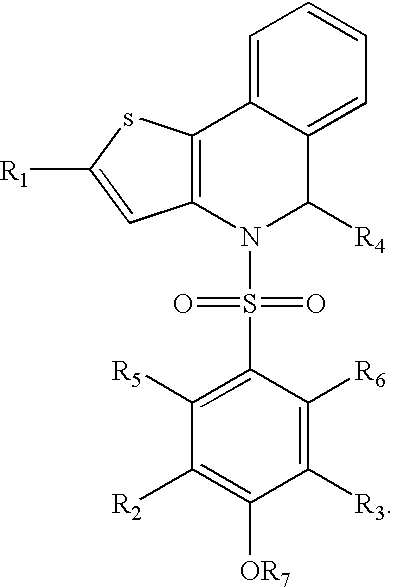
Polymorph of ALK tyrosine kinase inhibitor and preparation method thereof
Owner:SHOUYAO HLDG BEIJING CO LTD
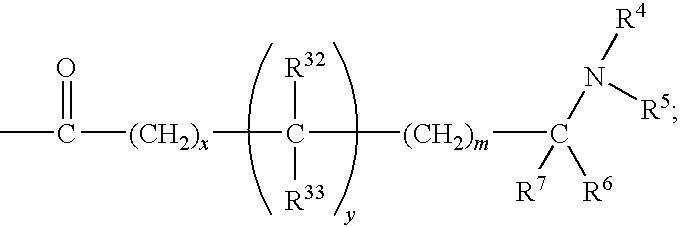
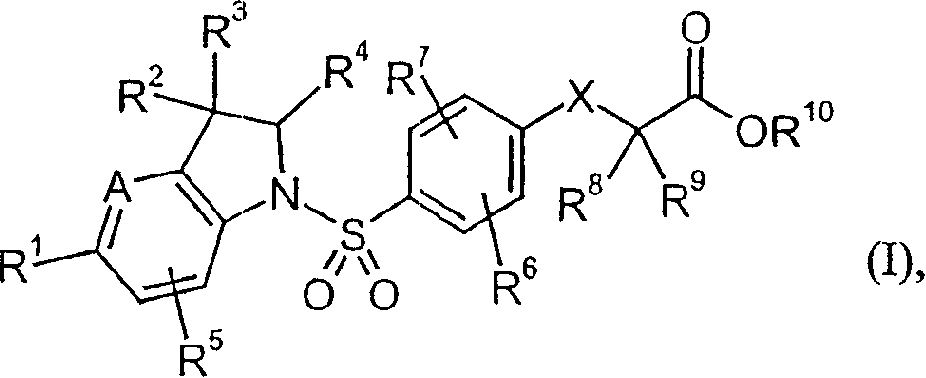
Heteroaryl-substituted sulfonamide compounds and their use as therapeutic agents
ActiveUS20200157089A1Eliminate side effectsUseful in treatmentOrganic active ingredientsNervous disorderDiseaseAryl
This invention is directed to benzenesulfonamide compounds, as stereoisomers, enantiomers, tautomers thereof or mixtures thereof; or pharmaceutically acceptable salts, solvates or prodrugs thereof, for the treatment of diseases or conditions associated with voltage-gated sodium channels, such as epilepsy and / or epileptic seizure disorders.
Owner:XENON PHARMACEUTICALS INC
Application of dihydrofolate synthetase and derivative thereof as well as preparation method and detection method of dihydrofolate synthetase
InactiveCN104212773AWide variety of sourcesGet fastLigasesVector-based foreign material introductionSulfur drugPhenylsulfonamide
The invention aims at providing an application of dihydrofolate synthetase in detection of sulphonamide residue and a rapid sulphonamide detection method constructed by utilizing the dihydrofolate synthetase. The dihydrofolate synthetase capable of detecting the sulphonamide residue is an amino acid sequence shown in a sequence SEQ ID NO.1. Compared with the traditional detection method, the dihydrofolate synthetase has the advantages that firstly the dihydrofolate synthetase is wide in source, protein fragments just containing a dihydrofolate synthetase core sequence can be applied, and the dihydrofolate synthetase can be rapidly and stably obtained by adopting a genetic engineering method; and secondly, a rapid detection method for all the sulphonamides containing a p-aminobenzene sulphonamide parent core structure can be constructed by utilizing the new application of the dihydrofolate synthetase.
Owner:NBGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
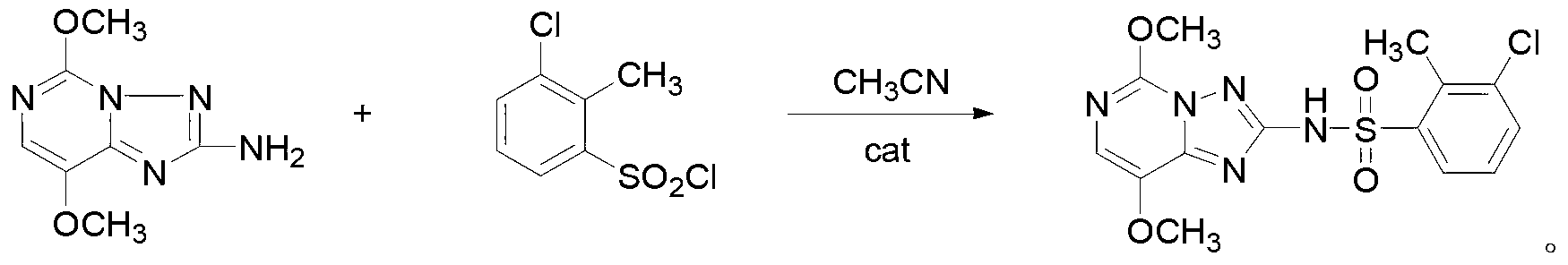
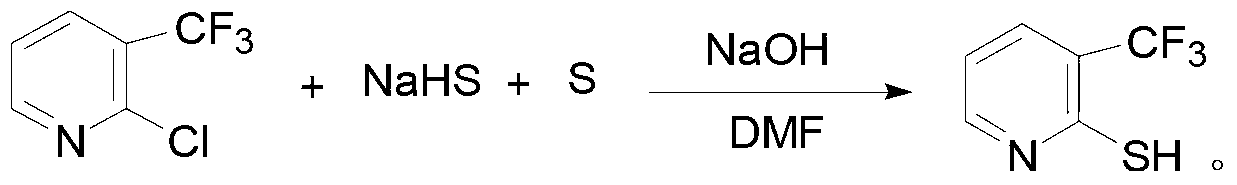

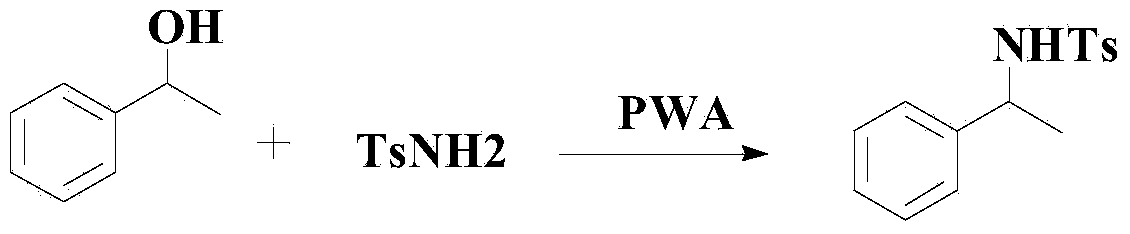
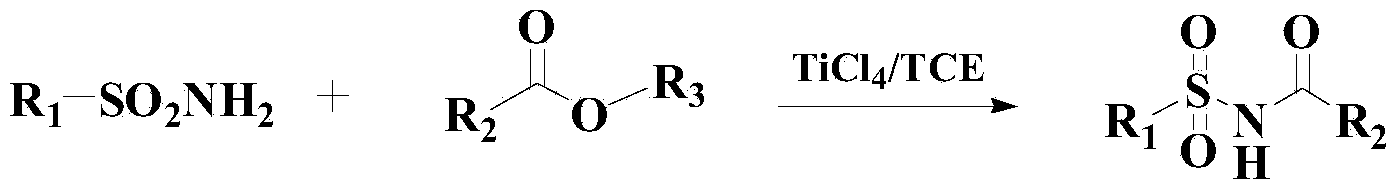
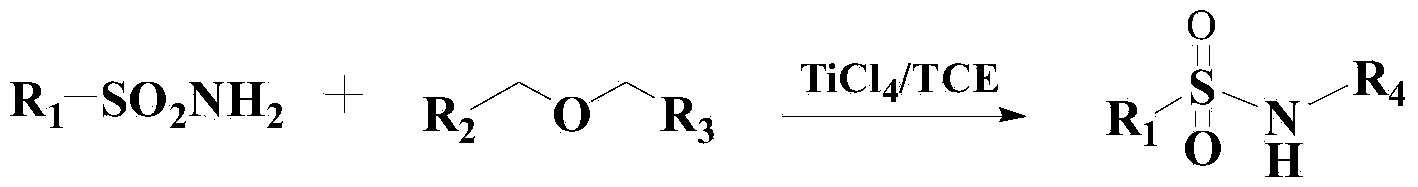
![Method for preparing N-[6-chloro-5-(2-methoxyphenoxy)[2,2'-dipyridine]-4-yl]-4-tertiary butyl-benzsulfamide Method for preparing N-[6-chloro-5-(2-methoxyphenoxy)[2,2'-dipyridine]-4-yl]-4-tertiary butyl-benzsulfamide](https://images-eureka.patsnap.com/patent_img/9c236e90-eddd-4a6a-b49b-7d3b7b97cd7a/710777DEST_PATH_IMAGE001.PNG)